User login
Chronic myeloid leukemia (CML) is a rare myeloproliferative neoplasm that is characterized by the presence of the Philadelphia (Ph) chromosome and uninhibited expansion of bone marrow stem cells. The Ph chromosome arises from a reciprocal translocation between the Abelson (ABL) region on chromosome 9 and the breakpoint cluster region (BCR) of chromosome 22 (t(9;22)(q34;q11.2), resulting in the BCR-ABL1 fusion gene.1BCR-ABL1 encodes an oncoprotein with constitutive tyrosine kinase activity that promotes growth and replication through downstream pathways, which is the driving factor in the pathogenesis of CML.1
Typical treatment for CML involves life-long use of oral BCR-ABL tyrosine kinase inhibitors (TKI). Currently, 5 TKIs have regulatory approval for treatment of this disease. With the introduction of imatinib in 2001 and the subsequent development of second- (dasatinib, nilotinib, bosutinib) and third-generation (ponatinib) TKIs, CML has become a chronic disease with a life-expectancy that is similar to that of the general population. This article reviews the diagnosis of CML and the parameters used for monitoring response to TKI therapy; the selection of initial TKI therapy is reviewed in a separate follow-up article.
Epidemiology
According to SEER data estimates, 8430 new cases of CML were diagnosed in the United States in 2018. CML is a disease of older adults, with a median age of 65 years at diagnosis, and there is a slight male predominance. Between 2011 and 2015, the number of new CML cases was 1.8 per 100,000 persons. The median overall survival (OS) in patients with newly diagnosed chronic-phase CML (CP-CML) has not been reached.2 Given the effective treatments available for managing CML, it is estimated that the prevalence of CML in the United States will plateau at 180,000 patients by 2050.3
Diagnosis
Case Presentation
A 53-year-old woman presents to her primary care physician with complaints of fatigue, early satiety, left upper quadrant abdominal pain, and an 8-lb unintentional weight loss over the prior month. Her past medical history is significant for uncontrolled diabetes, coronary artery disease requiring placement of 3 cardiac stents 2 years prior, and chronic obstructive pulmonary disease (COPD) related to a 30-pack-year history of smoking. On physicial exam her spleen is palpated 8 cm below the left costal margin. A complete blood count (CBC) with differential identifies a total white blood cell (WBC) count of 124,000/μL, with a left-shifted differential including 6% basophils, 3% eosinophils, and 3% blasts; hemoglobin is 12.4 g/dL and platelet count is 801 × 103/µL.
- How is the diagnosis of CML made?
Clinical Features
The diagnosis of CML is often suspected based on an incidental finding of leukocytosis and, in some cases, thrombocytosis. In many cases, this is an incidental finding on routine blood work, but approximately 50% of patients will present with constitutional symptoms associated with the disease. Characteristic features of the WBC differential include left-shifted maturation with neutrophilia and immature circulating myeloid cells. Basophilia and eosinophilia are often present as well. Splenomegaly is a common sign, present in 50% to 90% of patients at diagnosis. In those patients with symptoms related to CML at diagnosis, the most common presentation includes increasing fatigue, fevers, night sweats, early satiety, and weight loss. The diagnosis is confirmed by cytogenetic studies showing the Ph chromosome abnormality, t(9; 22)(q3.4;q1.1), and/or reverse transcriptase polymerase chain reaction (PCR) showing BCR-ABL1 transcripts.
- What further testing is needed when evaluating a patient for CML?
There are 3 distinct phases of CML: chronic phase (CP), accelerated phase (AP), and blast phase (BP). Bone marrow biopsy and aspiration at diagnosis are mandatory in order to determine the phase of the disease at diagnosis. This distinction is based on the percentage of blasts, promyelocytes, and basophils present as well as the platelet count and presence or absence of extramedullary disease.4 The vast majority of patients at diagnosis have CML that is in the chronic phase. The typical appearance in CP-CML is a hypercellular marrow with granulocytic and occasionally megakaryocytic hyperplasia. In many cases, basophilia and/or eosinophilia are noted as well. Dysplasia is not a typical finding in CML.5 Bone marrow fibrosis can be seen in up to one-third of patients at diagnosis, and may indicate a slightly worse prognosis.6 Although a diagnosis of CML can be made without a bone marrow biopsy, complete staging and prognostication are only possible with information gained from this test, including baseline karyotype and confirmation of CP versus a more advanced phase of CML.
The criteria for diagnosing AP-CML has not been agreed upon by various groups, but the modified MD Anderson Cancer Center (MDACC) criteria are used in the majority of clinical trials evaluating the efficacy of TKIs in preventing progression to advanced phases of CML. MDACC criteria define AP-CML as the presence of one of the following: 15% to 29% blasts in the peripheral blood or bone marrow, ≥ 30% peripheral blasts plus promyelocytes, ≥ 20% basophils in the blood or bone marrow, platelet count ≤ 100 × 103/μL unrelated to therapy, and clonal cytogenetic evolution in Ph-positive metaphases (Table).7
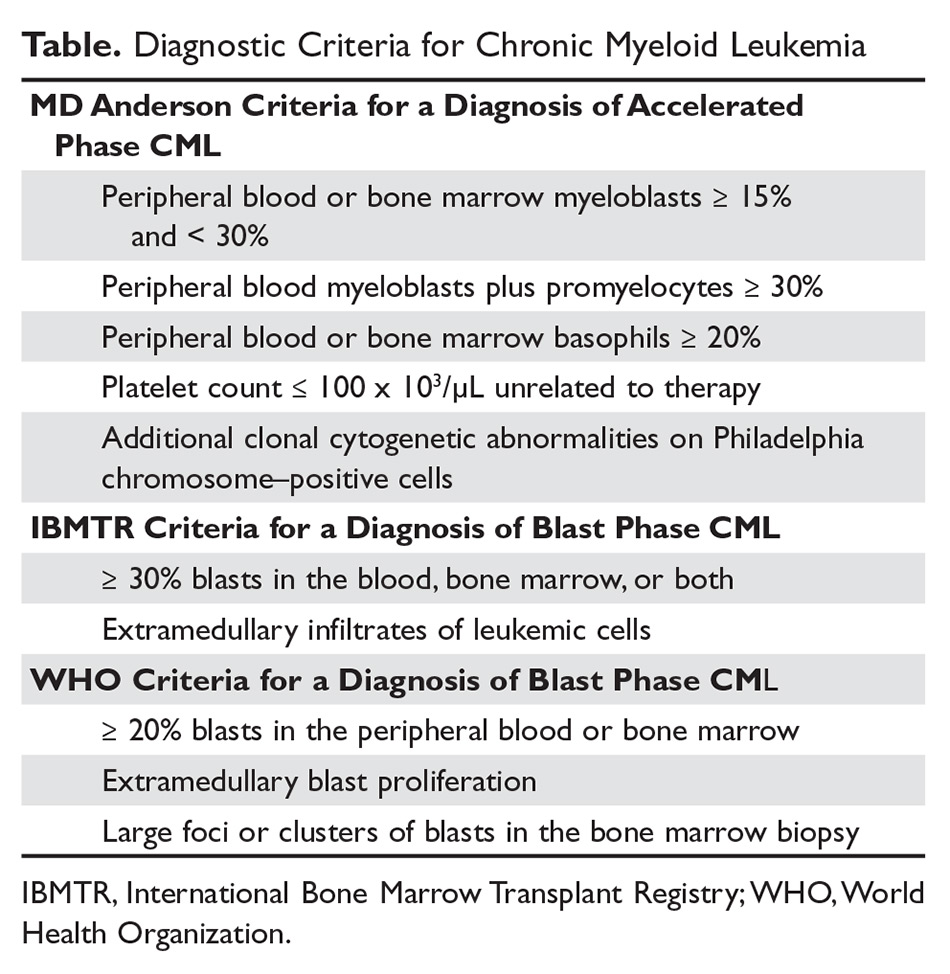
BP-CML is typically defined using the criteria developed by the International Bone Marrow Transplant Registry (IBMTR): ≥ 30% blasts in the peripheral blood and/or the bone marrow or the presence of extramedullary disease.8 Although not typically used in clinical trials, the revised World Health Organization (WHO) criteria for BP-CML include ≥ 20% blasts in the peripheral blood or bone marrow, extramedullary blast proliferation, and large foci or clusters of blasts in the bone marrow biopsy (Table).9 The defining feature of CML is the presence of the Ph chromosome abnormality. In a small subset of patients, additional chromosomal abnormalities (ACA) in the Ph-positive cells may be identified at diagnosis. Some reports indicate that the presence of “major route” ACA (trisomy 8, isochromosome 17q, a second Ph chromosome, or trisomy 19) at diagnosis may negatively impact prognosis, but other reports contradict these findings.10,11
The typical BCR breakpoint in CML is the major breakpoint cluster region (M-BCR), which results in a 210-kDa protein (p210). Alternate breakpoints that are less frequently identified are the minor BCR (mBCR or p190), which is more commonly found in Ph-positive acute lymphoblastic leukemia (ALL), and the micro BCR (µBCR or p230), which is much less common and is often characterized by chronic neutrophilia.12 Identifying which BCR-ABL1 transcript is present in each patient using qualitative PCR is crucial in order to ensure proper monitoring during treatment.
The most sensitive method for detecting BCR-ABL1 mRNA transcripts is the quantitative real-time PCR (RQ-PCR) assay, which is typically done on peripheral blood. RQ-PCR is capable of detecting a single CML cell in the presence of ≥ 100,000 normal cells. This test should be done during the initial diagnostic workup in order to confirm the presence of BCR-ABL1 transcripts, and it is used as a standard method for monitoring response to TKI therapy.13 The International Scale (IS) is a standardized approach to reporting RQ-PCR results that was developed to allow comparison of results across various laboratories and has become the gold standard for reporting BCR-ABL1 transcript values.14
Determining Risk Scores
Calculating a patient’s Sokal score or EURO risk score at diagnosis remains an important component of the diagnostic workup in CP-CML, as this information has prognostic and therapeutic implications (an online calculator is available through European LeukemiaNet [ELN]). The risk for disease progression to the accelerated or blast phases is higher in patients with intermediate- or high-risk scores compared to those with a low-risk score at diagnosis. The risk of progression in intermediate- or high-risk patients is lower when a second-generation TKI (dasatinib, nilotinib, or bosutinib) is used as frontline therapy compared to imatinib, and therefore, the National Comprehensive Cancer Network (NCCN) CML Panel recommends starting with a second-generation TKI in these patients.15-19
Monitoring Response to Therapy
Case Continued
Fluorescent in-situ hybridization using a peripheral blood sample to detect the BCR-ABL gene rearrangement is performed and is positive in 87% of cells. Bone marrow biopsy and aspiration show a 95% cellular bone marrow with granulocytic hyperplasia and 1% blasts. Cytogenetics are 46,XX,t(9;22)(q34;q11.2).20 RQ-PCR assay performed to measure BCR-ABL1 transcripts in the peripheral blood shows a value of 98% IS. The patient is ultimately given a diagnosis of CP-CML. Her Sokal risk score is 1.42, making her disease high risk.
How is response to TKI therapy measured and monitored?
After confirming a diagnosis of CML and selecting the most appropriate TKI for first-line therapy, the successful management of a CML patient relies on close monitoring and follow-up to ensure patients are meeting the desired treatment milestones. Responses in CML can be assessed based on hematologic parameters, cytogenetic results, and molecular responses. A complete hematologic response (CHR) implies complete normalization of peripheral blood counts (with the exception of TKI-induced cytopenias) and resolution of any palpable splenomegaly. The majority of patients will achieve a CHR within 4 to 6 weeks after initiating CML-directed therapy.21
Cytogenetic Response
Cytogenetic responses are defined by the decrease in the number of Ph chromosome–positive metaphases when assessed on bone marrow cytogenetics. A partial cytogenetic response (PCyR) is defined as having 1% to 35% Ph-positive metaphases, a major cytogenetic response (MCyR) as having 0% to 35% Ph-positive metaphases, and a CCyR implies that no Ph-positive metaphases are identified on bone marrow cytogenetics. An ideal response is the achievement of PCyR after 3 months on a TKI and a CCyR after 12 months on a TKI.22
Molecular Response
Once a patient has achieved a CCyR, monitoring their response to therapy can only be done using RQ-PCR to measure BCR-ABL1 transcripts in the peripheral blood. The NCCN and the ELN recommend monitoring RQ-PCR from the peripheral blood every 3 months in order to assess response to TKIs.19,23 As noted, the International Scale (IS) has become the gold standard reporting system for all BCR-ABL1 transcript levels in the majority of laboratories worldwide.14,24 Molecular responses are based on a log-reduction in BCR-ABL1 transcripts from a standardized baseline. Many molecular responses can be correlated with cytogenetic responses such that if reliable RQ-PCR testing is available, monitoring can be done using only peripheral blood RQ-PCR rather than repeat bone marrow biopsies. For example, an early molecular response (EMR) is defined as a RQ-PCR value of ≤ 10% IS, which is approximately equivalent to a PCyR.25 A value of 1% IS is approximately equivalent to CCyR. A major molecular response (MMR) is a ≥ 3-log reduction in BCR-ABL1 transcripts from baseline and is a value of ≤ 0.1% IS. Deeper levels of molecular response are best described by the log-reduction in BCR-ABL1 transcripts, with a 4-log reduction denoted as MR4.0, a 4.5-log reduction as MR4.5, and so forth. Complete molecular response (CMR) is defined by the level of sensitivity of the RQ-PCR assay being used.14
The definition of relapsed disease in CML is dependent on the type of response the patient had previously achieved. Relapse could be the loss of a hematologic or cytogenetic response, but fluctuations in BCR-ABL1 transcripts on routine RQ-PCR do not necessarily indicate relapsed CML. A 1-log increase in the level of BCR-ABL1 transcripts with a concurrent loss of MMR should prompt a bone marrow biopsy in order to assess for the loss of CCyR, and thus a cytogenetic relapse; however, this loss of MMR does not define relapse in and of itself. In the setting of relapsed disease, testing should be done to look for possible ABL kinase domain mutations, and alternate therapy should be selected.19
Multiple reports have identified the prognostic relevance of achieving an EMR at 3 and 6 months after starting TKI therapy. Marin and colleagues reported that in 282 imatinib-treated patients, there was a significant improvement in 8-year OS, progression-free survival, and cumulative incidence of CCyR and CMR in patients who had BCR-ABL1 transcripts < 9.84% IS after 3 months on treatment.25 This data highlights the importance of early molecular monitoring in order to ensure the best outcomes for patients with CP-CML.
The NCCN CML guidelines and ELN recommendations both agree that an ideal response after 3 months on a TKI is BCR-ABL1 transcripts < 10% IS, but treatment is not considered to be failing at this point if the patient marginally misses this milestone. After 6 months on treatment, an ideal response is considered BCR-ABL1 transcripts < 1%–10% IS. Ideally, patients will have BCR-ABL1 transcripts < 0.1%–1% IS by the time they complete 12 months of TKI therapy, suggesting that these patients have at least achieved a CCyR.19,23 Even after patients achieve these early milestones, frequent monitoring by RQ-PCR is required to ensure that they are maintaining their response to treatment. This will help to ensure patient compliance with treatment and will also help to identify a select subset of patients who could potentially be considered for an attempt at TKI cessation (not discussed in detail here) after a minimum of 3 years on therapy.19,26
Conclusion
Given the successful treatments available for patients with CML, it is crucial to identify patients with this disease, ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing, and select the best therapy for each individual patient. Once on treatment, the importance of frequent monitoring cannot be overstated.
1. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
2. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Leukemia - Chronic Myeloid Leukemia (CML). 2018.
3. Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123-3127.
4. Savage DG, Szydlo RM, Chase A, et al. Bone marrow transplantation for chronic myeloid leukaemia: the effects of differing criteria for defining chronic phase on probabilities of survival and relapse. Br J Haematol. 1997;99:30-35.
5. Knox WF, Bhavnani M, Davson J, Geary CG. Histological classification of chronic granulocytic leukaemia. Clin Lab Haematol. 1984;6:171-175.
6. Kvasnicka HM, Thiele J, Schmitt-Graeff A, et al. Impact of bone marrow morphology on multivariate risk classification in chronic myelogenous leukemia. Acta Haematol. 2003;109:53-56.
7. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
8. Druker BJ. Chronic myeloid leukemia In: DeVita VT, Lawrence TS, Rosenburg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007:2267-2304.
9. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
10. Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760-6768.
11. Alhuraiji A, Kantarjian H, Boddu P, et al. Prognostic significance of additional chromosomal abnormalities at the time of diagnosis in patients with chronic myeloid leukemia treated with frontline tyrosine kinase inhibitors. Am J Hematol. 2018;93:84-90.
12. Melo JV. BCR-ABL gene variants. Baillieres Clin Haematol. 1997;10:203-222.
13. Kantarjian HM, Talpaz M, Cortes J, et al. Quantitative polymerase chain reaction monitoring of BCR-ABL during therapy with imatinib mesylate (STI571; gleevec) in chronic-phase chronic myelogenous leukemia. Clin Cancer Res. 2003;9:160-166.
14. Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28-37.
15. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917-927.
16. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333-3340.
17. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044-1054.
18. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231-237.
19. Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108-1135.
20. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
21. Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207-219.
22. O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
23. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-884.
24. Larripa I, Ruiz MS, Gutierrez M, Bianchini M. [Guidelines for molecular monitoring of BCR-ABL1 in chronic myeloid leukemia patients by RT-qPCR.] Medicina (B Aires). 2017;77:61-72.
25. Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232-238.
26. Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17-23.
Chronic myeloid leukemia (CML) is a rare myeloproliferative neoplasm that is characterized by the presence of the Philadelphia (Ph) chromosome and uninhibited expansion of bone marrow stem cells. The Ph chromosome arises from a reciprocal translocation between the Abelson (ABL) region on chromosome 9 and the breakpoint cluster region (BCR) of chromosome 22 (t(9;22)(q34;q11.2), resulting in the BCR-ABL1 fusion gene.1BCR-ABL1 encodes an oncoprotein with constitutive tyrosine kinase activity that promotes growth and replication through downstream pathways, which is the driving factor in the pathogenesis of CML.1
Typical treatment for CML involves life-long use of oral BCR-ABL tyrosine kinase inhibitors (TKI). Currently, 5 TKIs have regulatory approval for treatment of this disease. With the introduction of imatinib in 2001 and the subsequent development of second- (dasatinib, nilotinib, bosutinib) and third-generation (ponatinib) TKIs, CML has become a chronic disease with a life-expectancy that is similar to that of the general population. This article reviews the diagnosis of CML and the parameters used for monitoring response to TKI therapy; the selection of initial TKI therapy is reviewed in a separate follow-up article.
Epidemiology
According to SEER data estimates, 8430 new cases of CML were diagnosed in the United States in 2018. CML is a disease of older adults, with a median age of 65 years at diagnosis, and there is a slight male predominance. Between 2011 and 2015, the number of new CML cases was 1.8 per 100,000 persons. The median overall survival (OS) in patients with newly diagnosed chronic-phase CML (CP-CML) has not been reached.2 Given the effective treatments available for managing CML, it is estimated that the prevalence of CML in the United States will plateau at 180,000 patients by 2050.3
Diagnosis
Case Presentation
A 53-year-old woman presents to her primary care physician with complaints of fatigue, early satiety, left upper quadrant abdominal pain, and an 8-lb unintentional weight loss over the prior month. Her past medical history is significant for uncontrolled diabetes, coronary artery disease requiring placement of 3 cardiac stents 2 years prior, and chronic obstructive pulmonary disease (COPD) related to a 30-pack-year history of smoking. On physicial exam her spleen is palpated 8 cm below the left costal margin. A complete blood count (CBC) with differential identifies a total white blood cell (WBC) count of 124,000/μL, with a left-shifted differential including 6% basophils, 3% eosinophils, and 3% blasts; hemoglobin is 12.4 g/dL and platelet count is 801 × 103/µL.
- How is the diagnosis of CML made?
Clinical Features
The diagnosis of CML is often suspected based on an incidental finding of leukocytosis and, in some cases, thrombocytosis. In many cases, this is an incidental finding on routine blood work, but approximately 50% of patients will present with constitutional symptoms associated with the disease. Characteristic features of the WBC differential include left-shifted maturation with neutrophilia and immature circulating myeloid cells. Basophilia and eosinophilia are often present as well. Splenomegaly is a common sign, present in 50% to 90% of patients at diagnosis. In those patients with symptoms related to CML at diagnosis, the most common presentation includes increasing fatigue, fevers, night sweats, early satiety, and weight loss. The diagnosis is confirmed by cytogenetic studies showing the Ph chromosome abnormality, t(9; 22)(q3.4;q1.1), and/or reverse transcriptase polymerase chain reaction (PCR) showing BCR-ABL1 transcripts.
- What further testing is needed when evaluating a patient for CML?
There are 3 distinct phases of CML: chronic phase (CP), accelerated phase (AP), and blast phase (BP). Bone marrow biopsy and aspiration at diagnosis are mandatory in order to determine the phase of the disease at diagnosis. This distinction is based on the percentage of blasts, promyelocytes, and basophils present as well as the platelet count and presence or absence of extramedullary disease.4 The vast majority of patients at diagnosis have CML that is in the chronic phase. The typical appearance in CP-CML is a hypercellular marrow with granulocytic and occasionally megakaryocytic hyperplasia. In many cases, basophilia and/or eosinophilia are noted as well. Dysplasia is not a typical finding in CML.5 Bone marrow fibrosis can be seen in up to one-third of patients at diagnosis, and may indicate a slightly worse prognosis.6 Although a diagnosis of CML can be made without a bone marrow biopsy, complete staging and prognostication are only possible with information gained from this test, including baseline karyotype and confirmation of CP versus a more advanced phase of CML.
The criteria for diagnosing AP-CML has not been agreed upon by various groups, but the modified MD Anderson Cancer Center (MDACC) criteria are used in the majority of clinical trials evaluating the efficacy of TKIs in preventing progression to advanced phases of CML. MDACC criteria define AP-CML as the presence of one of the following: 15% to 29% blasts in the peripheral blood or bone marrow, ≥ 30% peripheral blasts plus promyelocytes, ≥ 20% basophils in the blood or bone marrow, platelet count ≤ 100 × 103/μL unrelated to therapy, and clonal cytogenetic evolution in Ph-positive metaphases (Table).7

BP-CML is typically defined using the criteria developed by the International Bone Marrow Transplant Registry (IBMTR): ≥ 30% blasts in the peripheral blood and/or the bone marrow or the presence of extramedullary disease.8 Although not typically used in clinical trials, the revised World Health Organization (WHO) criteria for BP-CML include ≥ 20% blasts in the peripheral blood or bone marrow, extramedullary blast proliferation, and large foci or clusters of blasts in the bone marrow biopsy (Table).9 The defining feature of CML is the presence of the Ph chromosome abnormality. In a small subset of patients, additional chromosomal abnormalities (ACA) in the Ph-positive cells may be identified at diagnosis. Some reports indicate that the presence of “major route” ACA (trisomy 8, isochromosome 17q, a second Ph chromosome, or trisomy 19) at diagnosis may negatively impact prognosis, but other reports contradict these findings.10,11
The typical BCR breakpoint in CML is the major breakpoint cluster region (M-BCR), which results in a 210-kDa protein (p210). Alternate breakpoints that are less frequently identified are the minor BCR (mBCR or p190), which is more commonly found in Ph-positive acute lymphoblastic leukemia (ALL), and the micro BCR (µBCR or p230), which is much less common and is often characterized by chronic neutrophilia.12 Identifying which BCR-ABL1 transcript is present in each patient using qualitative PCR is crucial in order to ensure proper monitoring during treatment.
The most sensitive method for detecting BCR-ABL1 mRNA transcripts is the quantitative real-time PCR (RQ-PCR) assay, which is typically done on peripheral blood. RQ-PCR is capable of detecting a single CML cell in the presence of ≥ 100,000 normal cells. This test should be done during the initial diagnostic workup in order to confirm the presence of BCR-ABL1 transcripts, and it is used as a standard method for monitoring response to TKI therapy.13 The International Scale (IS) is a standardized approach to reporting RQ-PCR results that was developed to allow comparison of results across various laboratories and has become the gold standard for reporting BCR-ABL1 transcript values.14
Determining Risk Scores
Calculating a patient’s Sokal score or EURO risk score at diagnosis remains an important component of the diagnostic workup in CP-CML, as this information has prognostic and therapeutic implications (an online calculator is available through European LeukemiaNet [ELN]). The risk for disease progression to the accelerated or blast phases is higher in patients with intermediate- or high-risk scores compared to those with a low-risk score at diagnosis. The risk of progression in intermediate- or high-risk patients is lower when a second-generation TKI (dasatinib, nilotinib, or bosutinib) is used as frontline therapy compared to imatinib, and therefore, the National Comprehensive Cancer Network (NCCN) CML Panel recommends starting with a second-generation TKI in these patients.15-19
Monitoring Response to Therapy
Case Continued
Fluorescent in-situ hybridization using a peripheral blood sample to detect the BCR-ABL gene rearrangement is performed and is positive in 87% of cells. Bone marrow biopsy and aspiration show a 95% cellular bone marrow with granulocytic hyperplasia and 1% blasts. Cytogenetics are 46,XX,t(9;22)(q34;q11.2).20 RQ-PCR assay performed to measure BCR-ABL1 transcripts in the peripheral blood shows a value of 98% IS. The patient is ultimately given a diagnosis of CP-CML. Her Sokal risk score is 1.42, making her disease high risk.
How is response to TKI therapy measured and monitored?
After confirming a diagnosis of CML and selecting the most appropriate TKI for first-line therapy, the successful management of a CML patient relies on close monitoring and follow-up to ensure patients are meeting the desired treatment milestones. Responses in CML can be assessed based on hematologic parameters, cytogenetic results, and molecular responses. A complete hematologic response (CHR) implies complete normalization of peripheral blood counts (with the exception of TKI-induced cytopenias) and resolution of any palpable splenomegaly. The majority of patients will achieve a CHR within 4 to 6 weeks after initiating CML-directed therapy.21
Cytogenetic Response
Cytogenetic responses are defined by the decrease in the number of Ph chromosome–positive metaphases when assessed on bone marrow cytogenetics. A partial cytogenetic response (PCyR) is defined as having 1% to 35% Ph-positive metaphases, a major cytogenetic response (MCyR) as having 0% to 35% Ph-positive metaphases, and a CCyR implies that no Ph-positive metaphases are identified on bone marrow cytogenetics. An ideal response is the achievement of PCyR after 3 months on a TKI and a CCyR after 12 months on a TKI.22
Molecular Response
Once a patient has achieved a CCyR, monitoring their response to therapy can only be done using RQ-PCR to measure BCR-ABL1 transcripts in the peripheral blood. The NCCN and the ELN recommend monitoring RQ-PCR from the peripheral blood every 3 months in order to assess response to TKIs.19,23 As noted, the International Scale (IS) has become the gold standard reporting system for all BCR-ABL1 transcript levels in the majority of laboratories worldwide.14,24 Molecular responses are based on a log-reduction in BCR-ABL1 transcripts from a standardized baseline. Many molecular responses can be correlated with cytogenetic responses such that if reliable RQ-PCR testing is available, monitoring can be done using only peripheral blood RQ-PCR rather than repeat bone marrow biopsies. For example, an early molecular response (EMR) is defined as a RQ-PCR value of ≤ 10% IS, which is approximately equivalent to a PCyR.25 A value of 1% IS is approximately equivalent to CCyR. A major molecular response (MMR) is a ≥ 3-log reduction in BCR-ABL1 transcripts from baseline and is a value of ≤ 0.1% IS. Deeper levels of molecular response are best described by the log-reduction in BCR-ABL1 transcripts, with a 4-log reduction denoted as MR4.0, a 4.5-log reduction as MR4.5, and so forth. Complete molecular response (CMR) is defined by the level of sensitivity of the RQ-PCR assay being used.14
The definition of relapsed disease in CML is dependent on the type of response the patient had previously achieved. Relapse could be the loss of a hematologic or cytogenetic response, but fluctuations in BCR-ABL1 transcripts on routine RQ-PCR do not necessarily indicate relapsed CML. A 1-log increase in the level of BCR-ABL1 transcripts with a concurrent loss of MMR should prompt a bone marrow biopsy in order to assess for the loss of CCyR, and thus a cytogenetic relapse; however, this loss of MMR does not define relapse in and of itself. In the setting of relapsed disease, testing should be done to look for possible ABL kinase domain mutations, and alternate therapy should be selected.19
Multiple reports have identified the prognostic relevance of achieving an EMR at 3 and 6 months after starting TKI therapy. Marin and colleagues reported that in 282 imatinib-treated patients, there was a significant improvement in 8-year OS, progression-free survival, and cumulative incidence of CCyR and CMR in patients who had BCR-ABL1 transcripts < 9.84% IS after 3 months on treatment.25 This data highlights the importance of early molecular monitoring in order to ensure the best outcomes for patients with CP-CML.
The NCCN CML guidelines and ELN recommendations both agree that an ideal response after 3 months on a TKI is BCR-ABL1 transcripts < 10% IS, but treatment is not considered to be failing at this point if the patient marginally misses this milestone. After 6 months on treatment, an ideal response is considered BCR-ABL1 transcripts < 1%–10% IS. Ideally, patients will have BCR-ABL1 transcripts < 0.1%–1% IS by the time they complete 12 months of TKI therapy, suggesting that these patients have at least achieved a CCyR.19,23 Even after patients achieve these early milestones, frequent monitoring by RQ-PCR is required to ensure that they are maintaining their response to treatment. This will help to ensure patient compliance with treatment and will also help to identify a select subset of patients who could potentially be considered for an attempt at TKI cessation (not discussed in detail here) after a minimum of 3 years on therapy.19,26
Conclusion
Given the successful treatments available for patients with CML, it is crucial to identify patients with this disease, ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing, and select the best therapy for each individual patient. Once on treatment, the importance of frequent monitoring cannot be overstated.
Chronic myeloid leukemia (CML) is a rare myeloproliferative neoplasm that is characterized by the presence of the Philadelphia (Ph) chromosome and uninhibited expansion of bone marrow stem cells. The Ph chromosome arises from a reciprocal translocation between the Abelson (ABL) region on chromosome 9 and the breakpoint cluster region (BCR) of chromosome 22 (t(9;22)(q34;q11.2), resulting in the BCR-ABL1 fusion gene.1BCR-ABL1 encodes an oncoprotein with constitutive tyrosine kinase activity that promotes growth and replication through downstream pathways, which is the driving factor in the pathogenesis of CML.1
Typical treatment for CML involves life-long use of oral BCR-ABL tyrosine kinase inhibitors (TKI). Currently, 5 TKIs have regulatory approval for treatment of this disease. With the introduction of imatinib in 2001 and the subsequent development of second- (dasatinib, nilotinib, bosutinib) and third-generation (ponatinib) TKIs, CML has become a chronic disease with a life-expectancy that is similar to that of the general population. This article reviews the diagnosis of CML and the parameters used for monitoring response to TKI therapy; the selection of initial TKI therapy is reviewed in a separate follow-up article.
Epidemiology
According to SEER data estimates, 8430 new cases of CML were diagnosed in the United States in 2018. CML is a disease of older adults, with a median age of 65 years at diagnosis, and there is a slight male predominance. Between 2011 and 2015, the number of new CML cases was 1.8 per 100,000 persons. The median overall survival (OS) in patients with newly diagnosed chronic-phase CML (CP-CML) has not been reached.2 Given the effective treatments available for managing CML, it is estimated that the prevalence of CML in the United States will plateau at 180,000 patients by 2050.3
Diagnosis
Case Presentation
A 53-year-old woman presents to her primary care physician with complaints of fatigue, early satiety, left upper quadrant abdominal pain, and an 8-lb unintentional weight loss over the prior month. Her past medical history is significant for uncontrolled diabetes, coronary artery disease requiring placement of 3 cardiac stents 2 years prior, and chronic obstructive pulmonary disease (COPD) related to a 30-pack-year history of smoking. On physicial exam her spleen is palpated 8 cm below the left costal margin. A complete blood count (CBC) with differential identifies a total white blood cell (WBC) count of 124,000/μL, with a left-shifted differential including 6% basophils, 3% eosinophils, and 3% blasts; hemoglobin is 12.4 g/dL and platelet count is 801 × 103/µL.
- How is the diagnosis of CML made?
Clinical Features
The diagnosis of CML is often suspected based on an incidental finding of leukocytosis and, in some cases, thrombocytosis. In many cases, this is an incidental finding on routine blood work, but approximately 50% of patients will present with constitutional symptoms associated with the disease. Characteristic features of the WBC differential include left-shifted maturation with neutrophilia and immature circulating myeloid cells. Basophilia and eosinophilia are often present as well. Splenomegaly is a common sign, present in 50% to 90% of patients at diagnosis. In those patients with symptoms related to CML at diagnosis, the most common presentation includes increasing fatigue, fevers, night sweats, early satiety, and weight loss. The diagnosis is confirmed by cytogenetic studies showing the Ph chromosome abnormality, t(9; 22)(q3.4;q1.1), and/or reverse transcriptase polymerase chain reaction (PCR) showing BCR-ABL1 transcripts.
- What further testing is needed when evaluating a patient for CML?
There are 3 distinct phases of CML: chronic phase (CP), accelerated phase (AP), and blast phase (BP). Bone marrow biopsy and aspiration at diagnosis are mandatory in order to determine the phase of the disease at diagnosis. This distinction is based on the percentage of blasts, promyelocytes, and basophils present as well as the platelet count and presence or absence of extramedullary disease.4 The vast majority of patients at diagnosis have CML that is in the chronic phase. The typical appearance in CP-CML is a hypercellular marrow with granulocytic and occasionally megakaryocytic hyperplasia. In many cases, basophilia and/or eosinophilia are noted as well. Dysplasia is not a typical finding in CML.5 Bone marrow fibrosis can be seen in up to one-third of patients at diagnosis, and may indicate a slightly worse prognosis.6 Although a diagnosis of CML can be made without a bone marrow biopsy, complete staging and prognostication are only possible with information gained from this test, including baseline karyotype and confirmation of CP versus a more advanced phase of CML.
The criteria for diagnosing AP-CML has not been agreed upon by various groups, but the modified MD Anderson Cancer Center (MDACC) criteria are used in the majority of clinical trials evaluating the efficacy of TKIs in preventing progression to advanced phases of CML. MDACC criteria define AP-CML as the presence of one of the following: 15% to 29% blasts in the peripheral blood or bone marrow, ≥ 30% peripheral blasts plus promyelocytes, ≥ 20% basophils in the blood or bone marrow, platelet count ≤ 100 × 103/μL unrelated to therapy, and clonal cytogenetic evolution in Ph-positive metaphases (Table).7

BP-CML is typically defined using the criteria developed by the International Bone Marrow Transplant Registry (IBMTR): ≥ 30% blasts in the peripheral blood and/or the bone marrow or the presence of extramedullary disease.8 Although not typically used in clinical trials, the revised World Health Organization (WHO) criteria for BP-CML include ≥ 20% blasts in the peripheral blood or bone marrow, extramedullary blast proliferation, and large foci or clusters of blasts in the bone marrow biopsy (Table).9 The defining feature of CML is the presence of the Ph chromosome abnormality. In a small subset of patients, additional chromosomal abnormalities (ACA) in the Ph-positive cells may be identified at diagnosis. Some reports indicate that the presence of “major route” ACA (trisomy 8, isochromosome 17q, a second Ph chromosome, or trisomy 19) at diagnosis may negatively impact prognosis, but other reports contradict these findings.10,11
The typical BCR breakpoint in CML is the major breakpoint cluster region (M-BCR), which results in a 210-kDa protein (p210). Alternate breakpoints that are less frequently identified are the minor BCR (mBCR or p190), which is more commonly found in Ph-positive acute lymphoblastic leukemia (ALL), and the micro BCR (µBCR or p230), which is much less common and is often characterized by chronic neutrophilia.12 Identifying which BCR-ABL1 transcript is present in each patient using qualitative PCR is crucial in order to ensure proper monitoring during treatment.
The most sensitive method for detecting BCR-ABL1 mRNA transcripts is the quantitative real-time PCR (RQ-PCR) assay, which is typically done on peripheral blood. RQ-PCR is capable of detecting a single CML cell in the presence of ≥ 100,000 normal cells. This test should be done during the initial diagnostic workup in order to confirm the presence of BCR-ABL1 transcripts, and it is used as a standard method for monitoring response to TKI therapy.13 The International Scale (IS) is a standardized approach to reporting RQ-PCR results that was developed to allow comparison of results across various laboratories and has become the gold standard for reporting BCR-ABL1 transcript values.14
Determining Risk Scores
Calculating a patient’s Sokal score or EURO risk score at diagnosis remains an important component of the diagnostic workup in CP-CML, as this information has prognostic and therapeutic implications (an online calculator is available through European LeukemiaNet [ELN]). The risk for disease progression to the accelerated or blast phases is higher in patients with intermediate- or high-risk scores compared to those with a low-risk score at diagnosis. The risk of progression in intermediate- or high-risk patients is lower when a second-generation TKI (dasatinib, nilotinib, or bosutinib) is used as frontline therapy compared to imatinib, and therefore, the National Comprehensive Cancer Network (NCCN) CML Panel recommends starting with a second-generation TKI in these patients.15-19
Monitoring Response to Therapy
Case Continued
Fluorescent in-situ hybridization using a peripheral blood sample to detect the BCR-ABL gene rearrangement is performed and is positive in 87% of cells. Bone marrow biopsy and aspiration show a 95% cellular bone marrow with granulocytic hyperplasia and 1% blasts. Cytogenetics are 46,XX,t(9;22)(q34;q11.2).20 RQ-PCR assay performed to measure BCR-ABL1 transcripts in the peripheral blood shows a value of 98% IS. The patient is ultimately given a diagnosis of CP-CML. Her Sokal risk score is 1.42, making her disease high risk.
How is response to TKI therapy measured and monitored?
After confirming a diagnosis of CML and selecting the most appropriate TKI for first-line therapy, the successful management of a CML patient relies on close monitoring and follow-up to ensure patients are meeting the desired treatment milestones. Responses in CML can be assessed based on hematologic parameters, cytogenetic results, and molecular responses. A complete hematologic response (CHR) implies complete normalization of peripheral blood counts (with the exception of TKI-induced cytopenias) and resolution of any palpable splenomegaly. The majority of patients will achieve a CHR within 4 to 6 weeks after initiating CML-directed therapy.21
Cytogenetic Response
Cytogenetic responses are defined by the decrease in the number of Ph chromosome–positive metaphases when assessed on bone marrow cytogenetics. A partial cytogenetic response (PCyR) is defined as having 1% to 35% Ph-positive metaphases, a major cytogenetic response (MCyR) as having 0% to 35% Ph-positive metaphases, and a CCyR implies that no Ph-positive metaphases are identified on bone marrow cytogenetics. An ideal response is the achievement of PCyR after 3 months on a TKI and a CCyR after 12 months on a TKI.22
Molecular Response
Once a patient has achieved a CCyR, monitoring their response to therapy can only be done using RQ-PCR to measure BCR-ABL1 transcripts in the peripheral blood. The NCCN and the ELN recommend monitoring RQ-PCR from the peripheral blood every 3 months in order to assess response to TKIs.19,23 As noted, the International Scale (IS) has become the gold standard reporting system for all BCR-ABL1 transcript levels in the majority of laboratories worldwide.14,24 Molecular responses are based on a log-reduction in BCR-ABL1 transcripts from a standardized baseline. Many molecular responses can be correlated with cytogenetic responses such that if reliable RQ-PCR testing is available, monitoring can be done using only peripheral blood RQ-PCR rather than repeat bone marrow biopsies. For example, an early molecular response (EMR) is defined as a RQ-PCR value of ≤ 10% IS, which is approximately equivalent to a PCyR.25 A value of 1% IS is approximately equivalent to CCyR. A major molecular response (MMR) is a ≥ 3-log reduction in BCR-ABL1 transcripts from baseline and is a value of ≤ 0.1% IS. Deeper levels of molecular response are best described by the log-reduction in BCR-ABL1 transcripts, with a 4-log reduction denoted as MR4.0, a 4.5-log reduction as MR4.5, and so forth. Complete molecular response (CMR) is defined by the level of sensitivity of the RQ-PCR assay being used.14
The definition of relapsed disease in CML is dependent on the type of response the patient had previously achieved. Relapse could be the loss of a hematologic or cytogenetic response, but fluctuations in BCR-ABL1 transcripts on routine RQ-PCR do not necessarily indicate relapsed CML. A 1-log increase in the level of BCR-ABL1 transcripts with a concurrent loss of MMR should prompt a bone marrow biopsy in order to assess for the loss of CCyR, and thus a cytogenetic relapse; however, this loss of MMR does not define relapse in and of itself. In the setting of relapsed disease, testing should be done to look for possible ABL kinase domain mutations, and alternate therapy should be selected.19
Multiple reports have identified the prognostic relevance of achieving an EMR at 3 and 6 months after starting TKI therapy. Marin and colleagues reported that in 282 imatinib-treated patients, there was a significant improvement in 8-year OS, progression-free survival, and cumulative incidence of CCyR and CMR in patients who had BCR-ABL1 transcripts < 9.84% IS after 3 months on treatment.25 This data highlights the importance of early molecular monitoring in order to ensure the best outcomes for patients with CP-CML.
The NCCN CML guidelines and ELN recommendations both agree that an ideal response after 3 months on a TKI is BCR-ABL1 transcripts < 10% IS, but treatment is not considered to be failing at this point if the patient marginally misses this milestone. After 6 months on treatment, an ideal response is considered BCR-ABL1 transcripts < 1%–10% IS. Ideally, patients will have BCR-ABL1 transcripts < 0.1%–1% IS by the time they complete 12 months of TKI therapy, suggesting that these patients have at least achieved a CCyR.19,23 Even after patients achieve these early milestones, frequent monitoring by RQ-PCR is required to ensure that they are maintaining their response to treatment. This will help to ensure patient compliance with treatment and will also help to identify a select subset of patients who could potentially be considered for an attempt at TKI cessation (not discussed in detail here) after a minimum of 3 years on therapy.19,26
Conclusion
Given the successful treatments available for patients with CML, it is crucial to identify patients with this disease, ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing, and select the best therapy for each individual patient. Once on treatment, the importance of frequent monitoring cannot be overstated.
1. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
2. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Leukemia - Chronic Myeloid Leukemia (CML). 2018.
3. Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123-3127.
4. Savage DG, Szydlo RM, Chase A, et al. Bone marrow transplantation for chronic myeloid leukaemia: the effects of differing criteria for defining chronic phase on probabilities of survival and relapse. Br J Haematol. 1997;99:30-35.
5. Knox WF, Bhavnani M, Davson J, Geary CG. Histological classification of chronic granulocytic leukaemia. Clin Lab Haematol. 1984;6:171-175.
6. Kvasnicka HM, Thiele J, Schmitt-Graeff A, et al. Impact of bone marrow morphology on multivariate risk classification in chronic myelogenous leukemia. Acta Haematol. 2003;109:53-56.
7. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
8. Druker BJ. Chronic myeloid leukemia In: DeVita VT, Lawrence TS, Rosenburg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007:2267-2304.
9. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
10. Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760-6768.
11. Alhuraiji A, Kantarjian H, Boddu P, et al. Prognostic significance of additional chromosomal abnormalities at the time of diagnosis in patients with chronic myeloid leukemia treated with frontline tyrosine kinase inhibitors. Am J Hematol. 2018;93:84-90.
12. Melo JV. BCR-ABL gene variants. Baillieres Clin Haematol. 1997;10:203-222.
13. Kantarjian HM, Talpaz M, Cortes J, et al. Quantitative polymerase chain reaction monitoring of BCR-ABL during therapy with imatinib mesylate (STI571; gleevec) in chronic-phase chronic myelogenous leukemia. Clin Cancer Res. 2003;9:160-166.
14. Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28-37.
15. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917-927.
16. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333-3340.
17. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044-1054.
18. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231-237.
19. Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108-1135.
20. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
21. Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207-219.
22. O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
23. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-884.
24. Larripa I, Ruiz MS, Gutierrez M, Bianchini M. [Guidelines for molecular monitoring of BCR-ABL1 in chronic myeloid leukemia patients by RT-qPCR.] Medicina (B Aires). 2017;77:61-72.
25. Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232-238.
26. Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17-23.
1. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
2. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Leukemia - Chronic Myeloid Leukemia (CML). 2018.
3. Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123-3127.
4. Savage DG, Szydlo RM, Chase A, et al. Bone marrow transplantation for chronic myeloid leukaemia: the effects of differing criteria for defining chronic phase on probabilities of survival and relapse. Br J Haematol. 1997;99:30-35.
5. Knox WF, Bhavnani M, Davson J, Geary CG. Histological classification of chronic granulocytic leukaemia. Clin Lab Haematol. 1984;6:171-175.
6. Kvasnicka HM, Thiele J, Schmitt-Graeff A, et al. Impact of bone marrow morphology on multivariate risk classification in chronic myelogenous leukemia. Acta Haematol. 2003;109:53-56.
7. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
8. Druker BJ. Chronic myeloid leukemia In: DeVita VT, Lawrence TS, Rosenburg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007:2267-2304.
9. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
10. Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760-6768.
11. Alhuraiji A, Kantarjian H, Boddu P, et al. Prognostic significance of additional chromosomal abnormalities at the time of diagnosis in patients with chronic myeloid leukemia treated with frontline tyrosine kinase inhibitors. Am J Hematol. 2018;93:84-90.
12. Melo JV. BCR-ABL gene variants. Baillieres Clin Haematol. 1997;10:203-222.
13. Kantarjian HM, Talpaz M, Cortes J, et al. Quantitative polymerase chain reaction monitoring of BCR-ABL during therapy with imatinib mesylate (STI571; gleevec) in chronic-phase chronic myelogenous leukemia. Clin Cancer Res. 2003;9:160-166.
14. Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28-37.
15. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917-927.
16. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333-3340.
17. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044-1054.
18. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231-237.
19. Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108-1135.
20. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
21. Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207-219.
22. O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
23. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-884.
24. Larripa I, Ruiz MS, Gutierrez M, Bianchini M. [Guidelines for molecular monitoring of BCR-ABL1 in chronic myeloid leukemia patients by RT-qPCR.] Medicina (B Aires). 2017;77:61-72.
25. Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232-238.
26. Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17-23.