User login
The burden of chronic liver disease (CLD) in the United States is growing, and it is currently the fourth leading cause of death in adults aged 45 to 64 years.1 From 2012 to 2016, there were 538,720 hospitalizations in the United States for patients with cirrhosis, with almost a quarter having at least one cirrhosis-related complication. Inpatient hospitalizations for cirrhosis contribute to healthcare resource utilization, with a mean cost per CLD-related hospitalization of $16,271, and the presence of cirrhosis results in higher mortality and cost burden.1
In hospitalized patients with decompensated cirrhosis with ascites, intravenous human albumin (HA) infusion has been utilized for decades for a variety of indications. Current guidance by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommends the use of albumin for the prevention of paracentesis-induced circulatory dysfunction (PICD) for the prevention of kidney injury in spontaneous bacterial peritonitis (SBP) and for the diagnosis and treatment of hepatorenal syndrome (HRS).2,3 There have been several major trials in recent years studying the use of HA for other indications in patients with cirrhosis, and the Society of Critical Care Medicine (SCCM) updated their guidelines in 2020 to recommend HA administration in resuscitation of critically ill patients with liver failure with hypoalbuminemia.4This Clinical Progress Note addresses the use of albumin in hospitalized patients with cirrhosis, focusing on current indications and discussing potential uses published after the 2018 EASL guidelines. We conducted a literature search via the PubMed database. The authors began by using the Medical Subject Heading (MeSH) terms albumins/administration AND dosage; organization AND administration; adverse effects; and therapeutic use combined with liver cirrhosis as a MeSH major topic, which yielded 107 English-language articles published in the previous 10 years, and MeSH major topics of albumins and liver cirrhosis, which yielded 461 English-language articles, with 178 published in the previous 10 years. The search results were reviewed for applicability to albumin strategies for patients with cirrhosis.
CURRENT EVIDENCE-BASED INDICATIONS FOR USE OF ALBUMIN IN PATIENTS WITH CIRRHOSIS
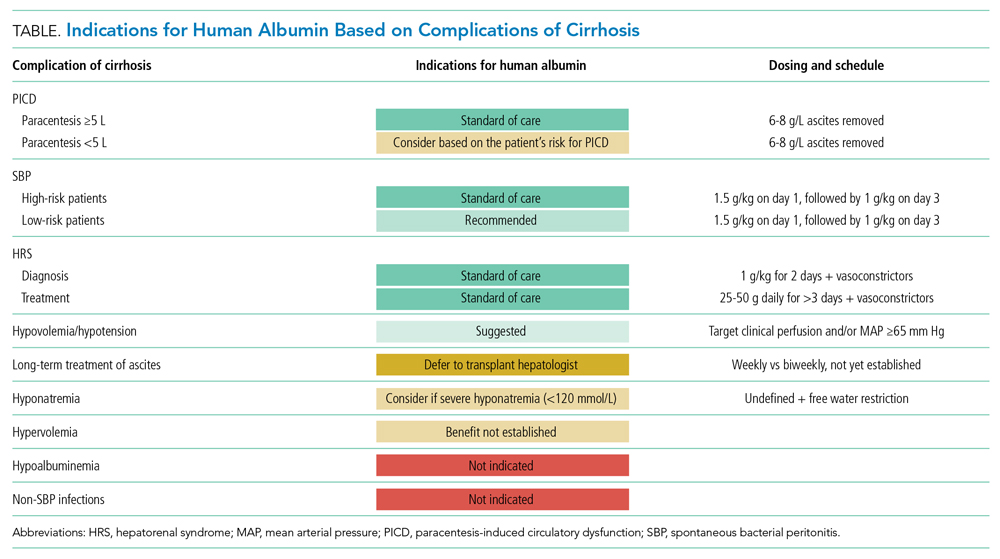
There are three widely accepted and evidence-based indications for HA infusion in patients with cirrhosis, considered standard of care (Table).
Prevention of PICD
Therapeutic large-volume paracentesis (LVP) leads to a rise in plasma renin activity (PRA) centrally through several mechanisms and is not impacted by the rate of ascites removal.5 LVP relieves abdominal pressure, increasing venous return to the heart and cardiac output, and the corresponding drop in systemic vascular resistance with splanchnic vasodilation decreases effective circulating volume and activates the renin-angiotensin system. This PRA activation and circulatory dysfunction are associated with reaccumulating ascites, renal impairment, hypervolemic hyponatremia, and increased mortality.6 A large meta-analysis of 17 trials with 1225 patients found that HA infusion improves outcomes and reduces mortality for patients undergoing LVP (odds ratio [OR], 0.64; 95% CI, 0.41-0.98), reduces the risk of PICD more than other volume expanders tested, and lowers the incidence of hyponatremia.6 More recently, in 2017, Kütting et al7 analyzed 21 trials with 1277 patients and did not observe a significant mortality benefit for HA after LVP (OR, 0.78; 95% CI, 0.55-1.11). However, negative outcomes such as rise in PRA (OR, 0.53; 95% CI, 0.29-0.97) and hyponatremia (OR, 0.62; 95% CI, 0.42-0.94) were prevented. Guidelines recommend HA after LVP ≥5 L to prevent PICD, with a replacement volume of 6 to 8 g of albumin per liter of ascitic fluid removed.2,3 Some patients may be at higher risk for PICD with less ascites removed, and the AASLD supports the use of HA to prevent PICD after smaller-volume paracentesis in patients who are already hypotensive (systolic blood pressure <90 mm Hg) or hyponatremic (<130 mmol/L), or have acute kidney injury.3
Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis is diagnosed by paracentesis, defined as ascitic neutrophil count ≥250 cells/µL with or without bacterascites (positive bacteriological culture). Bacterascites may be a precursor to the development of SBP, with the fluid neutrophil count of ≥250 determining the need for SBP treatment.2 SBP can lead to circulatory dysfunction, hepatic encephalopathy, and HRS. Treating SBP with HA in addition to antibiotics reduces the risk of kidney injury compared with antibiotics alone (OR for kidney injury with antibiotics alone, 4.6; 95% CI, 1.3-16.1) and also reduces the risk of death (OR for mortality with antibiotics alone, 4.5; 95% CI, 1.0-20.9).8 The AASLD recommends albumin in addition to antibiotics in SBP to prevent HRS and acute kidney injury, and high-risk patients who already have kidney dysfunction (creatinine >1 mg/dL) or jaundice (total bilirubin >5 mg/dL) are more likely to benefit from albumin. The treatment schedule is 25% HA at 1.5 g/kg on day 1 and 1 g/kg on day 3.3 The EASL recommends administering HA to all patients with cirrhosis with SBP regardless of renal or liver indices. They acknowledge, however, that the incidence of SBP-associated acute kidney injury will be low in patients without severe hepatic disease or baseline renal impairment.2
Hepatorenal Syndrome
Albumin combined with vasoconstrictors is effective in treating HRS with a response rate of 20% to 80% (average, 50%).3 Vasoactive medications can include combination midodrine and octreotide or norepinephrine (or terlipressin outside of the United States). In patients with suspected HRS, the recommended dosing of 25% HA is 1 g/kg (to a maximum of 100 g of albumin) on day 1 and then 40 to 50 g daily for at least 3 days after the diagnosis is confirmed.3 The optimal duration of therapy beyond 3 days of combined therapy with midodrine, albumin, and octreotide is not established. Terlipressin treatment is recommended for a maximum of 14 days in cases of partial response or nonresponse in renal recovery.2
INDICATIONS FOR ALBUMIN WITHOUT CLEAR EVIDENCE OF EFFICACY
Hypoalbuminemia
Albumin administration to raise serum albumin levels in hospitalized patients has been a common practice. However, new evidence suggests that treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis does not protect patients from risk and causes harm. The Albumin To prevenT Infection in chronic liveR (ATTIRE) trial, published in 2021, randomly assigned 777 patients across 35 centers in the United Kingdom to receive daily 20% HA to target a serum albumin level of 3.0 g/dL vs standard care, including HA for established indications.2,3 The primary end point was a composite of infection, kidney dysfunction, and death within 3 to 15 days of initiating treatment. There were no differences in the primary end point; secondary end points of death at 28 days, 3 months, or 6 months; or duration of hospitalization. The treatment group received 10 times more albumin than the control group and reported more adverse events, including pulmonary edema.9
Long-Term Treatment in Patients With Ascites
The human Albumin for the treatmeNt of aScites in patients With hEpatic ciRrhosis (ANSWER) trial, published in 2018, found improved 18-month survival in patients with cirrhosis and ascites treated with diuretics who received long-term albumin. This was an open-label trial of 431 patients at 33 sites in Italy, and the treatment arm received weekly infusions of 40 g of 20% HA. They observed a 38% reduction in mortality hazard ratio and half the number of hospital days annually.10 Based on these data and those from a 2006 Italian study with similar design and results, the Italian Association for the Study of the Liver (AISF) strongly recommends long-term albumin treatment in patients with cirrhosis with ascites.11 The lead author on the ANSWER trial also authored the AISF statement, although this recommendation has not been adopted by the EASL or the AASLD.
Conversely, the Midodrine and Albumin for CirrHoTic patients (MACHT) trial, also published in 2018, randomly assigned 173 patients with ascites awaiting liver transplant to receive 40 g of HA every 15 days and midodrine in addition to standard care vs placebo. MACHT found no difference in mortality or complications at 1 year.12
Long-term albumin therapy as a preventive measure may be a disease modifier, taking advantage of the pleiotropic effects of albumin, though the differing conclusions from ANSWER and MACHT necessitate additional trials. The ongoing PRECIOSA study in Spain is assessing dosage and schedule for this therapy.13
Augmenting Diuresis
Loop diuretics are highly protein-bound, and, with hypoalbuminemia, there is less effective drug delivered to the site of action. One clinical approach is to augment diuretics with concomitant HA infusion. This approach is not supported by strong evidence or guidelines.
Hyponatremia
In a retrospective cohort study of 2435 hospitalized patients with cirrhosis, 1126 of whom had hyponatremia, those patients with sodium <130 mmol/L who received HA were more likely to have resolution of hyponatremia to >135 mmol/L. This was associated with improved 30-day survival.14 From this observational data, the AASLD supports the use of albumin combined with extreme fluid restriction (<1000 mL/d) for patients with severe hyponatremia (<120 mmol/L).3
Non-SBP Infections
A 2019 meta-analysis found no evidence of a benefit of HA for bacterial infections other than SBP. However, only three trials encompassing 407 patients met the inclusion criteria.15
NEW GUIDELINE-SUGGESTED USE FOR ALBUMIN IN PATIENTS WITH CIRRHOSIS
SCCM Guideline Update: Hypoalbuminemia and Hypotension
The 2020 SCCM Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU “suggest using albumin for resuscitation of patients [with liver failure] over other fluids, especially when serum albumin is low (<3 g/dL).” Acute-on-chronic liver failure is decompensation of cirrhosis combined with organ dysfunction (eg, coagulopathy, encephalopathy, kidney injury), a scenario that is frequently encountered by hospitalists outside of intensive care settings. In hypotensive patients with cirrhosis, the SCCM recommends administering albumin to a target mean arterial pressure of 65 mm Hg or otherwise adequate perfusion. This new recommendation is conditional, based on expert consensus, and derives from low-quality evidence, with acknowledgement that “costs may be prohibitive.”4
While the ATTIRE study demonstrated no benefit in treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis, the 2020 SCCM guidelines, released prior to the publication of the ATTIRE study, focused on more acutely ill patients. In the ATTIRE study, only 2% to 3% of the study population was in an intensive care unit.4,9 The use of albumin infusion in the critically ill, hypoalbuminemic, hypotensive patient is not well studied, and the SCCM acknowledges the lack of supportive evidence for this practice in their guideline statement.
CONCLUSION
The three cardinal clinical indications for human albumin in patients with cirrhosis—prevention of PICD after LVP, in SBP, and for HRS—remain supported by the literature and guidelines, with the most recent guidance adding more nuance in patient selection based on individual risk (Table). With the publication of several large-scale studies in the past few years and a 2021 update to the AASLD guidance statement, clinicians have more evidence to guide their use of HA in patients with cirrhosis. In particular, the practice of treating isolated hypoalbuminemia with HA is no longer supported by the best evidence and is potentially harmful. A professional society recommendation to preferentially use albumin as a resuscitation fluid in hypoalbuminemia was made without the benefit of the results of the 2021 ATTIRE trial. On the horizon, additional results from ongoing and upcoming studies exploring concepts of effective albumin concentration and the pleiotropic properties of HA will impact the use of this therapy in hospitalized patients with cirrhosis.
1. Hirode G, Saab S, Wong RJ. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. 2020;3(4):e201997. https://doi.org/10.1001/jamanetworkopen.2020.1997
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460. https://doi.org/10.1016/j.jhep.2018.03.024
3. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
4. Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020;48(3):e173-e191. https://doi.org/10.1097/CCM.0000000000004192
5. Elsabaawy MM, Abdelhamid SR, Alsebaey A, et al. The impact of paracentesis flow rate in patients with liver cirrhosis on the development of paracentesis induced circulatory dysfunction. Clin Mol Hepatol. 2015;21(4):365-371. https://doi.org/10.3350/cmh.2015.21.4.365
6. Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(4):1172-1181. https://doi.org/10.1002/hep.24786
7. Kütting F, Schubert J, Franklin J, et al. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC-free cirrhotic patients undergoing large volume paracentesis. J Gastroenterol Hepatol. 2017;32(2):327-338. https://doi.org/10.1111/jgh.13421
8. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
9. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. https://doi.org/10.1056/NEJMoa2022166
10. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417-2429. https://doi.org/10.1016/S0140-6736(18)30840-7
11. Caraceni P, Angeli P, Prati D, et al. AISF-SIMTI position paper on the appropriate use of albumin in patients with liver cirrhosis: a 2020 update. Blood Transfus. 2021;19(1):9-13. https://doi.org/10.2450/2020.0414-20
12. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69(6):1250-1259. https://doi.org/10.1016/j.jhep.2018.08.006
13. Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157(1):149-162. https://doi.org/10.1053/j.gastro.2019.03.021
14. Bajaj JS, Tandon P, O’Leary JG, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol. 2018;113(9):1339. https://doi.org/10.1038/s41395-018-0119-3
15. Leão GS, Neto GJ, Jotz RdF, de Mattos AA, de Mattos ÂZ. Albumin for cirrhotic patients with extraperitoneal infections: a meta-analysis. J Gastroenterol Hepatol. 2019;34(12):2071-2076. https://doi.org/10.1111/jgh.14791
The burden of chronic liver disease (CLD) in the United States is growing, and it is currently the fourth leading cause of death in adults aged 45 to 64 years.1 From 2012 to 2016, there were 538,720 hospitalizations in the United States for patients with cirrhosis, with almost a quarter having at least one cirrhosis-related complication. Inpatient hospitalizations for cirrhosis contribute to healthcare resource utilization, with a mean cost per CLD-related hospitalization of $16,271, and the presence of cirrhosis results in higher mortality and cost burden.1
In hospitalized patients with decompensated cirrhosis with ascites, intravenous human albumin (HA) infusion has been utilized for decades for a variety of indications. Current guidance by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommends the use of albumin for the prevention of paracentesis-induced circulatory dysfunction (PICD) for the prevention of kidney injury in spontaneous bacterial peritonitis (SBP) and for the diagnosis and treatment of hepatorenal syndrome (HRS).2,3 There have been several major trials in recent years studying the use of HA for other indications in patients with cirrhosis, and the Society of Critical Care Medicine (SCCM) updated their guidelines in 2020 to recommend HA administration in resuscitation of critically ill patients with liver failure with hypoalbuminemia.4This Clinical Progress Note addresses the use of albumin in hospitalized patients with cirrhosis, focusing on current indications and discussing potential uses published after the 2018 EASL guidelines. We conducted a literature search via the PubMed database. The authors began by using the Medical Subject Heading (MeSH) terms albumins/administration AND dosage; organization AND administration; adverse effects; and therapeutic use combined with liver cirrhosis as a MeSH major topic, which yielded 107 English-language articles published in the previous 10 years, and MeSH major topics of albumins and liver cirrhosis, which yielded 461 English-language articles, with 178 published in the previous 10 years. The search results were reviewed for applicability to albumin strategies for patients with cirrhosis.
CURRENT EVIDENCE-BASED INDICATIONS FOR USE OF ALBUMIN IN PATIENTS WITH CIRRHOSIS
There are three widely accepted and evidence-based indications for HA infusion in patients with cirrhosis, considered standard of care (Table).

Prevention of PICD
Therapeutic large-volume paracentesis (LVP) leads to a rise in plasma renin activity (PRA) centrally through several mechanisms and is not impacted by the rate of ascites removal.5 LVP relieves abdominal pressure, increasing venous return to the heart and cardiac output, and the corresponding drop in systemic vascular resistance with splanchnic vasodilation decreases effective circulating volume and activates the renin-angiotensin system. This PRA activation and circulatory dysfunction are associated with reaccumulating ascites, renal impairment, hypervolemic hyponatremia, and increased mortality.6 A large meta-analysis of 17 trials with 1225 patients found that HA infusion improves outcomes and reduces mortality for patients undergoing LVP (odds ratio [OR], 0.64; 95% CI, 0.41-0.98), reduces the risk of PICD more than other volume expanders tested, and lowers the incidence of hyponatremia.6 More recently, in 2017, Kütting et al7 analyzed 21 trials with 1277 patients and did not observe a significant mortality benefit for HA after LVP (OR, 0.78; 95% CI, 0.55-1.11). However, negative outcomes such as rise in PRA (OR, 0.53; 95% CI, 0.29-0.97) and hyponatremia (OR, 0.62; 95% CI, 0.42-0.94) were prevented. Guidelines recommend HA after LVP ≥5 L to prevent PICD, with a replacement volume of 6 to 8 g of albumin per liter of ascitic fluid removed.2,3 Some patients may be at higher risk for PICD with less ascites removed, and the AASLD supports the use of HA to prevent PICD after smaller-volume paracentesis in patients who are already hypotensive (systolic blood pressure <90 mm Hg) or hyponatremic (<130 mmol/L), or have acute kidney injury.3
Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis is diagnosed by paracentesis, defined as ascitic neutrophil count ≥250 cells/µL with or without bacterascites (positive bacteriological culture). Bacterascites may be a precursor to the development of SBP, with the fluid neutrophil count of ≥250 determining the need for SBP treatment.2 SBP can lead to circulatory dysfunction, hepatic encephalopathy, and HRS. Treating SBP with HA in addition to antibiotics reduces the risk of kidney injury compared with antibiotics alone (OR for kidney injury with antibiotics alone, 4.6; 95% CI, 1.3-16.1) and also reduces the risk of death (OR for mortality with antibiotics alone, 4.5; 95% CI, 1.0-20.9).8 The AASLD recommends albumin in addition to antibiotics in SBP to prevent HRS and acute kidney injury, and high-risk patients who already have kidney dysfunction (creatinine >1 mg/dL) or jaundice (total bilirubin >5 mg/dL) are more likely to benefit from albumin. The treatment schedule is 25% HA at 1.5 g/kg on day 1 and 1 g/kg on day 3.3 The EASL recommends administering HA to all patients with cirrhosis with SBP regardless of renal or liver indices. They acknowledge, however, that the incidence of SBP-associated acute kidney injury will be low in patients without severe hepatic disease or baseline renal impairment.2
Hepatorenal Syndrome
Albumin combined with vasoconstrictors is effective in treating HRS with a response rate of 20% to 80% (average, 50%).3 Vasoactive medications can include combination midodrine and octreotide or norepinephrine (or terlipressin outside of the United States). In patients with suspected HRS, the recommended dosing of 25% HA is 1 g/kg (to a maximum of 100 g of albumin) on day 1 and then 40 to 50 g daily for at least 3 days after the diagnosis is confirmed.3 The optimal duration of therapy beyond 3 days of combined therapy with midodrine, albumin, and octreotide is not established. Terlipressin treatment is recommended for a maximum of 14 days in cases of partial response or nonresponse in renal recovery.2
INDICATIONS FOR ALBUMIN WITHOUT CLEAR EVIDENCE OF EFFICACY
Hypoalbuminemia
Albumin administration to raise serum albumin levels in hospitalized patients has been a common practice. However, new evidence suggests that treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis does not protect patients from risk and causes harm. The Albumin To prevenT Infection in chronic liveR (ATTIRE) trial, published in 2021, randomly assigned 777 patients across 35 centers in the United Kingdom to receive daily 20% HA to target a serum albumin level of 3.0 g/dL vs standard care, including HA for established indications.2,3 The primary end point was a composite of infection, kidney dysfunction, and death within 3 to 15 days of initiating treatment. There were no differences in the primary end point; secondary end points of death at 28 days, 3 months, or 6 months; or duration of hospitalization. The treatment group received 10 times more albumin than the control group and reported more adverse events, including pulmonary edema.9
Long-Term Treatment in Patients With Ascites
The human Albumin for the treatmeNt of aScites in patients With hEpatic ciRrhosis (ANSWER) trial, published in 2018, found improved 18-month survival in patients with cirrhosis and ascites treated with diuretics who received long-term albumin. This was an open-label trial of 431 patients at 33 sites in Italy, and the treatment arm received weekly infusions of 40 g of 20% HA. They observed a 38% reduction in mortality hazard ratio and half the number of hospital days annually.10 Based on these data and those from a 2006 Italian study with similar design and results, the Italian Association for the Study of the Liver (AISF) strongly recommends long-term albumin treatment in patients with cirrhosis with ascites.11 The lead author on the ANSWER trial also authored the AISF statement, although this recommendation has not been adopted by the EASL or the AASLD.
Conversely, the Midodrine and Albumin for CirrHoTic patients (MACHT) trial, also published in 2018, randomly assigned 173 patients with ascites awaiting liver transplant to receive 40 g of HA every 15 days and midodrine in addition to standard care vs placebo. MACHT found no difference in mortality or complications at 1 year.12
Long-term albumin therapy as a preventive measure may be a disease modifier, taking advantage of the pleiotropic effects of albumin, though the differing conclusions from ANSWER and MACHT necessitate additional trials. The ongoing PRECIOSA study in Spain is assessing dosage and schedule for this therapy.13
Augmenting Diuresis
Loop diuretics are highly protein-bound, and, with hypoalbuminemia, there is less effective drug delivered to the site of action. One clinical approach is to augment diuretics with concomitant HA infusion. This approach is not supported by strong evidence or guidelines.
Hyponatremia
In a retrospective cohort study of 2435 hospitalized patients with cirrhosis, 1126 of whom had hyponatremia, those patients with sodium <130 mmol/L who received HA were more likely to have resolution of hyponatremia to >135 mmol/L. This was associated with improved 30-day survival.14 From this observational data, the AASLD supports the use of albumin combined with extreme fluid restriction (<1000 mL/d) for patients with severe hyponatremia (<120 mmol/L).3
Non-SBP Infections
A 2019 meta-analysis found no evidence of a benefit of HA for bacterial infections other than SBP. However, only three trials encompassing 407 patients met the inclusion criteria.15
NEW GUIDELINE-SUGGESTED USE FOR ALBUMIN IN PATIENTS WITH CIRRHOSIS
SCCM Guideline Update: Hypoalbuminemia and Hypotension
The 2020 SCCM Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU “suggest using albumin for resuscitation of patients [with liver failure] over other fluids, especially when serum albumin is low (<3 g/dL).” Acute-on-chronic liver failure is decompensation of cirrhosis combined with organ dysfunction (eg, coagulopathy, encephalopathy, kidney injury), a scenario that is frequently encountered by hospitalists outside of intensive care settings. In hypotensive patients with cirrhosis, the SCCM recommends administering albumin to a target mean arterial pressure of 65 mm Hg or otherwise adequate perfusion. This new recommendation is conditional, based on expert consensus, and derives from low-quality evidence, with acknowledgement that “costs may be prohibitive.”4
While the ATTIRE study demonstrated no benefit in treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis, the 2020 SCCM guidelines, released prior to the publication of the ATTIRE study, focused on more acutely ill patients. In the ATTIRE study, only 2% to 3% of the study population was in an intensive care unit.4,9 The use of albumin infusion in the critically ill, hypoalbuminemic, hypotensive patient is not well studied, and the SCCM acknowledges the lack of supportive evidence for this practice in their guideline statement.
CONCLUSION
The three cardinal clinical indications for human albumin in patients with cirrhosis—prevention of PICD after LVP, in SBP, and for HRS—remain supported by the literature and guidelines, with the most recent guidance adding more nuance in patient selection based on individual risk (Table). With the publication of several large-scale studies in the past few years and a 2021 update to the AASLD guidance statement, clinicians have more evidence to guide their use of HA in patients with cirrhosis. In particular, the practice of treating isolated hypoalbuminemia with HA is no longer supported by the best evidence and is potentially harmful. A professional society recommendation to preferentially use albumin as a resuscitation fluid in hypoalbuminemia was made without the benefit of the results of the 2021 ATTIRE trial. On the horizon, additional results from ongoing and upcoming studies exploring concepts of effective albumin concentration and the pleiotropic properties of HA will impact the use of this therapy in hospitalized patients with cirrhosis.
The burden of chronic liver disease (CLD) in the United States is growing, and it is currently the fourth leading cause of death in adults aged 45 to 64 years.1 From 2012 to 2016, there were 538,720 hospitalizations in the United States for patients with cirrhosis, with almost a quarter having at least one cirrhosis-related complication. Inpatient hospitalizations for cirrhosis contribute to healthcare resource utilization, with a mean cost per CLD-related hospitalization of $16,271, and the presence of cirrhosis results in higher mortality and cost burden.1
In hospitalized patients with decompensated cirrhosis with ascites, intravenous human albumin (HA) infusion has been utilized for decades for a variety of indications. Current guidance by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommends the use of albumin for the prevention of paracentesis-induced circulatory dysfunction (PICD) for the prevention of kidney injury in spontaneous bacterial peritonitis (SBP) and for the diagnosis and treatment of hepatorenal syndrome (HRS).2,3 There have been several major trials in recent years studying the use of HA for other indications in patients with cirrhosis, and the Society of Critical Care Medicine (SCCM) updated their guidelines in 2020 to recommend HA administration in resuscitation of critically ill patients with liver failure with hypoalbuminemia.4This Clinical Progress Note addresses the use of albumin in hospitalized patients with cirrhosis, focusing on current indications and discussing potential uses published after the 2018 EASL guidelines. We conducted a literature search via the PubMed database. The authors began by using the Medical Subject Heading (MeSH) terms albumins/administration AND dosage; organization AND administration; adverse effects; and therapeutic use combined with liver cirrhosis as a MeSH major topic, which yielded 107 English-language articles published in the previous 10 years, and MeSH major topics of albumins and liver cirrhosis, which yielded 461 English-language articles, with 178 published in the previous 10 years. The search results were reviewed for applicability to albumin strategies for patients with cirrhosis.
CURRENT EVIDENCE-BASED INDICATIONS FOR USE OF ALBUMIN IN PATIENTS WITH CIRRHOSIS
There are three widely accepted and evidence-based indications for HA infusion in patients with cirrhosis, considered standard of care (Table).

Prevention of PICD
Therapeutic large-volume paracentesis (LVP) leads to a rise in plasma renin activity (PRA) centrally through several mechanisms and is not impacted by the rate of ascites removal.5 LVP relieves abdominal pressure, increasing venous return to the heart and cardiac output, and the corresponding drop in systemic vascular resistance with splanchnic vasodilation decreases effective circulating volume and activates the renin-angiotensin system. This PRA activation and circulatory dysfunction are associated with reaccumulating ascites, renal impairment, hypervolemic hyponatremia, and increased mortality.6 A large meta-analysis of 17 trials with 1225 patients found that HA infusion improves outcomes and reduces mortality for patients undergoing LVP (odds ratio [OR], 0.64; 95% CI, 0.41-0.98), reduces the risk of PICD more than other volume expanders tested, and lowers the incidence of hyponatremia.6 More recently, in 2017, Kütting et al7 analyzed 21 trials with 1277 patients and did not observe a significant mortality benefit for HA after LVP (OR, 0.78; 95% CI, 0.55-1.11). However, negative outcomes such as rise in PRA (OR, 0.53; 95% CI, 0.29-0.97) and hyponatremia (OR, 0.62; 95% CI, 0.42-0.94) were prevented. Guidelines recommend HA after LVP ≥5 L to prevent PICD, with a replacement volume of 6 to 8 g of albumin per liter of ascitic fluid removed.2,3 Some patients may be at higher risk for PICD with less ascites removed, and the AASLD supports the use of HA to prevent PICD after smaller-volume paracentesis in patients who are already hypotensive (systolic blood pressure <90 mm Hg) or hyponatremic (<130 mmol/L), or have acute kidney injury.3
Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis is diagnosed by paracentesis, defined as ascitic neutrophil count ≥250 cells/µL with or without bacterascites (positive bacteriological culture). Bacterascites may be a precursor to the development of SBP, with the fluid neutrophil count of ≥250 determining the need for SBP treatment.2 SBP can lead to circulatory dysfunction, hepatic encephalopathy, and HRS. Treating SBP with HA in addition to antibiotics reduces the risk of kidney injury compared with antibiotics alone (OR for kidney injury with antibiotics alone, 4.6; 95% CI, 1.3-16.1) and also reduces the risk of death (OR for mortality with antibiotics alone, 4.5; 95% CI, 1.0-20.9).8 The AASLD recommends albumin in addition to antibiotics in SBP to prevent HRS and acute kidney injury, and high-risk patients who already have kidney dysfunction (creatinine >1 mg/dL) or jaundice (total bilirubin >5 mg/dL) are more likely to benefit from albumin. The treatment schedule is 25% HA at 1.5 g/kg on day 1 and 1 g/kg on day 3.3 The EASL recommends administering HA to all patients with cirrhosis with SBP regardless of renal or liver indices. They acknowledge, however, that the incidence of SBP-associated acute kidney injury will be low in patients without severe hepatic disease or baseline renal impairment.2
Hepatorenal Syndrome
Albumin combined with vasoconstrictors is effective in treating HRS with a response rate of 20% to 80% (average, 50%).3 Vasoactive medications can include combination midodrine and octreotide or norepinephrine (or terlipressin outside of the United States). In patients with suspected HRS, the recommended dosing of 25% HA is 1 g/kg (to a maximum of 100 g of albumin) on day 1 and then 40 to 50 g daily for at least 3 days after the diagnosis is confirmed.3 The optimal duration of therapy beyond 3 days of combined therapy with midodrine, albumin, and octreotide is not established. Terlipressin treatment is recommended for a maximum of 14 days in cases of partial response or nonresponse in renal recovery.2
INDICATIONS FOR ALBUMIN WITHOUT CLEAR EVIDENCE OF EFFICACY
Hypoalbuminemia
Albumin administration to raise serum albumin levels in hospitalized patients has been a common practice. However, new evidence suggests that treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis does not protect patients from risk and causes harm. The Albumin To prevenT Infection in chronic liveR (ATTIRE) trial, published in 2021, randomly assigned 777 patients across 35 centers in the United Kingdom to receive daily 20% HA to target a serum albumin level of 3.0 g/dL vs standard care, including HA for established indications.2,3 The primary end point was a composite of infection, kidney dysfunction, and death within 3 to 15 days of initiating treatment. There were no differences in the primary end point; secondary end points of death at 28 days, 3 months, or 6 months; or duration of hospitalization. The treatment group received 10 times more albumin than the control group and reported more adverse events, including pulmonary edema.9
Long-Term Treatment in Patients With Ascites
The human Albumin for the treatmeNt of aScites in patients With hEpatic ciRrhosis (ANSWER) trial, published in 2018, found improved 18-month survival in patients with cirrhosis and ascites treated with diuretics who received long-term albumin. This was an open-label trial of 431 patients at 33 sites in Italy, and the treatment arm received weekly infusions of 40 g of 20% HA. They observed a 38% reduction in mortality hazard ratio and half the number of hospital days annually.10 Based on these data and those from a 2006 Italian study with similar design and results, the Italian Association for the Study of the Liver (AISF) strongly recommends long-term albumin treatment in patients with cirrhosis with ascites.11 The lead author on the ANSWER trial also authored the AISF statement, although this recommendation has not been adopted by the EASL or the AASLD.
Conversely, the Midodrine and Albumin for CirrHoTic patients (MACHT) trial, also published in 2018, randomly assigned 173 patients with ascites awaiting liver transplant to receive 40 g of HA every 15 days and midodrine in addition to standard care vs placebo. MACHT found no difference in mortality or complications at 1 year.12
Long-term albumin therapy as a preventive measure may be a disease modifier, taking advantage of the pleiotropic effects of albumin, though the differing conclusions from ANSWER and MACHT necessitate additional trials. The ongoing PRECIOSA study in Spain is assessing dosage and schedule for this therapy.13
Augmenting Diuresis
Loop diuretics are highly protein-bound, and, with hypoalbuminemia, there is less effective drug delivered to the site of action. One clinical approach is to augment diuretics with concomitant HA infusion. This approach is not supported by strong evidence or guidelines.
Hyponatremia
In a retrospective cohort study of 2435 hospitalized patients with cirrhosis, 1126 of whom had hyponatremia, those patients with sodium <130 mmol/L who received HA were more likely to have resolution of hyponatremia to >135 mmol/L. This was associated with improved 30-day survival.14 From this observational data, the AASLD supports the use of albumin combined with extreme fluid restriction (<1000 mL/d) for patients with severe hyponatremia (<120 mmol/L).3
Non-SBP Infections
A 2019 meta-analysis found no evidence of a benefit of HA for bacterial infections other than SBP. However, only three trials encompassing 407 patients met the inclusion criteria.15
NEW GUIDELINE-SUGGESTED USE FOR ALBUMIN IN PATIENTS WITH CIRRHOSIS
SCCM Guideline Update: Hypoalbuminemia and Hypotension
The 2020 SCCM Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU “suggest using albumin for resuscitation of patients [with liver failure] over other fluids, especially when serum albumin is low (<3 g/dL).” Acute-on-chronic liver failure is decompensation of cirrhosis combined with organ dysfunction (eg, coagulopathy, encephalopathy, kidney injury), a scenario that is frequently encountered by hospitalists outside of intensive care settings. In hypotensive patients with cirrhosis, the SCCM recommends administering albumin to a target mean arterial pressure of 65 mm Hg or otherwise adequate perfusion. This new recommendation is conditional, based on expert consensus, and derives from low-quality evidence, with acknowledgement that “costs may be prohibitive.”4
While the ATTIRE study demonstrated no benefit in treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis, the 2020 SCCM guidelines, released prior to the publication of the ATTIRE study, focused on more acutely ill patients. In the ATTIRE study, only 2% to 3% of the study population was in an intensive care unit.4,9 The use of albumin infusion in the critically ill, hypoalbuminemic, hypotensive patient is not well studied, and the SCCM acknowledges the lack of supportive evidence for this practice in their guideline statement.
CONCLUSION
The three cardinal clinical indications for human albumin in patients with cirrhosis—prevention of PICD after LVP, in SBP, and for HRS—remain supported by the literature and guidelines, with the most recent guidance adding more nuance in patient selection based on individual risk (Table). With the publication of several large-scale studies in the past few years and a 2021 update to the AASLD guidance statement, clinicians have more evidence to guide their use of HA in patients with cirrhosis. In particular, the practice of treating isolated hypoalbuminemia with HA is no longer supported by the best evidence and is potentially harmful. A professional society recommendation to preferentially use albumin as a resuscitation fluid in hypoalbuminemia was made without the benefit of the results of the 2021 ATTIRE trial. On the horizon, additional results from ongoing and upcoming studies exploring concepts of effective albumin concentration and the pleiotropic properties of HA will impact the use of this therapy in hospitalized patients with cirrhosis.
1. Hirode G, Saab S, Wong RJ. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. 2020;3(4):e201997. https://doi.org/10.1001/jamanetworkopen.2020.1997
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460. https://doi.org/10.1016/j.jhep.2018.03.024
3. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
4. Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020;48(3):e173-e191. https://doi.org/10.1097/CCM.0000000000004192
5. Elsabaawy MM, Abdelhamid SR, Alsebaey A, et al. The impact of paracentesis flow rate in patients with liver cirrhosis on the development of paracentesis induced circulatory dysfunction. Clin Mol Hepatol. 2015;21(4):365-371. https://doi.org/10.3350/cmh.2015.21.4.365
6. Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(4):1172-1181. https://doi.org/10.1002/hep.24786
7. Kütting F, Schubert J, Franklin J, et al. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC-free cirrhotic patients undergoing large volume paracentesis. J Gastroenterol Hepatol. 2017;32(2):327-338. https://doi.org/10.1111/jgh.13421
8. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
9. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. https://doi.org/10.1056/NEJMoa2022166
10. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417-2429. https://doi.org/10.1016/S0140-6736(18)30840-7
11. Caraceni P, Angeli P, Prati D, et al. AISF-SIMTI position paper on the appropriate use of albumin in patients with liver cirrhosis: a 2020 update. Blood Transfus. 2021;19(1):9-13. https://doi.org/10.2450/2020.0414-20
12. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69(6):1250-1259. https://doi.org/10.1016/j.jhep.2018.08.006
13. Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157(1):149-162. https://doi.org/10.1053/j.gastro.2019.03.021
14. Bajaj JS, Tandon P, O’Leary JG, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol. 2018;113(9):1339. https://doi.org/10.1038/s41395-018-0119-3
15. Leão GS, Neto GJ, Jotz RdF, de Mattos AA, de Mattos ÂZ. Albumin for cirrhotic patients with extraperitoneal infections: a meta-analysis. J Gastroenterol Hepatol. 2019;34(12):2071-2076. https://doi.org/10.1111/jgh.14791
1. Hirode G, Saab S, Wong RJ. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. 2020;3(4):e201997. https://doi.org/10.1001/jamanetworkopen.2020.1997
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460. https://doi.org/10.1016/j.jhep.2018.03.024
3. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
4. Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020;48(3):e173-e191. https://doi.org/10.1097/CCM.0000000000004192
5. Elsabaawy MM, Abdelhamid SR, Alsebaey A, et al. The impact of paracentesis flow rate in patients with liver cirrhosis on the development of paracentesis induced circulatory dysfunction. Clin Mol Hepatol. 2015;21(4):365-371. https://doi.org/10.3350/cmh.2015.21.4.365
6. Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(4):1172-1181. https://doi.org/10.1002/hep.24786
7. Kütting F, Schubert J, Franklin J, et al. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC-free cirrhotic patients undergoing large volume paracentesis. J Gastroenterol Hepatol. 2017;32(2):327-338. https://doi.org/10.1111/jgh.13421
8. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
9. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. https://doi.org/10.1056/NEJMoa2022166
10. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417-2429. https://doi.org/10.1016/S0140-6736(18)30840-7
11. Caraceni P, Angeli P, Prati D, et al. AISF-SIMTI position paper on the appropriate use of albumin in patients with liver cirrhosis: a 2020 update. Blood Transfus. 2021;19(1):9-13. https://doi.org/10.2450/2020.0414-20
12. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69(6):1250-1259. https://doi.org/10.1016/j.jhep.2018.08.006
13. Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157(1):149-162. https://doi.org/10.1053/j.gastro.2019.03.021
14. Bajaj JS, Tandon P, O’Leary JG, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol. 2018;113(9):1339. https://doi.org/10.1038/s41395-018-0119-3
15. Leão GS, Neto GJ, Jotz RdF, de Mattos AA, de Mattos ÂZ. Albumin for cirrhotic patients with extraperitoneal infections: a meta-analysis. J Gastroenterol Hepatol. 2019;34(12):2071-2076. https://doi.org/10.1111/jgh.14791
© 2021 Society of Hospital Medicine