User login
Advances in the management of soft-tissue and bone sarcomas—referred to collectively as “musculoskeletal sarcomas” hereafter—have resulted in significant improvements in survival and quality of life.1–3 Several factors have likely contributed to these advances, including improved surgical technique and the development of referral centers for sarcoma treatment that have embraced a multidisciplinary approach.1,2
The goal of treatment for musculoskeletal sarcomas is to optimize oncologic outcome and maximize functional restoration.2,3 Surgical resection has been the mainstay of therapy,1–7 as detailed earlier in this supplement. In patients with musculoskeletal sarcomas of the extremities, limb-sparing resection has been shown to be significantly superior to amputation.1,7–9 Wide local excision of the tumor along with its muscle compartment, followed by adjuvant chemotherapy and radiation therapy, has allowed limb salvage without an increased risk of recurrence in many patients.3 However, wide tumor resection can leave large defects that are not amenable to coverage by mobilization of the surrounding tissues, particularly if those tissues have been irradiated. As a result, resection can expose neurovascular structures, bone without periosteum, alloplastic materials, and internal fixation devices.
GOALS OF RECONSTRUCTION
Reconstructive surgery after musculoskeletal sarcoma resection aims to provide adequate wound coverage, preserve function, and optimize the cosmetic outcome.1–3 Tumors can be found on areas crucial to limb movement or may involve tissues vital to limb function. Reconstruction to repair these deficits can take many forms. In certain situations, amputation is still inevitable. In those cases, the reconstruction should provide stable stump coverage with durability and the ability to fit well with an external prosthesis.3
TIMING OF RECONSTRUCTION
Immediate reconstruction should be pursued if possible
Immediate reconstruction after a negative margin should always be considered and should be attempted when possible. Immediate reconstruction allows the reconstructive surgeon to benefit from better evaluation of the defect and exposed structures, as no scar tissue is present to distort the anatomy. Likewise, patients benefit from faster recovery and can receive adjuvant treatment (if necessary) sooner, as well as earlier rehabilitation. Patients may also benefit psychologically from immediate reconstruction.1,3
Indications for delayed reconstruction
Delayed reconstruction is primarily indicated when there are wound healing problems or there is uncertainty about the tumor margins. Secondary indications for delayed reconstruction are wound dehiscence and unstable soft-tissue coverage. If hardware is exposed, the recommendation is for early intervention and wound coverage with well-vascularized tissue to protect and cover the implant or prosthesis.
What about radiation therapy?
A very important consideration in reconstruction is the need for neoadjuvant or adjuvant radiation therapy.3,10,11 Irradiated wounds have a higher incidence of complications, including a tendency to dehisce. In patients who have been previously irradiated, the best practice is to perform immediate reconstruction with well-vascularized tissue, most likely a free tissue transfer.4,6,11,12 This practice reduces hospital stay, costs, and morbidity and increases limb salvage and patient satisfaction.13
SYSTEMATIC PREOPERATIVE PLANNING NEEDED
Reconstruction after musculoskeletal sarcoma resection should be planned systematically within a process that involves preoperative anticipation of the defect size and the resulting functional and cosmetic deficits that might need to be addressed. A preoperative visit to the reconstructive surgeon can be very helpful for presurgical planning.
During surgery it is usually preferable to allow the surgeon doing the tumor resection (eg, surgical oncologist or orthopedic oncologist) to complete the resection because the dimensions of the defect are not certain until negative margins are obtained.14 If tumor margins are unclear at the time of initial resection, the surgeon should consider delaying the definitive reconstruction until the permanent sections confirm negative margins. Temporary closure can be achieved with wound dressings, skin grafts (either allograft or autograft), or negative-pressure wound therapy. In the same context, if neurovascular structures are exposed it is reasonable to use a muscle flap without “tailoring” the flap to the defect. This approach allows the flap to be advanced or repositioned in case of positive margins, and the skin graft can be applied to the muscle surface in a second procedure.3
RECONSTRUCTIVE METHODS: A BRIEF OVERVIEW
Several methods can be used to close musculoskeletal sarcoma excision defects. Smaller defects can be closed primarily, although most defects are large and not amenable to primary closure. If fascia or muscle is preserved with only the skin coverage missing, the wound can be covered with either split-thickness or full-thickness skin grafts.1,4,6 Split-thickness skin grafts can be obtained in larger amounts and often heal faster than full-thickness skin grafts. However, most resections will require durable tissue coverage, particularly if adjuvant radiation therapy is planned.
In the case of long bone sarcoma resection, the resulting defect is usually large and complex and the traditional reconstruction is based on avascular allografts and local tissue flaps. However, allografts are associated with high rates of infection, nonunion, and fracture, leading to failure in about 50% of cases. Microvascular free flaps that contain bone, such as free fibula flaps, have been used instead of allografts with good success rates.2
Lately there has been growing interest in the use of the vacuum-assisted closure device (a form of negative-pressure wound therapy) to promote wound healing. It has been shown to improve the granulation and healing of open wounds by absorbing moisture, as well as to promote adherence after skin grafting, thereby reducing the risk of graft displacement.1,3 This device can be used immediately after musculoskeletal sarcoma resection while definitive tumor margin results are pending. It also can be used to prepare the wound bed for grafting in high-risk patients who would not tolerate more complex reconstructions.
Local or adjacent fascial, fasciocutaneous, and dermal flaps can also be used in lower-extremity reconstruction. However, muscle or musculocutaneous flaps are the mainstay of reconstruction after resection of musculoskeletal sarcomas. This group also includes perforator flaps, which have grown in popularity in the last few years.1,3
LOCATION-BASED WOUND RECONSTRUCTION
Musculoskeletal sarcomas can occur in virtually any region of the body, and myriad reconstructive options are available for various body sites. Since lower-extremity musculoskeletal sarcomas represent about 75% of cases,1 we will focus mainly on reconstruction of the lower extremity.
Factors driving choice of flap
Selection of an appropriate flap is essential to an optimal outcome. Flaps should be chosen with regard to donor site morbidity, functional requirements, length and diameter of the vascular pedicle, and aesthetic outcome.3 Usually physical examination, palpation of peripheral pulses, and Doppler ultrasonography are sufficient to evaluate the circulation. A preoperative angiogram should be considered in patients with severe peripheral vascular disease or previous trauma, which can potentially compromise the reconstructive outcome.15
Each region of the lower extremity possesses unique anatomic and functional characteristics that must be evaluated. It is useful to categorize the thigh, lower leg, and foot into separate anatomic units when planning reconstruction. We further divided these units into several subunits, as previously proposed by Sherman and Law15 and as outlined below.
Thigh
The thigh is usually well perfused and has several muscle groups, which facilitates reconstruction. Primary closure, skin grafts, or local flaps are acceptable options in most cases. The remaining musculature can be rotated or advanced to cover defects in the anterior or posterior thigh, providing bulk and adequate blood supply.
Hip and proximal/lateral thigh. Local muscle or myocutaneous flap options include tensor fascia lata, vastus lateralis, and rectus femoris flaps, all of which are based on the lateral circumflex femoral artery.
The tensor fascia lata flap is thin but has a long fascia extension that can be elevated from above the knee and can include a large skin paddle that is innervated by the lateral femoral cutaneous nerve. Some patients may experience knee instability after tensor fascia lata harvest.
The vastus lateralis muscle flap provides good bulk. Its arc of rotation reaches most of the inferior and posterior pelvis. It has little effect on ambulation.
The rectus femoris muscle flap is not so bulky, is easily mobilized, and has a wide arc of rotation. The donor site can be closed primarily. Harvest of this muscle can be associated with some strength loss during knee extension. For large defects of the upper third of the leg, a pedicled rectus abdominis muscle flap based on the deep inferior epigastric artery can be used. A vertically oriented skin island can be extended up to the costal margin, improving the reach. When the nature of the wound precludes use of pedicle flaps, free tissue transfer is indicated, with the latissimus dorsi muscle flap being used most commonly.15,16
Mid-thigh. Wounds in this location often can be closed with skin grafts or fasciocutaneous flaps. If the femur is exposed, however, a muscle flap will be required. As above, the tensor fascia lata, vastus lateralis, and rectus femoris can be used as flap options. If the lateral circumflex artery is unavailable, other flap options include the gracilis, vastus medialis, and rectus abdominis muscles. The gracilis muscle flap is based on the medial circumflex femoral artery and is useful for covering the medial aspect of the mid-thigh. Although this is a thin muscle, it can be used to cover long defects. The vastus medialis muscle flap is supplied by perforators from the profunda femoris and superficial femoral arteries. It can be rotated medially and advanced distally to cover patellar defects.
Supracondylar knee. The knee is a location where sarcoma resection is particularly likely to leave a defect with exposed bone, tendons, or ligaments that will need coverage. The gastrocnemius muscle flap combined with a split-thickness skin graft remains a consistent and reliable reconstructive option for this area. Other options are an extended medial gastrocnemius muscle flap or myocutaneous flap, which incorporates a random fasciocutaneous extension. For larger defects, free flaps should be considered, such as the anterior thigh flap, rectus abdominis muscle flap, or latissimus dorsi muscle flap. If tendons or ligaments need to be reconstructed, we favor autologous tissue, such as the fascia lata and plantaris tendons. These are easy to harvest and provide long-lasting joint stability.
Lower leg
Proximal third of the tibia. Defects here can usually be covered with a medial or lateral gastrocnemius muscle or myocutaneous flap, or a combination of the two. These muscles have a dominant vascular pedicle—the medial and lateral sural arteries. They can be harvested as an island for better reach, and they are reliable and have minimal donor site morbidity.15 The soleus muscle flap is another option that can be used alone or in combination with the medial or lateral gastrocnemius. Defects that are not amenable to closure by these flaps will most likely require free tissue transfer. The rectus abdominis or latissimus dorsi muscles are the first options. The latter can be combined with the serratus muscle if more bulk is needed.
Middle and lower thirds of the tibia. The soleus flap is frequently used for small or medium-sized mid-tibial defects. It is based on branches of the popliteal artery and posterior tibial artery. Larger defects require a combination of soleus and gastrocnemius muscle flaps or free tissue transfer.
Foot
Ideal reconstruction of the foot should provide thin and durable skin that will tolerate mechanical stress, and achieving this can be quite difficult. Skin grafts are seldom used for the foot, and are limited to non–weight-bearing portions with good underlying soft tissue.
Proximal non–weight-bearing areas (Achilles tendon and malleolar area). Local fasciocutaneous flaps are preferred. The lateral calcaneal artery flap, which is based on the peroneal artery branch, can cover exposed Achilles tendon, providing sensate coverage (sural nerve). The dorsalis pedis flap can be mobilized to cover the malleolar region and distal Achilles tendon, but donor site morbidity limits its use. Free tissue transfer is required for larger defects, and the the main options are flaps from the radial forearm, temporoparietal fascia, or lateral arm.
Heel and midplantar area. For heel reconstruction, the medial plantar artery flap, dorsalis pedis flap, abductor myocutaneous flap, peroneal artery flap, or anterior tibial artery flap can be used. The most versatile flap of the foot is the medial plantar artery flap, which is available only when the posterior tibial artery is intact. If local flaps are not suitable, microvascular tissue transfer is indicated. The radial forearm flap, scapular flap, lateral arm flap, or anterolateral thigh flap can be used. The radial forearm flap is usually the first choice because it is thin, has a long pedicle, and is easy to harvest.
If the foot defect is associated with a large cavity, muscle flaps are the first choices, specifically the gracilis or anterior serratus. A split latissimus muscle can also be applied. The full latissimus or the rectus abdominis are often too large for the type of defects observed.
Distal plantar area and forefoot. Most wounds in this region will require free tissue transfer. Free muscle flaps with split-thickness skin grafts provide the most stable and durable coverage.
Amputation vs limb salvage
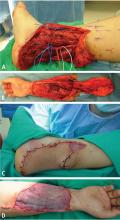
It is important to evaluate the effects of lower-extremity salvage on ambulation. Salvage of a nonfunctional limb is of little value for the patient. Likewise, patients with severe medical problems may not be good candidates for limb salvage procedures. In those situations, amputation of the lower extremity is indicated. Adequate soft-tissue coverage and good distal perfusion are necessary to ensure healing of an amputation. If possible, local tissue rearrangement may be enough to provide a good amputation stump to fit an external prosthesis. In the case of radiation damage to the tissue, a free tissue transfer is necessary. The calcaneal-plantar unit from the amputated limb is frequently used as a free flap. Other flaps from the amputated limb, called fillet flaps, are harvested immediately and converted to flaps transferred to the defect site. Studies show that they are oncologically safe and reliable.17 Other flaps that provide good coverage for amputation defects are the latissimus dorsi muscle flap, the radial forearm flap, and the anterolateral thigh flap.
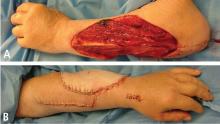
Upper extremities
POSTOPERATIVE CARE
Postoperative care following reconstruction after sarcoma resection requires a dedicated and trained team, particularly if a free flap is used for reconstruction.
Clinical evaluation of flaps includes color, temperature, and capillary refill. In cases of microsurgical reconstruction, postoperative care should include hourly examination of audible Doppler signals, at least for the first 36 hours. Free flap complications develop primarily in the first 24 hours, but they can occur during initial mobilization of the patient after a long period of bed rest. The surgical team should be aware of the potential problems and be able to act fast if necessary to reestablish blood flow to the flap.
In addition to flap monitoring, immobilization of the patient after surgery is extremely important. Postoperative swelling to the extremity should be avoided. Patients should be placed on bed rest until the postoperative swelling has subsided and the flap has adhered to the wound bed. Our protocol includes strict bed rest for about 7 days, followed by several days of dangling the extremity for short periods to ensure that dependent positioning will not alter the blood supply. A physical therapist should be involved to assist with crutches or a wheelchair. The patient should receive prophylactic anticoagulation during the resting period, in light of the high risk of deep vein thrombosis and pulmonary embolism. A compressive garment should be used to prevent lymphedema.
COMPLICATIONS ASSOCIATED WITH FLAPS
Once the flap is raised, it can still fail as a result of tension at insetting, inadequate blood flow, twisting of the pedicle, hematoma and/or infection, or the patient’s condition (eg, coagulopathy, poor nutritional status, anemia). Failure to correctly evaluate the direction of arterial flow, whether anterograde or retrograde, can cause flap loss. Instruments such as Doppler ultrasonographic equipment can be used to help to determine the flow. Partial or complete occlusion of the vascular pedicle can occur for several reasons (eg, twisting of the pedicle), and the consequences are disastrous if not recognized in time. If a pedicle problem is suspected in the case of a free flap, the patient should be taken to the operating room immediately and the flap should be explored. Rupture of the vascular anastomosis can occur as a result of technical problems, tension, and (in rare cases) infection.
Hematomas can cause mass effect, limit the venous return, and lead to flap necrosis. Hematoma formation also releases free radicals that can contribute to flap necrosis. Prevention is achieved through meticulous hemostasis. If a hematoma is suspected, the wound should be explored and the hematoma evacuated and washed out with normal saline.
The presence of an infected wound bed can also damage a flap by increasing its metabolic demand and causing the flap to be compromised by the infection itself. It is usually best to wait until the infection is controlled before planning the reconstruction.
Partial flap losses, skin graft losses, and wound dehiscence also are possible. Most of the time these require wound care, and patients’ nutrition and general health should be optimized to help the healing process. In the case of partial or complete flap loss, a new flap is often required and should be planned at a proper time.
CONCLUSIONS
Soft-tissue reconstruction following musculoskeletal sarcoma resection can be as simple as allowing the wound to heal by itself, which is less ideal, or as complex as coverage with a microsurgical osteocutaneous free flap. Limb salvage for sarcomas of the lower extremity has demonstrated good final functional outcomes without adversely affecting the oncologic results. Moreover, patients feel better psychologically and have higher quality of life.18,19
We believe that soft-tissue coverage after a wide resection is the most critical factor for avoiding postoperative complications of the tumor resection, such as infection or fractures. For this reason, we recommend the use of well-vascularized coverage at the time of the initial operation, if possible. Careful preoperative planning is especially important. We believe that reconstruction following musculoskeletal sarcoma resection can be done effectively only by using a team approach. Every such team should include, at minimum, an orthopedic surgeon and a reconstructive surgeon, with the mix of other providers dictated by the individual case.
- Misra A, Mistry N, Grimer R, Peart F. The management of soft tissue sarcoma. J Plast Reconstr Aesthet Surg 2009; 62:161–174.
- Morii T, Mochizuki K, Takushima A, Okazaki M, Satomi K. Soft tissue reconstruction using vascularized tissue transplantation following resection of musculoskeletal sarcoma: evaluation of oncologic and functional outcomes in 55 cases. Ann Plast Surg 2009; 62:252–257.
- Heller L, Kronowitz SJ. Lower extremity reconstruction. J Surg Oncol 2006; 94:479–489.
- Bannasch H, Haivas I, Momeni A, Stark GB. Oncosurgical and reconstructive concepts in the treatment of soft tissue sarcomas: a retrospective analysis. Arch Orthop Trauma Surg 2009; 129:43–49.
- Muramatsu K, Ihara K, Doi K, Hashimoto T, Taguchi T. Sarcoma in the forearm and hand: clinical outcomes and microsurgical reconstruction for limb salvage. Ann Plast Surg 2009; 62:28–33.
- Tukiainen E, Böhling T, Huuhtanen R. Soft tissue sarcoma of the trunk and extremities. Scand J Surg 2003; 92:257–263.
- Adelani MA, Holt GE, Dittus RS, Passman MA, Schwartz HS. Revascularization after segmental resection of lower extremity soft tissue sarcomas. J Surg Oncol 2007; 95:455–460.
- Lohman RF, Nabawi AS, Reece GP, Pollock RE, Evans GR. Soft tissue sarcoma of the upper extremity: a 5-year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer 2002; 94:2256–2264.
- Davis AM, Sennik S, Griffin AM, et al. Predictors of functional outcomes following limb salvage surgery for lower-extremity soft tissue sarcoma. J Surg Oncol 2000; 73:206–211.
- Heller L, Ballo MT, Cormier JN, Oates SD, Butler CE. Staged reconstruction for resection wounds in sarcoma patients treated with brachytherapy. Ann Plast Surg 2008; 60:58–63.
- Evans GR, Black JJ, Robb GL, et al. Adjuvant therapy: the effects on microvascular lower extremity reconstruction. Ann Plast Surg 1997; 39:141–144.
- Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg 1994; 93:980–987.
- Barwick WJ, Goldberg JA, Scully SP, Harrelson JM. Vascularized tissue transfer for closure of irradiated wounds after soft tissue sarcoma resection. Ann Surg 1992; 216:591–595.
- Masquelet AC, Romana MC. The medialis pedis flap: a new fasciocutaneous flap. Plast Reconstr Surg 1990; 85:765–772.
- Sherman R, Law M. Lower extremity reconstruction. In: Achauer BM, Eriksson E, Guyuron B, Coleman III JJ, Russell RC, Vander Kolk CA, eds. Plastic Surgery: Indications, Operations, and Outcomes. Vol 1. St. Louis, MO: Mosby; 2000:475–496.
- Innocenti M, Abed YY, Beltrami G, Delcroix L, Balatri A, Capanna R. Quadriceps muscle reconstruction with free functioning latissimus dorsi muscle flap after oncological resection. Microsurgery 2009; 29:189–198.
- Chiang YC, Wei FC, Wang JW, Chen WS. Reconstruction of below-knee stump using the salvaged foot fillet flap. Plast Reconstr Surg 1995; 96:731–738.
- Serletti JM, Carras AJ, O’Keefe RJ, Rosier RN. Functional outcome after soft-tissue reconstruction for limb salvage after sarcoma surgery. Plast Reconstr Surg 1998; 102:1576–1583.
- Niimi R, Matsumine A, Kusuzaki K, et al. Usefulness of limb salvage surgery for bone and soft tissue sarcomas of the distal lower leg. J Cancer Res Clin Oncol 2008; 134:1087–1095.
Advances in the management of soft-tissue and bone sarcomas—referred to collectively as “musculoskeletal sarcomas” hereafter—have resulted in significant improvements in survival and quality of life.1–3 Several factors have likely contributed to these advances, including improved surgical technique and the development of referral centers for sarcoma treatment that have embraced a multidisciplinary approach.1,2
The goal of treatment for musculoskeletal sarcomas is to optimize oncologic outcome and maximize functional restoration.2,3 Surgical resection has been the mainstay of therapy,1–7 as detailed earlier in this supplement. In patients with musculoskeletal sarcomas of the extremities, limb-sparing resection has been shown to be significantly superior to amputation.1,7–9 Wide local excision of the tumor along with its muscle compartment, followed by adjuvant chemotherapy and radiation therapy, has allowed limb salvage without an increased risk of recurrence in many patients.3 However, wide tumor resection can leave large defects that are not amenable to coverage by mobilization of the surrounding tissues, particularly if those tissues have been irradiated. As a result, resection can expose neurovascular structures, bone without periosteum, alloplastic materials, and internal fixation devices.
GOALS OF RECONSTRUCTION
Reconstructive surgery after musculoskeletal sarcoma resection aims to provide adequate wound coverage, preserve function, and optimize the cosmetic outcome.1–3 Tumors can be found on areas crucial to limb movement or may involve tissues vital to limb function. Reconstruction to repair these deficits can take many forms. In certain situations, amputation is still inevitable. In those cases, the reconstruction should provide stable stump coverage with durability and the ability to fit well with an external prosthesis.3
TIMING OF RECONSTRUCTION
Immediate reconstruction should be pursued if possible
Immediate reconstruction after a negative margin should always be considered and should be attempted when possible. Immediate reconstruction allows the reconstructive surgeon to benefit from better evaluation of the defect and exposed structures, as no scar tissue is present to distort the anatomy. Likewise, patients benefit from faster recovery and can receive adjuvant treatment (if necessary) sooner, as well as earlier rehabilitation. Patients may also benefit psychologically from immediate reconstruction.1,3
Indications for delayed reconstruction
Delayed reconstruction is primarily indicated when there are wound healing problems or there is uncertainty about the tumor margins. Secondary indications for delayed reconstruction are wound dehiscence and unstable soft-tissue coverage. If hardware is exposed, the recommendation is for early intervention and wound coverage with well-vascularized tissue to protect and cover the implant or prosthesis.
What about radiation therapy?
A very important consideration in reconstruction is the need for neoadjuvant or adjuvant radiation therapy.3,10,11 Irradiated wounds have a higher incidence of complications, including a tendency to dehisce. In patients who have been previously irradiated, the best practice is to perform immediate reconstruction with well-vascularized tissue, most likely a free tissue transfer.4,6,11,12 This practice reduces hospital stay, costs, and morbidity and increases limb salvage and patient satisfaction.13
SYSTEMATIC PREOPERATIVE PLANNING NEEDED
Reconstruction after musculoskeletal sarcoma resection should be planned systematically within a process that involves preoperative anticipation of the defect size and the resulting functional and cosmetic deficits that might need to be addressed. A preoperative visit to the reconstructive surgeon can be very helpful for presurgical planning.
During surgery it is usually preferable to allow the surgeon doing the tumor resection (eg, surgical oncologist or orthopedic oncologist) to complete the resection because the dimensions of the defect are not certain until negative margins are obtained.14 If tumor margins are unclear at the time of initial resection, the surgeon should consider delaying the definitive reconstruction until the permanent sections confirm negative margins. Temporary closure can be achieved with wound dressings, skin grafts (either allograft or autograft), or negative-pressure wound therapy. In the same context, if neurovascular structures are exposed it is reasonable to use a muscle flap without “tailoring” the flap to the defect. This approach allows the flap to be advanced or repositioned in case of positive margins, and the skin graft can be applied to the muscle surface in a second procedure.3
RECONSTRUCTIVE METHODS: A BRIEF OVERVIEW
Several methods can be used to close musculoskeletal sarcoma excision defects. Smaller defects can be closed primarily, although most defects are large and not amenable to primary closure. If fascia or muscle is preserved with only the skin coverage missing, the wound can be covered with either split-thickness or full-thickness skin grafts.1,4,6 Split-thickness skin grafts can be obtained in larger amounts and often heal faster than full-thickness skin grafts. However, most resections will require durable tissue coverage, particularly if adjuvant radiation therapy is planned.
In the case of long bone sarcoma resection, the resulting defect is usually large and complex and the traditional reconstruction is based on avascular allografts and local tissue flaps. However, allografts are associated with high rates of infection, nonunion, and fracture, leading to failure in about 50% of cases. Microvascular free flaps that contain bone, such as free fibula flaps, have been used instead of allografts with good success rates.2
Lately there has been growing interest in the use of the vacuum-assisted closure device (a form of negative-pressure wound therapy) to promote wound healing. It has been shown to improve the granulation and healing of open wounds by absorbing moisture, as well as to promote adherence after skin grafting, thereby reducing the risk of graft displacement.1,3 This device can be used immediately after musculoskeletal sarcoma resection while definitive tumor margin results are pending. It also can be used to prepare the wound bed for grafting in high-risk patients who would not tolerate more complex reconstructions.
Local or adjacent fascial, fasciocutaneous, and dermal flaps can also be used in lower-extremity reconstruction. However, muscle or musculocutaneous flaps are the mainstay of reconstruction after resection of musculoskeletal sarcomas. This group also includes perforator flaps, which have grown in popularity in the last few years.1,3
LOCATION-BASED WOUND RECONSTRUCTION
Musculoskeletal sarcomas can occur in virtually any region of the body, and myriad reconstructive options are available for various body sites. Since lower-extremity musculoskeletal sarcomas represent about 75% of cases,1 we will focus mainly on reconstruction of the lower extremity.
Factors driving choice of flap
Selection of an appropriate flap is essential to an optimal outcome. Flaps should be chosen with regard to donor site morbidity, functional requirements, length and diameter of the vascular pedicle, and aesthetic outcome.3 Usually physical examination, palpation of peripheral pulses, and Doppler ultrasonography are sufficient to evaluate the circulation. A preoperative angiogram should be considered in patients with severe peripheral vascular disease or previous trauma, which can potentially compromise the reconstructive outcome.15
Each region of the lower extremity possesses unique anatomic and functional characteristics that must be evaluated. It is useful to categorize the thigh, lower leg, and foot into separate anatomic units when planning reconstruction. We further divided these units into several subunits, as previously proposed by Sherman and Law15 and as outlined below.
Thigh
The thigh is usually well perfused and has several muscle groups, which facilitates reconstruction. Primary closure, skin grafts, or local flaps are acceptable options in most cases. The remaining musculature can be rotated or advanced to cover defects in the anterior or posterior thigh, providing bulk and adequate blood supply.
Hip and proximal/lateral thigh. Local muscle or myocutaneous flap options include tensor fascia lata, vastus lateralis, and rectus femoris flaps, all of which are based on the lateral circumflex femoral artery.
The tensor fascia lata flap is thin but has a long fascia extension that can be elevated from above the knee and can include a large skin paddle that is innervated by the lateral femoral cutaneous nerve. Some patients may experience knee instability after tensor fascia lata harvest.
The vastus lateralis muscle flap provides good bulk. Its arc of rotation reaches most of the inferior and posterior pelvis. It has little effect on ambulation.
The rectus femoris muscle flap is not so bulky, is easily mobilized, and has a wide arc of rotation. The donor site can be closed primarily. Harvest of this muscle can be associated with some strength loss during knee extension. For large defects of the upper third of the leg, a pedicled rectus abdominis muscle flap based on the deep inferior epigastric artery can be used. A vertically oriented skin island can be extended up to the costal margin, improving the reach. When the nature of the wound precludes use of pedicle flaps, free tissue transfer is indicated, with the latissimus dorsi muscle flap being used most commonly.15,16
Mid-thigh. Wounds in this location often can be closed with skin grafts or fasciocutaneous flaps. If the femur is exposed, however, a muscle flap will be required. As above, the tensor fascia lata, vastus lateralis, and rectus femoris can be used as flap options. If the lateral circumflex artery is unavailable, other flap options include the gracilis, vastus medialis, and rectus abdominis muscles. The gracilis muscle flap is based on the medial circumflex femoral artery and is useful for covering the medial aspect of the mid-thigh. Although this is a thin muscle, it can be used to cover long defects. The vastus medialis muscle flap is supplied by perforators from the profunda femoris and superficial femoral arteries. It can be rotated medially and advanced distally to cover patellar defects.
Supracondylar knee. The knee is a location where sarcoma resection is particularly likely to leave a defect with exposed bone, tendons, or ligaments that will need coverage. The gastrocnemius muscle flap combined with a split-thickness skin graft remains a consistent and reliable reconstructive option for this area. Other options are an extended medial gastrocnemius muscle flap or myocutaneous flap, which incorporates a random fasciocutaneous extension. For larger defects, free flaps should be considered, such as the anterior thigh flap, rectus abdominis muscle flap, or latissimus dorsi muscle flap. If tendons or ligaments need to be reconstructed, we favor autologous tissue, such as the fascia lata and plantaris tendons. These are easy to harvest and provide long-lasting joint stability.
Lower leg
Proximal third of the tibia. Defects here can usually be covered with a medial or lateral gastrocnemius muscle or myocutaneous flap, or a combination of the two. These muscles have a dominant vascular pedicle—the medial and lateral sural arteries. They can be harvested as an island for better reach, and they are reliable and have minimal donor site morbidity.15 The soleus muscle flap is another option that can be used alone or in combination with the medial or lateral gastrocnemius. Defects that are not amenable to closure by these flaps will most likely require free tissue transfer. The rectus abdominis or latissimus dorsi muscles are the first options. The latter can be combined with the serratus muscle if more bulk is needed.
Middle and lower thirds of the tibia. The soleus flap is frequently used for small or medium-sized mid-tibial defects. It is based on branches of the popliteal artery and posterior tibial artery. Larger defects require a combination of soleus and gastrocnemius muscle flaps or free tissue transfer.
Foot
Ideal reconstruction of the foot should provide thin and durable skin that will tolerate mechanical stress, and achieving this can be quite difficult. Skin grafts are seldom used for the foot, and are limited to non–weight-bearing portions with good underlying soft tissue.
Proximal non–weight-bearing areas (Achilles tendon and malleolar area). Local fasciocutaneous flaps are preferred. The lateral calcaneal artery flap, which is based on the peroneal artery branch, can cover exposed Achilles tendon, providing sensate coverage (sural nerve). The dorsalis pedis flap can be mobilized to cover the malleolar region and distal Achilles tendon, but donor site morbidity limits its use. Free tissue transfer is required for larger defects, and the the main options are flaps from the radial forearm, temporoparietal fascia, or lateral arm.
Heel and midplantar area. For heel reconstruction, the medial plantar artery flap, dorsalis pedis flap, abductor myocutaneous flap, peroneal artery flap, or anterior tibial artery flap can be used. The most versatile flap of the foot is the medial plantar artery flap, which is available only when the posterior tibial artery is intact. If local flaps are not suitable, microvascular tissue transfer is indicated. The radial forearm flap, scapular flap, lateral arm flap, or anterolateral thigh flap can be used. The radial forearm flap is usually the first choice because it is thin, has a long pedicle, and is easy to harvest.
If the foot defect is associated with a large cavity, muscle flaps are the first choices, specifically the gracilis or anterior serratus. A split latissimus muscle can also be applied. The full latissimus or the rectus abdominis are often too large for the type of defects observed.
Distal plantar area and forefoot. Most wounds in this region will require free tissue transfer. Free muscle flaps with split-thickness skin grafts provide the most stable and durable coverage.
Amputation vs limb salvage
It is important to evaluate the effects of lower-extremity salvage on ambulation. Salvage of a nonfunctional limb is of little value for the patient. Likewise, patients with severe medical problems may not be good candidates for limb salvage procedures. In those situations, amputation of the lower extremity is indicated. Adequate soft-tissue coverage and good distal perfusion are necessary to ensure healing of an amputation. If possible, local tissue rearrangement may be enough to provide a good amputation stump to fit an external prosthesis. In the case of radiation damage to the tissue, a free tissue transfer is necessary. The calcaneal-plantar unit from the amputated limb is frequently used as a free flap. Other flaps from the amputated limb, called fillet flaps, are harvested immediately and converted to flaps transferred to the defect site. Studies show that they are oncologically safe and reliable.17 Other flaps that provide good coverage for amputation defects are the latissimus dorsi muscle flap, the radial forearm flap, and the anterolateral thigh flap.
Upper extremities
POSTOPERATIVE CARE
Postoperative care following reconstruction after sarcoma resection requires a dedicated and trained team, particularly if a free flap is used for reconstruction.
Clinical evaluation of flaps includes color, temperature, and capillary refill. In cases of microsurgical reconstruction, postoperative care should include hourly examination of audible Doppler signals, at least for the first 36 hours. Free flap complications develop primarily in the first 24 hours, but they can occur during initial mobilization of the patient after a long period of bed rest. The surgical team should be aware of the potential problems and be able to act fast if necessary to reestablish blood flow to the flap.
In addition to flap monitoring, immobilization of the patient after surgery is extremely important. Postoperative swelling to the extremity should be avoided. Patients should be placed on bed rest until the postoperative swelling has subsided and the flap has adhered to the wound bed. Our protocol includes strict bed rest for about 7 days, followed by several days of dangling the extremity for short periods to ensure that dependent positioning will not alter the blood supply. A physical therapist should be involved to assist with crutches or a wheelchair. The patient should receive prophylactic anticoagulation during the resting period, in light of the high risk of deep vein thrombosis and pulmonary embolism. A compressive garment should be used to prevent lymphedema.
COMPLICATIONS ASSOCIATED WITH FLAPS
Once the flap is raised, it can still fail as a result of tension at insetting, inadequate blood flow, twisting of the pedicle, hematoma and/or infection, or the patient’s condition (eg, coagulopathy, poor nutritional status, anemia). Failure to correctly evaluate the direction of arterial flow, whether anterograde or retrograde, can cause flap loss. Instruments such as Doppler ultrasonographic equipment can be used to help to determine the flow. Partial or complete occlusion of the vascular pedicle can occur for several reasons (eg, twisting of the pedicle), and the consequences are disastrous if not recognized in time. If a pedicle problem is suspected in the case of a free flap, the patient should be taken to the operating room immediately and the flap should be explored. Rupture of the vascular anastomosis can occur as a result of technical problems, tension, and (in rare cases) infection.
Hematomas can cause mass effect, limit the venous return, and lead to flap necrosis. Hematoma formation also releases free radicals that can contribute to flap necrosis. Prevention is achieved through meticulous hemostasis. If a hematoma is suspected, the wound should be explored and the hematoma evacuated and washed out with normal saline.
The presence of an infected wound bed can also damage a flap by increasing its metabolic demand and causing the flap to be compromised by the infection itself. It is usually best to wait until the infection is controlled before planning the reconstruction.
Partial flap losses, skin graft losses, and wound dehiscence also are possible. Most of the time these require wound care, and patients’ nutrition and general health should be optimized to help the healing process. In the case of partial or complete flap loss, a new flap is often required and should be planned at a proper time.
CONCLUSIONS
Soft-tissue reconstruction following musculoskeletal sarcoma resection can be as simple as allowing the wound to heal by itself, which is less ideal, or as complex as coverage with a microsurgical osteocutaneous free flap. Limb salvage for sarcomas of the lower extremity has demonstrated good final functional outcomes without adversely affecting the oncologic results. Moreover, patients feel better psychologically and have higher quality of life.18,19
We believe that soft-tissue coverage after a wide resection is the most critical factor for avoiding postoperative complications of the tumor resection, such as infection or fractures. For this reason, we recommend the use of well-vascularized coverage at the time of the initial operation, if possible. Careful preoperative planning is especially important. We believe that reconstruction following musculoskeletal sarcoma resection can be done effectively only by using a team approach. Every such team should include, at minimum, an orthopedic surgeon and a reconstructive surgeon, with the mix of other providers dictated by the individual case.
Advances in the management of soft-tissue and bone sarcomas—referred to collectively as “musculoskeletal sarcomas” hereafter—have resulted in significant improvements in survival and quality of life.1–3 Several factors have likely contributed to these advances, including improved surgical technique and the development of referral centers for sarcoma treatment that have embraced a multidisciplinary approach.1,2
The goal of treatment for musculoskeletal sarcomas is to optimize oncologic outcome and maximize functional restoration.2,3 Surgical resection has been the mainstay of therapy,1–7 as detailed earlier in this supplement. In patients with musculoskeletal sarcomas of the extremities, limb-sparing resection has been shown to be significantly superior to amputation.1,7–9 Wide local excision of the tumor along with its muscle compartment, followed by adjuvant chemotherapy and radiation therapy, has allowed limb salvage without an increased risk of recurrence in many patients.3 However, wide tumor resection can leave large defects that are not amenable to coverage by mobilization of the surrounding tissues, particularly if those tissues have been irradiated. As a result, resection can expose neurovascular structures, bone without periosteum, alloplastic materials, and internal fixation devices.
GOALS OF RECONSTRUCTION
Reconstructive surgery after musculoskeletal sarcoma resection aims to provide adequate wound coverage, preserve function, and optimize the cosmetic outcome.1–3 Tumors can be found on areas crucial to limb movement or may involve tissues vital to limb function. Reconstruction to repair these deficits can take many forms. In certain situations, amputation is still inevitable. In those cases, the reconstruction should provide stable stump coverage with durability and the ability to fit well with an external prosthesis.3
TIMING OF RECONSTRUCTION
Immediate reconstruction should be pursued if possible
Immediate reconstruction after a negative margin should always be considered and should be attempted when possible. Immediate reconstruction allows the reconstructive surgeon to benefit from better evaluation of the defect and exposed structures, as no scar tissue is present to distort the anatomy. Likewise, patients benefit from faster recovery and can receive adjuvant treatment (if necessary) sooner, as well as earlier rehabilitation. Patients may also benefit psychologically from immediate reconstruction.1,3
Indications for delayed reconstruction
Delayed reconstruction is primarily indicated when there are wound healing problems or there is uncertainty about the tumor margins. Secondary indications for delayed reconstruction are wound dehiscence and unstable soft-tissue coverage. If hardware is exposed, the recommendation is for early intervention and wound coverage with well-vascularized tissue to protect and cover the implant or prosthesis.
What about radiation therapy?
A very important consideration in reconstruction is the need for neoadjuvant or adjuvant radiation therapy.3,10,11 Irradiated wounds have a higher incidence of complications, including a tendency to dehisce. In patients who have been previously irradiated, the best practice is to perform immediate reconstruction with well-vascularized tissue, most likely a free tissue transfer.4,6,11,12 This practice reduces hospital stay, costs, and morbidity and increases limb salvage and patient satisfaction.13
SYSTEMATIC PREOPERATIVE PLANNING NEEDED
Reconstruction after musculoskeletal sarcoma resection should be planned systematically within a process that involves preoperative anticipation of the defect size and the resulting functional and cosmetic deficits that might need to be addressed. A preoperative visit to the reconstructive surgeon can be very helpful for presurgical planning.
During surgery it is usually preferable to allow the surgeon doing the tumor resection (eg, surgical oncologist or orthopedic oncologist) to complete the resection because the dimensions of the defect are not certain until negative margins are obtained.14 If tumor margins are unclear at the time of initial resection, the surgeon should consider delaying the definitive reconstruction until the permanent sections confirm negative margins. Temporary closure can be achieved with wound dressings, skin grafts (either allograft or autograft), or negative-pressure wound therapy. In the same context, if neurovascular structures are exposed it is reasonable to use a muscle flap without “tailoring” the flap to the defect. This approach allows the flap to be advanced or repositioned in case of positive margins, and the skin graft can be applied to the muscle surface in a second procedure.3
RECONSTRUCTIVE METHODS: A BRIEF OVERVIEW
Several methods can be used to close musculoskeletal sarcoma excision defects. Smaller defects can be closed primarily, although most defects are large and not amenable to primary closure. If fascia or muscle is preserved with only the skin coverage missing, the wound can be covered with either split-thickness or full-thickness skin grafts.1,4,6 Split-thickness skin grafts can be obtained in larger amounts and often heal faster than full-thickness skin grafts. However, most resections will require durable tissue coverage, particularly if adjuvant radiation therapy is planned.
In the case of long bone sarcoma resection, the resulting defect is usually large and complex and the traditional reconstruction is based on avascular allografts and local tissue flaps. However, allografts are associated with high rates of infection, nonunion, and fracture, leading to failure in about 50% of cases. Microvascular free flaps that contain bone, such as free fibula flaps, have been used instead of allografts with good success rates.2
Lately there has been growing interest in the use of the vacuum-assisted closure device (a form of negative-pressure wound therapy) to promote wound healing. It has been shown to improve the granulation and healing of open wounds by absorbing moisture, as well as to promote adherence after skin grafting, thereby reducing the risk of graft displacement.1,3 This device can be used immediately after musculoskeletal sarcoma resection while definitive tumor margin results are pending. It also can be used to prepare the wound bed for grafting in high-risk patients who would not tolerate more complex reconstructions.
Local or adjacent fascial, fasciocutaneous, and dermal flaps can also be used in lower-extremity reconstruction. However, muscle or musculocutaneous flaps are the mainstay of reconstruction after resection of musculoskeletal sarcomas. This group also includes perforator flaps, which have grown in popularity in the last few years.1,3
LOCATION-BASED WOUND RECONSTRUCTION
Musculoskeletal sarcomas can occur in virtually any region of the body, and myriad reconstructive options are available for various body sites. Since lower-extremity musculoskeletal sarcomas represent about 75% of cases,1 we will focus mainly on reconstruction of the lower extremity.
Factors driving choice of flap
Selection of an appropriate flap is essential to an optimal outcome. Flaps should be chosen with regard to donor site morbidity, functional requirements, length and diameter of the vascular pedicle, and aesthetic outcome.3 Usually physical examination, palpation of peripheral pulses, and Doppler ultrasonography are sufficient to evaluate the circulation. A preoperative angiogram should be considered in patients with severe peripheral vascular disease or previous trauma, which can potentially compromise the reconstructive outcome.15
Each region of the lower extremity possesses unique anatomic and functional characteristics that must be evaluated. It is useful to categorize the thigh, lower leg, and foot into separate anatomic units when planning reconstruction. We further divided these units into several subunits, as previously proposed by Sherman and Law15 and as outlined below.
Thigh
The thigh is usually well perfused and has several muscle groups, which facilitates reconstruction. Primary closure, skin grafts, or local flaps are acceptable options in most cases. The remaining musculature can be rotated or advanced to cover defects in the anterior or posterior thigh, providing bulk and adequate blood supply.
Hip and proximal/lateral thigh. Local muscle or myocutaneous flap options include tensor fascia lata, vastus lateralis, and rectus femoris flaps, all of which are based on the lateral circumflex femoral artery.
The tensor fascia lata flap is thin but has a long fascia extension that can be elevated from above the knee and can include a large skin paddle that is innervated by the lateral femoral cutaneous nerve. Some patients may experience knee instability after tensor fascia lata harvest.
The vastus lateralis muscle flap provides good bulk. Its arc of rotation reaches most of the inferior and posterior pelvis. It has little effect on ambulation.
The rectus femoris muscle flap is not so bulky, is easily mobilized, and has a wide arc of rotation. The donor site can be closed primarily. Harvest of this muscle can be associated with some strength loss during knee extension. For large defects of the upper third of the leg, a pedicled rectus abdominis muscle flap based on the deep inferior epigastric artery can be used. A vertically oriented skin island can be extended up to the costal margin, improving the reach. When the nature of the wound precludes use of pedicle flaps, free tissue transfer is indicated, with the latissimus dorsi muscle flap being used most commonly.15,16
Mid-thigh. Wounds in this location often can be closed with skin grafts or fasciocutaneous flaps. If the femur is exposed, however, a muscle flap will be required. As above, the tensor fascia lata, vastus lateralis, and rectus femoris can be used as flap options. If the lateral circumflex artery is unavailable, other flap options include the gracilis, vastus medialis, and rectus abdominis muscles. The gracilis muscle flap is based on the medial circumflex femoral artery and is useful for covering the medial aspect of the mid-thigh. Although this is a thin muscle, it can be used to cover long defects. The vastus medialis muscle flap is supplied by perforators from the profunda femoris and superficial femoral arteries. It can be rotated medially and advanced distally to cover patellar defects.
Supracondylar knee. The knee is a location where sarcoma resection is particularly likely to leave a defect with exposed bone, tendons, or ligaments that will need coverage. The gastrocnemius muscle flap combined with a split-thickness skin graft remains a consistent and reliable reconstructive option for this area. Other options are an extended medial gastrocnemius muscle flap or myocutaneous flap, which incorporates a random fasciocutaneous extension. For larger defects, free flaps should be considered, such as the anterior thigh flap, rectus abdominis muscle flap, or latissimus dorsi muscle flap. If tendons or ligaments need to be reconstructed, we favor autologous tissue, such as the fascia lata and plantaris tendons. These are easy to harvest and provide long-lasting joint stability.
Lower leg
Proximal third of the tibia. Defects here can usually be covered with a medial or lateral gastrocnemius muscle or myocutaneous flap, or a combination of the two. These muscles have a dominant vascular pedicle—the medial and lateral sural arteries. They can be harvested as an island for better reach, and they are reliable and have minimal donor site morbidity.15 The soleus muscle flap is another option that can be used alone or in combination with the medial or lateral gastrocnemius. Defects that are not amenable to closure by these flaps will most likely require free tissue transfer. The rectus abdominis or latissimus dorsi muscles are the first options. The latter can be combined with the serratus muscle if more bulk is needed.
Middle and lower thirds of the tibia. The soleus flap is frequently used for small or medium-sized mid-tibial defects. It is based on branches of the popliteal artery and posterior tibial artery. Larger defects require a combination of soleus and gastrocnemius muscle flaps or free tissue transfer.
Foot
Ideal reconstruction of the foot should provide thin and durable skin that will tolerate mechanical stress, and achieving this can be quite difficult. Skin grafts are seldom used for the foot, and are limited to non–weight-bearing portions with good underlying soft tissue.
Proximal non–weight-bearing areas (Achilles tendon and malleolar area). Local fasciocutaneous flaps are preferred. The lateral calcaneal artery flap, which is based on the peroneal artery branch, can cover exposed Achilles tendon, providing sensate coverage (sural nerve). The dorsalis pedis flap can be mobilized to cover the malleolar region and distal Achilles tendon, but donor site morbidity limits its use. Free tissue transfer is required for larger defects, and the the main options are flaps from the radial forearm, temporoparietal fascia, or lateral arm.
Heel and midplantar area. For heel reconstruction, the medial plantar artery flap, dorsalis pedis flap, abductor myocutaneous flap, peroneal artery flap, or anterior tibial artery flap can be used. The most versatile flap of the foot is the medial plantar artery flap, which is available only when the posterior tibial artery is intact. If local flaps are not suitable, microvascular tissue transfer is indicated. The radial forearm flap, scapular flap, lateral arm flap, or anterolateral thigh flap can be used. The radial forearm flap is usually the first choice because it is thin, has a long pedicle, and is easy to harvest.
If the foot defect is associated with a large cavity, muscle flaps are the first choices, specifically the gracilis or anterior serratus. A split latissimus muscle can also be applied. The full latissimus or the rectus abdominis are often too large for the type of defects observed.
Distal plantar area and forefoot. Most wounds in this region will require free tissue transfer. Free muscle flaps with split-thickness skin grafts provide the most stable and durable coverage.
Amputation vs limb salvage
It is important to evaluate the effects of lower-extremity salvage on ambulation. Salvage of a nonfunctional limb is of little value for the patient. Likewise, patients with severe medical problems may not be good candidates for limb salvage procedures. In those situations, amputation of the lower extremity is indicated. Adequate soft-tissue coverage and good distal perfusion are necessary to ensure healing of an amputation. If possible, local tissue rearrangement may be enough to provide a good amputation stump to fit an external prosthesis. In the case of radiation damage to the tissue, a free tissue transfer is necessary. The calcaneal-plantar unit from the amputated limb is frequently used as a free flap. Other flaps from the amputated limb, called fillet flaps, are harvested immediately and converted to flaps transferred to the defect site. Studies show that they are oncologically safe and reliable.17 Other flaps that provide good coverage for amputation defects are the latissimus dorsi muscle flap, the radial forearm flap, and the anterolateral thigh flap.
Upper extremities
POSTOPERATIVE CARE
Postoperative care following reconstruction after sarcoma resection requires a dedicated and trained team, particularly if a free flap is used for reconstruction.
Clinical evaluation of flaps includes color, temperature, and capillary refill. In cases of microsurgical reconstruction, postoperative care should include hourly examination of audible Doppler signals, at least for the first 36 hours. Free flap complications develop primarily in the first 24 hours, but they can occur during initial mobilization of the patient after a long period of bed rest. The surgical team should be aware of the potential problems and be able to act fast if necessary to reestablish blood flow to the flap.
In addition to flap monitoring, immobilization of the patient after surgery is extremely important. Postoperative swelling to the extremity should be avoided. Patients should be placed on bed rest until the postoperative swelling has subsided and the flap has adhered to the wound bed. Our protocol includes strict bed rest for about 7 days, followed by several days of dangling the extremity for short periods to ensure that dependent positioning will not alter the blood supply. A physical therapist should be involved to assist with crutches or a wheelchair. The patient should receive prophylactic anticoagulation during the resting period, in light of the high risk of deep vein thrombosis and pulmonary embolism. A compressive garment should be used to prevent lymphedema.
COMPLICATIONS ASSOCIATED WITH FLAPS
Once the flap is raised, it can still fail as a result of tension at insetting, inadequate blood flow, twisting of the pedicle, hematoma and/or infection, or the patient’s condition (eg, coagulopathy, poor nutritional status, anemia). Failure to correctly evaluate the direction of arterial flow, whether anterograde or retrograde, can cause flap loss. Instruments such as Doppler ultrasonographic equipment can be used to help to determine the flow. Partial or complete occlusion of the vascular pedicle can occur for several reasons (eg, twisting of the pedicle), and the consequences are disastrous if not recognized in time. If a pedicle problem is suspected in the case of a free flap, the patient should be taken to the operating room immediately and the flap should be explored. Rupture of the vascular anastomosis can occur as a result of technical problems, tension, and (in rare cases) infection.
Hematomas can cause mass effect, limit the venous return, and lead to flap necrosis. Hematoma formation also releases free radicals that can contribute to flap necrosis. Prevention is achieved through meticulous hemostasis. If a hematoma is suspected, the wound should be explored and the hematoma evacuated and washed out with normal saline.
The presence of an infected wound bed can also damage a flap by increasing its metabolic demand and causing the flap to be compromised by the infection itself. It is usually best to wait until the infection is controlled before planning the reconstruction.
Partial flap losses, skin graft losses, and wound dehiscence also are possible. Most of the time these require wound care, and patients’ nutrition and general health should be optimized to help the healing process. In the case of partial or complete flap loss, a new flap is often required and should be planned at a proper time.
CONCLUSIONS
Soft-tissue reconstruction following musculoskeletal sarcoma resection can be as simple as allowing the wound to heal by itself, which is less ideal, or as complex as coverage with a microsurgical osteocutaneous free flap. Limb salvage for sarcomas of the lower extremity has demonstrated good final functional outcomes without adversely affecting the oncologic results. Moreover, patients feel better psychologically and have higher quality of life.18,19
We believe that soft-tissue coverage after a wide resection is the most critical factor for avoiding postoperative complications of the tumor resection, such as infection or fractures. For this reason, we recommend the use of well-vascularized coverage at the time of the initial operation, if possible. Careful preoperative planning is especially important. We believe that reconstruction following musculoskeletal sarcoma resection can be done effectively only by using a team approach. Every such team should include, at minimum, an orthopedic surgeon and a reconstructive surgeon, with the mix of other providers dictated by the individual case.
- Misra A, Mistry N, Grimer R, Peart F. The management of soft tissue sarcoma. J Plast Reconstr Aesthet Surg 2009; 62:161–174.
- Morii T, Mochizuki K, Takushima A, Okazaki M, Satomi K. Soft tissue reconstruction using vascularized tissue transplantation following resection of musculoskeletal sarcoma: evaluation of oncologic and functional outcomes in 55 cases. Ann Plast Surg 2009; 62:252–257.
- Heller L, Kronowitz SJ. Lower extremity reconstruction. J Surg Oncol 2006; 94:479–489.
- Bannasch H, Haivas I, Momeni A, Stark GB. Oncosurgical and reconstructive concepts in the treatment of soft tissue sarcomas: a retrospective analysis. Arch Orthop Trauma Surg 2009; 129:43–49.
- Muramatsu K, Ihara K, Doi K, Hashimoto T, Taguchi T. Sarcoma in the forearm and hand: clinical outcomes and microsurgical reconstruction for limb salvage. Ann Plast Surg 2009; 62:28–33.
- Tukiainen E, Böhling T, Huuhtanen R. Soft tissue sarcoma of the trunk and extremities. Scand J Surg 2003; 92:257–263.
- Adelani MA, Holt GE, Dittus RS, Passman MA, Schwartz HS. Revascularization after segmental resection of lower extremity soft tissue sarcomas. J Surg Oncol 2007; 95:455–460.
- Lohman RF, Nabawi AS, Reece GP, Pollock RE, Evans GR. Soft tissue sarcoma of the upper extremity: a 5-year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer 2002; 94:2256–2264.
- Davis AM, Sennik S, Griffin AM, et al. Predictors of functional outcomes following limb salvage surgery for lower-extremity soft tissue sarcoma. J Surg Oncol 2000; 73:206–211.
- Heller L, Ballo MT, Cormier JN, Oates SD, Butler CE. Staged reconstruction for resection wounds in sarcoma patients treated with brachytherapy. Ann Plast Surg 2008; 60:58–63.
- Evans GR, Black JJ, Robb GL, et al. Adjuvant therapy: the effects on microvascular lower extremity reconstruction. Ann Plast Surg 1997; 39:141–144.
- Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg 1994; 93:980–987.
- Barwick WJ, Goldberg JA, Scully SP, Harrelson JM. Vascularized tissue transfer for closure of irradiated wounds after soft tissue sarcoma resection. Ann Surg 1992; 216:591–595.
- Masquelet AC, Romana MC. The medialis pedis flap: a new fasciocutaneous flap. Plast Reconstr Surg 1990; 85:765–772.
- Sherman R, Law M. Lower extremity reconstruction. In: Achauer BM, Eriksson E, Guyuron B, Coleman III JJ, Russell RC, Vander Kolk CA, eds. Plastic Surgery: Indications, Operations, and Outcomes. Vol 1. St. Louis, MO: Mosby; 2000:475–496.
- Innocenti M, Abed YY, Beltrami G, Delcroix L, Balatri A, Capanna R. Quadriceps muscle reconstruction with free functioning latissimus dorsi muscle flap after oncological resection. Microsurgery 2009; 29:189–198.
- Chiang YC, Wei FC, Wang JW, Chen WS. Reconstruction of below-knee stump using the salvaged foot fillet flap. Plast Reconstr Surg 1995; 96:731–738.
- Serletti JM, Carras AJ, O’Keefe RJ, Rosier RN. Functional outcome after soft-tissue reconstruction for limb salvage after sarcoma surgery. Plast Reconstr Surg 1998; 102:1576–1583.
- Niimi R, Matsumine A, Kusuzaki K, et al. Usefulness of limb salvage surgery for bone and soft tissue sarcomas of the distal lower leg. J Cancer Res Clin Oncol 2008; 134:1087–1095.
- Misra A, Mistry N, Grimer R, Peart F. The management of soft tissue sarcoma. J Plast Reconstr Aesthet Surg 2009; 62:161–174.
- Morii T, Mochizuki K, Takushima A, Okazaki M, Satomi K. Soft tissue reconstruction using vascularized tissue transplantation following resection of musculoskeletal sarcoma: evaluation of oncologic and functional outcomes in 55 cases. Ann Plast Surg 2009; 62:252–257.
- Heller L, Kronowitz SJ. Lower extremity reconstruction. J Surg Oncol 2006; 94:479–489.
- Bannasch H, Haivas I, Momeni A, Stark GB. Oncosurgical and reconstructive concepts in the treatment of soft tissue sarcomas: a retrospective analysis. Arch Orthop Trauma Surg 2009; 129:43–49.
- Muramatsu K, Ihara K, Doi K, Hashimoto T, Taguchi T. Sarcoma in the forearm and hand: clinical outcomes and microsurgical reconstruction for limb salvage. Ann Plast Surg 2009; 62:28–33.
- Tukiainen E, Böhling T, Huuhtanen R. Soft tissue sarcoma of the trunk and extremities. Scand J Surg 2003; 92:257–263.
- Adelani MA, Holt GE, Dittus RS, Passman MA, Schwartz HS. Revascularization after segmental resection of lower extremity soft tissue sarcomas. J Surg Oncol 2007; 95:455–460.
- Lohman RF, Nabawi AS, Reece GP, Pollock RE, Evans GR. Soft tissue sarcoma of the upper extremity: a 5-year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer 2002; 94:2256–2264.
- Davis AM, Sennik S, Griffin AM, et al. Predictors of functional outcomes following limb salvage surgery for lower-extremity soft tissue sarcoma. J Surg Oncol 2000; 73:206–211.
- Heller L, Ballo MT, Cormier JN, Oates SD, Butler CE. Staged reconstruction for resection wounds in sarcoma patients treated with brachytherapy. Ann Plast Surg 2008; 60:58–63.
- Evans GR, Black JJ, Robb GL, et al. Adjuvant therapy: the effects on microvascular lower extremity reconstruction. Ann Plast Surg 1997; 39:141–144.
- Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg 1994; 93:980–987.
- Barwick WJ, Goldberg JA, Scully SP, Harrelson JM. Vascularized tissue transfer for closure of irradiated wounds after soft tissue sarcoma resection. Ann Surg 1992; 216:591–595.
- Masquelet AC, Romana MC. The medialis pedis flap: a new fasciocutaneous flap. Plast Reconstr Surg 1990; 85:765–772.
- Sherman R, Law M. Lower extremity reconstruction. In: Achauer BM, Eriksson E, Guyuron B, Coleman III JJ, Russell RC, Vander Kolk CA, eds. Plastic Surgery: Indications, Operations, and Outcomes. Vol 1. St. Louis, MO: Mosby; 2000:475–496.
- Innocenti M, Abed YY, Beltrami G, Delcroix L, Balatri A, Capanna R. Quadriceps muscle reconstruction with free functioning latissimus dorsi muscle flap after oncological resection. Microsurgery 2009; 29:189–198.
- Chiang YC, Wei FC, Wang JW, Chen WS. Reconstruction of below-knee stump using the salvaged foot fillet flap. Plast Reconstr Surg 1995; 96:731–738.
- Serletti JM, Carras AJ, O’Keefe RJ, Rosier RN. Functional outcome after soft-tissue reconstruction for limb salvage after sarcoma surgery. Plast Reconstr Surg 1998; 102:1576–1583.
- Niimi R, Matsumine A, Kusuzaki K, et al. Usefulness of limb salvage surgery for bone and soft tissue sarcomas of the distal lower leg. J Cancer Res Clin Oncol 2008; 134:1087–1095.