User login
Sarcomas are rare neoplasms arising from connective tissue elements of the body. Approximately 80% arise in soft tissue, while the remainder originate in bone.1 Soft-tissue sarcomas are tumors of the mesenchymal system, and about half develop in the extremities. Bone sarcomas are characterized by their location in bone and sometimes produce osteoid, or immature bone.
The most common types of soft-tissue sarcomas are malignant fibrous histiocytoma (although this term has lost favor with some pathologists) and liposarcoma.
The most common types of bone sarcomas are osteosarcoma (a sarcoma that makes osteoid or bone), Ewing sarcoma (composed of small round blue cells with a characteristic chromosomal translocation), and chondrosarcoma (a sarcoma that makes chondroid tissue, or cartilage).
EPIDEMIOLOGY AND PRIMARY BODY SITES
Approximately 10,600 new cases of soft-tissue sarcoma and 2,570 new cases of bone sarcoma were estimated to have been diagnosed in the United States in 2009.2,3 For perspective, the annual incidence of soft-tissue sarcoma is approximately 5.5% that of breast cancer and approximately 5% that of lung cancer.3
Most sarcomas develop in the lower extremities, although the age groups at greatest risk vary among sarcoma types.4
Soft-tissue sarcomas develop most commonly in the thigh and occur primarily in adults.2
Osteosarcomas are the most common bone sarcoma and develop most frequently in 10- to 20-year-olds; their most common location is the distal femur.5–8 Metastatic osteosarcoma is found in approximately 20% of patients at the time of osteosarcoma diagnosis. Osteosarcomas mainly spread hematogenously, and the lungs are the most common initial site of metastases, being affected in up to 90% of patients with metastatic disease.9
Ewing sarcomas develop most often in the long bones of the extremities or bones of the pelvis. The large majority of cases develop in patients aged 10 to 15 years. 5–8
Chondrosarcomas represent approximately 20% of all bone sarcomas and primarily affect older adults, with a peak incidence in the sixth decade of life.10
OVERVIEW OF PRESENTATION AND EVALUATION
Presentation is highly variable
The clinical presentation of patients with bone or soft-tissue sarcoma is highly variable. Patients often present with a mass, typically one that is increasing in size. In general, bone sarcomas are painful and soft-tissue sarcomas are not, but there are exceptions to this general rule. Constitutional symptoms are rare in patients with bone or soft-tissue sarcomas, but symptoms such as fever, malaise, and weight loss can be seen, especially in patients with Ewing sarcoma.11
Delayed presentation and diagnosis are common
Particularly when a sarcoma is painless, patients sometimes do not seek medical attention until a suspicious mass becomes quite large. Certain tumors, such as synovial sarcoma, a high-grade soft-tissue sarcoma often seen in young adults, may present as a slowly growing or stable-appearing mass over several years. In one study of 33 children with synovial sarcoma, the mean duration of symptoms was 98 weeks (range, 2–364), the mean patient delay before a doctor was seen was 43 weeks (0–156), the mean doctor delay before a correct diagnosis was made was 50 weeks (0–362), and the mean number of doctors seen before referral was 3 (1–6).12 For nearly half the patients in this study (15), the diagnosis was obtained only after unplanned excision, meaning that the surgeon did not expect a malignancy at the time of biopsy. Because delayed presentation is not uncommon in cases of bone or soft-tissue sarcoma, every patient with a mass with indeterminate imaging findings should be referred to or reviewed by an orthopedic or musculoskeletal oncologist.
Biopsy is gold standard for diagnosis
A comprehensive medical history and physical examination are essential at the initial presentation of patients with masses and/or pain suggestive of bone or soft-tissue sarcoma. Sarcoma simulators such as hematoma, metastatic disease, or infection can sometimes be ruled out by careful clinical examination, laboratory work-up, and appropriate imaging, but the gold standard for diagnosis is a biopsy. Moreover, an index of suspicion is required to rule out primary malignancy in any soft-tissue or bone lesion, and this index of suspicion will allow for referral or appropriate selection of the site for biopsy.
Biopsy considerations, as well as further detail on clinical presentation, are provided in the second and third articles in this supplement, which focus, respectively, on bone sarcoma and soft-tissue sarcoma. The remainder of this article reviews the use of imaging for the evaluation of suspected sarcomas, as imaging findings typically prompt or guide biopsy of a suspicious mass. Choosing the right imaging modality is critical to the diagnosis and management of patients with suspected sarcoma.
CONVENTIONAL IMAGING MODALITIES
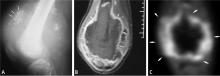
Despite their utility for evaluating osseous lesions, radiographs have limited to no value in the evaluation of soft-tissue sarcomas but can demonstrate matrix mineralization and erosion or destruction of adjacent bone.
Angiography. In the past, angiography was frequently used to assess the vascularity of sarcomas preoperatively. Diagnostic angiography has been replaced by conventional MRI and magnetic resonance angiography, but some vascular sarcomas may require presurgical embolization to prevent excessive bleeding during surgery.
Radionuclide bone scans have long been a reliable tool for detecting multifocal or disseminated osseous lesions and remain the mainstay for evaluation of osseous metastases. They also are helpful in identifying skip lesions of osteosarcoma (ie, smaller discrete foci of osteosarcoma occurring in the same bone or on the opposing side of a joint).14 Advantages of this modality include whole-body scanning and low radiation at relatively low cost. Radionuclide bone scans demonstrate areas of bony repair and thus could be negative in purely lytic/destructive processes such as renal cell carcinoma metastases and multiple myeloma.
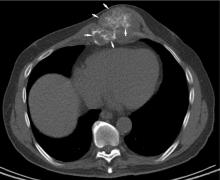
Chest radiographs are typically obtained in the initial stages of patient evaluation and are helpful in demonstrating large nodules or masses resulting from metastatic disease. In a patient with known sarcoma, a negative or equivocal chest radiograph should be followed by chest CT to definitively assess for metastasis.
CROSS-SECTIONAL IMAGING WITH MRI AND CT
MRI preferred for evaluation of most masses
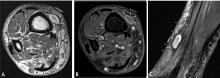
MRI is the examination of choice in the evaluation of soft-tissue masses in light of its superior contrast resolution and ability to demonstrate subtle changes in soft tissues.
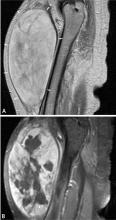
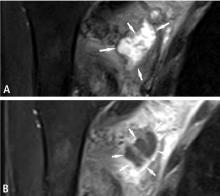
Predicting the histology of most soft-tissue masses is difficult, with the exception of some benign vascular lesions (eg, hemangioma), ganglia, neurogenic lesions, and well-differentiated lipomatous lesions. Aggressive features of a soft-tissue neoplasm include size greater than 5 cm,15 deep location, and absence of central enhancement, which is suggestive of necrosis (Figure 1). Yet one third of soft-tissue sarcomas are either superficial or smaller than 5 cm, which highlights the relative nonspecificity of these features.15
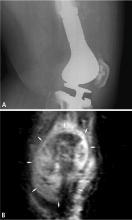
MRI is also the preferred modality in the evaluation of the majority of bone sarcomas, given its ability to accurately define the extent of marrow changes and soft-tissue involvement. MRI should be performed prior to a biopsy to prevent misinterpretation of biopsy-related signal changes in the surrounding tissues, which may negate the value of MRI in sarcoma staging.
Several distinct roles for CT
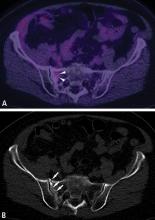
Chest CT should be obtained in all cases of known malignant neoplasms to evaluate for pulmonary nodules, masses, and lymphadenopathy. Despite the recent advances in MRI, CT remains the imaging modality of choice to evaluate the retroperitoneum, abdomen, and pelvis for masses, lymphadenopathy, or other signs of metastatic disease.
Post-treatment monitoring for recurrence
ULTRASONOGRAPHY
Ultrasonography has a limited role in the initial diagnosis and follow-up of musculoskeletal tumors. Its main advantages are a lack of ionizing radiation and dynamic imaging capabilities. Doppler ultrasonography allows direct visualization of tumor vascularity, which may be important for diagnosis and presurgical planning. Unfortunately, bone lesions cannot be evaluated with ultrasonography, owing to the inability of sound waves to penetrate the bony cortex. Poor sound wave penetration may prevent visualization of deep-seated lesions, such as retroperitoneal sarcomas.
Ultrasonography is best used for differentiating solid masses from cystic structures and can provide image guidance in solid tumor biopsy and cyst aspiration. It also may play a role in detecting suspected tumor recurrence in patients in whom artifact from implanted hardware precludes cross-sectional imaging, and it can be reliably used for following up unequivocal soft-tissue masses such as ganglia near joints.
POSITRON EMISSION TOMOGRAPHY
IMAGING-GUIDED INTERVENTIONS
Percutaneous imaging-guided procedures have increasingly replaced open surgical biopsies for bone and soft-tissue tumors. CT guidance is commonly used for percutaneous biopsy, whereas ultrasonographic guidance is sometimes used for superficial soft-tissue lesions. Although the shortest and most direct approach is desirable, this may not be possible in all cases due to the presence of nearby vital structures or the risk of contamination. Seeding of malignant cells along the biopsy tract is a well-known possible complication of image-guided biopsies, and en bloc resection of the needle tract is typically performed at the definitive surgery.
Knowledge of compartmental anatomy is paramount in planning the approach for these biopsies, and consultation with the referring orthopedic surgeon is recommended for optimal management. Expert histopathological interpretation of bone and soft-tissue specimens is essential for the efficacy and high success rates of percutaneous imaging-guided biopsies. Such expertise is integral to the broader interdisciplinary collaboration that is needed to arrive at the most plausible diagnosis, especially in the setting of uncommon or atypical neoplasms.
Currently, MRI-guided interventions are in the initial stage of evolution and could provide valuable guidance for subtle marrow or soft-tissue lesions visible on MRI but not well seen on CT.22 In the future, MRI could play an increasingly important role in imaging-guided procedures because of its lack of ionizing radiation and its ability to demonstrate subtle soft-tissue and bone marrow changes. Imaging-guided therapeutics are growing in their applications in musculoskeletal oncology. CT-guided radiofrequency ablation and cryoablation have been used in the treatment of a variety of tumors23 as well as in the palliation of metastatic bone pain.24
SUMMARY AND CONCLUSION
Bone and soft-tissue sarcomas are rare neoplasms with variable clinical presentations. A high index of suspicion is required for any unexplained mass with indeterminate imaging findings. Recent advances in imaging technology, including cross-sectional MRI and CT, have significantly refined the diagnosis and management of bone and soft-tissue sarcomas. When faced with a possible sarcoma, the clinician’s selection of imaging modalities has a direct impact on diagnosis, staging, and patient management.
- American Cancer Society. Cancer facts & figures 2009. Atlanta, GA: American Cancer Society; 2009.
- Weiss SW, Goldblum JR, Enzinger FM. Enzinger and Weiss’ Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249.
- Simon MA, Springfield DS, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8,542 Cases. 4th ed. Springfield, IL: Thomas; 1986.
- Unni KK. Bone Tumors. New York, NY: Churchill Livingstone; 1988.
- Unni KK. Atlas of Bone Pathology. New York, NY: Chapman & Hall; 1996:1 computer optical disc.
- Unni KK, Dahlin DC. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
- Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer 1999; 86:1602–1608.
- Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res 1986; 204:119–129.
- Kissane JM, Askin FB, Foulkes M, Stratton LB, Shirley SF. Ewing’s sarcoma of bone: clinicopathologic aspects of 303 cases from the Intergroup Ewing’s Sarcoma Study. Hum Pathol 1983; 14:773–779.
- Chotel F, Unnithan A, Chandrasekar CR, et al. Variability in the presentation of synovial sarcoma in children: a plea for greater awareness. J Bone Joint Surg Br 2008; 90:1090–1096.
- Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology 2008; 246:662–674.
- Richardson ML, Gillespy T. Magnetic resonance imaging. In: Kricun ME, ed. Imaging of Bone Tumors. Philadelphia, PA: WB Saunders; 1993:365.
- Fisher C. Soft tissue sarcomas: diagnosis, classification and prognostic factors. Br J Plast Surg 1996; 49:27–33.
- White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 2005; 61:1439–1445.
- White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6:5–17.
- Blodgett TM, Casagranda B, Townsend DW, Meltzer CC. Issues, controversies, and clinical utility of combined PET/CT imaging: what is the interpreting physician facing? AJR Am J Roentgenol 2005; 184(suppl 5):S138–S145.
- Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med 2008; 22:603–609.
- Benz MR, Czernin J, Allen-Auerbach MS, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res 2009; 15:2856–2863.
- Aoki J, Watanabe H, Shinozaki T, et al. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal Radiol 2003; 32:133–138.
- Blanco Sequeiros R, Klemola R, Ojala R, et al. MRI-guided trephine biopsy and fine-needle aspiration in the diagnosis of bone lesions in low-field (0.23 T) MRI system using optical instrument tracking. Eur Radiol 2002; 12:830–835.
- Rosenthal DI. Radiofrequency treatment. Orthop Clin North Am 2006; 37:475–484.
- Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol 2007; 10:120–131.
Sarcomas are rare neoplasms arising from connective tissue elements of the body. Approximately 80% arise in soft tissue, while the remainder originate in bone.1 Soft-tissue sarcomas are tumors of the mesenchymal system, and about half develop in the extremities. Bone sarcomas are characterized by their location in bone and sometimes produce osteoid, or immature bone.
The most common types of soft-tissue sarcomas are malignant fibrous histiocytoma (although this term has lost favor with some pathologists) and liposarcoma.
The most common types of bone sarcomas are osteosarcoma (a sarcoma that makes osteoid or bone), Ewing sarcoma (composed of small round blue cells with a characteristic chromosomal translocation), and chondrosarcoma (a sarcoma that makes chondroid tissue, or cartilage).
EPIDEMIOLOGY AND PRIMARY BODY SITES
Approximately 10,600 new cases of soft-tissue sarcoma and 2,570 new cases of bone sarcoma were estimated to have been diagnosed in the United States in 2009.2,3 For perspective, the annual incidence of soft-tissue sarcoma is approximately 5.5% that of breast cancer and approximately 5% that of lung cancer.3
Most sarcomas develop in the lower extremities, although the age groups at greatest risk vary among sarcoma types.4
Soft-tissue sarcomas develop most commonly in the thigh and occur primarily in adults.2
Osteosarcomas are the most common bone sarcoma and develop most frequently in 10- to 20-year-olds; their most common location is the distal femur.5–8 Metastatic osteosarcoma is found in approximately 20% of patients at the time of osteosarcoma diagnosis. Osteosarcomas mainly spread hematogenously, and the lungs are the most common initial site of metastases, being affected in up to 90% of patients with metastatic disease.9
Ewing sarcomas develop most often in the long bones of the extremities or bones of the pelvis. The large majority of cases develop in patients aged 10 to 15 years. 5–8
Chondrosarcomas represent approximately 20% of all bone sarcomas and primarily affect older adults, with a peak incidence in the sixth decade of life.10
OVERVIEW OF PRESENTATION AND EVALUATION
Presentation is highly variable
The clinical presentation of patients with bone or soft-tissue sarcoma is highly variable. Patients often present with a mass, typically one that is increasing in size. In general, bone sarcomas are painful and soft-tissue sarcomas are not, but there are exceptions to this general rule. Constitutional symptoms are rare in patients with bone or soft-tissue sarcomas, but symptoms such as fever, malaise, and weight loss can be seen, especially in patients with Ewing sarcoma.11
Delayed presentation and diagnosis are common
Particularly when a sarcoma is painless, patients sometimes do not seek medical attention until a suspicious mass becomes quite large. Certain tumors, such as synovial sarcoma, a high-grade soft-tissue sarcoma often seen in young adults, may present as a slowly growing or stable-appearing mass over several years. In one study of 33 children with synovial sarcoma, the mean duration of symptoms was 98 weeks (range, 2–364), the mean patient delay before a doctor was seen was 43 weeks (0–156), the mean doctor delay before a correct diagnosis was made was 50 weeks (0–362), and the mean number of doctors seen before referral was 3 (1–6).12 For nearly half the patients in this study (15), the diagnosis was obtained only after unplanned excision, meaning that the surgeon did not expect a malignancy at the time of biopsy. Because delayed presentation is not uncommon in cases of bone or soft-tissue sarcoma, every patient with a mass with indeterminate imaging findings should be referred to or reviewed by an orthopedic or musculoskeletal oncologist.
Biopsy is gold standard for diagnosis
A comprehensive medical history and physical examination are essential at the initial presentation of patients with masses and/or pain suggestive of bone or soft-tissue sarcoma. Sarcoma simulators such as hematoma, metastatic disease, or infection can sometimes be ruled out by careful clinical examination, laboratory work-up, and appropriate imaging, but the gold standard for diagnosis is a biopsy. Moreover, an index of suspicion is required to rule out primary malignancy in any soft-tissue or bone lesion, and this index of suspicion will allow for referral or appropriate selection of the site for biopsy.
Biopsy considerations, as well as further detail on clinical presentation, are provided in the second and third articles in this supplement, which focus, respectively, on bone sarcoma and soft-tissue sarcoma. The remainder of this article reviews the use of imaging for the evaluation of suspected sarcomas, as imaging findings typically prompt or guide biopsy of a suspicious mass. Choosing the right imaging modality is critical to the diagnosis and management of patients with suspected sarcoma.
CONVENTIONAL IMAGING MODALITIES
Despite their utility for evaluating osseous lesions, radiographs have limited to no value in the evaluation of soft-tissue sarcomas but can demonstrate matrix mineralization and erosion or destruction of adjacent bone.
Angiography. In the past, angiography was frequently used to assess the vascularity of sarcomas preoperatively. Diagnostic angiography has been replaced by conventional MRI and magnetic resonance angiography, but some vascular sarcomas may require presurgical embolization to prevent excessive bleeding during surgery.
Radionuclide bone scans have long been a reliable tool for detecting multifocal or disseminated osseous lesions and remain the mainstay for evaluation of osseous metastases. They also are helpful in identifying skip lesions of osteosarcoma (ie, smaller discrete foci of osteosarcoma occurring in the same bone or on the opposing side of a joint).14 Advantages of this modality include whole-body scanning and low radiation at relatively low cost. Radionuclide bone scans demonstrate areas of bony repair and thus could be negative in purely lytic/destructive processes such as renal cell carcinoma metastases and multiple myeloma.
Chest radiographs are typically obtained in the initial stages of patient evaluation and are helpful in demonstrating large nodules or masses resulting from metastatic disease. In a patient with known sarcoma, a negative or equivocal chest radiograph should be followed by chest CT to definitively assess for metastasis.
CROSS-SECTIONAL IMAGING WITH MRI AND CT
MRI preferred for evaluation of most masses
MRI is the examination of choice in the evaluation of soft-tissue masses in light of its superior contrast resolution and ability to demonstrate subtle changes in soft tissues.
Predicting the histology of most soft-tissue masses is difficult, with the exception of some benign vascular lesions (eg, hemangioma), ganglia, neurogenic lesions, and well-differentiated lipomatous lesions. Aggressive features of a soft-tissue neoplasm include size greater than 5 cm,15 deep location, and absence of central enhancement, which is suggestive of necrosis (Figure 1). Yet one third of soft-tissue sarcomas are either superficial or smaller than 5 cm, which highlights the relative nonspecificity of these features.15
MRI is also the preferred modality in the evaluation of the majority of bone sarcomas, given its ability to accurately define the extent of marrow changes and soft-tissue involvement. MRI should be performed prior to a biopsy to prevent misinterpretation of biopsy-related signal changes in the surrounding tissues, which may negate the value of MRI in sarcoma staging.
Several distinct roles for CT
Chest CT should be obtained in all cases of known malignant neoplasms to evaluate for pulmonary nodules, masses, and lymphadenopathy. Despite the recent advances in MRI, CT remains the imaging modality of choice to evaluate the retroperitoneum, abdomen, and pelvis for masses, lymphadenopathy, or other signs of metastatic disease.
Post-treatment monitoring for recurrence
ULTRASONOGRAPHY
Ultrasonography has a limited role in the initial diagnosis and follow-up of musculoskeletal tumors. Its main advantages are a lack of ionizing radiation and dynamic imaging capabilities. Doppler ultrasonography allows direct visualization of tumor vascularity, which may be important for diagnosis and presurgical planning. Unfortunately, bone lesions cannot be evaluated with ultrasonography, owing to the inability of sound waves to penetrate the bony cortex. Poor sound wave penetration may prevent visualization of deep-seated lesions, such as retroperitoneal sarcomas.
Ultrasonography is best used for differentiating solid masses from cystic structures and can provide image guidance in solid tumor biopsy and cyst aspiration. It also may play a role in detecting suspected tumor recurrence in patients in whom artifact from implanted hardware precludes cross-sectional imaging, and it can be reliably used for following up unequivocal soft-tissue masses such as ganglia near joints.
POSITRON EMISSION TOMOGRAPHY
IMAGING-GUIDED INTERVENTIONS
Percutaneous imaging-guided procedures have increasingly replaced open surgical biopsies for bone and soft-tissue tumors. CT guidance is commonly used for percutaneous biopsy, whereas ultrasonographic guidance is sometimes used for superficial soft-tissue lesions. Although the shortest and most direct approach is desirable, this may not be possible in all cases due to the presence of nearby vital structures or the risk of contamination. Seeding of malignant cells along the biopsy tract is a well-known possible complication of image-guided biopsies, and en bloc resection of the needle tract is typically performed at the definitive surgery.
Knowledge of compartmental anatomy is paramount in planning the approach for these biopsies, and consultation with the referring orthopedic surgeon is recommended for optimal management. Expert histopathological interpretation of bone and soft-tissue specimens is essential for the efficacy and high success rates of percutaneous imaging-guided biopsies. Such expertise is integral to the broader interdisciplinary collaboration that is needed to arrive at the most plausible diagnosis, especially in the setting of uncommon or atypical neoplasms.
Currently, MRI-guided interventions are in the initial stage of evolution and could provide valuable guidance for subtle marrow or soft-tissue lesions visible on MRI but not well seen on CT.22 In the future, MRI could play an increasingly important role in imaging-guided procedures because of its lack of ionizing radiation and its ability to demonstrate subtle soft-tissue and bone marrow changes. Imaging-guided therapeutics are growing in their applications in musculoskeletal oncology. CT-guided radiofrequency ablation and cryoablation have been used in the treatment of a variety of tumors23 as well as in the palliation of metastatic bone pain.24
SUMMARY AND CONCLUSION
Bone and soft-tissue sarcomas are rare neoplasms with variable clinical presentations. A high index of suspicion is required for any unexplained mass with indeterminate imaging findings. Recent advances in imaging technology, including cross-sectional MRI and CT, have significantly refined the diagnosis and management of bone and soft-tissue sarcomas. When faced with a possible sarcoma, the clinician’s selection of imaging modalities has a direct impact on diagnosis, staging, and patient management.
Sarcomas are rare neoplasms arising from connective tissue elements of the body. Approximately 80% arise in soft tissue, while the remainder originate in bone.1 Soft-tissue sarcomas are tumors of the mesenchymal system, and about half develop in the extremities. Bone sarcomas are characterized by their location in bone and sometimes produce osteoid, or immature bone.
The most common types of soft-tissue sarcomas are malignant fibrous histiocytoma (although this term has lost favor with some pathologists) and liposarcoma.
The most common types of bone sarcomas are osteosarcoma (a sarcoma that makes osteoid or bone), Ewing sarcoma (composed of small round blue cells with a characteristic chromosomal translocation), and chondrosarcoma (a sarcoma that makes chondroid tissue, or cartilage).
EPIDEMIOLOGY AND PRIMARY BODY SITES
Approximately 10,600 new cases of soft-tissue sarcoma and 2,570 new cases of bone sarcoma were estimated to have been diagnosed in the United States in 2009.2,3 For perspective, the annual incidence of soft-tissue sarcoma is approximately 5.5% that of breast cancer and approximately 5% that of lung cancer.3
Most sarcomas develop in the lower extremities, although the age groups at greatest risk vary among sarcoma types.4
Soft-tissue sarcomas develop most commonly in the thigh and occur primarily in adults.2
Osteosarcomas are the most common bone sarcoma and develop most frequently in 10- to 20-year-olds; their most common location is the distal femur.5–8 Metastatic osteosarcoma is found in approximately 20% of patients at the time of osteosarcoma diagnosis. Osteosarcomas mainly spread hematogenously, and the lungs are the most common initial site of metastases, being affected in up to 90% of patients with metastatic disease.9
Ewing sarcomas develop most often in the long bones of the extremities or bones of the pelvis. The large majority of cases develop in patients aged 10 to 15 years. 5–8
Chondrosarcomas represent approximately 20% of all bone sarcomas and primarily affect older adults, with a peak incidence in the sixth decade of life.10
OVERVIEW OF PRESENTATION AND EVALUATION
Presentation is highly variable
The clinical presentation of patients with bone or soft-tissue sarcoma is highly variable. Patients often present with a mass, typically one that is increasing in size. In general, bone sarcomas are painful and soft-tissue sarcomas are not, but there are exceptions to this general rule. Constitutional symptoms are rare in patients with bone or soft-tissue sarcomas, but symptoms such as fever, malaise, and weight loss can be seen, especially in patients with Ewing sarcoma.11
Delayed presentation and diagnosis are common
Particularly when a sarcoma is painless, patients sometimes do not seek medical attention until a suspicious mass becomes quite large. Certain tumors, such as synovial sarcoma, a high-grade soft-tissue sarcoma often seen in young adults, may present as a slowly growing or stable-appearing mass over several years. In one study of 33 children with synovial sarcoma, the mean duration of symptoms was 98 weeks (range, 2–364), the mean patient delay before a doctor was seen was 43 weeks (0–156), the mean doctor delay before a correct diagnosis was made was 50 weeks (0–362), and the mean number of doctors seen before referral was 3 (1–6).12 For nearly half the patients in this study (15), the diagnosis was obtained only after unplanned excision, meaning that the surgeon did not expect a malignancy at the time of biopsy. Because delayed presentation is not uncommon in cases of bone or soft-tissue sarcoma, every patient with a mass with indeterminate imaging findings should be referred to or reviewed by an orthopedic or musculoskeletal oncologist.
Biopsy is gold standard for diagnosis
A comprehensive medical history and physical examination are essential at the initial presentation of patients with masses and/or pain suggestive of bone or soft-tissue sarcoma. Sarcoma simulators such as hematoma, metastatic disease, or infection can sometimes be ruled out by careful clinical examination, laboratory work-up, and appropriate imaging, but the gold standard for diagnosis is a biopsy. Moreover, an index of suspicion is required to rule out primary malignancy in any soft-tissue or bone lesion, and this index of suspicion will allow for referral or appropriate selection of the site for biopsy.
Biopsy considerations, as well as further detail on clinical presentation, are provided in the second and third articles in this supplement, which focus, respectively, on bone sarcoma and soft-tissue sarcoma. The remainder of this article reviews the use of imaging for the evaluation of suspected sarcomas, as imaging findings typically prompt or guide biopsy of a suspicious mass. Choosing the right imaging modality is critical to the diagnosis and management of patients with suspected sarcoma.
CONVENTIONAL IMAGING MODALITIES
Despite their utility for evaluating osseous lesions, radiographs have limited to no value in the evaluation of soft-tissue sarcomas but can demonstrate matrix mineralization and erosion or destruction of adjacent bone.
Angiography. In the past, angiography was frequently used to assess the vascularity of sarcomas preoperatively. Diagnostic angiography has been replaced by conventional MRI and magnetic resonance angiography, but some vascular sarcomas may require presurgical embolization to prevent excessive bleeding during surgery.
Radionuclide bone scans have long been a reliable tool for detecting multifocal or disseminated osseous lesions and remain the mainstay for evaluation of osseous metastases. They also are helpful in identifying skip lesions of osteosarcoma (ie, smaller discrete foci of osteosarcoma occurring in the same bone or on the opposing side of a joint).14 Advantages of this modality include whole-body scanning and low radiation at relatively low cost. Radionuclide bone scans demonstrate areas of bony repair and thus could be negative in purely lytic/destructive processes such as renal cell carcinoma metastases and multiple myeloma.
Chest radiographs are typically obtained in the initial stages of patient evaluation and are helpful in demonstrating large nodules or masses resulting from metastatic disease. In a patient with known sarcoma, a negative or equivocal chest radiograph should be followed by chest CT to definitively assess for metastasis.
CROSS-SECTIONAL IMAGING WITH MRI AND CT
MRI preferred for evaluation of most masses
MRI is the examination of choice in the evaluation of soft-tissue masses in light of its superior contrast resolution and ability to demonstrate subtle changes in soft tissues.
Predicting the histology of most soft-tissue masses is difficult, with the exception of some benign vascular lesions (eg, hemangioma), ganglia, neurogenic lesions, and well-differentiated lipomatous lesions. Aggressive features of a soft-tissue neoplasm include size greater than 5 cm,15 deep location, and absence of central enhancement, which is suggestive of necrosis (Figure 1). Yet one third of soft-tissue sarcomas are either superficial or smaller than 5 cm, which highlights the relative nonspecificity of these features.15
MRI is also the preferred modality in the evaluation of the majority of bone sarcomas, given its ability to accurately define the extent of marrow changes and soft-tissue involvement. MRI should be performed prior to a biopsy to prevent misinterpretation of biopsy-related signal changes in the surrounding tissues, which may negate the value of MRI in sarcoma staging.
Several distinct roles for CT
Chest CT should be obtained in all cases of known malignant neoplasms to evaluate for pulmonary nodules, masses, and lymphadenopathy. Despite the recent advances in MRI, CT remains the imaging modality of choice to evaluate the retroperitoneum, abdomen, and pelvis for masses, lymphadenopathy, or other signs of metastatic disease.
Post-treatment monitoring for recurrence
ULTRASONOGRAPHY
Ultrasonography has a limited role in the initial diagnosis and follow-up of musculoskeletal tumors. Its main advantages are a lack of ionizing radiation and dynamic imaging capabilities. Doppler ultrasonography allows direct visualization of tumor vascularity, which may be important for diagnosis and presurgical planning. Unfortunately, bone lesions cannot be evaluated with ultrasonography, owing to the inability of sound waves to penetrate the bony cortex. Poor sound wave penetration may prevent visualization of deep-seated lesions, such as retroperitoneal sarcomas.
Ultrasonography is best used for differentiating solid masses from cystic structures and can provide image guidance in solid tumor biopsy and cyst aspiration. It also may play a role in detecting suspected tumor recurrence in patients in whom artifact from implanted hardware precludes cross-sectional imaging, and it can be reliably used for following up unequivocal soft-tissue masses such as ganglia near joints.
POSITRON EMISSION TOMOGRAPHY
IMAGING-GUIDED INTERVENTIONS
Percutaneous imaging-guided procedures have increasingly replaced open surgical biopsies for bone and soft-tissue tumors. CT guidance is commonly used for percutaneous biopsy, whereas ultrasonographic guidance is sometimes used for superficial soft-tissue lesions. Although the shortest and most direct approach is desirable, this may not be possible in all cases due to the presence of nearby vital structures or the risk of contamination. Seeding of malignant cells along the biopsy tract is a well-known possible complication of image-guided biopsies, and en bloc resection of the needle tract is typically performed at the definitive surgery.
Knowledge of compartmental anatomy is paramount in planning the approach for these biopsies, and consultation with the referring orthopedic surgeon is recommended for optimal management. Expert histopathological interpretation of bone and soft-tissue specimens is essential for the efficacy and high success rates of percutaneous imaging-guided biopsies. Such expertise is integral to the broader interdisciplinary collaboration that is needed to arrive at the most plausible diagnosis, especially in the setting of uncommon or atypical neoplasms.
Currently, MRI-guided interventions are in the initial stage of evolution and could provide valuable guidance for subtle marrow or soft-tissue lesions visible on MRI but not well seen on CT.22 In the future, MRI could play an increasingly important role in imaging-guided procedures because of its lack of ionizing radiation and its ability to demonstrate subtle soft-tissue and bone marrow changes. Imaging-guided therapeutics are growing in their applications in musculoskeletal oncology. CT-guided radiofrequency ablation and cryoablation have been used in the treatment of a variety of tumors23 as well as in the palliation of metastatic bone pain.24
SUMMARY AND CONCLUSION
Bone and soft-tissue sarcomas are rare neoplasms with variable clinical presentations. A high index of suspicion is required for any unexplained mass with indeterminate imaging findings. Recent advances in imaging technology, including cross-sectional MRI and CT, have significantly refined the diagnosis and management of bone and soft-tissue sarcomas. When faced with a possible sarcoma, the clinician’s selection of imaging modalities has a direct impact on diagnosis, staging, and patient management.
- American Cancer Society. Cancer facts & figures 2009. Atlanta, GA: American Cancer Society; 2009.
- Weiss SW, Goldblum JR, Enzinger FM. Enzinger and Weiss’ Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249.
- Simon MA, Springfield DS, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8,542 Cases. 4th ed. Springfield, IL: Thomas; 1986.
- Unni KK. Bone Tumors. New York, NY: Churchill Livingstone; 1988.
- Unni KK. Atlas of Bone Pathology. New York, NY: Chapman & Hall; 1996:1 computer optical disc.
- Unni KK, Dahlin DC. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
- Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer 1999; 86:1602–1608.
- Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res 1986; 204:119–129.
- Kissane JM, Askin FB, Foulkes M, Stratton LB, Shirley SF. Ewing’s sarcoma of bone: clinicopathologic aspects of 303 cases from the Intergroup Ewing’s Sarcoma Study. Hum Pathol 1983; 14:773–779.
- Chotel F, Unnithan A, Chandrasekar CR, et al. Variability in the presentation of synovial sarcoma in children: a plea for greater awareness. J Bone Joint Surg Br 2008; 90:1090–1096.
- Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology 2008; 246:662–674.
- Richardson ML, Gillespy T. Magnetic resonance imaging. In: Kricun ME, ed. Imaging of Bone Tumors. Philadelphia, PA: WB Saunders; 1993:365.
- Fisher C. Soft tissue sarcomas: diagnosis, classification and prognostic factors. Br J Plast Surg 1996; 49:27–33.
- White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 2005; 61:1439–1445.
- White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6:5–17.
- Blodgett TM, Casagranda B, Townsend DW, Meltzer CC. Issues, controversies, and clinical utility of combined PET/CT imaging: what is the interpreting physician facing? AJR Am J Roentgenol 2005; 184(suppl 5):S138–S145.
- Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med 2008; 22:603–609.
- Benz MR, Czernin J, Allen-Auerbach MS, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res 2009; 15:2856–2863.
- Aoki J, Watanabe H, Shinozaki T, et al. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal Radiol 2003; 32:133–138.
- Blanco Sequeiros R, Klemola R, Ojala R, et al. MRI-guided trephine biopsy and fine-needle aspiration in the diagnosis of bone lesions in low-field (0.23 T) MRI system using optical instrument tracking. Eur Radiol 2002; 12:830–835.
- Rosenthal DI. Radiofrequency treatment. Orthop Clin North Am 2006; 37:475–484.
- Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol 2007; 10:120–131.
- American Cancer Society. Cancer facts & figures 2009. Atlanta, GA: American Cancer Society; 2009.
- Weiss SW, Goldblum JR, Enzinger FM. Enzinger and Weiss’ Soft Tissue Tumors. 5th ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225–249.
- Simon MA, Springfield DS, eds. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8,542 Cases. 4th ed. Springfield, IL: Thomas; 1986.
- Unni KK. Bone Tumors. New York, NY: Churchill Livingstone; 1988.
- Unni KK. Atlas of Bone Pathology. New York, NY: Chapman & Hall; 1996:1 computer optical disc.
- Unni KK, Dahlin DC. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
- Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer 1999; 86:1602–1608.
- Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res 1986; 204:119–129.
- Kissane JM, Askin FB, Foulkes M, Stratton LB, Shirley SF. Ewing’s sarcoma of bone: clinicopathologic aspects of 303 cases from the Intergroup Ewing’s Sarcoma Study. Hum Pathol 1983; 14:773–779.
- Chotel F, Unnithan A, Chandrasekar CR, et al. Variability in the presentation of synovial sarcoma in children: a plea for greater awareness. J Bone Joint Surg Br 2008; 90:1090–1096.
- Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology 2008; 246:662–674.
- Richardson ML, Gillespy T. Magnetic resonance imaging. In: Kricun ME, ed. Imaging of Bone Tumors. Philadelphia, PA: WB Saunders; 1993:365.
- Fisher C. Soft tissue sarcomas: diagnosis, classification and prognostic factors. Br J Plast Surg 1996; 49:27–33.
- White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 2005; 61:1439–1445.
- White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol 2002; 6:5–17.
- Blodgett TM, Casagranda B, Townsend DW, Meltzer CC. Issues, controversies, and clinical utility of combined PET/CT imaging: what is the interpreting physician facing? AJR Am J Roentgenol 2005; 184(suppl 5):S138–S145.
- Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med 2008; 22:603–609.
- Benz MR, Czernin J, Allen-Auerbach MS, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res 2009; 15:2856–2863.
- Aoki J, Watanabe H, Shinozaki T, et al. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal Radiol 2003; 32:133–138.
- Blanco Sequeiros R, Klemola R, Ojala R, et al. MRI-guided trephine biopsy and fine-needle aspiration in the diagnosis of bone lesions in low-field (0.23 T) MRI system using optical instrument tracking. Eur Radiol 2002; 12:830–835.
- Rosenthal DI. Radiofrequency treatment. Orthop Clin North Am 2006; 37:475–484.
- Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol 2007; 10:120–131.