User login
Managing the Multiple Symptoms of Benign Prostatic Hyperplasia — CME
Managing Type 2 Diabetes in Men
Meeting New Challenges with Antiplatelet Therapy in Primary Care
Dr. Cobble has disclosed that he is on the advisory boards and speakers’ bureaus for AstraZeneca and Bristol-Myers Squibb and is on the speakers’ bureaus for Eli Lilly, Forest, and Kowa.
SUPPORT
This program is sponsored by the PCEC and is supported by funding from AstraZeneca.
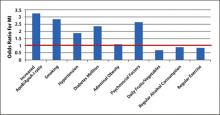
The death rate from coronary heart disease (CHD) declined by 59% from 1950 to 1999 in the United States, yet CHD remains a major cause of morbidity and mortality, resulting in an estimated 1.5 million heart attacks in 2011.1 Better recognition and treatment of the 9 modifiable risk factors for CHD identified by the INTERHEART study ( FIGURE 1 ), as well as changes in lifestyle practices, undoubtedly contributed to the decline in CHD mortality, but further improvement is possible.2 Estimates derived from the Second National Health and Nutrition Examination Survey (NHANES II) baseline data and 17-year mortality follow-up data indicate that 45% of CHD deaths in men and 64% in women could be avoided by eliminating 3 major risk factors: elevated total cholesterol (≥240 mg/dL), hypertension, and smoking.3
The evidence indicates that these 3 risk factors are not well controlled. Data from the National Cholesterol Education Program (NCEP) Evaluation ProjecT Utilizing Novel E-Technology (NEPTUNE) II survey and the Lipid Treatment Assessment Project 2 (L-TAP 2), as well as more recent evidence, indicate that many patients do not achieve low-density lipoprotein cholesterol (LDL-C) and triglyceride targets.4-10 Similarly, although there has been significant improvement in blood pressure (BP) control over the past 2 decades, BP is controlled in only half of all hypertensive patients.11,12 Finally, the sharp declines in the prevalence of cigarette smoking seen in the past have slowed in recent years, such that approximately 20% of US adults still smoke cigarettes.13
These trends are a concern since a greater risk factor burden in middle age is associated with poorer quality of life and higher medical costs, as well as a higher incidence of cardiovascular events in older age.1 A recent meta-analysis of 18 cohort studies involving 257,384 adults showed a higher incidence of cardiovascular events in later life with an increasing number of risk factors.14 For example, adults 55 years of age with an optimal risk factor profile (ie, total cholesterol <180 mg/dL, BP <120/80 mm Hg, nonsmoker, nondiabetic) had much lower risks of death from cardiovascular disease (CVD) through the age of 80 years than those with 2 or more risk factors (4.7% vs 29.6% among men, 6.4% vs 20.5% among women). This translates into a relative risk (RR) of cardiovascular death of 6 times for men and 3 times for women without optimal risk profiles. Similar trends were observed for risk of fatal CHD/nonfatal myocardial infarction (MI) (3.6% vs 37.5% among men, <1% vs 18.3% among women). These findings point to the critical importance of modifying multiple risk factors early in adulthood, well in advance of symptoms. However, the Study to Help Improve Early Evaluation and Management of Risk Factors Leading to Diabetes (SHIELD) showed that about half of patients with CHD are not diagnosed until symptoms become apparent, and fewer than one quarter are diagnosed as a result of screening.15
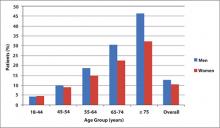
This review focuses on patient assessment and treatment strategies to modify abnormal lipid levels and high BP for primary prevention. Addressing other modifiable risk factors is also important, especially since risk factors such as abdominal obesity impact other risk factors ( FIGURE 1 ). An emphasis is placed on strategies in men, since the prevalence of CHD among patients aged 45 years and older is higher in men than in women ( FIGURE 2 ).16 Furthermore, men experience a first cardiovascular event a decade earlier than women, and a more serious CHD event, such as MI or sudden death, 2 decades earlier.1
FIGURE 1
Modifiable risk factors for myocardial infarction (MI)2
ApoB/ApoA-I, apolipoprotein B/apolipoprotein A-I.
FIGURE 2
Prevalence of heart disease by age and gender16
Assessment
The assessment of CHD risk in men need not be complicated and should be made practical so that it is applied consistently. A family and personal medical history and physical examination combined with laboratory determination of lipid levels and glycosylated hemoglobin can help assess modifiable risk factors. The assessment of CHD risk can be facilitated by using 1 of 2 risk calculators. The Framingham Risk Score [www.framinghamheartstudy.org/risk/gencardio.html] is widely used but may underestimate risk, especially in younger persons or those who appear to be healthy but may have other risk factors for CHD.17-19 The Reynolds Risk Score [www.reynoldsriskscore.org/] includes other risk factors, such as parental history of MI before age 60 years, low levels of apolipoprotein A (apoA), high levels of apolipoprotein B (apoB), and increased levels of high-sensitivity C-reactive protein (hs-CRP).19 The Reynolds Risk Score has been validated in healthy, nondiabetic men.20
The relevance of apolipoprotein levels, particularly apoB, to cardiovascular risk is increasingly appreciated.21 ApoB concentration represents the sum of atherogenic particles found on all atherogenic lipoproteins, including very-low-density lipoprotein, intermediate-density lipoprotein, low-density lipoprotein, and lipoprotein(a) cholesterol, whereas apoA represents the sum of antiatherogenic particles found on high-density lipoprotein cholesterol (HDL-C), the antiatherogenic lipoprotein.22 The ratio of apoB/apoA-I has, in fact, been shown to be a good predictor of cardiovascular events in young men without hypertension and diabetes but with chest pain.23 High-sensitivity C-reactive protein is a sensitive marker of acute inflammation and is associated with coronary risk.24 Measuring hs-CRP is a recommended option to determine enhanced absolute risk in people with an intermediate 10-year CHD risk of 10% to 20%.25
There remains some uncertainty regarding which lipid levels should be measured when screening for cardiovascular risk. The National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) advises that total cholesterol, LDL-C, HDL-C, and triglycerides be measured.26 More recent results from The Emerging Risk Factors Collaboration suggest that a more simplified approach may be reasonable.27 Review of data from 68 long-term prospective studies involving 302,430 people without initial vascular disease and 2.79 million person-years of follow-up showed that lipid assessment of vascular risk could be accomplished by measuring either total cholesterol and HDL-C levels or apolipoprotein levels; measuring the triglyceride level was of no added benefit in assessing vascular risk. In addition, fasting and nonfasting lipid levels were found to be of similar value in assessing risk. Other evidence shows that the combination of a triglyceride level ≥178 mg/dL and waist circumference ≥35.4 inches—the hypertriglyceridemic waist phenotype—is as discriminatory a screening tool as the NCEP ATP III guidelines to identify individuals at increased cardiometabolic risk.28 The use of more comprehensive lipoprotein and apolipoprotein testing, as well as noninvasive imaging, may have value in future cardiovascular risk assessment.
Treatment
The main goal of treatment in persons with 1 or more modifiable risk factors is to prevent an incident or primary cardiovascular event. Treatment strategies to achieve this goal in men and women are the same. Prevention of recurrent or secondary events will not be addressed here.
Lipids
Numerous clinical trials, such as the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS),29 Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA),30 and West of Scotland Coronary Prevention Study (WOSCOPS),31 definitively established the benefit of cardiovascular risk reduction with lipid-lowering treatment, particularly LDL-C-lowering treatment. Low-density lipoprotein cholesterol is the principal lipid target in most patients, with the treatment goal based on the presence of additional risk factors.32 Discussion of treatments for low HDL-C and elevated triglyceride levels is beyond the scope of this review but is expected to be included in the NCEP ATP IV guidelines scheduled for release later in 2012.
The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) also established significant benefits of statin therapy in primary prevention, compared with placebo, in persons with normal or modestly elevated LDL-C (<130 mg/dL) and elevated hs-CRP (≥2 mg/L).33 Rates of the primary end point (MI, stroke, arterial revascularization, hospitalization for unstable angina, or cardiovascular death) were 0.77 and 1.36 per 100 person-years of follow-up in the rosuvastatin and placebo groups, respectively (hazard ratio [HR], 0.56; 95% CI, 0.46-0.69; P < .00001). Further analysis showed that patients who achieved LDL-C <70 mg/dL had a 55% lower rate of vascular events compared with placebo.34
Results from large primary prevention clinical trials such as JUPITER have led to recommendations over the past decade or so for progressively lower LDL-C goals. A meta-analysis of 25 large clinical trials involving 155,613 subjects showed that for every 25 mg/dL reduction in LDL-C, the RR for several cardiovascular outcomes was reduced: vascular mortality, 0.89; major vascular events, 0.86; major coronary events, 0.84; and stroke, 0.90. Put differently, there was a 20% reduction in major coronary events for every 39 mg/dL LDL-C reduction.35
Recent trials support the benefits of intensive high-dose statin therapy in greatly reducing lipid levels, with associated benefits in terms of cardiovascular events. A meta-analysis of 7 trials involving 50,972 high-risk patients with a mean follow-up of 3.1 years showed significant reductions in the risk for cardiovascular events with intensive statin therapy. Those who achieved LDL-C <82 mg/dL with intensive statin therapy had lower cardiovascular risks compared with those with LDL-C ≥82 mg/dL: stroke, odds ratio (OR): 0.80; major coronary events, OR: 0.74; and CVD or CHD death, OR: 0.84.36 Significantly higher liver enzyme abnormalities were observed in patients treated with high-dose statin therapy. [See also Addressing Key Questions with Statin Therapy in this supplement.] The benefits of intensive statin therapy on the progression of coronary atherosclerosis have also been investigated. The Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin versus Atorvastatin (SATURN) by Nicholls et al37 included patients (N = 1039) with documented coronary vessel stenosis of at least 20% and a target vessel for imaging with less than 50% obstruction. Patients received either atorvastatin 80 mg daily or rosu-vastatin 40 mg daily for 104 weeks. In the rosuvastatin group, end-of-study LDL-C levels were lower (62.6 vs 70.2 mg/dL; P < .001) and HDL-C levels higher (50.4 vs 48.6 mg/dL; P = .01) compared with the atorvastatin group, respectively. The percent atheroma volume decreased by 1.22% with rosuvastatin and 0.99% with atorvastatin (P = .17). The normalized total atheroma volume decreased 6.39 mm3 with rosuvastatin and 4.42 mm3 with atorvastatin (P = .01). Atheroma regression was induced in the majority of patients in both groups.
Further support for treating with statin doses higher than those recommended for initial therapy comes from a prospective trial involving 1337 consecutive patients followed over a median of 33 months.10 Although 83% of these patients were on statin therapy, only 51% had an LDL-C <100 mg/dL, and only 15% of the very high-risk patients (n = 941) had an LDL-C <70 mg/dL. The use of intensive statin therapy was associated with a 12-fold higher possibility of achieving an LDL-C <70 mg/dL. Very high-risk patients who achieved an LDL-C <70 mg/dL had a significantly lower risk of all cardiovascular events (HR, 0.34; P = .003).
Blood pressure
As with dyslipidemia, the cardiovascular benefits of lowering elevated BP are well established. While the usual BP goal is <140/90 mm Hg, in those with hypertension and concomitant diabetes or renal disease, the goal is <130/80 mm Hg.38 It is not clear how best to achieve these goals, but therapy must be individualized based on patient comorbidities and drug side effects as recommended in current guidelines.38-40 With these guidelines as a basis, a simplified ABCD approach can be considered in selecting initial antihypertensive therapy ( FIGURE 3 ).
FIGURE 3
ABCD approach to initial antihypertensive therapy38-40
ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; MI, myocardial infarction. Monotherapy, however, does not result in BP control in most patients. As shown by the Antihypertensive Lipid-Lowering Treatment to Prevent Heart Attacks Trial (ALLHAT), BP control typically requires at least 2 different classes of drugs, with 3 or more drugs required in about 1 in 6 patients within 3 years and 1 in 4 patients within 5 years. A higher percentage of patients with diabetes mellitus or kidney impairment (creatinine ≥1.5 mg/dL) require 3 or more antihypertensive drugs after 5 years (33% and 40%, respectively).41
Several meta-analyses have been conducted recently to assess the magnitude of BP (systolic/diastolic) lowering in the different classes of antihypertensive drugs. While these meta-analyses have important limitations, such as differences in study design and the lack of a clear description of outcomes, some general impressions can be made. In 1 meta-analysis, thiazide diuretics were found to lower BP by 6/3 and 8/4 mm Hg at doses of 1 and 2 times the recommended starting dose, respectively. A BP-lowering effect of 6/3 mm Hg was observed with starting doses of loop diuretics.42 Another meta-analysis failed to find a statistically or clinically significant BP-lowering effect with potassium-sparing diuretics at low doses.43 For spironolactone, a review of 5 crossover studies found a reduction in BP of 21/7 mm Hg. In this review, daily doses of 25 to 100 mg were found to provide the best balance between BP reduction and safety and tolerability.44
Several meta-analyses of angiotensin receptor blockers (ARBs) have found BP reductions to be similar among the various ARB drugs. Generally, at maximum recommended doses, a BP reduction of 8/5 mm Hg is observed with these drugs, except for losartan, which produces a smaller BP reduction.45-49 Heran et al45 found a BP reduction of 12/7 mm Hg among the ARBs 1 to 12 hours after the dose was taken. When cost per quality-adjusted life-year gained was considered, 1 meta-analysis found that the slightly greater BP reduction with candesartan compared with losartan was not cost-effective.46 However, other benefits of candesartan compared with losartan therapy (eg, lower risk for cardiovascular disease, heart failure, dysrhythmias, and peripheral artery disease) should be considered.50 Adverse events were generally found to be similar among the ARBs.
No differences in BP lowering were observed among 92 trials of 14 different angiotensin-converting enzyme inhibitors. As a class, these drugs were found to produce a reduction in BP of 8/5 mm Hg.51
Because of the modest BP-lowering effects of each of the antihypertensive drugs currently available, consideration should be given to starting antihypertensive therapy with 2 agents for patients with stage 2 hypertension (ie, BP ≥160/100 mm Hg).
Summary
Elimination of key risk factors such as dyslipidemia and hypertension is important for reducing cardiovascular events later in life. A medical history, physical examination, and laboratory determination of lipid and glycosylated hemoglobin levels provide a good assessment of cardiovascular risk. A statin is first-line therapy for reducing LDL-C, which is the primary lipid target in most patients. High-dose statin therapy may be required to reach desired target levels. The choice of initial antihypertensive therapy is based on patient comorbidities and drug side effects; however, most patients require combination antihypertensive therapy to reach goal. The combination of this multifactorial risk approach along with smoking cessation and modification of other risk factors should complement current and future cardiovascular care for men.
1. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2-e220.
2. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937-952.
3. Mensah GA, Brown DW, Croft JB, Greenlund KJ. Major coronary risk factors and death from coronary heart disease: baseline and follow-up mortality data from the Second National Health and Nutrition Examination Survey (NHANES II). Am J Prev Med. 2005;29(5 suppl 1):68-74.
4. Davidson MH, Maki KC, Pearson TA, et al. Results of the National Cholesterol Education (NCEP) Program Evaluation ProjecT Utilizing Novel E-Technology (NEPTUNE) II survey and implications for treatment under the recent NCEP Writing Group recommendations. Am J Cardiol. 2005;96(4):556-563.
5. Waters DD, Brotons C, Chiang CW, et al. Lipid treatment assessment project 2: a multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation. 2009;120(1):28-34.
6. Vande Griend JP, Saseen JJ. Low-density lipoprotein cholesterol goal attainment in high-risk family medicine patients. J Clin Lipidol. 2009;3(3):195-200.
7. Barham AH, Goff DC, Jr, Chen H, et al. Appropriateness of cholesterol management in primary care by sex and level of cardiovascular risk. Prev Cardiol. 2009;12(2):95-101.
8. Kitkungvan D, Lynn Fillipon NM, Dani SS, Downey BC. Low-density lipoprotein cholesterol target achievement in patients at high risk for coronary heart disease. J Clin Lipidol. 2010;4(4):293-297.
9. Leiter LA, Lundman P, da Silva PM, et al. Persistent lipid abnormalities in statin- treated patients with diabetes mellitus in Europe and Canada: results of the Dyslipidaemia International Study. Diabet Med. 2011;28(11):1343-1351.
10. Rallidis LS, Kotakos C, Sourides V, et al. Attainment of optional low-density lipoprotein cholesterol goal of less than 70 mg/dl and impact on prognosis of very high risk stable coronary patients: a 3-year follow-up. Expert Opin Pharmacother. 2011;12(10):1481-1489.
11. Centers for Disease Control and Prevention. High blood pressure facts. http://www.cdc.gov/bloodpressure/facts.htm. Published 2012. Accessed May 2, 2012.
12. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303(20):2043-2050.
13. McClave A, Rock V, Thorne S, Malarcher A. State-specific prevalence of cigarette smoking and smokeless tobacco use among adults—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(43):1400-1406.
14. Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366(4):321-329.
15. Lewis SJ, Fox KM, Grandy S. Shield Study Group. Self-reported diagnosis of heart disease: results from the SHIELD study. Int J Clin Pract. 2009;63(5):726-734.
16. National Center for Health Statistics. Health, United States, 2010: with special feature on death and dying. http://www.cdc.gov/nchs/data/hus/hus10.pdf. Published 2011. Accessed May 2, 2012.
17. Hemann BA, Bimson WF, Taylor AJ. The Framingham Risk Score: an appraisal of its benefits and limitations. Am Heart Hosp J. 2007;5(2):91-96.
18. Karim R, Hodis HN, Detrano R, Liu CR, Liu CH, Mack WJ. Relation of Framingham risk score to subclinical atherosclerosis evaluated across three arterial sites. Am J Cardiol. 2008;102(7):825-830.
19. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611-619.
20. Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118(22):2243-2251.
21. Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51(15):1512-1524.
22. Fruchart JC, Sacks FM, Hermans MP, et al. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in dyslipidaemic patient. Diab Vasc Dis Res. 2008;5(4):319-335.
23. Koz C, Baysan O, Hasimi A, et al. Conventional and non-conventional coronary risk factors in male premature coronary artery disease patients already having a low Framingham risk score. Acta Cardiol. 2008;63(5):623-628.
24. Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol. 2007;27(1):15-26.
25. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499-511.
26. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421.
27. Di Angelantonio E, Sarwar N, Perry P, et al. Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000.
28. Blackburn P, Lemieux I, Alméras N, et al. The hypertriglyceridemic waist phenotype versus the National Cholesterol Education Program-Adult Treatment Panel III and International Diabetes Federation clinical criteria to identify high-risk men with an altered cardiometabolic risk profile. Metabolism. 2009;58(8):1123-1130.
29. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622.
30. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158.
31. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301-1307.
32. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239.
33. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207.
34. Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373(9670):1175-1182.
35. Delahoy PJ, Magliano DJ, Webb K, Grobler M, Liew D. The relationship between reduction in low-density lipoprotein cholesterol by statins and reduction in risk of cardiovascular outcomes: an updated meta-analysis. Clin Ther. 2009;31(2):236-244.
36. Chan DK, O’Rourke F, Shen Q, Mak JC, Hung WT. Meta-analysis of the cardiovascular benefits of intensive lipid lowering with statins. Acta Neurol Scand. 2011;124(3):188-195.
37. Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365(22):2078-2087.
38. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572.
39. Whitworth JA. World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983-1992.
40. National Clinical Guideline Centre. Hypertension: the clinical management of primary hypertension in adults. http://www.nice.org.uk/nicemedia/live/12167/54727/54727.pdf. Published 2011. Accessed May 2, 2012.
41. Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4(6):393-404.
42. Chen JM, Heran BS, Wright JM. Blood pressure lowering efficacy of diuretics as second-line therapy for primary hypertension. Cochrane Database Syst Rev. 2009;(4):CD007187.-
43. Heran BS, Chen JM, Wang JJ, Wright JM. Blood pressure lowering efficacy of potassium-sparing diuretics (that block the epithelial sodium channel) for primary hypertension. Cochrane Database Syst Rev. 2010;(1):CD008167.-
44. Batterink J, Stabler SN, Tejani AM, Fowkes CT. Spironolactone for hypertension. Cochrane Database Syst Rev. 2010;(8):CD008169.-
45. Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2008;(4):CD003822.-
46. Grosso AM, Bodalia PN, Macallister RJ, Hingorani AD, Moon JC, Scott MA. Comparative clinical-and cost-effectiveness of candesartan and losartan in the management of hypertension and heart failure: a systematic review, meta-and cost-utility analysis. Int J Clin Pract. 2011;65(3):253-263.
47. Nixon RM, Müller E, Lowy A, Falvey H. Valsartan vs. other angiotensin II receptor blockers in the treatment of hypertension: a meta-analytical approach. Int J Clin Pract. 2009;63(5):766-775.
48. Zhenfeng Z, Huilan S, Junya J, Dong L, Shan L. A systematic review and meta-analysis of candesartan and losartan in the management of essential hypertension. J Renin Angiotensin Aldosterone Syst. 2011;12(3):365-374.
49. Zheng Z, Lin S, Shi H. A systematic review and meta-analysis of telmisartan versus valsartan in the management of essential hypertension. J Clin Hypertens (Greenwich). 2010;12(6):414-421.
50. Kjeldsen SE, Stålhammar J, Hasvole P, Bodegard J, Olsson U, Russell D. Effects of losartan vs candesartan in reducing cardiovascular events in the primary treatment of hypertension. J Hum Hypertens. 2010;24(4):263-273.
51. Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database Syst Rev. 2008;(4):CD003823.-
Managing the Multiple Symptoms of Benign Prostatic Hyperplasia — CME
Managing Type 2 Diabetes in Men
Meeting New Challenges with Antiplatelet Therapy in Primary Care
Dr. Cobble has disclosed that he is on the advisory boards and speakers’ bureaus for AstraZeneca and Bristol-Myers Squibb and is on the speakers’ bureaus for Eli Lilly, Forest, and Kowa.
SUPPORT
This program is sponsored by the PCEC and is supported by funding from AstraZeneca.
The death rate from coronary heart disease (CHD) declined by 59% from 1950 to 1999 in the United States, yet CHD remains a major cause of morbidity and mortality, resulting in an estimated 1.5 million heart attacks in 2011.1 Better recognition and treatment of the 9 modifiable risk factors for CHD identified by the INTERHEART study ( FIGURE 1 ), as well as changes in lifestyle practices, undoubtedly contributed to the decline in CHD mortality, but further improvement is possible.2 Estimates derived from the Second National Health and Nutrition Examination Survey (NHANES II) baseline data and 17-year mortality follow-up data indicate that 45% of CHD deaths in men and 64% in women could be avoided by eliminating 3 major risk factors: elevated total cholesterol (≥240 mg/dL), hypertension, and smoking.3
The evidence indicates that these 3 risk factors are not well controlled. Data from the National Cholesterol Education Program (NCEP) Evaluation ProjecT Utilizing Novel E-Technology (NEPTUNE) II survey and the Lipid Treatment Assessment Project 2 (L-TAP 2), as well as more recent evidence, indicate that many patients do not achieve low-density lipoprotein cholesterol (LDL-C) and triglyceride targets.4-10 Similarly, although there has been significant improvement in blood pressure (BP) control over the past 2 decades, BP is controlled in only half of all hypertensive patients.11,12 Finally, the sharp declines in the prevalence of cigarette smoking seen in the past have slowed in recent years, such that approximately 20% of US adults still smoke cigarettes.13
These trends are a concern since a greater risk factor burden in middle age is associated with poorer quality of life and higher medical costs, as well as a higher incidence of cardiovascular events in older age.1 A recent meta-analysis of 18 cohort studies involving 257,384 adults showed a higher incidence of cardiovascular events in later life with an increasing number of risk factors.14 For example, adults 55 years of age with an optimal risk factor profile (ie, total cholesterol <180 mg/dL, BP <120/80 mm Hg, nonsmoker, nondiabetic) had much lower risks of death from cardiovascular disease (CVD) through the age of 80 years than those with 2 or more risk factors (4.7% vs 29.6% among men, 6.4% vs 20.5% among women). This translates into a relative risk (RR) of cardiovascular death of 6 times for men and 3 times for women without optimal risk profiles. Similar trends were observed for risk of fatal CHD/nonfatal myocardial infarction (MI) (3.6% vs 37.5% among men, <1% vs 18.3% among women). These findings point to the critical importance of modifying multiple risk factors early in adulthood, well in advance of symptoms. However, the Study to Help Improve Early Evaluation and Management of Risk Factors Leading to Diabetes (SHIELD) showed that about half of patients with CHD are not diagnosed until symptoms become apparent, and fewer than one quarter are diagnosed as a result of screening.15
This review focuses on patient assessment and treatment strategies to modify abnormal lipid levels and high BP for primary prevention. Addressing other modifiable risk factors is also important, especially since risk factors such as abdominal obesity impact other risk factors ( FIGURE 1 ). An emphasis is placed on strategies in men, since the prevalence of CHD among patients aged 45 years and older is higher in men than in women ( FIGURE 2 ).16 Furthermore, men experience a first cardiovascular event a decade earlier than women, and a more serious CHD event, such as MI or sudden death, 2 decades earlier.1
FIGURE 1
Modifiable risk factors for myocardial infarction (MI)2
ApoB/ApoA-I, apolipoprotein B/apolipoprotein A-I.
FIGURE 2
Prevalence of heart disease by age and gender16
Assessment
The assessment of CHD risk in men need not be complicated and should be made practical so that it is applied consistently. A family and personal medical history and physical examination combined with laboratory determination of lipid levels and glycosylated hemoglobin can help assess modifiable risk factors. The assessment of CHD risk can be facilitated by using 1 of 2 risk calculators. The Framingham Risk Score [www.framinghamheartstudy.org/risk/gencardio.html] is widely used but may underestimate risk, especially in younger persons or those who appear to be healthy but may have other risk factors for CHD.17-19 The Reynolds Risk Score [www.reynoldsriskscore.org/] includes other risk factors, such as parental history of MI before age 60 years, low levels of apolipoprotein A (apoA), high levels of apolipoprotein B (apoB), and increased levels of high-sensitivity C-reactive protein (hs-CRP).19 The Reynolds Risk Score has been validated in healthy, nondiabetic men.20
The relevance of apolipoprotein levels, particularly apoB, to cardiovascular risk is increasingly appreciated.21 ApoB concentration represents the sum of atherogenic particles found on all atherogenic lipoproteins, including very-low-density lipoprotein, intermediate-density lipoprotein, low-density lipoprotein, and lipoprotein(a) cholesterol, whereas apoA represents the sum of antiatherogenic particles found on high-density lipoprotein cholesterol (HDL-C), the antiatherogenic lipoprotein.22 The ratio of apoB/apoA-I has, in fact, been shown to be a good predictor of cardiovascular events in young men without hypertension and diabetes but with chest pain.23 High-sensitivity C-reactive protein is a sensitive marker of acute inflammation and is associated with coronary risk.24 Measuring hs-CRP is a recommended option to determine enhanced absolute risk in people with an intermediate 10-year CHD risk of 10% to 20%.25
There remains some uncertainty regarding which lipid levels should be measured when screening for cardiovascular risk. The National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) advises that total cholesterol, LDL-C, HDL-C, and triglycerides be measured.26 More recent results from The Emerging Risk Factors Collaboration suggest that a more simplified approach may be reasonable.27 Review of data from 68 long-term prospective studies involving 302,430 people without initial vascular disease and 2.79 million person-years of follow-up showed that lipid assessment of vascular risk could be accomplished by measuring either total cholesterol and HDL-C levels or apolipoprotein levels; measuring the triglyceride level was of no added benefit in assessing vascular risk. In addition, fasting and nonfasting lipid levels were found to be of similar value in assessing risk. Other evidence shows that the combination of a triglyceride level ≥178 mg/dL and waist circumference ≥35.4 inches—the hypertriglyceridemic waist phenotype—is as discriminatory a screening tool as the NCEP ATP III guidelines to identify individuals at increased cardiometabolic risk.28 The use of more comprehensive lipoprotein and apolipoprotein testing, as well as noninvasive imaging, may have value in future cardiovascular risk assessment.
Treatment
The main goal of treatment in persons with 1 or more modifiable risk factors is to prevent an incident or primary cardiovascular event. Treatment strategies to achieve this goal in men and women are the same. Prevention of recurrent or secondary events will not be addressed here.
Lipids
Numerous clinical trials, such as the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS),29 Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA),30 and West of Scotland Coronary Prevention Study (WOSCOPS),31 definitively established the benefit of cardiovascular risk reduction with lipid-lowering treatment, particularly LDL-C-lowering treatment. Low-density lipoprotein cholesterol is the principal lipid target in most patients, with the treatment goal based on the presence of additional risk factors.32 Discussion of treatments for low HDL-C and elevated triglyceride levels is beyond the scope of this review but is expected to be included in the NCEP ATP IV guidelines scheduled for release later in 2012.
The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) also established significant benefits of statin therapy in primary prevention, compared with placebo, in persons with normal or modestly elevated LDL-C (<130 mg/dL) and elevated hs-CRP (≥2 mg/L).33 Rates of the primary end point (MI, stroke, arterial revascularization, hospitalization for unstable angina, or cardiovascular death) were 0.77 and 1.36 per 100 person-years of follow-up in the rosuvastatin and placebo groups, respectively (hazard ratio [HR], 0.56; 95% CI, 0.46-0.69; P < .00001). Further analysis showed that patients who achieved LDL-C <70 mg/dL had a 55% lower rate of vascular events compared with placebo.34
Results from large primary prevention clinical trials such as JUPITER have led to recommendations over the past decade or so for progressively lower LDL-C goals. A meta-analysis of 25 large clinical trials involving 155,613 subjects showed that for every 25 mg/dL reduction in LDL-C, the RR for several cardiovascular outcomes was reduced: vascular mortality, 0.89; major vascular events, 0.86; major coronary events, 0.84; and stroke, 0.90. Put differently, there was a 20% reduction in major coronary events for every 39 mg/dL LDL-C reduction.35
Recent trials support the benefits of intensive high-dose statin therapy in greatly reducing lipid levels, with associated benefits in terms of cardiovascular events. A meta-analysis of 7 trials involving 50,972 high-risk patients with a mean follow-up of 3.1 years showed significant reductions in the risk for cardiovascular events with intensive statin therapy. Those who achieved LDL-C <82 mg/dL with intensive statin therapy had lower cardiovascular risks compared with those with LDL-C ≥82 mg/dL: stroke, odds ratio (OR): 0.80; major coronary events, OR: 0.74; and CVD or CHD death, OR: 0.84.36 Significantly higher liver enzyme abnormalities were observed in patients treated with high-dose statin therapy. [See also Addressing Key Questions with Statin Therapy in this supplement.] The benefits of intensive statin therapy on the progression of coronary atherosclerosis have also been investigated. The Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin versus Atorvastatin (SATURN) by Nicholls et al37 included patients (N = 1039) with documented coronary vessel stenosis of at least 20% and a target vessel for imaging with less than 50% obstruction. Patients received either atorvastatin 80 mg daily or rosu-vastatin 40 mg daily for 104 weeks. In the rosuvastatin group, end-of-study LDL-C levels were lower (62.6 vs 70.2 mg/dL; P < .001) and HDL-C levels higher (50.4 vs 48.6 mg/dL; P = .01) compared with the atorvastatin group, respectively. The percent atheroma volume decreased by 1.22% with rosuvastatin and 0.99% with atorvastatin (P = .17). The normalized total atheroma volume decreased 6.39 mm3 with rosuvastatin and 4.42 mm3 with atorvastatin (P = .01). Atheroma regression was induced in the majority of patients in both groups.
Further support for treating with statin doses higher than those recommended for initial therapy comes from a prospective trial involving 1337 consecutive patients followed over a median of 33 months.10 Although 83% of these patients were on statin therapy, only 51% had an LDL-C <100 mg/dL, and only 15% of the very high-risk patients (n = 941) had an LDL-C <70 mg/dL. The use of intensive statin therapy was associated with a 12-fold higher possibility of achieving an LDL-C <70 mg/dL. Very high-risk patients who achieved an LDL-C <70 mg/dL had a significantly lower risk of all cardiovascular events (HR, 0.34; P = .003).
Blood pressure
As with dyslipidemia, the cardiovascular benefits of lowering elevated BP are well established. While the usual BP goal is <140/90 mm Hg, in those with hypertension and concomitant diabetes or renal disease, the goal is <130/80 mm Hg.38 It is not clear how best to achieve these goals, but therapy must be individualized based on patient comorbidities and drug side effects as recommended in current guidelines.38-40 With these guidelines as a basis, a simplified ABCD approach can be considered in selecting initial antihypertensive therapy ( FIGURE 3 ).
FIGURE 3
ABCD approach to initial antihypertensive therapy38-40
ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; MI, myocardial infarction. Monotherapy, however, does not result in BP control in most patients. As shown by the Antihypertensive Lipid-Lowering Treatment to Prevent Heart Attacks Trial (ALLHAT), BP control typically requires at least 2 different classes of drugs, with 3 or more drugs required in about 1 in 6 patients within 3 years and 1 in 4 patients within 5 years. A higher percentage of patients with diabetes mellitus or kidney impairment (creatinine ≥1.5 mg/dL) require 3 or more antihypertensive drugs after 5 years (33% and 40%, respectively).41
Several meta-analyses have been conducted recently to assess the magnitude of BP (systolic/diastolic) lowering in the different classes of antihypertensive drugs. While these meta-analyses have important limitations, such as differences in study design and the lack of a clear description of outcomes, some general impressions can be made. In 1 meta-analysis, thiazide diuretics were found to lower BP by 6/3 and 8/4 mm Hg at doses of 1 and 2 times the recommended starting dose, respectively. A BP-lowering effect of 6/3 mm Hg was observed with starting doses of loop diuretics.42 Another meta-analysis failed to find a statistically or clinically significant BP-lowering effect with potassium-sparing diuretics at low doses.43 For spironolactone, a review of 5 crossover studies found a reduction in BP of 21/7 mm Hg. In this review, daily doses of 25 to 100 mg were found to provide the best balance between BP reduction and safety and tolerability.44
Several meta-analyses of angiotensin receptor blockers (ARBs) have found BP reductions to be similar among the various ARB drugs. Generally, at maximum recommended doses, a BP reduction of 8/5 mm Hg is observed with these drugs, except for losartan, which produces a smaller BP reduction.45-49 Heran et al45 found a BP reduction of 12/7 mm Hg among the ARBs 1 to 12 hours after the dose was taken. When cost per quality-adjusted life-year gained was considered, 1 meta-analysis found that the slightly greater BP reduction with candesartan compared with losartan was not cost-effective.46 However, other benefits of candesartan compared with losartan therapy (eg, lower risk for cardiovascular disease, heart failure, dysrhythmias, and peripheral artery disease) should be considered.50 Adverse events were generally found to be similar among the ARBs.
No differences in BP lowering were observed among 92 trials of 14 different angiotensin-converting enzyme inhibitors. As a class, these drugs were found to produce a reduction in BP of 8/5 mm Hg.51
Because of the modest BP-lowering effects of each of the antihypertensive drugs currently available, consideration should be given to starting antihypertensive therapy with 2 agents for patients with stage 2 hypertension (ie, BP ≥160/100 mm Hg).
Summary
Elimination of key risk factors such as dyslipidemia and hypertension is important for reducing cardiovascular events later in life. A medical history, physical examination, and laboratory determination of lipid and glycosylated hemoglobin levels provide a good assessment of cardiovascular risk. A statin is first-line therapy for reducing LDL-C, which is the primary lipid target in most patients. High-dose statin therapy may be required to reach desired target levels. The choice of initial antihypertensive therapy is based on patient comorbidities and drug side effects; however, most patients require combination antihypertensive therapy to reach goal. The combination of this multifactorial risk approach along with smoking cessation and modification of other risk factors should complement current and future cardiovascular care for men.
Managing the Multiple Symptoms of Benign Prostatic Hyperplasia — CME
Managing Type 2 Diabetes in Men
Meeting New Challenges with Antiplatelet Therapy in Primary Care
Dr. Cobble has disclosed that he is on the advisory boards and speakers’ bureaus for AstraZeneca and Bristol-Myers Squibb and is on the speakers’ bureaus for Eli Lilly, Forest, and Kowa.
SUPPORT
This program is sponsored by the PCEC and is supported by funding from AstraZeneca.
The death rate from coronary heart disease (CHD) declined by 59% from 1950 to 1999 in the United States, yet CHD remains a major cause of morbidity and mortality, resulting in an estimated 1.5 million heart attacks in 2011.1 Better recognition and treatment of the 9 modifiable risk factors for CHD identified by the INTERHEART study ( FIGURE 1 ), as well as changes in lifestyle practices, undoubtedly contributed to the decline in CHD mortality, but further improvement is possible.2 Estimates derived from the Second National Health and Nutrition Examination Survey (NHANES II) baseline data and 17-year mortality follow-up data indicate that 45% of CHD deaths in men and 64% in women could be avoided by eliminating 3 major risk factors: elevated total cholesterol (≥240 mg/dL), hypertension, and smoking.3
The evidence indicates that these 3 risk factors are not well controlled. Data from the National Cholesterol Education Program (NCEP) Evaluation ProjecT Utilizing Novel E-Technology (NEPTUNE) II survey and the Lipid Treatment Assessment Project 2 (L-TAP 2), as well as more recent evidence, indicate that many patients do not achieve low-density lipoprotein cholesterol (LDL-C) and triglyceride targets.4-10 Similarly, although there has been significant improvement in blood pressure (BP) control over the past 2 decades, BP is controlled in only half of all hypertensive patients.11,12 Finally, the sharp declines in the prevalence of cigarette smoking seen in the past have slowed in recent years, such that approximately 20% of US adults still smoke cigarettes.13
These trends are a concern since a greater risk factor burden in middle age is associated with poorer quality of life and higher medical costs, as well as a higher incidence of cardiovascular events in older age.1 A recent meta-analysis of 18 cohort studies involving 257,384 adults showed a higher incidence of cardiovascular events in later life with an increasing number of risk factors.14 For example, adults 55 years of age with an optimal risk factor profile (ie, total cholesterol <180 mg/dL, BP <120/80 mm Hg, nonsmoker, nondiabetic) had much lower risks of death from cardiovascular disease (CVD) through the age of 80 years than those with 2 or more risk factors (4.7% vs 29.6% among men, 6.4% vs 20.5% among women). This translates into a relative risk (RR) of cardiovascular death of 6 times for men and 3 times for women without optimal risk profiles. Similar trends were observed for risk of fatal CHD/nonfatal myocardial infarction (MI) (3.6% vs 37.5% among men, <1% vs 18.3% among women). These findings point to the critical importance of modifying multiple risk factors early in adulthood, well in advance of symptoms. However, the Study to Help Improve Early Evaluation and Management of Risk Factors Leading to Diabetes (SHIELD) showed that about half of patients with CHD are not diagnosed until symptoms become apparent, and fewer than one quarter are diagnosed as a result of screening.15
This review focuses on patient assessment and treatment strategies to modify abnormal lipid levels and high BP for primary prevention. Addressing other modifiable risk factors is also important, especially since risk factors such as abdominal obesity impact other risk factors ( FIGURE 1 ). An emphasis is placed on strategies in men, since the prevalence of CHD among patients aged 45 years and older is higher in men than in women ( FIGURE 2 ).16 Furthermore, men experience a first cardiovascular event a decade earlier than women, and a more serious CHD event, such as MI or sudden death, 2 decades earlier.1
FIGURE 1
Modifiable risk factors for myocardial infarction (MI)2
ApoB/ApoA-I, apolipoprotein B/apolipoprotein A-I.
FIGURE 2
Prevalence of heart disease by age and gender16
Assessment
The assessment of CHD risk in men need not be complicated and should be made practical so that it is applied consistently. A family and personal medical history and physical examination combined with laboratory determination of lipid levels and glycosylated hemoglobin can help assess modifiable risk factors. The assessment of CHD risk can be facilitated by using 1 of 2 risk calculators. The Framingham Risk Score [www.framinghamheartstudy.org/risk/gencardio.html] is widely used but may underestimate risk, especially in younger persons or those who appear to be healthy but may have other risk factors for CHD.17-19 The Reynolds Risk Score [www.reynoldsriskscore.org/] includes other risk factors, such as parental history of MI before age 60 years, low levels of apolipoprotein A (apoA), high levels of apolipoprotein B (apoB), and increased levels of high-sensitivity C-reactive protein (hs-CRP).19 The Reynolds Risk Score has been validated in healthy, nondiabetic men.20
The relevance of apolipoprotein levels, particularly apoB, to cardiovascular risk is increasingly appreciated.21 ApoB concentration represents the sum of atherogenic particles found on all atherogenic lipoproteins, including very-low-density lipoprotein, intermediate-density lipoprotein, low-density lipoprotein, and lipoprotein(a) cholesterol, whereas apoA represents the sum of antiatherogenic particles found on high-density lipoprotein cholesterol (HDL-C), the antiatherogenic lipoprotein.22 The ratio of apoB/apoA-I has, in fact, been shown to be a good predictor of cardiovascular events in young men without hypertension and diabetes but with chest pain.23 High-sensitivity C-reactive protein is a sensitive marker of acute inflammation and is associated with coronary risk.24 Measuring hs-CRP is a recommended option to determine enhanced absolute risk in people with an intermediate 10-year CHD risk of 10% to 20%.25
There remains some uncertainty regarding which lipid levels should be measured when screening for cardiovascular risk. The National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) advises that total cholesterol, LDL-C, HDL-C, and triglycerides be measured.26 More recent results from The Emerging Risk Factors Collaboration suggest that a more simplified approach may be reasonable.27 Review of data from 68 long-term prospective studies involving 302,430 people without initial vascular disease and 2.79 million person-years of follow-up showed that lipid assessment of vascular risk could be accomplished by measuring either total cholesterol and HDL-C levels or apolipoprotein levels; measuring the triglyceride level was of no added benefit in assessing vascular risk. In addition, fasting and nonfasting lipid levels were found to be of similar value in assessing risk. Other evidence shows that the combination of a triglyceride level ≥178 mg/dL and waist circumference ≥35.4 inches—the hypertriglyceridemic waist phenotype—is as discriminatory a screening tool as the NCEP ATP III guidelines to identify individuals at increased cardiometabolic risk.28 The use of more comprehensive lipoprotein and apolipoprotein testing, as well as noninvasive imaging, may have value in future cardiovascular risk assessment.
Treatment
The main goal of treatment in persons with 1 or more modifiable risk factors is to prevent an incident or primary cardiovascular event. Treatment strategies to achieve this goal in men and women are the same. Prevention of recurrent or secondary events will not be addressed here.
Lipids
Numerous clinical trials, such as the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS),29 Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA),30 and West of Scotland Coronary Prevention Study (WOSCOPS),31 definitively established the benefit of cardiovascular risk reduction with lipid-lowering treatment, particularly LDL-C-lowering treatment. Low-density lipoprotein cholesterol is the principal lipid target in most patients, with the treatment goal based on the presence of additional risk factors.32 Discussion of treatments for low HDL-C and elevated triglyceride levels is beyond the scope of this review but is expected to be included in the NCEP ATP IV guidelines scheduled for release later in 2012.
The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) also established significant benefits of statin therapy in primary prevention, compared with placebo, in persons with normal or modestly elevated LDL-C (<130 mg/dL) and elevated hs-CRP (≥2 mg/L).33 Rates of the primary end point (MI, stroke, arterial revascularization, hospitalization for unstable angina, or cardiovascular death) were 0.77 and 1.36 per 100 person-years of follow-up in the rosuvastatin and placebo groups, respectively (hazard ratio [HR], 0.56; 95% CI, 0.46-0.69; P < .00001). Further analysis showed that patients who achieved LDL-C <70 mg/dL had a 55% lower rate of vascular events compared with placebo.34
Results from large primary prevention clinical trials such as JUPITER have led to recommendations over the past decade or so for progressively lower LDL-C goals. A meta-analysis of 25 large clinical trials involving 155,613 subjects showed that for every 25 mg/dL reduction in LDL-C, the RR for several cardiovascular outcomes was reduced: vascular mortality, 0.89; major vascular events, 0.86; major coronary events, 0.84; and stroke, 0.90. Put differently, there was a 20% reduction in major coronary events for every 39 mg/dL LDL-C reduction.35
Recent trials support the benefits of intensive high-dose statin therapy in greatly reducing lipid levels, with associated benefits in terms of cardiovascular events. A meta-analysis of 7 trials involving 50,972 high-risk patients with a mean follow-up of 3.1 years showed significant reductions in the risk for cardiovascular events with intensive statin therapy. Those who achieved LDL-C <82 mg/dL with intensive statin therapy had lower cardiovascular risks compared with those with LDL-C ≥82 mg/dL: stroke, odds ratio (OR): 0.80; major coronary events, OR: 0.74; and CVD or CHD death, OR: 0.84.36 Significantly higher liver enzyme abnormalities were observed in patients treated with high-dose statin therapy. [See also Addressing Key Questions with Statin Therapy in this supplement.] The benefits of intensive statin therapy on the progression of coronary atherosclerosis have also been investigated. The Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin versus Atorvastatin (SATURN) by Nicholls et al37 included patients (N = 1039) with documented coronary vessel stenosis of at least 20% and a target vessel for imaging with less than 50% obstruction. Patients received either atorvastatin 80 mg daily or rosu-vastatin 40 mg daily for 104 weeks. In the rosuvastatin group, end-of-study LDL-C levels were lower (62.6 vs 70.2 mg/dL; P < .001) and HDL-C levels higher (50.4 vs 48.6 mg/dL; P = .01) compared with the atorvastatin group, respectively. The percent atheroma volume decreased by 1.22% with rosuvastatin and 0.99% with atorvastatin (P = .17). The normalized total atheroma volume decreased 6.39 mm3 with rosuvastatin and 4.42 mm3 with atorvastatin (P = .01). Atheroma regression was induced in the majority of patients in both groups.
Further support for treating with statin doses higher than those recommended for initial therapy comes from a prospective trial involving 1337 consecutive patients followed over a median of 33 months.10 Although 83% of these patients were on statin therapy, only 51% had an LDL-C <100 mg/dL, and only 15% of the very high-risk patients (n = 941) had an LDL-C <70 mg/dL. The use of intensive statin therapy was associated with a 12-fold higher possibility of achieving an LDL-C <70 mg/dL. Very high-risk patients who achieved an LDL-C <70 mg/dL had a significantly lower risk of all cardiovascular events (HR, 0.34; P = .003).
Blood pressure
As with dyslipidemia, the cardiovascular benefits of lowering elevated BP are well established. While the usual BP goal is <140/90 mm Hg, in those with hypertension and concomitant diabetes or renal disease, the goal is <130/80 mm Hg.38 It is not clear how best to achieve these goals, but therapy must be individualized based on patient comorbidities and drug side effects as recommended in current guidelines.38-40 With these guidelines as a basis, a simplified ABCD approach can be considered in selecting initial antihypertensive therapy ( FIGURE 3 ).
FIGURE 3
ABCD approach to initial antihypertensive therapy38-40
ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; MI, myocardial infarction. Monotherapy, however, does not result in BP control in most patients. As shown by the Antihypertensive Lipid-Lowering Treatment to Prevent Heart Attacks Trial (ALLHAT), BP control typically requires at least 2 different classes of drugs, with 3 or more drugs required in about 1 in 6 patients within 3 years and 1 in 4 patients within 5 years. A higher percentage of patients with diabetes mellitus or kidney impairment (creatinine ≥1.5 mg/dL) require 3 or more antihypertensive drugs after 5 years (33% and 40%, respectively).41
Several meta-analyses have been conducted recently to assess the magnitude of BP (systolic/diastolic) lowering in the different classes of antihypertensive drugs. While these meta-analyses have important limitations, such as differences in study design and the lack of a clear description of outcomes, some general impressions can be made. In 1 meta-analysis, thiazide diuretics were found to lower BP by 6/3 and 8/4 mm Hg at doses of 1 and 2 times the recommended starting dose, respectively. A BP-lowering effect of 6/3 mm Hg was observed with starting doses of loop diuretics.42 Another meta-analysis failed to find a statistically or clinically significant BP-lowering effect with potassium-sparing diuretics at low doses.43 For spironolactone, a review of 5 crossover studies found a reduction in BP of 21/7 mm Hg. In this review, daily doses of 25 to 100 mg were found to provide the best balance between BP reduction and safety and tolerability.44
Several meta-analyses of angiotensin receptor blockers (ARBs) have found BP reductions to be similar among the various ARB drugs. Generally, at maximum recommended doses, a BP reduction of 8/5 mm Hg is observed with these drugs, except for losartan, which produces a smaller BP reduction.45-49 Heran et al45 found a BP reduction of 12/7 mm Hg among the ARBs 1 to 12 hours after the dose was taken. When cost per quality-adjusted life-year gained was considered, 1 meta-analysis found that the slightly greater BP reduction with candesartan compared with losartan was not cost-effective.46 However, other benefits of candesartan compared with losartan therapy (eg, lower risk for cardiovascular disease, heart failure, dysrhythmias, and peripheral artery disease) should be considered.50 Adverse events were generally found to be similar among the ARBs.
No differences in BP lowering were observed among 92 trials of 14 different angiotensin-converting enzyme inhibitors. As a class, these drugs were found to produce a reduction in BP of 8/5 mm Hg.51
Because of the modest BP-lowering effects of each of the antihypertensive drugs currently available, consideration should be given to starting antihypertensive therapy with 2 agents for patients with stage 2 hypertension (ie, BP ≥160/100 mm Hg).
Summary
Elimination of key risk factors such as dyslipidemia and hypertension is important for reducing cardiovascular events later in life. A medical history, physical examination, and laboratory determination of lipid and glycosylated hemoglobin levels provide a good assessment of cardiovascular risk. A statin is first-line therapy for reducing LDL-C, which is the primary lipid target in most patients. High-dose statin therapy may be required to reach desired target levels. The choice of initial antihypertensive therapy is based on patient comorbidities and drug side effects; however, most patients require combination antihypertensive therapy to reach goal. The combination of this multifactorial risk approach along with smoking cessation and modification of other risk factors should complement current and future cardiovascular care for men.
1. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2-e220.
2. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937-952.
3. Mensah GA, Brown DW, Croft JB, Greenlund KJ. Major coronary risk factors and death from coronary heart disease: baseline and follow-up mortality data from the Second National Health and Nutrition Examination Survey (NHANES II). Am J Prev Med. 2005;29(5 suppl 1):68-74.
4. Davidson MH, Maki KC, Pearson TA, et al. Results of the National Cholesterol Education (NCEP) Program Evaluation ProjecT Utilizing Novel E-Technology (NEPTUNE) II survey and implications for treatment under the recent NCEP Writing Group recommendations. Am J Cardiol. 2005;96(4):556-563.
5. Waters DD, Brotons C, Chiang CW, et al. Lipid treatment assessment project 2: a multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation. 2009;120(1):28-34.
6. Vande Griend JP, Saseen JJ. Low-density lipoprotein cholesterol goal attainment in high-risk family medicine patients. J Clin Lipidol. 2009;3(3):195-200.
7. Barham AH, Goff DC, Jr, Chen H, et al. Appropriateness of cholesterol management in primary care by sex and level of cardiovascular risk. Prev Cardiol. 2009;12(2):95-101.
8. Kitkungvan D, Lynn Fillipon NM, Dani SS, Downey BC. Low-density lipoprotein cholesterol target achievement in patients at high risk for coronary heart disease. J Clin Lipidol. 2010;4(4):293-297.
9. Leiter LA, Lundman P, da Silva PM, et al. Persistent lipid abnormalities in statin- treated patients with diabetes mellitus in Europe and Canada: results of the Dyslipidaemia International Study. Diabet Med. 2011;28(11):1343-1351.
10. Rallidis LS, Kotakos C, Sourides V, et al. Attainment of optional low-density lipoprotein cholesterol goal of less than 70 mg/dl and impact on prognosis of very high risk stable coronary patients: a 3-year follow-up. Expert Opin Pharmacother. 2011;12(10):1481-1489.
11. Centers for Disease Control and Prevention. High blood pressure facts. http://www.cdc.gov/bloodpressure/facts.htm. Published 2012. Accessed May 2, 2012.
12. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303(20):2043-2050.
13. McClave A, Rock V, Thorne S, Malarcher A. State-specific prevalence of cigarette smoking and smokeless tobacco use among adults—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(43):1400-1406.
14. Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366(4):321-329.
15. Lewis SJ, Fox KM, Grandy S. Shield Study Group. Self-reported diagnosis of heart disease: results from the SHIELD study. Int J Clin Pract. 2009;63(5):726-734.
16. National Center for Health Statistics. Health, United States, 2010: with special feature on death and dying. http://www.cdc.gov/nchs/data/hus/hus10.pdf. Published 2011. Accessed May 2, 2012.
17. Hemann BA, Bimson WF, Taylor AJ. The Framingham Risk Score: an appraisal of its benefits and limitations. Am Heart Hosp J. 2007;5(2):91-96.
18. Karim R, Hodis HN, Detrano R, Liu CR, Liu CH, Mack WJ. Relation of Framingham risk score to subclinical atherosclerosis evaluated across three arterial sites. Am J Cardiol. 2008;102(7):825-830.
19. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611-619.
20. Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118(22):2243-2251.
21. Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51(15):1512-1524.
22. Fruchart JC, Sacks FM, Hermans MP, et al. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in dyslipidaemic patient. Diab Vasc Dis Res. 2008;5(4):319-335.
23. Koz C, Baysan O, Hasimi A, et al. Conventional and non-conventional coronary risk factors in male premature coronary artery disease patients already having a low Framingham risk score. Acta Cardiol. 2008;63(5):623-628.
24. Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol. 2007;27(1):15-26.
25. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499-511.
26. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421.
27. Di Angelantonio E, Sarwar N, Perry P, et al. Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000.
28. Blackburn P, Lemieux I, Alméras N, et al. The hypertriglyceridemic waist phenotype versus the National Cholesterol Education Program-Adult Treatment Panel III and International Diabetes Federation clinical criteria to identify high-risk men with an altered cardiometabolic risk profile. Metabolism. 2009;58(8):1123-1130.
29. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622.
30. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158.
31. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301-1307.
32. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239.
33. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207.
34. Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373(9670):1175-1182.
35. Delahoy PJ, Magliano DJ, Webb K, Grobler M, Liew D. The relationship between reduction in low-density lipoprotein cholesterol by statins and reduction in risk of cardiovascular outcomes: an updated meta-analysis. Clin Ther. 2009;31(2):236-244.
36. Chan DK, O’Rourke F, Shen Q, Mak JC, Hung WT. Meta-analysis of the cardiovascular benefits of intensive lipid lowering with statins. Acta Neurol Scand. 2011;124(3):188-195.
37. Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365(22):2078-2087.
38. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572.
39. Whitworth JA. World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983-1992.
40. National Clinical Guideline Centre. Hypertension: the clinical management of primary hypertension in adults. http://www.nice.org.uk/nicemedia/live/12167/54727/54727.pdf. Published 2011. Accessed May 2, 2012.
41. Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4(6):393-404.
42. Chen JM, Heran BS, Wright JM. Blood pressure lowering efficacy of diuretics as second-line therapy for primary hypertension. Cochrane Database Syst Rev. 2009;(4):CD007187.-
43. Heran BS, Chen JM, Wang JJ, Wright JM. Blood pressure lowering efficacy of potassium-sparing diuretics (that block the epithelial sodium channel) for primary hypertension. Cochrane Database Syst Rev. 2010;(1):CD008167.-
44. Batterink J, Stabler SN, Tejani AM, Fowkes CT. Spironolactone for hypertension. Cochrane Database Syst Rev. 2010;(8):CD008169.-
45. Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2008;(4):CD003822.-
46. Grosso AM, Bodalia PN, Macallister RJ, Hingorani AD, Moon JC, Scott MA. Comparative clinical-and cost-effectiveness of candesartan and losartan in the management of hypertension and heart failure: a systematic review, meta-and cost-utility analysis. Int J Clin Pract. 2011;65(3):253-263.
47. Nixon RM, Müller E, Lowy A, Falvey H. Valsartan vs. other angiotensin II receptor blockers in the treatment of hypertension: a meta-analytical approach. Int J Clin Pract. 2009;63(5):766-775.
48. Zhenfeng Z, Huilan S, Junya J, Dong L, Shan L. A systematic review and meta-analysis of candesartan and losartan in the management of essential hypertension. J Renin Angiotensin Aldosterone Syst. 2011;12(3):365-374.
49. Zheng Z, Lin S, Shi H. A systematic review and meta-analysis of telmisartan versus valsartan in the management of essential hypertension. J Clin Hypertens (Greenwich). 2010;12(6):414-421.
50. Kjeldsen SE, Stålhammar J, Hasvole P, Bodegard J, Olsson U, Russell D. Effects of losartan vs candesartan in reducing cardiovascular events in the primary treatment of hypertension. J Hum Hypertens. 2010;24(4):263-273.
51. Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database Syst Rev. 2008;(4):CD003823.-
1. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2-e220.
2. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937-952.
3. Mensah GA, Brown DW, Croft JB, Greenlund KJ. Major coronary risk factors and death from coronary heart disease: baseline and follow-up mortality data from the Second National Health and Nutrition Examination Survey (NHANES II). Am J Prev Med. 2005;29(5 suppl 1):68-74.
4. Davidson MH, Maki KC, Pearson TA, et al. Results of the National Cholesterol Education (NCEP) Program Evaluation ProjecT Utilizing Novel E-Technology (NEPTUNE) II survey and implications for treatment under the recent NCEP Writing Group recommendations. Am J Cardiol. 2005;96(4):556-563.
5. Waters DD, Brotons C, Chiang CW, et al. Lipid treatment assessment project 2: a multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation. 2009;120(1):28-34.
6. Vande Griend JP, Saseen JJ. Low-density lipoprotein cholesterol goal attainment in high-risk family medicine patients. J Clin Lipidol. 2009;3(3):195-200.
7. Barham AH, Goff DC, Jr, Chen H, et al. Appropriateness of cholesterol management in primary care by sex and level of cardiovascular risk. Prev Cardiol. 2009;12(2):95-101.
8. Kitkungvan D, Lynn Fillipon NM, Dani SS, Downey BC. Low-density lipoprotein cholesterol target achievement in patients at high risk for coronary heart disease. J Clin Lipidol. 2010;4(4):293-297.
9. Leiter LA, Lundman P, da Silva PM, et al. Persistent lipid abnormalities in statin- treated patients with diabetes mellitus in Europe and Canada: results of the Dyslipidaemia International Study. Diabet Med. 2011;28(11):1343-1351.
10. Rallidis LS, Kotakos C, Sourides V, et al. Attainment of optional low-density lipoprotein cholesterol goal of less than 70 mg/dl and impact on prognosis of very high risk stable coronary patients: a 3-year follow-up. Expert Opin Pharmacother. 2011;12(10):1481-1489.
11. Centers for Disease Control and Prevention. High blood pressure facts. http://www.cdc.gov/bloodpressure/facts.htm. Published 2012. Accessed May 2, 2012.
12. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303(20):2043-2050.
13. McClave A, Rock V, Thorne S, Malarcher A. State-specific prevalence of cigarette smoking and smokeless tobacco use among adults—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(43):1400-1406.
14. Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366(4):321-329.
15. Lewis SJ, Fox KM, Grandy S. Shield Study Group. Self-reported diagnosis of heart disease: results from the SHIELD study. Int J Clin Pract. 2009;63(5):726-734.
16. National Center for Health Statistics. Health, United States, 2010: with special feature on death and dying. http://www.cdc.gov/nchs/data/hus/hus10.pdf. Published 2011. Accessed May 2, 2012.
17. Hemann BA, Bimson WF, Taylor AJ. The Framingham Risk Score: an appraisal of its benefits and limitations. Am Heart Hosp J. 2007;5(2):91-96.
18. Karim R, Hodis HN, Detrano R, Liu CR, Liu CH, Mack WJ. Relation of Framingham risk score to subclinical atherosclerosis evaluated across three arterial sites. Am J Cardiol. 2008;102(7):825-830.
19. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611-619.
20. Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118(22):2243-2251.
21. Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51(15):1512-1524.
22. Fruchart JC, Sacks FM, Hermans MP, et al. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in dyslipidaemic patient. Diab Vasc Dis Res. 2008;5(4):319-335.
23. Koz C, Baysan O, Hasimi A, et al. Conventional and non-conventional coronary risk factors in male premature coronary artery disease patients already having a low Framingham risk score. Acta Cardiol. 2008;63(5):623-628.
24. Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol. 2007;27(1):15-26.
25. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499-511.
26. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421.
27. Di Angelantonio E, Sarwar N, Perry P, et al. Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000.
28. Blackburn P, Lemieux I, Alméras N, et al. The hypertriglyceridemic waist phenotype versus the National Cholesterol Education Program-Adult Treatment Panel III and International Diabetes Federation clinical criteria to identify high-risk men with an altered cardiometabolic risk profile. Metabolism. 2009;58(8):1123-1130.
29. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622.
30. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158.
31. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301-1307.
32. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239.
33. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207.
34. Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373(9670):1175-1182.
35. Delahoy PJ, Magliano DJ, Webb K, Grobler M, Liew D. The relationship between reduction in low-density lipoprotein cholesterol by statins and reduction in risk of cardiovascular outcomes: an updated meta-analysis. Clin Ther. 2009;31(2):236-244.
36. Chan DK, O’Rourke F, Shen Q, Mak JC, Hung WT. Meta-analysis of the cardiovascular benefits of intensive lipid lowering with statins. Acta Neurol Scand. 2011;124(3):188-195.
37. Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365(22):2078-2087.
38. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572.
39. Whitworth JA. World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983-1992.
40. National Clinical Guideline Centre. Hypertension: the clinical management of primary hypertension in adults. http://www.nice.org.uk/nicemedia/live/12167/54727/54727.pdf. Published 2011. Accessed May 2, 2012.
41. Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4(6):393-404.
42. Chen JM, Heran BS, Wright JM. Blood pressure lowering efficacy of diuretics as second-line therapy for primary hypertension. Cochrane Database Syst Rev. 2009;(4):CD007187.-
43. Heran BS, Chen JM, Wang JJ, Wright JM. Blood pressure lowering efficacy of potassium-sparing diuretics (that block the epithelial sodium channel) for primary hypertension. Cochrane Database Syst Rev. 2010;(1):CD008167.-
44. Batterink J, Stabler SN, Tejani AM, Fowkes CT. Spironolactone for hypertension. Cochrane Database Syst Rev. 2010;(8):CD008169.-
45. Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2008;(4):CD003822.-
46. Grosso AM, Bodalia PN, Macallister RJ, Hingorani AD, Moon JC, Scott MA. Comparative clinical-and cost-effectiveness of candesartan and losartan in the management of hypertension and heart failure: a systematic review, meta-and cost-utility analysis. Int J Clin Pract. 2011;65(3):253-263.
47. Nixon RM, Müller E, Lowy A, Falvey H. Valsartan vs. other angiotensin II receptor blockers in the treatment of hypertension: a meta-analytical approach. Int J Clin Pract. 2009;63(5):766-775.
48. Zhenfeng Z, Huilan S, Junya J, Dong L, Shan L. A systematic review and meta-analysis of candesartan and losartan in the management of essential hypertension. J Renin Angiotensin Aldosterone Syst. 2011;12(3):365-374.
49. Zheng Z, Lin S, Shi H. A systematic review and meta-analysis of telmisartan versus valsartan in the management of essential hypertension. J Clin Hypertens (Greenwich). 2010;12(6):414-421.
50. Kjeldsen SE, Stålhammar J, Hasvole P, Bodegard J, Olsson U, Russell D. Effects of losartan vs candesartan in reducing cardiovascular events in the primary treatment of hypertension. J Hum Hypertens. 2010;24(4):263-273.
51. Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database Syst Rev. 2008;(4):CD003823.-