User login
CASE Psychotic and reclusive
Ms. A, age 51, has schizophrenia and has been doing well living at a supervised residential facility. She was stable on haloperidol, 10 mg twice a day, for years but recently became agitated, threatening her roommate and yelling during the night. Ms. A begins to refuse to take her haloperidol. She also refuses to attend several outpatient appointments. As a result, Ms. A is admitted to the psychiatric unit on an involuntary basis.
In the hospital, Ms. A rarely comes out of her room. When she does come out, she usually sits in a chair, talking to herself and occasionally yelling or crying in apparent distress. Ms. A refuses to engage with her treatment team and lies mute in her bed when they attempt to interview her. Her records indicate that previous medication trials have included
Over the next week, Ms. A begins to interact more appropriately with nursing sta
[polldaddy:9945425]
The authors’ observations
As a class, antipsychotics lead to symptom reduction in approximately 70% of patients.1 However, the degree of response can vary markedly between individuals; although some patients may experience almost complete resolution of symptoms, others are still markedly impaired, as in Ms. A’s case.
A substantial amount of literature suggests that although the practice is common, use of >1 antipsychotic does not significantly increase efficacy but increases risk of adverse effects, such as type 2 diabetes mellitus, metabolic syndrome, cognitive impairment, and extrapyramidal symptoms.2-4 One exception is augmentation of clozapine with a second antipsychotic, which in certain cases appears to offer greater efficacy than clozapine alone.1 Practice guidelines and evidence generally do not support the use of multiple antipsychotics, but 20% of patients take >1 antipsychotic.5,6 Although antipsychotic polypharmacy may be appropriate for some patients, current literature suggests it is being done more often than recommended.
Clozapine is considered the most efficacious option for treatment-resistant schizophrenia.7 Because of Ms. A’s history of recurrent hospitalizations, her extensive list of trialed medications, and her ongoing symptoms despite a sufficient trial of haloperidol, the treatment team gives serious consideration to switching Ms. A to clozapine. However, Ms. A is not able to tolerate blood draws without significant support from nursing staff, and it is likely she would be unable to tolerate the frequent blood monitoring required of patients receiving clozapine.
Because many of Ms. A’s symptoms were negative or depressive, including hypersomnia, psychomotor retardation, sadness with frequent crying spells, and reduced interest in activities, adding an antidepressant to Ms. A’s medication regimen was considered. A recent systematic review and meta-analysis showed that adding an antidepressant to an antipsychotic in patients with schizophrenia had small but beneficial effects on depressive and negative symptoms and a low risk of adverse effects.8 However, Ms. A declined this option.
TREATMENT Adding long-acting haloperidol
Ms. A had previously achieved therapeutic blood levels9 with oral haloperidol. Data suggest that compared with the oral form, long-acting injectable antipsychotics can both improve compliance and decrease rehospitalization rates.10-12 Because Ms. A previously had done well with haloperidol decanoate, 200 mg every 2 weeks, achieving a blood level of 16.2 ng/mL, and because she had a partial response to oral haloperidol, we add haloperidol decanoate, 100 mg every 2 weeks, to her regimen, with the intention of transitioning her to all-depot dosing. In addition, the treatment team tries to engage Ms. A in a discussion of potential psychological contributions to her current presentation. They note that Ms. A has her basic needs met on the unit and reports feeling safe there; thus, a fear of discharge may be contributing to her lack of engagement with the team. However, because of her limited communication, it is challenging to investigate this hypothesis or explore other possible psychological issues.
Despite increasing the dosing of haloperidol, Ms. A shows minimal improvement. She continues to stonewall her treatment team, and is unwilling or unable to engage in meaningful conversation. A review of her chart suggests that this hospital course is different from previous ones in which her average stay was a few weeks, and she generally was able to converse with the treatment team, participate in discussions about her care, and make decisions about her desire for discharge.
The team considers if additional factors could be impacting Ms. A’s current presentation. They raise the possibility that she could be going through menopause, and hormonal fluctuations may be contributing to her symptoms. Despite being on the unit for nearly 2 months, Ms. A has not required the use of sanitary products. She also reports to nursing staff that at times she feels flushed and sweaty, but she is afebrile and does not have other signs or symptoms of infection.
[polldaddy:9945428]
The authors’ observations
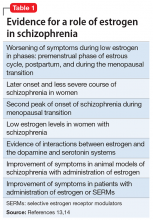
Evidence suggests that estrogen levels can influence the development and severity of symptoms of schizophrenia (Table 113,14). Rates of schizophrenia are lower in women, and women typically have a later onset of illness with less severe symptoms.13 Women also have a second peak incidence of schizophrenia between ages 45 and 50, corresponding with the hormonal changes associated with menopause and the associated drop in estrogen.14 Symptoms also fluctuate with hormonal cycles—women experience worsening symptoms during the premenstrual phase of the menstrual cycle, when estrogen levels are low, and an improvement of symptoms during high-estrogen phases of the cycle.14 Overall, low levels of estrogen also have been observed in women with schizophrenia relative to controls, although this may be partially attributable to treatment with antipsychotics.14
Estrogen affects various regions of the brain implicated in schizophrenia and likely imparts its behavioral effects through several different mechanisms. Estrogen can act on cells to directly impact intracellular signaling and to alter gene expression.15 Although most often thought of as being related to reproductive functions, estrogen receptors can be found in many cortical and subcortical regions of the brain, such as the hippocampus, substantia nigra, and prefrontal cortex. Estrogen receptor expression levels in certain brain regions have been found to be altered in individuals with schizophrenia.15 Estrogen also enhances neurogenesis and neuroplasticity, playing a role in learning and memory.16 Particularly relevant, estrogen has been shown to directly impact both the dopaminergic and serotonergic systems.15,17 In animal models, estrogen has been shown to decrease the behavioral effects induced by dopamine agonists and decrease symptoms of schizophrenia.18 The underlying molecular mechanisms by which estrogen has these effects are uncertain.
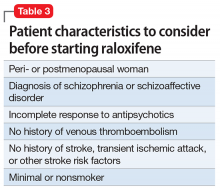
Given estrogen’s potentially protective effects, clinical trials have explored the role of estrogen as an adjuvant to antipsychotics for treating schizophrenia. Studies have shown that estrogen can improve psychotic symptoms in patients with schizophrenia.19,20 However, because estrogen administration can increase the risk of breast and uterine cancer, researchers are instead investigating selective estrogen receptor modulators (SERMs).14 These medications have mixed agonist and antagonist effects, with different effects on different tissues. Raloxifene is a SERM that acts as an estrogen agonist in some tissues, but an antagonist in uterine and breast tissue, which may minimize potential deleterious adverse effects (Table 221-24). Repeated randomized controlled trials have found promising results for use of raloxifene as an adjunctive treatment in peri- and postmenopausal women with schizophrenia, including those refractory to antipsychotic treatment.13,25-27
TREATMENT Address symptoms
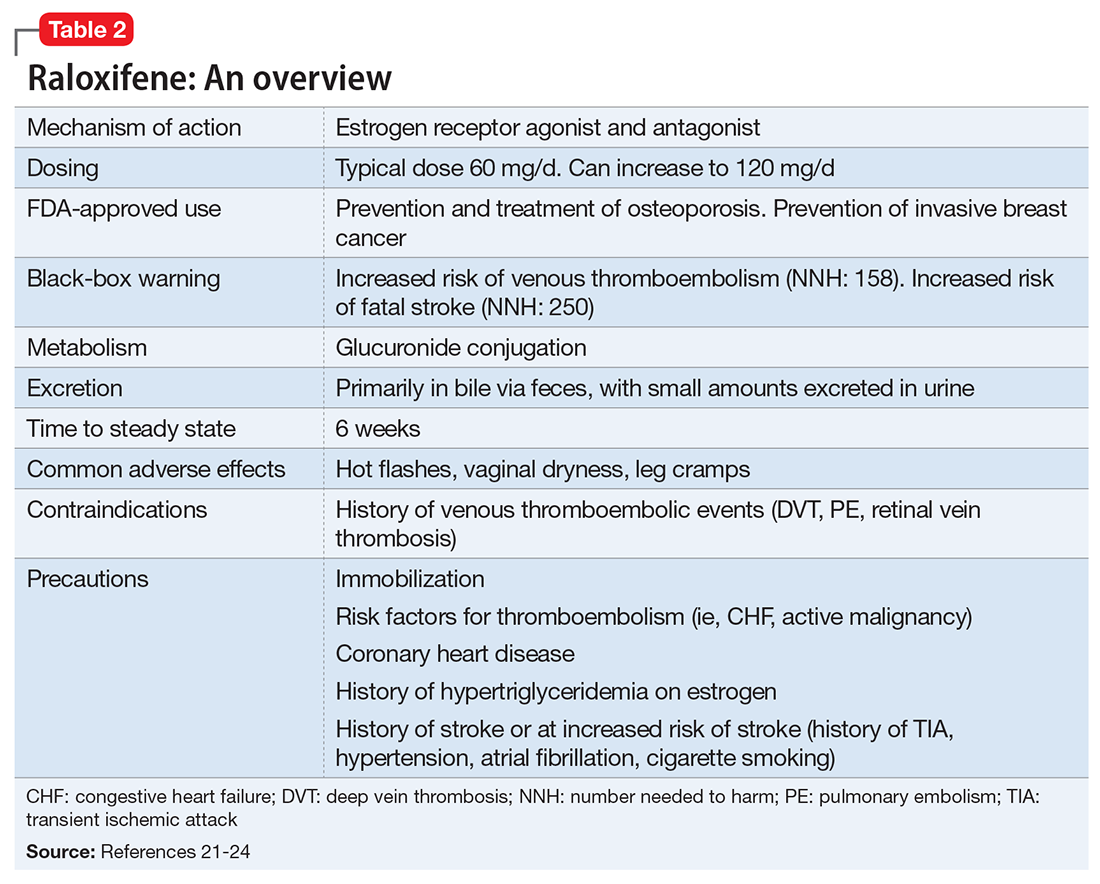
The treatment team takes steps to address Ms. A’s perimenopausal symptoms. For mild to moderate hot flashes, primary interventions are nonpharmacologic.28 Because Ms. A primarily reports her hot flashes at night, she is given lightweight pajamas and moved to the coolest room on the unit. Both bring some relief, and her hot flashes appear to be less distressing. The treatment team decides to consult Endocrinology to further investigate the feasibility of starting raloxifene (Table 3) because of their experience using this medication to manage osteoporosis.
[polldaddy:9945429]
The authors’ observations
Raloxifene is FDA-approved for treating osteoporosis and preventing invasive breast cancer.29 Because it is an estrogen antagonist in both breast and uterine tissues, raloxifene does not increase the risk of uterine or breast cancer. Large studies have shown rates of cardiovascular events are similar for raloxifene and placebo, and some studies have found that raloxifene treatment is associated with improvement in cardiovascular risk factors, including lower blood pressure, lower low-density lipoprotein cholesterol, and increased high-density lipoprotein cholesterol.29 Raloxifene does, however, increase risk of venous thromboembolism, including deep vein thrombosis and pulmonary embolism, and fatal stroke.29,30 Overall, the risk remains relatively low, with an absolute risk increase of fatal stroke of 0.7 per 1,000 woman-years (number needed to harm [NNH]: 250) and an absolute risk increase of venous thromboembolic events of 1.88 per 1,000 women-years (NNH: 158).31 However, raloxifene may not be appropriate for patients with independent risk factors for these events. Despite this, a large meta-analysis found a 10% decrease in mortality for patients taking raloxifene compared with those receiving placebo.32 Raloxifene also can cause hot flashes, muscle cramps, and flu-like symptoms.29
Diagnosis of menopause and perimenopause is largely clinical, with hormone testing generally recommended for women age <45 in whom the diagnosis may be unclear.28 Thus, Ms. A’s vasomotor symptoms and absence of a menstrual cycle for at least 2 months were diagnostic of perimenopause; a 12-month cessation in menstrual cycles is required for a diagnosis of menopause.28
OUTCOME Improvement with raloxifene
Because Ms. A is at relatively low risk for a thromboembolism or stroke, the benefit of raloxifene is thought to outweigh the risk, and she is started on raloxifene, 60 mg/d. Over the next 2 weeks, Ms. A becomes increasingly interactive, and is seen sitting at a table talking with other patients on multiple occasions. She spends time looking at fashion magazines, and engages in conversation about fashion with staff and other patients. She participates in group therapy for the first time during this hospital stay and begins to talk about discharge. She occasionally smiles and waves at her treatment team and participates more in the daily interview, although these interactions remain limited and on her terms. She maintains this improvement and is transferred to a psychiatric facility in her home county for ongoing care and discharge planning.
2. Citrome L, Jaffe A, Levine J, et al. Relationship between antipsychotic medication treatment and new cases of diabetes among psychiatric inpatients. Psychiatr Serv. 2004;55(9):1006-1013.
3. Correll CU, Frederickson AM, Kane JM, et al. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1-3):91-100.
4. Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527-542.
5. Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18-28.
6. Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-378.
7. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
8. Helfer B, Samara MT, Huhn M, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(9);876-886.
9. Ulrich S, Neuhof S, Braun V, et al. Therapeutic window of serum haloperidol concentration in acute schizophrenia and schizoaffective disorder. Pharmacopsychiatry. 1998;31(5):163-169.
10. Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014;30(8):1643-1655.
11. MacEwan JP, Kamat SA, Duffy RA, et al. Hospital readmission rates among patients with schizophrenia treated with long-acting injectables or oral antipsychotics. Psychiatr Serv. 2016;67(11):1183-1188.
12. Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768.
13. Usall J, Huerta-Ramos E, Iniesta R, et al; RALOPSYCAT Group. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2011;72(11):1552-1557.
14. Seeman MV. Treating schizophrenia at the time of menopause. Maturitas. 2012;72(2):117-120.
15. Gogos A, Sbisa AM, Sun J, et al. A role for estrogen in schizophrenia: clinical and preclinical findings. Int J Endocrinol. 2015;2015:615356. doi: 10.1155/2015/615356.
16. Khan MM. Neurocognitive, neuroprotective, and cardiometabolic effects of raloxifene: potential for improving therapeutic outcomes in schizophrenia. CNS Drugs. 2016;30(7):589-601.
17. Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015;9:37.
18. Häfner H, Behrens S, De Vry J, et al. An animal model for the effects of estradiol on dopamine-mediated behavior: implications for sex differences in schizophrenia. Psychiatry Res. 1991;38(2):125-134.
19. Akhondzadeh S, Nejatisafa AA, Amini H, et al. Adjunctive estrogen treatment in women with chronic schizophrenia: a double-blind, randomized, and placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(6):1007-1012.
20. Kulkarni J, de Castella A, Fitzgerald PB, et al. Estrogen in severe mental illness: a potential new treatment approach. Arch Gen Psychiatry. 2008;65(8):955-960.
21. Ellis AJ, Hendrick VM, Williams R, Komm BS. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf. 2015;14(6):921-934.
22. Morello KC, Wurz GT, DeGregorio MW. Pharmacokinetics of selective estrogen receptor modulators. Clin pharmacokinet. 2003;42(4):361-372.
23. Lewiecki EM, Miller PD, Harris ST, et al. Understanding and communicating the benefits and risks of denosumab, raloxifene, and teriparatide for the treatment of osteoporosis. J Clin Densitom. 2014;17(4):490-495.
24. Raloxifene Hydrochloride. Micromedex 2.0. Truven Health Analytics. www.micromedexsolutions.com. Accessed July 24, 2016.
25. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):947-954.
26. Huerta-Ramos E, Iniesta R, Ochoa S, et al. Effects of raloxifene on cognition in postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. Eur Neuropsychopharmacol. 2014;24(2):223-231.
27. Kianimehr G, Fatehi F, Hashempoor S, et al. Raloxifene adjunctive therapy for postmenopausal women suffering from chronic schizophrenia: a randomized double-blind and placebo controlled trial. Daru. 2014;22:55.
28. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975-4011.
29. Ellis AJ, Hendrick VM, Williams R, et al. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf. 2015;14(6):921-934.
30. Adomaityte J, Farooq M, Qayyum R. Effect of raloxifene therapy on venous thromboembolism in postmenopausal women. A meta-analysis. Thromb Haemost. 2008;99(2):338-342.
31. Lewiecki EM, Miller PD, Harris ST, et al. Understanding and communicating the benefits and risks of denosumab, raloxifene, and teriparatide for the treatment of osteoporosis. J Clin Densitom. 2014;17(4):490-495.
32. Grady D, Cauley JA, Stock JL, et al. Effect of raloxifene on all-cause mortality. Am J Med. 2010;123(5):469.e1-461.e7.
CASE Psychotic and reclusive
Ms. A, age 51, has schizophrenia and has been doing well living at a supervised residential facility. She was stable on haloperidol, 10 mg twice a day, for years but recently became agitated, threatening her roommate and yelling during the night. Ms. A begins to refuse to take her haloperidol. She also refuses to attend several outpatient appointments. As a result, Ms. A is admitted to the psychiatric unit on an involuntary basis.
In the hospital, Ms. A rarely comes out of her room. When she does come out, she usually sits in a chair, talking to herself and occasionally yelling or crying in apparent distress. Ms. A refuses to engage with her treatment team and lies mute in her bed when they attempt to interview her. Her records indicate that previous medication trials have included
Over the next week, Ms. A begins to interact more appropriately with nursing sta
[polldaddy:9945425]
The authors’ observations
As a class, antipsychotics lead to symptom reduction in approximately 70% of patients.1 However, the degree of response can vary markedly between individuals; although some patients may experience almost complete resolution of symptoms, others are still markedly impaired, as in Ms. A’s case.
A substantial amount of literature suggests that although the practice is common, use of >1 antipsychotic does not significantly increase efficacy but increases risk of adverse effects, such as type 2 diabetes mellitus, metabolic syndrome, cognitive impairment, and extrapyramidal symptoms.2-4 One exception is augmentation of clozapine with a second antipsychotic, which in certain cases appears to offer greater efficacy than clozapine alone.1 Practice guidelines and evidence generally do not support the use of multiple antipsychotics, but 20% of patients take >1 antipsychotic.5,6 Although antipsychotic polypharmacy may be appropriate for some patients, current literature suggests it is being done more often than recommended.
Clozapine is considered the most efficacious option for treatment-resistant schizophrenia.7 Because of Ms. A’s history of recurrent hospitalizations, her extensive list of trialed medications, and her ongoing symptoms despite a sufficient trial of haloperidol, the treatment team gives serious consideration to switching Ms. A to clozapine. However, Ms. A is not able to tolerate blood draws without significant support from nursing staff, and it is likely she would be unable to tolerate the frequent blood monitoring required of patients receiving clozapine.
Because many of Ms. A’s symptoms were negative or depressive, including hypersomnia, psychomotor retardation, sadness with frequent crying spells, and reduced interest in activities, adding an antidepressant to Ms. A’s medication regimen was considered. A recent systematic review and meta-analysis showed that adding an antidepressant to an antipsychotic in patients with schizophrenia had small but beneficial effects on depressive and negative symptoms and a low risk of adverse effects.8 However, Ms. A declined this option.
TREATMENT Adding long-acting haloperidol
Ms. A had previously achieved therapeutic blood levels9 with oral haloperidol. Data suggest that compared with the oral form, long-acting injectable antipsychotics can both improve compliance and decrease rehospitalization rates.10-12 Because Ms. A previously had done well with haloperidol decanoate, 200 mg every 2 weeks, achieving a blood level of 16.2 ng/mL, and because she had a partial response to oral haloperidol, we add haloperidol decanoate, 100 mg every 2 weeks, to her regimen, with the intention of transitioning her to all-depot dosing. In addition, the treatment team tries to engage Ms. A in a discussion of potential psychological contributions to her current presentation. They note that Ms. A has her basic needs met on the unit and reports feeling safe there; thus, a fear of discharge may be contributing to her lack of engagement with the team. However, because of her limited communication, it is challenging to investigate this hypothesis or explore other possible psychological issues.
Despite increasing the dosing of haloperidol, Ms. A shows minimal improvement. She continues to stonewall her treatment team, and is unwilling or unable to engage in meaningful conversation. A review of her chart suggests that this hospital course is different from previous ones in which her average stay was a few weeks, and she generally was able to converse with the treatment team, participate in discussions about her care, and make decisions about her desire for discharge.
The team considers if additional factors could be impacting Ms. A’s current presentation. They raise the possibility that she could be going through menopause, and hormonal fluctuations may be contributing to her symptoms. Despite being on the unit for nearly 2 months, Ms. A has not required the use of sanitary products. She also reports to nursing staff that at times she feels flushed and sweaty, but she is afebrile and does not have other signs or symptoms of infection.
[polldaddy:9945428]
The authors’ observations
Evidence suggests that estrogen levels can influence the development and severity of symptoms of schizophrenia (Table 113,14). Rates of schizophrenia are lower in women, and women typically have a later onset of illness with less severe symptoms.13 Women also have a second peak incidence of schizophrenia between ages 45 and 50, corresponding with the hormonal changes associated with menopause and the associated drop in estrogen.14 Symptoms also fluctuate with hormonal cycles—women experience worsening symptoms during the premenstrual phase of the menstrual cycle, when estrogen levels are low, and an improvement of symptoms during high-estrogen phases of the cycle.14 Overall, low levels of estrogen also have been observed in women with schizophrenia relative to controls, although this may be partially attributable to treatment with antipsychotics.14
Estrogen affects various regions of the brain implicated in schizophrenia and likely imparts its behavioral effects through several different mechanisms. Estrogen can act on cells to directly impact intracellular signaling and to alter gene expression.15 Although most often thought of as being related to reproductive functions, estrogen receptors can be found in many cortical and subcortical regions of the brain, such as the hippocampus, substantia nigra, and prefrontal cortex. Estrogen receptor expression levels in certain brain regions have been found to be altered in individuals with schizophrenia.15 Estrogen also enhances neurogenesis and neuroplasticity, playing a role in learning and memory.16 Particularly relevant, estrogen has been shown to directly impact both the dopaminergic and serotonergic systems.15,17 In animal models, estrogen has been shown to decrease the behavioral effects induced by dopamine agonists and decrease symptoms of schizophrenia.18 The underlying molecular mechanisms by which estrogen has these effects are uncertain.
Given estrogen’s potentially protective effects, clinical trials have explored the role of estrogen as an adjuvant to antipsychotics for treating schizophrenia. Studies have shown that estrogen can improve psychotic symptoms in patients with schizophrenia.19,20 However, because estrogen administration can increase the risk of breast and uterine cancer, researchers are instead investigating selective estrogen receptor modulators (SERMs).14 These medications have mixed agonist and antagonist effects, with different effects on different tissues. Raloxifene is a SERM that acts as an estrogen agonist in some tissues, but an antagonist in uterine and breast tissue, which may minimize potential deleterious adverse effects (Table 221-24). Repeated randomized controlled trials have found promising results for use of raloxifene as an adjunctive treatment in peri- and postmenopausal women with schizophrenia, including those refractory to antipsychotic treatment.13,25-27

TREATMENT Address symptoms
The treatment team takes steps to address Ms. A’s perimenopausal symptoms. For mild to moderate hot flashes, primary interventions are nonpharmacologic.28 Because Ms. A primarily reports her hot flashes at night, she is given lightweight pajamas and moved to the coolest room on the unit. Both bring some relief, and her hot flashes appear to be less distressing. The treatment team decides to consult Endocrinology to further investigate the feasibility of starting raloxifene (Table 3) because of their experience using this medication to manage osteoporosis.
[polldaddy:9945429]
The authors’ observations
Raloxifene is FDA-approved for treating osteoporosis and preventing invasive breast cancer.29 Because it is an estrogen antagonist in both breast and uterine tissues, raloxifene does not increase the risk of uterine or breast cancer. Large studies have shown rates of cardiovascular events are similar for raloxifene and placebo, and some studies have found that raloxifene treatment is associated with improvement in cardiovascular risk factors, including lower blood pressure, lower low-density lipoprotein cholesterol, and increased high-density lipoprotein cholesterol.29 Raloxifene does, however, increase risk of venous thromboembolism, including deep vein thrombosis and pulmonary embolism, and fatal stroke.29,30 Overall, the risk remains relatively low, with an absolute risk increase of fatal stroke of 0.7 per 1,000 woman-years (number needed to harm [NNH]: 250) and an absolute risk increase of venous thromboembolic events of 1.88 per 1,000 women-years (NNH: 158).31 However, raloxifene may not be appropriate for patients with independent risk factors for these events. Despite this, a large meta-analysis found a 10% decrease in mortality for patients taking raloxifene compared with those receiving placebo.32 Raloxifene also can cause hot flashes, muscle cramps, and flu-like symptoms.29
Diagnosis of menopause and perimenopause is largely clinical, with hormone testing generally recommended for women age <45 in whom the diagnosis may be unclear.28 Thus, Ms. A’s vasomotor symptoms and absence of a menstrual cycle for at least 2 months were diagnostic of perimenopause; a 12-month cessation in menstrual cycles is required for a diagnosis of menopause.28
OUTCOME Improvement with raloxifene
Because Ms. A is at relatively low risk for a thromboembolism or stroke, the benefit of raloxifene is thought to outweigh the risk, and she is started on raloxifene, 60 mg/d. Over the next 2 weeks, Ms. A becomes increasingly interactive, and is seen sitting at a table talking with other patients on multiple occasions. She spends time looking at fashion magazines, and engages in conversation about fashion with staff and other patients. She participates in group therapy for the first time during this hospital stay and begins to talk about discharge. She occasionally smiles and waves at her treatment team and participates more in the daily interview, although these interactions remain limited and on her terms. She maintains this improvement and is transferred to a psychiatric facility in her home county for ongoing care and discharge planning.
CASE Psychotic and reclusive
Ms. A, age 51, has schizophrenia and has been doing well living at a supervised residential facility. She was stable on haloperidol, 10 mg twice a day, for years but recently became agitated, threatening her roommate and yelling during the night. Ms. A begins to refuse to take her haloperidol. She also refuses to attend several outpatient appointments. As a result, Ms. A is admitted to the psychiatric unit on an involuntary basis.
In the hospital, Ms. A rarely comes out of her room. When she does come out, she usually sits in a chair, talking to herself and occasionally yelling or crying in apparent distress. Ms. A refuses to engage with her treatment team and lies mute in her bed when they attempt to interview her. Her records indicate that previous medication trials have included
Over the next week, Ms. A begins to interact more appropriately with nursing sta
[polldaddy:9945425]
The authors’ observations
As a class, antipsychotics lead to symptom reduction in approximately 70% of patients.1 However, the degree of response can vary markedly between individuals; although some patients may experience almost complete resolution of symptoms, others are still markedly impaired, as in Ms. A’s case.
A substantial amount of literature suggests that although the practice is common, use of >1 antipsychotic does not significantly increase efficacy but increases risk of adverse effects, such as type 2 diabetes mellitus, metabolic syndrome, cognitive impairment, and extrapyramidal symptoms.2-4 One exception is augmentation of clozapine with a second antipsychotic, which in certain cases appears to offer greater efficacy than clozapine alone.1 Practice guidelines and evidence generally do not support the use of multiple antipsychotics, but 20% of patients take >1 antipsychotic.5,6 Although antipsychotic polypharmacy may be appropriate for some patients, current literature suggests it is being done more often than recommended.
Clozapine is considered the most efficacious option for treatment-resistant schizophrenia.7 Because of Ms. A’s history of recurrent hospitalizations, her extensive list of trialed medications, and her ongoing symptoms despite a sufficient trial of haloperidol, the treatment team gives serious consideration to switching Ms. A to clozapine. However, Ms. A is not able to tolerate blood draws without significant support from nursing staff, and it is likely she would be unable to tolerate the frequent blood monitoring required of patients receiving clozapine.
Because many of Ms. A’s symptoms were negative or depressive, including hypersomnia, psychomotor retardation, sadness with frequent crying spells, and reduced interest in activities, adding an antidepressant to Ms. A’s medication regimen was considered. A recent systematic review and meta-analysis showed that adding an antidepressant to an antipsychotic in patients with schizophrenia had small but beneficial effects on depressive and negative symptoms and a low risk of adverse effects.8 However, Ms. A declined this option.
TREATMENT Adding long-acting haloperidol
Ms. A had previously achieved therapeutic blood levels9 with oral haloperidol. Data suggest that compared with the oral form, long-acting injectable antipsychotics can both improve compliance and decrease rehospitalization rates.10-12 Because Ms. A previously had done well with haloperidol decanoate, 200 mg every 2 weeks, achieving a blood level of 16.2 ng/mL, and because she had a partial response to oral haloperidol, we add haloperidol decanoate, 100 mg every 2 weeks, to her regimen, with the intention of transitioning her to all-depot dosing. In addition, the treatment team tries to engage Ms. A in a discussion of potential psychological contributions to her current presentation. They note that Ms. A has her basic needs met on the unit and reports feeling safe there; thus, a fear of discharge may be contributing to her lack of engagement with the team. However, because of her limited communication, it is challenging to investigate this hypothesis or explore other possible psychological issues.
Despite increasing the dosing of haloperidol, Ms. A shows minimal improvement. She continues to stonewall her treatment team, and is unwilling or unable to engage in meaningful conversation. A review of her chart suggests that this hospital course is different from previous ones in which her average stay was a few weeks, and she generally was able to converse with the treatment team, participate in discussions about her care, and make decisions about her desire for discharge.
The team considers if additional factors could be impacting Ms. A’s current presentation. They raise the possibility that she could be going through menopause, and hormonal fluctuations may be contributing to her symptoms. Despite being on the unit for nearly 2 months, Ms. A has not required the use of sanitary products. She also reports to nursing staff that at times she feels flushed and sweaty, but she is afebrile and does not have other signs or symptoms of infection.
[polldaddy:9945428]
The authors’ observations
Evidence suggests that estrogen levels can influence the development and severity of symptoms of schizophrenia (Table 113,14). Rates of schizophrenia are lower in women, and women typically have a later onset of illness with less severe symptoms.13 Women also have a second peak incidence of schizophrenia between ages 45 and 50, corresponding with the hormonal changes associated with menopause and the associated drop in estrogen.14 Symptoms also fluctuate with hormonal cycles—women experience worsening symptoms during the premenstrual phase of the menstrual cycle, when estrogen levels are low, and an improvement of symptoms during high-estrogen phases of the cycle.14 Overall, low levels of estrogen also have been observed in women with schizophrenia relative to controls, although this may be partially attributable to treatment with antipsychotics.14
Estrogen affects various regions of the brain implicated in schizophrenia and likely imparts its behavioral effects through several different mechanisms. Estrogen can act on cells to directly impact intracellular signaling and to alter gene expression.15 Although most often thought of as being related to reproductive functions, estrogen receptors can be found in many cortical and subcortical regions of the brain, such as the hippocampus, substantia nigra, and prefrontal cortex. Estrogen receptor expression levels in certain brain regions have been found to be altered in individuals with schizophrenia.15 Estrogen also enhances neurogenesis and neuroplasticity, playing a role in learning and memory.16 Particularly relevant, estrogen has been shown to directly impact both the dopaminergic and serotonergic systems.15,17 In animal models, estrogen has been shown to decrease the behavioral effects induced by dopamine agonists and decrease symptoms of schizophrenia.18 The underlying molecular mechanisms by which estrogen has these effects are uncertain.
Given estrogen’s potentially protective effects, clinical trials have explored the role of estrogen as an adjuvant to antipsychotics for treating schizophrenia. Studies have shown that estrogen can improve psychotic symptoms in patients with schizophrenia.19,20 However, because estrogen administration can increase the risk of breast and uterine cancer, researchers are instead investigating selective estrogen receptor modulators (SERMs).14 These medications have mixed agonist and antagonist effects, with different effects on different tissues. Raloxifene is a SERM that acts as an estrogen agonist in some tissues, but an antagonist in uterine and breast tissue, which may minimize potential deleterious adverse effects (Table 221-24). Repeated randomized controlled trials have found promising results for use of raloxifene as an adjunctive treatment in peri- and postmenopausal women with schizophrenia, including those refractory to antipsychotic treatment.13,25-27

TREATMENT Address symptoms
The treatment team takes steps to address Ms. A’s perimenopausal symptoms. For mild to moderate hot flashes, primary interventions are nonpharmacologic.28 Because Ms. A primarily reports her hot flashes at night, she is given lightweight pajamas and moved to the coolest room on the unit. Both bring some relief, and her hot flashes appear to be less distressing. The treatment team decides to consult Endocrinology to further investigate the feasibility of starting raloxifene (Table 3) because of their experience using this medication to manage osteoporosis.
[polldaddy:9945429]
The authors’ observations
Raloxifene is FDA-approved for treating osteoporosis and preventing invasive breast cancer.29 Because it is an estrogen antagonist in both breast and uterine tissues, raloxifene does not increase the risk of uterine or breast cancer. Large studies have shown rates of cardiovascular events are similar for raloxifene and placebo, and some studies have found that raloxifene treatment is associated with improvement in cardiovascular risk factors, including lower blood pressure, lower low-density lipoprotein cholesterol, and increased high-density lipoprotein cholesterol.29 Raloxifene does, however, increase risk of venous thromboembolism, including deep vein thrombosis and pulmonary embolism, and fatal stroke.29,30 Overall, the risk remains relatively low, with an absolute risk increase of fatal stroke of 0.7 per 1,000 woman-years (number needed to harm [NNH]: 250) and an absolute risk increase of venous thromboembolic events of 1.88 per 1,000 women-years (NNH: 158).31 However, raloxifene may not be appropriate for patients with independent risk factors for these events. Despite this, a large meta-analysis found a 10% decrease in mortality for patients taking raloxifene compared with those receiving placebo.32 Raloxifene also can cause hot flashes, muscle cramps, and flu-like symptoms.29
Diagnosis of menopause and perimenopause is largely clinical, with hormone testing generally recommended for women age <45 in whom the diagnosis may be unclear.28 Thus, Ms. A’s vasomotor symptoms and absence of a menstrual cycle for at least 2 months were diagnostic of perimenopause; a 12-month cessation in menstrual cycles is required for a diagnosis of menopause.28
OUTCOME Improvement with raloxifene
Because Ms. A is at relatively low risk for a thromboembolism or stroke, the benefit of raloxifene is thought to outweigh the risk, and she is started on raloxifene, 60 mg/d. Over the next 2 weeks, Ms. A becomes increasingly interactive, and is seen sitting at a table talking with other patients on multiple occasions. She spends time looking at fashion magazines, and engages in conversation about fashion with staff and other patients. She participates in group therapy for the first time during this hospital stay and begins to talk about discharge. She occasionally smiles and waves at her treatment team and participates more in the daily interview, although these interactions remain limited and on her terms. She maintains this improvement and is transferred to a psychiatric facility in her home county for ongoing care and discharge planning.
2. Citrome L, Jaffe A, Levine J, et al. Relationship between antipsychotic medication treatment and new cases of diabetes among psychiatric inpatients. Psychiatr Serv. 2004;55(9):1006-1013.
3. Correll CU, Frederickson AM, Kane JM, et al. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1-3):91-100.
4. Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527-542.
5. Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18-28.
6. Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-378.
7. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
8. Helfer B, Samara MT, Huhn M, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(9);876-886.
9. Ulrich S, Neuhof S, Braun V, et al. Therapeutic window of serum haloperidol concentration in acute schizophrenia and schizoaffective disorder. Pharmacopsychiatry. 1998;31(5):163-169.
10. Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014;30(8):1643-1655.
11. MacEwan JP, Kamat SA, Duffy RA, et al. Hospital readmission rates among patients with schizophrenia treated with long-acting injectables or oral antipsychotics. Psychiatr Serv. 2016;67(11):1183-1188.
12. Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768.
13. Usall J, Huerta-Ramos E, Iniesta R, et al; RALOPSYCAT Group. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2011;72(11):1552-1557.
14. Seeman MV. Treating schizophrenia at the time of menopause. Maturitas. 2012;72(2):117-120.
15. Gogos A, Sbisa AM, Sun J, et al. A role for estrogen in schizophrenia: clinical and preclinical findings. Int J Endocrinol. 2015;2015:615356. doi: 10.1155/2015/615356.
16. Khan MM. Neurocognitive, neuroprotective, and cardiometabolic effects of raloxifene: potential for improving therapeutic outcomes in schizophrenia. CNS Drugs. 2016;30(7):589-601.
17. Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015;9:37.
18. Häfner H, Behrens S, De Vry J, et al. An animal model for the effects of estradiol on dopamine-mediated behavior: implications for sex differences in schizophrenia. Psychiatry Res. 1991;38(2):125-134.
19. Akhondzadeh S, Nejatisafa AA, Amini H, et al. Adjunctive estrogen treatment in women with chronic schizophrenia: a double-blind, randomized, and placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(6):1007-1012.
20. Kulkarni J, de Castella A, Fitzgerald PB, et al. Estrogen in severe mental illness: a potential new treatment approach. Arch Gen Psychiatry. 2008;65(8):955-960.
21. Ellis AJ, Hendrick VM, Williams R, Komm BS. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf. 2015;14(6):921-934.
22. Morello KC, Wurz GT, DeGregorio MW. Pharmacokinetics of selective estrogen receptor modulators. Clin pharmacokinet. 2003;42(4):361-372.
23. Lewiecki EM, Miller PD, Harris ST, et al. Understanding and communicating the benefits and risks of denosumab, raloxifene, and teriparatide for the treatment of osteoporosis. J Clin Densitom. 2014;17(4):490-495.
24. Raloxifene Hydrochloride. Micromedex 2.0. Truven Health Analytics. www.micromedexsolutions.com. Accessed July 24, 2016.
25. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):947-954.
26. Huerta-Ramos E, Iniesta R, Ochoa S, et al. Effects of raloxifene on cognition in postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. Eur Neuropsychopharmacol. 2014;24(2):223-231.
27. Kianimehr G, Fatehi F, Hashempoor S, et al. Raloxifene adjunctive therapy for postmenopausal women suffering from chronic schizophrenia: a randomized double-blind and placebo controlled trial. Daru. 2014;22:55.
28. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975-4011.
29. Ellis AJ, Hendrick VM, Williams R, et al. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf. 2015;14(6):921-934.
30. Adomaityte J, Farooq M, Qayyum R. Effect of raloxifene therapy on venous thromboembolism in postmenopausal women. A meta-analysis. Thromb Haemost. 2008;99(2):338-342.
31. Lewiecki EM, Miller PD, Harris ST, et al. Understanding and communicating the benefits and risks of denosumab, raloxifene, and teriparatide for the treatment of osteoporosis. J Clin Densitom. 2014;17(4):490-495.
32. Grady D, Cauley JA, Stock JL, et al. Effect of raloxifene on all-cause mortality. Am J Med. 2010;123(5):469.e1-461.e7.
2. Citrome L, Jaffe A, Levine J, et al. Relationship between antipsychotic medication treatment and new cases of diabetes among psychiatric inpatients. Psychiatr Serv. 2004;55(9):1006-1013.
3. Correll CU, Frederickson AM, Kane JM, et al. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1-3):91-100.
4. Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527-542.
5. Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18-28.
6. Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-378.
7. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610.
8. Helfer B, Samara MT, Huhn M, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(9);876-886.
9. Ulrich S, Neuhof S, Braun V, et al. Therapeutic window of serum haloperidol concentration in acute schizophrenia and schizoaffective disorder. Pharmacopsychiatry. 1998;31(5):163-169.
10. Lafeuille MH, Dean J, Carter V, et al. Systematic review of long-acting injectables versus oral atypical antipsychotics on hospitalization in schizophrenia. Curr Med Res Opin. 2014;30(8):1643-1655.
11. MacEwan JP, Kamat SA, Duffy RA, et al. Hospital readmission rates among patients with schizophrenia treated with long-acting injectables or oral antipsychotics. Psychiatr Serv. 2016;67(11):1183-1188.
12. Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768.
13. Usall J, Huerta-Ramos E, Iniesta R, et al; RALOPSYCAT Group. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2011;72(11):1552-1557.
14. Seeman MV. Treating schizophrenia at the time of menopause. Maturitas. 2012;72(2):117-120.
15. Gogos A, Sbisa AM, Sun J, et al. A role for estrogen in schizophrenia: clinical and preclinical findings. Int J Endocrinol. 2015;2015:615356. doi: 10.1155/2015/615356.
16. Khan MM. Neurocognitive, neuroprotective, and cardiometabolic effects of raloxifene: potential for improving therapeutic outcomes in schizophrenia. CNS Drugs. 2016;30(7):589-601.
17. Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015;9:37.
18. Häfner H, Behrens S, De Vry J, et al. An animal model for the effects of estradiol on dopamine-mediated behavior: implications for sex differences in schizophrenia. Psychiatry Res. 1991;38(2):125-134.
19. Akhondzadeh S, Nejatisafa AA, Amini H, et al. Adjunctive estrogen treatment in women with chronic schizophrenia: a double-blind, randomized, and placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(6):1007-1012.
20. Kulkarni J, de Castella A, Fitzgerald PB, et al. Estrogen in severe mental illness: a potential new treatment approach. Arch Gen Psychiatry. 2008;65(8):955-960.
21. Ellis AJ, Hendrick VM, Williams R, Komm BS. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf. 2015;14(6):921-934.
22. Morello KC, Wurz GT, DeGregorio MW. Pharmacokinetics of selective estrogen receptor modulators. Clin pharmacokinet. 2003;42(4):361-372.
23. Lewiecki EM, Miller PD, Harris ST, et al. Understanding and communicating the benefits and risks of denosumab, raloxifene, and teriparatide for the treatment of osteoporosis. J Clin Densitom. 2014;17(4):490-495.
24. Raloxifene Hydrochloride. Micromedex 2.0. Truven Health Analytics. www.micromedexsolutions.com. Accessed July 24, 2016.
25. Kulkarni J, Gavrilidis E, Gwini SM, et al. Effect of adjunctive raloxifene therapy on severity of refractory schizophrenia in women: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):947-954.
26. Huerta-Ramos E, Iniesta R, Ochoa S, et al. Effects of raloxifene on cognition in postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. Eur Neuropsychopharmacol. 2014;24(2):223-231.
27. Kianimehr G, Fatehi F, Hashempoor S, et al. Raloxifene adjunctive therapy for postmenopausal women suffering from chronic schizophrenia: a randomized double-blind and placebo controlled trial. Daru. 2014;22:55.
28. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975-4011.
29. Ellis AJ, Hendrick VM, Williams R, et al. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf. 2015;14(6):921-934.
30. Adomaityte J, Farooq M, Qayyum R. Effect of raloxifene therapy on venous thromboembolism in postmenopausal women. A meta-analysis. Thromb Haemost. 2008;99(2):338-342.
31. Lewiecki EM, Miller PD, Harris ST, et al. Understanding and communicating the benefits and risks of denosumab, raloxifene, and teriparatide for the treatment of osteoporosis. J Clin Densitom. 2014;17(4):490-495.
32. Grady D, Cauley JA, Stock JL, et al. Effect of raloxifene on all-cause mortality. Am J Med. 2010;123(5):469.e1-461.e7.