User login
MADRID – Is there a need for an enterovirus-A71 vaccine?
This is a new question for North American and European physicians, but not so new in Asia.
“China says yes, with more than 15 million cases of hand, foot, and mouth disease resulting in 3,500 deaths since surveillance started in 2009,” Heli Harvala, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
Seroconversion rates 28 days after the second dose of these vaccines, both directed specifically against viral subgenotype C-4, are 92%-100%. Vaccine efficacy is 91%-97%, according to Dr. Harvala, a consultant medical virologist at University College London.
It remains a mystery why major outbreaks of severe EV-A71 disease have mostly occurred in Asia, with the notable exception of a Spanish outbreak of EV-A71 encephalitis in 2016. The possibility of much wider spread is concerning.
The Chinese monovalent EV-A71 vaccines, however, are seen as a stopgap. For one thing, recent evidence suggests that it’s probably not the specific EV-A71 C-4 viral subgenotype that accounts for all severe disease.
“I think we have to aim for a multivalent vaccine,” Dr. Harvala said.
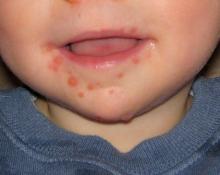
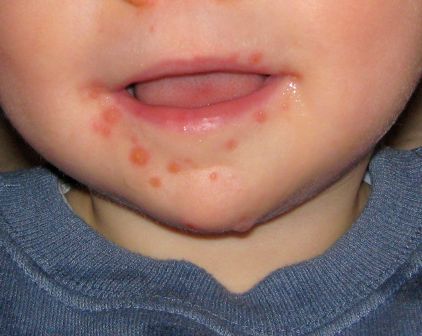
Now in clinical trials, investigational bivalent vaccines are directed against other EV-A71 subgenotypes in addition to C-4, and also against another enterovirus, coxsackievirus serotype A16, the most common cause of classic hand, foot, and mouth disease in the United States. But that’s probably not enough, according to Dr. Harvala. She noted that coxsackievirus A6, which was first identified more than 50 years ago, abruptly became the main cause of mild hand, foot, and mouth disease in China in 2013 and again in 2015. Moreover, its role in severe cases is growing, and there have been important outbreaks in the United States in recent years. These severe cases come in three main presentations, resembling either erythema multiforme, chicken pox, or eczema herpeticum.
Dr. Harvala added that a next-generation vaccine probably also should offer protection against enterovirus-D68. In 2014, there were 1,153 laboratory-confirmed EV-D68 infections and 14 deaths in the United States and Canada. This infection poses a diagnostic challenge: while the virus is readily detectable on throat swabs, it’s only rarely present in stool or cerebrospinal fluid samples.
“It’s important to keep in mind that this infection is still underdiagnosed. We are not really looking for it,” she said.
No specific treatment for enterovirus infections is available. Three capsid-binding antiviral agents now are in clinical trials: pleconaril, vapendavir, and pocapavir. In addition, translational studies have demonstrated that the SSRI fluoxetine inhibits enterovirus replication, but there have been no clinical trials as yet.
Although development of antivirals effective against enterovirus is an active area of research, Dr. Harvala thinks drug resistance will be an issue, underscoring the importance of vaccine development.
She reported having no financial conflicts of interest regarding her presentation.
MADRID – Is there a need for an enterovirus-A71 vaccine?
This is a new question for North American and European physicians, but not so new in Asia.
“China says yes, with more than 15 million cases of hand, foot, and mouth disease resulting in 3,500 deaths since surveillance started in 2009,” Heli Harvala, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
Seroconversion rates 28 days after the second dose of these vaccines, both directed specifically against viral subgenotype C-4, are 92%-100%. Vaccine efficacy is 91%-97%, according to Dr. Harvala, a consultant medical virologist at University College London.
It remains a mystery why major outbreaks of severe EV-A71 disease have mostly occurred in Asia, with the notable exception of a Spanish outbreak of EV-A71 encephalitis in 2016. The possibility of much wider spread is concerning.
The Chinese monovalent EV-A71 vaccines, however, are seen as a stopgap. For one thing, recent evidence suggests that it’s probably not the specific EV-A71 C-4 viral subgenotype that accounts for all severe disease.
“I think we have to aim for a multivalent vaccine,” Dr. Harvala said.
Now in clinical trials, investigational bivalent vaccines are directed against other EV-A71 subgenotypes in addition to C-4, and also against another enterovirus, coxsackievirus serotype A16, the most common cause of classic hand, foot, and mouth disease in the United States. But that’s probably not enough, according to Dr. Harvala. She noted that coxsackievirus A6, which was first identified more than 50 years ago, abruptly became the main cause of mild hand, foot, and mouth disease in China in 2013 and again in 2015. Moreover, its role in severe cases is growing, and there have been important outbreaks in the United States in recent years. These severe cases come in three main presentations, resembling either erythema multiforme, chicken pox, or eczema herpeticum.
Dr. Harvala added that a next-generation vaccine probably also should offer protection against enterovirus-D68. In 2014, there were 1,153 laboratory-confirmed EV-D68 infections and 14 deaths in the United States and Canada. This infection poses a diagnostic challenge: while the virus is readily detectable on throat swabs, it’s only rarely present in stool or cerebrospinal fluid samples.
“It’s important to keep in mind that this infection is still underdiagnosed. We are not really looking for it,” she said.
No specific treatment for enterovirus infections is available. Three capsid-binding antiviral agents now are in clinical trials: pleconaril, vapendavir, and pocapavir. In addition, translational studies have demonstrated that the SSRI fluoxetine inhibits enterovirus replication, but there have been no clinical trials as yet.
Although development of antivirals effective against enterovirus is an active area of research, Dr. Harvala thinks drug resistance will be an issue, underscoring the importance of vaccine development.
She reported having no financial conflicts of interest regarding her presentation.
MADRID – Is there a need for an enterovirus-A71 vaccine?
This is a new question for North American and European physicians, but not so new in Asia.
“China says yes, with more than 15 million cases of hand, foot, and mouth disease resulting in 3,500 deaths since surveillance started in 2009,” Heli Harvala, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
Seroconversion rates 28 days after the second dose of these vaccines, both directed specifically against viral subgenotype C-4, are 92%-100%. Vaccine efficacy is 91%-97%, according to Dr. Harvala, a consultant medical virologist at University College London.
It remains a mystery why major outbreaks of severe EV-A71 disease have mostly occurred in Asia, with the notable exception of a Spanish outbreak of EV-A71 encephalitis in 2016. The possibility of much wider spread is concerning.
The Chinese monovalent EV-A71 vaccines, however, are seen as a stopgap. For one thing, recent evidence suggests that it’s probably not the specific EV-A71 C-4 viral subgenotype that accounts for all severe disease.
“I think we have to aim for a multivalent vaccine,” Dr. Harvala said.
Now in clinical trials, investigational bivalent vaccines are directed against other EV-A71 subgenotypes in addition to C-4, and also against another enterovirus, coxsackievirus serotype A16, the most common cause of classic hand, foot, and mouth disease in the United States. But that’s probably not enough, according to Dr. Harvala. She noted that coxsackievirus A6, which was first identified more than 50 years ago, abruptly became the main cause of mild hand, foot, and mouth disease in China in 2013 and again in 2015. Moreover, its role in severe cases is growing, and there have been important outbreaks in the United States in recent years. These severe cases come in three main presentations, resembling either erythema multiforme, chicken pox, or eczema herpeticum.
Dr. Harvala added that a next-generation vaccine probably also should offer protection against enterovirus-D68. In 2014, there were 1,153 laboratory-confirmed EV-D68 infections and 14 deaths in the United States and Canada. This infection poses a diagnostic challenge: while the virus is readily detectable on throat swabs, it’s only rarely present in stool or cerebrospinal fluid samples.
“It’s important to keep in mind that this infection is still underdiagnosed. We are not really looking for it,” she said.
No specific treatment for enterovirus infections is available. Three capsid-binding antiviral agents now are in clinical trials: pleconaril, vapendavir, and pocapavir. In addition, translational studies have demonstrated that the SSRI fluoxetine inhibits enterovirus replication, but there have been no clinical trials as yet.
Although development of antivirals effective against enterovirus is an active area of research, Dr. Harvala thinks drug resistance will be an issue, underscoring the importance of vaccine development.
She reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM ESPID 2017