User login
A 29-year-old nulliparous woman presents for a routine checkup. She has hypertension and type 2 diabetes mellitus. Her current medications are chlorpropamide 500 mg daily, metformin 500 mg twice a day, lisinopril 40 mg daily, simvastatin 40 mg daily, and aspirin 81 mg daily. Her body mass index is 37 kg/m2 and her blood pressure is 130/80 mm Hg. Her hemoglobin A1c level is 7.8% and her low-density lipoprotein cholesterol 90 mg/dL.
She is considering pregnancy. How would you counsel her?
DEFINING DIABETES IN PREGNANCY
Diabetes in pregnant women, both gestational and pregestational, is the most common medical complication associated with pregnancy.1
- Gestational diabetes is defined as diabetes that is diagnosed during the second or third trimester of pregnancy and that is not clearly pregestational.2
- Pregestational diabetes exists before pregnancy and can be either type 1 or type 2.
Most cases of diabetes diagnosed during the first trimester reflect pregestational diabetes, as gestational diabetes occurs when insulin resistance increases in the later trimesters.
Type 1 diabetes involves autoimmune destruction of pancreatic islet cells, leading to insulin deficiency and the need for insulin therapy. Type 2 diabetes is characterized by insulin resistance rather than overall insulin deficiency. Type 2 diabetes tends to be associated with comorbidities such as obesity and hypertension, which are independent risk factors for adverse perinatal outcomes.3,4
Gestational diabetes accounts for most cases of diabetes during pregnancy. Although both pregestational and gestational diabetes increase the risk of maternal and fetal complications, pregestational diabetes is associated with significantly greater risks.1
IMPACT OF DIABETES ON THE MOTHER
Pregnancy increases the risk of maternal hypoglycemia, especially during the first trimester in patients with type 1 diabetes, as insulin sensitivity increases in early pregnancy.1 Pregnant women with diabetes may also have an altered counterregulatory response and less hypoglycemic awareness.1 Insulin resistance rises during the second and early third trimesters, increasing the risk of hyperglycemia in women with diabetes.1
Glycemic control during pregnancy is usually easier to achieve in patients with type 2 diabetes than with type 1, but it may require much higher insulin doses.
Because pregnancy is inherently a ketogenic state, women with type 1 diabetes are at higher risk of diabetic ketoacidosis, particularly during the second and third trimesters.1 There are reports of euglycemic diabetic ketoacidosis in pregnant women with either gestational or pregestational diabetes.5
Diabetes is associated with a risk of preeclampsia 4 times higher than in nondiabetic women.6 Other potential pregnancy-related complications include infections, polyhydramnios, spontaneous abortion, and cesarean delivery.1,7 The risk of pregnancy loss is similar in women with either type 1 or type 2 diabetes (2.6% and 3.7%, respectively), but the causes are different.8 Although preexisting diabetic complications such as retinopathy, nephropathy, and gastroparesis can be exacerbated during pregnancy,1 only severe gastroparesis and advanced renal disease are considered relative contraindications to pregnancy.
IMPACT OF DIABETES ON THE FETUS
Fetal complications of maternal diabetes include embryopathy (fetal malformations) and fetopathy (overgrowth, ie, fetus large for gestational age, and increased risk of fetal death or distress). Maternal hyperglycemia is associated with diabetic embryopathy, resulting in major birth defects in 5% to 25% of pregnancies and spontaneous abortions in 15% to 20%.9,10 There is a 2- to 6-fold increase in risk of congenital malformations.6
The most common diabetes-associated congenital malformations affect the cardiovascular system. Congenital heart disease includes tetralogy of Fallot, transposition of the great vessels, septal defects, and anomalous pulmonary venous return. Other relatively common defects involve the fetal central nervous system, spine, orofacial system, kidneys, urogenital system, gastrointestinal tract, and skeleton.11
The risk of fetopathy is proportional to the degree of maternal hyperglycemia. Excess maternal glucose and fatty acid levels can lead to fetal hyperglycemia and overgrowth, which increases fetal oxygen requirements. Erythropoietin levels rise, causing an increase in red cell mass, with subsequent hyperviscosity within the placenta and higher risk of fetal death.
Other complications include intrauterine growth restriction, prematurity, and preterm delivery. Fetal macrosomia (birth weight > 90th percentile or 4 kg, approximately 8 lb, 13 oz) occurs in 27% to 62% of children born to mothers with diabetes, a rate 10 times higher than in patients without diabetes. It contributes to shoulder dystocia (risk 2 to 4 times higher in diabetic pregnancies) and cesarean delivery.6 Infants born to mothers with diabetes also have higher risks of neonatal hypoglycemia, erythrocytosis, hyperbilirubinemia, hypocalcemia, respiratory distress, cardiomyopathy, and death, as well as for developing diabetes, obesity, and other adverse cardiometabolic outcomes later in life.11
GET GLUCOSE UNDER CONTROL BEFORE PREGNANCY
Nearly half of pregnancies in the general population are unplanned,15 so preconception diabetes assessment needs to be part of routine medical care for all reproductive-age women. Because most organogenesis occurs during the first 5 to 8 weeks after fertilization—potentially before a woman realizes she is pregnant—achieving optimal glycemic control before conception is necessary to improve pregnancy outcomes.1
EVERY VISIT IS AN OPPORTUNITY
Every medical visit with a reproductive-age woman with diabetes is an opportunity for counseling about pregnancy. Topics that need to be discussed include the risks of unplanned pregnancy and of poor metabolic control, and the benefits of improved maternal and fetal outcomes with appropriate pregnancy planning and diabetes management.
Referral to a registered dietitian for individualized counseling about proper nutrition, particularly during pregnancy, has been associated with positive outcomes.16 Patients with diabetes and at high risk of pregnancy complications should be referred to a clinic that specializes in high-risk pregnancies.
Practitioners also should emphasize the importance of regular exercise and encourage patients to maintain or achieve a medically optimal weight before conception. Ideally, this would be a normal body mass index; however, this is not always possible.
In women who are planning pregnancy or are not on effective contraception, medications should be reviewed for potential teratogenicity. If needed, discuss alternative medications or switch to safer ones. However, these changes should not interrupt diabetes treatment.
In addition, ensure that the patient is up to date on age- and disease-appropriate preventive care (eg, immunizations, screening for sexually transmitted disease and malignancy). Counseling and intervention for use of tobacco, alcohol, and recreational drugs are also important. As with any preconception counseling, the patient (and her partner, if possible) should be advised to avoid travel to areas where Zika virus is endemic, and informed about the availability of expanded carrier genetic screening through her obstetric provider.
Finally, pregnant women with diabetes benefit from screening for diabetic complications including hypertension, retinopathy, cardiovascular disease, neuropathy, and nephropathy.
ASSESSING RISKS
Blood pressure
Chronic (preexisting) hypertension is defined as a systolic pressure 140 mm Hg or higher or a diastolic pressure 90 mm Hg or higher, or both, that antedates pregnancy or is present before the 20th week of pregnancy.3 Chronic hypertension has been reported in up to 5% of pregnant women and is associated with increased risk of preterm delivery, superimposed preeclampsia, low birth weight, and perinatal death.3
Reproductive-age women with diabetes and high blood pressure benefit from lifestyle and behavioral modifications.17 If drug therapy is needed, antihypertensive drugs that are safe for the fetus should be used. Treatment of mild or moderate hypertension during pregnancy reduces the risk of progression to severe hypertension but may not improve obstetric outcomes.
Diabetic retinopathy
Diabetic retinopathy can significantly worsen during pregnancy: the risk of progression is double that in the nonpregnant state.18 Women with diabetes who are contemplating pregnancy should have a comprehensive eye examination before conception, and any active proliferative retinopathy needs to be treated. These patients may require ophthalmologic monitoring and treatment during pregnancy. (Note: laser photocoagulation is not contraindicated during pregnancy.)
Cardiovascular disease
Cardiovascular physiology changes dramatically during pregnancy. Cardiovascular disease, especially when superimposed on diabetes, can increase the risk of maternal death. Thus, evaluation for cardiovascular risk factors as well as cardiovascular system integrity before conception is important. Listen for arterial bruits and murmurs, and assess peripheral pulses. Consideration should be given to obtaining a preconception resting electrocardiogram in women with diabetes who are over age 35 or who are suspected of having cardiovascular disease.16
Neurologic disorders
Peripheral neuropathy, the most common neurologic complication of diabetes, is associated with injury and infection.19
Autonomic neuropathy is associated with decreased cardiac responsiveness and orthostatic hypotension.19 Diabetic gastroparesis alone can precipitate serious complications during pregnancy, including extreme hypoglycemia and hyperglycemia, increased risk of diabetic ketoacidosis, weight loss, malnutrition, frequent hospitalizations, and increased requirement for parenteral nutrition.20
Although diabetic neuropathy does not significantly worsen during pregnancy, women with preexisting gastroparesis should be counseled on the substantial risks associated with pregnancy. Screening for neuropathy should be part of all diabetic preconception examinations.
Renal complications
Pregnancy in women with diabetes and preexisting renal dysfunction increases their risk of accelerated progression of diabetic kidney disease.21 Preexisting renal dysfunction also increases the risk of pregnancy-related complications, such as stillbirth, intrauterine growth restriction, gestational hypertension, preeclampsia, and preterm delivery.19,21,22 Further, the risk of pregnancy complications correlates directly with the severity of renal dysfunction.22
Psychiatric disorders
Emotional wellness is essential for optimal diabetes management. It is important to recognize the emotional impact of diabetes in pregnant women and to conduct routine screening for depression, anxiety, stress, and eating disorders.16
LABORATORY TESTS TO CONSIDER
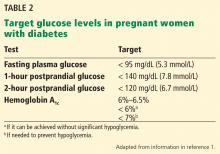
Hemoglobin A1c. The general consensus is to achieve the lowest hemoglobin A1c level possible that does not increase the risk of hypoglycemia. The American Diabetes Association (ADA) recommends that, before attempting to conceive, women should lower their hemoglobin A1c to below 6.5%.1
Thyroid measures. Autoimmune thyroid disease is the most common autoimmune disorder associated with diabetes and has been reported in 35% to 40% of women with type 1 diabetes.23 Recommendations are to check thyroid-stimulating hormone and thyroid peroxidase antibody levels before conception or early in pregnancy in all women with diabetes.1,24 Overt hypothyroidism should be treated before conception, given that early fetal brain development depends on maternal thyroxine.
Renal function testing. Preconception assessment of renal function is important for counseling and risk stratification. This assessment should include serum creatinine level, estimated glomerular filtration rate, and urinary albumin excretion.21
Celiac screening. Because women with type 1 diabetes are more susceptible to autoimmune diseases, they should be screened for celiac disease before conception, with testing for immunoglobulin A (IgA) and tissue transglutaminase antibodies, with or without IgA endomysial antibodies.16,25,26 An estimated 6% of patients with type 1 diabetes have celiac disease vs 1% of the general population.25 Celiac disease is 2 to 3 times more common in women, and asymptomatic people with type 1 diabetes are considered at increased risk for celiac disease.26
The association between type 1 diabetes and celiac disease most likely relates to the overlap in human leukocyte antigens of the diseases. There is no established link between type 2 diabetes and celiac disease.25
Undiagnosed celiac disease increases a woman’s risk of obstetric complications such as preterm birth, low birth weight, and stillbirth.26 The most likely explanation for these adverse effects is nutrient malabsorption, which is characteristic of celiac disease. Adherence to a gluten-free diet before and during gestation may reduce the risk of preterm delivery by as much as 20%.26
Vitamin B12 level. Celiac disease interferes with the absorption of vitamin B12-instrinsic factor in the ileum, which can lead to vitamin B12 deficiency. Therefore, baseline vitamin B12 levels should be checked before conception in women with celiac disease. Levels should also be checked in women taking metformin, which also decreases vitamin B12 absorption. Of note, increased folate levels due to taking supplements can potentially mask vitamin B12 deficiency.
MEDICATIONS TO REVIEW FOR PREGNANCY INTERACTIONS
Diabetic medications
Insulin is the first-line pharmacotherapy for pregnant patients with type 1, type 2, or gestational diabetes. Insulin does not cross the placenta to a measurable extent, and most insulin preparations have been classified as category B,1 meaning no risks to the fetus have been found in humans.
Insulin dosing during pregnancy is not static. Beginning around mid-gestation, insulin requirements increase,28,29 but after 32 weeks the need may decrease. These changes require practitioners to closely monitor blood glucose throughout pregnancy.
Both basal-bolus injections and continuous subcutaneous infusion are reasonable options during pregnancy.30 However, the need for multiple and potentially painful insulin injections daily can lead to poor compliance. This inconvenience has led to studies using oral hypoglycemic medications instead of insulin for patients with gestational and type 2 diabetes.
Metformin is an oral biguanide that decreases hepatic gluconeogenesis and intestinal glucose absorption while peripherally increasing glucose utilization and uptake. Metformin does not pose a risk of hypoglycemia because its mechanism of action does not involve increased insulin production.7
Metformin does cross the placenta, resulting in umbilical cord blood levels higher than maternal levels. Nevertheless, studies support the efficacy and short-term safety of metformin use during a pregnancy complicated by gestational or type 2 diabetes.7,31 Moreover, metformin has been associated with a lower risk of neonatal hypoglycemia and maternal weight gain than insulin.32 However, this agent should be used with caution, as long-term data are not yet available, and it may slightly increase the risk of premature delivery.
Glyburide is another oral hypoglycemic medication that has been used during pregnancy. This second-generation sulfonylurea enhances the release of insulin from the pancreas by binding beta islet cell ATP-calcium channel receptors. Compared with other sulfonylureas, glyburide has the lowest rate of maternal-to-fetal transfer, with umbilical cord plasma concentrations 70% of maternal levels.33 Although some trials support the efficacy and short-term safety of glyburide treatment for gestational diabetes,34 recent studies have associated glyburide use during pregnancy with a higher rate of neonatal hypoglycemia, neonatal respiratory distress, macrosomia, and neonatal intensive care unit admissions than insulin and metformin.1,35
Patients treated with oral agents should be informed that these drugs cross the placenta, and that although no adverse effects on the fetus have been demonstrated, long-term safety data are lacking. In addition, oral agents are ineffective in type 1 diabetes and may be insufficient to overcome the insulin resistance in type 2 diabetes.
Antihypertensive drugs
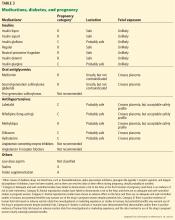
All antihypertensive drugs cross the placenta, but several have an acceptable safety profile in pregnancy, including methyldopa, labetalol, clonidine, prazosin, and nifedipine. Hydralazine and labetalol are short-acting, come in intravenous formulations, and can be used for urgent blood pressure control during pregnancy. Diltiazem may be used for heart rate control during pregnancy, and it has been shown to lower blood pressure and proteinuria in pregnant patients with underlying renal disease.36,37 The ADA recommends against chronic use of diuretics during pregnancy because of potential reductions in maternal plasma volume and uteroplacental perfusion.1
Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and direct renin inhibitors are contraindicated during pregnancy because of the risk of fetal defects, particularly in the renal system.21,38 Although there is evidence to question the association between first semester exposure and fetotoxicity,39 we avoid these drugs during pregnancy and switch to a different agent in women planning pregnancy.
Other drugs
Statins are contraindicated in pregnancy because they interfere with the development of the fetal nervous system.21 Although preliminary data from a small study did not identify safety risks associated with pravastatin use after 12 weeks of gestation,40 we recommend discontinuing statins in women attempting pregnancy.
Aspirin. The US Preventive Services Task Force41 recommends low-dose aspirin (81 mg/day) after 12 weeks of gestation for women with type 1 or type 2 diabetes, as well as those with renal disease or chronic hypertension, to prevent preeclampsia. Of note, higher doses need to be used with caution during pregnancy because fetal abnormalities have been reported, such as disruption of fetal vasculature (mesenteric vessels), gastroschisis, and small intestinal atresia.16
Folate supplementation (0.6–4 mg/day) is recommended in women with celiac disease to prevent neural tube defects in the offspring, and the US Preventive Services Task Force recommends 0.4 mg daily of folic acid supplementation for all women planning or capable of pregnancy.42–44 Higher doses, ranging from 0.6 to 5 mg/day, have been proposed for patients with diabetes,13 and we recommend at least 1 mg for this group, based on data suggesting that higher doses further reduce the risk of neural tube defects.43
IS BREASTFEEDING AFFECTED?
Maternal diabetes, insulin therapy, and oral hypoglycemic agents are not contraindications to breastfeeding. The US Preventive Services Task Force recommends interventions by primary care physicians to promote and support breastfeeding.45 Breastfeeding is encouraged based on various short- and long-term health benefits for both breastfed infants and breastfeeding mothers. Breastfeeding decreases a woman’s insulin requirements and increases the risk for hypoglycemia, especially in patients with insulin-dependent type 1 diabetes.1
Additionally, insulin sensitivity increases immediately following delivery of the placenta.1 Therefore, it is prudent to adjust insulin doses postpartum, especially while a patient is breastfeeding, or to suggest high-carbohydrate snacks before feeds.9,29
Antihypertensive drugs considered safe to use during lactation include captopril, enalapril, quinapril, labetalol, propranolol, nifedipine, and hydralazine.21,46 Methyldopa is not contraindicated, but it causes fatigue and worsened postpartum depression and should not be used as first-line therapy. Diuretics and ARBs are not recommended during lactation.21 Both metformin and glyburide enter breast milk in small enough amounts that they are not contraindicated during breastfeeding.16 The Lactmed database (www.toxnet.nlm.nih.gov) provides information about drugs and breastfeeding.
WHAT ABOUT CONTRACEPTIVES?
The ADA recommends contraception for women with diabetes because, just as in women without diabetes, the risks of unplanned pregnancy outweigh those of contraceptives.1
We recommend low-dose combination estrogen-progestin oral contraceptives to normotensive women under age 35 with diabetes but without underlying microvascular disease. For women over age 35 or for those with microvascular disease, additional options include intrauterine devices or progestin implants. We prefer not to use injectable depot medroxyprogesterone acetate because of its side effects of insulin resistance and weight gain.47
CASE DISCUSSION: NEXT STEPS
Our patient’s interest in family planning presents an opportunity for preconception counseling. We recommend a prenatal folic acid supplement, diet and regular exercise for weight loss, and screening tests including a comprehensive metabolic panel, hemoglobin A1c, thyroid-stimulating hormone, and dilated eye examination. We make sure she is up to date on her indicated health maintenance (eg, immunizations, disease screening), and we review her medications for potential teratogens. She denies any recreational drug use. Also, she has no plans for long-distance travel.
Our counseling includes discussions of pregnancy risks associated with pregestational diabetes and suboptimal glycemic control. We encourage her to use effective contraception until she is “medically optimized” for pregnancy—ie, until her hemoglobin A1c is lower than 6.5% and she has achieved a medically optimal weight. If feasible, a reduction of weight (7% or so) through lifestyle modification should be attempted, and if her hemoglobin A1c remains elevated, adding insulin would be recommended.
Pregnant patients or patients contemplating pregnancy are usually motivated to modify their behavior, making this a good time to reinforce lifestyle modifications. Many patients benefit from individualized counseling by a registered dietitian to help achieve the recommended weight and glycemic control.
Our physical examination in this patient includes screening for micro- and macrovascular complications of diabetes, and the test results are negative. Patients with active proliferative retinopathy should be referred to an ophthalmologist for assessment and treatment.
We review her medications for potential teratogenic effects and stop her ACE inhibitor (lisinopril) and statin (simvastatin). We switch her from a first-generation sulfonylurea (chlorpropamide) to glyburide, a second-generation sulfonylurea. Second-generation sulfonylureas are considered more “fetus-friendly” because first-generation sulfonylureas cross the placenta more easily and can cause fetal hyperinsulinemia, leading to macrosomia and neonatal hypoglycemia.7
The management of diabetes during pregnancy leans toward insulin use, given the lack of information regarding long-term outcomes with oral agents. If insulin is needed, it is best to initiate it before the patient conceives, and then to stop other diabetes medications. We would not make any changes to her aspirin or metformin use.
Educating the patient and her family about prevention, recognition, and treatment of hypoglycemia is important to prevent and manage the increased risk of hypoglycemia with insulin therapy and in early pregnancy.1 Consideration should be given to providing ketone strips as well as education on diabetic ketoacidosis prevention and detection.1 If the patient conceives, begin prenatal care early to allow adequate planning for care of her disease and evaluation of the fetus. Because of the complexity of insulin management in pregnancy, the ADA recommends referral, if possible, to a center offering team-based care, including an obstetrician specialized in high-risk pregnancies, an endocrinologist, and a dietitian.1
- American Diabetes Association. 13. Management of diabetes in pregnancy: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S137–S143. doi:10.2337/dc18-S013
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S13–S27. doi:10.2337/dc18-S002
- Lawler J, Osman M, Shelton JA, Yeh J. Population-based analysis of hypertensive disorders in pregnancy. Hypertens Pregnancy 2007; 26(1):67–76. doi:10.1080/10641950601147945
- Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015; 16(8):621–638. doi:10.1111/obr.12288
- Garrison EA, Jagasia S. Inpatient management of women with gestational and pregestational diabetes in pregnancy. Curr Diab Rep 2014; 14(2):457. doi:10.1007/s11892-013-0457-x
- Ballas J, Moore TR, Ramos GA. Management of diabetes in pregnancy. Curr Diab Rep 2012; 12(1):33–42. doi:10.1007/s11892-011-0249-0
- Ryu RJ, Hays KE, Hebert MF. Gestational diabetes mellitus management with oral hypoglycemic agents. Semin Perinatol 2014; 38(8):508–515. doi:10.1053/j.semperi.2014.08.012
- Cundy T, Gamble G, Neale L, et al. Differing causes of pregnancy loss in type 1 and type 2 diabetes. Diabetes Care 2007; 30(10):2603–2607. doi:10.2337/dc07-0555
- Castorino K, Jovanovic L. Pregnancy and diabetes management: advances and controversies. Clin Chem 2011; 57(2):221–230. doi:10.1373/clinchem.2010.155382
- Hammouda SA, Hakeem R. Role of HbA1c in predicting risk for congenital malformations. Prim Care Diabetes 2015; 9(6):458–464. doi:10.1016/j.pcd.2015.01.004
- Chen CP. Congenital malformations associated with maternal diabetes. Taiwanese J Obstet Gynecol 2005; 44(1):1–7. doi:10.1016/S1028-4559(09)60099-1
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33(3):676–682. doi:10.2337/dc09-1848
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36(5):1384–1395. doi:10.2337/dc12-2480
- HAPO Study Cooperative Research Group; Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358(19):1991–2002. doi:10.1056/NEJMoa0707943
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health 2014; 104(suppl 1):S43–S48. doi:10.2105/AJPH.2013.301416
- Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care 2008; 31(5):1060–1079. doi:10.2337/dc08-9020
- Webster LM, Conti-Ramsden F, Seed PT, Webb AJ, Nelson-Piercy C, Chappell LC. Impact of antihypertensive treatment on maternal and perinatal outcomes in pregnancy complicated by chronic hypertension: a systematic review and meta-analysis. J Am Heart Assoc 2017; 6(5).pii:e005526. doi:10.1161/JAHA.117.005526
- Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy: the Diabetes in Early Pregnancy Study. Diabetes Care 1995; 18(5):631–637. pmid:8586000
- American Diabetes Association. Standards of medical care in diabetes—2016. Diabetes Care 2016; 39 (suppl 1):S1–S109.
- Hawthorne, G. Maternal complications in diabetic pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):77–90. doi:10.1016/j.bpobgyn.2010.10.015
- Ringholm L, Damm JA, Vestgaard M, Damm P, Mathiesen ER. Diabetic nephropathy in women with preexisting diabetes: from pregnancy planning to breastfeeding. Curr Diab Rep 2016; 16(2):12. doi:10.1007/s11892-015-0705-3
- Zhang JJ, Ma XX, Hao L, Liu LJ, Lv JC, Zhang H. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol 2015; 10(11):1964–1978. doi:10.2215/CJN.09250914
- Umpierrez GE, Latif KA, Murphy MB, et al. Thyroid dysfunction in patients with type 1 diabetes: a longitudinal study. Diabetes Care 2003; 26(4):1181–1185. pmid:12663594
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Akirov A, Pinhas-Hamiel O. Co-occurrence of type 1 diabetes mellitus and celiac disease. World J Diabetes 2015; 6(5):707–714. doi:10.4239/wjd.v6.i5.707
- Saccone G, Berghella V, Sarno L, et al. Celiac disease and obstetric complications: a systematic review and metaanalysis. Am J Obstet Gynecol 2016; 214(2):225–234. doi:10.1016/j.ajog.2015.09.080
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
- de Valk HW, Visser GH. Insulin during pregnancy, labour and delivery. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):65–76. doi:10.1016/j.bpobgyn.2010.10.002
- Morello CM. Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus. Int J Gen Med 2011; 4:827–835. doi:10.2147/IJGM.S26889
- Farrar D, Tuffnell DJ, West J, West HM. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev 2016; (6):CD005542. doi:10.1002/14651858.CD005542.pub2
- Charles B, Norris R, Xiao X, Hague W. Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit 2006; 28(1):67–72. pmid:16418696
- Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ 2015; 350:h102. doi:10.1136/bmj.h102
- Hebert MF, Ma X, Naraharisetti SB, et al; Obstetric-Fetal Pharmacology Research Unit Network. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 2009; 85(6):607–614. doi:10.1038/clpt.2009.5
- Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000; 343(16):1134–1138. doi:10.1056/NEJM200010193431601
- Camelo Castillo W, Boggess K, Stürmer T, Brookhart MA, Benjamin DK Jr, Jonsson Funk M. Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes. JAMA Pediatr 2015; 169:452–458. doi:10.1001/jamapediatrics.2015.74
- Gowda RM, Khan IA, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac arrhythmias in pregnancy: clinical and therapeutic considerations. Int J Cardiol 2003; 88(2):129–133. pmid:12714190
- Khandelwal M, Kumanova M, Gaughan JP, Reece EA. Role of diltiazem in pregnant women with chronic renal disease. J Matern Fetal Neonatal Med 2002; 12(6):408–412. doi:10.1080/jmf.12.6.408.412
- Magee LA, Abalos E, von Dadelszen P, Sibai B, Easterling T, Walkinshaw S; CHIPS Study Group. How to manage hypertension in pregnancy effectively. Br J Clin Pharmacol 2011; 72(3):394–401. doi:10.1111/j.1365-2125.2011.04002.x
- Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 2006; 354(23):2443–2451. doi:10.1056/NEJMoa055202
- Costantine MM, Cleary K, Hebert MF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric-Fetal Pharmacology Research Units Network. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol 2016; 214(6):720.e1–720.e17. doi:10.1016/j.ajog.2015.12.038
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161(11):819–826. doi:10.7326/M14-1884
- Curry SJ, Grossman DC, Whitlock EP, Cantu A. Behavioral counseling research and evidence-based practice recommendations: US Preventive Services Task Force perspectives. Ann Intern Med 2014; 160(6):407–413. doi:10.7326/M13-2128
- Wald N, Law M, Morris J, Wald D. Quantifying the effect of folic acid. Lancet 2001; 358(9298):2069–2073. pmid:11755633
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. JAMA 2017; 317(2):183–189. doi:10.1001/jama.2016.19438
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Primary care interventions to support breastfeeding: US Preventive Services Task Force recommendation statement. JAMA 2016; 316(16):1688–1693. doi:10.1001/jama.2016.14697
- Newton ER, Hale TW. Drugs in breast milk. Clin Obstet Gynecol 2015; 58(4):868–884. doi:10.1097/GRF.0000000000000142
- Xiang AH, Kawakubo M, Kjos SL, Buchanan TA. Long-acting injectable progestin contraception and risk of type 2 diabetes in Latino women with prior gestational diabetes mellitus. Diabetes Care 2006; 29(3):613–617. pmid:16505515
A 29-year-old nulliparous woman presents for a routine checkup. She has hypertension and type 2 diabetes mellitus. Her current medications are chlorpropamide 500 mg daily, metformin 500 mg twice a day, lisinopril 40 mg daily, simvastatin 40 mg daily, and aspirin 81 mg daily. Her body mass index is 37 kg/m2 and her blood pressure is 130/80 mm Hg. Her hemoglobin A1c level is 7.8% and her low-density lipoprotein cholesterol 90 mg/dL.
She is considering pregnancy. How would you counsel her?
DEFINING DIABETES IN PREGNANCY
Diabetes in pregnant women, both gestational and pregestational, is the most common medical complication associated with pregnancy.1
- Gestational diabetes is defined as diabetes that is diagnosed during the second or third trimester of pregnancy and that is not clearly pregestational.2
- Pregestational diabetes exists before pregnancy and can be either type 1 or type 2.
Most cases of diabetes diagnosed during the first trimester reflect pregestational diabetes, as gestational diabetes occurs when insulin resistance increases in the later trimesters.
Type 1 diabetes involves autoimmune destruction of pancreatic islet cells, leading to insulin deficiency and the need for insulin therapy. Type 2 diabetes is characterized by insulin resistance rather than overall insulin deficiency. Type 2 diabetes tends to be associated with comorbidities such as obesity and hypertension, which are independent risk factors for adverse perinatal outcomes.3,4
Gestational diabetes accounts for most cases of diabetes during pregnancy. Although both pregestational and gestational diabetes increase the risk of maternal and fetal complications, pregestational diabetes is associated with significantly greater risks.1
IMPACT OF DIABETES ON THE MOTHER
Pregnancy increases the risk of maternal hypoglycemia, especially during the first trimester in patients with type 1 diabetes, as insulin sensitivity increases in early pregnancy.1 Pregnant women with diabetes may also have an altered counterregulatory response and less hypoglycemic awareness.1 Insulin resistance rises during the second and early third trimesters, increasing the risk of hyperglycemia in women with diabetes.1
Glycemic control during pregnancy is usually easier to achieve in patients with type 2 diabetes than with type 1, but it may require much higher insulin doses.
Because pregnancy is inherently a ketogenic state, women with type 1 diabetes are at higher risk of diabetic ketoacidosis, particularly during the second and third trimesters.1 There are reports of euglycemic diabetic ketoacidosis in pregnant women with either gestational or pregestational diabetes.5
Diabetes is associated with a risk of preeclampsia 4 times higher than in nondiabetic women.6 Other potential pregnancy-related complications include infections, polyhydramnios, spontaneous abortion, and cesarean delivery.1,7 The risk of pregnancy loss is similar in women with either type 1 or type 2 diabetes (2.6% and 3.7%, respectively), but the causes are different.8 Although preexisting diabetic complications such as retinopathy, nephropathy, and gastroparesis can be exacerbated during pregnancy,1 only severe gastroparesis and advanced renal disease are considered relative contraindications to pregnancy.
IMPACT OF DIABETES ON THE FETUS
Fetal complications of maternal diabetes include embryopathy (fetal malformations) and fetopathy (overgrowth, ie, fetus large for gestational age, and increased risk of fetal death or distress). Maternal hyperglycemia is associated with diabetic embryopathy, resulting in major birth defects in 5% to 25% of pregnancies and spontaneous abortions in 15% to 20%.9,10 There is a 2- to 6-fold increase in risk of congenital malformations.6
The most common diabetes-associated congenital malformations affect the cardiovascular system. Congenital heart disease includes tetralogy of Fallot, transposition of the great vessels, septal defects, and anomalous pulmonary venous return. Other relatively common defects involve the fetal central nervous system, spine, orofacial system, kidneys, urogenital system, gastrointestinal tract, and skeleton.11
The risk of fetopathy is proportional to the degree of maternal hyperglycemia. Excess maternal glucose and fatty acid levels can lead to fetal hyperglycemia and overgrowth, which increases fetal oxygen requirements. Erythropoietin levels rise, causing an increase in red cell mass, with subsequent hyperviscosity within the placenta and higher risk of fetal death.
Other complications include intrauterine growth restriction, prematurity, and preterm delivery. Fetal macrosomia (birth weight > 90th percentile or 4 kg, approximately 8 lb, 13 oz) occurs in 27% to 62% of children born to mothers with diabetes, a rate 10 times higher than in patients without diabetes. It contributes to shoulder dystocia (risk 2 to 4 times higher in diabetic pregnancies) and cesarean delivery.6 Infants born to mothers with diabetes also have higher risks of neonatal hypoglycemia, erythrocytosis, hyperbilirubinemia, hypocalcemia, respiratory distress, cardiomyopathy, and death, as well as for developing diabetes, obesity, and other adverse cardiometabolic outcomes later in life.11
GET GLUCOSE UNDER CONTROL BEFORE PREGNANCY
Nearly half of pregnancies in the general population are unplanned,15 so preconception diabetes assessment needs to be part of routine medical care for all reproductive-age women. Because most organogenesis occurs during the first 5 to 8 weeks after fertilization—potentially before a woman realizes she is pregnant—achieving optimal glycemic control before conception is necessary to improve pregnancy outcomes.1
EVERY VISIT IS AN OPPORTUNITY
Every medical visit with a reproductive-age woman with diabetes is an opportunity for counseling about pregnancy. Topics that need to be discussed include the risks of unplanned pregnancy and of poor metabolic control, and the benefits of improved maternal and fetal outcomes with appropriate pregnancy planning and diabetes management.
Referral to a registered dietitian for individualized counseling about proper nutrition, particularly during pregnancy, has been associated with positive outcomes.16 Patients with diabetes and at high risk of pregnancy complications should be referred to a clinic that specializes in high-risk pregnancies.
Practitioners also should emphasize the importance of regular exercise and encourage patients to maintain or achieve a medically optimal weight before conception. Ideally, this would be a normal body mass index; however, this is not always possible.
In women who are planning pregnancy or are not on effective contraception, medications should be reviewed for potential teratogenicity. If needed, discuss alternative medications or switch to safer ones. However, these changes should not interrupt diabetes treatment.
In addition, ensure that the patient is up to date on age- and disease-appropriate preventive care (eg, immunizations, screening for sexually transmitted disease and malignancy). Counseling and intervention for use of tobacco, alcohol, and recreational drugs are also important. As with any preconception counseling, the patient (and her partner, if possible) should be advised to avoid travel to areas where Zika virus is endemic, and informed about the availability of expanded carrier genetic screening through her obstetric provider.
Finally, pregnant women with diabetes benefit from screening for diabetic complications including hypertension, retinopathy, cardiovascular disease, neuropathy, and nephropathy.
ASSESSING RISKS
Blood pressure
Chronic (preexisting) hypertension is defined as a systolic pressure 140 mm Hg or higher or a diastolic pressure 90 mm Hg or higher, or both, that antedates pregnancy or is present before the 20th week of pregnancy.3 Chronic hypertension has been reported in up to 5% of pregnant women and is associated with increased risk of preterm delivery, superimposed preeclampsia, low birth weight, and perinatal death.3
Reproductive-age women with diabetes and high blood pressure benefit from lifestyle and behavioral modifications.17 If drug therapy is needed, antihypertensive drugs that are safe for the fetus should be used. Treatment of mild or moderate hypertension during pregnancy reduces the risk of progression to severe hypertension but may not improve obstetric outcomes.
Diabetic retinopathy
Diabetic retinopathy can significantly worsen during pregnancy: the risk of progression is double that in the nonpregnant state.18 Women with diabetes who are contemplating pregnancy should have a comprehensive eye examination before conception, and any active proliferative retinopathy needs to be treated. These patients may require ophthalmologic monitoring and treatment during pregnancy. (Note: laser photocoagulation is not contraindicated during pregnancy.)
Cardiovascular disease
Cardiovascular physiology changes dramatically during pregnancy. Cardiovascular disease, especially when superimposed on diabetes, can increase the risk of maternal death. Thus, evaluation for cardiovascular risk factors as well as cardiovascular system integrity before conception is important. Listen for arterial bruits and murmurs, and assess peripheral pulses. Consideration should be given to obtaining a preconception resting electrocardiogram in women with diabetes who are over age 35 or who are suspected of having cardiovascular disease.16
Neurologic disorders
Peripheral neuropathy, the most common neurologic complication of diabetes, is associated with injury and infection.19
Autonomic neuropathy is associated with decreased cardiac responsiveness and orthostatic hypotension.19 Diabetic gastroparesis alone can precipitate serious complications during pregnancy, including extreme hypoglycemia and hyperglycemia, increased risk of diabetic ketoacidosis, weight loss, malnutrition, frequent hospitalizations, and increased requirement for parenteral nutrition.20
Although diabetic neuropathy does not significantly worsen during pregnancy, women with preexisting gastroparesis should be counseled on the substantial risks associated with pregnancy. Screening for neuropathy should be part of all diabetic preconception examinations.
Renal complications
Pregnancy in women with diabetes and preexisting renal dysfunction increases their risk of accelerated progression of diabetic kidney disease.21 Preexisting renal dysfunction also increases the risk of pregnancy-related complications, such as stillbirth, intrauterine growth restriction, gestational hypertension, preeclampsia, and preterm delivery.19,21,22 Further, the risk of pregnancy complications correlates directly with the severity of renal dysfunction.22
Psychiatric disorders
Emotional wellness is essential for optimal diabetes management. It is important to recognize the emotional impact of diabetes in pregnant women and to conduct routine screening for depression, anxiety, stress, and eating disorders.16
LABORATORY TESTS TO CONSIDER
Hemoglobin A1c. The general consensus is to achieve the lowest hemoglobin A1c level possible that does not increase the risk of hypoglycemia. The American Diabetes Association (ADA) recommends that, before attempting to conceive, women should lower their hemoglobin A1c to below 6.5%.1
Thyroid measures. Autoimmune thyroid disease is the most common autoimmune disorder associated with diabetes and has been reported in 35% to 40% of women with type 1 diabetes.23 Recommendations are to check thyroid-stimulating hormone and thyroid peroxidase antibody levels before conception or early in pregnancy in all women with diabetes.1,24 Overt hypothyroidism should be treated before conception, given that early fetal brain development depends on maternal thyroxine.
Renal function testing. Preconception assessment of renal function is important for counseling and risk stratification. This assessment should include serum creatinine level, estimated glomerular filtration rate, and urinary albumin excretion.21
Celiac screening. Because women with type 1 diabetes are more susceptible to autoimmune diseases, they should be screened for celiac disease before conception, with testing for immunoglobulin A (IgA) and tissue transglutaminase antibodies, with or without IgA endomysial antibodies.16,25,26 An estimated 6% of patients with type 1 diabetes have celiac disease vs 1% of the general population.25 Celiac disease is 2 to 3 times more common in women, and asymptomatic people with type 1 diabetes are considered at increased risk for celiac disease.26
The association between type 1 diabetes and celiac disease most likely relates to the overlap in human leukocyte antigens of the diseases. There is no established link between type 2 diabetes and celiac disease.25
Undiagnosed celiac disease increases a woman’s risk of obstetric complications such as preterm birth, low birth weight, and stillbirth.26 The most likely explanation for these adverse effects is nutrient malabsorption, which is characteristic of celiac disease. Adherence to a gluten-free diet before and during gestation may reduce the risk of preterm delivery by as much as 20%.26
Vitamin B12 level. Celiac disease interferes with the absorption of vitamin B12-instrinsic factor in the ileum, which can lead to vitamin B12 deficiency. Therefore, baseline vitamin B12 levels should be checked before conception in women with celiac disease. Levels should also be checked in women taking metformin, which also decreases vitamin B12 absorption. Of note, increased folate levels due to taking supplements can potentially mask vitamin B12 deficiency.
MEDICATIONS TO REVIEW FOR PREGNANCY INTERACTIONS
Diabetic medications
Insulin is the first-line pharmacotherapy for pregnant patients with type 1, type 2, or gestational diabetes. Insulin does not cross the placenta to a measurable extent, and most insulin preparations have been classified as category B,1 meaning no risks to the fetus have been found in humans.
Insulin dosing during pregnancy is not static. Beginning around mid-gestation, insulin requirements increase,28,29 but after 32 weeks the need may decrease. These changes require practitioners to closely monitor blood glucose throughout pregnancy.
Both basal-bolus injections and continuous subcutaneous infusion are reasonable options during pregnancy.30 However, the need for multiple and potentially painful insulin injections daily can lead to poor compliance. This inconvenience has led to studies using oral hypoglycemic medications instead of insulin for patients with gestational and type 2 diabetes.
Metformin is an oral biguanide that decreases hepatic gluconeogenesis and intestinal glucose absorption while peripherally increasing glucose utilization and uptake. Metformin does not pose a risk of hypoglycemia because its mechanism of action does not involve increased insulin production.7
Metformin does cross the placenta, resulting in umbilical cord blood levels higher than maternal levels. Nevertheless, studies support the efficacy and short-term safety of metformin use during a pregnancy complicated by gestational or type 2 diabetes.7,31 Moreover, metformin has been associated with a lower risk of neonatal hypoglycemia and maternal weight gain than insulin.32 However, this agent should be used with caution, as long-term data are not yet available, and it may slightly increase the risk of premature delivery.
Glyburide is another oral hypoglycemic medication that has been used during pregnancy. This second-generation sulfonylurea enhances the release of insulin from the pancreas by binding beta islet cell ATP-calcium channel receptors. Compared with other sulfonylureas, glyburide has the lowest rate of maternal-to-fetal transfer, with umbilical cord plasma concentrations 70% of maternal levels.33 Although some trials support the efficacy and short-term safety of glyburide treatment for gestational diabetes,34 recent studies have associated glyburide use during pregnancy with a higher rate of neonatal hypoglycemia, neonatal respiratory distress, macrosomia, and neonatal intensive care unit admissions than insulin and metformin.1,35
Patients treated with oral agents should be informed that these drugs cross the placenta, and that although no adverse effects on the fetus have been demonstrated, long-term safety data are lacking. In addition, oral agents are ineffective in type 1 diabetes and may be insufficient to overcome the insulin resistance in type 2 diabetes.
Antihypertensive drugs
All antihypertensive drugs cross the placenta, but several have an acceptable safety profile in pregnancy, including methyldopa, labetalol, clonidine, prazosin, and nifedipine. Hydralazine and labetalol are short-acting, come in intravenous formulations, and can be used for urgent blood pressure control during pregnancy. Diltiazem may be used for heart rate control during pregnancy, and it has been shown to lower blood pressure and proteinuria in pregnant patients with underlying renal disease.36,37 The ADA recommends against chronic use of diuretics during pregnancy because of potential reductions in maternal plasma volume and uteroplacental perfusion.1
Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and direct renin inhibitors are contraindicated during pregnancy because of the risk of fetal defects, particularly in the renal system.21,38 Although there is evidence to question the association between first semester exposure and fetotoxicity,39 we avoid these drugs during pregnancy and switch to a different agent in women planning pregnancy.
Other drugs
Statins are contraindicated in pregnancy because they interfere with the development of the fetal nervous system.21 Although preliminary data from a small study did not identify safety risks associated with pravastatin use after 12 weeks of gestation,40 we recommend discontinuing statins in women attempting pregnancy.
Aspirin. The US Preventive Services Task Force41 recommends low-dose aspirin (81 mg/day) after 12 weeks of gestation for women with type 1 or type 2 diabetes, as well as those with renal disease or chronic hypertension, to prevent preeclampsia. Of note, higher doses need to be used with caution during pregnancy because fetal abnormalities have been reported, such as disruption of fetal vasculature (mesenteric vessels), gastroschisis, and small intestinal atresia.16
Folate supplementation (0.6–4 mg/day) is recommended in women with celiac disease to prevent neural tube defects in the offspring, and the US Preventive Services Task Force recommends 0.4 mg daily of folic acid supplementation for all women planning or capable of pregnancy.42–44 Higher doses, ranging from 0.6 to 5 mg/day, have been proposed for patients with diabetes,13 and we recommend at least 1 mg for this group, based on data suggesting that higher doses further reduce the risk of neural tube defects.43
IS BREASTFEEDING AFFECTED?
Maternal diabetes, insulin therapy, and oral hypoglycemic agents are not contraindications to breastfeeding. The US Preventive Services Task Force recommends interventions by primary care physicians to promote and support breastfeeding.45 Breastfeeding is encouraged based on various short- and long-term health benefits for both breastfed infants and breastfeeding mothers. Breastfeeding decreases a woman’s insulin requirements and increases the risk for hypoglycemia, especially in patients with insulin-dependent type 1 diabetes.1
Additionally, insulin sensitivity increases immediately following delivery of the placenta.1 Therefore, it is prudent to adjust insulin doses postpartum, especially while a patient is breastfeeding, or to suggest high-carbohydrate snacks before feeds.9,29
Antihypertensive drugs considered safe to use during lactation include captopril, enalapril, quinapril, labetalol, propranolol, nifedipine, and hydralazine.21,46 Methyldopa is not contraindicated, but it causes fatigue and worsened postpartum depression and should not be used as first-line therapy. Diuretics and ARBs are not recommended during lactation.21 Both metformin and glyburide enter breast milk in small enough amounts that they are not contraindicated during breastfeeding.16 The Lactmed database (www.toxnet.nlm.nih.gov) provides information about drugs and breastfeeding.
WHAT ABOUT CONTRACEPTIVES?
The ADA recommends contraception for women with diabetes because, just as in women without diabetes, the risks of unplanned pregnancy outweigh those of contraceptives.1
We recommend low-dose combination estrogen-progestin oral contraceptives to normotensive women under age 35 with diabetes but without underlying microvascular disease. For women over age 35 or for those with microvascular disease, additional options include intrauterine devices or progestin implants. We prefer not to use injectable depot medroxyprogesterone acetate because of its side effects of insulin resistance and weight gain.47
CASE DISCUSSION: NEXT STEPS
Our patient’s interest in family planning presents an opportunity for preconception counseling. We recommend a prenatal folic acid supplement, diet and regular exercise for weight loss, and screening tests including a comprehensive metabolic panel, hemoglobin A1c, thyroid-stimulating hormone, and dilated eye examination. We make sure she is up to date on her indicated health maintenance (eg, immunizations, disease screening), and we review her medications for potential teratogens. She denies any recreational drug use. Also, she has no plans for long-distance travel.
Our counseling includes discussions of pregnancy risks associated with pregestational diabetes and suboptimal glycemic control. We encourage her to use effective contraception until she is “medically optimized” for pregnancy—ie, until her hemoglobin A1c is lower than 6.5% and she has achieved a medically optimal weight. If feasible, a reduction of weight (7% or so) through lifestyle modification should be attempted, and if her hemoglobin A1c remains elevated, adding insulin would be recommended.
Pregnant patients or patients contemplating pregnancy are usually motivated to modify their behavior, making this a good time to reinforce lifestyle modifications. Many patients benefit from individualized counseling by a registered dietitian to help achieve the recommended weight and glycemic control.
Our physical examination in this patient includes screening for micro- and macrovascular complications of diabetes, and the test results are negative. Patients with active proliferative retinopathy should be referred to an ophthalmologist for assessment and treatment.
We review her medications for potential teratogenic effects and stop her ACE inhibitor (lisinopril) and statin (simvastatin). We switch her from a first-generation sulfonylurea (chlorpropamide) to glyburide, a second-generation sulfonylurea. Second-generation sulfonylureas are considered more “fetus-friendly” because first-generation sulfonylureas cross the placenta more easily and can cause fetal hyperinsulinemia, leading to macrosomia and neonatal hypoglycemia.7
The management of diabetes during pregnancy leans toward insulin use, given the lack of information regarding long-term outcomes with oral agents. If insulin is needed, it is best to initiate it before the patient conceives, and then to stop other diabetes medications. We would not make any changes to her aspirin or metformin use.
Educating the patient and her family about prevention, recognition, and treatment of hypoglycemia is important to prevent and manage the increased risk of hypoglycemia with insulin therapy and in early pregnancy.1 Consideration should be given to providing ketone strips as well as education on diabetic ketoacidosis prevention and detection.1 If the patient conceives, begin prenatal care early to allow adequate planning for care of her disease and evaluation of the fetus. Because of the complexity of insulin management in pregnancy, the ADA recommends referral, if possible, to a center offering team-based care, including an obstetrician specialized in high-risk pregnancies, an endocrinologist, and a dietitian.1
A 29-year-old nulliparous woman presents for a routine checkup. She has hypertension and type 2 diabetes mellitus. Her current medications are chlorpropamide 500 mg daily, metformin 500 mg twice a day, lisinopril 40 mg daily, simvastatin 40 mg daily, and aspirin 81 mg daily. Her body mass index is 37 kg/m2 and her blood pressure is 130/80 mm Hg. Her hemoglobin A1c level is 7.8% and her low-density lipoprotein cholesterol 90 mg/dL.
She is considering pregnancy. How would you counsel her?
DEFINING DIABETES IN PREGNANCY
Diabetes in pregnant women, both gestational and pregestational, is the most common medical complication associated with pregnancy.1
- Gestational diabetes is defined as diabetes that is diagnosed during the second or third trimester of pregnancy and that is not clearly pregestational.2
- Pregestational diabetes exists before pregnancy and can be either type 1 or type 2.
Most cases of diabetes diagnosed during the first trimester reflect pregestational diabetes, as gestational diabetes occurs when insulin resistance increases in the later trimesters.
Type 1 diabetes involves autoimmune destruction of pancreatic islet cells, leading to insulin deficiency and the need for insulin therapy. Type 2 diabetes is characterized by insulin resistance rather than overall insulin deficiency. Type 2 diabetes tends to be associated with comorbidities such as obesity and hypertension, which are independent risk factors for adverse perinatal outcomes.3,4
Gestational diabetes accounts for most cases of diabetes during pregnancy. Although both pregestational and gestational diabetes increase the risk of maternal and fetal complications, pregestational diabetes is associated with significantly greater risks.1
IMPACT OF DIABETES ON THE MOTHER
Pregnancy increases the risk of maternal hypoglycemia, especially during the first trimester in patients with type 1 diabetes, as insulin sensitivity increases in early pregnancy.1 Pregnant women with diabetes may also have an altered counterregulatory response and less hypoglycemic awareness.1 Insulin resistance rises during the second and early third trimesters, increasing the risk of hyperglycemia in women with diabetes.1
Glycemic control during pregnancy is usually easier to achieve in patients with type 2 diabetes than with type 1, but it may require much higher insulin doses.
Because pregnancy is inherently a ketogenic state, women with type 1 diabetes are at higher risk of diabetic ketoacidosis, particularly during the second and third trimesters.1 There are reports of euglycemic diabetic ketoacidosis in pregnant women with either gestational or pregestational diabetes.5
Diabetes is associated with a risk of preeclampsia 4 times higher than in nondiabetic women.6 Other potential pregnancy-related complications include infections, polyhydramnios, spontaneous abortion, and cesarean delivery.1,7 The risk of pregnancy loss is similar in women with either type 1 or type 2 diabetes (2.6% and 3.7%, respectively), but the causes are different.8 Although preexisting diabetic complications such as retinopathy, nephropathy, and gastroparesis can be exacerbated during pregnancy,1 only severe gastroparesis and advanced renal disease are considered relative contraindications to pregnancy.
IMPACT OF DIABETES ON THE FETUS
Fetal complications of maternal diabetes include embryopathy (fetal malformations) and fetopathy (overgrowth, ie, fetus large for gestational age, and increased risk of fetal death or distress). Maternal hyperglycemia is associated with diabetic embryopathy, resulting in major birth defects in 5% to 25% of pregnancies and spontaneous abortions in 15% to 20%.9,10 There is a 2- to 6-fold increase in risk of congenital malformations.6
The most common diabetes-associated congenital malformations affect the cardiovascular system. Congenital heart disease includes tetralogy of Fallot, transposition of the great vessels, septal defects, and anomalous pulmonary venous return. Other relatively common defects involve the fetal central nervous system, spine, orofacial system, kidneys, urogenital system, gastrointestinal tract, and skeleton.11
The risk of fetopathy is proportional to the degree of maternal hyperglycemia. Excess maternal glucose and fatty acid levels can lead to fetal hyperglycemia and overgrowth, which increases fetal oxygen requirements. Erythropoietin levels rise, causing an increase in red cell mass, with subsequent hyperviscosity within the placenta and higher risk of fetal death.
Other complications include intrauterine growth restriction, prematurity, and preterm delivery. Fetal macrosomia (birth weight > 90th percentile or 4 kg, approximately 8 lb, 13 oz) occurs in 27% to 62% of children born to mothers with diabetes, a rate 10 times higher than in patients without diabetes. It contributes to shoulder dystocia (risk 2 to 4 times higher in diabetic pregnancies) and cesarean delivery.6 Infants born to mothers with diabetes also have higher risks of neonatal hypoglycemia, erythrocytosis, hyperbilirubinemia, hypocalcemia, respiratory distress, cardiomyopathy, and death, as well as for developing diabetes, obesity, and other adverse cardiometabolic outcomes later in life.11
GET GLUCOSE UNDER CONTROL BEFORE PREGNANCY
Nearly half of pregnancies in the general population are unplanned,15 so preconception diabetes assessment needs to be part of routine medical care for all reproductive-age women. Because most organogenesis occurs during the first 5 to 8 weeks after fertilization—potentially before a woman realizes she is pregnant—achieving optimal glycemic control before conception is necessary to improve pregnancy outcomes.1
EVERY VISIT IS AN OPPORTUNITY
Every medical visit with a reproductive-age woman with diabetes is an opportunity for counseling about pregnancy. Topics that need to be discussed include the risks of unplanned pregnancy and of poor metabolic control, and the benefits of improved maternal and fetal outcomes with appropriate pregnancy planning and diabetes management.
Referral to a registered dietitian for individualized counseling about proper nutrition, particularly during pregnancy, has been associated with positive outcomes.16 Patients with diabetes and at high risk of pregnancy complications should be referred to a clinic that specializes in high-risk pregnancies.
Practitioners also should emphasize the importance of regular exercise and encourage patients to maintain or achieve a medically optimal weight before conception. Ideally, this would be a normal body mass index; however, this is not always possible.
In women who are planning pregnancy or are not on effective contraception, medications should be reviewed for potential teratogenicity. If needed, discuss alternative medications or switch to safer ones. However, these changes should not interrupt diabetes treatment.
In addition, ensure that the patient is up to date on age- and disease-appropriate preventive care (eg, immunizations, screening for sexually transmitted disease and malignancy). Counseling and intervention for use of tobacco, alcohol, and recreational drugs are also important. As with any preconception counseling, the patient (and her partner, if possible) should be advised to avoid travel to areas where Zika virus is endemic, and informed about the availability of expanded carrier genetic screening through her obstetric provider.
Finally, pregnant women with diabetes benefit from screening for diabetic complications including hypertension, retinopathy, cardiovascular disease, neuropathy, and nephropathy.
ASSESSING RISKS
Blood pressure
Chronic (preexisting) hypertension is defined as a systolic pressure 140 mm Hg or higher or a diastolic pressure 90 mm Hg or higher, or both, that antedates pregnancy or is present before the 20th week of pregnancy.3 Chronic hypertension has been reported in up to 5% of pregnant women and is associated with increased risk of preterm delivery, superimposed preeclampsia, low birth weight, and perinatal death.3
Reproductive-age women with diabetes and high blood pressure benefit from lifestyle and behavioral modifications.17 If drug therapy is needed, antihypertensive drugs that are safe for the fetus should be used. Treatment of mild or moderate hypertension during pregnancy reduces the risk of progression to severe hypertension but may not improve obstetric outcomes.
Diabetic retinopathy
Diabetic retinopathy can significantly worsen during pregnancy: the risk of progression is double that in the nonpregnant state.18 Women with diabetes who are contemplating pregnancy should have a comprehensive eye examination before conception, and any active proliferative retinopathy needs to be treated. These patients may require ophthalmologic monitoring and treatment during pregnancy. (Note: laser photocoagulation is not contraindicated during pregnancy.)
Cardiovascular disease
Cardiovascular physiology changes dramatically during pregnancy. Cardiovascular disease, especially when superimposed on diabetes, can increase the risk of maternal death. Thus, evaluation for cardiovascular risk factors as well as cardiovascular system integrity before conception is important. Listen for arterial bruits and murmurs, and assess peripheral pulses. Consideration should be given to obtaining a preconception resting electrocardiogram in women with diabetes who are over age 35 or who are suspected of having cardiovascular disease.16
Neurologic disorders
Peripheral neuropathy, the most common neurologic complication of diabetes, is associated with injury and infection.19
Autonomic neuropathy is associated with decreased cardiac responsiveness and orthostatic hypotension.19 Diabetic gastroparesis alone can precipitate serious complications during pregnancy, including extreme hypoglycemia and hyperglycemia, increased risk of diabetic ketoacidosis, weight loss, malnutrition, frequent hospitalizations, and increased requirement for parenteral nutrition.20
Although diabetic neuropathy does not significantly worsen during pregnancy, women with preexisting gastroparesis should be counseled on the substantial risks associated with pregnancy. Screening for neuropathy should be part of all diabetic preconception examinations.
Renal complications
Pregnancy in women with diabetes and preexisting renal dysfunction increases their risk of accelerated progression of diabetic kidney disease.21 Preexisting renal dysfunction also increases the risk of pregnancy-related complications, such as stillbirth, intrauterine growth restriction, gestational hypertension, preeclampsia, and preterm delivery.19,21,22 Further, the risk of pregnancy complications correlates directly with the severity of renal dysfunction.22
Psychiatric disorders
Emotional wellness is essential for optimal diabetes management. It is important to recognize the emotional impact of diabetes in pregnant women and to conduct routine screening for depression, anxiety, stress, and eating disorders.16
LABORATORY TESTS TO CONSIDER
Hemoglobin A1c. The general consensus is to achieve the lowest hemoglobin A1c level possible that does not increase the risk of hypoglycemia. The American Diabetes Association (ADA) recommends that, before attempting to conceive, women should lower their hemoglobin A1c to below 6.5%.1
Thyroid measures. Autoimmune thyroid disease is the most common autoimmune disorder associated with diabetes and has been reported in 35% to 40% of women with type 1 diabetes.23 Recommendations are to check thyroid-stimulating hormone and thyroid peroxidase antibody levels before conception or early in pregnancy in all women with diabetes.1,24 Overt hypothyroidism should be treated before conception, given that early fetal brain development depends on maternal thyroxine.
Renal function testing. Preconception assessment of renal function is important for counseling and risk stratification. This assessment should include serum creatinine level, estimated glomerular filtration rate, and urinary albumin excretion.21
Celiac screening. Because women with type 1 diabetes are more susceptible to autoimmune diseases, they should be screened for celiac disease before conception, with testing for immunoglobulin A (IgA) and tissue transglutaminase antibodies, with or without IgA endomysial antibodies.16,25,26 An estimated 6% of patients with type 1 diabetes have celiac disease vs 1% of the general population.25 Celiac disease is 2 to 3 times more common in women, and asymptomatic people with type 1 diabetes are considered at increased risk for celiac disease.26
The association between type 1 diabetes and celiac disease most likely relates to the overlap in human leukocyte antigens of the diseases. There is no established link between type 2 diabetes and celiac disease.25
Undiagnosed celiac disease increases a woman’s risk of obstetric complications such as preterm birth, low birth weight, and stillbirth.26 The most likely explanation for these adverse effects is nutrient malabsorption, which is characteristic of celiac disease. Adherence to a gluten-free diet before and during gestation may reduce the risk of preterm delivery by as much as 20%.26
Vitamin B12 level. Celiac disease interferes with the absorption of vitamin B12-instrinsic factor in the ileum, which can lead to vitamin B12 deficiency. Therefore, baseline vitamin B12 levels should be checked before conception in women with celiac disease. Levels should also be checked in women taking metformin, which also decreases vitamin B12 absorption. Of note, increased folate levels due to taking supplements can potentially mask vitamin B12 deficiency.
MEDICATIONS TO REVIEW FOR PREGNANCY INTERACTIONS
Diabetic medications
Insulin is the first-line pharmacotherapy for pregnant patients with type 1, type 2, or gestational diabetes. Insulin does not cross the placenta to a measurable extent, and most insulin preparations have been classified as category B,1 meaning no risks to the fetus have been found in humans.
Insulin dosing during pregnancy is not static. Beginning around mid-gestation, insulin requirements increase,28,29 but after 32 weeks the need may decrease. These changes require practitioners to closely monitor blood glucose throughout pregnancy.
Both basal-bolus injections and continuous subcutaneous infusion are reasonable options during pregnancy.30 However, the need for multiple and potentially painful insulin injections daily can lead to poor compliance. This inconvenience has led to studies using oral hypoglycemic medications instead of insulin for patients with gestational and type 2 diabetes.
Metformin is an oral biguanide that decreases hepatic gluconeogenesis and intestinal glucose absorption while peripherally increasing glucose utilization and uptake. Metformin does not pose a risk of hypoglycemia because its mechanism of action does not involve increased insulin production.7
Metformin does cross the placenta, resulting in umbilical cord blood levels higher than maternal levels. Nevertheless, studies support the efficacy and short-term safety of metformin use during a pregnancy complicated by gestational or type 2 diabetes.7,31 Moreover, metformin has been associated with a lower risk of neonatal hypoglycemia and maternal weight gain than insulin.32 However, this agent should be used with caution, as long-term data are not yet available, and it may slightly increase the risk of premature delivery.
Glyburide is another oral hypoglycemic medication that has been used during pregnancy. This second-generation sulfonylurea enhances the release of insulin from the pancreas by binding beta islet cell ATP-calcium channel receptors. Compared with other sulfonylureas, glyburide has the lowest rate of maternal-to-fetal transfer, with umbilical cord plasma concentrations 70% of maternal levels.33 Although some trials support the efficacy and short-term safety of glyburide treatment for gestational diabetes,34 recent studies have associated glyburide use during pregnancy with a higher rate of neonatal hypoglycemia, neonatal respiratory distress, macrosomia, and neonatal intensive care unit admissions than insulin and metformin.1,35
Patients treated with oral agents should be informed that these drugs cross the placenta, and that although no adverse effects on the fetus have been demonstrated, long-term safety data are lacking. In addition, oral agents are ineffective in type 1 diabetes and may be insufficient to overcome the insulin resistance in type 2 diabetes.
Antihypertensive drugs
All antihypertensive drugs cross the placenta, but several have an acceptable safety profile in pregnancy, including methyldopa, labetalol, clonidine, prazosin, and nifedipine. Hydralazine and labetalol are short-acting, come in intravenous formulations, and can be used for urgent blood pressure control during pregnancy. Diltiazem may be used for heart rate control during pregnancy, and it has been shown to lower blood pressure and proteinuria in pregnant patients with underlying renal disease.36,37 The ADA recommends against chronic use of diuretics during pregnancy because of potential reductions in maternal plasma volume and uteroplacental perfusion.1
Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and direct renin inhibitors are contraindicated during pregnancy because of the risk of fetal defects, particularly in the renal system.21,38 Although there is evidence to question the association between first semester exposure and fetotoxicity,39 we avoid these drugs during pregnancy and switch to a different agent in women planning pregnancy.
Other drugs
Statins are contraindicated in pregnancy because they interfere with the development of the fetal nervous system.21 Although preliminary data from a small study did not identify safety risks associated with pravastatin use after 12 weeks of gestation,40 we recommend discontinuing statins in women attempting pregnancy.
Aspirin. The US Preventive Services Task Force41 recommends low-dose aspirin (81 mg/day) after 12 weeks of gestation for women with type 1 or type 2 diabetes, as well as those with renal disease or chronic hypertension, to prevent preeclampsia. Of note, higher doses need to be used with caution during pregnancy because fetal abnormalities have been reported, such as disruption of fetal vasculature (mesenteric vessels), gastroschisis, and small intestinal atresia.16
Folate supplementation (0.6–4 mg/day) is recommended in women with celiac disease to prevent neural tube defects in the offspring, and the US Preventive Services Task Force recommends 0.4 mg daily of folic acid supplementation for all women planning or capable of pregnancy.42–44 Higher doses, ranging from 0.6 to 5 mg/day, have been proposed for patients with diabetes,13 and we recommend at least 1 mg for this group, based on data suggesting that higher doses further reduce the risk of neural tube defects.43
IS BREASTFEEDING AFFECTED?
Maternal diabetes, insulin therapy, and oral hypoglycemic agents are not contraindications to breastfeeding. The US Preventive Services Task Force recommends interventions by primary care physicians to promote and support breastfeeding.45 Breastfeeding is encouraged based on various short- and long-term health benefits for both breastfed infants and breastfeeding mothers. Breastfeeding decreases a woman’s insulin requirements and increases the risk for hypoglycemia, especially in patients with insulin-dependent type 1 diabetes.1
Additionally, insulin sensitivity increases immediately following delivery of the placenta.1 Therefore, it is prudent to adjust insulin doses postpartum, especially while a patient is breastfeeding, or to suggest high-carbohydrate snacks before feeds.9,29
Antihypertensive drugs considered safe to use during lactation include captopril, enalapril, quinapril, labetalol, propranolol, nifedipine, and hydralazine.21,46 Methyldopa is not contraindicated, but it causes fatigue and worsened postpartum depression and should not be used as first-line therapy. Diuretics and ARBs are not recommended during lactation.21 Both metformin and glyburide enter breast milk in small enough amounts that they are not contraindicated during breastfeeding.16 The Lactmed database (www.toxnet.nlm.nih.gov) provides information about drugs and breastfeeding.
WHAT ABOUT CONTRACEPTIVES?
The ADA recommends contraception for women with diabetes because, just as in women without diabetes, the risks of unplanned pregnancy outweigh those of contraceptives.1
We recommend low-dose combination estrogen-progestin oral contraceptives to normotensive women under age 35 with diabetes but without underlying microvascular disease. For women over age 35 or for those with microvascular disease, additional options include intrauterine devices or progestin implants. We prefer not to use injectable depot medroxyprogesterone acetate because of its side effects of insulin resistance and weight gain.47
CASE DISCUSSION: NEXT STEPS
Our patient’s interest in family planning presents an opportunity for preconception counseling. We recommend a prenatal folic acid supplement, diet and regular exercise for weight loss, and screening tests including a comprehensive metabolic panel, hemoglobin A1c, thyroid-stimulating hormone, and dilated eye examination. We make sure she is up to date on her indicated health maintenance (eg, immunizations, disease screening), and we review her medications for potential teratogens. She denies any recreational drug use. Also, she has no plans for long-distance travel.
Our counseling includes discussions of pregnancy risks associated with pregestational diabetes and suboptimal glycemic control. We encourage her to use effective contraception until she is “medically optimized” for pregnancy—ie, until her hemoglobin A1c is lower than 6.5% and she has achieved a medically optimal weight. If feasible, a reduction of weight (7% or so) through lifestyle modification should be attempted, and if her hemoglobin A1c remains elevated, adding insulin would be recommended.
Pregnant patients or patients contemplating pregnancy are usually motivated to modify their behavior, making this a good time to reinforce lifestyle modifications. Many patients benefit from individualized counseling by a registered dietitian to help achieve the recommended weight and glycemic control.
Our physical examination in this patient includes screening for micro- and macrovascular complications of diabetes, and the test results are negative. Patients with active proliferative retinopathy should be referred to an ophthalmologist for assessment and treatment.
We review her medications for potential teratogenic effects and stop her ACE inhibitor (lisinopril) and statin (simvastatin). We switch her from a first-generation sulfonylurea (chlorpropamide) to glyburide, a second-generation sulfonylurea. Second-generation sulfonylureas are considered more “fetus-friendly” because first-generation sulfonylureas cross the placenta more easily and can cause fetal hyperinsulinemia, leading to macrosomia and neonatal hypoglycemia.7
The management of diabetes during pregnancy leans toward insulin use, given the lack of information regarding long-term outcomes with oral agents. If insulin is needed, it is best to initiate it before the patient conceives, and then to stop other diabetes medications. We would not make any changes to her aspirin or metformin use.
Educating the patient and her family about prevention, recognition, and treatment of hypoglycemia is important to prevent and manage the increased risk of hypoglycemia with insulin therapy and in early pregnancy.1 Consideration should be given to providing ketone strips as well as education on diabetic ketoacidosis prevention and detection.1 If the patient conceives, begin prenatal care early to allow adequate planning for care of her disease and evaluation of the fetus. Because of the complexity of insulin management in pregnancy, the ADA recommends referral, if possible, to a center offering team-based care, including an obstetrician specialized in high-risk pregnancies, an endocrinologist, and a dietitian.1
- American Diabetes Association. 13. Management of diabetes in pregnancy: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S137–S143. doi:10.2337/dc18-S013
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S13–S27. doi:10.2337/dc18-S002
- Lawler J, Osman M, Shelton JA, Yeh J. Population-based analysis of hypertensive disorders in pregnancy. Hypertens Pregnancy 2007; 26(1):67–76. doi:10.1080/10641950601147945
- Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015; 16(8):621–638. doi:10.1111/obr.12288
- Garrison EA, Jagasia S. Inpatient management of women with gestational and pregestational diabetes in pregnancy. Curr Diab Rep 2014; 14(2):457. doi:10.1007/s11892-013-0457-x
- Ballas J, Moore TR, Ramos GA. Management of diabetes in pregnancy. Curr Diab Rep 2012; 12(1):33–42. doi:10.1007/s11892-011-0249-0
- Ryu RJ, Hays KE, Hebert MF. Gestational diabetes mellitus management with oral hypoglycemic agents. Semin Perinatol 2014; 38(8):508–515. doi:10.1053/j.semperi.2014.08.012
- Cundy T, Gamble G, Neale L, et al. Differing causes of pregnancy loss in type 1 and type 2 diabetes. Diabetes Care 2007; 30(10):2603–2607. doi:10.2337/dc07-0555
- Castorino K, Jovanovic L. Pregnancy and diabetes management: advances and controversies. Clin Chem 2011; 57(2):221–230. doi:10.1373/clinchem.2010.155382
- Hammouda SA, Hakeem R. Role of HbA1c in predicting risk for congenital malformations. Prim Care Diabetes 2015; 9(6):458–464. doi:10.1016/j.pcd.2015.01.004
- Chen CP. Congenital malformations associated with maternal diabetes. Taiwanese J Obstet Gynecol 2005; 44(1):1–7. doi:10.1016/S1028-4559(09)60099-1
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33(3):676–682. doi:10.2337/dc09-1848
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36(5):1384–1395. doi:10.2337/dc12-2480
- HAPO Study Cooperative Research Group; Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358(19):1991–2002. doi:10.1056/NEJMoa0707943
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health 2014; 104(suppl 1):S43–S48. doi:10.2105/AJPH.2013.301416
- Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care 2008; 31(5):1060–1079. doi:10.2337/dc08-9020
- Webster LM, Conti-Ramsden F, Seed PT, Webb AJ, Nelson-Piercy C, Chappell LC. Impact of antihypertensive treatment on maternal and perinatal outcomes in pregnancy complicated by chronic hypertension: a systematic review and meta-analysis. J Am Heart Assoc 2017; 6(5).pii:e005526. doi:10.1161/JAHA.117.005526
- Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy: the Diabetes in Early Pregnancy Study. Diabetes Care 1995; 18(5):631–637. pmid:8586000
- American Diabetes Association. Standards of medical care in diabetes—2016. Diabetes Care 2016; 39 (suppl 1):S1–S109.
- Hawthorne, G. Maternal complications in diabetic pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):77–90. doi:10.1016/j.bpobgyn.2010.10.015
- Ringholm L, Damm JA, Vestgaard M, Damm P, Mathiesen ER. Diabetic nephropathy in women with preexisting diabetes: from pregnancy planning to breastfeeding. Curr Diab Rep 2016; 16(2):12. doi:10.1007/s11892-015-0705-3
- Zhang JJ, Ma XX, Hao L, Liu LJ, Lv JC, Zhang H. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol 2015; 10(11):1964–1978. doi:10.2215/CJN.09250914
- Umpierrez GE, Latif KA, Murphy MB, et al. Thyroid dysfunction in patients with type 1 diabetes: a longitudinal study. Diabetes Care 2003; 26(4):1181–1185. pmid:12663594
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Akirov A, Pinhas-Hamiel O. Co-occurrence of type 1 diabetes mellitus and celiac disease. World J Diabetes 2015; 6(5):707–714. doi:10.4239/wjd.v6.i5.707
- Saccone G, Berghella V, Sarno L, et al. Celiac disease and obstetric complications: a systematic review and metaanalysis. Am J Obstet Gynecol 2016; 214(2):225–234. doi:10.1016/j.ajog.2015.09.080
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
- de Valk HW, Visser GH. Insulin during pregnancy, labour and delivery. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):65–76. doi:10.1016/j.bpobgyn.2010.10.002
- Morello CM. Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus. Int J Gen Med 2011; 4:827–835. doi:10.2147/IJGM.S26889
- Farrar D, Tuffnell DJ, West J, West HM. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev 2016; (6):CD005542. doi:10.1002/14651858.CD005542.pub2
- Charles B, Norris R, Xiao X, Hague W. Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit 2006; 28(1):67–72. pmid:16418696
- Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ 2015; 350:h102. doi:10.1136/bmj.h102
- Hebert MF, Ma X, Naraharisetti SB, et al; Obstetric-Fetal Pharmacology Research Unit Network. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 2009; 85(6):607–614. doi:10.1038/clpt.2009.5
- Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000; 343(16):1134–1138. doi:10.1056/NEJM200010193431601
- Camelo Castillo W, Boggess K, Stürmer T, Brookhart MA, Benjamin DK Jr, Jonsson Funk M. Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes. JAMA Pediatr 2015; 169:452–458. doi:10.1001/jamapediatrics.2015.74
- Gowda RM, Khan IA, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac arrhythmias in pregnancy: clinical and therapeutic considerations. Int J Cardiol 2003; 88(2):129–133. pmid:12714190
- Khandelwal M, Kumanova M, Gaughan JP, Reece EA. Role of diltiazem in pregnant women with chronic renal disease. J Matern Fetal Neonatal Med 2002; 12(6):408–412. doi:10.1080/jmf.12.6.408.412
- Magee LA, Abalos E, von Dadelszen P, Sibai B, Easterling T, Walkinshaw S; CHIPS Study Group. How to manage hypertension in pregnancy effectively. Br J Clin Pharmacol 2011; 72(3):394–401. doi:10.1111/j.1365-2125.2011.04002.x
- Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 2006; 354(23):2443–2451. doi:10.1056/NEJMoa055202
- Costantine MM, Cleary K, Hebert MF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric-Fetal Pharmacology Research Units Network. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol 2016; 214(6):720.e1–720.e17. doi:10.1016/j.ajog.2015.12.038
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161(11):819–826. doi:10.7326/M14-1884
- Curry SJ, Grossman DC, Whitlock EP, Cantu A. Behavioral counseling research and evidence-based practice recommendations: US Preventive Services Task Force perspectives. Ann Intern Med 2014; 160(6):407–413. doi:10.7326/M13-2128
- Wald N, Law M, Morris J, Wald D. Quantifying the effect of folic acid. Lancet 2001; 358(9298):2069–2073. pmid:11755633
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. JAMA 2017; 317(2):183–189. doi:10.1001/jama.2016.19438
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Primary care interventions to support breastfeeding: US Preventive Services Task Force recommendation statement. JAMA 2016; 316(16):1688–1693. doi:10.1001/jama.2016.14697
- Newton ER, Hale TW. Drugs in breast milk. Clin Obstet Gynecol 2015; 58(4):868–884. doi:10.1097/GRF.0000000000000142
- Xiang AH, Kawakubo M, Kjos SL, Buchanan TA. Long-acting injectable progestin contraception and risk of type 2 diabetes in Latino women with prior gestational diabetes mellitus. Diabetes Care 2006; 29(3):613–617. pmid:16505515
- American Diabetes Association. 13. Management of diabetes in pregnancy: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S137–S143. doi:10.2337/dc18-S013
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S13–S27. doi:10.2337/dc18-S002
- Lawler J, Osman M, Shelton JA, Yeh J. Population-based analysis of hypertensive disorders in pregnancy. Hypertens Pregnancy 2007; 26(1):67–76. doi:10.1080/10641950601147945
- Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015; 16(8):621–638. doi:10.1111/obr.12288
- Garrison EA, Jagasia S. Inpatient management of women with gestational and pregestational diabetes in pregnancy. Curr Diab Rep 2014; 14(2):457. doi:10.1007/s11892-013-0457-x
- Ballas J, Moore TR, Ramos GA. Management of diabetes in pregnancy. Curr Diab Rep 2012; 12(1):33–42. doi:10.1007/s11892-011-0249-0
- Ryu RJ, Hays KE, Hebert MF. Gestational diabetes mellitus management with oral hypoglycemic agents. Semin Perinatol 2014; 38(8):508–515. doi:10.1053/j.semperi.2014.08.012
- Cundy T, Gamble G, Neale L, et al. Differing causes of pregnancy loss in type 1 and type 2 diabetes. Diabetes Care 2007; 30(10):2603–2607. doi:10.2337/dc07-0555
- Castorino K, Jovanovic L. Pregnancy and diabetes management: advances and controversies. Clin Chem 2011; 57(2):221–230. doi:10.1373/clinchem.2010.155382
- Hammouda SA, Hakeem R. Role of HbA1c in predicting risk for congenital malformations. Prim Care Diabetes 2015; 9(6):458–464. doi:10.1016/j.pcd.2015.01.004
- Chen CP. Congenital malformations associated with maternal diabetes. Taiwanese J Obstet Gynecol 2005; 44(1):1–7. doi:10.1016/S1028-4559(09)60099-1
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33(3):676–682. doi:10.2337/dc09-1848
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36(5):1384–1395. doi:10.2337/dc12-2480
- HAPO Study Cooperative Research Group; Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358(19):1991–2002. doi:10.1056/NEJMoa0707943
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health 2014; 104(suppl 1):S43–S48. doi:10.2105/AJPH.2013.301416
- Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care 2008; 31(5):1060–1079. doi:10.2337/dc08-9020
- Webster LM, Conti-Ramsden F, Seed PT, Webb AJ, Nelson-Piercy C, Chappell LC. Impact of antihypertensive treatment on maternal and perinatal outcomes in pregnancy complicated by chronic hypertension: a systematic review and meta-analysis. J Am Heart Assoc 2017; 6(5).pii:e005526. doi:10.1161/JAHA.117.005526
- Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy: the Diabetes in Early Pregnancy Study. Diabetes Care 1995; 18(5):631–637. pmid:8586000
- American Diabetes Association. Standards of medical care in diabetes—2016. Diabetes Care 2016; 39 (suppl 1):S1–S109.
- Hawthorne, G. Maternal complications in diabetic pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):77–90. doi:10.1016/j.bpobgyn.2010.10.015
- Ringholm L, Damm JA, Vestgaard M, Damm P, Mathiesen ER. Diabetic nephropathy in women with preexisting diabetes: from pregnancy planning to breastfeeding. Curr Diab Rep 2016; 16(2):12. doi:10.1007/s11892-015-0705-3
- Zhang JJ, Ma XX, Hao L, Liu LJ, Lv JC, Zhang H. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol 2015; 10(11):1964–1978. doi:10.2215/CJN.09250914
- Umpierrez GE, Latif KA, Murphy MB, et al. Thyroid dysfunction in patients with type 1 diabetes: a longitudinal study. Diabetes Care 2003; 26(4):1181–1185. pmid:12663594
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Akirov A, Pinhas-Hamiel O. Co-occurrence of type 1 diabetes mellitus and celiac disease. World J Diabetes 2015; 6(5):707–714. doi:10.4239/wjd.v6.i5.707
- Saccone G, Berghella V, Sarno L, et al. Celiac disease and obstetric complications: a systematic review and metaanalysis. Am J Obstet Gynecol 2016; 214(2):225–234. doi:10.1016/j.ajog.2015.09.080
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
- de Valk HW, Visser GH. Insulin during pregnancy, labour and delivery. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):65–76. doi:10.1016/j.bpobgyn.2010.10.002
- Morello CM. Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus. Int J Gen Med 2011; 4:827–835. doi:10.2147/IJGM.S26889
- Farrar D, Tuffnell DJ, West J, West HM. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev 2016; (6):CD005542. doi:10.1002/14651858.CD005542.pub2
- Charles B, Norris R, Xiao X, Hague W. Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit 2006; 28(1):67–72. pmid:16418696
- Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ 2015; 350:h102. doi:10.1136/bmj.h102
- Hebert MF, Ma X, Naraharisetti SB, et al; Obstetric-Fetal Pharmacology Research Unit Network. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 2009; 85(6):607–614. doi:10.1038/clpt.2009.5
- Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000; 343(16):1134–1138. doi:10.1056/NEJM200010193431601
- Camelo Castillo W, Boggess K, Stürmer T, Brookhart MA, Benjamin DK Jr, Jonsson Funk M. Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes. JAMA Pediatr 2015; 169:452–458. doi:10.1001/jamapediatrics.2015.74
- Gowda RM, Khan IA, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac arrhythmias in pregnancy: clinical and therapeutic considerations. Int J Cardiol 2003; 88(2):129–133. pmid:12714190
- Khandelwal M, Kumanova M, Gaughan JP, Reece EA. Role of diltiazem in pregnant women with chronic renal disease. J Matern Fetal Neonatal Med 2002; 12(6):408–412. doi:10.1080/jmf.12.6.408.412
- Magee LA, Abalos E, von Dadelszen P, Sibai B, Easterling T, Walkinshaw S; CHIPS Study Group. How to manage hypertension in pregnancy effectively. Br J Clin Pharmacol 2011; 72(3):394–401. doi:10.1111/j.1365-2125.2011.04002.x
- Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 2006; 354(23):2443–2451. doi:10.1056/NEJMoa055202
- Costantine MM, Cleary K, Hebert MF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric-Fetal Pharmacology Research Units Network. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol 2016; 214(6):720.e1–720.e17. doi:10.1016/j.ajog.2015.12.038
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161(11):819–826. doi:10.7326/M14-1884
- Curry SJ, Grossman DC, Whitlock EP, Cantu A. Behavioral counseling research and evidence-based practice recommendations: US Preventive Services Task Force perspectives. Ann Intern Med 2014; 160(6):407–413. doi:10.7326/M13-2128
- Wald N, Law M, Morris J, Wald D. Quantifying the effect of folic acid. Lancet 2001; 358(9298):2069–2073. pmid:11755633
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. JAMA 2017; 317(2):183–189. doi:10.1001/jama.2016.19438
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Primary care interventions to support breastfeeding: US Preventive Services Task Force recommendation statement. JAMA 2016; 316(16):1688–1693. doi:10.1001/jama.2016.14697
- Newton ER, Hale TW. Drugs in breast milk. Clin Obstet Gynecol 2015; 58(4):868–884. doi:10.1097/GRF.0000000000000142
- Xiang AH, Kawakubo M, Kjos SL, Buchanan TA. Long-acting injectable progestin contraception and risk of type 2 diabetes in Latino women with prior gestational diabetes mellitus. Diabetes Care 2006; 29(3):613–617. pmid:16505515
KEY POINTS
- Aim for a hemoglobin A1c of 6.5% or lower, if it is attainable without increasing the risk of hypoglycemia.
- Avoid teratogenic drugs in sexually active women of childbearing age unless the patient uses effective contraception.
- Because about half of pregnancies are unplanned, it is important to routinely discuss family planning and provide preconception counseling that includes reducing risks associated with pregnancy.
- Screen for diabetic end-organ damage, especially retinopathy and nephropathy.