User login
• Treat burning foot pain in patients with diabetes with tricyclic antidepressants or anticonvulsants. A
• Prescribe custom-fitted extra-depth shoes for patients with diabetes and neuropathy or foot deformity. B
• Consider hyperbaric oxygen therapy for ulcers that fail to respond to standard therapy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE: AN OBESE PATIENT WITH BURNING FOOT PAIN
Mr. F., an obese 47-year-old with hypertension and type 2 diabetes, is trying to lose weight, and comes in to discuss a new exercise program. He recently had a negative cardiac workup for chest pain, which was ultimately diagnosed as gastroesophageal reflux disease.
The patient, whose most recent glycosylated hemoglobin (HbA1c) is 7.5, reports painful burning in his feet at night. A foot exam reveals no ulcers, lesions, or calluses; 2+ dorsalis pedis pulses bilaterally; and loss of sensation to 10-g monofilament testing at 3 sites on the bottom of each foot.
Based on Mr. F.’s presentation, it seems safe from a cardiac standpoint for him to embark on an exercise program, but what about his risk for a foot ulcer? An accurate assessment would enable you to tailor your recommendations and take the appropriate steps to modify the patient’s risk now—and to minimize complications down the road.
The incidence of diabetic foot ulcers—like that of diabetes itself—has risen in recent years.1 More than 15% of the approximately 23.5 million US adults with diabetes are expected to develop a foot ulcer at some point in their lives.2 Improvements in wound care and increased use of revascularization techniques have led to a decline in the number of ulcer-related amputations.3 But for 7% of those who develop diabetic foot ulcers, amputation is still the end result.4
As a family physician, you can play a key role in guarding against that outcome. This review—and the risk classification, ulcer grading, and treatment mnemonic tools that are detailed in the pages that follow—will help you optimize the foot care you provide to patients with diabetes.
Simple classification system accurately predicts risk
The longer an individual lives with diabetes and the poorer the level of blood glucose control, the greater the risk of ulceration.1,5 Other major risk factors include neuropathy, peripheral arterial disease, and a previous foot ulcer or amputation. All patients with diabetes should undergo an annual risk assessment for foot ulcers, which can be easily incorporated into their yearly physical. Although the causes of ulceration are numerous and complex, classification of risk based on simple measures has been found to accurately predict risk.6
Risk stratification tool. Medical history and in-office assessment of pulses, sensation, and foot deformities form the basis for a handy assessment tool (TABLE 1). In a study of 3256 patients, these simple criteria successfully predicted ulcer risk. During the nearly 2 years of the study, fewer than 1% of those categorized as low risk (n=2253) developed ulcers, while more than 29% of the high-risk group (n=477) did.6
To assess a patient’s risk, visually examine both feet, inspecting the skin, nails, and structure. Autonomic neuropathy may cause decreased sweating, leading to dry, breakable skin, while motor neuropathy can cause deformities such as hammer or claw toes, which also increase ulcer risk. Palpate for dorsalis pedis and posterior tibialis pulses. Although posterior tibialis pulses may be congenitally absent, the absence of a dorsalis pedis pulse is associated with a 6-fold increased risk of foot ulcer.1
Compared with patients with diabetes who have never had a foot ulcer, patients with a history of foot ulcer have 13 times the risk.7 As already noted, glucose control is also a key risk factor. For every 2-point increase in HbA1c, the odds ratio for ulcer development increases by 1.6,5 which is likely the result of hyperglycemia’s contribution to microvascular disease and peripheral neuropathy.
TABLE 1
Diabetic foot ulcer: Risk stratification
| Risk level/criteria | Incidence of ulcer* |
|---|---|
| Low risk All of the following: No history of ulcer At least 1 pulse per foot ≤1 of 10 sites insensate to monofilament testing No foot deformity or physical or visual impairment | 0.36% |
| Moderate risk 1 or more of the following: Missing both pulses in either foot ≥2 insensate sites to monofilament testing Foot deformity Unable to see or reach foot | 2.3% |
| High risk 1 or more of the following: Prior ulcer or amputation Neuropathy and absent pulses Neuropathy or absent pulses and calluses or foot deformity | 29.4% |
| * Percentage of patients in each risk category who developed foot ulcers over the nearly 2-year study period. | |
| Source: Leese GP, et al. Int J Clin Pract. 2006.6 | |
Screening for neuropathy, ulceration’s most consistent risk factor
Forty percent of patients with diabetes develop distal peripheral neuropathy.8 Damage to sensory nerves often results in the burning foot pain described by Mr. F., which can significantly affect quality of life. It may also present simply as decreased sensation or vibration sense. While decreased sensation is not painful and may not be troubling to the patient, it substantially heightens the risk of foot injury.
To screen for neuropathy, place a 10-g monofilament on noncallused plantar surfaces of the distal hallux and metatarsal heads, with enough pressure to slightly bend the filament. Instructing patients to close their eyes and report any sensation is more effective than prompting them for a response.
Recent guidelines from the American Diabetes Association and American Association of Clinical Endocrinologists recommend 1 additional screening test for neuropathy,9 such as pinprick or vibration sense.
Patients with neuropathy or other risk factors require more frequent follow-up to check for early signs of ulceration. Take the opportunity to provide education about the importance of self-examination of the feet, among other preventive measures (See “Preventing ulcers in high-risk feet”). Be alert to evidence of Charcot neuroarthropathy (CN). This progressive and irreversible condition of bone and joint slippage, dislocation, or fracture can affect any part of the foot, although it is typically found in the midfoot.
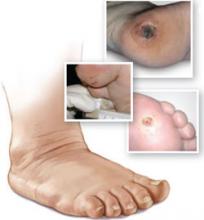
Acute CN is a clinical emergency characterized by pain, warmth, and erythema—making it clinically indistinguishable from cellulitis, osteomyelitis, or gout. Blood tests, including a white blood cell count, sedimentary rate, and uric acid level, may be necessary for diagnosis, as well as radiographs or magnetic resonance imaging. Treatment is long-term (4-6 months) immobilization of the joint to prevent further destruction,10 and bisphosphonates for pain during the acute phase.11 Untreated CN typically results in a rocker-bottom foot deformity (FIGURE) that puts patients at greater risk for plantar ulceration.
Although it is possible to successfully treat the majority of diabetic foot ulcers, the wounds result in considerable morbidity, lower quality of life, and increased health care costs. A far better approach is to focus on prevention, with appropriate interventions and frequent follow-up for those at high risk.
ENSURE THAT THE PATIENT HAS THE RIGHT FOOTWEAR.
Properly fitting shoes with ample room for the toes is a priority for all patients with diabetes, but “high-risk feet” need therapeutic footwear, which Medicare covers as a yearly benefit. For those with a history of foot ulcer or amputation, custom insoles, rigid rocker shoes, and orthotics can help prevent re-ulceration.34 For socks, synthetic blends are preferable to cotton, which can chafe when wet with sweat.
PROVIDE EDUCATIONAL MATERIALS; EMPHASIZE FOOT INSPECTION.
All patients with diabetes should receive general education regarding foot care, as there is evidence that it improves behavior and may prevent injuries.7,35 Educational materials emphasizing the importance of nightly foot inspection, overall foot care, and physician inspection are crucial for patients at high risk for developing foot ulcers. Excellent patient education materials are available from the National Diabetes Education Program (See “Take Care of Your Feet for a Lifetime”).
STRESS FREQUENT FOLLOW-UP.
Patients should be informed of their risk level for diabetic foot ulcers after screening. Advise those at high risk to have their feet inspected by a podiatrist or other knowledgeable clinician every 1 to 2 months.9
ENCOURAGE EXERCISE.
Non-weight-bearing exercise programs, including swimming, and a consistent level of daily weight-bearing activity should be encouraged. Caution patients to increase weight-bearing exercise gradually, however, ideally in a closely supervised setting, and to do everything possible to avoid even minimal foot trauma.
REVIEW MEDICATIONS AND ADJUST THERAPY, AS NEEDED.
Tight glycemic control and the use of angiotensin-converting enzyme inhibitors may help prevent the development of neuropathy.36,37 For those who already have neuropathy, tricyclic antidepressants38 or anticonvulsants may bring pain relief.
GUARD AGAINST CHARCOT NEUROARTHROPATHY.
Be alert to this diagnosis in patients who present with a warm, red, painful midfoot. Patients with long-standing neuropathy may benefit from preventive bracing to limit joint movement and lower the risk of Charcot neuroarthropathy.
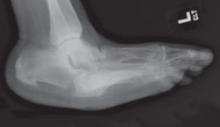
FIGURE
Rocker-bottom foot deformity
This radiograph reveals extensive collapse of the inner arch and a “rocker-bottom” foot deformity, the result of untreated Charcot neuroarthropathy.
CASE: MR. F.’S RISK BECOMES A REALITY
Mr. F. has a moderate risk of foot ulceration, based on evidence of neuropathy with 6 insensate sites (TABLE 1). You emphasize the importance of foot care, including appropriate footwear, and refer him to a podiatrist. You strongly support his decision to begin an exercise program to improve his glycemic control, decrease his cardiovascular mortality risk, and possibly help him lose weight.
Non-weight-bearing activity can be safely recommended to patients with diabetic neuropathy. So can daily weight-bearing activity, which actually decreases the risk of foot ulcer by maintaining leg muscle tone and plantar tissue tolerance to stress.12,13 Recent studies suggest that increasing weight-bearing activity slowly–walking daily and adding a total of 100 additional steps every 2 weeks, for example—in a carefully monitored program is not associated with increased risk of foot ulceration.14 You report these findings to Mr. F.
When he returns to your office in 2 months, Mr. F. has lost 7 pounds and his HbA1c has fallen to 6.5. Despite these positive developments, an examination of his feet reveals a full thickness ulcer on the left metatarsal head, which the patient had not noticed.
You recognize the clinical urgency of effectively treating Mr. F.’s diabetic foot ulcer, as size, duration, and grade are the greatest predictors of healing. Ulcers that are larger than 2 cm, have been present for more than 6 months,15 or have a higher grade on a scale such as the Wagner Foot Classification System16 (TABLE 2) are far less likely to heal than smaller, low-grade ulcers of shorter duration.
AIM DOC mnemonic guides ulcer care
Comprehensive, coordinated care improves outcomes for diabetic ulcers and has repeatedly been shown to reduce amputation rates.17-19 Large clinical trials have not evaluated each aspect of diabetic ulcer care, however, so the recommendations that follow are based on expert opinion and available evidence. These include assessing the limb’s arterial supply and ensuring that the patient undergoes revascularization, as needed; promptly treating infection; and providing optimal wound care, including debridement of callused and necrotic tissue, off-loading pressure, and applying moist wound dressings.20
AIM DOC, developed by 1 of the authors (JE), is a handy treatment tool. The mnemonic represents both the elements of treatment and the order in which they should be carried out. The letters stand for:
- Arterial disease
- Infection
- Measure
- Debride
- Off-load
- Cover
Here’s how to use AIM DOC, step by step:
Step 1: Assess for arterial disease
Start by assessing the vascular supply to the affected limb, which can be presumed to be adequate if pulses are palpable. If pulses cannot be palpated, the patient should undergo an ankle-brachial index (ABI) test and, if necessary, referred to a specialist to be evaluated for angioplasty or vascular bypass surgery. An ABI <0.9 is abnormal; 0.5 is considered the threshold for healing without such intervention.3 Keep in mind, however, that the ABI is falsely elevated in approximately 15% of patients with diabetes. If classic signs and symptoms of arterial disease are present, further evaluation is needed even if the ABI is normal.
Classic signs and symptoms of arterial disease include cool, hairless feet with shiny skin, and claudication. Location may provide another clue to etiology: Ulcers located on the heel, the outside of the foot, or between the toes tend to be associated with vascular disease, while ulcers with surrounding callus, such as the classic mal perforans ulcer on the metatarsal head, are neuropathic.
Step 2: Treat—or rule out—infection
While patients with diabetes have a 5-fold increase in risk of infection21 compared with individuals without the disease, there is no value in treating an uninfected ulcer with antibiotics. Diagnosing infection can be challenging because patients with diabetes may be less likely to demonstrate evidence of infection.21
An elevated white blood cell count, purulent drainage, foul odor, and/or erythema >2 cm around the wound clearly indicates a need for systemic antibiotics.22,23 Tissue necrosis, often assumed to represent ischemia, may result from neutrophilic vasculitis from soft tissue infection.24 Superficial cultures reflect colonization and should not be used to diagnose infection. In inspecting the wound for signs of infection, probe the ulcer and evaluate for osteomyelitis if it reaches bone.
Gram-positive cocci, especially Staphylococcus aureus, are the predominant pathogens in diabetic foot infections, and antibiotics effective against them may be sufficient for mild-to-moderate infections in patients who have not recently received antibiotic therapy. Gram-negative rods may be found in chronic wounds, however, and anaerobic pathogens may be present in patients with foot ischemia or gangrene; in both cases, broader-spectrum antimicrobials are required.22 Highly bioavailable oral antibiotics are indicated for infection, including some cases of osteomyelitis. Silver dressings may be helpful as a topical antimicrobial; there have been reports of successful treatment with honey, as well.25,26
Step 3: Measure (and grade) the wound
Accurate measurement of ulcer size is critical, both when you initially detect it and at each subsequent visit. To get an accurate measure, simply multiply the greatest length by the greatest width. The percent change in wound size after 4 weeks of treatment is a significant predictor of healing.25
Some clinicians also use photographs to track wound size, but these can be misleading. A better approach is to trace the wound on a sheet of acetate to document progress over time. If you see no improvement within 2 weeks, treatment should be modified.
Use a grading tool. There are a number of systems used for grading ulcers, none of which is universally accepted. One well-known tool is the Wagner Classification System16 (TABLE 2) referred to earlier. The higher the grade, the lower the likelihood that the ulcer will heal.
TABLE 2
Grading the ulcer: The Wagner system
| Grade | Description |
|---|---|
| 0 | No open lesions; may have deformity or cellulitis |
| 1 | Superficial diabetic ulcer (partial or full thickness) |
| 2 | Ulcer extension to ligament, tendon, joint capsule, or deep fascia (without abscess or osteomyelitis) |
| 3 | Deep ulcer with abscess, osteomyelitis, or joint sepsis |
| 4 | Gangrene localized to portion of forefoot or heel |
| 5 | Extensive gangrenous involvement of the entire foot |
| Adapted from: Wagner FW Jr. Orthopedics. 1987.16 | |
Step 4: Debride the ulcer
Frequent sharp debridement—to remove necrotic, callused, infected, and hypergranulation tissue—has long been considered essential in the treatment of neuropathic ulcers,17,21 especially for chronic or infected wounds. Debridement is thought to aid in healing by reducing pressure on the ulcer, decreasing bacterial contamination, enhancing platelet activation, releasing growth factors, and stimulating granulation tissue.
While some physicians are hesitant to perform debridement in the office, the process can actually be carried out without difficulty in an outpatient setting. Because of the neuropathy associated with most diabetic ulcers, no anesthesia is required. While the procedure is not typically painful, you will need to provide patient education to prepare the patient for the possibility of bleeding. Debride the wound to the outer edge of the hyperkeratotic tissue. If bleeding occurs, simply apply pressure until it stops.
When not to debride. Debridement is contraindicated under certain circumstances—if the limb has poor circulation, for example. Similarly, avoid debriding heel ulcers covered by eschar if there is no fluctuance in the underlying tissue, as the eschar provides a protective barrier.24 When sharp debridement is not possible, consider topical hydrogel or maggot therapy, an adjuvant treatment we’ll discuss in a bit.
Step 5: Off-load the wound
Mechanical load relief is vital for treating neuropathic ulcers, both to redistribute plantar pressures and protect granulation tissue. Total contact casting (TCC) is the gold standard, healing 90% of ulcers within 6 to 8 weeks.28 TCC is costly when applied weekly, however, and should only be done by a specialist, as an incorrectly applied cast can lead to the creation of new ulcers.
Because of the heaviness and inconvenience of the casts, many patients prefer removable devices, but these devices are much less effective. One study found that the average removable off-loading device is worn no more than 30% of the time that the patient is walking.29 Removable devices can be temporarily secured with plaster of Paris (a process that is sometimes referred to as instant contact casting) to ensure compliance.
Acceptable removable devices include a heel pressure relief shoe for heel ulcers and a CAM (controlled ankle motion) walker for metatarsal ulcers. Be sure off-loading devices are applied securely so no slippage can occur.
Step 6: Cover with moist dressings
The purpose of any topical dressing is to keep wounds moist, absorb exudate, and prevent contamination. A variety of moist dressings have been successfully used to treat ulcers, although evidence to recommend any particular dressing is insufficient.19 While wound vacuum-assisted closure (VAC) devices are widely used, there is little support for their use. A review of 7 trials comparing VAC devices with moistened gauze dressings or other topical agents found no evidence that topical negative pressure increases chronic wound healing.30
When foot ulcers do not heal
AIM DOC highlights the steps of diabetic ulcer care that are most likely to result in healing. When ulcers are slow to heal, review the 6 steps of treatment, paying particular attention to off-loading. If you establish that these have been appropriately applied and the wound is still not responding, consider alternative diagnoses such as venous insufficiency, vasculitis, or malignancy. Venous insufficiency ulcers may be difficult to differentiate from neuropathic ulcers, but they won’t heal without compression dressings. Diagnosis of vasculitis and malignancy can be made by biopsying the ulcer edge.
2 adjunctive therapies to consider
If there is no evidence to support an alternative diagnosis, consider adjunctive treatments, with hyperbaric oxygen therapy (HBOT) foremost among them.
A Cochrane review of HBOT found that it reduced the number of major amputations in diabetes patients with chronic foot ulcers, and improved healing at 1 year.31 Both Medicare and Medicaid cover HBOT for patients with diabetic ulcers classified as Wagner Grade 3 or higher that have not responded to 30 days of standard treatment.
Maggots, scientifically known as Lucilia sericata (Greenbottle) fly larvae, secrete proteolytic enzymes that debride necrotic tissue but are inactivated by living tissue. One meta-analysis found that neither surgical debridement nor larval therapy showed significant benefit over hydrogel.32 A subsequent small study did show statistically improved healing in ulcers debrided with maggots, compared with surgical debridement.33
CASE: MR. F.’S ULCER HEALS
Because Mr. F.’s ulcer is small and shallow and has been present for a short time, it has an excellent chance of healing if you follow the AIM DOC steps. You determine that he has adequate arterial supply and that the wound is uninfected (there is a strong dorsalis pedis pulse and no warmth, exudate, or erythema around the wound). Using a #15 blade, you pare away the callus surrounding the ulcer and document the length and width of the wound. You cover the ulcer with a moist dressing and instruct Mr. F to replace it twice a day, cautioning him against using alcohol or hydrogen peroxide, which could harm the healing skin. You discuss the importance of avoiding all weight bearing on the ulcerated foot, prescribe a CAM walker to wear at all times except while he’s sleeping, and schedule weekly follow-up visits to track progress.
In 2 weeks, the wound has resolved. You educate Mr. F. about his risk of ulcer recurrence and outline appropriate preventive steps. You also refer him for fitted extra-depth diabetic shoes and ongoing podiatry follow-up.
ACKNOWLEDGMENT
The authors wish to thank Gregory Mack, DPM, University of Wisconsin School of Medicine and Public Health, for his teaching and collaboration.
CORRESPONDENCE
Jennifer Eddy, MD, University of Wisconsin School of Medicine and Public Health, 617 W. Clairemont Avenue, Eau Claire, WI 54701; [email protected]
1. LeMaster JW, Reiber GE. Epidemiology and economic impact of foot ulcers. In: Boulton AJM, Cavanaugh PR, Rayman G, eds. The Foot in Diabetes. 4th ed. London, England: John Wiley & Sons Ltd; 2006:1–16.
2. Department of Health and Human Services Centers for Disease Control and Health Prevention. National Diabetes Fact Sheet 2007. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Accessed May 15, 2009.
3. Pinzur M. Amputations in the diabetic foot. In: Boulton AJM, Cavanaugh PR, Rayman G, eds. The Foot in Diabetes. 4th ed. London, England: John Wiley & Sons Ltd; 2006:308–322.
4. Margolis DJ, Allen-Taylor L, Hoffstad O, et al. Diabetic neuropathic foot ulcers and amputation. Wound Repair Regen. 2005;13:230-236.
5. Moss SE, Klein R, Kelin BE. The prevalence and incidence of lower extremity amputation in a diabetic population. Arch Intern Med. 1992;152:610-616.
6. Leese GP, Reid F, Green V, et al. Stratification of foot ulcer risk in patients with diabetes: a population based study. Int J Clin Pract. 2006;60:541-545.
7. Litzelman DR, Slemenda CW, Langefeld CD. Reduction of lower extremity clinical abnormalities in patient with non-insulin-dependent diabetes mellitus: a randomized controlled trial. Ann Intern Med. 1993;119:36-41.
8. Palumbo PJ, Melton LJ, III. Peripheral vascular disease and diabetes. In: National Diabetes Data Group, National Institute of Diabetes and Digestive and Kidney Disease, eds. Diabetes in America. 2nd ed. Bethesda, Md: National Institutes of Health, NIDDKD; 1995:401–408.
9. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31:1679-1685.
10. Jude EB. Charcot foot: what’s new in pathogensis and medical management? In: Boulton AJM, Cavanaugh PR, Rayman G, eds. The Foot in Diabetes. 4th ed. London, England: John Wiley & Sons Ltd; 2006: 265–273.
11. Pitocco D, Ruotolo V, Caputo S, et al. Six month treatment with alendronate in acute Charcot neuroarthropathy: a randomized controlled trial. Diabetes Care. 2005;28:1214-1215.
12. LeMaster JW, Reiber GE, Smith DG, et al. Daily weight-bearing activity does not increase the risk of diabetic foot ulcers. Med Sci Sports Exerc. 2003;35:1093-1099.
13. Lott DJ, Malug KS, Sinacore DR, et al. Relationship between changes in activity and plantar ulcer recurrence in a patient with diabetes mellitus. Phys Ther. 2005;85:579-588.
14. LeMaster JW, Mueller MJ, Reiber GE, et al. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: Feet First randomized controlled trial. Phys Ther. 2008;88:1-13.
15. Margolis DJ, Kantor J, Santanna J, et al. Risk factors for delayed healing of neuropathic foot ulcers: a pooled analysis. Arch Dermatol. 2000;136:1531-1535.
16. Wagner FW, Jr. The diabetic foot. Orthopedics. 1987;10:163-172.
17. Rith-Najarian S, Branchaid C, Beaulieu O, et al. Reducing lower-extremity amputations due to diabetes: application of the staged diabetes management approach in a primary care setting. J Fam Pract. 1998;47:127-132.
18. Canavan RJ, Unwin NC, Connolly VM, et al. Diabetes and non-diabetes related lower extremity amputation incidence before and after the introduction of better organized diabetic foot care. Diabetes Care. 2008;31.-
19. Krishnan St, Nash F, Baker NR, et al. Reduction in diabetes amputations over 11 years in a defined UK population. Diabetes Care. 2008;31:99-101.
20. Clayton W, Elasy TA. A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clin Diabetes. 2009;27:52-58.
21. Majno G, Joris I. Cells, Tissues, and Disease: Principles of General Pathology, 2nd ed. Oxford, England: Oxford University Press; 2004.
22. Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39:885-910.
23. Frykberg RG, Zgonis T, Armstrong DG, et al. Diabetic foot disorders: a clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(5 suppl):S2-S66.
24. Edmonds M, Foster A. The use of antibiotics in the diabetic foot. Am J Surg. 2004;187:S25-S28.
25. Eddy JJ, Gideonsen MD. Topical honey therapy for diabetic foot ulcers. J Fam Pract. 2005;54:533-535.
26. Remmen R, Coenen S, Seuntjens R, et al. Honey for refractory diabetic foot ulcers. J Fam Pract. 2005;54:863.-
27. Sheehan P, Jones P, Caselli A, et al. Percent change in wound area in diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26:1879-1882.
28. Lavery LA, Murdoch DP. Conventional offloading and activity monitoring. In: The Foot in Diabetes. 4th ed. Boulton AJM, Cavanaugh PR, Rayman G, eds. London, England: John Wiley & Sons Ltd; 2006: PG NUM.
29. Armstrong DG, Lavery LA, Kimbrel HR, et al. Activity patterns of patients with diabetic foot ulceration: patients with active ulceration may not adhere to a standard pressure off-loading regimen. Diabetes Care. 2003;26:2595-2597.
30. Ubbink DT, Westerbos SJ, Evans D, et al. H. Topical negative pressure for treating chronic wounds. Cochrane Database Syst Rev. 2008(3);CD001898.-
31. Kranke P, Bennett M, Roeckl-Wiedmann I, et al. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004(2);CD004123.-
32. Edwards J. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev. 2002(4);CD003556.-
33. Sherman RA. Maggot therapy for treating diabetic foot ulcers unresponsive to conventional therapy. Diabetes Care. 2003;26:446-451.
34. Spencer SA. Pressure relieving interventions for preventing and treating diabetic foot ulcers. Cochrane Database Syst Rev. 2000(3);CD002302.-
35. Valk GD, Kriegsman DM, Assendelft WJ. Patient education for preventing diabetic foot ulceration. Cochrane Database Syst Rev. 2001(4);CD001488.-(AU: 2005 update..?)
36. The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med. 1995;122:561-568.
37. Boulton AJM, Viniv AL, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956-962.
38. Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ. 2007;335:87.-
• Treat burning foot pain in patients with diabetes with tricyclic antidepressants or anticonvulsants. A
• Prescribe custom-fitted extra-depth shoes for patients with diabetes and neuropathy or foot deformity. B
• Consider hyperbaric oxygen therapy for ulcers that fail to respond to standard therapy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE: AN OBESE PATIENT WITH BURNING FOOT PAIN
Mr. F., an obese 47-year-old with hypertension and type 2 diabetes, is trying to lose weight, and comes in to discuss a new exercise program. He recently had a negative cardiac workup for chest pain, which was ultimately diagnosed as gastroesophageal reflux disease.
The patient, whose most recent glycosylated hemoglobin (HbA1c) is 7.5, reports painful burning in his feet at night. A foot exam reveals no ulcers, lesions, or calluses; 2+ dorsalis pedis pulses bilaterally; and loss of sensation to 10-g monofilament testing at 3 sites on the bottom of each foot.
Based on Mr. F.’s presentation, it seems safe from a cardiac standpoint for him to embark on an exercise program, but what about his risk for a foot ulcer? An accurate assessment would enable you to tailor your recommendations and take the appropriate steps to modify the patient’s risk now—and to minimize complications down the road.
The incidence of diabetic foot ulcers—like that of diabetes itself—has risen in recent years.1 More than 15% of the approximately 23.5 million US adults with diabetes are expected to develop a foot ulcer at some point in their lives.2 Improvements in wound care and increased use of revascularization techniques have led to a decline in the number of ulcer-related amputations.3 But for 7% of those who develop diabetic foot ulcers, amputation is still the end result.4
As a family physician, you can play a key role in guarding against that outcome. This review—and the risk classification, ulcer grading, and treatment mnemonic tools that are detailed in the pages that follow—will help you optimize the foot care you provide to patients with diabetes.
Simple classification system accurately predicts risk
The longer an individual lives with diabetes and the poorer the level of blood glucose control, the greater the risk of ulceration.1,5 Other major risk factors include neuropathy, peripheral arterial disease, and a previous foot ulcer or amputation. All patients with diabetes should undergo an annual risk assessment for foot ulcers, which can be easily incorporated into their yearly physical. Although the causes of ulceration are numerous and complex, classification of risk based on simple measures has been found to accurately predict risk.6
Risk stratification tool. Medical history and in-office assessment of pulses, sensation, and foot deformities form the basis for a handy assessment tool (TABLE 1). In a study of 3256 patients, these simple criteria successfully predicted ulcer risk. During the nearly 2 years of the study, fewer than 1% of those categorized as low risk (n=2253) developed ulcers, while more than 29% of the high-risk group (n=477) did.6
To assess a patient’s risk, visually examine both feet, inspecting the skin, nails, and structure. Autonomic neuropathy may cause decreased sweating, leading to dry, breakable skin, while motor neuropathy can cause deformities such as hammer or claw toes, which also increase ulcer risk. Palpate for dorsalis pedis and posterior tibialis pulses. Although posterior tibialis pulses may be congenitally absent, the absence of a dorsalis pedis pulse is associated with a 6-fold increased risk of foot ulcer.1
Compared with patients with diabetes who have never had a foot ulcer, patients with a history of foot ulcer have 13 times the risk.7 As already noted, glucose control is also a key risk factor. For every 2-point increase in HbA1c, the odds ratio for ulcer development increases by 1.6,5 which is likely the result of hyperglycemia’s contribution to microvascular disease and peripheral neuropathy.
TABLE 1
Diabetic foot ulcer: Risk stratification
| Risk level/criteria | Incidence of ulcer* |
|---|---|
| Low risk All of the following: No history of ulcer At least 1 pulse per foot ≤1 of 10 sites insensate to monofilament testing No foot deformity or physical or visual impairment | 0.36% |
| Moderate risk 1 or more of the following: Missing both pulses in either foot ≥2 insensate sites to monofilament testing Foot deformity Unable to see or reach foot | 2.3% |
| High risk 1 or more of the following: Prior ulcer or amputation Neuropathy and absent pulses Neuropathy or absent pulses and calluses or foot deformity | 29.4% |
| * Percentage of patients in each risk category who developed foot ulcers over the nearly 2-year study period. | |
| Source: Leese GP, et al. Int J Clin Pract. 2006.6 | |
Screening for neuropathy, ulceration’s most consistent risk factor
Forty percent of patients with diabetes develop distal peripheral neuropathy.8 Damage to sensory nerves often results in the burning foot pain described by Mr. F., which can significantly affect quality of life. It may also present simply as decreased sensation or vibration sense. While decreased sensation is not painful and may not be troubling to the patient, it substantially heightens the risk of foot injury.
To screen for neuropathy, place a 10-g monofilament on noncallused plantar surfaces of the distal hallux and metatarsal heads, with enough pressure to slightly bend the filament. Instructing patients to close their eyes and report any sensation is more effective than prompting them for a response.
Recent guidelines from the American Diabetes Association and American Association of Clinical Endocrinologists recommend 1 additional screening test for neuropathy,9 such as pinprick or vibration sense.
Patients with neuropathy or other risk factors require more frequent follow-up to check for early signs of ulceration. Take the opportunity to provide education about the importance of self-examination of the feet, among other preventive measures (See “Preventing ulcers in high-risk feet”). Be alert to evidence of Charcot neuroarthropathy (CN). This progressive and irreversible condition of bone and joint slippage, dislocation, or fracture can affect any part of the foot, although it is typically found in the midfoot.
Acute CN is a clinical emergency characterized by pain, warmth, and erythema—making it clinically indistinguishable from cellulitis, osteomyelitis, or gout. Blood tests, including a white blood cell count, sedimentary rate, and uric acid level, may be necessary for diagnosis, as well as radiographs or magnetic resonance imaging. Treatment is long-term (4-6 months) immobilization of the joint to prevent further destruction,10 and bisphosphonates for pain during the acute phase.11 Untreated CN typically results in a rocker-bottom foot deformity (FIGURE) that puts patients at greater risk for plantar ulceration.
Although it is possible to successfully treat the majority of diabetic foot ulcers, the wounds result in considerable morbidity, lower quality of life, and increased health care costs. A far better approach is to focus on prevention, with appropriate interventions and frequent follow-up for those at high risk.
ENSURE THAT THE PATIENT HAS THE RIGHT FOOTWEAR.
Properly fitting shoes with ample room for the toes is a priority for all patients with diabetes, but “high-risk feet” need therapeutic footwear, which Medicare covers as a yearly benefit. For those with a history of foot ulcer or amputation, custom insoles, rigid rocker shoes, and orthotics can help prevent re-ulceration.34 For socks, synthetic blends are preferable to cotton, which can chafe when wet with sweat.
PROVIDE EDUCATIONAL MATERIALS; EMPHASIZE FOOT INSPECTION.
All patients with diabetes should receive general education regarding foot care, as there is evidence that it improves behavior and may prevent injuries.7,35 Educational materials emphasizing the importance of nightly foot inspection, overall foot care, and physician inspection are crucial for patients at high risk for developing foot ulcers. Excellent patient education materials are available from the National Diabetes Education Program (See “Take Care of Your Feet for a Lifetime”).
STRESS FREQUENT FOLLOW-UP.
Patients should be informed of their risk level for diabetic foot ulcers after screening. Advise those at high risk to have their feet inspected by a podiatrist or other knowledgeable clinician every 1 to 2 months.9
ENCOURAGE EXERCISE.
Non-weight-bearing exercise programs, including swimming, and a consistent level of daily weight-bearing activity should be encouraged. Caution patients to increase weight-bearing exercise gradually, however, ideally in a closely supervised setting, and to do everything possible to avoid even minimal foot trauma.
REVIEW MEDICATIONS AND ADJUST THERAPY, AS NEEDED.
Tight glycemic control and the use of angiotensin-converting enzyme inhibitors may help prevent the development of neuropathy.36,37 For those who already have neuropathy, tricyclic antidepressants38 or anticonvulsants may bring pain relief.
GUARD AGAINST CHARCOT NEUROARTHROPATHY.
Be alert to this diagnosis in patients who present with a warm, red, painful midfoot. Patients with long-standing neuropathy may benefit from preventive bracing to limit joint movement and lower the risk of Charcot neuroarthropathy.
FIGURE
Rocker-bottom foot deformity
This radiograph reveals extensive collapse of the inner arch and a “rocker-bottom” foot deformity, the result of untreated Charcot neuroarthropathy.
CASE: MR. F.’S RISK BECOMES A REALITY
Mr. F. has a moderate risk of foot ulceration, based on evidence of neuropathy with 6 insensate sites (TABLE 1). You emphasize the importance of foot care, including appropriate footwear, and refer him to a podiatrist. You strongly support his decision to begin an exercise program to improve his glycemic control, decrease his cardiovascular mortality risk, and possibly help him lose weight.
Non-weight-bearing activity can be safely recommended to patients with diabetic neuropathy. So can daily weight-bearing activity, which actually decreases the risk of foot ulcer by maintaining leg muscle tone and plantar tissue tolerance to stress.12,13 Recent studies suggest that increasing weight-bearing activity slowly–walking daily and adding a total of 100 additional steps every 2 weeks, for example—in a carefully monitored program is not associated with increased risk of foot ulceration.14 You report these findings to Mr. F.
When he returns to your office in 2 months, Mr. F. has lost 7 pounds and his HbA1c has fallen to 6.5. Despite these positive developments, an examination of his feet reveals a full thickness ulcer on the left metatarsal head, which the patient had not noticed.
You recognize the clinical urgency of effectively treating Mr. F.’s diabetic foot ulcer, as size, duration, and grade are the greatest predictors of healing. Ulcers that are larger than 2 cm, have been present for more than 6 months,15 or have a higher grade on a scale such as the Wagner Foot Classification System16 (TABLE 2) are far less likely to heal than smaller, low-grade ulcers of shorter duration.
AIM DOC mnemonic guides ulcer care
Comprehensive, coordinated care improves outcomes for diabetic ulcers and has repeatedly been shown to reduce amputation rates.17-19 Large clinical trials have not evaluated each aspect of diabetic ulcer care, however, so the recommendations that follow are based on expert opinion and available evidence. These include assessing the limb’s arterial supply and ensuring that the patient undergoes revascularization, as needed; promptly treating infection; and providing optimal wound care, including debridement of callused and necrotic tissue, off-loading pressure, and applying moist wound dressings.20
AIM DOC, developed by 1 of the authors (JE), is a handy treatment tool. The mnemonic represents both the elements of treatment and the order in which they should be carried out. The letters stand for:
- Arterial disease
- Infection
- Measure
- Debride
- Off-load
- Cover
Here’s how to use AIM DOC, step by step:
Step 1: Assess for arterial disease
Start by assessing the vascular supply to the affected limb, which can be presumed to be adequate if pulses are palpable. If pulses cannot be palpated, the patient should undergo an ankle-brachial index (ABI) test and, if necessary, referred to a specialist to be evaluated for angioplasty or vascular bypass surgery. An ABI <0.9 is abnormal; 0.5 is considered the threshold for healing without such intervention.3 Keep in mind, however, that the ABI is falsely elevated in approximately 15% of patients with diabetes. If classic signs and symptoms of arterial disease are present, further evaluation is needed even if the ABI is normal.
Classic signs and symptoms of arterial disease include cool, hairless feet with shiny skin, and claudication. Location may provide another clue to etiology: Ulcers located on the heel, the outside of the foot, or between the toes tend to be associated with vascular disease, while ulcers with surrounding callus, such as the classic mal perforans ulcer on the metatarsal head, are neuropathic.
Step 2: Treat—or rule out—infection
While patients with diabetes have a 5-fold increase in risk of infection21 compared with individuals without the disease, there is no value in treating an uninfected ulcer with antibiotics. Diagnosing infection can be challenging because patients with diabetes may be less likely to demonstrate evidence of infection.21
An elevated white blood cell count, purulent drainage, foul odor, and/or erythema >2 cm around the wound clearly indicates a need for systemic antibiotics.22,23 Tissue necrosis, often assumed to represent ischemia, may result from neutrophilic vasculitis from soft tissue infection.24 Superficial cultures reflect colonization and should not be used to diagnose infection. In inspecting the wound for signs of infection, probe the ulcer and evaluate for osteomyelitis if it reaches bone.
Gram-positive cocci, especially Staphylococcus aureus, are the predominant pathogens in diabetic foot infections, and antibiotics effective against them may be sufficient for mild-to-moderate infections in patients who have not recently received antibiotic therapy. Gram-negative rods may be found in chronic wounds, however, and anaerobic pathogens may be present in patients with foot ischemia or gangrene; in both cases, broader-spectrum antimicrobials are required.22 Highly bioavailable oral antibiotics are indicated for infection, including some cases of osteomyelitis. Silver dressings may be helpful as a topical antimicrobial; there have been reports of successful treatment with honey, as well.25,26
Step 3: Measure (and grade) the wound
Accurate measurement of ulcer size is critical, both when you initially detect it and at each subsequent visit. To get an accurate measure, simply multiply the greatest length by the greatest width. The percent change in wound size after 4 weeks of treatment is a significant predictor of healing.25
Some clinicians also use photographs to track wound size, but these can be misleading. A better approach is to trace the wound on a sheet of acetate to document progress over time. If you see no improvement within 2 weeks, treatment should be modified.
Use a grading tool. There are a number of systems used for grading ulcers, none of which is universally accepted. One well-known tool is the Wagner Classification System16 (TABLE 2) referred to earlier. The higher the grade, the lower the likelihood that the ulcer will heal.
TABLE 2
Grading the ulcer: The Wagner system
| Grade | Description |
|---|---|
| 0 | No open lesions; may have deformity or cellulitis |
| 1 | Superficial diabetic ulcer (partial or full thickness) |
| 2 | Ulcer extension to ligament, tendon, joint capsule, or deep fascia (without abscess or osteomyelitis) |
| 3 | Deep ulcer with abscess, osteomyelitis, or joint sepsis |
| 4 | Gangrene localized to portion of forefoot or heel |
| 5 | Extensive gangrenous involvement of the entire foot |
| Adapted from: Wagner FW Jr. Orthopedics. 1987.16 | |
Step 4: Debride the ulcer
Frequent sharp debridement—to remove necrotic, callused, infected, and hypergranulation tissue—has long been considered essential in the treatment of neuropathic ulcers,17,21 especially for chronic or infected wounds. Debridement is thought to aid in healing by reducing pressure on the ulcer, decreasing bacterial contamination, enhancing platelet activation, releasing growth factors, and stimulating granulation tissue.
While some physicians are hesitant to perform debridement in the office, the process can actually be carried out without difficulty in an outpatient setting. Because of the neuropathy associated with most diabetic ulcers, no anesthesia is required. While the procedure is not typically painful, you will need to provide patient education to prepare the patient for the possibility of bleeding. Debride the wound to the outer edge of the hyperkeratotic tissue. If bleeding occurs, simply apply pressure until it stops.
When not to debride. Debridement is contraindicated under certain circumstances—if the limb has poor circulation, for example. Similarly, avoid debriding heel ulcers covered by eschar if there is no fluctuance in the underlying tissue, as the eschar provides a protective barrier.24 When sharp debridement is not possible, consider topical hydrogel or maggot therapy, an adjuvant treatment we’ll discuss in a bit.
Step 5: Off-load the wound
Mechanical load relief is vital for treating neuropathic ulcers, both to redistribute plantar pressures and protect granulation tissue. Total contact casting (TCC) is the gold standard, healing 90% of ulcers within 6 to 8 weeks.28 TCC is costly when applied weekly, however, and should only be done by a specialist, as an incorrectly applied cast can lead to the creation of new ulcers.
Because of the heaviness and inconvenience of the casts, many patients prefer removable devices, but these devices are much less effective. One study found that the average removable off-loading device is worn no more than 30% of the time that the patient is walking.29 Removable devices can be temporarily secured with plaster of Paris (a process that is sometimes referred to as instant contact casting) to ensure compliance.
Acceptable removable devices include a heel pressure relief shoe for heel ulcers and a CAM (controlled ankle motion) walker for metatarsal ulcers. Be sure off-loading devices are applied securely so no slippage can occur.
Step 6: Cover with moist dressings
The purpose of any topical dressing is to keep wounds moist, absorb exudate, and prevent contamination. A variety of moist dressings have been successfully used to treat ulcers, although evidence to recommend any particular dressing is insufficient.19 While wound vacuum-assisted closure (VAC) devices are widely used, there is little support for their use. A review of 7 trials comparing VAC devices with moistened gauze dressings or other topical agents found no evidence that topical negative pressure increases chronic wound healing.30
When foot ulcers do not heal
AIM DOC highlights the steps of diabetic ulcer care that are most likely to result in healing. When ulcers are slow to heal, review the 6 steps of treatment, paying particular attention to off-loading. If you establish that these have been appropriately applied and the wound is still not responding, consider alternative diagnoses such as venous insufficiency, vasculitis, or malignancy. Venous insufficiency ulcers may be difficult to differentiate from neuropathic ulcers, but they won’t heal without compression dressings. Diagnosis of vasculitis and malignancy can be made by biopsying the ulcer edge.
2 adjunctive therapies to consider
If there is no evidence to support an alternative diagnosis, consider adjunctive treatments, with hyperbaric oxygen therapy (HBOT) foremost among them.
A Cochrane review of HBOT found that it reduced the number of major amputations in diabetes patients with chronic foot ulcers, and improved healing at 1 year.31 Both Medicare and Medicaid cover HBOT for patients with diabetic ulcers classified as Wagner Grade 3 or higher that have not responded to 30 days of standard treatment.
Maggots, scientifically known as Lucilia sericata (Greenbottle) fly larvae, secrete proteolytic enzymes that debride necrotic tissue but are inactivated by living tissue. One meta-analysis found that neither surgical debridement nor larval therapy showed significant benefit over hydrogel.32 A subsequent small study did show statistically improved healing in ulcers debrided with maggots, compared with surgical debridement.33
CASE: MR. F.’S ULCER HEALS
Because Mr. F.’s ulcer is small and shallow and has been present for a short time, it has an excellent chance of healing if you follow the AIM DOC steps. You determine that he has adequate arterial supply and that the wound is uninfected (there is a strong dorsalis pedis pulse and no warmth, exudate, or erythema around the wound). Using a #15 blade, you pare away the callus surrounding the ulcer and document the length and width of the wound. You cover the ulcer with a moist dressing and instruct Mr. F to replace it twice a day, cautioning him against using alcohol or hydrogen peroxide, which could harm the healing skin. You discuss the importance of avoiding all weight bearing on the ulcerated foot, prescribe a CAM walker to wear at all times except while he’s sleeping, and schedule weekly follow-up visits to track progress.
In 2 weeks, the wound has resolved. You educate Mr. F. about his risk of ulcer recurrence and outline appropriate preventive steps. You also refer him for fitted extra-depth diabetic shoes and ongoing podiatry follow-up.
ACKNOWLEDGMENT
The authors wish to thank Gregory Mack, DPM, University of Wisconsin School of Medicine and Public Health, for his teaching and collaboration.
CORRESPONDENCE
Jennifer Eddy, MD, University of Wisconsin School of Medicine and Public Health, 617 W. Clairemont Avenue, Eau Claire, WI 54701; [email protected]
• Treat burning foot pain in patients with diabetes with tricyclic antidepressants or anticonvulsants. A
• Prescribe custom-fitted extra-depth shoes for patients with diabetes and neuropathy or foot deformity. B
• Consider hyperbaric oxygen therapy for ulcers that fail to respond to standard therapy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE: AN OBESE PATIENT WITH BURNING FOOT PAIN
Mr. F., an obese 47-year-old with hypertension and type 2 diabetes, is trying to lose weight, and comes in to discuss a new exercise program. He recently had a negative cardiac workup for chest pain, which was ultimately diagnosed as gastroesophageal reflux disease.
The patient, whose most recent glycosylated hemoglobin (HbA1c) is 7.5, reports painful burning in his feet at night. A foot exam reveals no ulcers, lesions, or calluses; 2+ dorsalis pedis pulses bilaterally; and loss of sensation to 10-g monofilament testing at 3 sites on the bottom of each foot.
Based on Mr. F.’s presentation, it seems safe from a cardiac standpoint for him to embark on an exercise program, but what about his risk for a foot ulcer? An accurate assessment would enable you to tailor your recommendations and take the appropriate steps to modify the patient’s risk now—and to minimize complications down the road.
The incidence of diabetic foot ulcers—like that of diabetes itself—has risen in recent years.1 More than 15% of the approximately 23.5 million US adults with diabetes are expected to develop a foot ulcer at some point in their lives.2 Improvements in wound care and increased use of revascularization techniques have led to a decline in the number of ulcer-related amputations.3 But for 7% of those who develop diabetic foot ulcers, amputation is still the end result.4
As a family physician, you can play a key role in guarding against that outcome. This review—and the risk classification, ulcer grading, and treatment mnemonic tools that are detailed in the pages that follow—will help you optimize the foot care you provide to patients with diabetes.
Simple classification system accurately predicts risk
The longer an individual lives with diabetes and the poorer the level of blood glucose control, the greater the risk of ulceration.1,5 Other major risk factors include neuropathy, peripheral arterial disease, and a previous foot ulcer or amputation. All patients with diabetes should undergo an annual risk assessment for foot ulcers, which can be easily incorporated into their yearly physical. Although the causes of ulceration are numerous and complex, classification of risk based on simple measures has been found to accurately predict risk.6
Risk stratification tool. Medical history and in-office assessment of pulses, sensation, and foot deformities form the basis for a handy assessment tool (TABLE 1). In a study of 3256 patients, these simple criteria successfully predicted ulcer risk. During the nearly 2 years of the study, fewer than 1% of those categorized as low risk (n=2253) developed ulcers, while more than 29% of the high-risk group (n=477) did.6
To assess a patient’s risk, visually examine both feet, inspecting the skin, nails, and structure. Autonomic neuropathy may cause decreased sweating, leading to dry, breakable skin, while motor neuropathy can cause deformities such as hammer or claw toes, which also increase ulcer risk. Palpate for dorsalis pedis and posterior tibialis pulses. Although posterior tibialis pulses may be congenitally absent, the absence of a dorsalis pedis pulse is associated with a 6-fold increased risk of foot ulcer.1
Compared with patients with diabetes who have never had a foot ulcer, patients with a history of foot ulcer have 13 times the risk.7 As already noted, glucose control is also a key risk factor. For every 2-point increase in HbA1c, the odds ratio for ulcer development increases by 1.6,5 which is likely the result of hyperglycemia’s contribution to microvascular disease and peripheral neuropathy.
TABLE 1
Diabetic foot ulcer: Risk stratification
| Risk level/criteria | Incidence of ulcer* |
|---|---|
| Low risk All of the following: No history of ulcer At least 1 pulse per foot ≤1 of 10 sites insensate to monofilament testing No foot deformity or physical or visual impairment | 0.36% |
| Moderate risk 1 or more of the following: Missing both pulses in either foot ≥2 insensate sites to monofilament testing Foot deformity Unable to see or reach foot | 2.3% |
| High risk 1 or more of the following: Prior ulcer or amputation Neuropathy and absent pulses Neuropathy or absent pulses and calluses or foot deformity | 29.4% |
| * Percentage of patients in each risk category who developed foot ulcers over the nearly 2-year study period. | |
| Source: Leese GP, et al. Int J Clin Pract. 2006.6 | |
Screening for neuropathy, ulceration’s most consistent risk factor
Forty percent of patients with diabetes develop distal peripheral neuropathy.8 Damage to sensory nerves often results in the burning foot pain described by Mr. F., which can significantly affect quality of life. It may also present simply as decreased sensation or vibration sense. While decreased sensation is not painful and may not be troubling to the patient, it substantially heightens the risk of foot injury.
To screen for neuropathy, place a 10-g monofilament on noncallused plantar surfaces of the distal hallux and metatarsal heads, with enough pressure to slightly bend the filament. Instructing patients to close their eyes and report any sensation is more effective than prompting them for a response.
Recent guidelines from the American Diabetes Association and American Association of Clinical Endocrinologists recommend 1 additional screening test for neuropathy,9 such as pinprick or vibration sense.
Patients with neuropathy or other risk factors require more frequent follow-up to check for early signs of ulceration. Take the opportunity to provide education about the importance of self-examination of the feet, among other preventive measures (See “Preventing ulcers in high-risk feet”). Be alert to evidence of Charcot neuroarthropathy (CN). This progressive and irreversible condition of bone and joint slippage, dislocation, or fracture can affect any part of the foot, although it is typically found in the midfoot.
Acute CN is a clinical emergency characterized by pain, warmth, and erythema—making it clinically indistinguishable from cellulitis, osteomyelitis, or gout. Blood tests, including a white blood cell count, sedimentary rate, and uric acid level, may be necessary for diagnosis, as well as radiographs or magnetic resonance imaging. Treatment is long-term (4-6 months) immobilization of the joint to prevent further destruction,10 and bisphosphonates for pain during the acute phase.11 Untreated CN typically results in a rocker-bottom foot deformity (FIGURE) that puts patients at greater risk for plantar ulceration.
Although it is possible to successfully treat the majority of diabetic foot ulcers, the wounds result in considerable morbidity, lower quality of life, and increased health care costs. A far better approach is to focus on prevention, with appropriate interventions and frequent follow-up for those at high risk.
ENSURE THAT THE PATIENT HAS THE RIGHT FOOTWEAR.
Properly fitting shoes with ample room for the toes is a priority for all patients with diabetes, but “high-risk feet” need therapeutic footwear, which Medicare covers as a yearly benefit. For those with a history of foot ulcer or amputation, custom insoles, rigid rocker shoes, and orthotics can help prevent re-ulceration.34 For socks, synthetic blends are preferable to cotton, which can chafe when wet with sweat.
PROVIDE EDUCATIONAL MATERIALS; EMPHASIZE FOOT INSPECTION.
All patients with diabetes should receive general education regarding foot care, as there is evidence that it improves behavior and may prevent injuries.7,35 Educational materials emphasizing the importance of nightly foot inspection, overall foot care, and physician inspection are crucial for patients at high risk for developing foot ulcers. Excellent patient education materials are available from the National Diabetes Education Program (See “Take Care of Your Feet for a Lifetime”).
STRESS FREQUENT FOLLOW-UP.
Patients should be informed of their risk level for diabetic foot ulcers after screening. Advise those at high risk to have their feet inspected by a podiatrist or other knowledgeable clinician every 1 to 2 months.9
ENCOURAGE EXERCISE.
Non-weight-bearing exercise programs, including swimming, and a consistent level of daily weight-bearing activity should be encouraged. Caution patients to increase weight-bearing exercise gradually, however, ideally in a closely supervised setting, and to do everything possible to avoid even minimal foot trauma.
REVIEW MEDICATIONS AND ADJUST THERAPY, AS NEEDED.
Tight glycemic control and the use of angiotensin-converting enzyme inhibitors may help prevent the development of neuropathy.36,37 For those who already have neuropathy, tricyclic antidepressants38 or anticonvulsants may bring pain relief.
GUARD AGAINST CHARCOT NEUROARTHROPATHY.
Be alert to this diagnosis in patients who present with a warm, red, painful midfoot. Patients with long-standing neuropathy may benefit from preventive bracing to limit joint movement and lower the risk of Charcot neuroarthropathy.
FIGURE
Rocker-bottom foot deformity
This radiograph reveals extensive collapse of the inner arch and a “rocker-bottom” foot deformity, the result of untreated Charcot neuroarthropathy.
CASE: MR. F.’S RISK BECOMES A REALITY
Mr. F. has a moderate risk of foot ulceration, based on evidence of neuropathy with 6 insensate sites (TABLE 1). You emphasize the importance of foot care, including appropriate footwear, and refer him to a podiatrist. You strongly support his decision to begin an exercise program to improve his glycemic control, decrease his cardiovascular mortality risk, and possibly help him lose weight.
Non-weight-bearing activity can be safely recommended to patients with diabetic neuropathy. So can daily weight-bearing activity, which actually decreases the risk of foot ulcer by maintaining leg muscle tone and plantar tissue tolerance to stress.12,13 Recent studies suggest that increasing weight-bearing activity slowly–walking daily and adding a total of 100 additional steps every 2 weeks, for example—in a carefully monitored program is not associated with increased risk of foot ulceration.14 You report these findings to Mr. F.
When he returns to your office in 2 months, Mr. F. has lost 7 pounds and his HbA1c has fallen to 6.5. Despite these positive developments, an examination of his feet reveals a full thickness ulcer on the left metatarsal head, which the patient had not noticed.
You recognize the clinical urgency of effectively treating Mr. F.’s diabetic foot ulcer, as size, duration, and grade are the greatest predictors of healing. Ulcers that are larger than 2 cm, have been present for more than 6 months,15 or have a higher grade on a scale such as the Wagner Foot Classification System16 (TABLE 2) are far less likely to heal than smaller, low-grade ulcers of shorter duration.
AIM DOC mnemonic guides ulcer care
Comprehensive, coordinated care improves outcomes for diabetic ulcers and has repeatedly been shown to reduce amputation rates.17-19 Large clinical trials have not evaluated each aspect of diabetic ulcer care, however, so the recommendations that follow are based on expert opinion and available evidence. These include assessing the limb’s arterial supply and ensuring that the patient undergoes revascularization, as needed; promptly treating infection; and providing optimal wound care, including debridement of callused and necrotic tissue, off-loading pressure, and applying moist wound dressings.20
AIM DOC, developed by 1 of the authors (JE), is a handy treatment tool. The mnemonic represents both the elements of treatment and the order in which they should be carried out. The letters stand for:
- Arterial disease
- Infection
- Measure
- Debride
- Off-load
- Cover
Here’s how to use AIM DOC, step by step:
Step 1: Assess for arterial disease
Start by assessing the vascular supply to the affected limb, which can be presumed to be adequate if pulses are palpable. If pulses cannot be palpated, the patient should undergo an ankle-brachial index (ABI) test and, if necessary, referred to a specialist to be evaluated for angioplasty or vascular bypass surgery. An ABI <0.9 is abnormal; 0.5 is considered the threshold for healing without such intervention.3 Keep in mind, however, that the ABI is falsely elevated in approximately 15% of patients with diabetes. If classic signs and symptoms of arterial disease are present, further evaluation is needed even if the ABI is normal.
Classic signs and symptoms of arterial disease include cool, hairless feet with shiny skin, and claudication. Location may provide another clue to etiology: Ulcers located on the heel, the outside of the foot, or between the toes tend to be associated with vascular disease, while ulcers with surrounding callus, such as the classic mal perforans ulcer on the metatarsal head, are neuropathic.
Step 2: Treat—or rule out—infection
While patients with diabetes have a 5-fold increase in risk of infection21 compared with individuals without the disease, there is no value in treating an uninfected ulcer with antibiotics. Diagnosing infection can be challenging because patients with diabetes may be less likely to demonstrate evidence of infection.21
An elevated white blood cell count, purulent drainage, foul odor, and/or erythema >2 cm around the wound clearly indicates a need for systemic antibiotics.22,23 Tissue necrosis, often assumed to represent ischemia, may result from neutrophilic vasculitis from soft tissue infection.24 Superficial cultures reflect colonization and should not be used to diagnose infection. In inspecting the wound for signs of infection, probe the ulcer and evaluate for osteomyelitis if it reaches bone.
Gram-positive cocci, especially Staphylococcus aureus, are the predominant pathogens in diabetic foot infections, and antibiotics effective against them may be sufficient for mild-to-moderate infections in patients who have not recently received antibiotic therapy. Gram-negative rods may be found in chronic wounds, however, and anaerobic pathogens may be present in patients with foot ischemia or gangrene; in both cases, broader-spectrum antimicrobials are required.22 Highly bioavailable oral antibiotics are indicated for infection, including some cases of osteomyelitis. Silver dressings may be helpful as a topical antimicrobial; there have been reports of successful treatment with honey, as well.25,26
Step 3: Measure (and grade) the wound
Accurate measurement of ulcer size is critical, both when you initially detect it and at each subsequent visit. To get an accurate measure, simply multiply the greatest length by the greatest width. The percent change in wound size after 4 weeks of treatment is a significant predictor of healing.25
Some clinicians also use photographs to track wound size, but these can be misleading. A better approach is to trace the wound on a sheet of acetate to document progress over time. If you see no improvement within 2 weeks, treatment should be modified.
Use a grading tool. There are a number of systems used for grading ulcers, none of which is universally accepted. One well-known tool is the Wagner Classification System16 (TABLE 2) referred to earlier. The higher the grade, the lower the likelihood that the ulcer will heal.
TABLE 2
Grading the ulcer: The Wagner system
| Grade | Description |
|---|---|
| 0 | No open lesions; may have deformity or cellulitis |
| 1 | Superficial diabetic ulcer (partial or full thickness) |
| 2 | Ulcer extension to ligament, tendon, joint capsule, or deep fascia (without abscess or osteomyelitis) |
| 3 | Deep ulcer with abscess, osteomyelitis, or joint sepsis |
| 4 | Gangrene localized to portion of forefoot or heel |
| 5 | Extensive gangrenous involvement of the entire foot |
| Adapted from: Wagner FW Jr. Orthopedics. 1987.16 | |
Step 4: Debride the ulcer
Frequent sharp debridement—to remove necrotic, callused, infected, and hypergranulation tissue—has long been considered essential in the treatment of neuropathic ulcers,17,21 especially for chronic or infected wounds. Debridement is thought to aid in healing by reducing pressure on the ulcer, decreasing bacterial contamination, enhancing platelet activation, releasing growth factors, and stimulating granulation tissue.
While some physicians are hesitant to perform debridement in the office, the process can actually be carried out without difficulty in an outpatient setting. Because of the neuropathy associated with most diabetic ulcers, no anesthesia is required. While the procedure is not typically painful, you will need to provide patient education to prepare the patient for the possibility of bleeding. Debride the wound to the outer edge of the hyperkeratotic tissue. If bleeding occurs, simply apply pressure until it stops.
When not to debride. Debridement is contraindicated under certain circumstances—if the limb has poor circulation, for example. Similarly, avoid debriding heel ulcers covered by eschar if there is no fluctuance in the underlying tissue, as the eschar provides a protective barrier.24 When sharp debridement is not possible, consider topical hydrogel or maggot therapy, an adjuvant treatment we’ll discuss in a bit.
Step 5: Off-load the wound
Mechanical load relief is vital for treating neuropathic ulcers, both to redistribute plantar pressures and protect granulation tissue. Total contact casting (TCC) is the gold standard, healing 90% of ulcers within 6 to 8 weeks.28 TCC is costly when applied weekly, however, and should only be done by a specialist, as an incorrectly applied cast can lead to the creation of new ulcers.
Because of the heaviness and inconvenience of the casts, many patients prefer removable devices, but these devices are much less effective. One study found that the average removable off-loading device is worn no more than 30% of the time that the patient is walking.29 Removable devices can be temporarily secured with plaster of Paris (a process that is sometimes referred to as instant contact casting) to ensure compliance.
Acceptable removable devices include a heel pressure relief shoe for heel ulcers and a CAM (controlled ankle motion) walker for metatarsal ulcers. Be sure off-loading devices are applied securely so no slippage can occur.
Step 6: Cover with moist dressings
The purpose of any topical dressing is to keep wounds moist, absorb exudate, and prevent contamination. A variety of moist dressings have been successfully used to treat ulcers, although evidence to recommend any particular dressing is insufficient.19 While wound vacuum-assisted closure (VAC) devices are widely used, there is little support for their use. A review of 7 trials comparing VAC devices with moistened gauze dressings or other topical agents found no evidence that topical negative pressure increases chronic wound healing.30
When foot ulcers do not heal
AIM DOC highlights the steps of diabetic ulcer care that are most likely to result in healing. When ulcers are slow to heal, review the 6 steps of treatment, paying particular attention to off-loading. If you establish that these have been appropriately applied and the wound is still not responding, consider alternative diagnoses such as venous insufficiency, vasculitis, or malignancy. Venous insufficiency ulcers may be difficult to differentiate from neuropathic ulcers, but they won’t heal without compression dressings. Diagnosis of vasculitis and malignancy can be made by biopsying the ulcer edge.
2 adjunctive therapies to consider
If there is no evidence to support an alternative diagnosis, consider adjunctive treatments, with hyperbaric oxygen therapy (HBOT) foremost among them.
A Cochrane review of HBOT found that it reduced the number of major amputations in diabetes patients with chronic foot ulcers, and improved healing at 1 year.31 Both Medicare and Medicaid cover HBOT for patients with diabetic ulcers classified as Wagner Grade 3 or higher that have not responded to 30 days of standard treatment.
Maggots, scientifically known as Lucilia sericata (Greenbottle) fly larvae, secrete proteolytic enzymes that debride necrotic tissue but are inactivated by living tissue. One meta-analysis found that neither surgical debridement nor larval therapy showed significant benefit over hydrogel.32 A subsequent small study did show statistically improved healing in ulcers debrided with maggots, compared with surgical debridement.33
CASE: MR. F.’S ULCER HEALS
Because Mr. F.’s ulcer is small and shallow and has been present for a short time, it has an excellent chance of healing if you follow the AIM DOC steps. You determine that he has adequate arterial supply and that the wound is uninfected (there is a strong dorsalis pedis pulse and no warmth, exudate, or erythema around the wound). Using a #15 blade, you pare away the callus surrounding the ulcer and document the length and width of the wound. You cover the ulcer with a moist dressing and instruct Mr. F to replace it twice a day, cautioning him against using alcohol or hydrogen peroxide, which could harm the healing skin. You discuss the importance of avoiding all weight bearing on the ulcerated foot, prescribe a CAM walker to wear at all times except while he’s sleeping, and schedule weekly follow-up visits to track progress.
In 2 weeks, the wound has resolved. You educate Mr. F. about his risk of ulcer recurrence and outline appropriate preventive steps. You also refer him for fitted extra-depth diabetic shoes and ongoing podiatry follow-up.
ACKNOWLEDGMENT
The authors wish to thank Gregory Mack, DPM, University of Wisconsin School of Medicine and Public Health, for his teaching and collaboration.
CORRESPONDENCE
Jennifer Eddy, MD, University of Wisconsin School of Medicine and Public Health, 617 W. Clairemont Avenue, Eau Claire, WI 54701; [email protected]
1. LeMaster JW, Reiber GE. Epidemiology and economic impact of foot ulcers. In: Boulton AJM, Cavanaugh PR, Rayman G, eds. The Foot in Diabetes. 4th ed. London, England: John Wiley & Sons Ltd; 2006:1–16.
2. Department of Health and Human Services Centers for Disease Control and Health Prevention. National Diabetes Fact Sheet 2007. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Accessed May 15, 2009.
3. Pinzur M. Amputations in the diabetic foot. In: Boulton AJM, Cavanaugh PR, Rayman G, eds. The Foot in Diabetes. 4th ed. London, England: John Wiley & Sons Ltd; 2006:308–322.
4. Margolis DJ, Allen-Taylor L, Hoffstad O, et al. Diabetic neuropathic foot ulcers and amputation. Wound Repair Regen. 2005;13:230-236.
5. Moss SE, Klein R, Kelin BE. The prevalence and incidence of lower extremity amputation in a diabetic population. Arch Intern Med. 1992;152:610-616.
6. Leese GP, Reid F, Green V, et al. Stratification of foot ulcer risk in patients with diabetes: a population based study. Int J Clin Pract. 2006;60:541-545.
7. Litzelman DR, Slemenda CW, Langefeld CD. Reduction of lower extremity clinical abnormalities in patient with non-insulin-dependent diabetes mellitus: a randomized controlled trial. Ann Intern Med. 1993;119:36-41.
8. Palumbo PJ, Melton LJ, III. Peripheral vascular disease and diabetes. In: National Diabetes Data Group, National Institute of Diabetes and Digestive and Kidney Disease, eds. Diabetes in America. 2nd ed. Bethesda, Md: National Institutes of Health, NIDDKD; 1995:401–408.
9. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31:1679-1685.
10. Jude EB. Charcot foot: what’s new in pathogensis and medical management? In: Boulton AJM, Cavanaugh PR, Rayman G, eds. The Foot in Diabetes. 4th ed. London, England: John Wiley & Sons Ltd; 2006: 265–273.
11. Pitocco D, Ruotolo V, Caputo S, et al. Six month treatment with alendronate in acute Charcot neuroarthropathy: a randomized controlled trial. Diabetes Care. 2005;28:1214-1215.
12. LeMaster JW, Reiber GE, Smith DG, et al. Daily weight-bearing activity does not increase the risk of diabetic foot ulcers. Med Sci Sports Exerc. 2003;35:1093-1099.
13. Lott DJ, Malug KS, Sinacore DR, et al. Relationship between changes in activity and plantar ulcer recurrence in a patient with diabetes mellitus. Phys Ther. 2005;85:579-588.
14. LeMaster JW, Mueller MJ, Reiber GE, et al. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: Feet First randomized controlled trial. Phys Ther. 2008;88:1-13.
15. Margolis DJ, Kantor J, Santanna J, et al. Risk factors for delayed healing of neuropathic foot ulcers: a pooled analysis. Arch Dermatol. 2000;136:1531-1535.
16. Wagner FW, Jr. The diabetic foot. Orthopedics. 1987;10:163-172.
17. Rith-Najarian S, Branchaid C, Beaulieu O, et al. Reducing lower-extremity amputations due to diabetes: application of the staged diabetes management approach in a primary care setting. J Fam Pract. 1998;47:127-132.
18. Canavan RJ, Unwin NC, Connolly VM, et al. Diabetes and non-diabetes related lower extremity amputation incidence before and after the introduction of better organized diabetic foot care. Diabetes Care. 2008;31.-
19. Krishnan St, Nash F, Baker NR, et al. Reduction in diabetes amputations over 11 years in a defined UK population. Diabetes Care. 2008;31:99-101.
20. Clayton W, Elasy TA. A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clin Diabetes. 2009;27:52-58.
21. Majno G, Joris I. Cells, Tissues, and Disease: Principles of General Pathology, 2nd ed. Oxford, England: Oxford University Press; 2004.
22. Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39:885-910.
23. Frykberg RG, Zgonis T, Armstrong DG, et al. Diabetic foot disorders: a clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(5 suppl):S2-S66.
24. Edmonds M, Foster A. The use of antibiotics in the diabetic foot. Am J Surg. 2004;187:S25-S28.
25. Eddy JJ, Gideonsen MD. Topical honey therapy for diabetic foot ulcers. J Fam Pract. 2005;54:533-535.
26. Remmen R, Coenen S, Seuntjens R, et al. Honey for refractory diabetic foot ulcers. J Fam Pract. 2005;54:863.-
27. Sheehan P, Jones P, Caselli A, et al. Percent change in wound area in diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26:1879-1882.
28. Lavery LA, Murdoch DP. Conventional offloading and activity monitoring. In: The Foot in Diabetes. 4th ed. Boulton AJM, Cavanaugh PR, Rayman G, eds. London, England: John Wiley & Sons Ltd; 2006: PG NUM.
29. Armstrong DG, Lavery LA, Kimbrel HR, et al. Activity patterns of patients with diabetic foot ulceration: patients with active ulceration may not adhere to a standard pressure off-loading regimen. Diabetes Care. 2003;26:2595-2597.
30. Ubbink DT, Westerbos SJ, Evans D, et al. H. Topical negative pressure for treating chronic wounds. Cochrane Database Syst Rev. 2008(3);CD001898.-
31. Kranke P, Bennett M, Roeckl-Wiedmann I, et al. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004(2);CD004123.-
32. Edwards J. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev. 2002(4);CD003556.-
33. Sherman RA. Maggot therapy for treating diabetic foot ulcers unresponsive to conventional therapy. Diabetes Care. 2003;26:446-451.
34. Spencer SA. Pressure relieving interventions for preventing and treating diabetic foot ulcers. Cochrane Database Syst Rev. 2000(3);CD002302.-
35. Valk GD, Kriegsman DM, Assendelft WJ. Patient education for preventing diabetic foot ulceration. Cochrane Database Syst Rev. 2001(4);CD001488.-(AU: 2005 update..?)
36. The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med. 1995;122:561-568.
37. Boulton AJM, Viniv AL, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956-962.
38. Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ. 2007;335:87.-
1. LeMaster JW, Reiber GE. Epidemiology and economic impact of foot ulcers. In: Boulton AJM, Cavanaugh PR, Rayman G, eds. The Foot in Diabetes. 4th ed. London, England: John Wiley & Sons Ltd; 2006:1–16.
2. Department of Health and Human Services Centers for Disease Control and Health Prevention. National Diabetes Fact Sheet 2007. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Accessed May 15, 2009.
3. Pinzur M. Amputations in the diabetic foot. In: Boulton AJM, Cavanaugh PR, Rayman G, eds. The Foot in Diabetes. 4th ed. London, England: John Wiley & Sons Ltd; 2006:308–322.
4. Margolis DJ, Allen-Taylor L, Hoffstad O, et al. Diabetic neuropathic foot ulcers and amputation. Wound Repair Regen. 2005;13:230-236.
5. Moss SE, Klein R, Kelin BE. The prevalence and incidence of lower extremity amputation in a diabetic population. Arch Intern Med. 1992;152:610-616.
6. Leese GP, Reid F, Green V, et al. Stratification of foot ulcer risk in patients with diabetes: a population based study. Int J Clin Pract. 2006;60:541-545.
7. Litzelman DR, Slemenda CW, Langefeld CD. Reduction of lower extremity clinical abnormalities in patient with non-insulin-dependent diabetes mellitus: a randomized controlled trial. Ann Intern Med. 1993;119:36-41.
8. Palumbo PJ, Melton LJ, III. Peripheral vascular disease and diabetes. In: National Diabetes Data Group, National Institute of Diabetes and Digestive and Kidney Disease, eds. Diabetes in America. 2nd ed. Bethesda, Md: National Institutes of Health, NIDDKD; 1995:401–408.
9. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31:1679-1685.
10. Jude EB. Charcot foot: what’s new in pathogensis and medical management? In: Boulton AJM, Cavanaugh PR, Rayman G, eds. The Foot in Diabetes. 4th ed. London, England: John Wiley & Sons Ltd; 2006: 265–273.
11. Pitocco D, Ruotolo V, Caputo S, et al. Six month treatment with alendronate in acute Charcot neuroarthropathy: a randomized controlled trial. Diabetes Care. 2005;28:1214-1215.
12. LeMaster JW, Reiber GE, Smith DG, et al. Daily weight-bearing activity does not increase the risk of diabetic foot ulcers. Med Sci Sports Exerc. 2003;35:1093-1099.
13. Lott DJ, Malug KS, Sinacore DR, et al. Relationship between changes in activity and plantar ulcer recurrence in a patient with diabetes mellitus. Phys Ther. 2005;85:579-588.
14. LeMaster JW, Mueller MJ, Reiber GE, et al. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: Feet First randomized controlled trial. Phys Ther. 2008;88:1-13.
15. Margolis DJ, Kantor J, Santanna J, et al. Risk factors for delayed healing of neuropathic foot ulcers: a pooled analysis. Arch Dermatol. 2000;136:1531-1535.
16. Wagner FW, Jr. The diabetic foot. Orthopedics. 1987;10:163-172.
17. Rith-Najarian S, Branchaid C, Beaulieu O, et al. Reducing lower-extremity amputations due to diabetes: application of the staged diabetes management approach in a primary care setting. J Fam Pract. 1998;47:127-132.
18. Canavan RJ, Unwin NC, Connolly VM, et al. Diabetes and non-diabetes related lower extremity amputation incidence before and after the introduction of better organized diabetic foot care. Diabetes Care. 2008;31.-
19. Krishnan St, Nash F, Baker NR, et al. Reduction in diabetes amputations over 11 years in a defined UK population. Diabetes Care. 2008;31:99-101.
20. Clayton W, Elasy TA. A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clin Diabetes. 2009;27:52-58.
21. Majno G, Joris I. Cells, Tissues, and Disease: Principles of General Pathology, 2nd ed. Oxford, England: Oxford University Press; 2004.
22. Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39:885-910.
23. Frykberg RG, Zgonis T, Armstrong DG, et al. Diabetic foot disorders: a clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(5 suppl):S2-S66.
24. Edmonds M, Foster A. The use of antibiotics in the diabetic foot. Am J Surg. 2004;187:S25-S28.
25. Eddy JJ, Gideonsen MD. Topical honey therapy for diabetic foot ulcers. J Fam Pract. 2005;54:533-535.
26. Remmen R, Coenen S, Seuntjens R, et al. Honey for refractory diabetic foot ulcers. J Fam Pract. 2005;54:863.-
27. Sheehan P, Jones P, Caselli A, et al. Percent change in wound area in diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26:1879-1882.
28. Lavery LA, Murdoch DP. Conventional offloading and activity monitoring. In: The Foot in Diabetes. 4th ed. Boulton AJM, Cavanaugh PR, Rayman G, eds. London, England: John Wiley & Sons Ltd; 2006: PG NUM.
29. Armstrong DG, Lavery LA, Kimbrel HR, et al. Activity patterns of patients with diabetic foot ulceration: patients with active ulceration may not adhere to a standard pressure off-loading regimen. Diabetes Care. 2003;26:2595-2597.
30. Ubbink DT, Westerbos SJ, Evans D, et al. H. Topical negative pressure for treating chronic wounds. Cochrane Database Syst Rev. 2008(3);CD001898.-
31. Kranke P, Bennett M, Roeckl-Wiedmann I, et al. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004(2);CD004123.-
32. Edwards J. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev. 2002(4);CD003556.-
33. Sherman RA. Maggot therapy for treating diabetic foot ulcers unresponsive to conventional therapy. Diabetes Care. 2003;26:446-451.
34. Spencer SA. Pressure relieving interventions for preventing and treating diabetic foot ulcers. Cochrane Database Syst Rev. 2000(3);CD002302.-
35. Valk GD, Kriegsman DM, Assendelft WJ. Patient education for preventing diabetic foot ulceration. Cochrane Database Syst Rev. 2001(4);CD001488.-(AU: 2005 update..?)
36. The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med. 1995;122:561-568.
37. Boulton AJM, Viniv AL, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956-962.
38. Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ. 2007;335:87.-