User login
Infantile hypertrophic pyloric stenosis (IHPS) is a common yet treatable condition in young infants, characterized by forceful vomiting after feeding as a result of hypertrophy of the pyloric muscle. Without proper diagnosis and surgical intervention, IHPS can eventually lead to dehydration, weight loss, and electrolyte disturbances, including the classic finding of hypochloremic alkalosis.1,2
IHPS occurs in two to four of every 1,000 births and is most common among white male infants.3,4 It develops between the first three to five weeks of life but rarely after age 12 weeks.1,2,5 IHPS is characterized by hypertrophy of the pyloric muscle (see figure), which eventually leads to gastric outlet obstruction.6,7
Because IHPS presents with symptoms that resemble those associated with other gastrointestinal disturbances, such as gastroesophageal reflux disease (GERD), it can be misdiagnosed in its early stages.1 Once it is identified, however, surgical correction by pyloromyotomy is considered curative, with a very low mortality rate (ie, 0.1%) and incidence recurrence in only 1% of patients. Typically, treated infants recover quickly and can begin feeding within hours after surgery.3,8
Although the etiology of IHPS remains unknown, providers can quickly recognize the signs and symptoms of IHPS in order for surgical treatment to be scheduled in a timely manner.
PATIENT PRESENTATION
Typically, an infant with IHPS will have a period of normal feeding for the first two to three weeks of life, followed by onset of nonbilious vomiting soon after feeding. Vomiting may become increasingly frequent and forceful—possibly described as “projectile.” Despite stomach distention, affected infants seem to have an insatiable appetite and may cry inconsolably. Depending on the duration of symptoms, patients may suffer significant weight loss, even falling below birth weight. In severe cases, a scaphoid abdomen and protruding ribcage may be present.1,2
Atypical Presentations
Premature infants with IHPS and those with certain medical or surgical conditions may present atypically. Vomiting may be less forceful, and the classic finding of visible gastric peristalsis may or may not be present.9 Researchers have documented cases in which hospitalized premature infants had nonprojectile vomiting, weight loss, and lethargy that were erroneously attributed to sepsis. IHPS should have been considered over sepsis when the infants’ clinical condition improved rapidly with rehydration, and when metabolic alkalosis (rather than acidosis) was identified.1,10,11
Projectile vomiting may not occur in infants with congenital anomalies that affect swallowing, such as cleft lip/palate or central nervous system disturbances. In infants who have recently undergone gastrointestinal (GI) surgery, IHPS may be misdiagnosed as adhesions or obstruction at an anastomotic site.1,10
RISK FACTORS
Despite the fact that IHPS is a relatively common condition, the etiology remains unknown. Research findings indicate that IHPS is not present at birth. Several hypotheses exist about potential risk factors, including genetics, use of macrolide antibiotics, and mechanical or environmental factors.6 IHPS has been associated with certain genetic conditions, including Cornelia de Lange syndrome and Smith-Lemli-Opitz syndrome, as well as chromosomal abnormalities, such as the translocation of chromosomes 8 and 17, and partial trisomy of chromosome 9.6,12
According to Chung,13 additional research has implicated the genetic loci IHPS1, also known as nitric oxide synthase 1 (NOS1), which encodes the gene for neuronal nitric oxide synthase (nNOS). This is the key enzyme for production of nitric oxide, which mediates relaxation of the pyloric smooth muscle.13
In a study by Mahon et al,12 an increased risk for IHPS was confirmed in young infants for whom systemic erythromycin had been prescribed as prophylactic treatment for pertussis. This may result from the agent’s motilin-like effects on antral smooth-muscle function.13
Mechanical defects, such as abnormal innervation of the pyloric muscle and neonatal hypergastrinemia and hyperacidity, have also been implicated.6 Infant sleeping position has been investigated as a possible environmental factor in the development of IHPS, correlating the decline in sudden infant death syndrome (attributed to decreased prone sleep position) with a decline in IHPS in recent years. However, no conclusive evidence has been shown to support this hypothesis.13
DIFFERENTIAL DIAGNOSIS
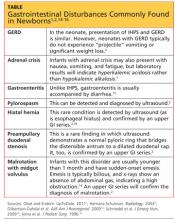
Typically, infants with nonbilious vomiting have either IHPS or GERD. Two factors to consider when evaluating the infant are its age and whether emesis is bilious or nonbilious. IHPS rarely causes bilious vomiting and most frequently occurs in infants between ages 3 and 6 weeks. Other GI disturbances that cause nonbilious emesis include adrenal crisis, gastroenteritis, pylorospasm, hiatal hernia, and preampullary duodenal stenosis.2 Malrotation or midgut volvulus may also be considered in the differential14 (see table1,2,14-16).
DIAGNOSIS
The diagnosis of IHPS is often made based on the history and physical exam. Imaging studies and labs can confirm the diagnosis, and surgery is usually deferred until dehydration has been addressed and any electrolyte disturbances corrected.1,17
Physical Examination
The infant may appear underweight and dehydrated, with visible peristaltic waves across the upper abdomen prior to emesis. Severe illness may be indicated in an underweight infant with the classic scaphoid abdomen.18
Palpation of an olive-shaped mass in the upper left quadrant of the epigastric region is pathognomonic for IHPS; the mass may be found more easily after emesis has occurred.19 To facilitate palpation, the hips can first be flexed to relax the abdominal wall. Next, the examiner should palpate gently for the “olive” in the space midway between the umbilicus and the xiphoid, between the two rectus muscles.3 All other findings in the physical exam should be within normal limits.
Imaging and Laboratory
Studies
An experienced provider can make a diagnosis of IHPS based on clinical examination alone in 60% to 80% of cases.20 However, most surgeons require the diagnosis to be confirmed with one of the following imaging studies before surgery.19
The diagnosis of IHPS can be confirmed by an upper GI series, but this is not commonly ordered as the primary diagnostic study. Ultrasound has become the modality of choice9; it can be used to quantify the size of an elongated, thickened pyloric muscle. The hypertrophied pylorus ranges in length from 14 to 16 mm, with thickness measuring more than 3.0 to 3.5 mm.1,19,21
An upper GI contrast study is rarely used for diagnosis of IHPS, but when this test is ordered to detect other gastrointestinal disease processes (eg, malrotation), IHPS may be identified incidentally.19 During an upper GI study, contrast material is propelled through the pyloric mucosa, and the string sign or the double-track sign may be visualized, revealing the mucosal filling defect.2
Abdominal x-ray may reveal a dilated stomach or a blockage, with a possible finding of gas in the gastric bubble but extending no further into the intestine.19
A study by Hernanz-Schulman2 was conducted to quantify the sensitivities and specificities associated with different diagnostic tools and the clinician’s experience and proficiency in using them. According to this study, palpation by a surgeon has a sensitivity of 31% to 99% and a specificity of 85% to 99% for detection of the pathognomonic olive-shaped mass. Ultrasound performed by an experienced technician has 97% to 100% sensitivity and 99% to 100% specificity for detecting IHPS. An upper GI series has 90% to 100% sensitivity and 99.5% specificity.
Venous blood gas and electrolyte levels are both helpful in making a diagnosis of IHPS.19,22 In a study by Oakley and Barnett,22 the following lab values were found useful in confirming the presence of pyloric stenosis: pH > 7.45; chloride 3 mEq/L. Sodium and potassium deficits may also be present.2,22
Before treatment is considered, the degree of dehydration must be determined by clinical examination and urinary output, as well as serum bicarbonate and chloride levels.3 Other laboratory tests to be ordered include urinalysis and a complete blood count (including platelets).
TREATMENT/MANAGEMENT
Initial management of vomiting in children should begin with fluid replacement if the patient appears dehydrated or if lab findings suggest an electrolyte imbalance. If surgery is later deemed necessary, this will reduce the risk for postoperative apnea.1,23
If IHPS is suspected, a pediatric surgery consult should be obtained. Before anesthesia is considered, serum bicarbonate should be measured with results no higher than 28 mEq/L, and the serum chloride level should be at least 100 mEq/L.3 Imaging, such as ultrasound or the upper GI series, may be ordered to confirm the diagnosis.
Surgical correction by a pyloromyotomy is curative. Presurgical gastric decompression via nasogastric tube placement will reduce the risk for postoperative vomiting and gastritis.20
Surgery
Although a variety of nonsurgical interventions, including oral and IV atropine and balloon dilation, have been described in the literature,3,24-26 the preferred treatment for IHPS is surgical intervention. Surgical correction has been so consistently successful (that is, provided the procedure is performed by a pediatric surgeon or other surgeon with appropriate experience3) that the treatment of choice for IHPS is the Ramstedt pyloromyotomy, which was first performed in 1912.6,27,28
Although the approach may differ based on the individual surgeon’s preference, the pyloric muscle is pulled through an incision in the abdominal wall. A longitudinal incision is made through the muscle with blunt dissection to the submucosa on the anterior surface of the pylorus. The pylorus is then returned to the abdominal cavity, and the abdominal incision is sutured.3,20
The laparoscopic approach, first described in the literature in the mid-1990s,29 is gaining popularity among surgeons, although recent studies have demonstrated that open and laparoscopic procedures are comparably safe and effective for the management of IHPS.30-33 Results from a recent study by Jia et al30 indicate that the laparoscopic approach results in reduced postoperative emesis, shorter length of hospital stay, and shorter recovery times; Hall et al31 emphasize the advantages of laparoscopic pyloromyotomy and recommend it over open surgery in facilities with experienced surgeons.
In follow-up ultrasound studies, hypertrophy of the pyloric muscle was reportedly resolved between two and 12 weeks after pyloromyotomy.34
Postoperative Care
The gastric tube can be removed and oral fluids reintroduced slowly at the surgeon’s discretion, between 8 and 12 hours after surgery. Postoperative vomiting is common (occurring in up to 80% of patients) but should resolve within 24 hours. Mild emesis should not delay the refeeding schedule.3
Patients should be evaluated by the surgeon one to two weeks following surgery unless the infant shows signs of infection (ie, fever, erythema, edema, bleeding, purulent drainage, excess pain, decreased fluid or nutritional intake). Follow-up imaging and laboratory studies are not indicated in an otherwise healthy infant.20
CONCLUSION
Early detection of IHPS, a condition characterized by hypertrophy of the pyloric muscle that results in gastric obstruction and projectile vomiting, can prevent complications of dehydration, malnutrition, and electrolyte disturbances. Surgery by open or laparoscopic pyloromyotomy is curative. Knowing the key physical exam findings, laboratory values, and typical patient history in IHPS enhances the clinician’s ability to make a timely diagnosis.
REFERENCES
1. Olivé AP, Endom EE. Infantile hypertrophic pyloric stenosis (2011). www.uptodate.com/contents/infan tile-hypertrophic-pyloric-stenosis. Accessed August 20, 2012.
2. Hernanz-Schulman M. Infantile hypertrophic pyloric stenosis. Radiology. 2003;227(2):319-331.
3. Aspelund G, Langer JC. Current management of hypertrophic pyloric stenosis. Semin Pediatr Surg. 2007;16(1):27-33.
4. To T, Wajja A, Wales PW, Langer JC. Population demographic indicators associated with incidence of pyloric stenosis. Arch Pediatr Adolesc Med. 2005;159(6):520-525.
5. MacMahon B. The continuing enigma of pyloric stenosis of infancy: a review. Epidemiology. 2006; 17(2):195-201.
6. Panteli C. New insights into the pathogenesis of infantile pyloric stenosis. Pediatr Surg Int. 2009;25 (12):1043-1052.
7. Stone CK, Humphries RL, eds. Current Medical Diagnosis and Treatment: Emergency Medicine. 7th ed. Chapter 50. Pediatric emergencies (2008). http://accessmedicine.com/resourceTOC.aspx?resource ID=718. Accessed August 20, 2012.
8. Hulka F, Harrison MW, Campbell TJ, Campbell JR. Complications of pyloromyotomy for infantile hypertrophic pyloric stenosis. Am J Surg. 1997;173 (5):450-452.
9. Shaoul R, Enav B, Steiner Z, et al. Clinical presentation of pyloric stenosis: the change is in our hands. Isr Med Assoc J. 2004;6(3):134-137.
10. Weinstein MM, Seibert JJ, Ehrenberg A. Six atypical presentations of congenital hypertrophic pyloric stenosis. Clin Pediatr. 1979;18(2):120-122.
11. Eyal O, Asia A, Yorgenson U, et al. Atypical infantile hypertrophic pyloric stenosis [in Hebrew]. Harefuah. 1999;136(2):113-114, 175.
12. Mahon BE, Rosenman MB, Kleiman MB. Maternal and infant use of erythromycin and other macrolide antibiotics as risk factors for infantile hypertrophic pyloric stenosis. J Pediatr. 2001;139(3):380-384.
13. Chung E. Infantile hypertrophic pyloric stenosis: genes and environment. Arch Dis Child. 2008;93 (12):1003-1004.
14. Gilbertson-Dahdal DL, Dutta S, Varich LJ, Barth RA. Neonatal malrotation with midgut volvulus mimicking duodenal atresia. AJR Am J Roentgenol. 2009;192(5):1269-1271.
15. Schmedel W, Ashe L, Kuznicki K. A 24-day-old child with projectile vomiting. J Emerg Nurs. 2009; 35(2):163-164.
16. Iijima T, Okamatsu T, Matsumura M, Yatsuzuka M. Hypertrophic pyloric stenosis associated with hiatal hernia. J Pediatr Surg. 1996;31(2):277-279.
17. Nakayama DK, Taylor LA. Hypertrophic pyloric stenosis in infancy: an analysis of treatment outcome. N C Med J. 1998;59(5):310-313.
18. Irish MS, Pearl RH, Caty M, Glick PL. The approach to common abdominal diagnoses in infants and children. Pediatr Clin North Am. 1998; 45(4):729-772.
19. Askew N. An overview of infantile hypertrophic pyloric stenosis: literature review. Paediatric Nurs. 2010;22(8):27-30.
20. Rudolph CD. Infantile hypertrophic pyloric stenosis. In: Rudolph CD, Rudolph AM, eds. Rudolph’s Pediatrics. 21st ed. New York, NY: McGraw-Hill; 2002:1402-1403.
21. Hallam D, Hansen B, Bødker B, et al. Pyloric size in normal infants and in infants suspected of having hypertrophic pyloric stenosis. Acta Radiol. 2005;36(3):261.
22. Oakley EA, Barnett PL. Is acid base determination an accurate predictor of pyloric stenosis? J Paediatr Child Health. 2000;36(6):587-589.
23. Steven IM, Allen TH, Sweeney DB. Congenital hypertrophic pyloric stenosis: the anaesthetist’s view. Anaesth Intensive Care. 1973;1(6):544-546.
24. Singh UK, Kumar R, Prasad R. Oral atropine sulfate for infantile hypertrophic pyloric stenosis. Indian Pediatr. 2005;42(5):473-476.
25. Nagita A, Yamaguchi J, Amemoto K, et al. Management and ultrasonographic appearance of infantile hypertrophic pyloric stenosis with intravenous atropine sulfate. J Pediatr Gastroenterol Nutr. 1996;23(2):172-177.
26. Ogawa Y, Higashimoto U, Nishijima E, et al. Successful endoscopic balloon dilatation for hypertrophic pyloric stenosis. J Pediatr Surg. 1996;31(12):1712-1714.
27. Langer JC, To T. Does pediatric surgical specialty training affect outcome after Ramstedt pyloromyotomy? A population-based study. Pediatrics. 2004;113(5):1342-1347.
28. Pollock WF, Norris WJ. Dr. Conrad Ramstedt and pyloromyotomy. Surgery. 1957;42(5):966-970.
29. Najmaldin A, Tan HL. Early experience with laparoscopic pyloromyotomy for infantile hypertrophic pyloric stenosis. J Pediatr Surg. 1995;30(1):37-38.
30. Jia WQ, Tian JH, Yang KH, et al. Open versus laparoscopic pyloromyotomy for pyloric stenosis: a meta-analysis of randomized controlled trials. Eur J Pediatr Surg. 2011;21(2):77-81.
31. Hall NJ, Pacilli M, Eaton S, et al. Recovery after open versus laparoscopic pyloromyotomy for pyloric stenosis: a double-blind multicentre randomised controlled trial. Lancet. 2009;373(9661):390-398.
32. Adibe OO, Nichol PF, Flake AAW, Mattei P. Comparison of outcomes after laparoscopic and open pyloromyotomy at a high-volume pediatric teaching hospital. J Pediatr Surg. 2006;41(10):1676-1678.
33. St. Peter SD, Holcomb GW, Calkins CM, et al. Open versus laparoscopic pyloromyotomy for pyloric stenosis: a prospective, randomized trial. Ann Surg. 2006;244(3):363-370.
34. Okorie NM, Dickson JA, Carver RA, Steiner GM. What happens to the pylorus after pyloromyotomy? Arch Dis Child. 1988;63(11):1339-1341.
Infantile hypertrophic pyloric stenosis (IHPS) is a common yet treatable condition in young infants, characterized by forceful vomiting after feeding as a result of hypertrophy of the pyloric muscle. Without proper diagnosis and surgical intervention, IHPS can eventually lead to dehydration, weight loss, and electrolyte disturbances, including the classic finding of hypochloremic alkalosis.1,2
IHPS occurs in two to four of every 1,000 births and is most common among white male infants.3,4 It develops between the first three to five weeks of life but rarely after age 12 weeks.1,2,5 IHPS is characterized by hypertrophy of the pyloric muscle (see figure), which eventually leads to gastric outlet obstruction.6,7
Because IHPS presents with symptoms that resemble those associated with other gastrointestinal disturbances, such as gastroesophageal reflux disease (GERD), it can be misdiagnosed in its early stages.1 Once it is identified, however, surgical correction by pyloromyotomy is considered curative, with a very low mortality rate (ie, 0.1%) and incidence recurrence in only 1% of patients. Typically, treated infants recover quickly and can begin feeding within hours after surgery.3,8
Although the etiology of IHPS remains unknown, providers can quickly recognize the signs and symptoms of IHPS in order for surgical treatment to be scheduled in a timely manner.
PATIENT PRESENTATION
Typically, an infant with IHPS will have a period of normal feeding for the first two to three weeks of life, followed by onset of nonbilious vomiting soon after feeding. Vomiting may become increasingly frequent and forceful—possibly described as “projectile.” Despite stomach distention, affected infants seem to have an insatiable appetite and may cry inconsolably. Depending on the duration of symptoms, patients may suffer significant weight loss, even falling below birth weight. In severe cases, a scaphoid abdomen and protruding ribcage may be present.1,2
Atypical Presentations
Premature infants with IHPS and those with certain medical or surgical conditions may present atypically. Vomiting may be less forceful, and the classic finding of visible gastric peristalsis may or may not be present.9 Researchers have documented cases in which hospitalized premature infants had nonprojectile vomiting, weight loss, and lethargy that were erroneously attributed to sepsis. IHPS should have been considered over sepsis when the infants’ clinical condition improved rapidly with rehydration, and when metabolic alkalosis (rather than acidosis) was identified.1,10,11
Projectile vomiting may not occur in infants with congenital anomalies that affect swallowing, such as cleft lip/palate or central nervous system disturbances. In infants who have recently undergone gastrointestinal (GI) surgery, IHPS may be misdiagnosed as adhesions or obstruction at an anastomotic site.1,10
RISK FACTORS
Despite the fact that IHPS is a relatively common condition, the etiology remains unknown. Research findings indicate that IHPS is not present at birth. Several hypotheses exist about potential risk factors, including genetics, use of macrolide antibiotics, and mechanical or environmental factors.6 IHPS has been associated with certain genetic conditions, including Cornelia de Lange syndrome and Smith-Lemli-Opitz syndrome, as well as chromosomal abnormalities, such as the translocation of chromosomes 8 and 17, and partial trisomy of chromosome 9.6,12
According to Chung,13 additional research has implicated the genetic loci IHPS1, also known as nitric oxide synthase 1 (NOS1), which encodes the gene for neuronal nitric oxide synthase (nNOS). This is the key enzyme for production of nitric oxide, which mediates relaxation of the pyloric smooth muscle.13
In a study by Mahon et al,12 an increased risk for IHPS was confirmed in young infants for whom systemic erythromycin had been prescribed as prophylactic treatment for pertussis. This may result from the agent’s motilin-like effects on antral smooth-muscle function.13
Mechanical defects, such as abnormal innervation of the pyloric muscle and neonatal hypergastrinemia and hyperacidity, have also been implicated.6 Infant sleeping position has been investigated as a possible environmental factor in the development of IHPS, correlating the decline in sudden infant death syndrome (attributed to decreased prone sleep position) with a decline in IHPS in recent years. However, no conclusive evidence has been shown to support this hypothesis.13
DIFFERENTIAL DIAGNOSIS
Typically, infants with nonbilious vomiting have either IHPS or GERD. Two factors to consider when evaluating the infant are its age and whether emesis is bilious or nonbilious. IHPS rarely causes bilious vomiting and most frequently occurs in infants between ages 3 and 6 weeks. Other GI disturbances that cause nonbilious emesis include adrenal crisis, gastroenteritis, pylorospasm, hiatal hernia, and preampullary duodenal stenosis.2 Malrotation or midgut volvulus may also be considered in the differential14 (see table1,2,14-16).
DIAGNOSIS
The diagnosis of IHPS is often made based on the history and physical exam. Imaging studies and labs can confirm the diagnosis, and surgery is usually deferred until dehydration has been addressed and any electrolyte disturbances corrected.1,17
Physical Examination
The infant may appear underweight and dehydrated, with visible peristaltic waves across the upper abdomen prior to emesis. Severe illness may be indicated in an underweight infant with the classic scaphoid abdomen.18
Palpation of an olive-shaped mass in the upper left quadrant of the epigastric region is pathognomonic for IHPS; the mass may be found more easily after emesis has occurred.19 To facilitate palpation, the hips can first be flexed to relax the abdominal wall. Next, the examiner should palpate gently for the “olive” in the space midway between the umbilicus and the xiphoid, between the two rectus muscles.3 All other findings in the physical exam should be within normal limits.
Imaging and Laboratory
Studies
An experienced provider can make a diagnosis of IHPS based on clinical examination alone in 60% to 80% of cases.20 However, most surgeons require the diagnosis to be confirmed with one of the following imaging studies before surgery.19
The diagnosis of IHPS can be confirmed by an upper GI series, but this is not commonly ordered as the primary diagnostic study. Ultrasound has become the modality of choice9; it can be used to quantify the size of an elongated, thickened pyloric muscle. The hypertrophied pylorus ranges in length from 14 to 16 mm, with thickness measuring more than 3.0 to 3.5 mm.1,19,21
An upper GI contrast study is rarely used for diagnosis of IHPS, but when this test is ordered to detect other gastrointestinal disease processes (eg, malrotation), IHPS may be identified incidentally.19 During an upper GI study, contrast material is propelled through the pyloric mucosa, and the string sign or the double-track sign may be visualized, revealing the mucosal filling defect.2
Abdominal x-ray may reveal a dilated stomach or a blockage, with a possible finding of gas in the gastric bubble but extending no further into the intestine.19
A study by Hernanz-Schulman2 was conducted to quantify the sensitivities and specificities associated with different diagnostic tools and the clinician’s experience and proficiency in using them. According to this study, palpation by a surgeon has a sensitivity of 31% to 99% and a specificity of 85% to 99% for detection of the pathognomonic olive-shaped mass. Ultrasound performed by an experienced technician has 97% to 100% sensitivity and 99% to 100% specificity for detecting IHPS. An upper GI series has 90% to 100% sensitivity and 99.5% specificity.
Venous blood gas and electrolyte levels are both helpful in making a diagnosis of IHPS.19,22 In a study by Oakley and Barnett,22 the following lab values were found useful in confirming the presence of pyloric stenosis: pH > 7.45; chloride 3 mEq/L. Sodium and potassium deficits may also be present.2,22
Before treatment is considered, the degree of dehydration must be determined by clinical examination and urinary output, as well as serum bicarbonate and chloride levels.3 Other laboratory tests to be ordered include urinalysis and a complete blood count (including platelets).
TREATMENT/MANAGEMENT
Initial management of vomiting in children should begin with fluid replacement if the patient appears dehydrated or if lab findings suggest an electrolyte imbalance. If surgery is later deemed necessary, this will reduce the risk for postoperative apnea.1,23
If IHPS is suspected, a pediatric surgery consult should be obtained. Before anesthesia is considered, serum bicarbonate should be measured with results no higher than 28 mEq/L, and the serum chloride level should be at least 100 mEq/L.3 Imaging, such as ultrasound or the upper GI series, may be ordered to confirm the diagnosis.
Surgical correction by a pyloromyotomy is curative. Presurgical gastric decompression via nasogastric tube placement will reduce the risk for postoperative vomiting and gastritis.20
Surgery
Although a variety of nonsurgical interventions, including oral and IV atropine and balloon dilation, have been described in the literature,3,24-26 the preferred treatment for IHPS is surgical intervention. Surgical correction has been so consistently successful (that is, provided the procedure is performed by a pediatric surgeon or other surgeon with appropriate experience3) that the treatment of choice for IHPS is the Ramstedt pyloromyotomy, which was first performed in 1912.6,27,28
Although the approach may differ based on the individual surgeon’s preference, the pyloric muscle is pulled through an incision in the abdominal wall. A longitudinal incision is made through the muscle with blunt dissection to the submucosa on the anterior surface of the pylorus. The pylorus is then returned to the abdominal cavity, and the abdominal incision is sutured.3,20
The laparoscopic approach, first described in the literature in the mid-1990s,29 is gaining popularity among surgeons, although recent studies have demonstrated that open and laparoscopic procedures are comparably safe and effective for the management of IHPS.30-33 Results from a recent study by Jia et al30 indicate that the laparoscopic approach results in reduced postoperative emesis, shorter length of hospital stay, and shorter recovery times; Hall et al31 emphasize the advantages of laparoscopic pyloromyotomy and recommend it over open surgery in facilities with experienced surgeons.
In follow-up ultrasound studies, hypertrophy of the pyloric muscle was reportedly resolved between two and 12 weeks after pyloromyotomy.34
Postoperative Care
The gastric tube can be removed and oral fluids reintroduced slowly at the surgeon’s discretion, between 8 and 12 hours after surgery. Postoperative vomiting is common (occurring in up to 80% of patients) but should resolve within 24 hours. Mild emesis should not delay the refeeding schedule.3
Patients should be evaluated by the surgeon one to two weeks following surgery unless the infant shows signs of infection (ie, fever, erythema, edema, bleeding, purulent drainage, excess pain, decreased fluid or nutritional intake). Follow-up imaging and laboratory studies are not indicated in an otherwise healthy infant.20
CONCLUSION
Early detection of IHPS, a condition characterized by hypertrophy of the pyloric muscle that results in gastric obstruction and projectile vomiting, can prevent complications of dehydration, malnutrition, and electrolyte disturbances. Surgery by open or laparoscopic pyloromyotomy is curative. Knowing the key physical exam findings, laboratory values, and typical patient history in IHPS enhances the clinician’s ability to make a timely diagnosis.
REFERENCES
1. Olivé AP, Endom EE. Infantile hypertrophic pyloric stenosis (2011). www.uptodate.com/contents/infan tile-hypertrophic-pyloric-stenosis. Accessed August 20, 2012.
2. Hernanz-Schulman M. Infantile hypertrophic pyloric stenosis. Radiology. 2003;227(2):319-331.
3. Aspelund G, Langer JC. Current management of hypertrophic pyloric stenosis. Semin Pediatr Surg. 2007;16(1):27-33.
4. To T, Wajja A, Wales PW, Langer JC. Population demographic indicators associated with incidence of pyloric stenosis. Arch Pediatr Adolesc Med. 2005;159(6):520-525.
5. MacMahon B. The continuing enigma of pyloric stenosis of infancy: a review. Epidemiology. 2006; 17(2):195-201.
6. Panteli C. New insights into the pathogenesis of infantile pyloric stenosis. Pediatr Surg Int. 2009;25 (12):1043-1052.
7. Stone CK, Humphries RL, eds. Current Medical Diagnosis and Treatment: Emergency Medicine. 7th ed. Chapter 50. Pediatric emergencies (2008). http://accessmedicine.com/resourceTOC.aspx?resource ID=718. Accessed August 20, 2012.
8. Hulka F, Harrison MW, Campbell TJ, Campbell JR. Complications of pyloromyotomy for infantile hypertrophic pyloric stenosis. Am J Surg. 1997;173 (5):450-452.
9. Shaoul R, Enav B, Steiner Z, et al. Clinical presentation of pyloric stenosis: the change is in our hands. Isr Med Assoc J. 2004;6(3):134-137.
10. Weinstein MM, Seibert JJ, Ehrenberg A. Six atypical presentations of congenital hypertrophic pyloric stenosis. Clin Pediatr. 1979;18(2):120-122.
11. Eyal O, Asia A, Yorgenson U, et al. Atypical infantile hypertrophic pyloric stenosis [in Hebrew]. Harefuah. 1999;136(2):113-114, 175.
12. Mahon BE, Rosenman MB, Kleiman MB. Maternal and infant use of erythromycin and other macrolide antibiotics as risk factors for infantile hypertrophic pyloric stenosis. J Pediatr. 2001;139(3):380-384.
13. Chung E. Infantile hypertrophic pyloric stenosis: genes and environment. Arch Dis Child. 2008;93 (12):1003-1004.
14. Gilbertson-Dahdal DL, Dutta S, Varich LJ, Barth RA. Neonatal malrotation with midgut volvulus mimicking duodenal atresia. AJR Am J Roentgenol. 2009;192(5):1269-1271.
15. Schmedel W, Ashe L, Kuznicki K. A 24-day-old child with projectile vomiting. J Emerg Nurs. 2009; 35(2):163-164.
16. Iijima T, Okamatsu T, Matsumura M, Yatsuzuka M. Hypertrophic pyloric stenosis associated with hiatal hernia. J Pediatr Surg. 1996;31(2):277-279.
17. Nakayama DK, Taylor LA. Hypertrophic pyloric stenosis in infancy: an analysis of treatment outcome. N C Med J. 1998;59(5):310-313.
18. Irish MS, Pearl RH, Caty M, Glick PL. The approach to common abdominal diagnoses in infants and children. Pediatr Clin North Am. 1998; 45(4):729-772.
19. Askew N. An overview of infantile hypertrophic pyloric stenosis: literature review. Paediatric Nurs. 2010;22(8):27-30.
20. Rudolph CD. Infantile hypertrophic pyloric stenosis. In: Rudolph CD, Rudolph AM, eds. Rudolph’s Pediatrics. 21st ed. New York, NY: McGraw-Hill; 2002:1402-1403.
21. Hallam D, Hansen B, Bødker B, et al. Pyloric size in normal infants and in infants suspected of having hypertrophic pyloric stenosis. Acta Radiol. 2005;36(3):261.
22. Oakley EA, Barnett PL. Is acid base determination an accurate predictor of pyloric stenosis? J Paediatr Child Health. 2000;36(6):587-589.
23. Steven IM, Allen TH, Sweeney DB. Congenital hypertrophic pyloric stenosis: the anaesthetist’s view. Anaesth Intensive Care. 1973;1(6):544-546.
24. Singh UK, Kumar R, Prasad R. Oral atropine sulfate for infantile hypertrophic pyloric stenosis. Indian Pediatr. 2005;42(5):473-476.
25. Nagita A, Yamaguchi J, Amemoto K, et al. Management and ultrasonographic appearance of infantile hypertrophic pyloric stenosis with intravenous atropine sulfate. J Pediatr Gastroenterol Nutr. 1996;23(2):172-177.
26. Ogawa Y, Higashimoto U, Nishijima E, et al. Successful endoscopic balloon dilatation for hypertrophic pyloric stenosis. J Pediatr Surg. 1996;31(12):1712-1714.
27. Langer JC, To T. Does pediatric surgical specialty training affect outcome after Ramstedt pyloromyotomy? A population-based study. Pediatrics. 2004;113(5):1342-1347.
28. Pollock WF, Norris WJ. Dr. Conrad Ramstedt and pyloromyotomy. Surgery. 1957;42(5):966-970.
29. Najmaldin A, Tan HL. Early experience with laparoscopic pyloromyotomy for infantile hypertrophic pyloric stenosis. J Pediatr Surg. 1995;30(1):37-38.
30. Jia WQ, Tian JH, Yang KH, et al. Open versus laparoscopic pyloromyotomy for pyloric stenosis: a meta-analysis of randomized controlled trials. Eur J Pediatr Surg. 2011;21(2):77-81.
31. Hall NJ, Pacilli M, Eaton S, et al. Recovery after open versus laparoscopic pyloromyotomy for pyloric stenosis: a double-blind multicentre randomised controlled trial. Lancet. 2009;373(9661):390-398.
32. Adibe OO, Nichol PF, Flake AAW, Mattei P. Comparison of outcomes after laparoscopic and open pyloromyotomy at a high-volume pediatric teaching hospital. J Pediatr Surg. 2006;41(10):1676-1678.
33. St. Peter SD, Holcomb GW, Calkins CM, et al. Open versus laparoscopic pyloromyotomy for pyloric stenosis: a prospective, randomized trial. Ann Surg. 2006;244(3):363-370.
34. Okorie NM, Dickson JA, Carver RA, Steiner GM. What happens to the pylorus after pyloromyotomy? Arch Dis Child. 1988;63(11):1339-1341.
Infantile hypertrophic pyloric stenosis (IHPS) is a common yet treatable condition in young infants, characterized by forceful vomiting after feeding as a result of hypertrophy of the pyloric muscle. Without proper diagnosis and surgical intervention, IHPS can eventually lead to dehydration, weight loss, and electrolyte disturbances, including the classic finding of hypochloremic alkalosis.1,2
IHPS occurs in two to four of every 1,000 births and is most common among white male infants.3,4 It develops between the first three to five weeks of life but rarely after age 12 weeks.1,2,5 IHPS is characterized by hypertrophy of the pyloric muscle (see figure), which eventually leads to gastric outlet obstruction.6,7
Because IHPS presents with symptoms that resemble those associated with other gastrointestinal disturbances, such as gastroesophageal reflux disease (GERD), it can be misdiagnosed in its early stages.1 Once it is identified, however, surgical correction by pyloromyotomy is considered curative, with a very low mortality rate (ie, 0.1%) and incidence recurrence in only 1% of patients. Typically, treated infants recover quickly and can begin feeding within hours after surgery.3,8
Although the etiology of IHPS remains unknown, providers can quickly recognize the signs and symptoms of IHPS in order for surgical treatment to be scheduled in a timely manner.
PATIENT PRESENTATION
Typically, an infant with IHPS will have a period of normal feeding for the first two to three weeks of life, followed by onset of nonbilious vomiting soon after feeding. Vomiting may become increasingly frequent and forceful—possibly described as “projectile.” Despite stomach distention, affected infants seem to have an insatiable appetite and may cry inconsolably. Depending on the duration of symptoms, patients may suffer significant weight loss, even falling below birth weight. In severe cases, a scaphoid abdomen and protruding ribcage may be present.1,2
Atypical Presentations
Premature infants with IHPS and those with certain medical or surgical conditions may present atypically. Vomiting may be less forceful, and the classic finding of visible gastric peristalsis may or may not be present.9 Researchers have documented cases in which hospitalized premature infants had nonprojectile vomiting, weight loss, and lethargy that were erroneously attributed to sepsis. IHPS should have been considered over sepsis when the infants’ clinical condition improved rapidly with rehydration, and when metabolic alkalosis (rather than acidosis) was identified.1,10,11
Projectile vomiting may not occur in infants with congenital anomalies that affect swallowing, such as cleft lip/palate or central nervous system disturbances. In infants who have recently undergone gastrointestinal (GI) surgery, IHPS may be misdiagnosed as adhesions or obstruction at an anastomotic site.1,10
RISK FACTORS
Despite the fact that IHPS is a relatively common condition, the etiology remains unknown. Research findings indicate that IHPS is not present at birth. Several hypotheses exist about potential risk factors, including genetics, use of macrolide antibiotics, and mechanical or environmental factors.6 IHPS has been associated with certain genetic conditions, including Cornelia de Lange syndrome and Smith-Lemli-Opitz syndrome, as well as chromosomal abnormalities, such as the translocation of chromosomes 8 and 17, and partial trisomy of chromosome 9.6,12
According to Chung,13 additional research has implicated the genetic loci IHPS1, also known as nitric oxide synthase 1 (NOS1), which encodes the gene for neuronal nitric oxide synthase (nNOS). This is the key enzyme for production of nitric oxide, which mediates relaxation of the pyloric smooth muscle.13
In a study by Mahon et al,12 an increased risk for IHPS was confirmed in young infants for whom systemic erythromycin had been prescribed as prophylactic treatment for pertussis. This may result from the agent’s motilin-like effects on antral smooth-muscle function.13
Mechanical defects, such as abnormal innervation of the pyloric muscle and neonatal hypergastrinemia and hyperacidity, have also been implicated.6 Infant sleeping position has been investigated as a possible environmental factor in the development of IHPS, correlating the decline in sudden infant death syndrome (attributed to decreased prone sleep position) with a decline in IHPS in recent years. However, no conclusive evidence has been shown to support this hypothesis.13
DIFFERENTIAL DIAGNOSIS
Typically, infants with nonbilious vomiting have either IHPS or GERD. Two factors to consider when evaluating the infant are its age and whether emesis is bilious or nonbilious. IHPS rarely causes bilious vomiting and most frequently occurs in infants between ages 3 and 6 weeks. Other GI disturbances that cause nonbilious emesis include adrenal crisis, gastroenteritis, pylorospasm, hiatal hernia, and preampullary duodenal stenosis.2 Malrotation or midgut volvulus may also be considered in the differential14 (see table1,2,14-16).
DIAGNOSIS
The diagnosis of IHPS is often made based on the history and physical exam. Imaging studies and labs can confirm the diagnosis, and surgery is usually deferred until dehydration has been addressed and any electrolyte disturbances corrected.1,17
Physical Examination
The infant may appear underweight and dehydrated, with visible peristaltic waves across the upper abdomen prior to emesis. Severe illness may be indicated in an underweight infant with the classic scaphoid abdomen.18
Palpation of an olive-shaped mass in the upper left quadrant of the epigastric region is pathognomonic for IHPS; the mass may be found more easily after emesis has occurred.19 To facilitate palpation, the hips can first be flexed to relax the abdominal wall. Next, the examiner should palpate gently for the “olive” in the space midway between the umbilicus and the xiphoid, between the two rectus muscles.3 All other findings in the physical exam should be within normal limits.
Imaging and Laboratory
Studies
An experienced provider can make a diagnosis of IHPS based on clinical examination alone in 60% to 80% of cases.20 However, most surgeons require the diagnosis to be confirmed with one of the following imaging studies before surgery.19
The diagnosis of IHPS can be confirmed by an upper GI series, but this is not commonly ordered as the primary diagnostic study. Ultrasound has become the modality of choice9; it can be used to quantify the size of an elongated, thickened pyloric muscle. The hypertrophied pylorus ranges in length from 14 to 16 mm, with thickness measuring more than 3.0 to 3.5 mm.1,19,21
An upper GI contrast study is rarely used for diagnosis of IHPS, but when this test is ordered to detect other gastrointestinal disease processes (eg, malrotation), IHPS may be identified incidentally.19 During an upper GI study, contrast material is propelled through the pyloric mucosa, and the string sign or the double-track sign may be visualized, revealing the mucosal filling defect.2
Abdominal x-ray may reveal a dilated stomach or a blockage, with a possible finding of gas in the gastric bubble but extending no further into the intestine.19
A study by Hernanz-Schulman2 was conducted to quantify the sensitivities and specificities associated with different diagnostic tools and the clinician’s experience and proficiency in using them. According to this study, palpation by a surgeon has a sensitivity of 31% to 99% and a specificity of 85% to 99% for detection of the pathognomonic olive-shaped mass. Ultrasound performed by an experienced technician has 97% to 100% sensitivity and 99% to 100% specificity for detecting IHPS. An upper GI series has 90% to 100% sensitivity and 99.5% specificity.
Venous blood gas and electrolyte levels are both helpful in making a diagnosis of IHPS.19,22 In a study by Oakley and Barnett,22 the following lab values were found useful in confirming the presence of pyloric stenosis: pH > 7.45; chloride 3 mEq/L. Sodium and potassium deficits may also be present.2,22
Before treatment is considered, the degree of dehydration must be determined by clinical examination and urinary output, as well as serum bicarbonate and chloride levels.3 Other laboratory tests to be ordered include urinalysis and a complete blood count (including platelets).
TREATMENT/MANAGEMENT
Initial management of vomiting in children should begin with fluid replacement if the patient appears dehydrated or if lab findings suggest an electrolyte imbalance. If surgery is later deemed necessary, this will reduce the risk for postoperative apnea.1,23
If IHPS is suspected, a pediatric surgery consult should be obtained. Before anesthesia is considered, serum bicarbonate should be measured with results no higher than 28 mEq/L, and the serum chloride level should be at least 100 mEq/L.3 Imaging, such as ultrasound or the upper GI series, may be ordered to confirm the diagnosis.
Surgical correction by a pyloromyotomy is curative. Presurgical gastric decompression via nasogastric tube placement will reduce the risk for postoperative vomiting and gastritis.20
Surgery
Although a variety of nonsurgical interventions, including oral and IV atropine and balloon dilation, have been described in the literature,3,24-26 the preferred treatment for IHPS is surgical intervention. Surgical correction has been so consistently successful (that is, provided the procedure is performed by a pediatric surgeon or other surgeon with appropriate experience3) that the treatment of choice for IHPS is the Ramstedt pyloromyotomy, which was first performed in 1912.6,27,28
Although the approach may differ based on the individual surgeon’s preference, the pyloric muscle is pulled through an incision in the abdominal wall. A longitudinal incision is made through the muscle with blunt dissection to the submucosa on the anterior surface of the pylorus. The pylorus is then returned to the abdominal cavity, and the abdominal incision is sutured.3,20
The laparoscopic approach, first described in the literature in the mid-1990s,29 is gaining popularity among surgeons, although recent studies have demonstrated that open and laparoscopic procedures are comparably safe and effective for the management of IHPS.30-33 Results from a recent study by Jia et al30 indicate that the laparoscopic approach results in reduced postoperative emesis, shorter length of hospital stay, and shorter recovery times; Hall et al31 emphasize the advantages of laparoscopic pyloromyotomy and recommend it over open surgery in facilities with experienced surgeons.
In follow-up ultrasound studies, hypertrophy of the pyloric muscle was reportedly resolved between two and 12 weeks after pyloromyotomy.34
Postoperative Care
The gastric tube can be removed and oral fluids reintroduced slowly at the surgeon’s discretion, between 8 and 12 hours after surgery. Postoperative vomiting is common (occurring in up to 80% of patients) but should resolve within 24 hours. Mild emesis should not delay the refeeding schedule.3
Patients should be evaluated by the surgeon one to two weeks following surgery unless the infant shows signs of infection (ie, fever, erythema, edema, bleeding, purulent drainage, excess pain, decreased fluid or nutritional intake). Follow-up imaging and laboratory studies are not indicated in an otherwise healthy infant.20
CONCLUSION
Early detection of IHPS, a condition characterized by hypertrophy of the pyloric muscle that results in gastric obstruction and projectile vomiting, can prevent complications of dehydration, malnutrition, and electrolyte disturbances. Surgery by open or laparoscopic pyloromyotomy is curative. Knowing the key physical exam findings, laboratory values, and typical patient history in IHPS enhances the clinician’s ability to make a timely diagnosis.
REFERENCES
1. Olivé AP, Endom EE. Infantile hypertrophic pyloric stenosis (2011). www.uptodate.com/contents/infan tile-hypertrophic-pyloric-stenosis. Accessed August 20, 2012.
2. Hernanz-Schulman M. Infantile hypertrophic pyloric stenosis. Radiology. 2003;227(2):319-331.
3. Aspelund G, Langer JC. Current management of hypertrophic pyloric stenosis. Semin Pediatr Surg. 2007;16(1):27-33.
4. To T, Wajja A, Wales PW, Langer JC. Population demographic indicators associated with incidence of pyloric stenosis. Arch Pediatr Adolesc Med. 2005;159(6):520-525.
5. MacMahon B. The continuing enigma of pyloric stenosis of infancy: a review. Epidemiology. 2006; 17(2):195-201.
6. Panteli C. New insights into the pathogenesis of infantile pyloric stenosis. Pediatr Surg Int. 2009;25 (12):1043-1052.
7. Stone CK, Humphries RL, eds. Current Medical Diagnosis and Treatment: Emergency Medicine. 7th ed. Chapter 50. Pediatric emergencies (2008). http://accessmedicine.com/resourceTOC.aspx?resource ID=718. Accessed August 20, 2012.
8. Hulka F, Harrison MW, Campbell TJ, Campbell JR. Complications of pyloromyotomy for infantile hypertrophic pyloric stenosis. Am J Surg. 1997;173 (5):450-452.
9. Shaoul R, Enav B, Steiner Z, et al. Clinical presentation of pyloric stenosis: the change is in our hands. Isr Med Assoc J. 2004;6(3):134-137.
10. Weinstein MM, Seibert JJ, Ehrenberg A. Six atypical presentations of congenital hypertrophic pyloric stenosis. Clin Pediatr. 1979;18(2):120-122.
11. Eyal O, Asia A, Yorgenson U, et al. Atypical infantile hypertrophic pyloric stenosis [in Hebrew]. Harefuah. 1999;136(2):113-114, 175.
12. Mahon BE, Rosenman MB, Kleiman MB. Maternal and infant use of erythromycin and other macrolide antibiotics as risk factors for infantile hypertrophic pyloric stenosis. J Pediatr. 2001;139(3):380-384.
13. Chung E. Infantile hypertrophic pyloric stenosis: genes and environment. Arch Dis Child. 2008;93 (12):1003-1004.
14. Gilbertson-Dahdal DL, Dutta S, Varich LJ, Barth RA. Neonatal malrotation with midgut volvulus mimicking duodenal atresia. AJR Am J Roentgenol. 2009;192(5):1269-1271.
15. Schmedel W, Ashe L, Kuznicki K. A 24-day-old child with projectile vomiting. J Emerg Nurs. 2009; 35(2):163-164.
16. Iijima T, Okamatsu T, Matsumura M, Yatsuzuka M. Hypertrophic pyloric stenosis associated with hiatal hernia. J Pediatr Surg. 1996;31(2):277-279.
17. Nakayama DK, Taylor LA. Hypertrophic pyloric stenosis in infancy: an analysis of treatment outcome. N C Med J. 1998;59(5):310-313.
18. Irish MS, Pearl RH, Caty M, Glick PL. The approach to common abdominal diagnoses in infants and children. Pediatr Clin North Am. 1998; 45(4):729-772.
19. Askew N. An overview of infantile hypertrophic pyloric stenosis: literature review. Paediatric Nurs. 2010;22(8):27-30.
20. Rudolph CD. Infantile hypertrophic pyloric stenosis. In: Rudolph CD, Rudolph AM, eds. Rudolph’s Pediatrics. 21st ed. New York, NY: McGraw-Hill; 2002:1402-1403.
21. Hallam D, Hansen B, Bødker B, et al. Pyloric size in normal infants and in infants suspected of having hypertrophic pyloric stenosis. Acta Radiol. 2005;36(3):261.
22. Oakley EA, Barnett PL. Is acid base determination an accurate predictor of pyloric stenosis? J Paediatr Child Health. 2000;36(6):587-589.
23. Steven IM, Allen TH, Sweeney DB. Congenital hypertrophic pyloric stenosis: the anaesthetist’s view. Anaesth Intensive Care. 1973;1(6):544-546.
24. Singh UK, Kumar R, Prasad R. Oral atropine sulfate for infantile hypertrophic pyloric stenosis. Indian Pediatr. 2005;42(5):473-476.
25. Nagita A, Yamaguchi J, Amemoto K, et al. Management and ultrasonographic appearance of infantile hypertrophic pyloric stenosis with intravenous atropine sulfate. J Pediatr Gastroenterol Nutr. 1996;23(2):172-177.
26. Ogawa Y, Higashimoto U, Nishijima E, et al. Successful endoscopic balloon dilatation for hypertrophic pyloric stenosis. J Pediatr Surg. 1996;31(12):1712-1714.
27. Langer JC, To T. Does pediatric surgical specialty training affect outcome after Ramstedt pyloromyotomy? A population-based study. Pediatrics. 2004;113(5):1342-1347.
28. Pollock WF, Norris WJ. Dr. Conrad Ramstedt and pyloromyotomy. Surgery. 1957;42(5):966-970.
29. Najmaldin A, Tan HL. Early experience with laparoscopic pyloromyotomy for infantile hypertrophic pyloric stenosis. J Pediatr Surg. 1995;30(1):37-38.
30. Jia WQ, Tian JH, Yang KH, et al. Open versus laparoscopic pyloromyotomy for pyloric stenosis: a meta-analysis of randomized controlled trials. Eur J Pediatr Surg. 2011;21(2):77-81.
31. Hall NJ, Pacilli M, Eaton S, et al. Recovery after open versus laparoscopic pyloromyotomy for pyloric stenosis: a double-blind multicentre randomised controlled trial. Lancet. 2009;373(9661):390-398.
32. Adibe OO, Nichol PF, Flake AAW, Mattei P. Comparison of outcomes after laparoscopic and open pyloromyotomy at a high-volume pediatric teaching hospital. J Pediatr Surg. 2006;41(10):1676-1678.
33. St. Peter SD, Holcomb GW, Calkins CM, et al. Open versus laparoscopic pyloromyotomy for pyloric stenosis: a prospective, randomized trial. Ann Surg. 2006;244(3):363-370.
34. Okorie NM, Dickson JA, Carver RA, Steiner GM. What happens to the pylorus after pyloromyotomy? Arch Dis Child. 1988;63(11):1339-1341.