User login
Esophageal adenocarcinoma is fatal to 90% of patients, indicating a profound need for new therapeutic agents, according to Zhuewn Wang and her colleagues.
The search for genes involved in oncogenesis provides one avenue for finding potential targets. Ms. Wang and her colleagues performed a laboratory study at the University of Michigan, Ann Arbor, to examine the results of the overexpression of DKK3 (the gene for the Dickkopf-3 protein [DKK3]) in DKK3-transfected tissue-cultured esophageal adenocarcinoma (EAC) cell lines. They found that DKK3 overexpression correlated with significantly increased proliferation and Matrigel invasion ability – both known to be important oncogenic traits (J. Thorac. Cardiovasc. Surg. 2015;150:377-85).
DKK3 was overexpressed (greater than twofold) in 76% (72/95) of esophageal adenocarcinomas tested. In addition, the DKK3 protein was present at moderate to high levels in 47% (29/62) of esophageal adenocarcinomas as shown by tissue microarray. Nodal metastases were also significantly increased in patients with esophageal adenocarcinomas highly overexpressing DKK3 (28/32) vs. non–highly expressing EAC tumors (42/63).
In vitro studies showed that stable transfection of DKK3 in an EAC cell line significantly increased proliferation and Matrigel invasion. The researchers also found that the levels of SMAD4, a key mediator of the transforming growth factor–beta pathway, increased after activin treatment of the transfected cell line, and siSMAD4 significantly decreased Matrigel invasion, suggesting that DKK3 acts through the transforming growth factor–beta pathway, according to the researchers.
Additionally, the transfected cells showed increased endothelial tube formation, and they were significantly more resistant to 5-fluorouracil and cisplatin. This finding correlates with the fact that DKK3 expression was found to be significantly higher in chemoresistant esophageal adenocarcinomas, the investigators reported.
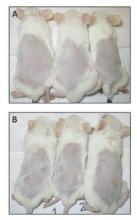
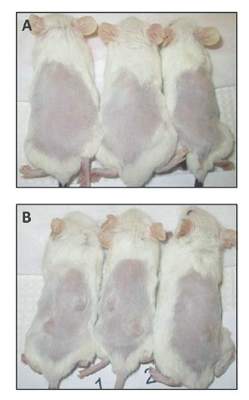
In their animal model system (NOD/SCIDg mice), injection of the transfected cells resulted in tumors at all sites (8/8), whereas vector-only cells grew in only one of eight sites.
“The results of the current study suggest that DKK3 may play an important role in tumor growth and invasion in EAC. DKK3 is overexpressed in a significant number of esophageal adenocarcinomas and targeting DKK3 and its downstream mediators may be beneficial in the prevention and treatment of micrometastatic disease and potentially decreasing disease recurrence,” the researchers concluded.
The authors reported that they had no relevant financial conflicts.
The number of EAC cell lines currently available for study is limited. Despite the fact that the majority of EAC human tumors overexpress Dickkopf-3 in vivo, none of the cell lines used in this study had significant expression. Therefore the authors had to use a forced overexpression transfection strategy to evaluate EAC tumor biology, according to Dr. David R. Jones (J. Thorac. Cardovasc. Surg. 2015;150:288).
This is less than optimal because of an inability to silence Dickkopf-3 to determine if it is sufficient or merely required to drive tumorigenesis or aggressiveness. A more glaring and overarching problem is the lack of an adequate animal model, Dr. Jones added. The xenograft flank model may not recapitulate EAC tumorigenesis or metastases. “Currently there are no well-characterized EAC genetically engineered mouse models that faithfully reproduce EAC,” he pointed out. “The lack of EAC animal models is a major barrier to the preclinical evaluation of novel therapies, target validation, and pathway discovery and confirmation.”
Dr. Jones is a cardiothoracic surgeon at Memorial Sloan Kettering Cancer Center, New York. He made these remarks in his invited commentary.
The number of EAC cell lines currently available for study is limited. Despite the fact that the majority of EAC human tumors overexpress Dickkopf-3 in vivo, none of the cell lines used in this study had significant expression. Therefore the authors had to use a forced overexpression transfection strategy to evaluate EAC tumor biology, according to Dr. David R. Jones (J. Thorac. Cardovasc. Surg. 2015;150:288).
This is less than optimal because of an inability to silence Dickkopf-3 to determine if it is sufficient or merely required to drive tumorigenesis or aggressiveness. A more glaring and overarching problem is the lack of an adequate animal model, Dr. Jones added. The xenograft flank model may not recapitulate EAC tumorigenesis or metastases. “Currently there are no well-characterized EAC genetically engineered mouse models that faithfully reproduce EAC,” he pointed out. “The lack of EAC animal models is a major barrier to the preclinical evaluation of novel therapies, target validation, and pathway discovery and confirmation.”
Dr. Jones is a cardiothoracic surgeon at Memorial Sloan Kettering Cancer Center, New York. He made these remarks in his invited commentary.
The number of EAC cell lines currently available for study is limited. Despite the fact that the majority of EAC human tumors overexpress Dickkopf-3 in vivo, none of the cell lines used in this study had significant expression. Therefore the authors had to use a forced overexpression transfection strategy to evaluate EAC tumor biology, according to Dr. David R. Jones (J. Thorac. Cardovasc. Surg. 2015;150:288).
This is less than optimal because of an inability to silence Dickkopf-3 to determine if it is sufficient or merely required to drive tumorigenesis or aggressiveness. A more glaring and overarching problem is the lack of an adequate animal model, Dr. Jones added. The xenograft flank model may not recapitulate EAC tumorigenesis or metastases. “Currently there are no well-characterized EAC genetically engineered mouse models that faithfully reproduce EAC,” he pointed out. “The lack of EAC animal models is a major barrier to the preclinical evaluation of novel therapies, target validation, and pathway discovery and confirmation.”
Dr. Jones is a cardiothoracic surgeon at Memorial Sloan Kettering Cancer Center, New York. He made these remarks in his invited commentary.
Esophageal adenocarcinoma is fatal to 90% of patients, indicating a profound need for new therapeutic agents, according to Zhuewn Wang and her colleagues.
The search for genes involved in oncogenesis provides one avenue for finding potential targets. Ms. Wang and her colleagues performed a laboratory study at the University of Michigan, Ann Arbor, to examine the results of the overexpression of DKK3 (the gene for the Dickkopf-3 protein [DKK3]) in DKK3-transfected tissue-cultured esophageal adenocarcinoma (EAC) cell lines. They found that DKK3 overexpression correlated with significantly increased proliferation and Matrigel invasion ability – both known to be important oncogenic traits (J. Thorac. Cardiovasc. Surg. 2015;150:377-85).
DKK3 was overexpressed (greater than twofold) in 76% (72/95) of esophageal adenocarcinomas tested. In addition, the DKK3 protein was present at moderate to high levels in 47% (29/62) of esophageal adenocarcinomas as shown by tissue microarray. Nodal metastases were also significantly increased in patients with esophageal adenocarcinomas highly overexpressing DKK3 (28/32) vs. non–highly expressing EAC tumors (42/63).
In vitro studies showed that stable transfection of DKK3 in an EAC cell line significantly increased proliferation and Matrigel invasion. The researchers also found that the levels of SMAD4, a key mediator of the transforming growth factor–beta pathway, increased after activin treatment of the transfected cell line, and siSMAD4 significantly decreased Matrigel invasion, suggesting that DKK3 acts through the transforming growth factor–beta pathway, according to the researchers.
Additionally, the transfected cells showed increased endothelial tube formation, and they were significantly more resistant to 5-fluorouracil and cisplatin. This finding correlates with the fact that DKK3 expression was found to be significantly higher in chemoresistant esophageal adenocarcinomas, the investigators reported.
In their animal model system (NOD/SCIDg mice), injection of the transfected cells resulted in tumors at all sites (8/8), whereas vector-only cells grew in only one of eight sites.
“The results of the current study suggest that DKK3 may play an important role in tumor growth and invasion in EAC. DKK3 is overexpressed in a significant number of esophageal adenocarcinomas and targeting DKK3 and its downstream mediators may be beneficial in the prevention and treatment of micrometastatic disease and potentially decreasing disease recurrence,” the researchers concluded.
The authors reported that they had no relevant financial conflicts.
Esophageal adenocarcinoma is fatal to 90% of patients, indicating a profound need for new therapeutic agents, according to Zhuewn Wang and her colleagues.
The search for genes involved in oncogenesis provides one avenue for finding potential targets. Ms. Wang and her colleagues performed a laboratory study at the University of Michigan, Ann Arbor, to examine the results of the overexpression of DKK3 (the gene for the Dickkopf-3 protein [DKK3]) in DKK3-transfected tissue-cultured esophageal adenocarcinoma (EAC) cell lines. They found that DKK3 overexpression correlated with significantly increased proliferation and Matrigel invasion ability – both known to be important oncogenic traits (J. Thorac. Cardiovasc. Surg. 2015;150:377-85).
DKK3 was overexpressed (greater than twofold) in 76% (72/95) of esophageal adenocarcinomas tested. In addition, the DKK3 protein was present at moderate to high levels in 47% (29/62) of esophageal adenocarcinomas as shown by tissue microarray. Nodal metastases were also significantly increased in patients with esophageal adenocarcinomas highly overexpressing DKK3 (28/32) vs. non–highly expressing EAC tumors (42/63).
In vitro studies showed that stable transfection of DKK3 in an EAC cell line significantly increased proliferation and Matrigel invasion. The researchers also found that the levels of SMAD4, a key mediator of the transforming growth factor–beta pathway, increased after activin treatment of the transfected cell line, and siSMAD4 significantly decreased Matrigel invasion, suggesting that DKK3 acts through the transforming growth factor–beta pathway, according to the researchers.
Additionally, the transfected cells showed increased endothelial tube formation, and they were significantly more resistant to 5-fluorouracil and cisplatin. This finding correlates with the fact that DKK3 expression was found to be significantly higher in chemoresistant esophageal adenocarcinomas, the investigators reported.
In their animal model system (NOD/SCIDg mice), injection of the transfected cells resulted in tumors at all sites (8/8), whereas vector-only cells grew in only one of eight sites.
“The results of the current study suggest that DKK3 may play an important role in tumor growth and invasion in EAC. DKK3 is overexpressed in a significant number of esophageal adenocarcinomas and targeting DKK3 and its downstream mediators may be beneficial in the prevention and treatment of micrometastatic disease and potentially decreasing disease recurrence,” the researchers concluded.
The authors reported that they had no relevant financial conflicts.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: DKK3 may be important in mediating invasion in esophageal adenocarcinoma and could be a novel target for treating and preventing metastasis.
Major finding: Dickkopf-3 overexpression correlated with tumorigenesis and aggressiveness traits in an esophageal adenocarcinoma cell line, and the gene was overexpressed in primary EAC tumors relative to normal esophageal tissue.
Data source: Laboratory studies using cell culture and a mouse model.
Disclosures: The authors reported that they had no relevant financial conflicts.