User login
Mycobacterium chelonae, along with Mycobacterium fortuitum and Mycobacterium abscessus, belongs to a rapidly growing group of nontuberculous mycobacteria (NTM), which are classified as environmental saprophytes found in soil, water, and dust. Under certain circumstances, NTM can cause infection in humans. Nontuberculous mycobacteria are known to cause infection in immunosuppressed patients (such as in the setting of AIDS or immunotherapy following solid organ transplantation); however, they can also cause serious morbidity in immunocompetent patients with certain predisposing factors (eg, recent history of a traumatic wound, recent drug injections, impaired cell-mediated immunity).1-4
We present the case of a patient who presented with multiple reddish blue, nodular, suppurative lesions on the bilateral legs of 1 month’s duration. The patient had a history of renal transplantation 6 years prior followed by immunosuppressive therapy. A punch biopsy of a sample nodule was performed, followed by histologic examination and culture of the biopsy specimen, but polymerase chain reaction (PCR) assay for genotyping of the specimen was necessary to determine the responsible Mycobacterium species.
Case Report
A 61-year-old woman was admitted to our hospital for evaluation and treatment of multiple subcutaneous nodules on the bilateral legs. The patient had undergone successful cadaveric renal transplantation 6 years prior due to polycystic kidney disease and was undergoing maintenance immunosuppressive combination therapy with tacrolimus 4 mg and methylprednisolone 4 mg daily. No other medications or concomitant diseases were reported.
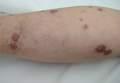
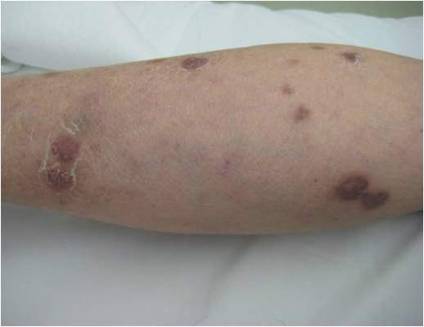
Physical examination revealed multiple slightly tender, brown to purple papules and nodules on the lower legs ranging in size from 2 mm to 1 cm in diameter (Figure 1), some of which exhibited central necrosis (Figure 2). The patient did not recall any previous trauma to the lower legs. Her body temperature was measured at 37.9°C and no regional lymphadenopathy or any other physical abnormalities were observed. Multiple blood culture samples were negative for bacteria, fungi, and mycobacteria.
| 
| |
Figure 1. Multiple slightly tender, brown to purple papules and nodules on the lower left leg. | Figure 2. A nodule on the lower right leg exhibited central necrosis. |
During her 2 weeks in the hospital, the patient’s tacrolimus and methylprednisolone dosages were decreased to 2 mg daily. Routine laboratory tests and serum chemistry were normal with the exception of elevated creatinine levels (1.88 mg/dL [reference range, 0.6 to 1.2 mg/dL]). Chest radiography and interferon-γ release assay were negative. A punch biopsy from a sample nodule was performed and revealed granulomatous inflammation surrounded by giant cells on histopathology. Microscopic examination of the specimen revealed alcohol- and acid-resistant bacilli on Ziehl-Neelsen staining. A biopsy specimen was cultured on Löwenstein-Jensen medium at 25°C, 37°C, and 42°C according to NTM detection protocol5 and showed growth of NTM at 37°C. On the basis of the positive culture, genetic analysis of the specimen was performed using a strip test that permits identification of 13 common species of NTM. The organism was identified as M chelonae.
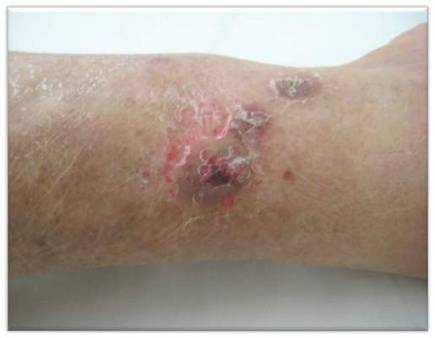
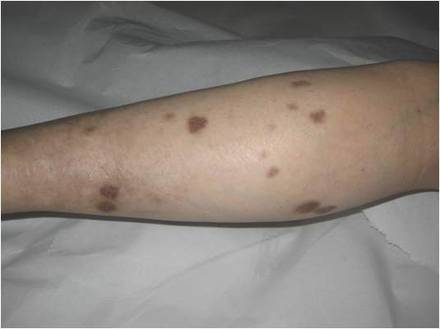
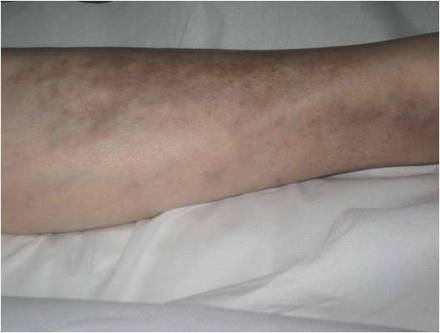
While awaiting species identification and results of drug susceptibility testing, treatment with oral clarithromycin 250 mg twice daily was initiated and continued for 10 days until the patient developed gastrointestinal adverse effects, at which point oral ciprofloxacin 250 mg twice daily was substituted. In laboratory testing, the isolated M chelonae strain showed sensitivity to ciprofloxacin, clarithromycin, tobramycin, and amikacin at minimum inhibitory concentrations of less than 1, 2, 4, and 16, respectively. Treatment with ciprofloxacin 250 mg twice daily was continued for 6 months, which resulted in slow resolution of the lesions until the end of treatment (Figure 3). No recurrence of the lesions was noted at 24-month follow-up, but areas of hyperpigmentation were noted at the lesion sites (Figure 4).
| 
| ||
Figure 3. Following 6 months of treatment with oral ciprofloxacin 250 mg twice daily, nodules on the left leg had resolved and papules had decreased in size. | Figure 4. Skin lesions had resolved without recurrence at 24-month follow-up, although hyperpigmented areas remained. |
Comment
Mycobacterium chelonae, a member of the NTM group, grows rapidly on Löwenstein-Jensen medium, usually following incubation for 5 to 7 days at temperatures of 28°C to 32°C, and is characterized by its lack of pigmentation. Nontuberculous mycobacteria, which are resistant to standard disinfectants such as chlorine, organomercurials, and alkaline glutaraldehydes, may cause nosocomial outbreaks, infecting otherwise healthy individuals receiving any type of injection (eg, in cosmetic procedures), as well as those with suppressed immunity.6
In addition to cutaneous manifestations, NTM may cause various extracutaneous diseases, such as osteomyelitis, infective bronchiectasis, endocarditis, pericarditis, lymphadenopathy, and ocular infections.1-4 The species M chelonae may cause localized skin infections, soft tissue lesions (eg, granulomatous nodules, ulcers, abscesses, sporotrichoid lesions), and cutaneous disseminated infections.
Immunosuppression associated with treatment following renal transplantation was the primary cause of M chelonae infection in our patient, as has previously been reported in the literature.3-4 This was further supported by the lack of prior trauma or invasive procedure (eg, mesotherapy) in the affected areas. Specifically, our patient had more than 5 lesions on the lower legs; in accordance with a previous comprehensive study,1 the presence of more than 5 lesions indicates a disseminated cutaneous infection, which usually is correlated with immunosuppression (such as in our patient). Localized infections generally are observed in immunocompetent hosts.1
The exact pathogenetic mechanism of M chelonae infection in our patient is not clear. In patients with suppressed immunity, the variable clinical presentation of infection with NTM often impedes diagnosis. Cutaneous M chelonae lesions may be mistakenly diagnosed as Kaposi sarcoma or rarely as pyoderma gangrenosum. The differential diagnosis of subcutaneous nodules includes histoplasmosis, cryptococcosis, blastomycosis, coccidioidomycosis, nocardiosis, mycetoma, sporotrichosis, actinomycosis, and tuberculosis. In our patient, approximately 2 months elapsed between presentation of symptoms and definitive diagnosis, which was less than that reported in previously published cases.2,7-9
Histology and tissue culture followed by proper genetic analysis remains the gold standard for diagnosing NTM infection.10,11 In the interest of patients, time-consuming biochemical analyses should be replaced by molecular genetic diagnostic strip tests, which are fast, exact, and available in commercial kits for both common mycobacteria and additional species.12
Once the diagnosis of NTM infection has been established, sensitivity testing is mandatory to guide targeted therapy; however, clinicians should bear in mind that susceptibility testing does not guarantee clinical success, as correlations of susceptibility testing and clinical response have not been assessed.8 Standard antituberculous drugs (eg, isoniazid, rifampin, pyrazinamide) have no role in the treatment of M chelonae infection. The first-line antibiotics are clarithromycin, tobramycin, and linezolid, followed by imipenem, amikacin, clofazimine, doxycycline, and ciprofloxacin.10 Optimal outcomes have been reported in patients treated both with antibiotics and with surgical debridement. Although monotherapy with quinolones is not recommended for treatment of infection with NTM due to the high risk of mutational resistance, our patient received long-term antibiotic treatment with ciprofloxacin over a 6-month period and showed no recurrence at 24-month follow-up.
Conclusion
Clinicians who treat patients with chronic skin or soft tissue infections should consider infection with NTM in the differential diagnosis, particularly in patients with suppressed immunity, but also in immunocompetent patients following any invasive procedure. Detailed medical history and skin biopsy followed by histology and culture are recommended for the diagnosis. Infection with NTM requires rapid action. Sensitivity testing is necessary in choosing an effective treatment. New molecular genetic diagnostic strip tests can differentiate species of NTM sooner than biochemical analyses, thereby helping clinicians initiate appropriate antimicrobial treatment in a timely fashion.
1. Wallace RJ Jr, Brown BA, Onyi GO. Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance of oral antimicrobials other than clarithromycin. J Infect Dis. 1992;166:405-412.
2. Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
3. Alexander S, John GT, Jesudason M, et al. Infections with atypical mycobacteria in renal transplant recipients. Indian J Pathol Microbiol. 2007;50:482-484.
4. Dorman S, Subramanian A; AST Infectious Diseases Community of Practice. Nontuberculous mycobacteria in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S63-S69.
5. Whitman WB, Goodfellow M, Kämpfer P, et al, eds. Bergey’s Manual of Systematic Bacteriology. 2nd ed. New York, NY: Springer-Verlag; 2012. The Actinobacteria; vol 5.
6. Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria [published online ahead of print September 5, 2001]. Clin Infect Dis. 2001;33:1363-1374.
7. Dodiuk-Gad R, Dyachenko P, Ziv M, et al. Nontuberculous mycobacterial infections of the skin: a retrospective study of 25 cases [published online ahead of print March 26, 2007]. J Am Acad Dermatol. 2007;57:413-420.
8. Regnier S, Cambau E, Meningaud JP, et al. Clinical management of rapidly growing mycobacterial cutaneous infections in patients after mesotherapy. Clin Infect Dis. 2009;49:1358-1364.
9. Somily AM, AL-Anazi AR, Babay HA, et al. Mycobacterium chelonae complex bacteremia from a post-renal transplant patient: case report and literature review. Jpn J Infect Dis. 2010;63:61-64.
10. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction in Am J Respir Crit Care Med. 2007;175:744-745]. Am J Respir Crit Care Med. 2007;175:367-416.
11. Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases [published online ahead of print September 6, 2010]. J Dermatol. 2010;37:965-972.
12. Lee AS, Jelfs P, Sintchenko V, et al. Identification of non-tuberculous mycobacteria: utility of the GenoType Mycobacterium CM/AS assay compared with HPLC and 16S rRNA gene sequencing [published online ahead of print June 5, 2009]. J of Med Microb. 2009;58(pt 7):900-904.
Mycobacterium chelonae, along with Mycobacterium fortuitum and Mycobacterium abscessus, belongs to a rapidly growing group of nontuberculous mycobacteria (NTM), which are classified as environmental saprophytes found in soil, water, and dust. Under certain circumstances, NTM can cause infection in humans. Nontuberculous mycobacteria are known to cause infection in immunosuppressed patients (such as in the setting of AIDS or immunotherapy following solid organ transplantation); however, they can also cause serious morbidity in immunocompetent patients with certain predisposing factors (eg, recent history of a traumatic wound, recent drug injections, impaired cell-mediated immunity).1-4
We present the case of a patient who presented with multiple reddish blue, nodular, suppurative lesions on the bilateral legs of 1 month’s duration. The patient had a history of renal transplantation 6 years prior followed by immunosuppressive therapy. A punch biopsy of a sample nodule was performed, followed by histologic examination and culture of the biopsy specimen, but polymerase chain reaction (PCR) assay for genotyping of the specimen was necessary to determine the responsible Mycobacterium species.
Case Report
A 61-year-old woman was admitted to our hospital for evaluation and treatment of multiple subcutaneous nodules on the bilateral legs. The patient had undergone successful cadaveric renal transplantation 6 years prior due to polycystic kidney disease and was undergoing maintenance immunosuppressive combination therapy with tacrolimus 4 mg and methylprednisolone 4 mg daily. No other medications or concomitant diseases were reported.
Physical examination revealed multiple slightly tender, brown to purple papules and nodules on the lower legs ranging in size from 2 mm to 1 cm in diameter (Figure 1), some of which exhibited central necrosis (Figure 2). The patient did not recall any previous trauma to the lower legs. Her body temperature was measured at 37.9°C and no regional lymphadenopathy or any other physical abnormalities were observed. Multiple blood culture samples were negative for bacteria, fungi, and mycobacteria.

| 
| |
Figure 1. Multiple slightly tender, brown to purple papules and nodules on the lower left leg. | Figure 2. A nodule on the lower right leg exhibited central necrosis. |
During her 2 weeks in the hospital, the patient’s tacrolimus and methylprednisolone dosages were decreased to 2 mg daily. Routine laboratory tests and serum chemistry were normal with the exception of elevated creatinine levels (1.88 mg/dL [reference range, 0.6 to 1.2 mg/dL]). Chest radiography and interferon-γ release assay were negative. A punch biopsy from a sample nodule was performed and revealed granulomatous inflammation surrounded by giant cells on histopathology. Microscopic examination of the specimen revealed alcohol- and acid-resistant bacilli on Ziehl-Neelsen staining. A biopsy specimen was cultured on Löwenstein-Jensen medium at 25°C, 37°C, and 42°C according to NTM detection protocol5 and showed growth of NTM at 37°C. On the basis of the positive culture, genetic analysis of the specimen was performed using a strip test that permits identification of 13 common species of NTM. The organism was identified as M chelonae.
While awaiting species identification and results of drug susceptibility testing, treatment with oral clarithromycin 250 mg twice daily was initiated and continued for 10 days until the patient developed gastrointestinal adverse effects, at which point oral ciprofloxacin 250 mg twice daily was substituted. In laboratory testing, the isolated M chelonae strain showed sensitivity to ciprofloxacin, clarithromycin, tobramycin, and amikacin at minimum inhibitory concentrations of less than 1, 2, 4, and 16, respectively. Treatment with ciprofloxacin 250 mg twice daily was continued for 6 months, which resulted in slow resolution of the lesions until the end of treatment (Figure 3). No recurrence of the lesions was noted at 24-month follow-up, but areas of hyperpigmentation were noted at the lesion sites (Figure 4).

| 
| ||
Figure 3. Following 6 months of treatment with oral ciprofloxacin 250 mg twice daily, nodules on the left leg had resolved and papules had decreased in size. | Figure 4. Skin lesions had resolved without recurrence at 24-month follow-up, although hyperpigmented areas remained. |
Comment
Mycobacterium chelonae, a member of the NTM group, grows rapidly on Löwenstein-Jensen medium, usually following incubation for 5 to 7 days at temperatures of 28°C to 32°C, and is characterized by its lack of pigmentation. Nontuberculous mycobacteria, which are resistant to standard disinfectants such as chlorine, organomercurials, and alkaline glutaraldehydes, may cause nosocomial outbreaks, infecting otherwise healthy individuals receiving any type of injection (eg, in cosmetic procedures), as well as those with suppressed immunity.6
In addition to cutaneous manifestations, NTM may cause various extracutaneous diseases, such as osteomyelitis, infective bronchiectasis, endocarditis, pericarditis, lymphadenopathy, and ocular infections.1-4 The species M chelonae may cause localized skin infections, soft tissue lesions (eg, granulomatous nodules, ulcers, abscesses, sporotrichoid lesions), and cutaneous disseminated infections.
Immunosuppression associated with treatment following renal transplantation was the primary cause of M chelonae infection in our patient, as has previously been reported in the literature.3-4 This was further supported by the lack of prior trauma or invasive procedure (eg, mesotherapy) in the affected areas. Specifically, our patient had more than 5 lesions on the lower legs; in accordance with a previous comprehensive study,1 the presence of more than 5 lesions indicates a disseminated cutaneous infection, which usually is correlated with immunosuppression (such as in our patient). Localized infections generally are observed in immunocompetent hosts.1
The exact pathogenetic mechanism of M chelonae infection in our patient is not clear. In patients with suppressed immunity, the variable clinical presentation of infection with NTM often impedes diagnosis. Cutaneous M chelonae lesions may be mistakenly diagnosed as Kaposi sarcoma or rarely as pyoderma gangrenosum. The differential diagnosis of subcutaneous nodules includes histoplasmosis, cryptococcosis, blastomycosis, coccidioidomycosis, nocardiosis, mycetoma, sporotrichosis, actinomycosis, and tuberculosis. In our patient, approximately 2 months elapsed between presentation of symptoms and definitive diagnosis, which was less than that reported in previously published cases.2,7-9
Histology and tissue culture followed by proper genetic analysis remains the gold standard for diagnosing NTM infection.10,11 In the interest of patients, time-consuming biochemical analyses should be replaced by molecular genetic diagnostic strip tests, which are fast, exact, and available in commercial kits for both common mycobacteria and additional species.12
Once the diagnosis of NTM infection has been established, sensitivity testing is mandatory to guide targeted therapy; however, clinicians should bear in mind that susceptibility testing does not guarantee clinical success, as correlations of susceptibility testing and clinical response have not been assessed.8 Standard antituberculous drugs (eg, isoniazid, rifampin, pyrazinamide) have no role in the treatment of M chelonae infection. The first-line antibiotics are clarithromycin, tobramycin, and linezolid, followed by imipenem, amikacin, clofazimine, doxycycline, and ciprofloxacin.10 Optimal outcomes have been reported in patients treated both with antibiotics and with surgical debridement. Although monotherapy with quinolones is not recommended for treatment of infection with NTM due to the high risk of mutational resistance, our patient received long-term antibiotic treatment with ciprofloxacin over a 6-month period and showed no recurrence at 24-month follow-up.
Conclusion
Clinicians who treat patients with chronic skin or soft tissue infections should consider infection with NTM in the differential diagnosis, particularly in patients with suppressed immunity, but also in immunocompetent patients following any invasive procedure. Detailed medical history and skin biopsy followed by histology and culture are recommended for the diagnosis. Infection with NTM requires rapid action. Sensitivity testing is necessary in choosing an effective treatment. New molecular genetic diagnostic strip tests can differentiate species of NTM sooner than biochemical analyses, thereby helping clinicians initiate appropriate antimicrobial treatment in a timely fashion.
Mycobacterium chelonae, along with Mycobacterium fortuitum and Mycobacterium abscessus, belongs to a rapidly growing group of nontuberculous mycobacteria (NTM), which are classified as environmental saprophytes found in soil, water, and dust. Under certain circumstances, NTM can cause infection in humans. Nontuberculous mycobacteria are known to cause infection in immunosuppressed patients (such as in the setting of AIDS or immunotherapy following solid organ transplantation); however, they can also cause serious morbidity in immunocompetent patients with certain predisposing factors (eg, recent history of a traumatic wound, recent drug injections, impaired cell-mediated immunity).1-4
We present the case of a patient who presented with multiple reddish blue, nodular, suppurative lesions on the bilateral legs of 1 month’s duration. The patient had a history of renal transplantation 6 years prior followed by immunosuppressive therapy. A punch biopsy of a sample nodule was performed, followed by histologic examination and culture of the biopsy specimen, but polymerase chain reaction (PCR) assay for genotyping of the specimen was necessary to determine the responsible Mycobacterium species.
Case Report
A 61-year-old woman was admitted to our hospital for evaluation and treatment of multiple subcutaneous nodules on the bilateral legs. The patient had undergone successful cadaveric renal transplantation 6 years prior due to polycystic kidney disease and was undergoing maintenance immunosuppressive combination therapy with tacrolimus 4 mg and methylprednisolone 4 mg daily. No other medications or concomitant diseases were reported.
Physical examination revealed multiple slightly tender, brown to purple papules and nodules on the lower legs ranging in size from 2 mm to 1 cm in diameter (Figure 1), some of which exhibited central necrosis (Figure 2). The patient did not recall any previous trauma to the lower legs. Her body temperature was measured at 37.9°C and no regional lymphadenopathy or any other physical abnormalities were observed. Multiple blood culture samples were negative for bacteria, fungi, and mycobacteria.

| 
| |
Figure 1. Multiple slightly tender, brown to purple papules and nodules on the lower left leg. | Figure 2. A nodule on the lower right leg exhibited central necrosis. |
During her 2 weeks in the hospital, the patient’s tacrolimus and methylprednisolone dosages were decreased to 2 mg daily. Routine laboratory tests and serum chemistry were normal with the exception of elevated creatinine levels (1.88 mg/dL [reference range, 0.6 to 1.2 mg/dL]). Chest radiography and interferon-γ release assay were negative. A punch biopsy from a sample nodule was performed and revealed granulomatous inflammation surrounded by giant cells on histopathology. Microscopic examination of the specimen revealed alcohol- and acid-resistant bacilli on Ziehl-Neelsen staining. A biopsy specimen was cultured on Löwenstein-Jensen medium at 25°C, 37°C, and 42°C according to NTM detection protocol5 and showed growth of NTM at 37°C. On the basis of the positive culture, genetic analysis of the specimen was performed using a strip test that permits identification of 13 common species of NTM. The organism was identified as M chelonae.
While awaiting species identification and results of drug susceptibility testing, treatment with oral clarithromycin 250 mg twice daily was initiated and continued for 10 days until the patient developed gastrointestinal adverse effects, at which point oral ciprofloxacin 250 mg twice daily was substituted. In laboratory testing, the isolated M chelonae strain showed sensitivity to ciprofloxacin, clarithromycin, tobramycin, and amikacin at minimum inhibitory concentrations of less than 1, 2, 4, and 16, respectively. Treatment with ciprofloxacin 250 mg twice daily was continued for 6 months, which resulted in slow resolution of the lesions until the end of treatment (Figure 3). No recurrence of the lesions was noted at 24-month follow-up, but areas of hyperpigmentation were noted at the lesion sites (Figure 4).

| 
| ||
Figure 3. Following 6 months of treatment with oral ciprofloxacin 250 mg twice daily, nodules on the left leg had resolved and papules had decreased in size. | Figure 4. Skin lesions had resolved without recurrence at 24-month follow-up, although hyperpigmented areas remained. |
Comment
Mycobacterium chelonae, a member of the NTM group, grows rapidly on Löwenstein-Jensen medium, usually following incubation for 5 to 7 days at temperatures of 28°C to 32°C, and is characterized by its lack of pigmentation. Nontuberculous mycobacteria, which are resistant to standard disinfectants such as chlorine, organomercurials, and alkaline glutaraldehydes, may cause nosocomial outbreaks, infecting otherwise healthy individuals receiving any type of injection (eg, in cosmetic procedures), as well as those with suppressed immunity.6
In addition to cutaneous manifestations, NTM may cause various extracutaneous diseases, such as osteomyelitis, infective bronchiectasis, endocarditis, pericarditis, lymphadenopathy, and ocular infections.1-4 The species M chelonae may cause localized skin infections, soft tissue lesions (eg, granulomatous nodules, ulcers, abscesses, sporotrichoid lesions), and cutaneous disseminated infections.
Immunosuppression associated with treatment following renal transplantation was the primary cause of M chelonae infection in our patient, as has previously been reported in the literature.3-4 This was further supported by the lack of prior trauma or invasive procedure (eg, mesotherapy) in the affected areas. Specifically, our patient had more than 5 lesions on the lower legs; in accordance with a previous comprehensive study,1 the presence of more than 5 lesions indicates a disseminated cutaneous infection, which usually is correlated with immunosuppression (such as in our patient). Localized infections generally are observed in immunocompetent hosts.1
The exact pathogenetic mechanism of M chelonae infection in our patient is not clear. In patients with suppressed immunity, the variable clinical presentation of infection with NTM often impedes diagnosis. Cutaneous M chelonae lesions may be mistakenly diagnosed as Kaposi sarcoma or rarely as pyoderma gangrenosum. The differential diagnosis of subcutaneous nodules includes histoplasmosis, cryptococcosis, blastomycosis, coccidioidomycosis, nocardiosis, mycetoma, sporotrichosis, actinomycosis, and tuberculosis. In our patient, approximately 2 months elapsed between presentation of symptoms and definitive diagnosis, which was less than that reported in previously published cases.2,7-9
Histology and tissue culture followed by proper genetic analysis remains the gold standard for diagnosing NTM infection.10,11 In the interest of patients, time-consuming biochemical analyses should be replaced by molecular genetic diagnostic strip tests, which are fast, exact, and available in commercial kits for both common mycobacteria and additional species.12
Once the diagnosis of NTM infection has been established, sensitivity testing is mandatory to guide targeted therapy; however, clinicians should bear in mind that susceptibility testing does not guarantee clinical success, as correlations of susceptibility testing and clinical response have not been assessed.8 Standard antituberculous drugs (eg, isoniazid, rifampin, pyrazinamide) have no role in the treatment of M chelonae infection. The first-line antibiotics are clarithromycin, tobramycin, and linezolid, followed by imipenem, amikacin, clofazimine, doxycycline, and ciprofloxacin.10 Optimal outcomes have been reported in patients treated both with antibiotics and with surgical debridement. Although monotherapy with quinolones is not recommended for treatment of infection with NTM due to the high risk of mutational resistance, our patient received long-term antibiotic treatment with ciprofloxacin over a 6-month period and showed no recurrence at 24-month follow-up.
Conclusion
Clinicians who treat patients with chronic skin or soft tissue infections should consider infection with NTM in the differential diagnosis, particularly in patients with suppressed immunity, but also in immunocompetent patients following any invasive procedure. Detailed medical history and skin biopsy followed by histology and culture are recommended for the diagnosis. Infection with NTM requires rapid action. Sensitivity testing is necessary in choosing an effective treatment. New molecular genetic diagnostic strip tests can differentiate species of NTM sooner than biochemical analyses, thereby helping clinicians initiate appropriate antimicrobial treatment in a timely fashion.
1. Wallace RJ Jr, Brown BA, Onyi GO. Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance of oral antimicrobials other than clarithromycin. J Infect Dis. 1992;166:405-412.
2. Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
3. Alexander S, John GT, Jesudason M, et al. Infections with atypical mycobacteria in renal transplant recipients. Indian J Pathol Microbiol. 2007;50:482-484.
4. Dorman S, Subramanian A; AST Infectious Diseases Community of Practice. Nontuberculous mycobacteria in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S63-S69.
5. Whitman WB, Goodfellow M, Kämpfer P, et al, eds. Bergey’s Manual of Systematic Bacteriology. 2nd ed. New York, NY: Springer-Verlag; 2012. The Actinobacteria; vol 5.
6. Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria [published online ahead of print September 5, 2001]. Clin Infect Dis. 2001;33:1363-1374.
7. Dodiuk-Gad R, Dyachenko P, Ziv M, et al. Nontuberculous mycobacterial infections of the skin: a retrospective study of 25 cases [published online ahead of print March 26, 2007]. J Am Acad Dermatol. 2007;57:413-420.
8. Regnier S, Cambau E, Meningaud JP, et al. Clinical management of rapidly growing mycobacterial cutaneous infections in patients after mesotherapy. Clin Infect Dis. 2009;49:1358-1364.
9. Somily AM, AL-Anazi AR, Babay HA, et al. Mycobacterium chelonae complex bacteremia from a post-renal transplant patient: case report and literature review. Jpn J Infect Dis. 2010;63:61-64.
10. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction in Am J Respir Crit Care Med. 2007;175:744-745]. Am J Respir Crit Care Med. 2007;175:367-416.
11. Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases [published online ahead of print September 6, 2010]. J Dermatol. 2010;37:965-972.
12. Lee AS, Jelfs P, Sintchenko V, et al. Identification of non-tuberculous mycobacteria: utility of the GenoType Mycobacterium CM/AS assay compared with HPLC and 16S rRNA gene sequencing [published online ahead of print June 5, 2009]. J of Med Microb. 2009;58(pt 7):900-904.
1. Wallace RJ Jr, Brown BA, Onyi GO. Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance of oral antimicrobials other than clarithromycin. J Infect Dis. 1992;166:405-412.
2. Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
3. Alexander S, John GT, Jesudason M, et al. Infections with atypical mycobacteria in renal transplant recipients. Indian J Pathol Microbiol. 2007;50:482-484.
4. Dorman S, Subramanian A; AST Infectious Diseases Community of Practice. Nontuberculous mycobacteria in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S63-S69.
5. Whitman WB, Goodfellow M, Kämpfer P, et al, eds. Bergey’s Manual of Systematic Bacteriology. 2nd ed. New York, NY: Springer-Verlag; 2012. The Actinobacteria; vol 5.
6. Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria [published online ahead of print September 5, 2001]. Clin Infect Dis. 2001;33:1363-1374.
7. Dodiuk-Gad R, Dyachenko P, Ziv M, et al. Nontuberculous mycobacterial infections of the skin: a retrospective study of 25 cases [published online ahead of print March 26, 2007]. J Am Acad Dermatol. 2007;57:413-420.
8. Regnier S, Cambau E, Meningaud JP, et al. Clinical management of rapidly growing mycobacterial cutaneous infections in patients after mesotherapy. Clin Infect Dis. 2009;49:1358-1364.
9. Somily AM, AL-Anazi AR, Babay HA, et al. Mycobacterium chelonae complex bacteremia from a post-renal transplant patient: case report and literature review. Jpn J Infect Dis. 2010;63:61-64.
10. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction in Am J Respir Crit Care Med. 2007;175:744-745]. Am J Respir Crit Care Med. 2007;175:367-416.
11. Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases [published online ahead of print September 6, 2010]. J Dermatol. 2010;37:965-972.
12. Lee AS, Jelfs P, Sintchenko V, et al. Identification of non-tuberculous mycobacteria: utility of the GenoType Mycobacterium CM/AS assay compared with HPLC and 16S rRNA gene sequencing [published online ahead of print June 5, 2009]. J of Med Microb. 2009;58(pt 7):900-904.
Practice Points
- Nontuberculous mycobacteria (NTM) are environmental saprophytes that can cause infection in immunosuppressed individuals as well as immunocompetent individuals with certain predisposing factors.
- It is important for clinicians to consider NTM in the differential diagnosis for patients who present with chronic skin or soft tissue infections.
- Histologic examination and culture of a biopsy specimen followed by polymerase chain reaction assay for genotyping of the specimen are recommended to determine the responsible Mycobacterium species.
- New molecular genetic strip tests can differentiate NTM species more quickly.