User login
Adults are sleeping less and less in our society. Yet sleep is no longer thought of as strictly a restorative process for the body. The importance of sleep for metabolic function and specifically glucose homeostasis is now widely accepted, as many studies have shown a correlation between sleep deprivation or poor sleep quality and an increased risk of diabetes.
Obesity and aging are both associated with worse sleep. As the prevalence of obesity and diabetes increases, and as the number of elderly people increases, it is imperative to target sleep in the overall treatment of our patients.
In the pages that follow, we examine the evidence of a link between sleep loss (both short sleep duration and poor-quality sleep) and the risk of diabetes. (For evidence linking short sleep duration and the related problem of obesity, we invite the reader to refer to previous publications on the topic.1,2)
SLEEP LOSS, OBESITY, AND DIABETES ARE ALL ON THE RISE
The prevalence of obesity and, consequently, of type 2 diabetes mellitus has increased alarmingly worldwide and particularly in the United States in the past few decades. Such a rapid increase cannot be explained simply by an alteration in the genetic pool; it is more likely due to environmental, socioeconomic, behavioral, and demographic factors and the interaction between genetics and these factors. Besides traditional lifestyle factors such as high-calorie diets and sedentary habits, other, nontraditional behavioral and environmental factors could be contributing to the epidemic of obesity and diabetes.
At the same time, people are sleeping less, and sleep disorders are on the rise. According to recent polls from the US Centers for Disease Control and Prevention, approximately 29% of US adults report sleeping less than 7 hours per night, and 50 to 70 million have chronic sleep and wakefulness disorders.3
The sleep curtailment of our times probably is partly self-imposed, as the pace and the opportunities of modern society place more demands on time for work and leisure activities and leave less time for sleep.
The quality of sleep has also declined as the population has aged and as the prevalence of obesity and its related sleep disorders has increased. Furthermore, patients with type 2 diabetes tend to sleep less, and to sleep poorly.4,5 Poor sleep quality generally results in overall sleep loss.
GLUCOSE TOLERANCE HAS A CIRCADIAN RHYTHM
The human body regulates blood levels of glucose within a narrow range.
Glucose tolerance refers to the ability to maintain euglycemia by disposing of exogenous glucose via insulin-mediated and non–insulin-mediated mechanisms. Normal glucose tolerance depends on the ability of the pancreatic beta cells to produce insulin. As insulin sensitivity declines, insulin secretion increases to maintain normal glucose levels. Diabetes becomes manifest when the pancreatic beta cells fail to compensate for the decreased insulin sensitivity.
Glucose tolerance varies in a circadian rhythm, including during the different stages of sleep.
HOW SLEEP AFFECTS METABOLISM AND HORMONES
Sleep has often been thought of as a “restorative” process for the mind and the body; however, many studies have shown that it also directly affects many metabolic and hormonal processes.6
Sleep has five stages: rapid eye movement (REM) sleep and stages 1, 2, 3, and 4 of non-REM sleep. The deeper stages of non-REM sleep, ie, stages 3 and 4, are also known as slow-wave sleep and are thought to be the most restorative.
Additionally, the onset of slow-wave sleep is temporally associated with transient metabolic, hormonal, and neurophysiologic changes, all of which can affect glucose homeostasis. The brain uses less glucose,7 the pituitary gland releases more growth hormone and less corticotropin,8 the sympathetic nervous system is less active, and conversely, vagal tone is increased.9
As a result, in the first part of the night, when slow-wave sleep predominates, glucose metabolism is slower. These effects are reversed in the second part of the night, when REM sleep, stage 1, and awakening are more likely.
In view of these important changes in glucose metabolism during sleep, it is not surprising that getting less sleep or poorer sleep on a regular basis could affect overall glucose homeostasis.
SHORT SLEEP DURATION AND RISK OF DIABETES
Laboratory and epidemiologic evidence supports an association between short sleep duration (< 7 hours per night) and the risk of diabetes, and also between poor sleep quality and the risk of diabetes. We will explore putative mechanisms for these relationships.
Laboratory studies of short sleep duration and glucose metabolism
Studies in small numbers of healthy volunteers who underwent experimental sleep restriction or disruption have revealed mechanisms by which sleep loss might increase the risk of diabetes.
Kuhn et al10 performed the very first laboratory study of the effect of sleep deprivation on metabolism. Published in 1969, it showed that total sleep deprivation led to a marked increase in glucose levels.
A caution in extrapolating such results to real-life conditions is that total sleep deprivation is uncommon in humans and is inevitably followed by sleep recovery, with normalization of glucose metabolism. However, people in modern society are experiencing recurrent partial sleep deprivation, and its effect on glucose metabolism may be different.
Spiegel et al,11 in landmark laboratory studies of partial sleep deprivation in healthy, lean adults, found that restricting sleep to 4 hours per night for 6 nights resulted in a 40% decrease in glucose tolerance, to levels similar to those seen in older adults with impaired glucose tolerance. This metabolic change was paralleled by an increase in the activity of the sympathetic nervous system, and both of these effects reversed with sleep recovery.
A criticism of these initial studies is that they restricted sleep to 4 hours, a restriction more severe than that seen in real life.
Nedeltcheva et al12 more recently examined the effects of less-severe sleep curtailment (5.5 hours per night for 14 nights) in sedentary middle-aged men and women. This degree of bedtime restriction led to a decrease in glucose tolerance due to decreased insulin sensitivity in the absence of adequate beta cell compensation.
Such recurrent bedtime restriction is closer to the short sleep duration experienced by many people in everyday life, and in people at risk it may facilitate the development of insulin resistance, reduced glucose tolerance, and ultimately diabetes. Indeed, epidemiologic studies suggest that people who sleep less than 6 hours per night are at higher risk of type 2 diabetes.
Epidemiologic studies of short sleep duration and glucose metabolism
Multiple cross-sectional epidemiologic studies have suggested an association between short sleep duration and diabetes, and several prospective epidemiologic studies have suggested that short sleep actually plays a causative role in diabetes.
The landmark observations of Spiegel et al11 led to a number of epidemiologic studies examining the relationships between sleep duration and sleep disturbances and diabetes risk.13
The Sleep Heart Study14 was a large, cross-sectional, community-based study of the cardiovascular consequences of sleep-disordered breathing. The authors assessed the relationship between reported sleep duration and impaired glucose tolerance or type 2 diabetes in more than 1,400 men and women who had no history of insomnia. After adjustment for age, sex, race, body habitus, and apnea-hypopnea index, the prevalence of impaired glucose tolerance and type 2 diabetes was higher in those who reported sleeping 6 hours or less per night—or 9 hours or more per night (more below about the possible effect of too much sleep on the risk of diabetes).
The major limitations of the study were that it was cross-sectional in design, sleep duration was self-reported, the reasons for sleep curtailment were unknown, and possible confounding variables as physical activity, diet, and socioeconomic status were not measured.
Knutson et al,4 in our medical center, examined the association between self-reported sleep duration and sleep quality on the one hand and hemoglobin A1c levels on the other in 161 black patients with type 2 diabetes. In patients without diabetic complications, glycemic control correlated with perceived sleep debt (calculated as the difference between self-reported actual and preferred weekday sleep duration); the authors calculated that a perceived sleep debt of 3 hours per night predicted a hemoglobin A1c value 1.1 absolute percentage points higher than the median value. The analyses controlled for age, sex, body mass index, insulin use, and the presence of major complications; it excluded patients whose sleep was frequently disrupted by pain. The effect size was comparable to (but opposite) that of oral antidiabetic drugs. However, the direction of causality cannot be confirmed from this association, as it is possible that poor glycemic control in diabetic patients could impair their ability to achieve sufficient sleep.
To date, several major prospective studies have looked at the association between short sleep duration and sleep problems and the risk of developing type 2 diabetes in adults.
The Nurses Health Study15 followed 70,000 nondiabetic women for 10 years. Compared with nurses who slept 7 to 8 hours per 24 hours, those who slept 5 hours or less had a relative risk of diabetes of 1.34 even after controlling for many covariables, such as body mass index, shift work, hypertension, exercise, and depression.
The first National Health and Nutrition Examination Survey (NHANES I)16 examined the effect of sleep duration on the risk of incident diabetes in roughly 9,000 men and women over a period of 8 to 10 years. The statistical model included body mass index and hypertension and adjusted for physical activity, depression, alcohol consumption, ethnicity, education, marital status, and age. Findings: those who slept 5 hours or less per night were significantly more likely to develop type 2 diabetes than were those who slept 7 hours per night (odds ratio 1.57, 95% confidence interval [CI] 1.11–2.22), and so were those who slept 9 or more hours per night (odds ratio 1.57, 95% CI 1.10–2.24).
Kawakami et al17 followed 2,649 Japanese men for 8 years. Those who had difficulty going to sleep and staying asleep, which are both likely to result in shorter sleep duration, had higher age-adjusted risks of developing type 2 diabetes, with hazard ratios of 2.98 and 2.23, respectively.
Björkelund et al18 followed 6,599 nondiabetic Swedish men for an average of 15 years. Self-reported difficulty sleeping predicted the development of diabetes with an odds ratio of 1.52 even after controlling for age, body mass index at screening, changes in body mass index at follow-up, baseline glucose level, follow-up time, physical activity, family history of type 2 diabetes, smoking, social class, and alcohol intake.19
Interestingly, the authors found that the resting heart rate was higher at baseline in the men who later developed diabetes. This finding could be interpreted as reflecting greater sympathetic nervous system activity, a putative mediator of the metabolic dysfunction associated with both short sleep duration and obstructive sleep apnea.20,21
Meisinger et al,22 in a study of more than 8,000 nondiabetic German men and women 25 to 74 years old, found a hazard ratio of developing diabetes of 1.60 (95% CI 1.05–2.45) in men and 1.98 (95% CI 1.20–3.29) in women who reported difficulty staying asleep, who thus would have shortened sleep duration. This effect was independent of other risk factors for diabetes.
Yaggi et al,23 in a prospective study of 1,139 US men, also found a U-shaped relationship between sleep duration and the incidence of diabetes, with higher rates in people who slept less than 5 or more than 8 hours per night.
Cappuccio et al24 performed a meta-analysis of all the prospective studies published to date. Their review included 10 prospective studies, with 107,756 participants followed for a median of 9.5 years. Sleep duration and sleep disturbances were self-reported in all the studies. They calculated that the risk of developing diabetes was 28% higher with short sleep duration (≤ 5 or < 6 hours in the different studies), 48% higher with long sleep duration (> 8 hours), 57% higher with difficulty going to sleep, and 84% higher with difficulty staying asleep.
Limitations of these studies. A consideration when trying to interpret the relationship between length of sleep and the incidence of diabetes is that sleep duration in these studies was self-reported, not measured. If a patient reports sleeping more than 8 hours per night, it could mean that he or she is not truly getting so much sleep, but rather is spending more time in bed trying to sleep.
Another possibility is that the higher incidence of type 2 diabetes in people who slept longer is due to undiagnosed obstructive sleep apnea, which is associated with daytime sleepiness and possibly longer sleep time to compensate for inefficient sleep.
Finally, depressive symptoms, unemployment, a low level of physical activity, and undiagnosed health conditions have all been associated with long sleep duration and could affect the relationship with diabetes risk.
In summary, epidemiologic studies from different geographic locations have consistently indicated that short sleep or poor sleep may increase the risk of developing type 2 diabetes mellitus and suggest that such an association spans different countries, cultures, and ethnic groups.
Therefore, there is a need for additional prospective epidemiologic studies that use objective measures of sleep. Furthermore, studies need to determine whether the cause of sleep restriction (eg, insomnia vs lifestyle choice) affects this relationship. Randomized, controlled, interventional studies would also be useful to determine whether lengthening sleep duration affects the development of impaired glucose tolerance or type 2 diabetes mellitus.
Putative mechanisms linking short sleep duration and the risk of diabetes
The effects of sleep loss on glucose metabolism are likely multifactorial, involving several interacting pathways.
Decreased brain glucose utilization has been shown on positron emission tomography in sleep-deprived subjects.25
Hormonal dysregulation. Sleep deprivation is associated with disturbances in the secretion of the counterregulatory hormones growth hormone26 and cortisol.11
Young, healthy volunteers who were allowed to sleep only 4 hours per night for 6 nights showed a change in their patterns of growth hormone release, from a normal single pulse to a biphasic pattern.26 They were exposed to a higher overall amount of growth hormone in the sleep-deprived condition, which could contribute to higher glucose levels.
Also, evening cortisol levels were significantly higher in young, healthy men who were allowed to sleep only 4 hours per night for 6 nights,11 as well as in young, healthy women who were allowed to sleep only 3 hours for 1 night.27 A cross-sectional analysis that included 2,751 men and women also demonstrated that short sleep duration and sleep disturbances are independently associated with more cortisol secretion in the evening.28 Elevated evening cortisol levels can lead to morning insulin resistance.29
Inflammation. Levels of inflammatory cytokines, inflammation, or both increase as sleep duration decreases, which in turn can also increase insulin resistance.30,31
Sympathetic nervous system activity. Patients who have been sleep-deprived have been shown to have higher sympathetic nervous system activity, lower parasympathetic activity, or both.11,32 The sympathetic nervous system inhibits insulin release while the parasympathetic system stimulates it, so these changes both increase glucose levels.33 Moreover, overactivity of the sympathetic nervous system results in insulin resistance.34
Excess weight is a well-established risk factor for type 2 diabetes mellitus, and several epidemiologic studies have suggested that sleep loss may increase the risk of becoming overweight or obese,1,35 which would ultimately increase the risk of type 2 diabetes.
A primary mechanism linking sleep deprivation and weight gain is likely to be hyperactivity of the orexin system. Orexigenic neurons play a central role in wakefulness, but, as suggested by the name, they also promote feeding.36 Studies in animals have indicated that the orexin system is overactive during sleep deprivation,37–39 and this could be in part mediated by the increase in sympathetic activity.
Increased sympathetic activity also affects the levels of peripheral appetite hormones, inhibiting leptin release40 and stimulating ghrelin release.41 Lower leptin levels and higher ghrelin levels act in concert to further activate orexin neurons,42,43 resulting in increased food intake.
One could also argue that less time sleeping also allows more opportunity to eat.44
Reduced energy expenditure. Sleep loss and its associated sleepiness and fatigue may result in reduced energy expenditure, partly due to less exercise but also due to less nonexercise activity thermogenesis. To date, reduced energy expenditure is an unexplored pathway that could link short sleep, the risk of obesity, and ultimately diabetes. In many overweight and obese people, this cascade of negative events is likely to be accelerated by sleep-disordered breathing, a reported independent risk factor for insulin resistance.45,46
SLEEP QUALITY AND THE RISK OF DIABETES
Slow-wave sleep and diabetes
Slow-wave sleep, the most restorative sleep, is associated with metabolic, hormonal, and neurophysiologic changes that affect glucose homeostasis. Its disturbance may have deleterious effects on glucose tolerance.
Shallow slow-wave sleep occurs in elderly people47 and in obese people, even in the absence of obstructive sleep apnea.48,49 Both groups are also at higher risk of diabetes.50 One wonders if the decreased slow-wave sleep could in part contribute to the risk of diabetes in these groups.
A few studies specifically tested the effect of experimental suppression of slow-wave sleep on glucose homeostasis.
Tasali et al51 evaluated nine young, lean, nondiabetic men and women after 2 consecutive nights of undisturbed sleep and after 3 consecutive nights of suppressed slow-wave sleep without a change in total sleep duration or in REM sleep duration. Slow-wave sleep was disturbed by “delivering acoustic stimuli of various frequencies and intensities” whenever the subjects started to go into stage 3 or stage 4 sleep. This decreased the amount of slow-wave sleep by nearly 90%, which is comparable to the degree of sleep fragmentation seen in moderate to severe obstructive sleep apnea. After 3 nights of slow-wave sleep suppression, insulin sensitivity decreased by 25%, without a compensatory increase in insulin release, which resulted in a reduction in glucose tolerance of 23%, a value seen in older adults with impaired glucose tolerance.52
Stamatakis et al53 confirmed these findings in a similar study of 11 healthy, normal volunteers whose sleep was fragmented for 2 nights across all stages of sleep using auditory and mechanical stimuli. Insulin sensitivity significantly decreased, as did glucose effectiveness (ability of glucose to dispose itself independently of an insulin response) after the 2 nights of disturbed sleep quality.
These results support the hypothesis that poor sleep quality with short durations of slow-wave sleep, as seen with aging and obesity, could contribute to the higher risk of type 2 diabetes in these populations. These data also suggest that more studies are needed to look at the relationship between amount and quality of slow-wave sleep and diabetes risk.
Obstructive sleep apnea and diabetes
The most robust evidence that not only short sleep duration but also poor sleep quality affects diabetes risk comes from studies of metabolic function in patients with obstructive sleep apnea, an increasingly common condition.
Obstructive sleep apnea is characterized by recurrent episodes of partial or complete upper airway obstruction with intermittent hypoxia and microarousals, resulting in low amounts of slow-wave sleep and overall decreased sleep quality.54
Obstructive sleep apnea is common in patients with type 2 diabetes, and several clinical and epidemiologic studies suggest that, untreated, it may worsen diabetes risk or control.21,45–46,55–59
The Sleep AHEAD (Action for Health in Diabetes) study60 revealed, in cross-sectional data, that more than 84% of obese patients with type 2 diabetes had obstructive sleep apnea (with an apnea-hypopnea index ≥ 5).
Aronsohn et al,5 in a study conducted in our laboratory in 60 patients with type 2 diabetes, found that 46 (77%) of them had obstructive sleep apnea. Furthermore, the worse the obstructive sleep apnea, the worse the glucose control. After controlling for age, sex, race, body mass index, number of diabetes medications, level of exercise, years of diabetes, and total sleep time, compared with patients without obstructive sleep apnea, the adjusted mean hemoglobin A1c was increased in a linear trend by (in absolute percentage points):
- 1.49% in patients with mild obstructive sleep apnea (P = .0028)
- 1.93% in patients with moderate obstructive sleep apnea (P = .0033)
- 3.69% in patients with severe obstructive sleep apnea (P < .0001).
Other epidemiologic studies. A growing number of epidemiologic studies, in various geographic regions, have suggested an independent link between obstructive sleep apnea and risk of type 2 diabetes.61 Most of the studies have been cross-sectional, and while most had positive findings, a criticism is that the methodology varied among the studies, both in how obstructive sleep apnea was assessed (snoring vs polysomnography) and in the metabolic assessment (oral glucose tolerance test, homeostatic model assessment, hemoglobin A1c, medical history, physician examination, or patient report).
A more recent prospective study of 544 nondiabetic patients65 showed that the risk of developing type 2 diabetes over an average of 2.7 years of follow-up was a function of the severity of obstructive sleep apnea expressed in quartiles: for each increased quartile of severity there was a 43% increase in the incidence of diabetes. Additionally, in patients with moderate to severe sleep apnea, regular use of continuous positive airway pressure (CPAP) was associated with an attenuated risk.65
Two prospective studies (not included in Table 1) used snoring as a marker of obstructive sleep apnea; at 10 years of follow-up, snoring was associated with a higher risk of developing diabetes in both men and women.73,74
Does CPAP improve glucose metabolism? Other studies have specifically examined the effects of CPAP treatment on glucose metabolism, in both diabetic and nondiabetic populations. Accumulating evidence suggests that metabolic abnormalities can be partially corrected by CPAP treatment, which supports the concept of a causal link between obstructive sleep apnea and altered glucose control. This topic is beyond the scope of this review; please see previously published literature61,75 for further information. Whether treating obstructive sleep apnea may delay the development or reduce the severity of type 2 diabetes is another important unanswered question.
Is obstructive sleep apnea a cause or consequence of diabetes? It may be a novel risk factor for type 2 diabetes, and its association with altered glucose metabolism is well supported by a large set of cross-sectional studies, but there are still insufficient longitudinal studies to indicate a direction of causality.
If obstructive sleep apnea is the cause, what is the mechanism? There are likely many. High levels of sympathetic nervous system activity, intermittent hypoxia, sleep fragmentation, and sleep loss in obstructive sleep apnea may all lead to dysregulation of the hypothalamic-pituitary axis, endothelial dysfunction, and alterations in cytokine and adipokine release and are all potential mechanisms of abnormal glucose metabolism in this population.
WHAT TO TELL PATIENTS
Taken together, the current evidence suggests that strategies to improve the duration and the quality of sleep should be considered as a potential intervention to prevent or delay the development of type 2 diabetes mellitus in at-risk populations. While further studies are needed to better elucidate the mechanisms of the relationship between sleep loss and diabetes risk and to determine if extending sleep and treating obstructive sleep apnea decreases the risk of diabetes, we urge clinicians to recommend at least 7 hours of uninterrupted sleep per night as a goal in maintaining a healthy lifestyle. Additionally, clinicians should systematically evaluate the risk of obstructive sleep apnea in their patients who have type 2 diabetes mellitus and the metabolic syndrome, and conversely, should assess for diabetes in patients with known obstructive sleep apnea.
- Pannain S, Van Cauter E. Sleep loss, obesity and diabetes: prevalence, association and emerging evidence for causation. Obesity Metab 2008; 4:28–41.
- Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol 2008; 159(suppl 1):S59–S66.
- US Centers for Disease Control and Prevention (CDC). Perceived insufficient rest or sleep among adults—United States, 2008. MMWR Morb Mortal Wkly Rep 2009; 58:1175–1179.
- Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med 2006; 166:1768–1774.
- Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med 2010; 181:507–513.
- Broussard J, Knutson KL. Sleep and metabolic risk and disease. In:Cappuccio FP, Miller MA, Lockley SW, editors. Sleep, Health and Society: From Aetiology to Public Health. Cary, NC: Oxford University Press; 2010:111–140.
- Zoccoli G, Walker AM, Lenzi P, Franzini C. The cerebral circulation during sleep: regulation mechanisms and functional implications. Sleep Med Rev 2002; 6:443–455.
- Pannain S, Van Cauter E. Modulation of endocrine function by sleepwake homeostasis and circadian rhythmicity. Sleep Med Clin 2007; 2:147–159.
- Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 1993; 328:303–307.
- Kuhn E, Brodan V, Brodanová M, Rysánek K. Metabolic reflection of sleep deprivation. Act Nerv Super (Praha) 1969; 11:165–174.
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999; 354:1435–1439.
- Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab 2009; 94:3242–3250.
- Zizi F, Jean-Louis G, Brown CD, Ogedegbe G, Boutin-Foster C, McFarlane SI. Sleep duration and the risk of diabetes mellitus: epidemiologic evidence and pathophysiologic insights. Curr Diab Rep 2010; 10:43–47.
- Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005; 165:863–867.
- Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of selfreported sleep duration and incident diabetes in women. Diabetes Care 2003; 26:380–384.
- Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep 2007; 30:1667–1673.
- Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care 2004; 27:282–283.
- Björkelund C, Bondyr-Carlsson D, Lapidus L, et al. Sleep disturbances in midlife unrelated to 32-year diabetes incidence: the prospective population study of women in Gothenburg. Diabetes Care 2005; 28:2739–2744.
- Nilsson PM, Rööst M, Engström G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care 2004; 27:2464–2469.
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141:846–850.
- Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol 2005; 99:1998–2007.
- Meisinger C, Heier M, Loewel H; MONICA/KORA Augsburg Cohort Study. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia 2005; 48:235–241.
- Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 2006; 29:657–661.
- Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2010; 33:414–420.
- Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 2000; 9:335–352.
- Spiegel K, Leproult R, Colecchia EF, et al. Adaptation of the 24-h growth hormone profile to a state of sleep debt. Am J Physiol Regul Integr Comp Physiol 2000; 279:R874–R883.
- Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav 2010; 99:651–656.
- Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Selfreported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab 2009; 94:4801–4809.
- Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev 1997; 18:716–738.
- Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab 1999; 84:2603–2607.
- Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 2004; 89:2119–2126.
- Spiegel K, Leproult R, L’hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004; 89:5762–5771.
- Teff KL. Visceral nerves: vagal and sympathetic innervation. JPEN J Parenter Enteral Nutr 2008; 32:569–571.
- Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens 2001; 14:304S–309S.
- Cappuccio F, Miller MA. The epidemiology of sleep and cardiovascular risk and disease. In:Cappuccio FP, Miller MA, Lockley SW, editors. Sleep, Health and Society: From Aetiology to Public Health. Cary, NC: Oxford University Press; 2010:111–140.
- Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med Rev 2005; 9:231–241.
- Wu MF, John J, Maidment N, Lam HA, Siegel JM. Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am J Physiol Regul Integr Comp Physiol 2002; 283:R1079–R1086.
- Estabrooke IV, McCarthy MT, Ko E, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci 2001; 21:1656–1662.
- Zeitzer JM, Buckmaster CL, Lyons DM, Mignot E. Increasing length of wakefulness and modulation of hypocretin-1 in the wake-consolidated squirrel monkey. Am J Physiol Regul Integr Comp Physiol 2007; 293:R1736–R1742.
- Rayner DV, Trayhurn P. Regulation of leptin production: sympathetic nervous system interactions. J Mol Med 2001; 79:8–20.
- van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 2004; 25:426–457.
- Samson WK, Taylor MM, Ferguson AV. Non-sleep effects of hypocretin/orexin. Sleep Med Rev 2005; 9:243–252.
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci 2001; 24:429–458.
- Qin LQ, Li J, Wang Y, Wang J, Xu JY, Kaneko T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci 2003; 73:2467–2475.
- Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 2002; 165:670–676.
- Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE; Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004; 160:521–530.
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 2000; 284:861–868.
- Resta O, Foschino Barbaro MP, Bonfitto P, et al. Low sleep quality and daytime sleepiness in obese patients without obstructive sleep apnoea syndrome. J Intern Med 2003; 253:536–543.
- Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med 1994; 154:1705–1711.
- Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003; 289:76–79.
- Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A 2008; 105:1044–1049.
- Prigeon RL, Kahn SE, Porte D. Changes in insulin sensitivity, glucose effectiveness, and B-cell function in regularly exercising subjects. Metabolism 1995; 44:1259–1263.
- Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010; 137:95–101.
- Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med 2005; 142:187–197.
- Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 2002; 165:677–682.
- Tassone F, Lanfranco F, Gianotti L, et al. Obstructive sleep apnoea syndrome impairs insulin sensitivity independently of anthropometric variables. Clin Endocrinol (Oxf) 2003; 59:374–379.
- Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J 2004; 25:735–741.
- Svatikova A, Wolk R, Gami AS, Pohanka M, Somers VK. Interactions between obstructive sleep apnea and the metabolic syndrome. Curr Diab Rep 2005; 5:53–58.
- Budhiraja R, Quan SF. Sleep-disordered breathing and cardiovascular health. Curr Opin Pulm Med 2005; 11:501–506.
- Foster GD, Sanders MH, Millman R, et al; Sleep AHEAD Research Group. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 2009; 32:1017–1019.
- Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 2008; 133:496–506.
- Stoohs RA, Facchini F, Guilleminault C. Insulin resistance and sleep-disordered breathing in healthy humans. Am J Respir Crit Care Med 1996; 154:170–174.
- Mahmood K, Akhter N, Eldeirawi K, et al. Prevalence of type 2 diabetes in patients with obstructive sleep apnea in a multi-ethnic sample. J Clin Sleep Med 2009; 5:215–221.
- Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med 2005; 172:1590–1595.
- Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med 2009; 122:1122–1127.
- Elmasry A, Lindberg E, Berne C, et al. Sleep-disordered breathing and glucose metabolism in hypertensive men: a population-based study. J Intern Med 2001; 249:153–161.
- Lam JC, Lam B, Lam CL, et al. Obstructive sleep apnea and the metabolic syndrome in community-based Chinese adults in Hong Kong. Respir Med 2006; 100:980–987.
- Okada M, Takamizawa A, Tsushima K, Urushihata K, Fujimoto K, Kubo K. Relationship between sleep-disordered breathing and lifestyle-related illnesses in subjects who have undergone health-screening. Intern Med 2006; 45:891–896.
- Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep 2006; 29:777–783.
- Seicean S, Kirchner HL, Gottlieb DJ, et al. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care 2008; 31:1001–1006.
- Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med 2009; 179:235–240.
- Steiropoulos P, Papanas N, Nena E, et al. Markers of glycemic control and insulin resistance in non-diabetic patients with obstructive sleep apnea hypopnea syndrome: does adherence to CPAP treatment improve glycemic control? Sleep Med 2009; 10:887–891.
- Al-Delaimy WK, Manson JE, Willett WC, Stampfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol 2002; 155:387–393.
- Elmasry A, Janson C, Lindberg E, Gislason T, Tageldin MA, Boman G. The role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male population. J Intern Med 2000; 248:13–20.
- Steiropoulos P, Papanas N, Nena E, Maltezos E, Bouros D. Continuous positive airway pressure treatment in patients with sleep apnoea: does it really improve glucose metabolism? Curr Diabetes Rev 2010; 6:156–166.
Adults are sleeping less and less in our society. Yet sleep is no longer thought of as strictly a restorative process for the body. The importance of sleep for metabolic function and specifically glucose homeostasis is now widely accepted, as many studies have shown a correlation between sleep deprivation or poor sleep quality and an increased risk of diabetes.
Obesity and aging are both associated with worse sleep. As the prevalence of obesity and diabetes increases, and as the number of elderly people increases, it is imperative to target sleep in the overall treatment of our patients.
In the pages that follow, we examine the evidence of a link between sleep loss (both short sleep duration and poor-quality sleep) and the risk of diabetes. (For evidence linking short sleep duration and the related problem of obesity, we invite the reader to refer to previous publications on the topic.1,2)
SLEEP LOSS, OBESITY, AND DIABETES ARE ALL ON THE RISE
The prevalence of obesity and, consequently, of type 2 diabetes mellitus has increased alarmingly worldwide and particularly in the United States in the past few decades. Such a rapid increase cannot be explained simply by an alteration in the genetic pool; it is more likely due to environmental, socioeconomic, behavioral, and demographic factors and the interaction between genetics and these factors. Besides traditional lifestyle factors such as high-calorie diets and sedentary habits, other, nontraditional behavioral and environmental factors could be contributing to the epidemic of obesity and diabetes.
At the same time, people are sleeping less, and sleep disorders are on the rise. According to recent polls from the US Centers for Disease Control and Prevention, approximately 29% of US adults report sleeping less than 7 hours per night, and 50 to 70 million have chronic sleep and wakefulness disorders.3
The sleep curtailment of our times probably is partly self-imposed, as the pace and the opportunities of modern society place more demands on time for work and leisure activities and leave less time for sleep.
The quality of sleep has also declined as the population has aged and as the prevalence of obesity and its related sleep disorders has increased. Furthermore, patients with type 2 diabetes tend to sleep less, and to sleep poorly.4,5 Poor sleep quality generally results in overall sleep loss.
GLUCOSE TOLERANCE HAS A CIRCADIAN RHYTHM
The human body regulates blood levels of glucose within a narrow range.
Glucose tolerance refers to the ability to maintain euglycemia by disposing of exogenous glucose via insulin-mediated and non–insulin-mediated mechanisms. Normal glucose tolerance depends on the ability of the pancreatic beta cells to produce insulin. As insulin sensitivity declines, insulin secretion increases to maintain normal glucose levels. Diabetes becomes manifest when the pancreatic beta cells fail to compensate for the decreased insulin sensitivity.
Glucose tolerance varies in a circadian rhythm, including during the different stages of sleep.
HOW SLEEP AFFECTS METABOLISM AND HORMONES
Sleep has often been thought of as a “restorative” process for the mind and the body; however, many studies have shown that it also directly affects many metabolic and hormonal processes.6
Sleep has five stages: rapid eye movement (REM) sleep and stages 1, 2, 3, and 4 of non-REM sleep. The deeper stages of non-REM sleep, ie, stages 3 and 4, are also known as slow-wave sleep and are thought to be the most restorative.
Additionally, the onset of slow-wave sleep is temporally associated with transient metabolic, hormonal, and neurophysiologic changes, all of which can affect glucose homeostasis. The brain uses less glucose,7 the pituitary gland releases more growth hormone and less corticotropin,8 the sympathetic nervous system is less active, and conversely, vagal tone is increased.9
As a result, in the first part of the night, when slow-wave sleep predominates, glucose metabolism is slower. These effects are reversed in the second part of the night, when REM sleep, stage 1, and awakening are more likely.
In view of these important changes in glucose metabolism during sleep, it is not surprising that getting less sleep or poorer sleep on a regular basis could affect overall glucose homeostasis.
SHORT SLEEP DURATION AND RISK OF DIABETES
Laboratory and epidemiologic evidence supports an association between short sleep duration (< 7 hours per night) and the risk of diabetes, and also between poor sleep quality and the risk of diabetes. We will explore putative mechanisms for these relationships.
Laboratory studies of short sleep duration and glucose metabolism
Studies in small numbers of healthy volunteers who underwent experimental sleep restriction or disruption have revealed mechanisms by which sleep loss might increase the risk of diabetes.
Kuhn et al10 performed the very first laboratory study of the effect of sleep deprivation on metabolism. Published in 1969, it showed that total sleep deprivation led to a marked increase in glucose levels.
A caution in extrapolating such results to real-life conditions is that total sleep deprivation is uncommon in humans and is inevitably followed by sleep recovery, with normalization of glucose metabolism. However, people in modern society are experiencing recurrent partial sleep deprivation, and its effect on glucose metabolism may be different.
Spiegel et al,11 in landmark laboratory studies of partial sleep deprivation in healthy, lean adults, found that restricting sleep to 4 hours per night for 6 nights resulted in a 40% decrease in glucose tolerance, to levels similar to those seen in older adults with impaired glucose tolerance. This metabolic change was paralleled by an increase in the activity of the sympathetic nervous system, and both of these effects reversed with sleep recovery.
A criticism of these initial studies is that they restricted sleep to 4 hours, a restriction more severe than that seen in real life.
Nedeltcheva et al12 more recently examined the effects of less-severe sleep curtailment (5.5 hours per night for 14 nights) in sedentary middle-aged men and women. This degree of bedtime restriction led to a decrease in glucose tolerance due to decreased insulin sensitivity in the absence of adequate beta cell compensation.
Such recurrent bedtime restriction is closer to the short sleep duration experienced by many people in everyday life, and in people at risk it may facilitate the development of insulin resistance, reduced glucose tolerance, and ultimately diabetes. Indeed, epidemiologic studies suggest that people who sleep less than 6 hours per night are at higher risk of type 2 diabetes.
Epidemiologic studies of short sleep duration and glucose metabolism
Multiple cross-sectional epidemiologic studies have suggested an association between short sleep duration and diabetes, and several prospective epidemiologic studies have suggested that short sleep actually plays a causative role in diabetes.
The landmark observations of Spiegel et al11 led to a number of epidemiologic studies examining the relationships between sleep duration and sleep disturbances and diabetes risk.13
The Sleep Heart Study14 was a large, cross-sectional, community-based study of the cardiovascular consequences of sleep-disordered breathing. The authors assessed the relationship between reported sleep duration and impaired glucose tolerance or type 2 diabetes in more than 1,400 men and women who had no history of insomnia. After adjustment for age, sex, race, body habitus, and apnea-hypopnea index, the prevalence of impaired glucose tolerance and type 2 diabetes was higher in those who reported sleeping 6 hours or less per night—or 9 hours or more per night (more below about the possible effect of too much sleep on the risk of diabetes).
The major limitations of the study were that it was cross-sectional in design, sleep duration was self-reported, the reasons for sleep curtailment were unknown, and possible confounding variables as physical activity, diet, and socioeconomic status were not measured.
Knutson et al,4 in our medical center, examined the association between self-reported sleep duration and sleep quality on the one hand and hemoglobin A1c levels on the other in 161 black patients with type 2 diabetes. In patients without diabetic complications, glycemic control correlated with perceived sleep debt (calculated as the difference between self-reported actual and preferred weekday sleep duration); the authors calculated that a perceived sleep debt of 3 hours per night predicted a hemoglobin A1c value 1.1 absolute percentage points higher than the median value. The analyses controlled for age, sex, body mass index, insulin use, and the presence of major complications; it excluded patients whose sleep was frequently disrupted by pain. The effect size was comparable to (but opposite) that of oral antidiabetic drugs. However, the direction of causality cannot be confirmed from this association, as it is possible that poor glycemic control in diabetic patients could impair their ability to achieve sufficient sleep.
To date, several major prospective studies have looked at the association between short sleep duration and sleep problems and the risk of developing type 2 diabetes in adults.
The Nurses Health Study15 followed 70,000 nondiabetic women for 10 years. Compared with nurses who slept 7 to 8 hours per 24 hours, those who slept 5 hours or less had a relative risk of diabetes of 1.34 even after controlling for many covariables, such as body mass index, shift work, hypertension, exercise, and depression.
The first National Health and Nutrition Examination Survey (NHANES I)16 examined the effect of sleep duration on the risk of incident diabetes in roughly 9,000 men and women over a period of 8 to 10 years. The statistical model included body mass index and hypertension and adjusted for physical activity, depression, alcohol consumption, ethnicity, education, marital status, and age. Findings: those who slept 5 hours or less per night were significantly more likely to develop type 2 diabetes than were those who slept 7 hours per night (odds ratio 1.57, 95% confidence interval [CI] 1.11–2.22), and so were those who slept 9 or more hours per night (odds ratio 1.57, 95% CI 1.10–2.24).
Kawakami et al17 followed 2,649 Japanese men for 8 years. Those who had difficulty going to sleep and staying asleep, which are both likely to result in shorter sleep duration, had higher age-adjusted risks of developing type 2 diabetes, with hazard ratios of 2.98 and 2.23, respectively.
Björkelund et al18 followed 6,599 nondiabetic Swedish men for an average of 15 years. Self-reported difficulty sleeping predicted the development of diabetes with an odds ratio of 1.52 even after controlling for age, body mass index at screening, changes in body mass index at follow-up, baseline glucose level, follow-up time, physical activity, family history of type 2 diabetes, smoking, social class, and alcohol intake.19
Interestingly, the authors found that the resting heart rate was higher at baseline in the men who later developed diabetes. This finding could be interpreted as reflecting greater sympathetic nervous system activity, a putative mediator of the metabolic dysfunction associated with both short sleep duration and obstructive sleep apnea.20,21
Meisinger et al,22 in a study of more than 8,000 nondiabetic German men and women 25 to 74 years old, found a hazard ratio of developing diabetes of 1.60 (95% CI 1.05–2.45) in men and 1.98 (95% CI 1.20–3.29) in women who reported difficulty staying asleep, who thus would have shortened sleep duration. This effect was independent of other risk factors for diabetes.
Yaggi et al,23 in a prospective study of 1,139 US men, also found a U-shaped relationship between sleep duration and the incidence of diabetes, with higher rates in people who slept less than 5 or more than 8 hours per night.
Cappuccio et al24 performed a meta-analysis of all the prospective studies published to date. Their review included 10 prospective studies, with 107,756 participants followed for a median of 9.5 years. Sleep duration and sleep disturbances were self-reported in all the studies. They calculated that the risk of developing diabetes was 28% higher with short sleep duration (≤ 5 or < 6 hours in the different studies), 48% higher with long sleep duration (> 8 hours), 57% higher with difficulty going to sleep, and 84% higher with difficulty staying asleep.
Limitations of these studies. A consideration when trying to interpret the relationship between length of sleep and the incidence of diabetes is that sleep duration in these studies was self-reported, not measured. If a patient reports sleeping more than 8 hours per night, it could mean that he or she is not truly getting so much sleep, but rather is spending more time in bed trying to sleep.
Another possibility is that the higher incidence of type 2 diabetes in people who slept longer is due to undiagnosed obstructive sleep apnea, which is associated with daytime sleepiness and possibly longer sleep time to compensate for inefficient sleep.
Finally, depressive symptoms, unemployment, a low level of physical activity, and undiagnosed health conditions have all been associated with long sleep duration and could affect the relationship with diabetes risk.
In summary, epidemiologic studies from different geographic locations have consistently indicated that short sleep or poor sleep may increase the risk of developing type 2 diabetes mellitus and suggest that such an association spans different countries, cultures, and ethnic groups.
Therefore, there is a need for additional prospective epidemiologic studies that use objective measures of sleep. Furthermore, studies need to determine whether the cause of sleep restriction (eg, insomnia vs lifestyle choice) affects this relationship. Randomized, controlled, interventional studies would also be useful to determine whether lengthening sleep duration affects the development of impaired glucose tolerance or type 2 diabetes mellitus.
Putative mechanisms linking short sleep duration and the risk of diabetes
The effects of sleep loss on glucose metabolism are likely multifactorial, involving several interacting pathways.
Decreased brain glucose utilization has been shown on positron emission tomography in sleep-deprived subjects.25
Hormonal dysregulation. Sleep deprivation is associated with disturbances in the secretion of the counterregulatory hormones growth hormone26 and cortisol.11
Young, healthy volunteers who were allowed to sleep only 4 hours per night for 6 nights showed a change in their patterns of growth hormone release, from a normal single pulse to a biphasic pattern.26 They were exposed to a higher overall amount of growth hormone in the sleep-deprived condition, which could contribute to higher glucose levels.
Also, evening cortisol levels were significantly higher in young, healthy men who were allowed to sleep only 4 hours per night for 6 nights,11 as well as in young, healthy women who were allowed to sleep only 3 hours for 1 night.27 A cross-sectional analysis that included 2,751 men and women also demonstrated that short sleep duration and sleep disturbances are independently associated with more cortisol secretion in the evening.28 Elevated evening cortisol levels can lead to morning insulin resistance.29
Inflammation. Levels of inflammatory cytokines, inflammation, or both increase as sleep duration decreases, which in turn can also increase insulin resistance.30,31
Sympathetic nervous system activity. Patients who have been sleep-deprived have been shown to have higher sympathetic nervous system activity, lower parasympathetic activity, or both.11,32 The sympathetic nervous system inhibits insulin release while the parasympathetic system stimulates it, so these changes both increase glucose levels.33 Moreover, overactivity of the sympathetic nervous system results in insulin resistance.34
Excess weight is a well-established risk factor for type 2 diabetes mellitus, and several epidemiologic studies have suggested that sleep loss may increase the risk of becoming overweight or obese,1,35 which would ultimately increase the risk of type 2 diabetes.
A primary mechanism linking sleep deprivation and weight gain is likely to be hyperactivity of the orexin system. Orexigenic neurons play a central role in wakefulness, but, as suggested by the name, they also promote feeding.36 Studies in animals have indicated that the orexin system is overactive during sleep deprivation,37–39 and this could be in part mediated by the increase in sympathetic activity.
Increased sympathetic activity also affects the levels of peripheral appetite hormones, inhibiting leptin release40 and stimulating ghrelin release.41 Lower leptin levels and higher ghrelin levels act in concert to further activate orexin neurons,42,43 resulting in increased food intake.
One could also argue that less time sleeping also allows more opportunity to eat.44
Reduced energy expenditure. Sleep loss and its associated sleepiness and fatigue may result in reduced energy expenditure, partly due to less exercise but also due to less nonexercise activity thermogenesis. To date, reduced energy expenditure is an unexplored pathway that could link short sleep, the risk of obesity, and ultimately diabetes. In many overweight and obese people, this cascade of negative events is likely to be accelerated by sleep-disordered breathing, a reported independent risk factor for insulin resistance.45,46
SLEEP QUALITY AND THE RISK OF DIABETES
Slow-wave sleep and diabetes
Slow-wave sleep, the most restorative sleep, is associated with metabolic, hormonal, and neurophysiologic changes that affect glucose homeostasis. Its disturbance may have deleterious effects on glucose tolerance.
Shallow slow-wave sleep occurs in elderly people47 and in obese people, even in the absence of obstructive sleep apnea.48,49 Both groups are also at higher risk of diabetes.50 One wonders if the decreased slow-wave sleep could in part contribute to the risk of diabetes in these groups.
A few studies specifically tested the effect of experimental suppression of slow-wave sleep on glucose homeostasis.
Tasali et al51 evaluated nine young, lean, nondiabetic men and women after 2 consecutive nights of undisturbed sleep and after 3 consecutive nights of suppressed slow-wave sleep without a change in total sleep duration or in REM sleep duration. Slow-wave sleep was disturbed by “delivering acoustic stimuli of various frequencies and intensities” whenever the subjects started to go into stage 3 or stage 4 sleep. This decreased the amount of slow-wave sleep by nearly 90%, which is comparable to the degree of sleep fragmentation seen in moderate to severe obstructive sleep apnea. After 3 nights of slow-wave sleep suppression, insulin sensitivity decreased by 25%, without a compensatory increase in insulin release, which resulted in a reduction in glucose tolerance of 23%, a value seen in older adults with impaired glucose tolerance.52
Stamatakis et al53 confirmed these findings in a similar study of 11 healthy, normal volunteers whose sleep was fragmented for 2 nights across all stages of sleep using auditory and mechanical stimuli. Insulin sensitivity significantly decreased, as did glucose effectiveness (ability of glucose to dispose itself independently of an insulin response) after the 2 nights of disturbed sleep quality.
These results support the hypothesis that poor sleep quality with short durations of slow-wave sleep, as seen with aging and obesity, could contribute to the higher risk of type 2 diabetes in these populations. These data also suggest that more studies are needed to look at the relationship between amount and quality of slow-wave sleep and diabetes risk.
Obstructive sleep apnea and diabetes
The most robust evidence that not only short sleep duration but also poor sleep quality affects diabetes risk comes from studies of metabolic function in patients with obstructive sleep apnea, an increasingly common condition.
Obstructive sleep apnea is characterized by recurrent episodes of partial or complete upper airway obstruction with intermittent hypoxia and microarousals, resulting in low amounts of slow-wave sleep and overall decreased sleep quality.54
Obstructive sleep apnea is common in patients with type 2 diabetes, and several clinical and epidemiologic studies suggest that, untreated, it may worsen diabetes risk or control.21,45–46,55–59
The Sleep AHEAD (Action for Health in Diabetes) study60 revealed, in cross-sectional data, that more than 84% of obese patients with type 2 diabetes had obstructive sleep apnea (with an apnea-hypopnea index ≥ 5).
Aronsohn et al,5 in a study conducted in our laboratory in 60 patients with type 2 diabetes, found that 46 (77%) of them had obstructive sleep apnea. Furthermore, the worse the obstructive sleep apnea, the worse the glucose control. After controlling for age, sex, race, body mass index, number of diabetes medications, level of exercise, years of diabetes, and total sleep time, compared with patients without obstructive sleep apnea, the adjusted mean hemoglobin A1c was increased in a linear trend by (in absolute percentage points):
- 1.49% in patients with mild obstructive sleep apnea (P = .0028)
- 1.93% in patients with moderate obstructive sleep apnea (P = .0033)
- 3.69% in patients with severe obstructive sleep apnea (P < .0001).
Other epidemiologic studies. A growing number of epidemiologic studies, in various geographic regions, have suggested an independent link between obstructive sleep apnea and risk of type 2 diabetes.61 Most of the studies have been cross-sectional, and while most had positive findings, a criticism is that the methodology varied among the studies, both in how obstructive sleep apnea was assessed (snoring vs polysomnography) and in the metabolic assessment (oral glucose tolerance test, homeostatic model assessment, hemoglobin A1c, medical history, physician examination, or patient report).
A more recent prospective study of 544 nondiabetic patients65 showed that the risk of developing type 2 diabetes over an average of 2.7 years of follow-up was a function of the severity of obstructive sleep apnea expressed in quartiles: for each increased quartile of severity there was a 43% increase in the incidence of diabetes. Additionally, in patients with moderate to severe sleep apnea, regular use of continuous positive airway pressure (CPAP) was associated with an attenuated risk.65
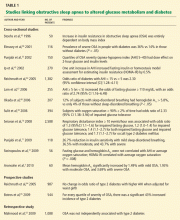
Two prospective studies (not included in Table 1) used snoring as a marker of obstructive sleep apnea; at 10 years of follow-up, snoring was associated with a higher risk of developing diabetes in both men and women.73,74
Does CPAP improve glucose metabolism? Other studies have specifically examined the effects of CPAP treatment on glucose metabolism, in both diabetic and nondiabetic populations. Accumulating evidence suggests that metabolic abnormalities can be partially corrected by CPAP treatment, which supports the concept of a causal link between obstructive sleep apnea and altered glucose control. This topic is beyond the scope of this review; please see previously published literature61,75 for further information. Whether treating obstructive sleep apnea may delay the development or reduce the severity of type 2 diabetes is another important unanswered question.
Is obstructive sleep apnea a cause or consequence of diabetes? It may be a novel risk factor for type 2 diabetes, and its association with altered glucose metabolism is well supported by a large set of cross-sectional studies, but there are still insufficient longitudinal studies to indicate a direction of causality.
If obstructive sleep apnea is the cause, what is the mechanism? There are likely many. High levels of sympathetic nervous system activity, intermittent hypoxia, sleep fragmentation, and sleep loss in obstructive sleep apnea may all lead to dysregulation of the hypothalamic-pituitary axis, endothelial dysfunction, and alterations in cytokine and adipokine release and are all potential mechanisms of abnormal glucose metabolism in this population.
WHAT TO TELL PATIENTS
Taken together, the current evidence suggests that strategies to improve the duration and the quality of sleep should be considered as a potential intervention to prevent or delay the development of type 2 diabetes mellitus in at-risk populations. While further studies are needed to better elucidate the mechanisms of the relationship between sleep loss and diabetes risk and to determine if extending sleep and treating obstructive sleep apnea decreases the risk of diabetes, we urge clinicians to recommend at least 7 hours of uninterrupted sleep per night as a goal in maintaining a healthy lifestyle. Additionally, clinicians should systematically evaluate the risk of obstructive sleep apnea in their patients who have type 2 diabetes mellitus and the metabolic syndrome, and conversely, should assess for diabetes in patients with known obstructive sleep apnea.
Adults are sleeping less and less in our society. Yet sleep is no longer thought of as strictly a restorative process for the body. The importance of sleep for metabolic function and specifically glucose homeostasis is now widely accepted, as many studies have shown a correlation between sleep deprivation or poor sleep quality and an increased risk of diabetes.
Obesity and aging are both associated with worse sleep. As the prevalence of obesity and diabetes increases, and as the number of elderly people increases, it is imperative to target sleep in the overall treatment of our patients.
In the pages that follow, we examine the evidence of a link between sleep loss (both short sleep duration and poor-quality sleep) and the risk of diabetes. (For evidence linking short sleep duration and the related problem of obesity, we invite the reader to refer to previous publications on the topic.1,2)
SLEEP LOSS, OBESITY, AND DIABETES ARE ALL ON THE RISE
The prevalence of obesity and, consequently, of type 2 diabetes mellitus has increased alarmingly worldwide and particularly in the United States in the past few decades. Such a rapid increase cannot be explained simply by an alteration in the genetic pool; it is more likely due to environmental, socioeconomic, behavioral, and demographic factors and the interaction between genetics and these factors. Besides traditional lifestyle factors such as high-calorie diets and sedentary habits, other, nontraditional behavioral and environmental factors could be contributing to the epidemic of obesity and diabetes.
At the same time, people are sleeping less, and sleep disorders are on the rise. According to recent polls from the US Centers for Disease Control and Prevention, approximately 29% of US adults report sleeping less than 7 hours per night, and 50 to 70 million have chronic sleep and wakefulness disorders.3
The sleep curtailment of our times probably is partly self-imposed, as the pace and the opportunities of modern society place more demands on time for work and leisure activities and leave less time for sleep.
The quality of sleep has also declined as the population has aged and as the prevalence of obesity and its related sleep disorders has increased. Furthermore, patients with type 2 diabetes tend to sleep less, and to sleep poorly.4,5 Poor sleep quality generally results in overall sleep loss.
GLUCOSE TOLERANCE HAS A CIRCADIAN RHYTHM
The human body regulates blood levels of glucose within a narrow range.
Glucose tolerance refers to the ability to maintain euglycemia by disposing of exogenous glucose via insulin-mediated and non–insulin-mediated mechanisms. Normal glucose tolerance depends on the ability of the pancreatic beta cells to produce insulin. As insulin sensitivity declines, insulin secretion increases to maintain normal glucose levels. Diabetes becomes manifest when the pancreatic beta cells fail to compensate for the decreased insulin sensitivity.
Glucose tolerance varies in a circadian rhythm, including during the different stages of sleep.
HOW SLEEP AFFECTS METABOLISM AND HORMONES
Sleep has often been thought of as a “restorative” process for the mind and the body; however, many studies have shown that it also directly affects many metabolic and hormonal processes.6
Sleep has five stages: rapid eye movement (REM) sleep and stages 1, 2, 3, and 4 of non-REM sleep. The deeper stages of non-REM sleep, ie, stages 3 and 4, are also known as slow-wave sleep and are thought to be the most restorative.
Additionally, the onset of slow-wave sleep is temporally associated with transient metabolic, hormonal, and neurophysiologic changes, all of which can affect glucose homeostasis. The brain uses less glucose,7 the pituitary gland releases more growth hormone and less corticotropin,8 the sympathetic nervous system is less active, and conversely, vagal tone is increased.9
As a result, in the first part of the night, when slow-wave sleep predominates, glucose metabolism is slower. These effects are reversed in the second part of the night, when REM sleep, stage 1, and awakening are more likely.
In view of these important changes in glucose metabolism during sleep, it is not surprising that getting less sleep or poorer sleep on a regular basis could affect overall glucose homeostasis.
SHORT SLEEP DURATION AND RISK OF DIABETES
Laboratory and epidemiologic evidence supports an association between short sleep duration (< 7 hours per night) and the risk of diabetes, and also between poor sleep quality and the risk of diabetes. We will explore putative mechanisms for these relationships.
Laboratory studies of short sleep duration and glucose metabolism
Studies in small numbers of healthy volunteers who underwent experimental sleep restriction or disruption have revealed mechanisms by which sleep loss might increase the risk of diabetes.
Kuhn et al10 performed the very first laboratory study of the effect of sleep deprivation on metabolism. Published in 1969, it showed that total sleep deprivation led to a marked increase in glucose levels.
A caution in extrapolating such results to real-life conditions is that total sleep deprivation is uncommon in humans and is inevitably followed by sleep recovery, with normalization of glucose metabolism. However, people in modern society are experiencing recurrent partial sleep deprivation, and its effect on glucose metabolism may be different.
Spiegel et al,11 in landmark laboratory studies of partial sleep deprivation in healthy, lean adults, found that restricting sleep to 4 hours per night for 6 nights resulted in a 40% decrease in glucose tolerance, to levels similar to those seen in older adults with impaired glucose tolerance. This metabolic change was paralleled by an increase in the activity of the sympathetic nervous system, and both of these effects reversed with sleep recovery.
A criticism of these initial studies is that they restricted sleep to 4 hours, a restriction more severe than that seen in real life.
Nedeltcheva et al12 more recently examined the effects of less-severe sleep curtailment (5.5 hours per night for 14 nights) in sedentary middle-aged men and women. This degree of bedtime restriction led to a decrease in glucose tolerance due to decreased insulin sensitivity in the absence of adequate beta cell compensation.
Such recurrent bedtime restriction is closer to the short sleep duration experienced by many people in everyday life, and in people at risk it may facilitate the development of insulin resistance, reduced glucose tolerance, and ultimately diabetes. Indeed, epidemiologic studies suggest that people who sleep less than 6 hours per night are at higher risk of type 2 diabetes.
Epidemiologic studies of short sleep duration and glucose metabolism
Multiple cross-sectional epidemiologic studies have suggested an association between short sleep duration and diabetes, and several prospective epidemiologic studies have suggested that short sleep actually plays a causative role in diabetes.
The landmark observations of Spiegel et al11 led to a number of epidemiologic studies examining the relationships between sleep duration and sleep disturbances and diabetes risk.13
The Sleep Heart Study14 was a large, cross-sectional, community-based study of the cardiovascular consequences of sleep-disordered breathing. The authors assessed the relationship between reported sleep duration and impaired glucose tolerance or type 2 diabetes in more than 1,400 men and women who had no history of insomnia. After adjustment for age, sex, race, body habitus, and apnea-hypopnea index, the prevalence of impaired glucose tolerance and type 2 diabetes was higher in those who reported sleeping 6 hours or less per night—or 9 hours or more per night (more below about the possible effect of too much sleep on the risk of diabetes).
The major limitations of the study were that it was cross-sectional in design, sleep duration was self-reported, the reasons for sleep curtailment were unknown, and possible confounding variables as physical activity, diet, and socioeconomic status were not measured.
Knutson et al,4 in our medical center, examined the association between self-reported sleep duration and sleep quality on the one hand and hemoglobin A1c levels on the other in 161 black patients with type 2 diabetes. In patients without diabetic complications, glycemic control correlated with perceived sleep debt (calculated as the difference between self-reported actual and preferred weekday sleep duration); the authors calculated that a perceived sleep debt of 3 hours per night predicted a hemoglobin A1c value 1.1 absolute percentage points higher than the median value. The analyses controlled for age, sex, body mass index, insulin use, and the presence of major complications; it excluded patients whose sleep was frequently disrupted by pain. The effect size was comparable to (but opposite) that of oral antidiabetic drugs. However, the direction of causality cannot be confirmed from this association, as it is possible that poor glycemic control in diabetic patients could impair their ability to achieve sufficient sleep.
To date, several major prospective studies have looked at the association between short sleep duration and sleep problems and the risk of developing type 2 diabetes in adults.
The Nurses Health Study15 followed 70,000 nondiabetic women for 10 years. Compared with nurses who slept 7 to 8 hours per 24 hours, those who slept 5 hours or less had a relative risk of diabetes of 1.34 even after controlling for many covariables, such as body mass index, shift work, hypertension, exercise, and depression.
The first National Health and Nutrition Examination Survey (NHANES I)16 examined the effect of sleep duration on the risk of incident diabetes in roughly 9,000 men and women over a period of 8 to 10 years. The statistical model included body mass index and hypertension and adjusted for physical activity, depression, alcohol consumption, ethnicity, education, marital status, and age. Findings: those who slept 5 hours or less per night were significantly more likely to develop type 2 diabetes than were those who slept 7 hours per night (odds ratio 1.57, 95% confidence interval [CI] 1.11–2.22), and so were those who slept 9 or more hours per night (odds ratio 1.57, 95% CI 1.10–2.24).
Kawakami et al17 followed 2,649 Japanese men for 8 years. Those who had difficulty going to sleep and staying asleep, which are both likely to result in shorter sleep duration, had higher age-adjusted risks of developing type 2 diabetes, with hazard ratios of 2.98 and 2.23, respectively.
Björkelund et al18 followed 6,599 nondiabetic Swedish men for an average of 15 years. Self-reported difficulty sleeping predicted the development of diabetes with an odds ratio of 1.52 even after controlling for age, body mass index at screening, changes in body mass index at follow-up, baseline glucose level, follow-up time, physical activity, family history of type 2 diabetes, smoking, social class, and alcohol intake.19
Interestingly, the authors found that the resting heart rate was higher at baseline in the men who later developed diabetes. This finding could be interpreted as reflecting greater sympathetic nervous system activity, a putative mediator of the metabolic dysfunction associated with both short sleep duration and obstructive sleep apnea.20,21
Meisinger et al,22 in a study of more than 8,000 nondiabetic German men and women 25 to 74 years old, found a hazard ratio of developing diabetes of 1.60 (95% CI 1.05–2.45) in men and 1.98 (95% CI 1.20–3.29) in women who reported difficulty staying asleep, who thus would have shortened sleep duration. This effect was independent of other risk factors for diabetes.
Yaggi et al,23 in a prospective study of 1,139 US men, also found a U-shaped relationship between sleep duration and the incidence of diabetes, with higher rates in people who slept less than 5 or more than 8 hours per night.
Cappuccio et al24 performed a meta-analysis of all the prospective studies published to date. Their review included 10 prospective studies, with 107,756 participants followed for a median of 9.5 years. Sleep duration and sleep disturbances were self-reported in all the studies. They calculated that the risk of developing diabetes was 28% higher with short sleep duration (≤ 5 or < 6 hours in the different studies), 48% higher with long sleep duration (> 8 hours), 57% higher with difficulty going to sleep, and 84% higher with difficulty staying asleep.
Limitations of these studies. A consideration when trying to interpret the relationship between length of sleep and the incidence of diabetes is that sleep duration in these studies was self-reported, not measured. If a patient reports sleeping more than 8 hours per night, it could mean that he or she is not truly getting so much sleep, but rather is spending more time in bed trying to sleep.
Another possibility is that the higher incidence of type 2 diabetes in people who slept longer is due to undiagnosed obstructive sleep apnea, which is associated with daytime sleepiness and possibly longer sleep time to compensate for inefficient sleep.
Finally, depressive symptoms, unemployment, a low level of physical activity, and undiagnosed health conditions have all been associated with long sleep duration and could affect the relationship with diabetes risk.
In summary, epidemiologic studies from different geographic locations have consistently indicated that short sleep or poor sleep may increase the risk of developing type 2 diabetes mellitus and suggest that such an association spans different countries, cultures, and ethnic groups.
Therefore, there is a need for additional prospective epidemiologic studies that use objective measures of sleep. Furthermore, studies need to determine whether the cause of sleep restriction (eg, insomnia vs lifestyle choice) affects this relationship. Randomized, controlled, interventional studies would also be useful to determine whether lengthening sleep duration affects the development of impaired glucose tolerance or type 2 diabetes mellitus.
Putative mechanisms linking short sleep duration and the risk of diabetes
The effects of sleep loss on glucose metabolism are likely multifactorial, involving several interacting pathways.
Decreased brain glucose utilization has been shown on positron emission tomography in sleep-deprived subjects.25
Hormonal dysregulation. Sleep deprivation is associated with disturbances in the secretion of the counterregulatory hormones growth hormone26 and cortisol.11
Young, healthy volunteers who were allowed to sleep only 4 hours per night for 6 nights showed a change in their patterns of growth hormone release, from a normal single pulse to a biphasic pattern.26 They were exposed to a higher overall amount of growth hormone in the sleep-deprived condition, which could contribute to higher glucose levels.
Also, evening cortisol levels were significantly higher in young, healthy men who were allowed to sleep only 4 hours per night for 6 nights,11 as well as in young, healthy women who were allowed to sleep only 3 hours for 1 night.27 A cross-sectional analysis that included 2,751 men and women also demonstrated that short sleep duration and sleep disturbances are independently associated with more cortisol secretion in the evening.28 Elevated evening cortisol levels can lead to morning insulin resistance.29
Inflammation. Levels of inflammatory cytokines, inflammation, or both increase as sleep duration decreases, which in turn can also increase insulin resistance.30,31
Sympathetic nervous system activity. Patients who have been sleep-deprived have been shown to have higher sympathetic nervous system activity, lower parasympathetic activity, or both.11,32 The sympathetic nervous system inhibits insulin release while the parasympathetic system stimulates it, so these changes both increase glucose levels.33 Moreover, overactivity of the sympathetic nervous system results in insulin resistance.34
Excess weight is a well-established risk factor for type 2 diabetes mellitus, and several epidemiologic studies have suggested that sleep loss may increase the risk of becoming overweight or obese,1,35 which would ultimately increase the risk of type 2 diabetes.
A primary mechanism linking sleep deprivation and weight gain is likely to be hyperactivity of the orexin system. Orexigenic neurons play a central role in wakefulness, but, as suggested by the name, they also promote feeding.36 Studies in animals have indicated that the orexin system is overactive during sleep deprivation,37–39 and this could be in part mediated by the increase in sympathetic activity.
Increased sympathetic activity also affects the levels of peripheral appetite hormones, inhibiting leptin release40 and stimulating ghrelin release.41 Lower leptin levels and higher ghrelin levels act in concert to further activate orexin neurons,42,43 resulting in increased food intake.
One could also argue that less time sleeping also allows more opportunity to eat.44
Reduced energy expenditure. Sleep loss and its associated sleepiness and fatigue may result in reduced energy expenditure, partly due to less exercise but also due to less nonexercise activity thermogenesis. To date, reduced energy expenditure is an unexplored pathway that could link short sleep, the risk of obesity, and ultimately diabetes. In many overweight and obese people, this cascade of negative events is likely to be accelerated by sleep-disordered breathing, a reported independent risk factor for insulin resistance.45,46
SLEEP QUALITY AND THE RISK OF DIABETES
Slow-wave sleep and diabetes
Slow-wave sleep, the most restorative sleep, is associated with metabolic, hormonal, and neurophysiologic changes that affect glucose homeostasis. Its disturbance may have deleterious effects on glucose tolerance.
Shallow slow-wave sleep occurs in elderly people47 and in obese people, even in the absence of obstructive sleep apnea.48,49 Both groups are also at higher risk of diabetes.50 One wonders if the decreased slow-wave sleep could in part contribute to the risk of diabetes in these groups.
A few studies specifically tested the effect of experimental suppression of slow-wave sleep on glucose homeostasis.
Tasali et al51 evaluated nine young, lean, nondiabetic men and women after 2 consecutive nights of undisturbed sleep and after 3 consecutive nights of suppressed slow-wave sleep without a change in total sleep duration or in REM sleep duration. Slow-wave sleep was disturbed by “delivering acoustic stimuli of various frequencies and intensities” whenever the subjects started to go into stage 3 or stage 4 sleep. This decreased the amount of slow-wave sleep by nearly 90%, which is comparable to the degree of sleep fragmentation seen in moderate to severe obstructive sleep apnea. After 3 nights of slow-wave sleep suppression, insulin sensitivity decreased by 25%, without a compensatory increase in insulin release, which resulted in a reduction in glucose tolerance of 23%, a value seen in older adults with impaired glucose tolerance.52
Stamatakis et al53 confirmed these findings in a similar study of 11 healthy, normal volunteers whose sleep was fragmented for 2 nights across all stages of sleep using auditory and mechanical stimuli. Insulin sensitivity significantly decreased, as did glucose effectiveness (ability of glucose to dispose itself independently of an insulin response) after the 2 nights of disturbed sleep quality.
These results support the hypothesis that poor sleep quality with short durations of slow-wave sleep, as seen with aging and obesity, could contribute to the higher risk of type 2 diabetes in these populations. These data also suggest that more studies are needed to look at the relationship between amount and quality of slow-wave sleep and diabetes risk.
Obstructive sleep apnea and diabetes
The most robust evidence that not only short sleep duration but also poor sleep quality affects diabetes risk comes from studies of metabolic function in patients with obstructive sleep apnea, an increasingly common condition.
Obstructive sleep apnea is characterized by recurrent episodes of partial or complete upper airway obstruction with intermittent hypoxia and microarousals, resulting in low amounts of slow-wave sleep and overall decreased sleep quality.54
Obstructive sleep apnea is common in patients with type 2 diabetes, and several clinical and epidemiologic studies suggest that, untreated, it may worsen diabetes risk or control.21,45–46,55–59
The Sleep AHEAD (Action for Health in Diabetes) study60 revealed, in cross-sectional data, that more than 84% of obese patients with type 2 diabetes had obstructive sleep apnea (with an apnea-hypopnea index ≥ 5).
Aronsohn et al,5 in a study conducted in our laboratory in 60 patients with type 2 diabetes, found that 46 (77%) of them had obstructive sleep apnea. Furthermore, the worse the obstructive sleep apnea, the worse the glucose control. After controlling for age, sex, race, body mass index, number of diabetes medications, level of exercise, years of diabetes, and total sleep time, compared with patients without obstructive sleep apnea, the adjusted mean hemoglobin A1c was increased in a linear trend by (in absolute percentage points):
- 1.49% in patients with mild obstructive sleep apnea (P = .0028)
- 1.93% in patients with moderate obstructive sleep apnea (P = .0033)
- 3.69% in patients with severe obstructive sleep apnea (P < .0001).
Other epidemiologic studies. A growing number of epidemiologic studies, in various geographic regions, have suggested an independent link between obstructive sleep apnea and risk of type 2 diabetes.61 Most of the studies have been cross-sectional, and while most had positive findings, a criticism is that the methodology varied among the studies, both in how obstructive sleep apnea was assessed (snoring vs polysomnography) and in the metabolic assessment (oral glucose tolerance test, homeostatic model assessment, hemoglobin A1c, medical history, physician examination, or patient report).
A more recent prospective study of 544 nondiabetic patients65 showed that the risk of developing type 2 diabetes over an average of 2.7 years of follow-up was a function of the severity of obstructive sleep apnea expressed in quartiles: for each increased quartile of severity there was a 43% increase in the incidence of diabetes. Additionally, in patients with moderate to severe sleep apnea, regular use of continuous positive airway pressure (CPAP) was associated with an attenuated risk.65
Two prospective studies (not included in Table 1) used snoring as a marker of obstructive sleep apnea; at 10 years of follow-up, snoring was associated with a higher risk of developing diabetes in both men and women.73,74
Does CPAP improve glucose metabolism? Other studies have specifically examined the effects of CPAP treatment on glucose metabolism, in both diabetic and nondiabetic populations. Accumulating evidence suggests that metabolic abnormalities can be partially corrected by CPAP treatment, which supports the concept of a causal link between obstructive sleep apnea and altered glucose control. This topic is beyond the scope of this review; please see previously published literature61,75 for further information. Whether treating obstructive sleep apnea may delay the development or reduce the severity of type 2 diabetes is another important unanswered question.
Is obstructive sleep apnea a cause or consequence of diabetes? It may be a novel risk factor for type 2 diabetes, and its association with altered glucose metabolism is well supported by a large set of cross-sectional studies, but there are still insufficient longitudinal studies to indicate a direction of causality.
If obstructive sleep apnea is the cause, what is the mechanism? There are likely many. High levels of sympathetic nervous system activity, intermittent hypoxia, sleep fragmentation, and sleep loss in obstructive sleep apnea may all lead to dysregulation of the hypothalamic-pituitary axis, endothelial dysfunction, and alterations in cytokine and adipokine release and are all potential mechanisms of abnormal glucose metabolism in this population.
WHAT TO TELL PATIENTS
Taken together, the current evidence suggests that strategies to improve the duration and the quality of sleep should be considered as a potential intervention to prevent or delay the development of type 2 diabetes mellitus in at-risk populations. While further studies are needed to better elucidate the mechanisms of the relationship between sleep loss and diabetes risk and to determine if extending sleep and treating obstructive sleep apnea decreases the risk of diabetes, we urge clinicians to recommend at least 7 hours of uninterrupted sleep per night as a goal in maintaining a healthy lifestyle. Additionally, clinicians should systematically evaluate the risk of obstructive sleep apnea in their patients who have type 2 diabetes mellitus and the metabolic syndrome, and conversely, should assess for diabetes in patients with known obstructive sleep apnea.
- Pannain S, Van Cauter E. Sleep loss, obesity and diabetes: prevalence, association and emerging evidence for causation. Obesity Metab 2008; 4:28–41.
- Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol 2008; 159(suppl 1):S59–S66.
- US Centers for Disease Control and Prevention (CDC). Perceived insufficient rest or sleep among adults—United States, 2008. MMWR Morb Mortal Wkly Rep 2009; 58:1175–1179.
- Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med 2006; 166:1768–1774.
- Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med 2010; 181:507–513.
- Broussard J, Knutson KL. Sleep and metabolic risk and disease. In:Cappuccio FP, Miller MA, Lockley SW, editors. Sleep, Health and Society: From Aetiology to Public Health. Cary, NC: Oxford University Press; 2010:111–140.
- Zoccoli G, Walker AM, Lenzi P, Franzini C. The cerebral circulation during sleep: regulation mechanisms and functional implications. Sleep Med Rev 2002; 6:443–455.
- Pannain S, Van Cauter E. Modulation of endocrine function by sleepwake homeostasis and circadian rhythmicity. Sleep Med Clin 2007; 2:147–159.
- Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 1993; 328:303–307.
- Kuhn E, Brodan V, Brodanová M, Rysánek K. Metabolic reflection of sleep deprivation. Act Nerv Super (Praha) 1969; 11:165–174.
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999; 354:1435–1439.
- Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab 2009; 94:3242–3250.
- Zizi F, Jean-Louis G, Brown CD, Ogedegbe G, Boutin-Foster C, McFarlane SI. Sleep duration and the risk of diabetes mellitus: epidemiologic evidence and pathophysiologic insights. Curr Diab Rep 2010; 10:43–47.
- Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005; 165:863–867.
- Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of selfreported sleep duration and incident diabetes in women. Diabetes Care 2003; 26:380–384.
- Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep 2007; 30:1667–1673.
- Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care 2004; 27:282–283.
- Björkelund C, Bondyr-Carlsson D, Lapidus L, et al. Sleep disturbances in midlife unrelated to 32-year diabetes incidence: the prospective population study of women in Gothenburg. Diabetes Care 2005; 28:2739–2744.
- Nilsson PM, Rööst M, Engström G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care 2004; 27:2464–2469.
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141:846–850.
- Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol 2005; 99:1998–2007.
- Meisinger C, Heier M, Loewel H; MONICA/KORA Augsburg Cohort Study. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia 2005; 48:235–241.
- Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 2006; 29:657–661.
- Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2010; 33:414–420.
- Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 2000; 9:335–352.
- Spiegel K, Leproult R, Colecchia EF, et al. Adaptation of the 24-h growth hormone profile to a state of sleep debt. Am J Physiol Regul Integr Comp Physiol 2000; 279:R874–R883.
- Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav 2010; 99:651–656.
- Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Selfreported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab 2009; 94:4801–4809.
- Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev 1997; 18:716–738.
- Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab 1999; 84:2603–2607.
- Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 2004; 89:2119–2126.
- Spiegel K, Leproult R, L’hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004; 89:5762–5771.
- Teff KL. Visceral nerves: vagal and sympathetic innervation. JPEN J Parenter Enteral Nutr 2008; 32:569–571.
- Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens 2001; 14:304S–309S.
- Cappuccio F, Miller MA. The epidemiology of sleep and cardiovascular risk and disease. In:Cappuccio FP, Miller MA, Lockley SW, editors. Sleep, Health and Society: From Aetiology to Public Health. Cary, NC: Oxford University Press; 2010:111–140.
- Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med Rev 2005; 9:231–241.
- Wu MF, John J, Maidment N, Lam HA, Siegel JM. Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am J Physiol Regul Integr Comp Physiol 2002; 283:R1079–R1086.
- Estabrooke IV, McCarthy MT, Ko E, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci 2001; 21:1656–1662.
- Zeitzer JM, Buckmaster CL, Lyons DM, Mignot E. Increasing length of wakefulness and modulation of hypocretin-1 in the wake-consolidated squirrel monkey. Am J Physiol Regul Integr Comp Physiol 2007; 293:R1736–R1742.
- Rayner DV, Trayhurn P. Regulation of leptin production: sympathetic nervous system interactions. J Mol Med 2001; 79:8–20.
- van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 2004; 25:426–457.
- Samson WK, Taylor MM, Ferguson AV. Non-sleep effects of hypocretin/orexin. Sleep Med Rev 2005; 9:243–252.
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci 2001; 24:429–458.
- Qin LQ, Li J, Wang Y, Wang J, Xu JY, Kaneko T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci 2003; 73:2467–2475.
- Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 2002; 165:670–676.
- Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE; Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004; 160:521–530.
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 2000; 284:861–868.
- Resta O, Foschino Barbaro MP, Bonfitto P, et al. Low sleep quality and daytime sleepiness in obese patients without obstructive sleep apnoea syndrome. J Intern Med 2003; 253:536–543.
- Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med 1994; 154:1705–1711.
- Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003; 289:76–79.
- Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A 2008; 105:1044–1049.
- Prigeon RL, Kahn SE, Porte D. Changes in insulin sensitivity, glucose effectiveness, and B-cell function in regularly exercising subjects. Metabolism 1995; 44:1259–1263.
- Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010; 137:95–101.
- Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med 2005; 142:187–197.
- Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 2002; 165:677–682.
- Tassone F, Lanfranco F, Gianotti L, et al. Obstructive sleep apnoea syndrome impairs insulin sensitivity independently of anthropometric variables. Clin Endocrinol (Oxf) 2003; 59:374–379.
- Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J 2004; 25:735–741.
- Svatikova A, Wolk R, Gami AS, Pohanka M, Somers VK. Interactions between obstructive sleep apnea and the metabolic syndrome. Curr Diab Rep 2005; 5:53–58.
- Budhiraja R, Quan SF. Sleep-disordered breathing and cardiovascular health. Curr Opin Pulm Med 2005; 11:501–506.
- Foster GD, Sanders MH, Millman R, et al; Sleep AHEAD Research Group. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 2009; 32:1017–1019.
- Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 2008; 133:496–506.
- Stoohs RA, Facchini F, Guilleminault C. Insulin resistance and sleep-disordered breathing in healthy humans. Am J Respir Crit Care Med 1996; 154:170–174.
- Mahmood K, Akhter N, Eldeirawi K, et al. Prevalence of type 2 diabetes in patients with obstructive sleep apnea in a multi-ethnic sample. J Clin Sleep Med 2009; 5:215–221.
- Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med 2005; 172:1590–1595.
- Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med 2009; 122:1122–1127.
- Elmasry A, Lindberg E, Berne C, et al. Sleep-disordered breathing and glucose metabolism in hypertensive men: a population-based study. J Intern Med 2001; 249:153–161.
- Lam JC, Lam B, Lam CL, et al. Obstructive sleep apnea and the metabolic syndrome in community-based Chinese adults in Hong Kong. Respir Med 2006; 100:980–987.
- Okada M, Takamizawa A, Tsushima K, Urushihata K, Fujimoto K, Kubo K. Relationship between sleep-disordered breathing and lifestyle-related illnesses in subjects who have undergone health-screening. Intern Med 2006; 45:891–896.
- Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep 2006; 29:777–783.
- Seicean S, Kirchner HL, Gottlieb DJ, et al. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care 2008; 31:1001–1006.
- Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med 2009; 179:235–240.
- Steiropoulos P, Papanas N, Nena E, et al. Markers of glycemic control and insulin resistance in non-diabetic patients with obstructive sleep apnea hypopnea syndrome: does adherence to CPAP treatment improve glycemic control? Sleep Med 2009; 10:887–891.
- Al-Delaimy WK, Manson JE, Willett WC, Stampfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol 2002; 155:387–393.
- Elmasry A, Janson C, Lindberg E, Gislason T, Tageldin MA, Boman G. The role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male population. J Intern Med 2000; 248:13–20.
- Steiropoulos P, Papanas N, Nena E, Maltezos E, Bouros D. Continuous positive airway pressure treatment in patients with sleep apnoea: does it really improve glucose metabolism? Curr Diabetes Rev 2010; 6:156–166.
- Pannain S, Van Cauter E. Sleep loss, obesity and diabetes: prevalence, association and emerging evidence for causation. Obesity Metab 2008; 4:28–41.
- Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol 2008; 159(suppl 1):S59–S66.
- US Centers for Disease Control and Prevention (CDC). Perceived insufficient rest or sleep among adults—United States, 2008. MMWR Morb Mortal Wkly Rep 2009; 58:1175–1179.
- Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med 2006; 166:1768–1774.
- Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med 2010; 181:507–513.
- Broussard J, Knutson KL. Sleep and metabolic risk and disease. In:Cappuccio FP, Miller MA, Lockley SW, editors. Sleep, Health and Society: From Aetiology to Public Health. Cary, NC: Oxford University Press; 2010:111–140.
- Zoccoli G, Walker AM, Lenzi P, Franzini C. The cerebral circulation during sleep: regulation mechanisms and functional implications. Sleep Med Rev 2002; 6:443–455.
- Pannain S, Van Cauter E. Modulation of endocrine function by sleepwake homeostasis and circadian rhythmicity. Sleep Med Clin 2007; 2:147–159.
- Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 1993; 328:303–307.
- Kuhn E, Brodan V, Brodanová M, Rysánek K. Metabolic reflection of sleep deprivation. Act Nerv Super (Praha) 1969; 11:165–174.
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999; 354:1435–1439.
- Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab 2009; 94:3242–3250.
- Zizi F, Jean-Louis G, Brown CD, Ogedegbe G, Boutin-Foster C, McFarlane SI. Sleep duration and the risk of diabetes mellitus: epidemiologic evidence and pathophysiologic insights. Curr Diab Rep 2010; 10:43–47.
- Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005; 165:863–867.
- Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of selfreported sleep duration and incident diabetes in women. Diabetes Care 2003; 26:380–384.
- Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep 2007; 30:1667–1673.
- Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care 2004; 27:282–283.
- Björkelund C, Bondyr-Carlsson D, Lapidus L, et al. Sleep disturbances in midlife unrelated to 32-year diabetes incidence: the prospective population study of women in Gothenburg. Diabetes Care 2005; 28:2739–2744.
- Nilsson PM, Rööst M, Engström G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care 2004; 27:2464–2469.
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141:846–850.
- Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol 2005; 99:1998–2007.
- Meisinger C, Heier M, Loewel H; MONICA/KORA Augsburg Cohort Study. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia 2005; 48:235–241.
- Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 2006; 29:657–661.
- Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2010; 33:414–420.
- Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 2000; 9:335–352.
- Spiegel K, Leproult R, Colecchia EF, et al. Adaptation of the 24-h growth hormone profile to a state of sleep debt. Am J Physiol Regul Integr Comp Physiol 2000; 279:R874–R883.
- Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav 2010; 99:651–656.
- Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Selfreported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab 2009; 94:4801–4809.
- Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev 1997; 18:716–738.
- Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab 1999; 84:2603–2607.
- Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 2004; 89:2119–2126.
- Spiegel K, Leproult R, L’hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004; 89:5762–5771.
- Teff KL. Visceral nerves: vagal and sympathetic innervation. JPEN J Parenter Enteral Nutr 2008; 32:569–571.
- Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens 2001; 14:304S–309S.
- Cappuccio F, Miller MA. The epidemiology of sleep and cardiovascular risk and disease. In:Cappuccio FP, Miller MA, Lockley SW, editors. Sleep, Health and Society: From Aetiology to Public Health. Cary, NC: Oxford University Press; 2010:111–140.
- Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med Rev 2005; 9:231–241.
- Wu MF, John J, Maidment N, Lam HA, Siegel JM. Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am J Physiol Regul Integr Comp Physiol 2002; 283:R1079–R1086.
- Estabrooke IV, McCarthy MT, Ko E, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci 2001; 21:1656–1662.
- Zeitzer JM, Buckmaster CL, Lyons DM, Mignot E. Increasing length of wakefulness and modulation of hypocretin-1 in the wake-consolidated squirrel monkey. Am J Physiol Regul Integr Comp Physiol 2007; 293:R1736–R1742.
- Rayner DV, Trayhurn P. Regulation of leptin production: sympathetic nervous system interactions. J Mol Med 2001; 79:8–20.
- van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 2004; 25:426–457.
- Samson WK, Taylor MM, Ferguson AV. Non-sleep effects of hypocretin/orexin. Sleep Med Rev 2005; 9:243–252.
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci 2001; 24:429–458.
- Qin LQ, Li J, Wang Y, Wang J, Xu JY, Kaneko T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci 2003; 73:2467–2475.
- Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 2002; 165:670–676.
- Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE; Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004; 160:521–530.
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 2000; 284:861–868.
- Resta O, Foschino Barbaro MP, Bonfitto P, et al. Low sleep quality and daytime sleepiness in obese patients without obstructive sleep apnoea syndrome. J Intern Med 2003; 253:536–543.
- Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med 1994; 154:1705–1711.
- Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003; 289:76–79.
- Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A 2008; 105:1044–1049.
- Prigeon RL, Kahn SE, Porte D. Changes in insulin sensitivity, glucose effectiveness, and B-cell function in regularly exercising subjects. Metabolism 1995; 44:1259–1263.
- Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010; 137:95–101.
- Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med 2005; 142:187–197.
- Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 2002; 165:677–682.
- Tassone F, Lanfranco F, Gianotti L, et al. Obstructive sleep apnoea syndrome impairs insulin sensitivity independently of anthropometric variables. Clin Endocrinol (Oxf) 2003; 59:374–379.
- Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J 2004; 25:735–741.
- Svatikova A, Wolk R, Gami AS, Pohanka M, Somers VK. Interactions between obstructive sleep apnea and the metabolic syndrome. Curr Diab Rep 2005; 5:53–58.
- Budhiraja R, Quan SF. Sleep-disordered breathing and cardiovascular health. Curr Opin Pulm Med 2005; 11:501–506.
- Foster GD, Sanders MH, Millman R, et al; Sleep AHEAD Research Group. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 2009; 32:1017–1019.
- Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 2008; 133:496–506.
- Stoohs RA, Facchini F, Guilleminault C. Insulin resistance and sleep-disordered breathing in healthy humans. Am J Respir Crit Care Med 1996; 154:170–174.
- Mahmood K, Akhter N, Eldeirawi K, et al. Prevalence of type 2 diabetes in patients with obstructive sleep apnea in a multi-ethnic sample. J Clin Sleep Med 2009; 5:215–221.
- Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med 2005; 172:1590–1595.
- Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med 2009; 122:1122–1127.
- Elmasry A, Lindberg E, Berne C, et al. Sleep-disordered breathing and glucose metabolism in hypertensive men: a population-based study. J Intern Med 2001; 249:153–161.
- Lam JC, Lam B, Lam CL, et al. Obstructive sleep apnea and the metabolic syndrome in community-based Chinese adults in Hong Kong. Respir Med 2006; 100:980–987.
- Okada M, Takamizawa A, Tsushima K, Urushihata K, Fujimoto K, Kubo K. Relationship between sleep-disordered breathing and lifestyle-related illnesses in subjects who have undergone health-screening. Intern Med 2006; 45:891–896.
- Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep 2006; 29:777–783.
- Seicean S, Kirchner HL, Gottlieb DJ, et al. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care 2008; 31:1001–1006.
- Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med 2009; 179:235–240.
- Steiropoulos P, Papanas N, Nena E, et al. Markers of glycemic control and insulin resistance in non-diabetic patients with obstructive sleep apnea hypopnea syndrome: does adherence to CPAP treatment improve glycemic control? Sleep Med 2009; 10:887–891.
- Al-Delaimy WK, Manson JE, Willett WC, Stampfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol 2002; 155:387–393.
- Elmasry A, Janson C, Lindberg E, Gislason T, Tageldin MA, Boman G. The role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male population. J Intern Med 2000; 248:13–20.
- Steiropoulos P, Papanas N, Nena E, Maltezos E, Bouros D. Continuous positive airway pressure treatment in patients with sleep apnoea: does it really improve glucose metabolism? Curr Diabetes Rev 2010; 6:156–166.
KEY POINTS
- Sleep loss and sleep disturbances have become very common in our society, and so have obesity and type 2 diabetes.
- In epidemiologic studies, people who reported sleeping less were at higher risk of diabetes or disordered glucose metabolism.
- In laboratory studies, short-term sleep deprivation caused measurable changes in glucose metabolism, hormone levels, autonomic nervous system activity, and other variables, which are plausible mechanisms by which loss of sleep could contribute to diabetes.
- Obstructive sleep apnea is very common in people with diabetes and may be directly linked to diabetes risk and worse diabetes control. Diabetic patients should be systematically assessed for obstructive sleep apnea, and patients with known obstructive sleep apnea should be screened for diabetes.