User login
Venetoclax might improve the treatment of certain patients with T-cell acute lymphoblastic leukemia (T-ALL), according to preclinical research published in Leukemia.
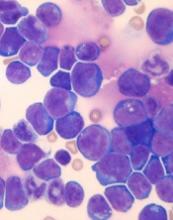
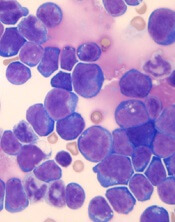
Researchers found that a ribosomal defect—the R98S mutation in ribosomal protein L10 (RPL10 R98S)—causes overexpression of BCL-2 in T-ALL.
The BCL-2 inhibitor venetoclax induced apoptosis of RPL10 R98S T-ALL cells and inhibited leukemia progression in mouse models of RPL10 R98S T-ALL.
The researchers therefore believe venetoclax could be used, in combination with other drugs, to treat T-ALL patients with RPL10 R98S.
“In the past couple of years, it has become clear that ribosome defects play a role in different types of cancer,” said study author Kim De Keersmaecker, PhD, of KU Leuven in Leuven, Belgium.
“In the case of a ribosome defect, the cells still produce proteins, but the balance between their quantities is slightly off, which leads to cancer.”
Dr De Keersmaecker and her colleagues noted that RPL10 R98S affects 8% of pediatric patients with T-ALL.
With this study, the researchers found that RPL10 R98S mutant cells were more resilient than wild-type (WT) cells. In overgrowth condition, Ba/F3 RPL10 R98S mutant cells “displayed a clear survival benefit” over RPL10 WT cells.
Likewise, RPL10 R98S Jurkat cells exhibited a survival benefit over WT Jurkat cells in overgrowth condition. And RPL10 R98S Jurkat cells were more resistant to treatment with doxorubicin.
Dr De Keersmaecker and her colleagues said the increased survival they observed in RPL10 R98S mutant cells is associated with enhanced BCL-2 expression. So the team decided to test a BCL-2 inhibitor in RPL10 R98S leukemic cells.
In vitro, venetoclax induced slightly more apoptosis in Jurkat RPL10 R98S cells than WT Jurkat cells. In vivo, venetoclax induced apoptosis in RPL10 R98S T-ALL cells but not WT T-ALL cells.
The researchers also found that venetoclax could re-sensitize RPL10 R98S cells to doxorubicin.
Finally, the team injected RPL10 WT and R98S samples from pediatric T-ALL patients into mice and treated the animals with DMSO or venetoclax (50 mg/kg) once a week.
Venetoclax had very little effect on the RPL10 WT mice. Percentages of human CD45 T-ALL cells in the peripheral blood were similar whether mice received DMSO or venetoclax.
However, in the RPL10 R98S mice, those that received DMSO experienced disease progression, while there were no signs of leukemia progression in the peripheral blood of mice that received venetoclax.
The splenomegaly observed in DMSO-treated mice was “almost completely suppressed” in mice that received venetoclax, according to the researchers.
The team also said they observed a 30% to 50% suppression of human CD45 leukemia cell engraftment in the bone marrow and the presence of 30% to 40% mouse CD45 cells in mice treated with venetoclax.
On the other hand, mice treated with DMSO had more than 95% human leukemia infiltration in the bone marrow and no mouse CD45-expressing cells.
Dr De Keersmaecker and her colleagues said these results suggest RPL10 R98S pediatric T-ALL is sensitive to BCL-2 targeted therapies such as venetoclax. However, venetoclax alone would not be sufficient to treat this type of T-ALL.
“Patients with leukemia often get a drug cocktail, while our study only tested the BCL-2 inhibitor,” Dr De Keersmaecker said. “That’s why our follow-up study will focus on a cocktail of this BCL-2 inhibitor and other drugs. For patients with the ribosome defect analyzed in our study, this avenue is definitely worth examining in greater detail.”
Venetoclax might improve the treatment of certain patients with T-cell acute lymphoblastic leukemia (T-ALL), according to preclinical research published in Leukemia.
Researchers found that a ribosomal defect—the R98S mutation in ribosomal protein L10 (RPL10 R98S)—causes overexpression of BCL-2 in T-ALL.
The BCL-2 inhibitor venetoclax induced apoptosis of RPL10 R98S T-ALL cells and inhibited leukemia progression in mouse models of RPL10 R98S T-ALL.
The researchers therefore believe venetoclax could be used, in combination with other drugs, to treat T-ALL patients with RPL10 R98S.
“In the past couple of years, it has become clear that ribosome defects play a role in different types of cancer,” said study author Kim De Keersmaecker, PhD, of KU Leuven in Leuven, Belgium.
“In the case of a ribosome defect, the cells still produce proteins, but the balance between their quantities is slightly off, which leads to cancer.”
Dr De Keersmaecker and her colleagues noted that RPL10 R98S affects 8% of pediatric patients with T-ALL.
With this study, the researchers found that RPL10 R98S mutant cells were more resilient than wild-type (WT) cells. In overgrowth condition, Ba/F3 RPL10 R98S mutant cells “displayed a clear survival benefit” over RPL10 WT cells.
Likewise, RPL10 R98S Jurkat cells exhibited a survival benefit over WT Jurkat cells in overgrowth condition. And RPL10 R98S Jurkat cells were more resistant to treatment with doxorubicin.
Dr De Keersmaecker and her colleagues said the increased survival they observed in RPL10 R98S mutant cells is associated with enhanced BCL-2 expression. So the team decided to test a BCL-2 inhibitor in RPL10 R98S leukemic cells.
In vitro, venetoclax induced slightly more apoptosis in Jurkat RPL10 R98S cells than WT Jurkat cells. In vivo, venetoclax induced apoptosis in RPL10 R98S T-ALL cells but not WT T-ALL cells.
The researchers also found that venetoclax could re-sensitize RPL10 R98S cells to doxorubicin.
Finally, the team injected RPL10 WT and R98S samples from pediatric T-ALL patients into mice and treated the animals with DMSO or venetoclax (50 mg/kg) once a week.
Venetoclax had very little effect on the RPL10 WT mice. Percentages of human CD45 T-ALL cells in the peripheral blood were similar whether mice received DMSO or venetoclax.
However, in the RPL10 R98S mice, those that received DMSO experienced disease progression, while there were no signs of leukemia progression in the peripheral blood of mice that received venetoclax.
The splenomegaly observed in DMSO-treated mice was “almost completely suppressed” in mice that received venetoclax, according to the researchers.
The team also said they observed a 30% to 50% suppression of human CD45 leukemia cell engraftment in the bone marrow and the presence of 30% to 40% mouse CD45 cells in mice treated with venetoclax.
On the other hand, mice treated with DMSO had more than 95% human leukemia infiltration in the bone marrow and no mouse CD45-expressing cells.
Dr De Keersmaecker and her colleagues said these results suggest RPL10 R98S pediatric T-ALL is sensitive to BCL-2 targeted therapies such as venetoclax. However, venetoclax alone would not be sufficient to treat this type of T-ALL.
“Patients with leukemia often get a drug cocktail, while our study only tested the BCL-2 inhibitor,” Dr De Keersmaecker said. “That’s why our follow-up study will focus on a cocktail of this BCL-2 inhibitor and other drugs. For patients with the ribosome defect analyzed in our study, this avenue is definitely worth examining in greater detail.”
Venetoclax might improve the treatment of certain patients with T-cell acute lymphoblastic leukemia (T-ALL), according to preclinical research published in Leukemia.
Researchers found that a ribosomal defect—the R98S mutation in ribosomal protein L10 (RPL10 R98S)—causes overexpression of BCL-2 in T-ALL.
The BCL-2 inhibitor venetoclax induced apoptosis of RPL10 R98S T-ALL cells and inhibited leukemia progression in mouse models of RPL10 R98S T-ALL.
The researchers therefore believe venetoclax could be used, in combination with other drugs, to treat T-ALL patients with RPL10 R98S.
“In the past couple of years, it has become clear that ribosome defects play a role in different types of cancer,” said study author Kim De Keersmaecker, PhD, of KU Leuven in Leuven, Belgium.
“In the case of a ribosome defect, the cells still produce proteins, but the balance between their quantities is slightly off, which leads to cancer.”
Dr De Keersmaecker and her colleagues noted that RPL10 R98S affects 8% of pediatric patients with T-ALL.
With this study, the researchers found that RPL10 R98S mutant cells were more resilient than wild-type (WT) cells. In overgrowth condition, Ba/F3 RPL10 R98S mutant cells “displayed a clear survival benefit” over RPL10 WT cells.
Likewise, RPL10 R98S Jurkat cells exhibited a survival benefit over WT Jurkat cells in overgrowth condition. And RPL10 R98S Jurkat cells were more resistant to treatment with doxorubicin.
Dr De Keersmaecker and her colleagues said the increased survival they observed in RPL10 R98S mutant cells is associated with enhanced BCL-2 expression. So the team decided to test a BCL-2 inhibitor in RPL10 R98S leukemic cells.
In vitro, venetoclax induced slightly more apoptosis in Jurkat RPL10 R98S cells than WT Jurkat cells. In vivo, venetoclax induced apoptosis in RPL10 R98S T-ALL cells but not WT T-ALL cells.
The researchers also found that venetoclax could re-sensitize RPL10 R98S cells to doxorubicin.
Finally, the team injected RPL10 WT and R98S samples from pediatric T-ALL patients into mice and treated the animals with DMSO or venetoclax (50 mg/kg) once a week.
Venetoclax had very little effect on the RPL10 WT mice. Percentages of human CD45 T-ALL cells in the peripheral blood were similar whether mice received DMSO or venetoclax.
However, in the RPL10 R98S mice, those that received DMSO experienced disease progression, while there were no signs of leukemia progression in the peripheral blood of mice that received venetoclax.
The splenomegaly observed in DMSO-treated mice was “almost completely suppressed” in mice that received venetoclax, according to the researchers.
The team also said they observed a 30% to 50% suppression of human CD45 leukemia cell engraftment in the bone marrow and the presence of 30% to 40% mouse CD45 cells in mice treated with venetoclax.
On the other hand, mice treated with DMSO had more than 95% human leukemia infiltration in the bone marrow and no mouse CD45-expressing cells.
Dr De Keersmaecker and her colleagues said these results suggest RPL10 R98S pediatric T-ALL is sensitive to BCL-2 targeted therapies such as venetoclax. However, venetoclax alone would not be sufficient to treat this type of T-ALL.
“Patients with leukemia often get a drug cocktail, while our study only tested the BCL-2 inhibitor,” Dr De Keersmaecker said. “That’s why our follow-up study will focus on a cocktail of this BCL-2 inhibitor and other drugs. For patients with the ribosome defect analyzed in our study, this avenue is definitely worth examining in greater detail.”