User login
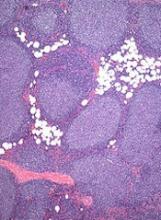
The US Food and Drug Administration (FDA) has granted 2 fast track designations to 5F9, an anti-CD47 antibody.
The designations are for 5F9 as a treatment for relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL).
Data supporting the fast track designations were derived from a phase 1b/2 trial of 5F9 in combination with rituximab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma, including DLBCL and FL.
Forty Seven, Inc., the company developing 5F9, expects to announce initial safety and efficacy data from the phase 1b portion of the trial in the second quarter of 2018.
About fast track designation
The FDA’s fast track drug development program is designed to expedite clinical development and submission of applications for drugs with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the drug’s development plan and written communications about issues such as trial design and use of biomarkers.
Drugs that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met.
Fast track drugs may also be eligible for rolling review, which allows a developer to submit individual sections of a drug’s application for review as they are ready, rather than waiting until all sections are complete.
The US Food and Drug Administration (FDA) has granted 2 fast track designations to 5F9, an anti-CD47 antibody.
The designations are for 5F9 as a treatment for relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL).
Data supporting the fast track designations were derived from a phase 1b/2 trial of 5F9 in combination with rituximab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma, including DLBCL and FL.
Forty Seven, Inc., the company developing 5F9, expects to announce initial safety and efficacy data from the phase 1b portion of the trial in the second quarter of 2018.
About fast track designation
The FDA’s fast track drug development program is designed to expedite clinical development and submission of applications for drugs with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the drug’s development plan and written communications about issues such as trial design and use of biomarkers.
Drugs that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met.
Fast track drugs may also be eligible for rolling review, which allows a developer to submit individual sections of a drug’s application for review as they are ready, rather than waiting until all sections are complete.
The US Food and Drug Administration (FDA) has granted 2 fast track designations to 5F9, an anti-CD47 antibody.
The designations are for 5F9 as a treatment for relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL).
Data supporting the fast track designations were derived from a phase 1b/2 trial of 5F9 in combination with rituximab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma, including DLBCL and FL.
Forty Seven, Inc., the company developing 5F9, expects to announce initial safety and efficacy data from the phase 1b portion of the trial in the second quarter of 2018.
About fast track designation
The FDA’s fast track drug development program is designed to expedite clinical development and submission of applications for drugs with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the drug’s development plan and written communications about issues such as trial design and use of biomarkers.
Drugs that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met.
Fast track drugs may also be eligible for rolling review, which allows a developer to submit individual sections of a drug’s application for review as they are ready, rather than waiting until all sections are complete.