User login
- Expectant management may be offered to asymptomatic patients with small adnexal masses (≤3 cm) lower beta-human chorionic gonadotropin (β-hCG) levels (<1000 mIU/mL), evidence of spontaneous resolution (eg, falling β-hCG levels) who are willing to accept the risk of tubal (A).
- Systemic methotrexate administration resolves ectopic pregnancy in 87% to 95% of cases, maintains tubal patency in 75% to 81%, and results in subsequent successful pregnancy in about 58% to 61% of patients. Hemodynamically stable patients with adnexal mass≤3.5 cm, β-hCG levels <5000 mIU/mL, no adnexal yolk sac and normal hematologic, liver, and kidney functions are ideal candidates for methotrexate therapy (A).
Management strategies for patients with ectopic pregnancy have evolved rapidly, with ambulatory medical therapy becoming an option for more patients.1 In part 1, published in the May 2006 JFP, using a practical decision protocol, we discuss the physical findings that most reliably suggest ectopic pregnancy, describe sensible use of laboratory and imaging studies, and explain what to do when results are equivocal.
Management choices
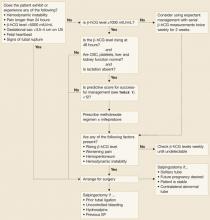
Once the diagnosis of ectopic pregnancy has been made, options include surgical, medical, or expectant management (FIGURE). The goal of treatment is to minimize disease-and treatment-related morbidity while maximizing reproductive potential.
Administer Rhogam to all Rh-negative women.
Clinical prediction tools have been developed to aid management decision making. Fernandez et al developed a score based on gestational age, β-hCG level, progesterone level, abdominal pain, hemoperitoneum volume, and hematosalpinx diameter.2 A score <12 predicts a >80% success with expectant or nonsurgical management (TABLE 1). Similarly, to predict response to a single-dose of methotrexate, Elito et al3 developed a score based on β-hCG level, ultrasound findings, have size of the mass (cm), and color Doppler image aspects (TABLE 1). In a small study of for 40 patients, those with scores >5 had a the 97% success rate.3
FIGURE
Deciding which management option is best for your patient with ectopic pregnancy
TABLE 1
Predictive score for successful treatment of ectopic pregnancy
| Predictive score for expectant management and several nonsurgical treatments (Fernandez 1991) | |||
| CRITERION | 1 POINT | 2 POINT | 3 POINT |
| β-hCG (mIU/mL) | <1000 | 1000–5000 | >5000 |
| Progesterone (ng/mL) | <5 | 5–10 | >10 |
| Abdominal pain | Absent | Induced | Spontaneous |
| Hematosalpinx (cm) | <1 | 1–3 | >3 |
| Hemoperitoneum (mL) | 0 | 1–100 | >100 |
| Score <12: 80% success with various nonsurgical treatments, including expectant management. | |||
| Predictive score for single dose methotrexate (50 mg/m2 IM) (Elito 1999) | |||
| PARAMETERS | 0 POINTS | 1 POINTS | 2 POINTS |
| β-hCG (mIU/mL) | >5000 | 1500–5000 | <1500 |
| Aspects of the image | Live embryo | Tubal ring | Hematosalpinx |
| Size of the mass | >3.0–3.5 | 2.6–3.0 | <2.5 |
| Color Doppler | High risk | Medium risk | Low risk |
| Score≥5: 97% success with single-dose methotrexate. | |||
| Sources: Fernandez et al 1991,2 Elito et al 1999.3 | |||
Surgical management
Surgery is preferred for ruptured ectopic pregnancy. Surgery is also indicated for patients with evidence of hemodynamic instability, anemia, pain for longer than 24 hours, β-hCG levels greater than 5000 mIU/mL, or with a gestational sac that measures more than 3.5 to 4 cm on ultrasound.1,4,5
Laparoscopic techniques minimize the trauma and morbidity of salpingectomy or salpingostomy. Compared with older procedures, they lessen blood loss, decrease the need for analgesia, and allow a shorter hospital stay and an earlier return to work.6
Salpingostomy removes the ectopic pregnancy while preserving the Fallopian tube. Weekly quantitative β-hCG testing is required to rule out persistent ectopic pregnancy, which occurs in 5% to 8% of patients following salpingostomy.7 The likelihood of persistent ectopic pregnancy following salpingostomy increases with an ectopic pregnancy <2 cm in diameter, salpingostomy performed <6 weeks from the last menstrual period, a β-hCG level >3000 mIU/mL, or progesterone level over 35 nmol/L combined with a daily change in β-hCG over 100 mIU/mL.8,9
Expectant management possible when β-hCG levels <1000 mIU/mL
Expectant management may be offered to asymptomatic women with small adnexal masses, lower β-hCG levels, and evidence of spontaneous resolution (eg, falling β-hCG levels) who are willing to accept the risk of tubal rupture.10 Rising β-hCG levels, pain, hemodynamic instability, or hemoperitoneum on ultrasound dictate switching to active management.11
Eighty percent of women with initial β-hCG levels <1000 mIU/mL experience spontaneous resolution (TABLE 2).1,4,5,11-17 In one study, women with initial β-hCG levels <1000 mIU/mL, adnexal masses <4 cm, no fetal heartbeat, and <100 mL of fluid in the pouch of Douglas were managed by serial ultrasound and β-hCG levels obtained twice-weekly for 2 weeks; the result was an 88% chance of spontaneous resolution.18 Women (n=9) with initial β-hCG levels ≤1000 mIU/mL with subsequent rising titers experienced no spontaneous resolution.
TABLE 2
Treatment options for ectopic pregnancy
| MODALITY | DESCRIPTION | EP RESOLUTION (%) | TUBAL PATENCY (%) | FUTURE IUP (%) | EP (%) | ADVERSE EVENTS | ||
| Salpingectomy Open or laparoscopic | Excision of ectopic pregnancy and tube | 100 | NA | 42–82* | 6–13 | Hemorrhage Infection Adhesions | ||
| Laparoscopic | Excision of ectopic pregnancy with repair of tube | 93 | 76 | 57 | 13 | Incomplete removal (persistent EP) Analgesic needed Lost work time | ||
| Expectant management | Twice-weekly β-hCG, ultrasound obtained for 2 weeks | 67–68 | 76–77 | 68–86 | 7–13 | Persistent EP 25% need medical or surgical management | ||
| β-hCG <1000 | 88 | |||||||
| β-hCG ≥1000 | 48 | |||||||
| Methotrexate Multidose | 1 mg/kg IV or IM with 0.1 mg/kg folic acid on alternating days. Stop when >15% drop in β-hCG observed or 4 doses administered | 93–95 | 75 | 58 | 7 | Mucositis (stomatitis, gastritis, diarrhea) Dermatitis Bone marrow suppression Hepatic dysfunction Pleuritis | ||
| Single dose | Injection of 50 mg/m2. β-hCG levels days 4 and 7. Repeat dose if no drop. | 87–90 | 81 | 61 | 8 | Reversible alopecia Photosensitivity Pulmonary fibrosis | ||
| Oral | 50 mg daily for 5 days or 60 mg/m2 (one time in 2 divided doses) | 86 | ||||||
| Direct injection | Ultrasound or laparoscopic guidance of 12.5–25 mg | 76 | 80 | 57 | 6 | |||
| EP, ectopic pregnancy; IUP, intrauterine pregnancy; NA, not applicable; IM, intramuscular; IV, intravenous. | ||||||||
| *82% if contralateral tube normal. | ||||||||
Medical management an option for about 25% of patients
Methotrexate depletes tetrahydrofolate cofactors required for DNA and RNA synthesis and cell replication, and thereby inhibits the rapidly growing trophoblasts in patients with ectopic pregnancy.
Methotrexate may be used for primary treatment of ectopic pregnancy, for persistent ectopic pregnancy following tubal sparing surgery, as prophylaxis to reduce persistent ectopic pregnancy following salpingostomy, and in cornual and cervical pregnancies.11,13,19
Who qualifies. Patients eligible for methotrexate administration are those without hemodynamic instability or evidence of tubal rupture (clinical or ultrasound), desiring future fertility, having a gestational sac <3.5 cm, a β-hCG level less than 5000 mIU/mL, no fetal cardiac motion on ultrasound, and the ability and willingness to comply with post-treatment monitoring.10,14
Systemic methotrexate successfully resolves ectopic pregnancy in 90% of patients. Subsequent tubal patency rates approximate 80% and pregnancy rates 60% with recurrent ectopic pregnancy rates 8%.10,13,15,16 The cost of treatment for systemic methotrexate was $5721 per patient compared with $4066 for salpingostomy.20 Hematologic, liver, and renal functions should be assessed before treatment.
Following methotrexate administration, some patients experience a transient increase in abdominal pain, vaginal bleeding, and rising β-hCG. Exclude a ruptured ectopic pregnancy in patients with worsening pain. Pelvic examinations, sexual intercourse, and TVUS should be avoided or minimized during treatment.10
Two regimens are used for the systemic administration of methotrexate (TABLE 2).
The single-dose regimen uses an intramuscular (IM) injection dose of 50 mg/m2 of methotrexate without leucovorin. It has an overall success rate of 87%. β-hCG levels are measured on days 4 and 7, and the medication is repeated if no drop is noticed.13 β-hCG levels are then repeated weekly until undetectable (usually 4 weeks). Serum progesterone levels drop significantly faster than β-hCG following methotrexate administration, and levels <1.5 ng/mL predict ectopic pregnancy resolution more accurately than β-hCG levels.21 Repeat dosing is needed in up to 14% of women.
In the multidose regimen, IM injections of 1 mg/kg of methotrexate are given followed by leucovorin (0.1 mg/kg) after 24 hours. β-hCG levels are checked every other day until there is a 15% or more drop on 2 consecutive days, or 4 doses of methotrexate are administered.1 β-hCG levels are then repeated weekly until undetectable. About half of the women receiving the multidose regimen require 4 or more doses (6.8% require >4 doses). On the other hand, 10% of women treated with this regimen require only 1 dose. Multidose methotrexate has also been used as first-line therapy in cervical, interstitial, ovarian, and abdominal gestation, but with substantially lower success.19
Single-dose vs multidose regimens. Although there are no direct comparison trials, a meta-analysis combining the results of 26 studies with 1067 women treated with a single-dose regimen and 267 women treated with a multidose regimen found that the multidose regimen was slightly more effective.16 The success rate for women with multidose treatment was 92.7% (95% confidence interval [CI], 89–96) vs a single-dose treatment success rate of 88.1% (95% CI, 86–90).
Women treated with single-dose regimens had fewer side effects (31.3% vs 41.2%). However, in both regimens, women who experienced adverse side effects were less likely to have failed treatment (single dose, odds ratio [OR]=0.27; multidose, OR=0.72). Another systematic review including 19 studies with 393 women treated with single-dose methotrexate and 338 women treated with multidose methotrexate had similar outcome findings; the multidose regimen was slightly more effective (93% vs 87%).13
A recent retrospective study comparing the multidose and single-dose regimens in 643 patients with ectopic pregnancy (single dose, n=555; multidose, n=97) from a single database also showed slightly better success with the multidose regimen (95% vs 90%, P=.18).22 Therefore, the multidose regimen may be preferred but a randomized controlled trial comparing the 2 regimens is needed.
In clinically stable women, the serum β-hCG level at presentation is probably the most important single factor determining failure of single-dose methotrexate; patients with low β-hCG levels (1000–2000 mIU/mL) have a high (~98%) response rate.23
Add mifepristone? Mifepristone 600 mg orally added to methotrexate increases successful resolution of unruptured ectopic pregnancy, decreases resolution time, and reduces the need for a second injection or laparotomy, without worsening side effects.24 A recent randomized controlled trial, however, showed that this combination had little advantage over methotrexate alone, and concluded that adding mifepristone be limited to patients with serum progesterone ≥10 ng/mL.25
Direct injection. When fetal cardiac activity is present, injection of 20% potassium chloride (KCl) 0.5 mL into the gestational sac under ultrasound guidance results in asystole and a slow resolution of ectopic pregnancy. Because KCl does not affect the trophoblast, trophoblastic tissue may continue to proliferate leading to tubal rupture.26 Hyperosmolar glucose 1 to 3 mL injected laparoscopically or under ultrasound guidance into the gestational sac maintains tubal patency, has few side effects, but its initial high success rates in resolving ectopic pregnancy (94% to 100%) has not been duplicated.11,13 Another option is prostaglandin F-2 alpha, which when injected into the Fallopian tube causes contractions and vasoconstriction, resulting in a resolution of ectopic pregnancy in 92% of patients.13 However, serious side effects have been reported, including severe abdominal discomfort, vomiting, and pulmonary edema.
CORRESPONDENCE
K. Ramakrishnan, MD, Department of Family and Preventive Medicine, University of Oklahoma Health Sciences Center, 900 NE 10th Street, Oklahoma City, OK 73104. E-mail: [email protected]
1. Carr RJ, Evans P. Ectopic pregnancy. Prim Care 2000;27:169-183.
2. Fernandez H, Lelaidier C, Thouvenez V, et al. The use of a pretherapeutic, predictive score to determine inclusion criteria for the non-surgical management of ectopic pregnancy. Hum Reprod 1991;6:995-998.
3. Elito J, Jr, Reichmann AP, Uchiyama MN, Camano L. Predictive score for the systemic treatment of unruptured ectopic pregnancy with a single dose of methotrexate. Int J Gynecol Obstet 1999;67:75-79.
4. Pisarska MD, Carson SA, Buster JE. Ectopic pregnancy. Lancet 1998;351:1115-1120.
5. Farquhar CM. Ectopic pregnancy. Lancet 2005;366:583-591.
6. Kelly AJ, Sowter MC, Trinder J. The management of tubal pregnancy. Royal College of Obstetricians and Gynecologists (RCOG). Guideline No. 21, May 2004. Available at: www.rcog.org.uk/resources/Public/pdf/management_tubal_pregnancy21.pdf.
7. Rulin MC. Is salpingostomy the surgical treatment of choice for unruptured tubal pregnancy? Obstet Gynecol 1995;86:1010-1013.
8. Seifer DB. Persistent ectopic pregnancy: an argument for heightened vigilance and patient compliance. Fertil Steril 1997;68:402-404.
9. Hagstrom HG, Hahlin M, Bennegard-Edèn B, Sjoblom P, Thorburn J, Lindblom B. Prediction of persistent ectopic pregnancy after laparoscopic salpingostomy. Obstet Gynecol 1994;84:798-802.
10. ACOG practice bulletin. Medical management of tubal pregnancy. Number 3, December 1998. Clinical management guidelines for obstetrician-gynecologists. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 1999;65:97-103.
11. Mittal S. Non-surgical management of ectopic pregnancy. Obs Gyn Com 1999;1:23-28.
12. Cohen MA, Sauer MV. Expectant management of ectopic pregnancy. Clin Obstet Gynecol 1999;42:48-54.
13. Buster JE, Pisarska MD. Medical management of ectopic pregnancy. Clin Obstet Gynecol 1999;42:23-30.
14. Murray H, Baakdah H, Bardell T, Tulandi T. Diagnosis and treatment of ectopic pregnancy. CMAJ 2005;173:905-912.
15. Pansky M. Methotrexate (MXT) treatment for ectopic pregnancy-systemic vs local injection. Scientific presentation at The First World Congress on Controversies in Obstetrics, Gynecology & Infertility. Prague, Czech Republic, 1999. Available at: www.obgyn.net/firstcontroversies/prague1999pansky.doc. Accessed December 4, 2005.
16. Barnhart KT, Gosman G, Ashby R, Sammel M. The medical management of ectopic pregnancy: a meta-analysis comparing “single dose” and “multidose” regimens. Obstet Gynecol 2003;101:778-784.
17. Lipscomb GH, Meyer NL, Flynn DE, Peterson M, Ling F. Oral methotrexate for treatment of ectopic pregnancy. Am J Obstet Gynecol 2002;186:1192-1195.
18. Trio D, Strobelt N, Picciolo C, Lapinski RH, Ghidini A. Prognostic factors for successful expectant management of ectopic pregnancy. Fertil Steril 1995;63:469-472.
19. Barnhart K, Esposito M, Coutifaris C. An update on the medical treatment of ectopic pregnancy. Obstet Gynecol Clin North Am 2000;27:653-667, viii.
20. Mol BW, Hajenius PJ, Engelsbel S, et al. Treatment of tubal pregnancy in the Netherlands: an economic comparison of systemic methotrexate administration and laparoscopic salpingostomy. Am J Obstet Gynecol 1999;181:945-951.
21. Saraj AJ, Wilcox JG, Najmabadi, Stein SM, Johnson MB, Paulson RJ. Resolution of hormonal markers of ectopic gestation: a randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol 1998;92:989-994.
22. Lipscomb GH, Givens VM, Meyer NL, Bran D. Comparison of multidose and single-dose methotrexate protocols for the treatment of ectopic pregnancy. Am J Obstet Gynecol 2005;192:1844-1847.
23. Sowter MC, Farquhar CM. Ectopic pregnancy: an update. Curr Opin Obstet Gynecol 2004;16:289-293.
24. Gazvani MR, Baruah DN, Alfirevic Z, Emery SJ. Mifepristone in combination with methotrexate for the medical treatment of tubal pregnancy: a randomized, controlled trial. Hum Reprod 1998;13:1987-1990.
25. Rozenberg P, Chevret S, Camus E, et al. Medical treatment of ectopic pregnancies: a randomized clinical trial comparing methotrexate-mifepristone and methotrexate-placebo. Hum Reprod 2003;18:1802-1808.
26. Pansky M, Golan A, Bukovsky I, Caspi E. Nonsurgical management of tubal pregnancy. Necessity in view of the changing clinical appearance. Am J Obstet Gynecol 1991;164:888-895.
- Expectant management may be offered to asymptomatic patients with small adnexal masses (≤3 cm) lower beta-human chorionic gonadotropin (β-hCG) levels (<1000 mIU/mL), evidence of spontaneous resolution (eg, falling β-hCG levels) who are willing to accept the risk of tubal (A).
- Systemic methotrexate administration resolves ectopic pregnancy in 87% to 95% of cases, maintains tubal patency in 75% to 81%, and results in subsequent successful pregnancy in about 58% to 61% of patients. Hemodynamically stable patients with adnexal mass≤3.5 cm, β-hCG levels <5000 mIU/mL, no adnexal yolk sac and normal hematologic, liver, and kidney functions are ideal candidates for methotrexate therapy (A).
Management strategies for patients with ectopic pregnancy have evolved rapidly, with ambulatory medical therapy becoming an option for more patients.1 In part 1, published in the May 2006 JFP, using a practical decision protocol, we discuss the physical findings that most reliably suggest ectopic pregnancy, describe sensible use of laboratory and imaging studies, and explain what to do when results are equivocal.
Management choices
Once the diagnosis of ectopic pregnancy has been made, options include surgical, medical, or expectant management (FIGURE). The goal of treatment is to minimize disease-and treatment-related morbidity while maximizing reproductive potential.
Administer Rhogam to all Rh-negative women.
Clinical prediction tools have been developed to aid management decision making. Fernandez et al developed a score based on gestational age, β-hCG level, progesterone level, abdominal pain, hemoperitoneum volume, and hematosalpinx diameter.2 A score <12 predicts a >80% success with expectant or nonsurgical management (TABLE 1). Similarly, to predict response to a single-dose of methotrexate, Elito et al3 developed a score based on β-hCG level, ultrasound findings, have size of the mass (cm), and color Doppler image aspects (TABLE 1). In a small study of for 40 patients, those with scores >5 had a the 97% success rate.3
FIGURE
Deciding which management option is best for your patient with ectopic pregnancy
TABLE 1
Predictive score for successful treatment of ectopic pregnancy
| Predictive score for expectant management and several nonsurgical treatments (Fernandez 1991) | |||
| CRITERION | 1 POINT | 2 POINT | 3 POINT |
| β-hCG (mIU/mL) | <1000 | 1000–5000 | >5000 |
| Progesterone (ng/mL) | <5 | 5–10 | >10 |
| Abdominal pain | Absent | Induced | Spontaneous |
| Hematosalpinx (cm) | <1 | 1–3 | >3 |
| Hemoperitoneum (mL) | 0 | 1–100 | >100 |
| Score <12: 80% success with various nonsurgical treatments, including expectant management. | |||
| Predictive score for single dose methotrexate (50 mg/m2 IM) (Elito 1999) | |||
| PARAMETERS | 0 POINTS | 1 POINTS | 2 POINTS |
| β-hCG (mIU/mL) | >5000 | 1500–5000 | <1500 |
| Aspects of the image | Live embryo | Tubal ring | Hematosalpinx |
| Size of the mass | >3.0–3.5 | 2.6–3.0 | <2.5 |
| Color Doppler | High risk | Medium risk | Low risk |
| Score≥5: 97% success with single-dose methotrexate. | |||
| Sources: Fernandez et al 1991,2 Elito et al 1999.3 | |||
Surgical management
Surgery is preferred for ruptured ectopic pregnancy. Surgery is also indicated for patients with evidence of hemodynamic instability, anemia, pain for longer than 24 hours, β-hCG levels greater than 5000 mIU/mL, or with a gestational sac that measures more than 3.5 to 4 cm on ultrasound.1,4,5
Laparoscopic techniques minimize the trauma and morbidity of salpingectomy or salpingostomy. Compared with older procedures, they lessen blood loss, decrease the need for analgesia, and allow a shorter hospital stay and an earlier return to work.6
Salpingostomy removes the ectopic pregnancy while preserving the Fallopian tube. Weekly quantitative β-hCG testing is required to rule out persistent ectopic pregnancy, which occurs in 5% to 8% of patients following salpingostomy.7 The likelihood of persistent ectopic pregnancy following salpingostomy increases with an ectopic pregnancy <2 cm in diameter, salpingostomy performed <6 weeks from the last menstrual period, a β-hCG level >3000 mIU/mL, or progesterone level over 35 nmol/L combined with a daily change in β-hCG over 100 mIU/mL.8,9
Expectant management possible when β-hCG levels <1000 mIU/mL
Expectant management may be offered to asymptomatic women with small adnexal masses, lower β-hCG levels, and evidence of spontaneous resolution (eg, falling β-hCG levels) who are willing to accept the risk of tubal rupture.10 Rising β-hCG levels, pain, hemodynamic instability, or hemoperitoneum on ultrasound dictate switching to active management.11
Eighty percent of women with initial β-hCG levels <1000 mIU/mL experience spontaneous resolution (TABLE 2).1,4,5,11-17 In one study, women with initial β-hCG levels <1000 mIU/mL, adnexal masses <4 cm, no fetal heartbeat, and <100 mL of fluid in the pouch of Douglas were managed by serial ultrasound and β-hCG levels obtained twice-weekly for 2 weeks; the result was an 88% chance of spontaneous resolution.18 Women (n=9) with initial β-hCG levels ≤1000 mIU/mL with subsequent rising titers experienced no spontaneous resolution.
TABLE 2
Treatment options for ectopic pregnancy
| MODALITY | DESCRIPTION | EP RESOLUTION (%) | TUBAL PATENCY (%) | FUTURE IUP (%) | EP (%) | ADVERSE EVENTS | ||
| Salpingectomy Open or laparoscopic | Excision of ectopic pregnancy and tube | 100 | NA | 42–82* | 6–13 | Hemorrhage Infection Adhesions | ||
| Laparoscopic | Excision of ectopic pregnancy with repair of tube | 93 | 76 | 57 | 13 | Incomplete removal (persistent EP) Analgesic needed Lost work time | ||
| Expectant management | Twice-weekly β-hCG, ultrasound obtained for 2 weeks | 67–68 | 76–77 | 68–86 | 7–13 | Persistent EP 25% need medical or surgical management | ||
| β-hCG <1000 | 88 | |||||||
| β-hCG ≥1000 | 48 | |||||||
| Methotrexate Multidose | 1 mg/kg IV or IM with 0.1 mg/kg folic acid on alternating days. Stop when >15% drop in β-hCG observed or 4 doses administered | 93–95 | 75 | 58 | 7 | Mucositis (stomatitis, gastritis, diarrhea) Dermatitis Bone marrow suppression Hepatic dysfunction Pleuritis | ||
| Single dose | Injection of 50 mg/m2. β-hCG levels days 4 and 7. Repeat dose if no drop. | 87–90 | 81 | 61 | 8 | Reversible alopecia Photosensitivity Pulmonary fibrosis | ||
| Oral | 50 mg daily for 5 days or 60 mg/m2 (one time in 2 divided doses) | 86 | ||||||
| Direct injection | Ultrasound or laparoscopic guidance of 12.5–25 mg | 76 | 80 | 57 | 6 | |||
| EP, ectopic pregnancy; IUP, intrauterine pregnancy; NA, not applicable; IM, intramuscular; IV, intravenous. | ||||||||
| *82% if contralateral tube normal. | ||||||||
Medical management an option for about 25% of patients
Methotrexate depletes tetrahydrofolate cofactors required for DNA and RNA synthesis and cell replication, and thereby inhibits the rapidly growing trophoblasts in patients with ectopic pregnancy.
Methotrexate may be used for primary treatment of ectopic pregnancy, for persistent ectopic pregnancy following tubal sparing surgery, as prophylaxis to reduce persistent ectopic pregnancy following salpingostomy, and in cornual and cervical pregnancies.11,13,19
Who qualifies. Patients eligible for methotrexate administration are those without hemodynamic instability or evidence of tubal rupture (clinical or ultrasound), desiring future fertility, having a gestational sac <3.5 cm, a β-hCG level less than 5000 mIU/mL, no fetal cardiac motion on ultrasound, and the ability and willingness to comply with post-treatment monitoring.10,14
Systemic methotrexate successfully resolves ectopic pregnancy in 90% of patients. Subsequent tubal patency rates approximate 80% and pregnancy rates 60% with recurrent ectopic pregnancy rates 8%.10,13,15,16 The cost of treatment for systemic methotrexate was $5721 per patient compared with $4066 for salpingostomy.20 Hematologic, liver, and renal functions should be assessed before treatment.
Following methotrexate administration, some patients experience a transient increase in abdominal pain, vaginal bleeding, and rising β-hCG. Exclude a ruptured ectopic pregnancy in patients with worsening pain. Pelvic examinations, sexual intercourse, and TVUS should be avoided or minimized during treatment.10
Two regimens are used for the systemic administration of methotrexate (TABLE 2).
The single-dose regimen uses an intramuscular (IM) injection dose of 50 mg/m2 of methotrexate without leucovorin. It has an overall success rate of 87%. β-hCG levels are measured on days 4 and 7, and the medication is repeated if no drop is noticed.13 β-hCG levels are then repeated weekly until undetectable (usually 4 weeks). Serum progesterone levels drop significantly faster than β-hCG following methotrexate administration, and levels <1.5 ng/mL predict ectopic pregnancy resolution more accurately than β-hCG levels.21 Repeat dosing is needed in up to 14% of women.
In the multidose regimen, IM injections of 1 mg/kg of methotrexate are given followed by leucovorin (0.1 mg/kg) after 24 hours. β-hCG levels are checked every other day until there is a 15% or more drop on 2 consecutive days, or 4 doses of methotrexate are administered.1 β-hCG levels are then repeated weekly until undetectable. About half of the women receiving the multidose regimen require 4 or more doses (6.8% require >4 doses). On the other hand, 10% of women treated with this regimen require only 1 dose. Multidose methotrexate has also been used as first-line therapy in cervical, interstitial, ovarian, and abdominal gestation, but with substantially lower success.19
Single-dose vs multidose regimens. Although there are no direct comparison trials, a meta-analysis combining the results of 26 studies with 1067 women treated with a single-dose regimen and 267 women treated with a multidose regimen found that the multidose regimen was slightly more effective.16 The success rate for women with multidose treatment was 92.7% (95% confidence interval [CI], 89–96) vs a single-dose treatment success rate of 88.1% (95% CI, 86–90).
Women treated with single-dose regimens had fewer side effects (31.3% vs 41.2%). However, in both regimens, women who experienced adverse side effects were less likely to have failed treatment (single dose, odds ratio [OR]=0.27; multidose, OR=0.72). Another systematic review including 19 studies with 393 women treated with single-dose methotrexate and 338 women treated with multidose methotrexate had similar outcome findings; the multidose regimen was slightly more effective (93% vs 87%).13
A recent retrospective study comparing the multidose and single-dose regimens in 643 patients with ectopic pregnancy (single dose, n=555; multidose, n=97) from a single database also showed slightly better success with the multidose regimen (95% vs 90%, P=.18).22 Therefore, the multidose regimen may be preferred but a randomized controlled trial comparing the 2 regimens is needed.
In clinically stable women, the serum β-hCG level at presentation is probably the most important single factor determining failure of single-dose methotrexate; patients with low β-hCG levels (1000–2000 mIU/mL) have a high (~98%) response rate.23
Add mifepristone? Mifepristone 600 mg orally added to methotrexate increases successful resolution of unruptured ectopic pregnancy, decreases resolution time, and reduces the need for a second injection or laparotomy, without worsening side effects.24 A recent randomized controlled trial, however, showed that this combination had little advantage over methotrexate alone, and concluded that adding mifepristone be limited to patients with serum progesterone ≥10 ng/mL.25
Direct injection. When fetal cardiac activity is present, injection of 20% potassium chloride (KCl) 0.5 mL into the gestational sac under ultrasound guidance results in asystole and a slow resolution of ectopic pregnancy. Because KCl does not affect the trophoblast, trophoblastic tissue may continue to proliferate leading to tubal rupture.26 Hyperosmolar glucose 1 to 3 mL injected laparoscopically or under ultrasound guidance into the gestational sac maintains tubal patency, has few side effects, but its initial high success rates in resolving ectopic pregnancy (94% to 100%) has not been duplicated.11,13 Another option is prostaglandin F-2 alpha, which when injected into the Fallopian tube causes contractions and vasoconstriction, resulting in a resolution of ectopic pregnancy in 92% of patients.13 However, serious side effects have been reported, including severe abdominal discomfort, vomiting, and pulmonary edema.
CORRESPONDENCE
K. Ramakrishnan, MD, Department of Family and Preventive Medicine, University of Oklahoma Health Sciences Center, 900 NE 10th Street, Oklahoma City, OK 73104. E-mail: [email protected]
- Expectant management may be offered to asymptomatic patients with small adnexal masses (≤3 cm) lower beta-human chorionic gonadotropin (β-hCG) levels (<1000 mIU/mL), evidence of spontaneous resolution (eg, falling β-hCG levels) who are willing to accept the risk of tubal (A).
- Systemic methotrexate administration resolves ectopic pregnancy in 87% to 95% of cases, maintains tubal patency in 75% to 81%, and results in subsequent successful pregnancy in about 58% to 61% of patients. Hemodynamically stable patients with adnexal mass≤3.5 cm, β-hCG levels <5000 mIU/mL, no adnexal yolk sac and normal hematologic, liver, and kidney functions are ideal candidates for methotrexate therapy (A).
Management strategies for patients with ectopic pregnancy have evolved rapidly, with ambulatory medical therapy becoming an option for more patients.1 In part 1, published in the May 2006 JFP, using a practical decision protocol, we discuss the physical findings that most reliably suggest ectopic pregnancy, describe sensible use of laboratory and imaging studies, and explain what to do when results are equivocal.
Management choices
Once the diagnosis of ectopic pregnancy has been made, options include surgical, medical, or expectant management (FIGURE). The goal of treatment is to minimize disease-and treatment-related morbidity while maximizing reproductive potential.
Administer Rhogam to all Rh-negative women.
Clinical prediction tools have been developed to aid management decision making. Fernandez et al developed a score based on gestational age, β-hCG level, progesterone level, abdominal pain, hemoperitoneum volume, and hematosalpinx diameter.2 A score <12 predicts a >80% success with expectant or nonsurgical management (TABLE 1). Similarly, to predict response to a single-dose of methotrexate, Elito et al3 developed a score based on β-hCG level, ultrasound findings, have size of the mass (cm), and color Doppler image aspects (TABLE 1). In a small study of for 40 patients, those with scores >5 had a the 97% success rate.3
FIGURE
Deciding which management option is best for your patient with ectopic pregnancy
TABLE 1
Predictive score for successful treatment of ectopic pregnancy
| Predictive score for expectant management and several nonsurgical treatments (Fernandez 1991) | |||
| CRITERION | 1 POINT | 2 POINT | 3 POINT |
| β-hCG (mIU/mL) | <1000 | 1000–5000 | >5000 |
| Progesterone (ng/mL) | <5 | 5–10 | >10 |
| Abdominal pain | Absent | Induced | Spontaneous |
| Hematosalpinx (cm) | <1 | 1–3 | >3 |
| Hemoperitoneum (mL) | 0 | 1–100 | >100 |
| Score <12: 80% success with various nonsurgical treatments, including expectant management. | |||
| Predictive score for single dose methotrexate (50 mg/m2 IM) (Elito 1999) | |||
| PARAMETERS | 0 POINTS | 1 POINTS | 2 POINTS |
| β-hCG (mIU/mL) | >5000 | 1500–5000 | <1500 |
| Aspects of the image | Live embryo | Tubal ring | Hematosalpinx |
| Size of the mass | >3.0–3.5 | 2.6–3.0 | <2.5 |
| Color Doppler | High risk | Medium risk | Low risk |
| Score≥5: 97% success with single-dose methotrexate. | |||
| Sources: Fernandez et al 1991,2 Elito et al 1999.3 | |||
Surgical management
Surgery is preferred for ruptured ectopic pregnancy. Surgery is also indicated for patients with evidence of hemodynamic instability, anemia, pain for longer than 24 hours, β-hCG levels greater than 5000 mIU/mL, or with a gestational sac that measures more than 3.5 to 4 cm on ultrasound.1,4,5
Laparoscopic techniques minimize the trauma and morbidity of salpingectomy or salpingostomy. Compared with older procedures, they lessen blood loss, decrease the need for analgesia, and allow a shorter hospital stay and an earlier return to work.6
Salpingostomy removes the ectopic pregnancy while preserving the Fallopian tube. Weekly quantitative β-hCG testing is required to rule out persistent ectopic pregnancy, which occurs in 5% to 8% of patients following salpingostomy.7 The likelihood of persistent ectopic pregnancy following salpingostomy increases with an ectopic pregnancy <2 cm in diameter, salpingostomy performed <6 weeks from the last menstrual period, a β-hCG level >3000 mIU/mL, or progesterone level over 35 nmol/L combined with a daily change in β-hCG over 100 mIU/mL.8,9
Expectant management possible when β-hCG levels <1000 mIU/mL
Expectant management may be offered to asymptomatic women with small adnexal masses, lower β-hCG levels, and evidence of spontaneous resolution (eg, falling β-hCG levels) who are willing to accept the risk of tubal rupture.10 Rising β-hCG levels, pain, hemodynamic instability, or hemoperitoneum on ultrasound dictate switching to active management.11
Eighty percent of women with initial β-hCG levels <1000 mIU/mL experience spontaneous resolution (TABLE 2).1,4,5,11-17 In one study, women with initial β-hCG levels <1000 mIU/mL, adnexal masses <4 cm, no fetal heartbeat, and <100 mL of fluid in the pouch of Douglas were managed by serial ultrasound and β-hCG levels obtained twice-weekly for 2 weeks; the result was an 88% chance of spontaneous resolution.18 Women (n=9) with initial β-hCG levels ≤1000 mIU/mL with subsequent rising titers experienced no spontaneous resolution.
TABLE 2
Treatment options for ectopic pregnancy
| MODALITY | DESCRIPTION | EP RESOLUTION (%) | TUBAL PATENCY (%) | FUTURE IUP (%) | EP (%) | ADVERSE EVENTS | ||
| Salpingectomy Open or laparoscopic | Excision of ectopic pregnancy and tube | 100 | NA | 42–82* | 6–13 | Hemorrhage Infection Adhesions | ||
| Laparoscopic | Excision of ectopic pregnancy with repair of tube | 93 | 76 | 57 | 13 | Incomplete removal (persistent EP) Analgesic needed Lost work time | ||
| Expectant management | Twice-weekly β-hCG, ultrasound obtained for 2 weeks | 67–68 | 76–77 | 68–86 | 7–13 | Persistent EP 25% need medical or surgical management | ||
| β-hCG <1000 | 88 | |||||||
| β-hCG ≥1000 | 48 | |||||||
| Methotrexate Multidose | 1 mg/kg IV or IM with 0.1 mg/kg folic acid on alternating days. Stop when >15% drop in β-hCG observed or 4 doses administered | 93–95 | 75 | 58 | 7 | Mucositis (stomatitis, gastritis, diarrhea) Dermatitis Bone marrow suppression Hepatic dysfunction Pleuritis | ||
| Single dose | Injection of 50 mg/m2. β-hCG levels days 4 and 7. Repeat dose if no drop. | 87–90 | 81 | 61 | 8 | Reversible alopecia Photosensitivity Pulmonary fibrosis | ||
| Oral | 50 mg daily for 5 days or 60 mg/m2 (one time in 2 divided doses) | 86 | ||||||
| Direct injection | Ultrasound or laparoscopic guidance of 12.5–25 mg | 76 | 80 | 57 | 6 | |||
| EP, ectopic pregnancy; IUP, intrauterine pregnancy; NA, not applicable; IM, intramuscular; IV, intravenous. | ||||||||
| *82% if contralateral tube normal. | ||||||||
Medical management an option for about 25% of patients
Methotrexate depletes tetrahydrofolate cofactors required for DNA and RNA synthesis and cell replication, and thereby inhibits the rapidly growing trophoblasts in patients with ectopic pregnancy.
Methotrexate may be used for primary treatment of ectopic pregnancy, for persistent ectopic pregnancy following tubal sparing surgery, as prophylaxis to reduce persistent ectopic pregnancy following salpingostomy, and in cornual and cervical pregnancies.11,13,19
Who qualifies. Patients eligible for methotrexate administration are those without hemodynamic instability or evidence of tubal rupture (clinical or ultrasound), desiring future fertility, having a gestational sac <3.5 cm, a β-hCG level less than 5000 mIU/mL, no fetal cardiac motion on ultrasound, and the ability and willingness to comply with post-treatment monitoring.10,14
Systemic methotrexate successfully resolves ectopic pregnancy in 90% of patients. Subsequent tubal patency rates approximate 80% and pregnancy rates 60% with recurrent ectopic pregnancy rates 8%.10,13,15,16 The cost of treatment for systemic methotrexate was $5721 per patient compared with $4066 for salpingostomy.20 Hematologic, liver, and renal functions should be assessed before treatment.
Following methotrexate administration, some patients experience a transient increase in abdominal pain, vaginal bleeding, and rising β-hCG. Exclude a ruptured ectopic pregnancy in patients with worsening pain. Pelvic examinations, sexual intercourse, and TVUS should be avoided or minimized during treatment.10
Two regimens are used for the systemic administration of methotrexate (TABLE 2).
The single-dose regimen uses an intramuscular (IM) injection dose of 50 mg/m2 of methotrexate without leucovorin. It has an overall success rate of 87%. β-hCG levels are measured on days 4 and 7, and the medication is repeated if no drop is noticed.13 β-hCG levels are then repeated weekly until undetectable (usually 4 weeks). Serum progesterone levels drop significantly faster than β-hCG following methotrexate administration, and levels <1.5 ng/mL predict ectopic pregnancy resolution more accurately than β-hCG levels.21 Repeat dosing is needed in up to 14% of women.
In the multidose regimen, IM injections of 1 mg/kg of methotrexate are given followed by leucovorin (0.1 mg/kg) after 24 hours. β-hCG levels are checked every other day until there is a 15% or more drop on 2 consecutive days, or 4 doses of methotrexate are administered.1 β-hCG levels are then repeated weekly until undetectable. About half of the women receiving the multidose regimen require 4 or more doses (6.8% require >4 doses). On the other hand, 10% of women treated with this regimen require only 1 dose. Multidose methotrexate has also been used as first-line therapy in cervical, interstitial, ovarian, and abdominal gestation, but with substantially lower success.19
Single-dose vs multidose regimens. Although there are no direct comparison trials, a meta-analysis combining the results of 26 studies with 1067 women treated with a single-dose regimen and 267 women treated with a multidose regimen found that the multidose regimen was slightly more effective.16 The success rate for women with multidose treatment was 92.7% (95% confidence interval [CI], 89–96) vs a single-dose treatment success rate of 88.1% (95% CI, 86–90).
Women treated with single-dose regimens had fewer side effects (31.3% vs 41.2%). However, in both regimens, women who experienced adverse side effects were less likely to have failed treatment (single dose, odds ratio [OR]=0.27; multidose, OR=0.72). Another systematic review including 19 studies with 393 women treated with single-dose methotrexate and 338 women treated with multidose methotrexate had similar outcome findings; the multidose regimen was slightly more effective (93% vs 87%).13
A recent retrospective study comparing the multidose and single-dose regimens in 643 patients with ectopic pregnancy (single dose, n=555; multidose, n=97) from a single database also showed slightly better success with the multidose regimen (95% vs 90%, P=.18).22 Therefore, the multidose regimen may be preferred but a randomized controlled trial comparing the 2 regimens is needed.
In clinically stable women, the serum β-hCG level at presentation is probably the most important single factor determining failure of single-dose methotrexate; patients with low β-hCG levels (1000–2000 mIU/mL) have a high (~98%) response rate.23
Add mifepristone? Mifepristone 600 mg orally added to methotrexate increases successful resolution of unruptured ectopic pregnancy, decreases resolution time, and reduces the need for a second injection or laparotomy, without worsening side effects.24 A recent randomized controlled trial, however, showed that this combination had little advantage over methotrexate alone, and concluded that adding mifepristone be limited to patients with serum progesterone ≥10 ng/mL.25
Direct injection. When fetal cardiac activity is present, injection of 20% potassium chloride (KCl) 0.5 mL into the gestational sac under ultrasound guidance results in asystole and a slow resolution of ectopic pregnancy. Because KCl does not affect the trophoblast, trophoblastic tissue may continue to proliferate leading to tubal rupture.26 Hyperosmolar glucose 1 to 3 mL injected laparoscopically or under ultrasound guidance into the gestational sac maintains tubal patency, has few side effects, but its initial high success rates in resolving ectopic pregnancy (94% to 100%) has not been duplicated.11,13 Another option is prostaglandin F-2 alpha, which when injected into the Fallopian tube causes contractions and vasoconstriction, resulting in a resolution of ectopic pregnancy in 92% of patients.13 However, serious side effects have been reported, including severe abdominal discomfort, vomiting, and pulmonary edema.
CORRESPONDENCE
K. Ramakrishnan, MD, Department of Family and Preventive Medicine, University of Oklahoma Health Sciences Center, 900 NE 10th Street, Oklahoma City, OK 73104. E-mail: [email protected]
1. Carr RJ, Evans P. Ectopic pregnancy. Prim Care 2000;27:169-183.
2. Fernandez H, Lelaidier C, Thouvenez V, et al. The use of a pretherapeutic, predictive score to determine inclusion criteria for the non-surgical management of ectopic pregnancy. Hum Reprod 1991;6:995-998.
3. Elito J, Jr, Reichmann AP, Uchiyama MN, Camano L. Predictive score for the systemic treatment of unruptured ectopic pregnancy with a single dose of methotrexate. Int J Gynecol Obstet 1999;67:75-79.
4. Pisarska MD, Carson SA, Buster JE. Ectopic pregnancy. Lancet 1998;351:1115-1120.
5. Farquhar CM. Ectopic pregnancy. Lancet 2005;366:583-591.
6. Kelly AJ, Sowter MC, Trinder J. The management of tubal pregnancy. Royal College of Obstetricians and Gynecologists (RCOG). Guideline No. 21, May 2004. Available at: www.rcog.org.uk/resources/Public/pdf/management_tubal_pregnancy21.pdf.
7. Rulin MC. Is salpingostomy the surgical treatment of choice for unruptured tubal pregnancy? Obstet Gynecol 1995;86:1010-1013.
8. Seifer DB. Persistent ectopic pregnancy: an argument for heightened vigilance and patient compliance. Fertil Steril 1997;68:402-404.
9. Hagstrom HG, Hahlin M, Bennegard-Edèn B, Sjoblom P, Thorburn J, Lindblom B. Prediction of persistent ectopic pregnancy after laparoscopic salpingostomy. Obstet Gynecol 1994;84:798-802.
10. ACOG practice bulletin. Medical management of tubal pregnancy. Number 3, December 1998. Clinical management guidelines for obstetrician-gynecologists. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 1999;65:97-103.
11. Mittal S. Non-surgical management of ectopic pregnancy. Obs Gyn Com 1999;1:23-28.
12. Cohen MA, Sauer MV. Expectant management of ectopic pregnancy. Clin Obstet Gynecol 1999;42:48-54.
13. Buster JE, Pisarska MD. Medical management of ectopic pregnancy. Clin Obstet Gynecol 1999;42:23-30.
14. Murray H, Baakdah H, Bardell T, Tulandi T. Diagnosis and treatment of ectopic pregnancy. CMAJ 2005;173:905-912.
15. Pansky M. Methotrexate (MXT) treatment for ectopic pregnancy-systemic vs local injection. Scientific presentation at The First World Congress on Controversies in Obstetrics, Gynecology & Infertility. Prague, Czech Republic, 1999. Available at: www.obgyn.net/firstcontroversies/prague1999pansky.doc. Accessed December 4, 2005.
16. Barnhart KT, Gosman G, Ashby R, Sammel M. The medical management of ectopic pregnancy: a meta-analysis comparing “single dose” and “multidose” regimens. Obstet Gynecol 2003;101:778-784.
17. Lipscomb GH, Meyer NL, Flynn DE, Peterson M, Ling F. Oral methotrexate for treatment of ectopic pregnancy. Am J Obstet Gynecol 2002;186:1192-1195.
18. Trio D, Strobelt N, Picciolo C, Lapinski RH, Ghidini A. Prognostic factors for successful expectant management of ectopic pregnancy. Fertil Steril 1995;63:469-472.
19. Barnhart K, Esposito M, Coutifaris C. An update on the medical treatment of ectopic pregnancy. Obstet Gynecol Clin North Am 2000;27:653-667, viii.
20. Mol BW, Hajenius PJ, Engelsbel S, et al. Treatment of tubal pregnancy in the Netherlands: an economic comparison of systemic methotrexate administration and laparoscopic salpingostomy. Am J Obstet Gynecol 1999;181:945-951.
21. Saraj AJ, Wilcox JG, Najmabadi, Stein SM, Johnson MB, Paulson RJ. Resolution of hormonal markers of ectopic gestation: a randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol 1998;92:989-994.
22. Lipscomb GH, Givens VM, Meyer NL, Bran D. Comparison of multidose and single-dose methotrexate protocols for the treatment of ectopic pregnancy. Am J Obstet Gynecol 2005;192:1844-1847.
23. Sowter MC, Farquhar CM. Ectopic pregnancy: an update. Curr Opin Obstet Gynecol 2004;16:289-293.
24. Gazvani MR, Baruah DN, Alfirevic Z, Emery SJ. Mifepristone in combination with methotrexate for the medical treatment of tubal pregnancy: a randomized, controlled trial. Hum Reprod 1998;13:1987-1990.
25. Rozenberg P, Chevret S, Camus E, et al. Medical treatment of ectopic pregnancies: a randomized clinical trial comparing methotrexate-mifepristone and methotrexate-placebo. Hum Reprod 2003;18:1802-1808.
26. Pansky M, Golan A, Bukovsky I, Caspi E. Nonsurgical management of tubal pregnancy. Necessity in view of the changing clinical appearance. Am J Obstet Gynecol 1991;164:888-895.
1. Carr RJ, Evans P. Ectopic pregnancy. Prim Care 2000;27:169-183.
2. Fernandez H, Lelaidier C, Thouvenez V, et al. The use of a pretherapeutic, predictive score to determine inclusion criteria for the non-surgical management of ectopic pregnancy. Hum Reprod 1991;6:995-998.
3. Elito J, Jr, Reichmann AP, Uchiyama MN, Camano L. Predictive score for the systemic treatment of unruptured ectopic pregnancy with a single dose of methotrexate. Int J Gynecol Obstet 1999;67:75-79.
4. Pisarska MD, Carson SA, Buster JE. Ectopic pregnancy. Lancet 1998;351:1115-1120.
5. Farquhar CM. Ectopic pregnancy. Lancet 2005;366:583-591.
6. Kelly AJ, Sowter MC, Trinder J. The management of tubal pregnancy. Royal College of Obstetricians and Gynecologists (RCOG). Guideline No. 21, May 2004. Available at: www.rcog.org.uk/resources/Public/pdf/management_tubal_pregnancy21.pdf.
7. Rulin MC. Is salpingostomy the surgical treatment of choice for unruptured tubal pregnancy? Obstet Gynecol 1995;86:1010-1013.
8. Seifer DB. Persistent ectopic pregnancy: an argument for heightened vigilance and patient compliance. Fertil Steril 1997;68:402-404.
9. Hagstrom HG, Hahlin M, Bennegard-Edèn B, Sjoblom P, Thorburn J, Lindblom B. Prediction of persistent ectopic pregnancy after laparoscopic salpingostomy. Obstet Gynecol 1994;84:798-802.
10. ACOG practice bulletin. Medical management of tubal pregnancy. Number 3, December 1998. Clinical management guidelines for obstetrician-gynecologists. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 1999;65:97-103.
11. Mittal S. Non-surgical management of ectopic pregnancy. Obs Gyn Com 1999;1:23-28.
12. Cohen MA, Sauer MV. Expectant management of ectopic pregnancy. Clin Obstet Gynecol 1999;42:48-54.
13. Buster JE, Pisarska MD. Medical management of ectopic pregnancy. Clin Obstet Gynecol 1999;42:23-30.
14. Murray H, Baakdah H, Bardell T, Tulandi T. Diagnosis and treatment of ectopic pregnancy. CMAJ 2005;173:905-912.
15. Pansky M. Methotrexate (MXT) treatment for ectopic pregnancy-systemic vs local injection. Scientific presentation at The First World Congress on Controversies in Obstetrics, Gynecology & Infertility. Prague, Czech Republic, 1999. Available at: www.obgyn.net/firstcontroversies/prague1999pansky.doc. Accessed December 4, 2005.
16. Barnhart KT, Gosman G, Ashby R, Sammel M. The medical management of ectopic pregnancy: a meta-analysis comparing “single dose” and “multidose” regimens. Obstet Gynecol 2003;101:778-784.
17. Lipscomb GH, Meyer NL, Flynn DE, Peterson M, Ling F. Oral methotrexate for treatment of ectopic pregnancy. Am J Obstet Gynecol 2002;186:1192-1195.
18. Trio D, Strobelt N, Picciolo C, Lapinski RH, Ghidini A. Prognostic factors for successful expectant management of ectopic pregnancy. Fertil Steril 1995;63:469-472.
19. Barnhart K, Esposito M, Coutifaris C. An update on the medical treatment of ectopic pregnancy. Obstet Gynecol Clin North Am 2000;27:653-667, viii.
20. Mol BW, Hajenius PJ, Engelsbel S, et al. Treatment of tubal pregnancy in the Netherlands: an economic comparison of systemic methotrexate administration and laparoscopic salpingostomy. Am J Obstet Gynecol 1999;181:945-951.
21. Saraj AJ, Wilcox JG, Najmabadi, Stein SM, Johnson MB, Paulson RJ. Resolution of hormonal markers of ectopic gestation: a randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol 1998;92:989-994.
22. Lipscomb GH, Givens VM, Meyer NL, Bran D. Comparison of multidose and single-dose methotrexate protocols for the treatment of ectopic pregnancy. Am J Obstet Gynecol 2005;192:1844-1847.
23. Sowter MC, Farquhar CM. Ectopic pregnancy: an update. Curr Opin Obstet Gynecol 2004;16:289-293.
24. Gazvani MR, Baruah DN, Alfirevic Z, Emery SJ. Mifepristone in combination with methotrexate for the medical treatment of tubal pregnancy: a randomized, controlled trial. Hum Reprod 1998;13:1987-1990.
25. Rozenberg P, Chevret S, Camus E, et al. Medical treatment of ectopic pregnancies: a randomized clinical trial comparing methotrexate-mifepristone and methotrexate-placebo. Hum Reprod 2003;18:1802-1808.
26. Pansky M, Golan A, Bukovsky I, Caspi E. Nonsurgical management of tubal pregnancy. Necessity in view of the changing clinical appearance. Am J Obstet Gynecol 1991;164:888-895.