User login
Ectopic pregnancy: Expectant management an immediate surgery?
- Expectant management may be offered to asymptomatic patients with small adnexal masses (≤3 cm) lower beta-human chorionic gonadotropin (β-hCG) levels (<1000 mIU/mL), evidence of spontaneous resolution (eg, falling β-hCG levels) who are willing to accept the risk of tubal (A).
- Systemic methotrexate administration resolves ectopic pregnancy in 87% to 95% of cases, maintains tubal patency in 75% to 81%, and results in subsequent successful pregnancy in about 58% to 61% of patients. Hemodynamically stable patients with adnexal mass≤3.5 cm, β-hCG levels <5000 mIU/mL, no adnexal yolk sac and normal hematologic, liver, and kidney functions are ideal candidates for methotrexate therapy (A).
Management strategies for patients with ectopic pregnancy have evolved rapidly, with ambulatory medical therapy becoming an option for more patients.1 In part 1, published in the May 2006 JFP, using a practical decision protocol, we discuss the physical findings that most reliably suggest ectopic pregnancy, describe sensible use of laboratory and imaging studies, and explain what to do when results are equivocal.
Management choices
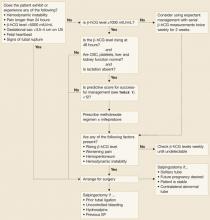
Once the diagnosis of ectopic pregnancy has been made, options include surgical, medical, or expectant management (FIGURE). The goal of treatment is to minimize disease-and treatment-related morbidity while maximizing reproductive potential.
Administer Rhogam to all Rh-negative women.
Clinical prediction tools have been developed to aid management decision making. Fernandez et al developed a score based on gestational age, β-hCG level, progesterone level, abdominal pain, hemoperitoneum volume, and hematosalpinx diameter.2 A score <12 predicts a >80% success with expectant or nonsurgical management (TABLE 1). Similarly, to predict response to a single-dose of methotrexate, Elito et al3 developed a score based on β-hCG level, ultrasound findings, have size of the mass (cm), and color Doppler image aspects (TABLE 1). In a small study of for 40 patients, those with scores >5 had a the 97% success rate.3
FIGURE
Deciding which management option is best for your patient with ectopic pregnancy
TABLE 1
Predictive score for successful treatment of ectopic pregnancy
| Predictive score for expectant management and several nonsurgical treatments (Fernandez 1991) | |||
| CRITERION | 1 POINT | 2 POINT | 3 POINT |
| β-hCG (mIU/mL) | <1000 | 1000–5000 | >5000 |
| Progesterone (ng/mL) | <5 | 5–10 | >10 |
| Abdominal pain | Absent | Induced | Spontaneous |
| Hematosalpinx (cm) | <1 | 1–3 | >3 |
| Hemoperitoneum (mL) | 0 | 1–100 | >100 |
| Score <12: 80% success with various nonsurgical treatments, including expectant management. | |||
| Predictive score for single dose methotrexate (50 mg/m2 IM) (Elito 1999) | |||
| PARAMETERS | 0 POINTS | 1 POINTS | 2 POINTS |
| β-hCG (mIU/mL) | >5000 | 1500–5000 | <1500 |
| Aspects of the image | Live embryo | Tubal ring | Hematosalpinx |
| Size of the mass | >3.0–3.5 | 2.6–3.0 | <2.5 |
| Color Doppler | High risk | Medium risk | Low risk |
| Score≥5: 97% success with single-dose methotrexate. | |||
| Sources: Fernandez et al 1991,2 Elito et al 1999.3 | |||
Surgical management
Surgery is preferred for ruptured ectopic pregnancy. Surgery is also indicated for patients with evidence of hemodynamic instability, anemia, pain for longer than 24 hours, β-hCG levels greater than 5000 mIU/mL, or with a gestational sac that measures more than 3.5 to 4 cm on ultrasound.1,4,5
Laparoscopic techniques minimize the trauma and morbidity of salpingectomy or salpingostomy. Compared with older procedures, they lessen blood loss, decrease the need for analgesia, and allow a shorter hospital stay and an earlier return to work.6
Salpingostomy removes the ectopic pregnancy while preserving the Fallopian tube. Weekly quantitative β-hCG testing is required to rule out persistent ectopic pregnancy, which occurs in 5% to 8% of patients following salpingostomy.7 The likelihood of persistent ectopic pregnancy following salpingostomy increases with an ectopic pregnancy <2 cm in diameter, salpingostomy performed <6 weeks from the last menstrual period, a β-hCG level >3000 mIU/mL, or progesterone level over 35 nmol/L combined with a daily change in β-hCG over 100 mIU/mL.8,9
Expectant management possible when β-hCG levels <1000 mIU/mL
Expectant management may be offered to asymptomatic women with small adnexal masses, lower β-hCG levels, and evidence of spontaneous resolution (eg, falling β-hCG levels) who are willing to accept the risk of tubal rupture.10 Rising β-hCG levels, pain, hemodynamic instability, or hemoperitoneum on ultrasound dictate switching to active management.11
Eighty percent of women with initial β-hCG levels <1000 mIU/mL experience spontaneous resolution (TABLE 2).1,4,5,11-17 In one study, women with initial β-hCG levels <1000 mIU/mL, adnexal masses <4 cm, no fetal heartbeat, and <100 mL of fluid in the pouch of Douglas were managed by serial ultrasound and β-hCG levels obtained twice-weekly for 2 weeks; the result was an 88% chance of spontaneous resolution.18 Women (n=9) with initial β-hCG levels ≤1000 mIU/mL with subsequent rising titers experienced no spontaneous resolution.
TABLE 2
Treatment options for ectopic pregnancy
| MODALITY | DESCRIPTION | EP RESOLUTION (%) | TUBAL PATENCY (%) | FUTURE IUP (%) | EP (%) | ADVERSE EVENTS | ||
| Salpingectomy Open or laparoscopic | Excision of ectopic pregnancy and tube | 100 | NA | 42–82* | 6–13 | Hemorrhage Infection Adhesions | ||
| Laparoscopic | Excision of ectopic pregnancy with repair of tube | 93 | 76 | 57 | 13 | Incomplete removal (persistent EP) Analgesic needed Lost work time | ||
| Expectant management | Twice-weekly β-hCG, ultrasound obtained for 2 weeks | 67–68 | 76–77 | 68–86 | 7–13 | Persistent EP 25% need medical or surgical management | ||
| β-hCG <1000 | 88 | |||||||
| β-hCG ≥1000 | 48 | |||||||
| Methotrexate Multidose | 1 mg/kg IV or IM with 0.1 mg/kg folic acid on alternating days. Stop when >15% drop in β-hCG observed or 4 doses administered | 93–95 | 75 | 58 | 7 | Mucositis (stomatitis, gastritis, diarrhea) Dermatitis Bone marrow suppression Hepatic dysfunction Pleuritis | ||
| Single dose | Injection of 50 mg/m2. β-hCG levels days 4 and 7. Repeat dose if no drop. | 87–90 | 81 | 61 | 8 | Reversible alopecia Photosensitivity Pulmonary fibrosis | ||
| Oral | 50 mg daily for 5 days or 60 mg/m2 (one time in 2 divided doses) | 86 | ||||||
| Direct injection | Ultrasound or laparoscopic guidance of 12.5–25 mg | 76 | 80 | 57 | 6 | |||
| EP, ectopic pregnancy; IUP, intrauterine pregnancy; NA, not applicable; IM, intramuscular; IV, intravenous. | ||||||||
| *82% if contralateral tube normal. | ||||||||
Medical management an option for about 25% of patients
Methotrexate depletes tetrahydrofolate cofactors required for DNA and RNA synthesis and cell replication, and thereby inhibits the rapidly growing trophoblasts in patients with ectopic pregnancy.
Methotrexate may be used for primary treatment of ectopic pregnancy, for persistent ectopic pregnancy following tubal sparing surgery, as prophylaxis to reduce persistent ectopic pregnancy following salpingostomy, and in cornual and cervical pregnancies.11,13,19
Who qualifies. Patients eligible for methotrexate administration are those without hemodynamic instability or evidence of tubal rupture (clinical or ultrasound), desiring future fertility, having a gestational sac <3.5 cm, a β-hCG level less than 5000 mIU/mL, no fetal cardiac motion on ultrasound, and the ability and willingness to comply with post-treatment monitoring.10,14
Systemic methotrexate successfully resolves ectopic pregnancy in 90% of patients. Subsequent tubal patency rates approximate 80% and pregnancy rates 60% with recurrent ectopic pregnancy rates 8%.10,13,15,16 The cost of treatment for systemic methotrexate was $5721 per patient compared with $4066 for salpingostomy.20 Hematologic, liver, and renal functions should be assessed before treatment.
Following methotrexate administration, some patients experience a transient increase in abdominal pain, vaginal bleeding, and rising β-hCG. Exclude a ruptured ectopic pregnancy in patients with worsening pain. Pelvic examinations, sexual intercourse, and TVUS should be avoided or minimized during treatment.10
Two regimens are used for the systemic administration of methotrexate (TABLE 2).
The single-dose regimen uses an intramuscular (IM) injection dose of 50 mg/m2 of methotrexate without leucovorin. It has an overall success rate of 87%. β-hCG levels are measured on days 4 and 7, and the medication is repeated if no drop is noticed.13 β-hCG levels are then repeated weekly until undetectable (usually 4 weeks). Serum progesterone levels drop significantly faster than β-hCG following methotrexate administration, and levels <1.5 ng/mL predict ectopic pregnancy resolution more accurately than β-hCG levels.21 Repeat dosing is needed in up to 14% of women.
In the multidose regimen, IM injections of 1 mg/kg of methotrexate are given followed by leucovorin (0.1 mg/kg) after 24 hours. β-hCG levels are checked every other day until there is a 15% or more drop on 2 consecutive days, or 4 doses of methotrexate are administered.1 β-hCG levels are then repeated weekly until undetectable. About half of the women receiving the multidose regimen require 4 or more doses (6.8% require >4 doses). On the other hand, 10% of women treated with this regimen require only 1 dose. Multidose methotrexate has also been used as first-line therapy in cervical, interstitial, ovarian, and abdominal gestation, but with substantially lower success.19
Single-dose vs multidose regimens. Although there are no direct comparison trials, a meta-analysis combining the results of 26 studies with 1067 women treated with a single-dose regimen and 267 women treated with a multidose regimen found that the multidose regimen was slightly more effective.16 The success rate for women with multidose treatment was 92.7% (95% confidence interval [CI], 89–96) vs a single-dose treatment success rate of 88.1% (95% CI, 86–90).
Women treated with single-dose regimens had fewer side effects (31.3% vs 41.2%). However, in both regimens, women who experienced adverse side effects were less likely to have failed treatment (single dose, odds ratio [OR]=0.27; multidose, OR=0.72). Another systematic review including 19 studies with 393 women treated with single-dose methotrexate and 338 women treated with multidose methotrexate had similar outcome findings; the multidose regimen was slightly more effective (93% vs 87%).13
A recent retrospective study comparing the multidose and single-dose regimens in 643 patients with ectopic pregnancy (single dose, n=555; multidose, n=97) from a single database also showed slightly better success with the multidose regimen (95% vs 90%, P=.18).22 Therefore, the multidose regimen may be preferred but a randomized controlled trial comparing the 2 regimens is needed.
In clinically stable women, the serum β-hCG level at presentation is probably the most important single factor determining failure of single-dose methotrexate; patients with low β-hCG levels (1000–2000 mIU/mL) have a high (~98%) response rate.23
Add mifepristone? Mifepristone 600 mg orally added to methotrexate increases successful resolution of unruptured ectopic pregnancy, decreases resolution time, and reduces the need for a second injection or laparotomy, without worsening side effects.24 A recent randomized controlled trial, however, showed that this combination had little advantage over methotrexate alone, and concluded that adding mifepristone be limited to patients with serum progesterone ≥10 ng/mL.25
Direct injection. When fetal cardiac activity is present, injection of 20% potassium chloride (KCl) 0.5 mL into the gestational sac under ultrasound guidance results in asystole and a slow resolution of ectopic pregnancy. Because KCl does not affect the trophoblast, trophoblastic tissue may continue to proliferate leading to tubal rupture.26 Hyperosmolar glucose 1 to 3 mL injected laparoscopically or under ultrasound guidance into the gestational sac maintains tubal patency, has few side effects, but its initial high success rates in resolving ectopic pregnancy (94% to 100%) has not been duplicated.11,13 Another option is prostaglandin F-2 alpha, which when injected into the Fallopian tube causes contractions and vasoconstriction, resulting in a resolution of ectopic pregnancy in 92% of patients.13 However, serious side effects have been reported, including severe abdominal discomfort, vomiting, and pulmonary edema.
CORRESPONDENCE
K. Ramakrishnan, MD, Department of Family and Preventive Medicine, University of Oklahoma Health Sciences Center, 900 NE 10th Street, Oklahoma City, OK 73104. E-mail: [email protected]
1. Carr RJ, Evans P. Ectopic pregnancy. Prim Care 2000;27:169-183.
2. Fernandez H, Lelaidier C, Thouvenez V, et al. The use of a pretherapeutic, predictive score to determine inclusion criteria for the non-surgical management of ectopic pregnancy. Hum Reprod 1991;6:995-998.
3. Elito J, Jr, Reichmann AP, Uchiyama MN, Camano L. Predictive score for the systemic treatment of unruptured ectopic pregnancy with a single dose of methotrexate. Int J Gynecol Obstet 1999;67:75-79.
4. Pisarska MD, Carson SA, Buster JE. Ectopic pregnancy. Lancet 1998;351:1115-1120.
5. Farquhar CM. Ectopic pregnancy. Lancet 2005;366:583-591.
6. Kelly AJ, Sowter MC, Trinder J. The management of tubal pregnancy. Royal College of Obstetricians and Gynecologists (RCOG). Guideline No. 21, May 2004. Available at: www.rcog.org.uk/resources/Public/pdf/management_tubal_pregnancy21.pdf.
7. Rulin MC. Is salpingostomy the surgical treatment of choice for unruptured tubal pregnancy? Obstet Gynecol 1995;86:1010-1013.
8. Seifer DB. Persistent ectopic pregnancy: an argument for heightened vigilance and patient compliance. Fertil Steril 1997;68:402-404.
9. Hagstrom HG, Hahlin M, Bennegard-Edèn B, Sjoblom P, Thorburn J, Lindblom B. Prediction of persistent ectopic pregnancy after laparoscopic salpingostomy. Obstet Gynecol 1994;84:798-802.
10. ACOG practice bulletin. Medical management of tubal pregnancy. Number 3, December 1998. Clinical management guidelines for obstetrician-gynecologists. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 1999;65:97-103.
11. Mittal S. Non-surgical management of ectopic pregnancy. Obs Gyn Com 1999;1:23-28.
12. Cohen MA, Sauer MV. Expectant management of ectopic pregnancy. Clin Obstet Gynecol 1999;42:48-54.
13. Buster JE, Pisarska MD. Medical management of ectopic pregnancy. Clin Obstet Gynecol 1999;42:23-30.
14. Murray H, Baakdah H, Bardell T, Tulandi T. Diagnosis and treatment of ectopic pregnancy. CMAJ 2005;173:905-912.
15. Pansky M. Methotrexate (MXT) treatment for ectopic pregnancy-systemic vs local injection. Scientific presentation at The First World Congress on Controversies in Obstetrics, Gynecology & Infertility. Prague, Czech Republic, 1999. Available at: www.obgyn.net/firstcontroversies/prague1999pansky.doc. Accessed December 4, 2005.
16. Barnhart KT, Gosman G, Ashby R, Sammel M. The medical management of ectopic pregnancy: a meta-analysis comparing “single dose” and “multidose” regimens. Obstet Gynecol 2003;101:778-784.
17. Lipscomb GH, Meyer NL, Flynn DE, Peterson M, Ling F. Oral methotrexate for treatment of ectopic pregnancy. Am J Obstet Gynecol 2002;186:1192-1195.
18. Trio D, Strobelt N, Picciolo C, Lapinski RH, Ghidini A. Prognostic factors for successful expectant management of ectopic pregnancy. Fertil Steril 1995;63:469-472.
19. Barnhart K, Esposito M, Coutifaris C. An update on the medical treatment of ectopic pregnancy. Obstet Gynecol Clin North Am 2000;27:653-667, viii.
20. Mol BW, Hajenius PJ, Engelsbel S, et al. Treatment of tubal pregnancy in the Netherlands: an economic comparison of systemic methotrexate administration and laparoscopic salpingostomy. Am J Obstet Gynecol 1999;181:945-951.
21. Saraj AJ, Wilcox JG, Najmabadi, Stein SM, Johnson MB, Paulson RJ. Resolution of hormonal markers of ectopic gestation: a randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol 1998;92:989-994.
22. Lipscomb GH, Givens VM, Meyer NL, Bran D. Comparison of multidose and single-dose methotrexate protocols for the treatment of ectopic pregnancy. Am J Obstet Gynecol 2005;192:1844-1847.
23. Sowter MC, Farquhar CM. Ectopic pregnancy: an update. Curr Opin Obstet Gynecol 2004;16:289-293.
24. Gazvani MR, Baruah DN, Alfirevic Z, Emery SJ. Mifepristone in combination with methotrexate for the medical treatment of tubal pregnancy: a randomized, controlled trial. Hum Reprod 1998;13:1987-1990.
25. Rozenberg P, Chevret S, Camus E, et al. Medical treatment of ectopic pregnancies: a randomized clinical trial comparing methotrexate-mifepristone and methotrexate-placebo. Hum Reprod 2003;18:1802-1808.
26. Pansky M, Golan A, Bukovsky I, Caspi E. Nonsurgical management of tubal pregnancy. Necessity in view of the changing clinical appearance. Am J Obstet Gynecol 1991;164:888-895.
- Expectant management may be offered to asymptomatic patients with small adnexal masses (≤3 cm) lower beta-human chorionic gonadotropin (β-hCG) levels (<1000 mIU/mL), evidence of spontaneous resolution (eg, falling β-hCG levels) who are willing to accept the risk of tubal (A).
- Systemic methotrexate administration resolves ectopic pregnancy in 87% to 95% of cases, maintains tubal patency in 75% to 81%, and results in subsequent successful pregnancy in about 58% to 61% of patients. Hemodynamically stable patients with adnexal mass≤3.5 cm, β-hCG levels <5000 mIU/mL, no adnexal yolk sac and normal hematologic, liver, and kidney functions are ideal candidates for methotrexate therapy (A).
Management strategies for patients with ectopic pregnancy have evolved rapidly, with ambulatory medical therapy becoming an option for more patients.1 In part 1, published in the May 2006 JFP, using a practical decision protocol, we discuss the physical findings that most reliably suggest ectopic pregnancy, describe sensible use of laboratory and imaging studies, and explain what to do when results are equivocal.
Management choices
Once the diagnosis of ectopic pregnancy has been made, options include surgical, medical, or expectant management (FIGURE). The goal of treatment is to minimize disease-and treatment-related morbidity while maximizing reproductive potential.
Administer Rhogam to all Rh-negative women.
Clinical prediction tools have been developed to aid management decision making. Fernandez et al developed a score based on gestational age, β-hCG level, progesterone level, abdominal pain, hemoperitoneum volume, and hematosalpinx diameter.2 A score <12 predicts a >80% success with expectant or nonsurgical management (TABLE 1). Similarly, to predict response to a single-dose of methotrexate, Elito et al3 developed a score based on β-hCG level, ultrasound findings, have size of the mass (cm), and color Doppler image aspects (TABLE 1). In a small study of for 40 patients, those with scores >5 had a the 97% success rate.3
FIGURE
Deciding which management option is best for your patient with ectopic pregnancy
TABLE 1
Predictive score for successful treatment of ectopic pregnancy
| Predictive score for expectant management and several nonsurgical treatments (Fernandez 1991) | |||
| CRITERION | 1 POINT | 2 POINT | 3 POINT |
| β-hCG (mIU/mL) | <1000 | 1000–5000 | >5000 |
| Progesterone (ng/mL) | <5 | 5–10 | >10 |
| Abdominal pain | Absent | Induced | Spontaneous |
| Hematosalpinx (cm) | <1 | 1–3 | >3 |
| Hemoperitoneum (mL) | 0 | 1–100 | >100 |
| Score <12: 80% success with various nonsurgical treatments, including expectant management. | |||
| Predictive score for single dose methotrexate (50 mg/m2 IM) (Elito 1999) | |||
| PARAMETERS | 0 POINTS | 1 POINTS | 2 POINTS |
| β-hCG (mIU/mL) | >5000 | 1500–5000 | <1500 |
| Aspects of the image | Live embryo | Tubal ring | Hematosalpinx |
| Size of the mass | >3.0–3.5 | 2.6–3.0 | <2.5 |
| Color Doppler | High risk | Medium risk | Low risk |
| Score≥5: 97% success with single-dose methotrexate. | |||
| Sources: Fernandez et al 1991,2 Elito et al 1999.3 | |||
Surgical management
Surgery is preferred for ruptured ectopic pregnancy. Surgery is also indicated for patients with evidence of hemodynamic instability, anemia, pain for longer than 24 hours, β-hCG levels greater than 5000 mIU/mL, or with a gestational sac that measures more than 3.5 to 4 cm on ultrasound.1,4,5
Laparoscopic techniques minimize the trauma and morbidity of salpingectomy or salpingostomy. Compared with older procedures, they lessen blood loss, decrease the need for analgesia, and allow a shorter hospital stay and an earlier return to work.6
Salpingostomy removes the ectopic pregnancy while preserving the Fallopian tube. Weekly quantitative β-hCG testing is required to rule out persistent ectopic pregnancy, which occurs in 5% to 8% of patients following salpingostomy.7 The likelihood of persistent ectopic pregnancy following salpingostomy increases with an ectopic pregnancy <2 cm in diameter, salpingostomy performed <6 weeks from the last menstrual period, a β-hCG level >3000 mIU/mL, or progesterone level over 35 nmol/L combined with a daily change in β-hCG over 100 mIU/mL.8,9
Expectant management possible when β-hCG levels <1000 mIU/mL
Expectant management may be offered to asymptomatic women with small adnexal masses, lower β-hCG levels, and evidence of spontaneous resolution (eg, falling β-hCG levels) who are willing to accept the risk of tubal rupture.10 Rising β-hCG levels, pain, hemodynamic instability, or hemoperitoneum on ultrasound dictate switching to active management.11
Eighty percent of women with initial β-hCG levels <1000 mIU/mL experience spontaneous resolution (TABLE 2).1,4,5,11-17 In one study, women with initial β-hCG levels <1000 mIU/mL, adnexal masses <4 cm, no fetal heartbeat, and <100 mL of fluid in the pouch of Douglas were managed by serial ultrasound and β-hCG levels obtained twice-weekly for 2 weeks; the result was an 88% chance of spontaneous resolution.18 Women (n=9) with initial β-hCG levels ≤1000 mIU/mL with subsequent rising titers experienced no spontaneous resolution.
TABLE 2
Treatment options for ectopic pregnancy
| MODALITY | DESCRIPTION | EP RESOLUTION (%) | TUBAL PATENCY (%) | FUTURE IUP (%) | EP (%) | ADVERSE EVENTS | ||
| Salpingectomy Open or laparoscopic | Excision of ectopic pregnancy and tube | 100 | NA | 42–82* | 6–13 | Hemorrhage Infection Adhesions | ||
| Laparoscopic | Excision of ectopic pregnancy with repair of tube | 93 | 76 | 57 | 13 | Incomplete removal (persistent EP) Analgesic needed Lost work time | ||
| Expectant management | Twice-weekly β-hCG, ultrasound obtained for 2 weeks | 67–68 | 76–77 | 68–86 | 7–13 | Persistent EP 25% need medical or surgical management | ||
| β-hCG <1000 | 88 | |||||||
| β-hCG ≥1000 | 48 | |||||||
| Methotrexate Multidose | 1 mg/kg IV or IM with 0.1 mg/kg folic acid on alternating days. Stop when >15% drop in β-hCG observed or 4 doses administered | 93–95 | 75 | 58 | 7 | Mucositis (stomatitis, gastritis, diarrhea) Dermatitis Bone marrow suppression Hepatic dysfunction Pleuritis | ||
| Single dose | Injection of 50 mg/m2. β-hCG levels days 4 and 7. Repeat dose if no drop. | 87–90 | 81 | 61 | 8 | Reversible alopecia Photosensitivity Pulmonary fibrosis | ||
| Oral | 50 mg daily for 5 days or 60 mg/m2 (one time in 2 divided doses) | 86 | ||||||
| Direct injection | Ultrasound or laparoscopic guidance of 12.5–25 mg | 76 | 80 | 57 | 6 | |||
| EP, ectopic pregnancy; IUP, intrauterine pregnancy; NA, not applicable; IM, intramuscular; IV, intravenous. | ||||||||
| *82% if contralateral tube normal. | ||||||||
Medical management an option for about 25% of patients
Methotrexate depletes tetrahydrofolate cofactors required for DNA and RNA synthesis and cell replication, and thereby inhibits the rapidly growing trophoblasts in patients with ectopic pregnancy.
Methotrexate may be used for primary treatment of ectopic pregnancy, for persistent ectopic pregnancy following tubal sparing surgery, as prophylaxis to reduce persistent ectopic pregnancy following salpingostomy, and in cornual and cervical pregnancies.11,13,19
Who qualifies. Patients eligible for methotrexate administration are those without hemodynamic instability or evidence of tubal rupture (clinical or ultrasound), desiring future fertility, having a gestational sac <3.5 cm, a β-hCG level less than 5000 mIU/mL, no fetal cardiac motion on ultrasound, and the ability and willingness to comply with post-treatment monitoring.10,14
Systemic methotrexate successfully resolves ectopic pregnancy in 90% of patients. Subsequent tubal patency rates approximate 80% and pregnancy rates 60% with recurrent ectopic pregnancy rates 8%.10,13,15,16 The cost of treatment for systemic methotrexate was $5721 per patient compared with $4066 for salpingostomy.20 Hematologic, liver, and renal functions should be assessed before treatment.
Following methotrexate administration, some patients experience a transient increase in abdominal pain, vaginal bleeding, and rising β-hCG. Exclude a ruptured ectopic pregnancy in patients with worsening pain. Pelvic examinations, sexual intercourse, and TVUS should be avoided or minimized during treatment.10
Two regimens are used for the systemic administration of methotrexate (TABLE 2).
The single-dose regimen uses an intramuscular (IM) injection dose of 50 mg/m2 of methotrexate without leucovorin. It has an overall success rate of 87%. β-hCG levels are measured on days 4 and 7, and the medication is repeated if no drop is noticed.13 β-hCG levels are then repeated weekly until undetectable (usually 4 weeks). Serum progesterone levels drop significantly faster than β-hCG following methotrexate administration, and levels <1.5 ng/mL predict ectopic pregnancy resolution more accurately than β-hCG levels.21 Repeat dosing is needed in up to 14% of women.
In the multidose regimen, IM injections of 1 mg/kg of methotrexate are given followed by leucovorin (0.1 mg/kg) after 24 hours. β-hCG levels are checked every other day until there is a 15% or more drop on 2 consecutive days, or 4 doses of methotrexate are administered.1 β-hCG levels are then repeated weekly until undetectable. About half of the women receiving the multidose regimen require 4 or more doses (6.8% require >4 doses). On the other hand, 10% of women treated with this regimen require only 1 dose. Multidose methotrexate has also been used as first-line therapy in cervical, interstitial, ovarian, and abdominal gestation, but with substantially lower success.19
Single-dose vs multidose regimens. Although there are no direct comparison trials, a meta-analysis combining the results of 26 studies with 1067 women treated with a single-dose regimen and 267 women treated with a multidose regimen found that the multidose regimen was slightly more effective.16 The success rate for women with multidose treatment was 92.7% (95% confidence interval [CI], 89–96) vs a single-dose treatment success rate of 88.1% (95% CI, 86–90).
Women treated with single-dose regimens had fewer side effects (31.3% vs 41.2%). However, in both regimens, women who experienced adverse side effects were less likely to have failed treatment (single dose, odds ratio [OR]=0.27; multidose, OR=0.72). Another systematic review including 19 studies with 393 women treated with single-dose methotrexate and 338 women treated with multidose methotrexate had similar outcome findings; the multidose regimen was slightly more effective (93% vs 87%).13
A recent retrospective study comparing the multidose and single-dose regimens in 643 patients with ectopic pregnancy (single dose, n=555; multidose, n=97) from a single database also showed slightly better success with the multidose regimen (95% vs 90%, P=.18).22 Therefore, the multidose regimen may be preferred but a randomized controlled trial comparing the 2 regimens is needed.
In clinically stable women, the serum β-hCG level at presentation is probably the most important single factor determining failure of single-dose methotrexate; patients with low β-hCG levels (1000–2000 mIU/mL) have a high (~98%) response rate.23
Add mifepristone? Mifepristone 600 mg orally added to methotrexate increases successful resolution of unruptured ectopic pregnancy, decreases resolution time, and reduces the need for a second injection or laparotomy, without worsening side effects.24 A recent randomized controlled trial, however, showed that this combination had little advantage over methotrexate alone, and concluded that adding mifepristone be limited to patients with serum progesterone ≥10 ng/mL.25
Direct injection. When fetal cardiac activity is present, injection of 20% potassium chloride (KCl) 0.5 mL into the gestational sac under ultrasound guidance results in asystole and a slow resolution of ectopic pregnancy. Because KCl does not affect the trophoblast, trophoblastic tissue may continue to proliferate leading to tubal rupture.26 Hyperosmolar glucose 1 to 3 mL injected laparoscopically or under ultrasound guidance into the gestational sac maintains tubal patency, has few side effects, but its initial high success rates in resolving ectopic pregnancy (94% to 100%) has not been duplicated.11,13 Another option is prostaglandin F-2 alpha, which when injected into the Fallopian tube causes contractions and vasoconstriction, resulting in a resolution of ectopic pregnancy in 92% of patients.13 However, serious side effects have been reported, including severe abdominal discomfort, vomiting, and pulmonary edema.
CORRESPONDENCE
K. Ramakrishnan, MD, Department of Family and Preventive Medicine, University of Oklahoma Health Sciences Center, 900 NE 10th Street, Oklahoma City, OK 73104. E-mail: [email protected]
- Expectant management may be offered to asymptomatic patients with small adnexal masses (≤3 cm) lower beta-human chorionic gonadotropin (β-hCG) levels (<1000 mIU/mL), evidence of spontaneous resolution (eg, falling β-hCG levels) who are willing to accept the risk of tubal (A).
- Systemic methotrexate administration resolves ectopic pregnancy in 87% to 95% of cases, maintains tubal patency in 75% to 81%, and results in subsequent successful pregnancy in about 58% to 61% of patients. Hemodynamically stable patients with adnexal mass≤3.5 cm, β-hCG levels <5000 mIU/mL, no adnexal yolk sac and normal hematologic, liver, and kidney functions are ideal candidates for methotrexate therapy (A).
Management strategies for patients with ectopic pregnancy have evolved rapidly, with ambulatory medical therapy becoming an option for more patients.1 In part 1, published in the May 2006 JFP, using a practical decision protocol, we discuss the physical findings that most reliably suggest ectopic pregnancy, describe sensible use of laboratory and imaging studies, and explain what to do when results are equivocal.
Management choices
Once the diagnosis of ectopic pregnancy has been made, options include surgical, medical, or expectant management (FIGURE). The goal of treatment is to minimize disease-and treatment-related morbidity while maximizing reproductive potential.
Administer Rhogam to all Rh-negative women.
Clinical prediction tools have been developed to aid management decision making. Fernandez et al developed a score based on gestational age, β-hCG level, progesterone level, abdominal pain, hemoperitoneum volume, and hematosalpinx diameter.2 A score <12 predicts a >80% success with expectant or nonsurgical management (TABLE 1). Similarly, to predict response to a single-dose of methotrexate, Elito et al3 developed a score based on β-hCG level, ultrasound findings, have size of the mass (cm), and color Doppler image aspects (TABLE 1). In a small study of for 40 patients, those with scores >5 had a the 97% success rate.3
FIGURE
Deciding which management option is best for your patient with ectopic pregnancy
TABLE 1
Predictive score for successful treatment of ectopic pregnancy
| Predictive score for expectant management and several nonsurgical treatments (Fernandez 1991) | |||
| CRITERION | 1 POINT | 2 POINT | 3 POINT |
| β-hCG (mIU/mL) | <1000 | 1000–5000 | >5000 |
| Progesterone (ng/mL) | <5 | 5–10 | >10 |
| Abdominal pain | Absent | Induced | Spontaneous |
| Hematosalpinx (cm) | <1 | 1–3 | >3 |
| Hemoperitoneum (mL) | 0 | 1–100 | >100 |
| Score <12: 80% success with various nonsurgical treatments, including expectant management. | |||
| Predictive score for single dose methotrexate (50 mg/m2 IM) (Elito 1999) | |||
| PARAMETERS | 0 POINTS | 1 POINTS | 2 POINTS |
| β-hCG (mIU/mL) | >5000 | 1500–5000 | <1500 |
| Aspects of the image | Live embryo | Tubal ring | Hematosalpinx |
| Size of the mass | >3.0–3.5 | 2.6–3.0 | <2.5 |
| Color Doppler | High risk | Medium risk | Low risk |
| Score≥5: 97% success with single-dose methotrexate. | |||
| Sources: Fernandez et al 1991,2 Elito et al 1999.3 | |||
Surgical management
Surgery is preferred for ruptured ectopic pregnancy. Surgery is also indicated for patients with evidence of hemodynamic instability, anemia, pain for longer than 24 hours, β-hCG levels greater than 5000 mIU/mL, or with a gestational sac that measures more than 3.5 to 4 cm on ultrasound.1,4,5
Laparoscopic techniques minimize the trauma and morbidity of salpingectomy or salpingostomy. Compared with older procedures, they lessen blood loss, decrease the need for analgesia, and allow a shorter hospital stay and an earlier return to work.6
Salpingostomy removes the ectopic pregnancy while preserving the Fallopian tube. Weekly quantitative β-hCG testing is required to rule out persistent ectopic pregnancy, which occurs in 5% to 8% of patients following salpingostomy.7 The likelihood of persistent ectopic pregnancy following salpingostomy increases with an ectopic pregnancy <2 cm in diameter, salpingostomy performed <6 weeks from the last menstrual period, a β-hCG level >3000 mIU/mL, or progesterone level over 35 nmol/L combined with a daily change in β-hCG over 100 mIU/mL.8,9
Expectant management possible when β-hCG levels <1000 mIU/mL
Expectant management may be offered to asymptomatic women with small adnexal masses, lower β-hCG levels, and evidence of spontaneous resolution (eg, falling β-hCG levels) who are willing to accept the risk of tubal rupture.10 Rising β-hCG levels, pain, hemodynamic instability, or hemoperitoneum on ultrasound dictate switching to active management.11
Eighty percent of women with initial β-hCG levels <1000 mIU/mL experience spontaneous resolution (TABLE 2).1,4,5,11-17 In one study, women with initial β-hCG levels <1000 mIU/mL, adnexal masses <4 cm, no fetal heartbeat, and <100 mL of fluid in the pouch of Douglas were managed by serial ultrasound and β-hCG levels obtained twice-weekly for 2 weeks; the result was an 88% chance of spontaneous resolution.18 Women (n=9) with initial β-hCG levels ≤1000 mIU/mL with subsequent rising titers experienced no spontaneous resolution.
TABLE 2
Treatment options for ectopic pregnancy
| MODALITY | DESCRIPTION | EP RESOLUTION (%) | TUBAL PATENCY (%) | FUTURE IUP (%) | EP (%) | ADVERSE EVENTS | ||
| Salpingectomy Open or laparoscopic | Excision of ectopic pregnancy and tube | 100 | NA | 42–82* | 6–13 | Hemorrhage Infection Adhesions | ||
| Laparoscopic | Excision of ectopic pregnancy with repair of tube | 93 | 76 | 57 | 13 | Incomplete removal (persistent EP) Analgesic needed Lost work time | ||
| Expectant management | Twice-weekly β-hCG, ultrasound obtained for 2 weeks | 67–68 | 76–77 | 68–86 | 7–13 | Persistent EP 25% need medical or surgical management | ||
| β-hCG <1000 | 88 | |||||||
| β-hCG ≥1000 | 48 | |||||||
| Methotrexate Multidose | 1 mg/kg IV or IM with 0.1 mg/kg folic acid on alternating days. Stop when >15% drop in β-hCG observed or 4 doses administered | 93–95 | 75 | 58 | 7 | Mucositis (stomatitis, gastritis, diarrhea) Dermatitis Bone marrow suppression Hepatic dysfunction Pleuritis | ||
| Single dose | Injection of 50 mg/m2. β-hCG levels days 4 and 7. Repeat dose if no drop. | 87–90 | 81 | 61 | 8 | Reversible alopecia Photosensitivity Pulmonary fibrosis | ||
| Oral | 50 mg daily for 5 days or 60 mg/m2 (one time in 2 divided doses) | 86 | ||||||
| Direct injection | Ultrasound or laparoscopic guidance of 12.5–25 mg | 76 | 80 | 57 | 6 | |||
| EP, ectopic pregnancy; IUP, intrauterine pregnancy; NA, not applicable; IM, intramuscular; IV, intravenous. | ||||||||
| *82% if contralateral tube normal. | ||||||||
Medical management an option for about 25% of patients
Methotrexate depletes tetrahydrofolate cofactors required for DNA and RNA synthesis and cell replication, and thereby inhibits the rapidly growing trophoblasts in patients with ectopic pregnancy.
Methotrexate may be used for primary treatment of ectopic pregnancy, for persistent ectopic pregnancy following tubal sparing surgery, as prophylaxis to reduce persistent ectopic pregnancy following salpingostomy, and in cornual and cervical pregnancies.11,13,19
Who qualifies. Patients eligible for methotrexate administration are those without hemodynamic instability or evidence of tubal rupture (clinical or ultrasound), desiring future fertility, having a gestational sac <3.5 cm, a β-hCG level less than 5000 mIU/mL, no fetal cardiac motion on ultrasound, and the ability and willingness to comply with post-treatment monitoring.10,14
Systemic methotrexate successfully resolves ectopic pregnancy in 90% of patients. Subsequent tubal patency rates approximate 80% and pregnancy rates 60% with recurrent ectopic pregnancy rates 8%.10,13,15,16 The cost of treatment for systemic methotrexate was $5721 per patient compared with $4066 for salpingostomy.20 Hematologic, liver, and renal functions should be assessed before treatment.
Following methotrexate administration, some patients experience a transient increase in abdominal pain, vaginal bleeding, and rising β-hCG. Exclude a ruptured ectopic pregnancy in patients with worsening pain. Pelvic examinations, sexual intercourse, and TVUS should be avoided or minimized during treatment.10
Two regimens are used for the systemic administration of methotrexate (TABLE 2).
The single-dose regimen uses an intramuscular (IM) injection dose of 50 mg/m2 of methotrexate without leucovorin. It has an overall success rate of 87%. β-hCG levels are measured on days 4 and 7, and the medication is repeated if no drop is noticed.13 β-hCG levels are then repeated weekly until undetectable (usually 4 weeks). Serum progesterone levels drop significantly faster than β-hCG following methotrexate administration, and levels <1.5 ng/mL predict ectopic pregnancy resolution more accurately than β-hCG levels.21 Repeat dosing is needed in up to 14% of women.
In the multidose regimen, IM injections of 1 mg/kg of methotrexate are given followed by leucovorin (0.1 mg/kg) after 24 hours. β-hCG levels are checked every other day until there is a 15% or more drop on 2 consecutive days, or 4 doses of methotrexate are administered.1 β-hCG levels are then repeated weekly until undetectable. About half of the women receiving the multidose regimen require 4 or more doses (6.8% require >4 doses). On the other hand, 10% of women treated with this regimen require only 1 dose. Multidose methotrexate has also been used as first-line therapy in cervical, interstitial, ovarian, and abdominal gestation, but with substantially lower success.19
Single-dose vs multidose regimens. Although there are no direct comparison trials, a meta-analysis combining the results of 26 studies with 1067 women treated with a single-dose regimen and 267 women treated with a multidose regimen found that the multidose regimen was slightly more effective.16 The success rate for women with multidose treatment was 92.7% (95% confidence interval [CI], 89–96) vs a single-dose treatment success rate of 88.1% (95% CI, 86–90).
Women treated with single-dose regimens had fewer side effects (31.3% vs 41.2%). However, in both regimens, women who experienced adverse side effects were less likely to have failed treatment (single dose, odds ratio [OR]=0.27; multidose, OR=0.72). Another systematic review including 19 studies with 393 women treated with single-dose methotrexate and 338 women treated with multidose methotrexate had similar outcome findings; the multidose regimen was slightly more effective (93% vs 87%).13
A recent retrospective study comparing the multidose and single-dose regimens in 643 patients with ectopic pregnancy (single dose, n=555; multidose, n=97) from a single database also showed slightly better success with the multidose regimen (95% vs 90%, P=.18).22 Therefore, the multidose regimen may be preferred but a randomized controlled trial comparing the 2 regimens is needed.
In clinically stable women, the serum β-hCG level at presentation is probably the most important single factor determining failure of single-dose methotrexate; patients with low β-hCG levels (1000–2000 mIU/mL) have a high (~98%) response rate.23
Add mifepristone? Mifepristone 600 mg orally added to methotrexate increases successful resolution of unruptured ectopic pregnancy, decreases resolution time, and reduces the need for a second injection or laparotomy, without worsening side effects.24 A recent randomized controlled trial, however, showed that this combination had little advantage over methotrexate alone, and concluded that adding mifepristone be limited to patients with serum progesterone ≥10 ng/mL.25
Direct injection. When fetal cardiac activity is present, injection of 20% potassium chloride (KCl) 0.5 mL into the gestational sac under ultrasound guidance results in asystole and a slow resolution of ectopic pregnancy. Because KCl does not affect the trophoblast, trophoblastic tissue may continue to proliferate leading to tubal rupture.26 Hyperosmolar glucose 1 to 3 mL injected laparoscopically or under ultrasound guidance into the gestational sac maintains tubal patency, has few side effects, but its initial high success rates in resolving ectopic pregnancy (94% to 100%) has not been duplicated.11,13 Another option is prostaglandin F-2 alpha, which when injected into the Fallopian tube causes contractions and vasoconstriction, resulting in a resolution of ectopic pregnancy in 92% of patients.13 However, serious side effects have been reported, including severe abdominal discomfort, vomiting, and pulmonary edema.
CORRESPONDENCE
K. Ramakrishnan, MD, Department of Family and Preventive Medicine, University of Oklahoma Health Sciences Center, 900 NE 10th Street, Oklahoma City, OK 73104. E-mail: [email protected]
1. Carr RJ, Evans P. Ectopic pregnancy. Prim Care 2000;27:169-183.
2. Fernandez H, Lelaidier C, Thouvenez V, et al. The use of a pretherapeutic, predictive score to determine inclusion criteria for the non-surgical management of ectopic pregnancy. Hum Reprod 1991;6:995-998.
3. Elito J, Jr, Reichmann AP, Uchiyama MN, Camano L. Predictive score for the systemic treatment of unruptured ectopic pregnancy with a single dose of methotrexate. Int J Gynecol Obstet 1999;67:75-79.
4. Pisarska MD, Carson SA, Buster JE. Ectopic pregnancy. Lancet 1998;351:1115-1120.
5. Farquhar CM. Ectopic pregnancy. Lancet 2005;366:583-591.
6. Kelly AJ, Sowter MC, Trinder J. The management of tubal pregnancy. Royal College of Obstetricians and Gynecologists (RCOG). Guideline No. 21, May 2004. Available at: www.rcog.org.uk/resources/Public/pdf/management_tubal_pregnancy21.pdf.
7. Rulin MC. Is salpingostomy the surgical treatment of choice for unruptured tubal pregnancy? Obstet Gynecol 1995;86:1010-1013.
8. Seifer DB. Persistent ectopic pregnancy: an argument for heightened vigilance and patient compliance. Fertil Steril 1997;68:402-404.
9. Hagstrom HG, Hahlin M, Bennegard-Edèn B, Sjoblom P, Thorburn J, Lindblom B. Prediction of persistent ectopic pregnancy after laparoscopic salpingostomy. Obstet Gynecol 1994;84:798-802.
10. ACOG practice bulletin. Medical management of tubal pregnancy. Number 3, December 1998. Clinical management guidelines for obstetrician-gynecologists. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 1999;65:97-103.
11. Mittal S. Non-surgical management of ectopic pregnancy. Obs Gyn Com 1999;1:23-28.
12. Cohen MA, Sauer MV. Expectant management of ectopic pregnancy. Clin Obstet Gynecol 1999;42:48-54.
13. Buster JE, Pisarska MD. Medical management of ectopic pregnancy. Clin Obstet Gynecol 1999;42:23-30.
14. Murray H, Baakdah H, Bardell T, Tulandi T. Diagnosis and treatment of ectopic pregnancy. CMAJ 2005;173:905-912.
15. Pansky M. Methotrexate (MXT) treatment for ectopic pregnancy-systemic vs local injection. Scientific presentation at The First World Congress on Controversies in Obstetrics, Gynecology & Infertility. Prague, Czech Republic, 1999. Available at: www.obgyn.net/firstcontroversies/prague1999pansky.doc. Accessed December 4, 2005.
16. Barnhart KT, Gosman G, Ashby R, Sammel M. The medical management of ectopic pregnancy: a meta-analysis comparing “single dose” and “multidose” regimens. Obstet Gynecol 2003;101:778-784.
17. Lipscomb GH, Meyer NL, Flynn DE, Peterson M, Ling F. Oral methotrexate for treatment of ectopic pregnancy. Am J Obstet Gynecol 2002;186:1192-1195.
18. Trio D, Strobelt N, Picciolo C, Lapinski RH, Ghidini A. Prognostic factors for successful expectant management of ectopic pregnancy. Fertil Steril 1995;63:469-472.
19. Barnhart K, Esposito M, Coutifaris C. An update on the medical treatment of ectopic pregnancy. Obstet Gynecol Clin North Am 2000;27:653-667, viii.
20. Mol BW, Hajenius PJ, Engelsbel S, et al. Treatment of tubal pregnancy in the Netherlands: an economic comparison of systemic methotrexate administration and laparoscopic salpingostomy. Am J Obstet Gynecol 1999;181:945-951.
21. Saraj AJ, Wilcox JG, Najmabadi, Stein SM, Johnson MB, Paulson RJ. Resolution of hormonal markers of ectopic gestation: a randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol 1998;92:989-994.
22. Lipscomb GH, Givens VM, Meyer NL, Bran D. Comparison of multidose and single-dose methotrexate protocols for the treatment of ectopic pregnancy. Am J Obstet Gynecol 2005;192:1844-1847.
23. Sowter MC, Farquhar CM. Ectopic pregnancy: an update. Curr Opin Obstet Gynecol 2004;16:289-293.
24. Gazvani MR, Baruah DN, Alfirevic Z, Emery SJ. Mifepristone in combination with methotrexate for the medical treatment of tubal pregnancy: a randomized, controlled trial. Hum Reprod 1998;13:1987-1990.
25. Rozenberg P, Chevret S, Camus E, et al. Medical treatment of ectopic pregnancies: a randomized clinical trial comparing methotrexate-mifepristone and methotrexate-placebo. Hum Reprod 2003;18:1802-1808.
26. Pansky M, Golan A, Bukovsky I, Caspi E. Nonsurgical management of tubal pregnancy. Necessity in view of the changing clinical appearance. Am J Obstet Gynecol 1991;164:888-895.
1. Carr RJ, Evans P. Ectopic pregnancy. Prim Care 2000;27:169-183.
2. Fernandez H, Lelaidier C, Thouvenez V, et al. The use of a pretherapeutic, predictive score to determine inclusion criteria for the non-surgical management of ectopic pregnancy. Hum Reprod 1991;6:995-998.
3. Elito J, Jr, Reichmann AP, Uchiyama MN, Camano L. Predictive score for the systemic treatment of unruptured ectopic pregnancy with a single dose of methotrexate. Int J Gynecol Obstet 1999;67:75-79.
4. Pisarska MD, Carson SA, Buster JE. Ectopic pregnancy. Lancet 1998;351:1115-1120.
5. Farquhar CM. Ectopic pregnancy. Lancet 2005;366:583-591.
6. Kelly AJ, Sowter MC, Trinder J. The management of tubal pregnancy. Royal College of Obstetricians and Gynecologists (RCOG). Guideline No. 21, May 2004. Available at: www.rcog.org.uk/resources/Public/pdf/management_tubal_pregnancy21.pdf.
7. Rulin MC. Is salpingostomy the surgical treatment of choice for unruptured tubal pregnancy? Obstet Gynecol 1995;86:1010-1013.
8. Seifer DB. Persistent ectopic pregnancy: an argument for heightened vigilance and patient compliance. Fertil Steril 1997;68:402-404.
9. Hagstrom HG, Hahlin M, Bennegard-Edèn B, Sjoblom P, Thorburn J, Lindblom B. Prediction of persistent ectopic pregnancy after laparoscopic salpingostomy. Obstet Gynecol 1994;84:798-802.
10. ACOG practice bulletin. Medical management of tubal pregnancy. Number 3, December 1998. Clinical management guidelines for obstetrician-gynecologists. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 1999;65:97-103.
11. Mittal S. Non-surgical management of ectopic pregnancy. Obs Gyn Com 1999;1:23-28.
12. Cohen MA, Sauer MV. Expectant management of ectopic pregnancy. Clin Obstet Gynecol 1999;42:48-54.
13. Buster JE, Pisarska MD. Medical management of ectopic pregnancy. Clin Obstet Gynecol 1999;42:23-30.
14. Murray H, Baakdah H, Bardell T, Tulandi T. Diagnosis and treatment of ectopic pregnancy. CMAJ 2005;173:905-912.
15. Pansky M. Methotrexate (MXT) treatment for ectopic pregnancy-systemic vs local injection. Scientific presentation at The First World Congress on Controversies in Obstetrics, Gynecology & Infertility. Prague, Czech Republic, 1999. Available at: www.obgyn.net/firstcontroversies/prague1999pansky.doc. Accessed December 4, 2005.
16. Barnhart KT, Gosman G, Ashby R, Sammel M. The medical management of ectopic pregnancy: a meta-analysis comparing “single dose” and “multidose” regimens. Obstet Gynecol 2003;101:778-784.
17. Lipscomb GH, Meyer NL, Flynn DE, Peterson M, Ling F. Oral methotrexate for treatment of ectopic pregnancy. Am J Obstet Gynecol 2002;186:1192-1195.
18. Trio D, Strobelt N, Picciolo C, Lapinski RH, Ghidini A. Prognostic factors for successful expectant management of ectopic pregnancy. Fertil Steril 1995;63:469-472.
19. Barnhart K, Esposito M, Coutifaris C. An update on the medical treatment of ectopic pregnancy. Obstet Gynecol Clin North Am 2000;27:653-667, viii.
20. Mol BW, Hajenius PJ, Engelsbel S, et al. Treatment of tubal pregnancy in the Netherlands: an economic comparison of systemic methotrexate administration and laparoscopic salpingostomy. Am J Obstet Gynecol 1999;181:945-951.
21. Saraj AJ, Wilcox JG, Najmabadi, Stein SM, Johnson MB, Paulson RJ. Resolution of hormonal markers of ectopic gestation: a randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol 1998;92:989-994.
22. Lipscomb GH, Givens VM, Meyer NL, Bran D. Comparison of multidose and single-dose methotrexate protocols for the treatment of ectopic pregnancy. Am J Obstet Gynecol 2005;192:1844-1847.
23. Sowter MC, Farquhar CM. Ectopic pregnancy: an update. Curr Opin Obstet Gynecol 2004;16:289-293.
24. Gazvani MR, Baruah DN, Alfirevic Z, Emery SJ. Mifepristone in combination with methotrexate for the medical treatment of tubal pregnancy: a randomized, controlled trial. Hum Reprod 1998;13:1987-1990.
25. Rozenberg P, Chevret S, Camus E, et al. Medical treatment of ectopic pregnancies: a randomized clinical trial comparing methotrexate-mifepristone and methotrexate-placebo. Hum Reprod 2003;18:1802-1808.
26. Pansky M, Golan A, Bukovsky I, Caspi E. Nonsurgical management of tubal pregnancy. Necessity in view of the changing clinical appearance. Am J Obstet Gynecol 1991;164:888-895.
Ectopic pregnancy: Forget the “classic presentation” if you want to catch it sooner
- Less than half of the patients with ectopic pregnancy present with the classic triad of a history of amenorrhea, abdominal pain, and irregular vaginal bleeding (C).
- Definite cervical motion tenderness and peritoneal signs are the most sensitive and specific examination findings for ectopic pregnancy—91% and 95%, respectively (A).
- Beta-human chorionic gonadotropin (β-hCG) levels can be used in combination with ultrasound findings to improve the accuracy of the diagnosis of ectopic pregnancy (A).
- Women with initial nondiagnostic transvaginal ultrasound should be followed with serial β-hCGs (B).
Despite advanced detection methods, ectopic pregnancy may be missed in 40% to 50% of patients on an initial visit.1 Most women with ectopic pregnancy have no risk factors (TABLE 1),2-5 and the classic triad of a history of amenorrhea, abdominal pain, and irregular vaginal bleeding is absent in more than half of cases.
Early diagnosis not only decreases maternal mortality and morbidity; it also helps preserve future reproductive capacity—only one third of women with ectopic pregnancy have subsequent live births.2
Management strategies for patients with ectopic pregnancy have evolved rapidly, with ambulatory medical therapy becoming an option for more patients.6 Using a practical decision protocol, we discuss the physical findings that most reliably suggest ectopic pregnancy, describe sensible use of laboratory and imaging studies, and explain what to do when results are equivocal. In part 2 of this article (coming in the June 2006 Journal of Family Practice), we provide a decision protocol for management of ectopic pregnancy.
TABLE 1
Risk factors for ectopic pregnancy
| DEGREE OF RISK | FACTOR | ODDS RATIO (95% CONFIDENCE INTERVALS) | ||
|---|---|---|---|---|
| ANKUM ET AL3 | MOL ET AL4 | BOUYER ET AL5 | ||
| High | Previous tubal surgery | 21 (9.3–47) | 4.0 (2.6–6.1) | |
| Tubal ligation | 9.3 (4.9–18) | |||
| Previous ectopic pregnancy | 8.3 (6.0–11.5) | |||
| In utero DES exposure | 5.6 (2.4–13) | |||
| Current IUD use | 4.2–45* | |||
| Tubal pathology/abnormality | 3.5–25* | |||
| Moderate | Infertility | 2.5–21* | 2.7 (1.8–4.2) | |
| History of PID | 2.5 (2.1–3.0) | 3.4 (2.4–5.0) | ||
| Previous chlamydial or gonococcal infection | 2.8–3.7* | |||
| Current smoking | 2.3 (2.0–2.8) | 3.9 (2.6–5.9) | ||
| Spontaneous abortions ≥3 | 3.0 (1.3–6.9) | |||
| Induced abortions (medical±surgical) | 2.8 (1.1–7.2) | |||
| Lifetime sexual partners >1 | 2.1 (1.4–4.8) | |||
| Low | Age first intercourse <18 years | 1.6 (1.1–2.5) | ||
| Previous pelvic/abdominal surgery | 0.93–3.8* | |||
| Vaginal douching | 1.1–3.1* | |||
| DES, diethylstilbestrol; PID, pelvic inflammatory disease; IUD, intrauterine device | ||||
| * Range; summary odds ratio not calculated owing to significant heterogeneity between studies. | ||||
Evaluation
Clinical features of ectopic pregnancy highly variable
No single clinical feature accurately indicates ectopic pregnancy. Less than half of women with ectopic pregnancy exhibit the classic triad of a history of amenorrhea, abdominal pain, and irregular vaginal bleeding.1 And, unfortunately, these features are seen commonly in patients with both viable (50%) and nonviable (25%) intrauterine pregnancies, as well as in threatened abortion, cervical irritation, infection, and trauma.7
Set a low threshold for suspicion. For any woman of childbearing age with abdominal pain and vaginal bleeding, consider pregnancy and take steps to rule out ectopic pregnancy. Abdominal pain and vaginal bleeding are highly sensitive for ectopic pregnancy but are not specific for the disorder (TABLE 2).8-17 Pain located in the hypogastrium or iliac fossa may be mild to severe. Vaginal bleeding, present in 50% to 80% of patients with ectopic pregnancy, can be mistaken for a normal menstrual period.2,6 Pregnancy-associated symptoms of nausea and vomiting, breast tenderness, and fatigue may be present.
Lower abdominal and adnexal tenderness can be elicited in most women with ectopic pregnancy. Cervical motion tenderness, peritoneal signs, and adnexal masses are most specific for ectopic pregnancy, but are not sensitive.8 An adnexal mass is palpable in less than 10% of cases; when it is detected, one third of patients will have a contralateral ectopic pregnancy on ultrasonography.18 Symptoms of hemodynamic compromise (orthostasis, hypotension, shock) are becoming uncommon with earlier diagnosis of ectopic pregnancy, facilitated by improved detection methods.
TABLE 2
Significance of features associated with ectopic pregnancy
| FEATURES | SN (%) | SP (%) | LR+ | LR– |
|---|---|---|---|---|
| Clinical features8 | ||||
| Any risk factors | 23 | 83 | 1.4 | 0.9 |
| Estimated gestational age <70 days | 95 | 27 | 1.3 | 0.2 |
| Vaginal bleeding | 69 | 26 | 0.9 | 1.2 |
| Abdominal pain | 97 | 15 | 1.1 | 0.2 |
| Abdominal tenderness | 85 | 50 | 1.7 | 0.3 |
| Peritoneal signs | 23 | 95 | 4.6 | 0.8 |
| Cervical motion tenderness | 33 | 91 | 3.7 | 0.7 |
| Adnexal tenderness | 69 | 62 | 1.8 | 0.5 |
| Adnexal mass | 5 | 96 | 1.3 | 1.0 |
| Transvaginal ultrasound | ||||
| No intrauterine gestational sac9 | 100 | 89 | 9.1 | <0.1 |
| Adnexal mass10 | ||||
| Separate from ovary | 93 | 99 | 93 | 0.1 |
| Cardiac activity | 20 | 100 | ∞ | 0.8 |
| Yolk sac or embryo | 37 | 100 | ∞ | 0.6 |
| Tubal ring/yolk sac or embryo | 65 | 99 | 65 | 0.4 |
| Fluid in pouch of Douglas11 | ||||
| Any | 63 | 69 | 2.0 | 0.5 |
| Echogenic | 56 | 96 | 14.0 | 0.5 |
| Color-flow Doppler12 | 95 | 98 | 47.5 | 0.1 |
| β-hCG combined with transvaginal ultrasound | ||||
| Empty uterus | ||||
| ≥1000 mIU/mL10,13,14 | 43–96 | 86–100 | 3.0–∞ | 0.04–0.66 |
| ≥1500 mIU/mL13,15 | 40–99 | 84–96 | 6.3–9.9 | 0.01–0.63 |
| ≥2000 mIU/mL13,16 | 38–48 | 80–98 | 2.3–25.3 | 0.63–0.68 |
| Adnexal mass* | ||||
| ≥1000 mIU/mL13 | 73 | 85 | 4.7 | 0.32 |
| ≥1500 mIU/mL13,15 | 46–64 | 92–96 | 5.9–16.5 | 0.38–0.58 |
| ≥2000 mIU/mL13 | 55 | 96 | 14.2 | 0.47 |
| β-hCG rise in 48 hours (empty uterus)† | ||||
| >66%17 | — | — | 1.1 | — |
| <66%17 | — | — | 7.4 | — |
| >50%13 | — | — | 2.8 | — |
| <50%13 | — | — | 3.3 | — |
| β-hCG fall in 48 hours (empty uterus)† | ||||
| >50%13,17 | — | — | 0.8–1.4 | — |
| <50%13,17 | — | — | 0–0.1 | — |
| * Mass or fluid in cul de sac for β-hCG ≥1500 mIU/mL and ≥2000 mIU/mL. | ||||
| † Strata-specific likelihood ratios reported, sensitivity and specificity not applicable. | ||||
| Sn, sensitivity; Sp, specificity; LR, likelihood ratio; β-hCG, beta-human chorionic gonadotropin. | ||||
Laboratory tests
In an early normal pregnancy, the beta-human chorionic gonadotropin (β-hCG) level doubles every 1.8 to 3 days, rising to 1000 mIU/mL IRP (International Reference Preparation, measured by radioimmunoassay) by 5 weeks, to 2500 mIU/mL by 6 weeks, and to 13,000 mIU/mL (±3000) by 7 weeks. A single quantitative β-hCG level is not always helpful, as it can range from less than 100 mIU/mL to greater than 50,000 mIU/mL with both ruptured and unruptured ectopic pregnancies.19
Rethinking the rate of rise in β-hCG. The oft-quoted maxim that ectopic pregnancy or impending miscarriage is associated with a rise in β-hCG of less than 66% in 48 hours arose from a 1981 study that included only 20 women and that was based on an 85% confidence interval (CI).20 A recent study of 287 women suggests that the minimal expected rise in 2 days for a viable intrauterine pregnancy (based on a 99% CI) should be at least 53%.21
Combining β-hCG levels and ultrasound findings to improve diagnosis. Because the exact gestational age is often unknown in women presenting with features of ectopic pregnancy, the β-hCG level is used to determine whether a gestational sac can be identified on ultrasound. With a β-hCG level of 6500 mIU/mL, a gestational sac should be visible on abdominal ultrasound in the uterus of a woman with an intrauterine pregnancy; the sac should be absent in ectopic pregnancy. This β-hCG level is designated the discriminatory zone.22
With a high-resolution transvaginal ultrasound (TVUS), the gestational sac of a normal single intrauterine pregnancy should be visible at a β-hCG level above 1000–1500 mIU/mL, depending on equipment and technical expertise.2,7 In multiple gestations the discriminatory zone is observed to be above 2300 mIU/mL.2 Various ultrasonography findings have been combined with β-hCG levels to more accurately diagnose ectopic pregnancy (TABLE 2 and FIGURE).
Serial β-hCG measurements. If TVUS findings are indeterminate, serial measurements of β-hCG levels at 48-hour intervals may be useful in managing suspected ectopic pregnancy. Dart et al17 found that a rise in β-hCG level of 66% or more was usually associated with a viable intrauterine pregnancy, though 22% of women in that study had an ectopic pregnancy. Mol et al13 found similar results using a cutoff of a β-hCG level rise of 50% or more; 35% had an ectopic pregnancy. Increases in the β-hCG level less than these cutoffs almost always indicated an abnormal pregnancy, and about half of these women had an ectopic pregnancy.13,17 Also a fall in the β-hCG level by less than 50% was almost always associated with an abnormal pregnancy, but only 19% of these women had an ectopic pregnancy. 13,17 Alternatively, decreases in β-hCG level of greater than 50% reduced the chance that patients had an ectopic pregnancy to less than 3%.13,17
Ectopic pregnancy is defined as the implantation of the fertilized egg that occurs outside the uterine cavity. It is practically synonymous with tubal pregnancy, as 97% occur in the Fallopian tube.6,23 Ectopic pregnancy occurs in 2% of all pregnancies in the United States, affecting more than 100,000 patients each year. It is a leading cause of pregnancy-related death in the first trimester,2 with a yearly monetary impact of greater than 1 billion dollars. The incidence has increased almost six-fold since 1970 due to delayed child-bearing, rising prevalence of sexually transmitted diseases, and sterilization procedures.6
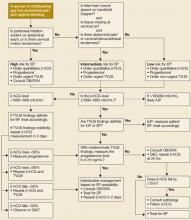
FIGURE
Diagnostic protocol with high sensitivity and specificity for ectopic pregnancy
* Other than midline suprapubic cramping.
† Risk may be higher in presence of these factors: previous EP, tubal surgery, tubal disease detected by hysterosalpingogram or laproscopy, diethylstilbestrol exposure, sterilization, intrauterine device.
‡ Progesterone is useful only when TVUS is indeterminate.
§ β-hCG level for criterion should be based on local values at which IUP visible on TVUS.
|| TVUS should be repeated when β-hCG has risen above the discrimination zone.
¶ Only 2/170 patients in this category had EP; therefore, D&C is safe but may not be cost-effective.11
β-hCG, beta-human chorionic gonadotropin; EP, ectopic pregnancy; TVUS, transvaginal ultrasound; OB/GYN, obstetrician/gynecologist;
IUP, intrauterine pregnancy; D&C, dilation and curettage.
Serum progesterone levels may have limited usefulness. Serum progesterone measurements are not considered accurate enough to diagnose ectopic pregnancy.24 However, progesterone levels may be helpful if they are either very high or very low. Levels less than 22 ng/mL have a sensitivity of 100% for ectopic pregnancy.25 Only 5 of 1615 patients (0.3%) in 13 studies with progesterone levels below 5 ng/mL had a viable intrauterine pregnancy.24 By establishing the low likelihood of a viable intrauterine pregnancy, serum progesterone measurements may also be useful when β-hCG levels have reached 1000–1500 mIU/mL and the TVUS is equivocal.25
When D&C may help. Dilation and curettage (D&C) is an option for patients with an indeterminate ultrasound result and progesterone levels less than 5 ng/mL, as it can rule out an ectopic pregnancy with only a small chance of interrupting a viable intrauterine pregnancy. The presence of chorionic villi rules out an ectopic pregnancy. In the absence of villi, if β-hCG levels subsequently plateau or increase, it is presumptive of ectopic pregnancy. Chorionic villous sampling by pipelle curette has both insufficient sensitivity and predictive value in diagnosing ectopic pregnancy, and should not be considered a substitute for D&C.26
Other tests that have limited use. Patients with ectopic pregnancy may have elevated serum markers of smooth muscle destruction such as creatinine phosphokinase (CPK), myoglobin, and smooth muscle heavy-chain myosin (SMHC). However, these tests are of limited value in diagnosing ectopic pregnancy.27 SMHC may be of use in evaluating pregnancies less than 5 weeks’ gestation when the TVUS is not diagnostic. Serum vascular endothelial growth factor is another marker elevated in women with ectopic pregnancy; levels greater than 200 pg/mL distinguish ectopic pregnancy from intrauterine pregnancy (sensitivity=60%, specificity=90%).28
Imaging
In normal pregnancies, a gestational sac is seen on TVUS between 4 and 5 weeks’ gestation;29 the yolk sac is visible at around 6 weeks; and an embryo can be detected between 6 and 7 weeks. A gestational sac greater than 10 mm in diameter without a yolk sac, or greater than 25 mm without an embryo, indicates an abnormal regnancy.9,29 Presence of an intrauterine pregnancy on ultrasound effectively rules out ectopic pregnancy because heterotopic pregnancy is rare (1 in 2600–30,000 pregnancies).30 Hormonal changes associated with pregnancy result in a uterine endometrial fluid collection (pseudo-sac) in 8% of ectopic pregnancies.31
Specific diagnostic finding for ectopic pregnancy. A gestational sac visible on ultrasound with a yolk sac or fetal pole outside the endometrial cavity (TABLE 2) is diagnostic of ectopic pregnancy. The initial ultrasound is indeterminate in 15% to 20% of women with clinical features suggesting ectopic pregnancy.14,15 Dart et al32 developed a subclassification system of indeterminate ultrasound including 5 categories that stratify the risk of ectopic pregnancy (TABLE 3).
Endometrial stripe thickness of dubious value. For patients with an empty uterus on TVUS and a β-hCG level below the discriminatory zone, endometrial stripe thickness may identify an abnormal pregnancy. In a retrospective study of 117 patients with β-hCG levels less than 1500 mIU/mL, stripe thickness <6 mm had a sensitivity of 100% in identifying ectopic pregnancy or miscarriage; at >13 mm, it had a specificity of 100%.33
Two recent studies, however, have called into question the value of stripe thickness in identifying ectopic pregnancy. One suggested it was of little value with β-hCG levels <1500 mIU/mL,34 and the other concluded its predictive value for ectopic pregnancy and the likelihood of obtaining chorionic villi on D&C is confined to β-hCG values at or below 1000 mIU/mL.35
Color-enhanced sonography. The addition of endovaginal color-flow imaging to sonography enables exclusion of ectopic pregnancy by establishing findings consistent with a nonviable intrauterine pregnancy: an intrauterine gestational sac, nonvisualization of an adnexal mass, and absent placental blood flow (sensitivity=95%, specificity=98%).12 Color-flow Doppler imaging shows enhanced blood flow to the affected tube. A cutoff value of 8% change in tubal blood flow has been used to diagnose ectopic pregnancy (sensitivity=85%, specificity=96%).36 In cases where the gestational sac is questionable or absent, color-flow Doppler may expedite diagnosis and possibly identify candidates for expectant or medical management.
When an MRI might help. Magnetic resonance imaging (MRI), in a small study of 37 patients, allowed recognition of tubal wall enhancements and fresh tubal hematoma, and was diagnostic in 21 patients.37 An MRI may be useful when precise and early diagnosis of ectopic pregnancy is imperative, such as pre-existing damage to the contralateral tube where preservation of tubal patency is paramount and when prior TVUS is inconclusive.37
TABLE 3
Detecting ectopic pregnancy with ultrasound and β-hCG levels
| SUBCLASS | β-hCG <1000 mIU/mL, % (95% CI) | β-hCG >1000 mIU/mL, % (95% CI) |
|---|---|---|
| Empty uterus | 17.9 (12.7–24.2) | 6.0 (2.2–12.8) |
| Nonspecific intrauterine fluid* | 12.2 (4.6–25.0) | 1.2 (0.1–5.9) |
| Echogenic intrauterine material | 10.5 (1.8–30.6) | 2.7 (0.5–8.4) |
| Abnormal intrauterine sac† | 0.0 (0.0–39.3) | 0.0 (0.0–3.1) |
| Normal intrauterine sac | 0.0 (0.0–34.8) | 0.0 (0.0–8.4) |
| * Anechoic intrauterine fluid collection >10 mm diameter with no echogenic border. | ||
| † Anechoic intrauterine fluid collection >10 mm diameter with no yolk sac or fetal pole or grossly irregular border. | ||
| Source: Dart et al 2002.32 | ||
A practical evaluation algorithm
Prediction models based on clinical presentation, which classify patients into high-, intermediate-, and low-risk groups, are useful for estimating the risk of ectopic pregnancy in first-trimester patients (FIGURE). Diagnostic pathways incorporating physical findings, quantitative β-hCG levels, and ultrasound results have been developed to manage possible ectopic pregnancy.38,39 A protocol created by Barnhart using β-hCG and TVUS was accurate and safe when applied to women presenting in emergency settings (sensitivity=100%, specificity=99.9%).30 If β-hCG levels were greater than 1500 mIU/mL, ultrasound was performed. Clinically stable patients with β-hCG levels below 1500 mIU/mL were followed with serial β-hCG levels.
A decision model comparing 6 possible diagnostic strategies that combined clinical examination, TVUS, β-hCG, serum progesterone, and D&C showed that TVUS followed by serial serum β-hCGs in those with initial nondiagnostic ultrasound scans was the most accurate and efficient model.40 By using this strategy, all ectopic pregnancies would be detected, fewer than 1 in 100 normal pregnancies would be interrupted, and diagnostic lag would average only 1.46 days.
CORRESPONDENCE
K. Ramakrishnan, MD, Department of Family and Preventive Medicine, University of Oklahoma Health Sciences Center, 900 NE 10th Street, Oklahoma City, OK 73104. E-mail: [email protected]
1. Stovall TG, Kellerman AL, Ling FW, Buster JE. Emergency department diagnosis of ectopic pregnancy. Ann Emerg Med 1990;19:1098-1103.
2. Fylstra DL. Tubal pregnancy: a review of current diagnosis and treatment. Obstet Gynecol Surv 1998;53:320-328.
3. Ankum WM, Mol BW, Van der Veen F, Bossuyt PM. Risk factors for ectopic pregnancy: a meta-analysis. Fertil Steril 1996;65:1093-1099.
4. Mol BW, Ankum WM, Bossuyt PM, Van der Veen F. Contraception and the risk of ectopic pregnancy: a meta-analysis. Contraception 1995;52:337-341.
5. Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiology 2003;157:185-194.
6. Carr RJ, Evans P. Ectopic pregnancy. Prim Care 2000;27:169-183.
7. Barnhart K, Esposito M, Coutifaris C. An update on the medical treatment of ectopic pregnancy. Obstet Gynecol Clin North Am 2000;27:653-667, viii.
8. Buckley RG, King KJ, Disney JD, Ambroz PK, Gorman JD, Klausen JH. Derivation of a clinical prediction model for the emergency department diagnosis of ectopic pregnancy. Acad Emerg Med 1998;5:951-960.
9. Cacciatore B, Tiitinen A, Stenman U, Ylostalo P. Normal early pregnancy: serum hCG levels and vaginal ultrasonography findings. Br J Obstet Gynecol 1990;97:899-903.
10. Brown DL, Doubilet PM. Transvaginal sonography for diagnosing ectopic pregnancy: positivity criteria and performance characteristics. J Ultrasound Med 1994;13:259-266.
11. Nyberg DA, Hughes MP, Mack LA, Wang KY. Extrauterine findings of ectopic pregnancy at transvaginal US: importance of echogenic fluid. Radiology 1991;178:823-826.
12. Pellerito JS, Taylor KJ, Quedens-Case C, et al. Ectopic pregnancy: evaluation with endovaginal color flow imaging. Radiology 1992;183:407-411.
13. Mol BW, Hajenius PJ, Engelsbel S, et al. Serum human chorionic gonadotropin measurement in the diagnosis of ectopic pregnancy when transvaginal sonography is inconclusive. Fertil Steril 1998;70:972-981.
14. Kaplan BC, Dart RG, Moskos M, et al. Ectopic pregnancy: prospective study with improved diagnostic accuracy. Ann Emerg Med 1996;28:10-17.
15. Braffman BH, Coleman BG, Ramchandani P, et al. Emergency department screening for ectopic pregnancy: a prospective US study. Radiology 1994;190:797-802.
16. Mateer JR, Aiman EJ, Brown MH, Olson DW. Ultrasonographic examination by emergency physicians of patients at risk for ectopic pregnancy. Acad Emerg Med 1995;2:867-873.
17. Dart RG, Mitterando J, Dart LM. Rate of change of serial beta-human chorionic gonadotropin values as a predictor of ectopic pregnancy in patients with indeterminate transvaginal ultrasound findings. Ann Emerg Med 1999;34:703-710.
18. Dart RG, Kaplan B, Varaklis K. Predictive value of history and physical examination in patients with suspected ectopic pregnancy. Ann Emerg Med 1999;33:283-290.
19. Carson SA, Buster JE. Ectopic pregnancy. N Engl J Med 1993;329:1174-1181.
20. Kadar N, Caldwell BV, Romero R. A method of screening for ectopic pregnancy and its indications. Obstet Gynecol 1981;58:162-166.
21. Barnhart KT, Sammel MD, Rinaudo PF, Zhou L, Hummel AC, Guo W. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol 2004;104:50-55.
22. Kadar N, DeVore G, Romero R. Discriminatory hCG zone: its use in the sonographic evaluation for ectopic pregnancy. Obstet Gynecol 1981;58:156-161.
23. Brennan DF. Ectopic pregnancy—Part I: Clinical and laboratory diagnosis. Acad Emerg Med 1995;2:1081-1089.
24. Mol BWJ, Lijmer JL, Ankum WM, Van der Veen F, Bossuyt PM. The accuracy of single serum progesterone measurement in the diagnosis of ectopic pregnancy: a meta-analysis. Hum Reprod 1998;13:3220-3227.
25. Buckley RG, King KJ, Disney JD, Riffenburgh RH, Gorman JD, Klausen JH. Serum progesterone testing to predict ectopic pregnancy in symptomatic first-trimester patients. Ann Emerg Med 2000;36:95-100.
26. Barnhart KT, Gracia CR, Reindl B, Wheeler JE. Usefulness of pipelle endometrial biopsy in the diagnosis of women at risk for ectopic pregnancy. Am J Obstet Gynecol 2003;188:906-909.
27. Birkhahn RH, Gaeta TJ, Paraschiv D, et al. Serum levels of myoglobin, creatine phosphokinase, and smooth muscle heavy-chain myosin in patients with ectopic pregnancy. Ann Emerg Med 2001;38:628-632.
28. Daniel Y, Geva E, Lerner-Geva L, et al. Levels of vascular endothelial growth factor are elevated in ectopic pregnancy: is this a novel marker? Fertil Steril 1999;72:1013-1017.
29. Bree RL, Edwards M, Bohm-Velez M, Beyler S, Roberts J, Mendelson EB. Transvaginal sonography in the evaluation of normal early pregnancy: correlation with hCG level. AJR Am J Roentgenol 1989;153:75-79.
30. Barnhart K, Mennuti MT, Benjamin I, Jacobson S, Goodman D, Coutifaris C. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol 1994;84:1010-1015.
31. Hill LM, Kislak S, Martin JG. Transvaginal sonographic detection of the pseudogestational sac associated with ectopic pregnancy. Obstet Gynecol 1990;75:986-988.
32. Dart R, Burke G, Dart L. Subclassification of indeterminate pelvic ultrasonography: prospective evaluation of the risk of ectopic pregnancy. Ann Emerg Med 2002;39:382-388.
33. Spandorfer SD, Barnhart KT. Endometrial stripe thickness as a predictor of ectopic pregnancy. Fertil Steril 1996;66:474-477.
34. Mol BW, Hajenius PJ, Engelsbel S, et al. Are gestational age and endometrial thickness alternatives for serum human chorionic gonadotropin as criteria for the diagnosis of ectopic pregnancy? Fertil Steril 1999;72:643-645.
35. Dart RG, Dart L, Mitchell P, Berty C. The predictive value of endometrial stripe thickness in patients with suspected ectopic pregnancy who have an empty uterus at ultrasonography. Acad Emerg Med 1999;6:602-608.
36. Kirchler H, Seebacher S, Alge AA, Muller-Holzner E, Fessler S, Kolle D. Early diagnosis of tubal pregnancy: changes in tubal blood flow evaluated by endovaginal color Doppler sonography. Obstet Gynecol 1993;82(4 Pt 1):561-565.
37. Kataoka ML, Togashi K, Kobayashi H, Inoue T, Fujii S, Konishi J. Evaluation of ectopic pregnancy by magnetic resonance imaging. Hum Reprod 1999;14:2644-2650.
38. Cacciatore B, Stenman U, Ylosato P. Diagnosis of ectopic pregnancy by vaginal ultrasonography in combination with a discriminatory serum hCG level of 1000 IU/l (IRP). Br J Obstet Gynecol 1990;97:904-908.
39. Mol BWJ, Van der Veen F, Bossuyt PM. Implementation of probabilistic decision rules improves the predictive values of algorithms in the diagnostic management of ectopic pregnancy. Hum Reprod 1999;14:2855-2862.
40. Gracia CR, Barnhart KT. Diagnosing ectopic pregnancy: decision analysis comparing six strategies. Obstet Gynecol 2001;97:464-470.
- Less than half of the patients with ectopic pregnancy present with the classic triad of a history of amenorrhea, abdominal pain, and irregular vaginal bleeding (C).
- Definite cervical motion tenderness and peritoneal signs are the most sensitive and specific examination findings for ectopic pregnancy—91% and 95%, respectively (A).
- Beta-human chorionic gonadotropin (β-hCG) levels can be used in combination with ultrasound findings to improve the accuracy of the diagnosis of ectopic pregnancy (A).
- Women with initial nondiagnostic transvaginal ultrasound should be followed with serial β-hCGs (B).
Despite advanced detection methods, ectopic pregnancy may be missed in 40% to 50% of patients on an initial visit.1 Most women with ectopic pregnancy have no risk factors (TABLE 1),2-5 and the classic triad of a history of amenorrhea, abdominal pain, and irregular vaginal bleeding is absent in more than half of cases.
Early diagnosis not only decreases maternal mortality and morbidity; it also helps preserve future reproductive capacity—only one third of women with ectopic pregnancy have subsequent live births.2
Management strategies for patients with ectopic pregnancy have evolved rapidly, with ambulatory medical therapy becoming an option for more patients.6 Using a practical decision protocol, we discuss the physical findings that most reliably suggest ectopic pregnancy, describe sensible use of laboratory and imaging studies, and explain what to do when results are equivocal. In part 2 of this article (coming in the June 2006 Journal of Family Practice), we provide a decision protocol for management of ectopic pregnancy.
TABLE 1
Risk factors for ectopic pregnancy
| DEGREE OF RISK | FACTOR | ODDS RATIO (95% CONFIDENCE INTERVALS) | ||
|---|---|---|---|---|
| ANKUM ET AL3 | MOL ET AL4 | BOUYER ET AL5 | ||
| High | Previous tubal surgery | 21 (9.3–47) | 4.0 (2.6–6.1) | |
| Tubal ligation | 9.3 (4.9–18) | |||
| Previous ectopic pregnancy | 8.3 (6.0–11.5) | |||
| In utero DES exposure | 5.6 (2.4–13) | |||
| Current IUD use | 4.2–45* | |||
| Tubal pathology/abnormality | 3.5–25* | |||
| Moderate | Infertility | 2.5–21* | 2.7 (1.8–4.2) | |
| History of PID | 2.5 (2.1–3.0) | 3.4 (2.4–5.0) | ||
| Previous chlamydial or gonococcal infection | 2.8–3.7* | |||
| Current smoking | 2.3 (2.0–2.8) | 3.9 (2.6–5.9) | ||
| Spontaneous abortions ≥3 | 3.0 (1.3–6.9) | |||
| Induced abortions (medical±surgical) | 2.8 (1.1–7.2) | |||
| Lifetime sexual partners >1 | 2.1 (1.4–4.8) | |||
| Low | Age first intercourse <18 years | 1.6 (1.1–2.5) | ||
| Previous pelvic/abdominal surgery | 0.93–3.8* | |||
| Vaginal douching | 1.1–3.1* | |||
| DES, diethylstilbestrol; PID, pelvic inflammatory disease; IUD, intrauterine device | ||||
| * Range; summary odds ratio not calculated owing to significant heterogeneity between studies. | ||||
Evaluation
Clinical features of ectopic pregnancy highly variable
No single clinical feature accurately indicates ectopic pregnancy. Less than half of women with ectopic pregnancy exhibit the classic triad of a history of amenorrhea, abdominal pain, and irregular vaginal bleeding.1 And, unfortunately, these features are seen commonly in patients with both viable (50%) and nonviable (25%) intrauterine pregnancies, as well as in threatened abortion, cervical irritation, infection, and trauma.7
Set a low threshold for suspicion. For any woman of childbearing age with abdominal pain and vaginal bleeding, consider pregnancy and take steps to rule out ectopic pregnancy. Abdominal pain and vaginal bleeding are highly sensitive for ectopic pregnancy but are not specific for the disorder (TABLE 2).8-17 Pain located in the hypogastrium or iliac fossa may be mild to severe. Vaginal bleeding, present in 50% to 80% of patients with ectopic pregnancy, can be mistaken for a normal menstrual period.2,6 Pregnancy-associated symptoms of nausea and vomiting, breast tenderness, and fatigue may be present.
Lower abdominal and adnexal tenderness can be elicited in most women with ectopic pregnancy. Cervical motion tenderness, peritoneal signs, and adnexal masses are most specific for ectopic pregnancy, but are not sensitive.8 An adnexal mass is palpable in less than 10% of cases; when it is detected, one third of patients will have a contralateral ectopic pregnancy on ultrasonography.18 Symptoms of hemodynamic compromise (orthostasis, hypotension, shock) are becoming uncommon with earlier diagnosis of ectopic pregnancy, facilitated by improved detection methods.
TABLE 2
Significance of features associated with ectopic pregnancy
| FEATURES | SN (%) | SP (%) | LR+ | LR– |
|---|---|---|---|---|
| Clinical features8 | ||||
| Any risk factors | 23 | 83 | 1.4 | 0.9 |
| Estimated gestational age <70 days | 95 | 27 | 1.3 | 0.2 |
| Vaginal bleeding | 69 | 26 | 0.9 | 1.2 |
| Abdominal pain | 97 | 15 | 1.1 | 0.2 |
| Abdominal tenderness | 85 | 50 | 1.7 | 0.3 |
| Peritoneal signs | 23 | 95 | 4.6 | 0.8 |
| Cervical motion tenderness | 33 | 91 | 3.7 | 0.7 |
| Adnexal tenderness | 69 | 62 | 1.8 | 0.5 |
| Adnexal mass | 5 | 96 | 1.3 | 1.0 |
| Transvaginal ultrasound | ||||
| No intrauterine gestational sac9 | 100 | 89 | 9.1 | <0.1 |
| Adnexal mass10 | ||||
| Separate from ovary | 93 | 99 | 93 | 0.1 |
| Cardiac activity | 20 | 100 | ∞ | 0.8 |
| Yolk sac or embryo | 37 | 100 | ∞ | 0.6 |
| Tubal ring/yolk sac or embryo | 65 | 99 | 65 | 0.4 |
| Fluid in pouch of Douglas11 | ||||
| Any | 63 | 69 | 2.0 | 0.5 |
| Echogenic | 56 | 96 | 14.0 | 0.5 |
| Color-flow Doppler12 | 95 | 98 | 47.5 | 0.1 |
| β-hCG combined with transvaginal ultrasound | ||||
| Empty uterus | ||||
| ≥1000 mIU/mL10,13,14 | 43–96 | 86–100 | 3.0–∞ | 0.04–0.66 |
| ≥1500 mIU/mL13,15 | 40–99 | 84–96 | 6.3–9.9 | 0.01–0.63 |
| ≥2000 mIU/mL13,16 | 38–48 | 80–98 | 2.3–25.3 | 0.63–0.68 |
| Adnexal mass* | ||||
| ≥1000 mIU/mL13 | 73 | 85 | 4.7 | 0.32 |
| ≥1500 mIU/mL13,15 | 46–64 | 92–96 | 5.9–16.5 | 0.38–0.58 |
| ≥2000 mIU/mL13 | 55 | 96 | 14.2 | 0.47 |
| β-hCG rise in 48 hours (empty uterus)† | ||||
| >66%17 | — | — | 1.1 | — |
| <66%17 | — | — | 7.4 | — |
| >50%13 | — | — | 2.8 | — |
| <50%13 | — | — | 3.3 | — |
| β-hCG fall in 48 hours (empty uterus)† | ||||
| >50%13,17 | — | — | 0.8–1.4 | — |
| <50%13,17 | — | — | 0–0.1 | — |
| * Mass or fluid in cul de sac for β-hCG ≥1500 mIU/mL and ≥2000 mIU/mL. | ||||
| † Strata-specific likelihood ratios reported, sensitivity and specificity not applicable. | ||||
| Sn, sensitivity; Sp, specificity; LR, likelihood ratio; β-hCG, beta-human chorionic gonadotropin. | ||||
Laboratory tests
In an early normal pregnancy, the beta-human chorionic gonadotropin (β-hCG) level doubles every 1.8 to 3 days, rising to 1000 mIU/mL IRP (International Reference Preparation, measured by radioimmunoassay) by 5 weeks, to 2500 mIU/mL by 6 weeks, and to 13,000 mIU/mL (±3000) by 7 weeks. A single quantitative β-hCG level is not always helpful, as it can range from less than 100 mIU/mL to greater than 50,000 mIU/mL with both ruptured and unruptured ectopic pregnancies.19
Rethinking the rate of rise in β-hCG. The oft-quoted maxim that ectopic pregnancy or impending miscarriage is associated with a rise in β-hCG of less than 66% in 48 hours arose from a 1981 study that included only 20 women and that was based on an 85% confidence interval (CI).20 A recent study of 287 women suggests that the minimal expected rise in 2 days for a viable intrauterine pregnancy (based on a 99% CI) should be at least 53%.21
Combining β-hCG levels and ultrasound findings to improve diagnosis. Because the exact gestational age is often unknown in women presenting with features of ectopic pregnancy, the β-hCG level is used to determine whether a gestational sac can be identified on ultrasound. With a β-hCG level of 6500 mIU/mL, a gestational sac should be visible on abdominal ultrasound in the uterus of a woman with an intrauterine pregnancy; the sac should be absent in ectopic pregnancy. This β-hCG level is designated the discriminatory zone.22
With a high-resolution transvaginal ultrasound (TVUS), the gestational sac of a normal single intrauterine pregnancy should be visible at a β-hCG level above 1000–1500 mIU/mL, depending on equipment and technical expertise.2,7 In multiple gestations the discriminatory zone is observed to be above 2300 mIU/mL.2 Various ultrasonography findings have been combined with β-hCG levels to more accurately diagnose ectopic pregnancy (TABLE 2 and FIGURE).
Serial β-hCG measurements. If TVUS findings are indeterminate, serial measurements of β-hCG levels at 48-hour intervals may be useful in managing suspected ectopic pregnancy. Dart et al17 found that a rise in β-hCG level of 66% or more was usually associated with a viable intrauterine pregnancy, though 22% of women in that study had an ectopic pregnancy. Mol et al13 found similar results using a cutoff of a β-hCG level rise of 50% or more; 35% had an ectopic pregnancy. Increases in the β-hCG level less than these cutoffs almost always indicated an abnormal pregnancy, and about half of these women had an ectopic pregnancy.13,17 Also a fall in the β-hCG level by less than 50% was almost always associated with an abnormal pregnancy, but only 19% of these women had an ectopic pregnancy. 13,17 Alternatively, decreases in β-hCG level of greater than 50% reduced the chance that patients had an ectopic pregnancy to less than 3%.13,17
Ectopic pregnancy is defined as the implantation of the fertilized egg that occurs outside the uterine cavity. It is practically synonymous with tubal pregnancy, as 97% occur in the Fallopian tube.6,23 Ectopic pregnancy occurs in 2% of all pregnancies in the United States, affecting more than 100,000 patients each year. It is a leading cause of pregnancy-related death in the first trimester,2 with a yearly monetary impact of greater than 1 billion dollars. The incidence has increased almost six-fold since 1970 due to delayed child-bearing, rising prevalence of sexually transmitted diseases, and sterilization procedures.6
FIGURE
Diagnostic protocol with high sensitivity and specificity for ectopic pregnancy
* Other than midline suprapubic cramping.
† Risk may be higher in presence of these factors: previous EP, tubal surgery, tubal disease detected by hysterosalpingogram or laproscopy, diethylstilbestrol exposure, sterilization, intrauterine device.
‡ Progesterone is useful only when TVUS is indeterminate.
§ β-hCG level for criterion should be based on local values at which IUP visible on TVUS.
|| TVUS should be repeated when β-hCG has risen above the discrimination zone.
¶ Only 2/170 patients in this category had EP; therefore, D&C is safe but may not be cost-effective.11
β-hCG, beta-human chorionic gonadotropin; EP, ectopic pregnancy; TVUS, transvaginal ultrasound; OB/GYN, obstetrician/gynecologist;
IUP, intrauterine pregnancy; D&C, dilation and curettage.
Serum progesterone levels may have limited usefulness. Serum progesterone measurements are not considered accurate enough to diagnose ectopic pregnancy.24 However, progesterone levels may be helpful if they are either very high or very low. Levels less than 22 ng/mL have a sensitivity of 100% for ectopic pregnancy.25 Only 5 of 1615 patients (0.3%) in 13 studies with progesterone levels below 5 ng/mL had a viable intrauterine pregnancy.24 By establishing the low likelihood of a viable intrauterine pregnancy, serum progesterone measurements may also be useful when β-hCG levels have reached 1000–1500 mIU/mL and the TVUS is equivocal.25
When D&C may help. Dilation and curettage (D&C) is an option for patients with an indeterminate ultrasound result and progesterone levels less than 5 ng/mL, as it can rule out an ectopic pregnancy with only a small chance of interrupting a viable intrauterine pregnancy. The presence of chorionic villi rules out an ectopic pregnancy. In the absence of villi, if β-hCG levels subsequently plateau or increase, it is presumptive of ectopic pregnancy. Chorionic villous sampling by pipelle curette has both insufficient sensitivity and predictive value in diagnosing ectopic pregnancy, and should not be considered a substitute for D&C.26
Other tests that have limited use. Patients with ectopic pregnancy may have elevated serum markers of smooth muscle destruction such as creatinine phosphokinase (CPK), myoglobin, and smooth muscle heavy-chain myosin (SMHC). However, these tests are of limited value in diagnosing ectopic pregnancy.27 SMHC may be of use in evaluating pregnancies less than 5 weeks’ gestation when the TVUS is not diagnostic. Serum vascular endothelial growth factor is another marker elevated in women with ectopic pregnancy; levels greater than 200 pg/mL distinguish ectopic pregnancy from intrauterine pregnancy (sensitivity=60%, specificity=90%).28
Imaging
In normal pregnancies, a gestational sac is seen on TVUS between 4 and 5 weeks’ gestation;29 the yolk sac is visible at around 6 weeks; and an embryo can be detected between 6 and 7 weeks. A gestational sac greater than 10 mm in diameter without a yolk sac, or greater than 25 mm without an embryo, indicates an abnormal regnancy.9,29 Presence of an intrauterine pregnancy on ultrasound effectively rules out ectopic pregnancy because heterotopic pregnancy is rare (1 in 2600–30,000 pregnancies).30 Hormonal changes associated with pregnancy result in a uterine endometrial fluid collection (pseudo-sac) in 8% of ectopic pregnancies.31
Specific diagnostic finding for ectopic pregnancy. A gestational sac visible on ultrasound with a yolk sac or fetal pole outside the endometrial cavity (TABLE 2) is diagnostic of ectopic pregnancy. The initial ultrasound is indeterminate in 15% to 20% of women with clinical features suggesting ectopic pregnancy.14,15 Dart et al32 developed a subclassification system of indeterminate ultrasound including 5 categories that stratify the risk of ectopic pregnancy (TABLE 3).
Endometrial stripe thickness of dubious value. For patients with an empty uterus on TVUS and a β-hCG level below the discriminatory zone, endometrial stripe thickness may identify an abnormal pregnancy. In a retrospective study of 117 patients with β-hCG levels less than 1500 mIU/mL, stripe thickness <6 mm had a sensitivity of 100% in identifying ectopic pregnancy or miscarriage; at >13 mm, it had a specificity of 100%.33
Two recent studies, however, have called into question the value of stripe thickness in identifying ectopic pregnancy. One suggested it was of little value with β-hCG levels <1500 mIU/mL,34 and the other concluded its predictive value for ectopic pregnancy and the likelihood of obtaining chorionic villi on D&C is confined to β-hCG values at or below 1000 mIU/mL.35
Color-enhanced sonography. The addition of endovaginal color-flow imaging to sonography enables exclusion of ectopic pregnancy by establishing findings consistent with a nonviable intrauterine pregnancy: an intrauterine gestational sac, nonvisualization of an adnexal mass, and absent placental blood flow (sensitivity=95%, specificity=98%).12 Color-flow Doppler imaging shows enhanced blood flow to the affected tube. A cutoff value of 8% change in tubal blood flow has been used to diagnose ectopic pregnancy (sensitivity=85%, specificity=96%).36 In cases where the gestational sac is questionable or absent, color-flow Doppler may expedite diagnosis and possibly identify candidates for expectant or medical management.
When an MRI might help. Magnetic resonance imaging (MRI), in a small study of 37 patients, allowed recognition of tubal wall enhancements and fresh tubal hematoma, and was diagnostic in 21 patients.37 An MRI may be useful when precise and early diagnosis of ectopic pregnancy is imperative, such as pre-existing damage to the contralateral tube where preservation of tubal patency is paramount and when prior TVUS is inconclusive.37
TABLE 3
Detecting ectopic pregnancy with ultrasound and β-hCG levels
| SUBCLASS | β-hCG <1000 mIU/mL, % (95% CI) | β-hCG >1000 mIU/mL, % (95% CI) |
|---|---|---|
| Empty uterus | 17.9 (12.7–24.2) | 6.0 (2.2–12.8) |
| Nonspecific intrauterine fluid* | 12.2 (4.6–25.0) | 1.2 (0.1–5.9) |
| Echogenic intrauterine material | 10.5 (1.8–30.6) | 2.7 (0.5–8.4) |
| Abnormal intrauterine sac† | 0.0 (0.0–39.3) | 0.0 (0.0–3.1) |
| Normal intrauterine sac | 0.0 (0.0–34.8) | 0.0 (0.0–8.4) |
| * Anechoic intrauterine fluid collection >10 mm diameter with no echogenic border. | ||
| † Anechoic intrauterine fluid collection >10 mm diameter with no yolk sac or fetal pole or grossly irregular border. | ||
| Source: Dart et al 2002.32 | ||
A practical evaluation algorithm
Prediction models based on clinical presentation, which classify patients into high-, intermediate-, and low-risk groups, are useful for estimating the risk of ectopic pregnancy in first-trimester patients (FIGURE). Diagnostic pathways incorporating physical findings, quantitative β-hCG levels, and ultrasound results have been developed to manage possible ectopic pregnancy.38,39 A protocol created by Barnhart using β-hCG and TVUS was accurate and safe when applied to women presenting in emergency settings (sensitivity=100%, specificity=99.9%).30 If β-hCG levels were greater than 1500 mIU/mL, ultrasound was performed. Clinically stable patients with β-hCG levels below 1500 mIU/mL were followed with serial β-hCG levels.
A decision model comparing 6 possible diagnostic strategies that combined clinical examination, TVUS, β-hCG, serum progesterone, and D&C showed that TVUS followed by serial serum β-hCGs in those with initial nondiagnostic ultrasound scans was the most accurate and efficient model.40 By using this strategy, all ectopic pregnancies would be detected, fewer than 1 in 100 normal pregnancies would be interrupted, and diagnostic lag would average only 1.46 days.
CORRESPONDENCE
K. Ramakrishnan, MD, Department of Family and Preventive Medicine, University of Oklahoma Health Sciences Center, 900 NE 10th Street, Oklahoma City, OK 73104. E-mail: [email protected]
- Less than half of the patients with ectopic pregnancy present with the classic triad of a history of amenorrhea, abdominal pain, and irregular vaginal bleeding (C).
- Definite cervical motion tenderness and peritoneal signs are the most sensitive and specific examination findings for ectopic pregnancy—91% and 95%, respectively (A).
- Beta-human chorionic gonadotropin (β-hCG) levels can be used in combination with ultrasound findings to improve the accuracy of the diagnosis of ectopic pregnancy (A).
- Women with initial nondiagnostic transvaginal ultrasound should be followed with serial β-hCGs (B).
Despite advanced detection methods, ectopic pregnancy may be missed in 40% to 50% of patients on an initial visit.1 Most women with ectopic pregnancy have no risk factors (TABLE 1),2-5 and the classic triad of a history of amenorrhea, abdominal pain, and irregular vaginal bleeding is absent in more than half of cases.
Early diagnosis not only decreases maternal mortality and morbidity; it also helps preserve future reproductive capacity—only one third of women with ectopic pregnancy have subsequent live births.2
Management strategies for patients with ectopic pregnancy have evolved rapidly, with ambulatory medical therapy becoming an option for more patients.6 Using a practical decision protocol, we discuss the physical findings that most reliably suggest ectopic pregnancy, describe sensible use of laboratory and imaging studies, and explain what to do when results are equivocal. In part 2 of this article (coming in the June 2006 Journal of Family Practice), we provide a decision protocol for management of ectopic pregnancy.
TABLE 1
Risk factors for ectopic pregnancy
| DEGREE OF RISK | FACTOR | ODDS RATIO (95% CONFIDENCE INTERVALS) | ||
|---|---|---|---|---|
| ANKUM ET AL3 | MOL ET AL4 | BOUYER ET AL5 | ||
| High | Previous tubal surgery | 21 (9.3–47) | 4.0 (2.6–6.1) | |
| Tubal ligation | 9.3 (4.9–18) | |||
| Previous ectopic pregnancy | 8.3 (6.0–11.5) | |||
| In utero DES exposure | 5.6 (2.4–13) | |||
| Current IUD use | 4.2–45* | |||
| Tubal pathology/abnormality | 3.5–25* | |||
| Moderate | Infertility | 2.5–21* | 2.7 (1.8–4.2) | |
| History of PID | 2.5 (2.1–3.0) | 3.4 (2.4–5.0) | ||
| Previous chlamydial or gonococcal infection | 2.8–3.7* | |||
| Current smoking | 2.3 (2.0–2.8) | 3.9 (2.6–5.9) | ||
| Spontaneous abortions ≥3 | 3.0 (1.3–6.9) | |||
| Induced abortions (medical±surgical) | 2.8 (1.1–7.2) | |||
| Lifetime sexual partners >1 | 2.1 (1.4–4.8) | |||
| Low | Age first intercourse <18 years | 1.6 (1.1–2.5) | ||
| Previous pelvic/abdominal surgery | 0.93–3.8* | |||
| Vaginal douching | 1.1–3.1* | |||
| DES, diethylstilbestrol; PID, pelvic inflammatory disease; IUD, intrauterine device | ||||
| * Range; summary odds ratio not calculated owing to significant heterogeneity between studies. | ||||
Evaluation
Clinical features of ectopic pregnancy highly variable
No single clinical feature accurately indicates ectopic pregnancy. Less than half of women with ectopic pregnancy exhibit the classic triad of a history of amenorrhea, abdominal pain, and irregular vaginal bleeding.1 And, unfortunately, these features are seen commonly in patients with both viable (50%) and nonviable (25%) intrauterine pregnancies, as well as in threatened abortion, cervical irritation, infection, and trauma.7
Set a low threshold for suspicion. For any woman of childbearing age with abdominal pain and vaginal bleeding, consider pregnancy and take steps to rule out ectopic pregnancy. Abdominal pain and vaginal bleeding are highly sensitive for ectopic pregnancy but are not specific for the disorder (TABLE 2).8-17 Pain located in the hypogastrium or iliac fossa may be mild to severe. Vaginal bleeding, present in 50% to 80% of patients with ectopic pregnancy, can be mistaken for a normal menstrual period.2,6 Pregnancy-associated symptoms of nausea and vomiting, breast tenderness, and fatigue may be present.
Lower abdominal and adnexal tenderness can be elicited in most women with ectopic pregnancy. Cervical motion tenderness, peritoneal signs, and adnexal masses are most specific for ectopic pregnancy, but are not sensitive.8 An adnexal mass is palpable in less than 10% of cases; when it is detected, one third of patients will have a contralateral ectopic pregnancy on ultrasonography.18 Symptoms of hemodynamic compromise (orthostasis, hypotension, shock) are becoming uncommon with earlier diagnosis of ectopic pregnancy, facilitated by improved detection methods.
TABLE 2
Significance of features associated with ectopic pregnancy
| FEATURES | SN (%) | SP (%) | LR+ | LR– |
|---|---|---|---|---|
| Clinical features8 | ||||
| Any risk factors | 23 | 83 | 1.4 | 0.9 |
| Estimated gestational age <70 days | 95 | 27 | 1.3 | 0.2 |
| Vaginal bleeding | 69 | 26 | 0.9 | 1.2 |
| Abdominal pain | 97 | 15 | 1.1 | 0.2 |
| Abdominal tenderness | 85 | 50 | 1.7 | 0.3 |
| Peritoneal signs | 23 | 95 | 4.6 | 0.8 |
| Cervical motion tenderness | 33 | 91 | 3.7 | 0.7 |
| Adnexal tenderness | 69 | 62 | 1.8 | 0.5 |
| Adnexal mass | 5 | 96 | 1.3 | 1.0 |
| Transvaginal ultrasound | ||||
| No intrauterine gestational sac9 | 100 | 89 | 9.1 | <0.1 |
| Adnexal mass10 | ||||
| Separate from ovary | 93 | 99 | 93 | 0.1 |
| Cardiac activity | 20 | 100 | ∞ | 0.8 |
| Yolk sac or embryo | 37 | 100 | ∞ | 0.6 |
| Tubal ring/yolk sac or embryo | 65 | 99 | 65 | 0.4 |
| Fluid in pouch of Douglas11 | ||||
| Any | 63 | 69 | 2.0 | 0.5 |
| Echogenic | 56 | 96 | 14.0 | 0.5 |
| Color-flow Doppler12 | 95 | 98 | 47.5 | 0.1 |
| β-hCG combined with transvaginal ultrasound | ||||
| Empty uterus | ||||
| ≥1000 mIU/mL10,13,14 | 43–96 | 86–100 | 3.0–∞ | 0.04–0.66 |
| ≥1500 mIU/mL13,15 | 40–99 | 84–96 | 6.3–9.9 | 0.01–0.63 |
| ≥2000 mIU/mL13,16 | 38–48 | 80–98 | 2.3–25.3 | 0.63–0.68 |
| Adnexal mass* | ||||
| ≥1000 mIU/mL13 | 73 | 85 | 4.7 | 0.32 |
| ≥1500 mIU/mL13,15 | 46–64 | 92–96 | 5.9–16.5 | 0.38–0.58 |
| ≥2000 mIU/mL13 | 55 | 96 | 14.2 | 0.47 |
| β-hCG rise in 48 hours (empty uterus)† | ||||
| >66%17 | — | — | 1.1 | — |
| <66%17 | — | — | 7.4 | — |
| >50%13 | — | — | 2.8 | — |
| <50%13 | — | — | 3.3 | — |
| β-hCG fall in 48 hours (empty uterus)† | ||||
| >50%13,17 | — | — | 0.8–1.4 | — |
| <50%13,17 | — | — | 0–0.1 | — |
| * Mass or fluid in cul de sac for β-hCG ≥1500 mIU/mL and ≥2000 mIU/mL. | ||||
| † Strata-specific likelihood ratios reported, sensitivity and specificity not applicable. | ||||
| Sn, sensitivity; Sp, specificity; LR, likelihood ratio; β-hCG, beta-human chorionic gonadotropin. | ||||
Laboratory tests
In an early normal pregnancy, the beta-human chorionic gonadotropin (β-hCG) level doubles every 1.8 to 3 days, rising to 1000 mIU/mL IRP (International Reference Preparation, measured by radioimmunoassay) by 5 weeks, to 2500 mIU/mL by 6 weeks, and to 13,000 mIU/mL (±3000) by 7 weeks. A single quantitative β-hCG level is not always helpful, as it can range from less than 100 mIU/mL to greater than 50,000 mIU/mL with both ruptured and unruptured ectopic pregnancies.19
Rethinking the rate of rise in β-hCG. The oft-quoted maxim that ectopic pregnancy or impending miscarriage is associated with a rise in β-hCG of less than 66% in 48 hours arose from a 1981 study that included only 20 women and that was based on an 85% confidence interval (CI).20 A recent study of 287 women suggests that the minimal expected rise in 2 days for a viable intrauterine pregnancy (based on a 99% CI) should be at least 53%.21
Combining β-hCG levels and ultrasound findings to improve diagnosis. Because the exact gestational age is often unknown in women presenting with features of ectopic pregnancy, the β-hCG level is used to determine whether a gestational sac can be identified on ultrasound. With a β-hCG level of 6500 mIU/mL, a gestational sac should be visible on abdominal ultrasound in the uterus of a woman with an intrauterine pregnancy; the sac should be absent in ectopic pregnancy. This β-hCG level is designated the discriminatory zone.22
With a high-resolution transvaginal ultrasound (TVUS), the gestational sac of a normal single intrauterine pregnancy should be visible at a β-hCG level above 1000–1500 mIU/mL, depending on equipment and technical expertise.2,7 In multiple gestations the discriminatory zone is observed to be above 2300 mIU/mL.2 Various ultrasonography findings have been combined with β-hCG levels to more accurately diagnose ectopic pregnancy (TABLE 2 and FIGURE).
Serial β-hCG measurements. If TVUS findings are indeterminate, serial measurements of β-hCG levels at 48-hour intervals may be useful in managing suspected ectopic pregnancy. Dart et al17 found that a rise in β-hCG level of 66% or more was usually associated with a viable intrauterine pregnancy, though 22% of women in that study had an ectopic pregnancy. Mol et al13 found similar results using a cutoff of a β-hCG level rise of 50% or more; 35% had an ectopic pregnancy. Increases in the β-hCG level less than these cutoffs almost always indicated an abnormal pregnancy, and about half of these women had an ectopic pregnancy.13,17 Also a fall in the β-hCG level by less than 50% was almost always associated with an abnormal pregnancy, but only 19% of these women had an ectopic pregnancy. 13,17 Alternatively, decreases in β-hCG level of greater than 50% reduced the chance that patients had an ectopic pregnancy to less than 3%.13,17
Ectopic pregnancy is defined as the implantation of the fertilized egg that occurs outside the uterine cavity. It is practically synonymous with tubal pregnancy, as 97% occur in the Fallopian tube.6,23 Ectopic pregnancy occurs in 2% of all pregnancies in the United States, affecting more than 100,000 patients each year. It is a leading cause of pregnancy-related death in the first trimester,2 with a yearly monetary impact of greater than 1 billion dollars. The incidence has increased almost six-fold since 1970 due to delayed child-bearing, rising prevalence of sexually transmitted diseases, and sterilization procedures.6
FIGURE
Diagnostic protocol with high sensitivity and specificity for ectopic pregnancy
* Other than midline suprapubic cramping.
† Risk may be higher in presence of these factors: previous EP, tubal surgery, tubal disease detected by hysterosalpingogram or laproscopy, diethylstilbestrol exposure, sterilization, intrauterine device.
‡ Progesterone is useful only when TVUS is indeterminate.
§ β-hCG level for criterion should be based on local values at which IUP visible on TVUS.
|| TVUS should be repeated when β-hCG has risen above the discrimination zone.
¶ Only 2/170 patients in this category had EP; therefore, D&C is safe but may not be cost-effective.11
β-hCG, beta-human chorionic gonadotropin; EP, ectopic pregnancy; TVUS, transvaginal ultrasound; OB/GYN, obstetrician/gynecologist;
IUP, intrauterine pregnancy; D&C, dilation and curettage.
Serum progesterone levels may have limited usefulness. Serum progesterone measurements are not considered accurate enough to diagnose ectopic pregnancy.24 However, progesterone levels may be helpful if they are either very high or very low. Levels less than 22 ng/mL have a sensitivity of 100% for ectopic pregnancy.25 Only 5 of 1615 patients (0.3%) in 13 studies with progesterone levels below 5 ng/mL had a viable intrauterine pregnancy.24 By establishing the low likelihood of a viable intrauterine pregnancy, serum progesterone measurements may also be useful when β-hCG levels have reached 1000–1500 mIU/mL and the TVUS is equivocal.25
When D&C may help. Dilation and curettage (D&C) is an option for patients with an indeterminate ultrasound result and progesterone levels less than 5 ng/mL, as it can rule out an ectopic pregnancy with only a small chance of interrupting a viable intrauterine pregnancy. The presence of chorionic villi rules out an ectopic pregnancy. In the absence of villi, if β-hCG levels subsequently plateau or increase, it is presumptive of ectopic pregnancy. Chorionic villous sampling by pipelle curette has both insufficient sensitivity and predictive value in diagnosing ectopic pregnancy, and should not be considered a substitute for D&C.26
Other tests that have limited use. Patients with ectopic pregnancy may have elevated serum markers of smooth muscle destruction such as creatinine phosphokinase (CPK), myoglobin, and smooth muscle heavy-chain myosin (SMHC). However, these tests are of limited value in diagnosing ectopic pregnancy.27 SMHC may be of use in evaluating pregnancies less than 5 weeks’ gestation when the TVUS is not diagnostic. Serum vascular endothelial growth factor is another marker elevated in women with ectopic pregnancy; levels greater than 200 pg/mL distinguish ectopic pregnancy from intrauterine pregnancy (sensitivity=60%, specificity=90%).28
Imaging
In normal pregnancies, a gestational sac is seen on TVUS between 4 and 5 weeks’ gestation;29 the yolk sac is visible at around 6 weeks; and an embryo can be detected between 6 and 7 weeks. A gestational sac greater than 10 mm in diameter without a yolk sac, or greater than 25 mm without an embryo, indicates an abnormal regnancy.9,29 Presence of an intrauterine pregnancy on ultrasound effectively rules out ectopic pregnancy because heterotopic pregnancy is rare (1 in 2600–30,000 pregnancies).30 Hormonal changes associated with pregnancy result in a uterine endometrial fluid collection (pseudo-sac) in 8% of ectopic pregnancies.31
Specific diagnostic finding for ectopic pregnancy. A gestational sac visible on ultrasound with a yolk sac or fetal pole outside the endometrial cavity (TABLE 2) is diagnostic of ectopic pregnancy. The initial ultrasound is indeterminate in 15% to 20% of women with clinical features suggesting ectopic pregnancy.14,15 Dart et al32 developed a subclassification system of indeterminate ultrasound including 5 categories that stratify the risk of ectopic pregnancy (TABLE 3).
Endometrial stripe thickness of dubious value. For patients with an empty uterus on TVUS and a β-hCG level below the discriminatory zone, endometrial stripe thickness may identify an abnormal pregnancy. In a retrospective study of 117 patients with β-hCG levels less than 1500 mIU/mL, stripe thickness <6 mm had a sensitivity of 100% in identifying ectopic pregnancy or miscarriage; at >13 mm, it had a specificity of 100%.33
Two recent studies, however, have called into question the value of stripe thickness in identifying ectopic pregnancy. One suggested it was of little value with β-hCG levels <1500 mIU/mL,34 and the other concluded its predictive value for ectopic pregnancy and the likelihood of obtaining chorionic villi on D&C is confined to β-hCG values at or below 1000 mIU/mL.35
Color-enhanced sonography. The addition of endovaginal color-flow imaging to sonography enables exclusion of ectopic pregnancy by establishing findings consistent with a nonviable intrauterine pregnancy: an intrauterine gestational sac, nonvisualization of an adnexal mass, and absent placental blood flow (sensitivity=95%, specificity=98%).12 Color-flow Doppler imaging shows enhanced blood flow to the affected tube. A cutoff value of 8% change in tubal blood flow has been used to diagnose ectopic pregnancy (sensitivity=85%, specificity=96%).36 In cases where the gestational sac is questionable or absent, color-flow Doppler may expedite diagnosis and possibly identify candidates for expectant or medical management.
When an MRI might help. Magnetic resonance imaging (MRI), in a small study of 37 patients, allowed recognition of tubal wall enhancements and fresh tubal hematoma, and was diagnostic in 21 patients.37 An MRI may be useful when precise and early diagnosis of ectopic pregnancy is imperative, such as pre-existing damage to the contralateral tube where preservation of tubal patency is paramount and when prior TVUS is inconclusive.37
TABLE 3
Detecting ectopic pregnancy with ultrasound and β-hCG levels
| SUBCLASS | β-hCG <1000 mIU/mL, % (95% CI) | β-hCG >1000 mIU/mL, % (95% CI) |
|---|---|---|
| Empty uterus | 17.9 (12.7–24.2) | 6.0 (2.2–12.8) |
| Nonspecific intrauterine fluid* | 12.2 (4.6–25.0) | 1.2 (0.1–5.9) |
| Echogenic intrauterine material | 10.5 (1.8–30.6) | 2.7 (0.5–8.4) |
| Abnormal intrauterine sac† | 0.0 (0.0–39.3) | 0.0 (0.0–3.1) |
| Normal intrauterine sac | 0.0 (0.0–34.8) | 0.0 (0.0–8.4) |
| * Anechoic intrauterine fluid collection >10 mm diameter with no echogenic border. | ||
| † Anechoic intrauterine fluid collection >10 mm diameter with no yolk sac or fetal pole or grossly irregular border. | ||
| Source: Dart et al 2002.32 | ||
A practical evaluation algorithm
Prediction models based on clinical presentation, which classify patients into high-, intermediate-, and low-risk groups, are useful for estimating the risk of ectopic pregnancy in first-trimester patients (FIGURE). Diagnostic pathways incorporating physical findings, quantitative β-hCG levels, and ultrasound results have been developed to manage possible ectopic pregnancy.38,39 A protocol created by Barnhart using β-hCG and TVUS was accurate and safe when applied to women presenting in emergency settings (sensitivity=100%, specificity=99.9%).30 If β-hCG levels were greater than 1500 mIU/mL, ultrasound was performed. Clinically stable patients with β-hCG levels below 1500 mIU/mL were followed with serial β-hCG levels.
A decision model comparing 6 possible diagnostic strategies that combined clinical examination, TVUS, β-hCG, serum progesterone, and D&C showed that TVUS followed by serial serum β-hCGs in those with initial nondiagnostic ultrasound scans was the most accurate and efficient model.40 By using this strategy, all ectopic pregnancies would be detected, fewer than 1 in 100 normal pregnancies would be interrupted, and diagnostic lag would average only 1.46 days.
CORRESPONDENCE
K. Ramakrishnan, MD, Department of Family and Preventive Medicine, University of Oklahoma Health Sciences Center, 900 NE 10th Street, Oklahoma City, OK 73104. E-mail: [email protected]
1. Stovall TG, Kellerman AL, Ling FW, Buster JE. Emergency department diagnosis of ectopic pregnancy. Ann Emerg Med 1990;19:1098-1103.
2. Fylstra DL. Tubal pregnancy: a review of current diagnosis and treatment. Obstet Gynecol Surv 1998;53:320-328.
3. Ankum WM, Mol BW, Van der Veen F, Bossuyt PM. Risk factors for ectopic pregnancy: a meta-analysis. Fertil Steril 1996;65:1093-1099.
4. Mol BW, Ankum WM, Bossuyt PM, Van der Veen F. Contraception and the risk of ectopic pregnancy: a meta-analysis. Contraception 1995;52:337-341.
5. Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiology 2003;157:185-194.
6. Carr RJ, Evans P. Ectopic pregnancy. Prim Care 2000;27:169-183.
7. Barnhart K, Esposito M, Coutifaris C. An update on the medical treatment of ectopic pregnancy. Obstet Gynecol Clin North Am 2000;27:653-667, viii.
8. Buckley RG, King KJ, Disney JD, Ambroz PK, Gorman JD, Klausen JH. Derivation of a clinical prediction model for the emergency department diagnosis of ectopic pregnancy. Acad Emerg Med 1998;5:951-960.
9. Cacciatore B, Tiitinen A, Stenman U, Ylostalo P. Normal early pregnancy: serum hCG levels and vaginal ultrasonography findings. Br J Obstet Gynecol 1990;97:899-903.
10. Brown DL, Doubilet PM. Transvaginal sonography for diagnosing ectopic pregnancy: positivity criteria and performance characteristics. J Ultrasound Med 1994;13:259-266.
11. Nyberg DA, Hughes MP, Mack LA, Wang KY. Extrauterine findings of ectopic pregnancy at transvaginal US: importance of echogenic fluid. Radiology 1991;178:823-826.
12. Pellerito JS, Taylor KJ, Quedens-Case C, et al. Ectopic pregnancy: evaluation with endovaginal color flow imaging. Radiology 1992;183:407-411.
13. Mol BW, Hajenius PJ, Engelsbel S, et al. Serum human chorionic gonadotropin measurement in the diagnosis of ectopic pregnancy when transvaginal sonography is inconclusive. Fertil Steril 1998;70:972-981.
14. Kaplan BC, Dart RG, Moskos M, et al. Ectopic pregnancy: prospective study with improved diagnostic accuracy. Ann Emerg Med 1996;28:10-17.
15. Braffman BH, Coleman BG, Ramchandani P, et al. Emergency department screening for ectopic pregnancy: a prospective US study. Radiology 1994;190:797-802.
16. Mateer JR, Aiman EJ, Brown MH, Olson DW. Ultrasonographic examination by emergency physicians of patients at risk for ectopic pregnancy. Acad Emerg Med 1995;2:867-873.
17. Dart RG, Mitterando J, Dart LM. Rate of change of serial beta-human chorionic gonadotropin values as a predictor of ectopic pregnancy in patients with indeterminate transvaginal ultrasound findings. Ann Emerg Med 1999;34:703-710.
18. Dart RG, Kaplan B, Varaklis K. Predictive value of history and physical examination in patients with suspected ectopic pregnancy. Ann Emerg Med 1999;33:283-290.
19. Carson SA, Buster JE. Ectopic pregnancy. N Engl J Med 1993;329:1174-1181.
20. Kadar N, Caldwell BV, Romero R. A method of screening for ectopic pregnancy and its indications. Obstet Gynecol 1981;58:162-166.
21. Barnhart KT, Sammel MD, Rinaudo PF, Zhou L, Hummel AC, Guo W. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol 2004;104:50-55.
22. Kadar N, DeVore G, Romero R. Discriminatory hCG zone: its use in the sonographic evaluation for ectopic pregnancy. Obstet Gynecol 1981;58:156-161.
23. Brennan DF. Ectopic pregnancy—Part I: Clinical and laboratory diagnosis. Acad Emerg Med 1995;2:1081-1089.
24. Mol BWJ, Lijmer JL, Ankum WM, Van der Veen F, Bossuyt PM. The accuracy of single serum progesterone measurement in the diagnosis of ectopic pregnancy: a meta-analysis. Hum Reprod 1998;13:3220-3227.
25. Buckley RG, King KJ, Disney JD, Riffenburgh RH, Gorman JD, Klausen JH. Serum progesterone testing to predict ectopic pregnancy in symptomatic first-trimester patients. Ann Emerg Med 2000;36:95-100.
26. Barnhart KT, Gracia CR, Reindl B, Wheeler JE. Usefulness of pipelle endometrial biopsy in the diagnosis of women at risk for ectopic pregnancy. Am J Obstet Gynecol 2003;188:906-909.
27. Birkhahn RH, Gaeta TJ, Paraschiv D, et al. Serum levels of myoglobin, creatine phosphokinase, and smooth muscle heavy-chain myosin in patients with ectopic pregnancy. Ann Emerg Med 2001;38:628-632.
28. Daniel Y, Geva E, Lerner-Geva L, et al. Levels of vascular endothelial growth factor are elevated in ectopic pregnancy: is this a novel marker? Fertil Steril 1999;72:1013-1017.
29. Bree RL, Edwards M, Bohm-Velez M, Beyler S, Roberts J, Mendelson EB. Transvaginal sonography in the evaluation of normal early pregnancy: correlation with hCG level. AJR Am J Roentgenol 1989;153:75-79.
30. Barnhart K, Mennuti MT, Benjamin I, Jacobson S, Goodman D, Coutifaris C. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol 1994;84:1010-1015.
31. Hill LM, Kislak S, Martin JG. Transvaginal sonographic detection of the pseudogestational sac associated with ectopic pregnancy. Obstet Gynecol 1990;75:986-988.
32. Dart R, Burke G, Dart L. Subclassification of indeterminate pelvic ultrasonography: prospective evaluation of the risk of ectopic pregnancy. Ann Emerg Med 2002;39:382-388.
33. Spandorfer SD, Barnhart KT. Endometrial stripe thickness as a predictor of ectopic pregnancy. Fertil Steril 1996;66:474-477.
34. Mol BW, Hajenius PJ, Engelsbel S, et al. Are gestational age and endometrial thickness alternatives for serum human chorionic gonadotropin as criteria for the diagnosis of ectopic pregnancy? Fertil Steril 1999;72:643-645.
35. Dart RG, Dart L, Mitchell P, Berty C. The predictive value of endometrial stripe thickness in patients with suspected ectopic pregnancy who have an empty uterus at ultrasonography. Acad Emerg Med 1999;6:602-608.
36. Kirchler H, Seebacher S, Alge AA, Muller-Holzner E, Fessler S, Kolle D. Early diagnosis of tubal pregnancy: changes in tubal blood flow evaluated by endovaginal color Doppler sonography. Obstet Gynecol 1993;82(4 Pt 1):561-565.
37. Kataoka ML, Togashi K, Kobayashi H, Inoue T, Fujii S, Konishi J. Evaluation of ectopic pregnancy by magnetic resonance imaging. Hum Reprod 1999;14:2644-2650.
38. Cacciatore B, Stenman U, Ylosato P. Diagnosis of ectopic pregnancy by vaginal ultrasonography in combination with a discriminatory serum hCG level of 1000 IU/l (IRP). Br J Obstet Gynecol 1990;97:904-908.
39. Mol BWJ, Van der Veen F, Bossuyt PM. Implementation of probabilistic decision rules improves the predictive values of algorithms in the diagnostic management of ectopic pregnancy. Hum Reprod 1999;14:2855-2862.
40. Gracia CR, Barnhart KT. Diagnosing ectopic pregnancy: decision analysis comparing six strategies. Obstet Gynecol 2001;97:464-470.
1. Stovall TG, Kellerman AL, Ling FW, Buster JE. Emergency department diagnosis of ectopic pregnancy. Ann Emerg Med 1990;19:1098-1103.
2. Fylstra DL. Tubal pregnancy: a review of current diagnosis and treatment. Obstet Gynecol Surv 1998;53:320-328.
3. Ankum WM, Mol BW, Van der Veen F, Bossuyt PM. Risk factors for ectopic pregnancy: a meta-analysis. Fertil Steril 1996;65:1093-1099.
4. Mol BW, Ankum WM, Bossuyt PM, Van der Veen F. Contraception and the risk of ectopic pregnancy: a meta-analysis. Contraception 1995;52:337-341.
5. Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiology 2003;157:185-194.
6. Carr RJ, Evans P. Ectopic pregnancy. Prim Care 2000;27:169-183.
7. Barnhart K, Esposito M, Coutifaris C. An update on the medical treatment of ectopic pregnancy. Obstet Gynecol Clin North Am 2000;27:653-667, viii.
8. Buckley RG, King KJ, Disney JD, Ambroz PK, Gorman JD, Klausen JH. Derivation of a clinical prediction model for the emergency department diagnosis of ectopic pregnancy. Acad Emerg Med 1998;5:951-960.
9. Cacciatore B, Tiitinen A, Stenman U, Ylostalo P. Normal early pregnancy: serum hCG levels and vaginal ultrasonography findings. Br J Obstet Gynecol 1990;97:899-903.
10. Brown DL, Doubilet PM. Transvaginal sonography for diagnosing ectopic pregnancy: positivity criteria and performance characteristics. J Ultrasound Med 1994;13:259-266.
11. Nyberg DA, Hughes MP, Mack LA, Wang KY. Extrauterine findings of ectopic pregnancy at transvaginal US: importance of echogenic fluid. Radiology 1991;178:823-826.
12. Pellerito JS, Taylor KJ, Quedens-Case C, et al. Ectopic pregnancy: evaluation with endovaginal color flow imaging. Radiology 1992;183:407-411.
13. Mol BW, Hajenius PJ, Engelsbel S, et al. Serum human chorionic gonadotropin measurement in the diagnosis of ectopic pregnancy when transvaginal sonography is inconclusive. Fertil Steril 1998;70:972-981.
14. Kaplan BC, Dart RG, Moskos M, et al. Ectopic pregnancy: prospective study with improved diagnostic accuracy. Ann Emerg Med 1996;28:10-17.
15. Braffman BH, Coleman BG, Ramchandani P, et al. Emergency department screening for ectopic pregnancy: a prospective US study. Radiology 1994;190:797-802.
16. Mateer JR, Aiman EJ, Brown MH, Olson DW. Ultrasonographic examination by emergency physicians of patients at risk for ectopic pregnancy. Acad Emerg Med 1995;2:867-873.
17. Dart RG, Mitterando J, Dart LM. Rate of change of serial beta-human chorionic gonadotropin values as a predictor of ectopic pregnancy in patients with indeterminate transvaginal ultrasound findings. Ann Emerg Med 1999;34:703-710.
18. Dart RG, Kaplan B, Varaklis K. Predictive value of history and physical examination in patients with suspected ectopic pregnancy. Ann Emerg Med 1999;33:283-290.
19. Carson SA, Buster JE. Ectopic pregnancy. N Engl J Med 1993;329:1174-1181.
20. Kadar N, Caldwell BV, Romero R. A method of screening for ectopic pregnancy and its indications. Obstet Gynecol 1981;58:162-166.
21. Barnhart KT, Sammel MD, Rinaudo PF, Zhou L, Hummel AC, Guo W. Symptomatic patients with an early viable intrauterine pregnancy: hCG curves redefined. Obstet Gynecol 2004;104:50-55.
22. Kadar N, DeVore G, Romero R. Discriminatory hCG zone: its use in the sonographic evaluation for ectopic pregnancy. Obstet Gynecol 1981;58:156-161.
23. Brennan DF. Ectopic pregnancy—Part I: Clinical and laboratory diagnosis. Acad Emerg Med 1995;2:1081-1089.
24. Mol BWJ, Lijmer JL, Ankum WM, Van der Veen F, Bossuyt PM. The accuracy of single serum progesterone measurement in the diagnosis of ectopic pregnancy: a meta-analysis. Hum Reprod 1998;13:3220-3227.
25. Buckley RG, King KJ, Disney JD, Riffenburgh RH, Gorman JD, Klausen JH. Serum progesterone testing to predict ectopic pregnancy in symptomatic first-trimester patients. Ann Emerg Med 2000;36:95-100.
26. Barnhart KT, Gracia CR, Reindl B, Wheeler JE. Usefulness of pipelle endometrial biopsy in the diagnosis of women at risk for ectopic pregnancy. Am J Obstet Gynecol 2003;188:906-909.
27. Birkhahn RH, Gaeta TJ, Paraschiv D, et al. Serum levels of myoglobin, creatine phosphokinase, and smooth muscle heavy-chain myosin in patients with ectopic pregnancy. Ann Emerg Med 2001;38:628-632.
28. Daniel Y, Geva E, Lerner-Geva L, et al. Levels of vascular endothelial growth factor are elevated in ectopic pregnancy: is this a novel marker? Fertil Steril 1999;72:1013-1017.
29. Bree RL, Edwards M, Bohm-Velez M, Beyler S, Roberts J, Mendelson EB. Transvaginal sonography in the evaluation of normal early pregnancy: correlation with hCG level. AJR Am J Roentgenol 1989;153:75-79.
30. Barnhart K, Mennuti MT, Benjamin I, Jacobson S, Goodman D, Coutifaris C. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol 1994;84:1010-1015.
31. Hill LM, Kislak S, Martin JG. Transvaginal sonographic detection of the pseudogestational sac associated with ectopic pregnancy. Obstet Gynecol 1990;75:986-988.
32. Dart R, Burke G, Dart L. Subclassification of indeterminate pelvic ultrasonography: prospective evaluation of the risk of ectopic pregnancy. Ann Emerg Med 2002;39:382-388.
33. Spandorfer SD, Barnhart KT. Endometrial stripe thickness as a predictor of ectopic pregnancy. Fertil Steril 1996;66:474-477.
34. Mol BW, Hajenius PJ, Engelsbel S, et al. Are gestational age and endometrial thickness alternatives for serum human chorionic gonadotropin as criteria for the diagnosis of ectopic pregnancy? Fertil Steril 1999;72:643-645.
35. Dart RG, Dart L, Mitchell P, Berty C. The predictive value of endometrial stripe thickness in patients with suspected ectopic pregnancy who have an empty uterus at ultrasonography. Acad Emerg Med 1999;6:602-608.
36. Kirchler H, Seebacher S, Alge AA, Muller-Holzner E, Fessler S, Kolle D. Early diagnosis of tubal pregnancy: changes in tubal blood flow evaluated by endovaginal color Doppler sonography. Obstet Gynecol 1993;82(4 Pt 1):561-565.
37. Kataoka ML, Togashi K, Kobayashi H, Inoue T, Fujii S, Konishi J. Evaluation of ectopic pregnancy by magnetic resonance imaging. Hum Reprod 1999;14:2644-2650.
38. Cacciatore B, Stenman U, Ylosato P. Diagnosis of ectopic pregnancy by vaginal ultrasonography in combination with a discriminatory serum hCG level of 1000 IU/l (IRP). Br J Obstet Gynecol 1990;97:904-908.
39. Mol BWJ, Van der Veen F, Bossuyt PM. Implementation of probabilistic decision rules improves the predictive values of algorithms in the diagnostic management of ectopic pregnancy. Hum Reprod 1999;14:2855-2862.
40. Gracia CR, Barnhart KT. Diagnosing ectopic pregnancy: decision analysis comparing six strategies. Obstet Gynecol 2001;97:464-470.