User login
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).
Steps to take before increasing the dose
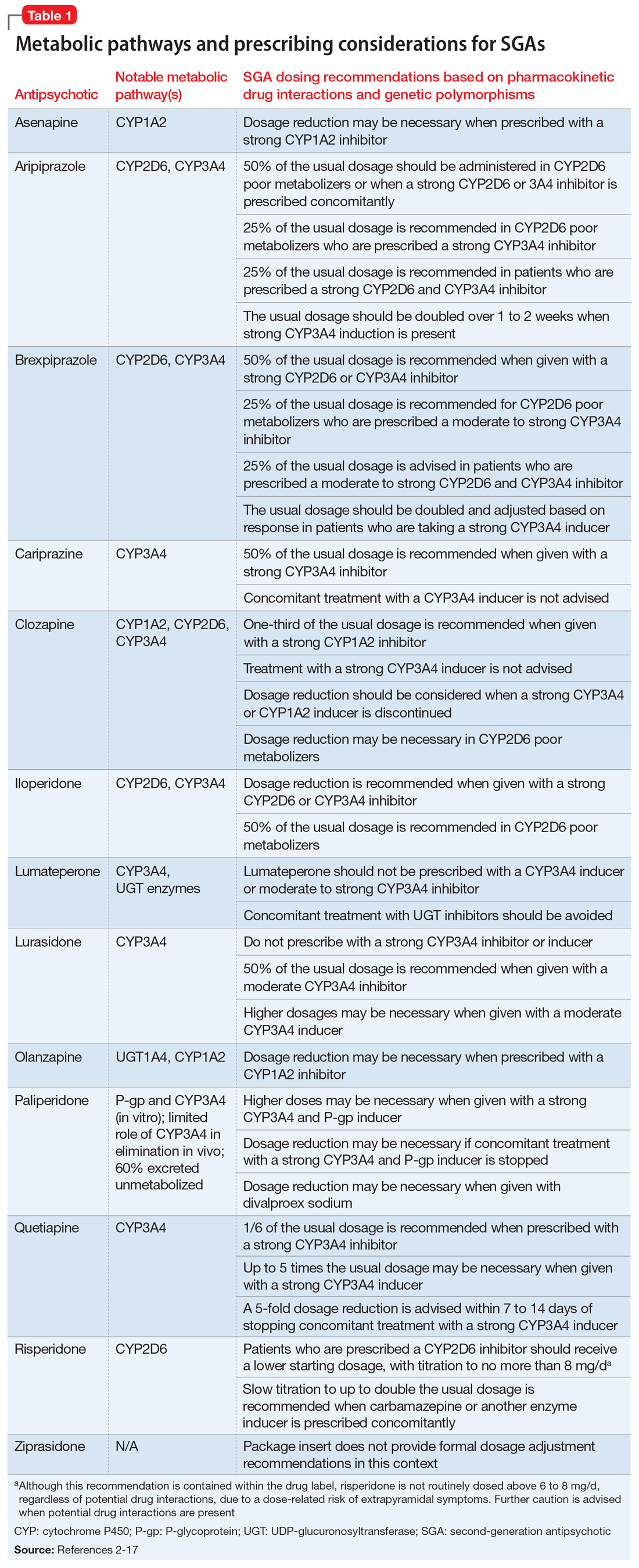
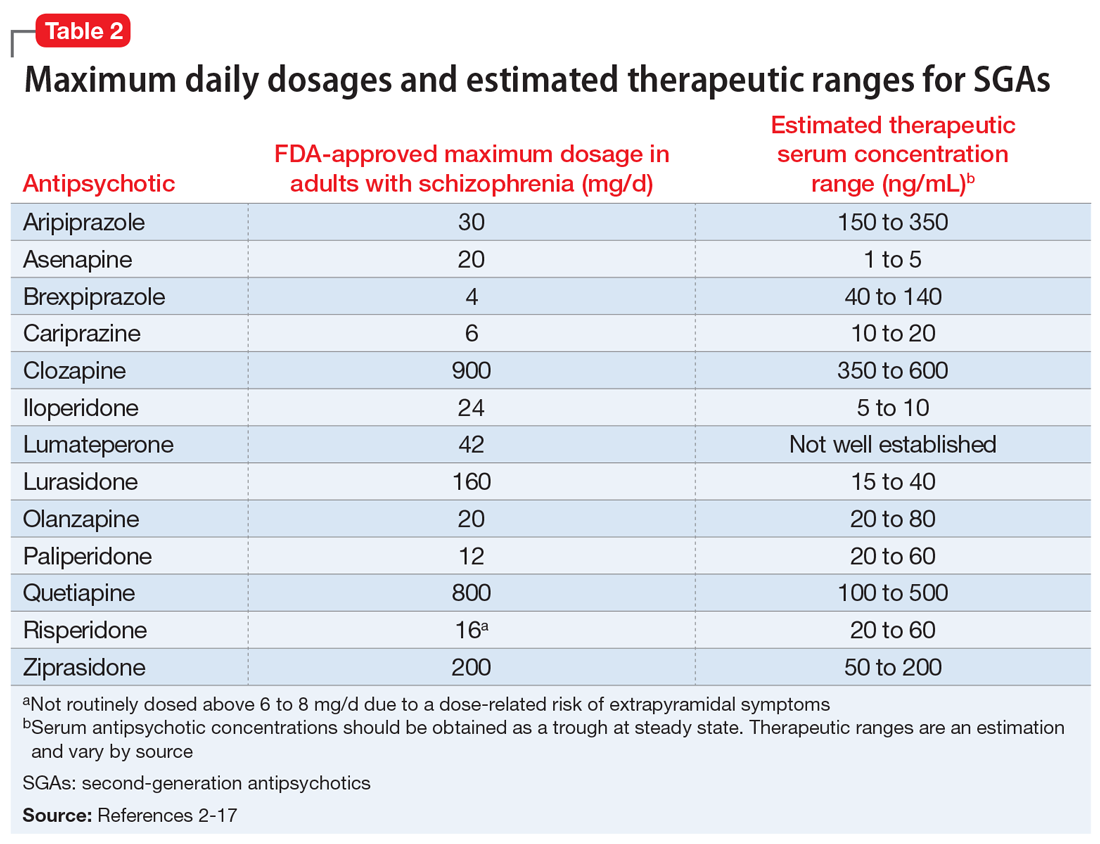
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.
SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).

Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.

SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
Mr. K, age 21, is admitted to the psychiatry unit with agitation, disorganized behavior, and paranoia. Upon presentation, he has no known medical history or current medications. He is diagnosed with schizophrenia and subsequently tolerates but does not respond to adequate durations of treatment with fluphenazine, 20 mg/d; aripiprazole, 30 mg/d; and risperidone, 6 mg/d. Medication adherence is verified, but Mr. K is reluctant to try a fourth antipsychotic. The treatment team suspects that Mr. K may be a cytochrome P450 (CYP) 2D6 ultra-rapid metabolizer, so they obtain a serum risperidone level. The serum risperidone concentration is subtherapeutic (10 ng/mL). What should be considered next?
Several factors must be considered when a patient with psychosis does not experience significant symptomatic improvement with an adequate antipsychotic trial. This article focuses on high-dose second-generation antipsychotic (SGA) therapy in adults with psychosis. “High-dose” antipsychotic therapy is dosing that exceeds the standard maximum dosage for a given antipsychotic. Existing evidence on the use of high-dose SGAs consists of open-label studies and case reports, as well as a handful of randomized controlled trials (RCTs) with small sample sizes and high dropout rates. In some studies, the use of concomitant interventions (eg, duplicate antipsychotic therapy) limit the interpretation of data. High-dose first-generation antipsychotic therapy is discouraged because of a heightened risk of extrapyramidal symptoms (EPS).

Steps to take before increasing the dose
When considering prescribing high-dose antipsychotic therapy, first confirm that the patient has been adherent to the current medication regimen. Also, screen for factors that might impair drug absorption, such as bariatric surgery or noncompliance with administration precautions.1 For example, administration of lurasidone with less than 350 calories may considerably decrease absorption.2 Dosage requirements may vary based on ethnicity, gender, CYP polymorphisms, and pharmacokinetic drug interactions (Table 12-17).1,18,19 Causes of inadequate efficacy should be addressed before considering the use of high-dose antipsychotic therapy.1 Under certain circumstances, serum drug concentrations may be used to guide antipsychotic dosing (Table 22-17). Inadequate response despite a therapeutic serum concentration may indicate pharmacodynamic failure.1 Inadequate response in the context of subtherapeutic serum concentrations, good medication adherence, and compliance to administration precautions may be indicative of a genetic polymorphism or drug interaction.1 Changes in antipsychotic dosing or selection may be warranted, depending on associated risks and benefits.

SGAs and high-dose administration
The SGA with the greatest evidence for high-dose administration is olanzapine, which is similar in structure and receptor pharmacology to clozapine.20,21 The use of high-dose olanzapine is controversial. High-dose olanzapine has been compared to clozapine in patients with treatment-resistant schizophrenia (TRS) and schizoaffective disorder. Meltzer et al22 reported similar efficacy with clozapine, 300 to 900 mg/d, and olanzapine, 25 to 45 mg/d. In this study, high-dose olanzapine caused more weight gain when compared to clozapine. Olanzapine dosages of up to 100 mg/d have been prescribed for TRS; however, this is not common practice.23 A study comparing 10, 20, and 40 mg/d in patients with non-TRS or schizoaffective disorder showed no advantage with higher dosages.24
There is limited data on high-dose treatment with other SGAs.17 Orthostasis may limit iloperidone’s safety at high doses, and single doses of asenapine should not exceed 10 mg.25 Limited sublingual surface area and saliva saturation result in decreased bioavailability with higher asenapine doses.25,26 In a small RCT of patients with stable schizophrenia or schizoaffective disorder, aripiprazole was relatively well-tolerated up to 75 mg/d, whereas akathisia and tachycardia occurred with 90 mg/d.27 Case reports have documented successful treatment with aripiprazole, 60 to 75 mg/d; however, dizziness and worsening psychosis, agitation, and confusion have been observed.28-31
There is a paucity of data on high-dose risperidone and paliperidone, possibly due to their potent dopamine-2 (D2) receptor antagonism and dose-related risk of EPS.1 At risperidone dosages >6 mg/d, the balance between D2 and serotonin-2A (5-HT2A) receptor potency is lost, which increases the potential for EPS.32 In one RCT, long-acting injectable (LAI) risperidone, up to 100 mg biweekly, was well-tolerated but no more effective for TRS than 50 mg biweekly.33 A case report suggested improvement of TRS in a patient administered risperidone LAI, 75 mg vs 37.5 mg biweekly, but it is unclear if a 50-mg dosage was tried.34 Another case report documented improvement in schizophrenia symptoms with risperidone LAI, 125 mg biweekly; however, anticholinergic therapy was required for EPS.35
Dose-dependent adverse effects, including EPS, sedation, anticholinergic effects, orthostasis, hyperprolactinemia, and QTc prolongation, may limit the safety of high-dose antipsychotic therapy.1,20,36 Two studies showed no correlation between QTc prolongation and ziprasidone dosages of up to 320 mg/d for psychosis.37,38 QTc prolongation was more likely at higher ziprasidone concentrations.37 Higher concentrations, but not higher dosages, also trended toward improvement in positive symptoms, and concentrations >100 ng/mL were associated with more negative symptoms.37 A case report described improvement in positive symptoms of schizoaffective disorder with ziprasidone, 320 mg/d, but activation, hostility, and depression worsened.39
Continue to: Compared with other antipsychotics...
Compared with other antipsychotics, high-dose clozapine and quetiapine may be less likely to cause EPS due to lower D2 receptor occupancies.40 Nevertheless, increased activity at other postsynaptic receptors may lead to constipation, metabolic effects, and sedation.1,41,42 Case reports suggest efficacy with quetiapine, 1,200 to 2,400 mg/d, vs lower dosages for patients with TRS.43,44 However, RCTs of quetiapine, 600 and 800 mg/d vs 1,200 mg/d, have not demonstrated an efficacy advantage with high-dose treatment in patients with schizophrenia or schizoaffective disorder.41,45 High-dose quetiapine has also resulted in photopsia, cardiotoxicity, orthostasis, dysphagia, and sedation.43,46,47
Proceed with caution
In light of safety concerns and a lack of high-quality evidence for high-dose antipsychotic therapy, alternative solutions for inadequate response to treatment should be considered. Underlying causes of poor response should be addressed, and alternative antipsychotics should be utilized, when appropriate. A clozapine trial remains first-line for TRS. Olanzapine may be the best-supported high-dose antipsychotic alternative when clozapine is not an option. High antipsychotic dosages are not well-studied in patients with genetic polymorphisms or unavoidable drug interactions. Serum antipsychotic concentrations may facilitate dosing in these patients.
If high-dose antipsychotic therapy is deemed necessary, its ongoing appropriateness should be continually re-evaluated. Higher antipsychotic dosages and D2 receptor occupancies may be required to manage acute psychosis, but efficacy may be maintained and adverse effects limited with the use of lower dosages during maintenance treatment.48,49 Long-term treatment with high-dose antipsychotic therapy should be avoided, when possible. If high-dose antipsychotic therapy is prescribed, the rationale should be well-documented. Dosage adjustments should not be made until steady state is reached on a given dosage. Electrocardiograms should be obtained at baseline, steady state, and routinely thereafter.3,20 Tolerability should be assessed regularly, and screening for drug interactions should be conducted when new medications are initiated.
Case CONTINUED
Because Mr. K’s serum risperidone level is subtherapeutic (10 ng/mL), his risperidone dosage is cautiously titrated to 10 mg/d, divided (serum concentration: 22 ng/mL). Mr. K develops mild orthostasis but denies other adverse effects. His psychotic symptoms resolve, and he is discharged with education on nonpharmacologic management of orthostasis. The rationale for high-dose risperidone is relayed to his outpatient psychiatrist, as well as a recommendation to monitor Mr. K closely for continued efficacy and tolerability.
Related Resource
- Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Clozapine • Clozaril
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Ziprasidone • Geodon
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.
1. Morrissette DA, Stahl SM. Treating the violence patient with psychosis or impulsivity utilizing antipsychotic polypharmacy and high-dose monotherapy. CNS Spectrums. 2014;19(5):439-448.
2. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2019.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.
4. Vyas P, Hwang BJ, Brasic JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Saphris [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
7. Abilify [package insert]. Tokyo, Japan: Otsuka America Pharmaceutical, Inc.; 2014.
8. Rexulti [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2020.
9. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
10. Clozaril [package insert]. Rosemont, PA: Novartis Pharmaceuticals Corporation; 2017.
11. Fanapt [package insert]. Washington, DC: Vanda Pharmaceuticals Inc.; 2016.
12. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
13. Zyprexa [package insert]. Indianapolis, IN: Lilly USA, LLC.; 2020.
14. Invega [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019.
15. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2016.
16. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2020.
17. Geodon [package insert]. New York, NY: Pfizer Inc.; 2020.
18. Chaudhry IB, Neelam K, Duddu V, et al. Ethnicity and psychopharmacology. J Psychopharmacol. 2008;22(6):673-680.
19. Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631. doi: 10.1016/j.neuropharm.2019.05.008
20. Barnes TRE, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3-78.
21. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsych Dis Treat. 2019;15:2559-2569.
22. Meltzer HY, Bobo WV, Ajanta R, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274-285.
23. Batail JM, Langree B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res. 2014;159(2-3):411-414.
24. Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2008;28(4):392-400.
25. Stahl SM. Stahl’s essential psychopharmacology prescriber’s guide. 6th ed. Cambridge University Press; 2017.
26. Bartlett JA, van der Voort Maarschalk K. Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech. 2012;13(4):1110-1115.
27. Auby P, Saha A, Ali M, et al. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharm. 2002;12(3):288.
28. Chavez B, Poveda R. Efficacy with high-dose aripiprazole after olanzapine-related metabolic disturbances. Ann Pharmacother. 2006;40(12):2265-2268.
29. Duggal HS, Mendhekar DN. High-dose aripiprazole in treatment-resistant schizophrenia. J Clin Psychiatry. 2006;67(4):674-675.
30. Thone J. Worsened agitation and confusion in schizophrenia subsequent to high-dose aripiprazole. J Neuropsychiatry Clin Neurosci. 2007;19(4):481-482.
31. Saatcioglu O, Gumus S, Kamberyan K, et al. Efficacy of high-dose aripiprazole for treatment-resistant schizoaffective disorder: a case report. Psychopharmacol Bull. 2010;43(4):70-72.
32. Thomson SR, Chogtu B, Bhattacharjee D, et al. Extrapyramidal symptoms probably related to risperidone treatment: a case series. Ann Neurosci. 2017;24(3):155-163.
33. Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1-3):14-22.
34. Hou Y, Lai C. The response of psychotic symptoms in a patient with resistant schizophrenia under treatment of high-dose risperidone long-acting injection. J Neuropsychiatry Clin Neurosci. 2014;26(3):E16-E17. doi: 10.1176/appi.neuropsych.13070150
35. Albrecht A, Morena PG, Baumann P, et al. High dose of depot risperidone in a nonresponder schizophrenic patient. J Clin Psychopharmacol. 2004;24(6):673-674.
36. Mace S, Taylor D. Reducing the rates of prescribing high-dose antipsychotics and polypharmacy on psychiatric inpatient and intensive care units: results of a 6-year quality improvement programme. Ther Adv Psychopharmacol. 2015;5(1):4-12.
37. Goff DC, McEvoy JP, Citrome L, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms. J Clin Psychopharmacol. 2013;33:485-490.
38. Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10(4):227-232.
39. Kaushik S, Maccabee N, Kaushik S, et al. Activation induced by high-dose ziprasidone: a case report. J Clin Psychiatry. 2009;70(9):1326-1327.
40. Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515-531.
41. Honer WG, MacEwan W, Gendron A, et al. A randomized, double-blind, placebo-controlled study of safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(1):13-20.
42. Sokolski KN, Brown BJ, Meldon M. Urinary retention following repeated high-dose quetiapine. Ann Pharmacother. 2004;38(5):899-890.
43. Chandrappa P, Ho L. Case reports of patients with treatment-resistant schizophrenia and related psychotic disorders intolerant to clozapine responding to high doses of quetiapine. Ther Adv Psychopharmacol. 2012;2(5):207-209.
44. Pierre JM, Wirshing DA, Wirshing WC, et al. High-dose quetiapine in treatment refractory schizophrenia. Schizophr Res. 2005;73:373-375.
45. Lindenmyer JP, Citrome L, Khan A, et al. A randomized, double-blind parallel-group, fixed-dose, clinical trial of quetiapine at 600 vs. 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 2011;31(2):160-168.
46. Hazra M, Culo S, Mamo D. High-dose quetiapine and photopsia. J Clin Psychopharmacol. 2006;26(5):546-547.
47. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017;17(4):478-481.
48. Takeuchi H, Suzuki T, Bies RR, et al. Dose reduction of risperidone and olanzapine and estimated D2 receptor occupancy in stable patients with schizophrenia: findings from an open-label, randomized, controlled study. J Clin Psychiatry. 2014;75(11):1209-1214.
49. Kumar V, Rao NP, Narasimha V, et al. Antipsychotic dose in maintenance treatment of schizophrenia: a retrospective study. Psychiatry Res. 2016;245:311-316.

