User login
One in 5 Americans are taking at least 1 psychotropic medication.1 Elderly dementia patients in extended care facilities are the most likely population to be prescribed psychotropic medication: 87% of these patients are on at least 1 psychotropic medication, 66% on at least 2, 36% on at least 3, and 11% on 4 or more.2 Psychotropic medications alleviate the symptoms of mental illness, such as depression, anxiety, and psychosis. Unfortunately, these medications often have adverse effects (AEs), including, but not limited to, excessive sedation, cardiac abnormalities, and tardive dyskinesia.
In 1987, Congress passed the Nursing Home Reform Act (NHRA) as part of the Omnibus Budget Reconciliation Act. The NHRA mandated that residents must remain free of “physical or chemical restraints imposed for the purpose of discipline or convenience.”3
In 1991, in order to meet the NHRA requirements, the Centers for Medicare and Medicaid Services (CMS) issued a guideline that nursing homes should use antipsychotic drug therapy only to treat a specific condition as diagnosed and documented in the clinical record. In 2006, CMS published guidance on the reduction of psychotropic medication usage. These CMS guidelines are the community-accepted standards for prescribing psychotropic medications, and accrediting bodies expect compliance with the guidelines. The guidelines also recommend that antipsychotics should be prescribed at the lowest possible dose, used for the shortest period, and continually undergo gradual dose reduction (GDR).4 To ensure these standards are met, a review of the use of psychotropic medications should be performed regularly.
The purpose of this project was to improve documentation of GDR and review of psychotropic medication based on CMS guidelines in the community living centers (CLCs) at the Carl Vinson Veterans Affairs Medical Center (CVVAMC) in Dublin, Georgia.
Background
The CVVAMC provides long-term care to veterans living in CLCs in a comfortable, homelike environment with a person-centered, nursing-home level of care. During a patient’s stay, clinical pharmacy services reviews patient medication use regularly. Additionally, an interdisciplinary team (IDT) of physicians, pharmacists, nurses, recreation therapists, dieticians, and chaplains meet to discuss the medical, psychosocial, and spiritual needs of the residents at the time of admission and quarterly thereafter. Family and caregivers are invited to attend as well.
During the meeting, the IDT care plan is updated and reviewed with the veteran. Currently at the CVVAMC, care plans include discussion of pain management; however, the plan does not address psychotropic medication use. To be compliant with CMS guidelines, during IDT meetings discussion of psychotropic medication use would be advantageous, because physicians could receive input and feedback from other health care team members in determining whether psychotropic medication changes are needed.
Methods
All documentation and chart notes are recorded and stored in the Computerized Patient Record System (CPRS). The purpose of an electronic template within CPRS is to provide standardized, guided documentation in an easy-to-use format that can be integrated smoothly into the existing system. Template utilization has been shown to improve documentation of medication reconciliation, health records, and coding in the VA health system.5-7 A review of CPRS showed that no template existed for documenting psychotropic medication initiation and GDR.
Objectives
The authors’ primary objective was to improve patient record documentation in CPRS through implementation of staff education and an electronic template for physicians to document the management of psychotropic medications and patients’ GDRs. The secondary objective was to implement a discussion involving the physician and other members of the team during IDT meetings about patient safety and effectiveness of psychotropic medications.
Template
To improve the suboptimal compliance with the CMS guidelines, an electronic template was created to evaluate GDR attempts and facilitate review of prescribed psychotropic medications. The pharmacy and therapeutics committee approved the template, and the clinical applications coordinator implemented the electronic template. The template was then revised as necessary for ease of use, based on health care provider (HCP) and pharmacist feedback.
The use of the electronic template was phased in over a 1-month period. Complete implementation was achieved in January 2014. An in-service was provided for nurses, HCPs, and directors on the importance of psychotropic medication review and GDRs. A second in-service was conducted for the HCPs to learn how to properly complete the electronic template. Providers were instructed to use the template during IDT meetings when completing monthly chart reviews and changing psychotropic medications.
Additionally, resident assessment coordinators who attend all IDT meetings were requested to remind the team to discuss psychotropic medication use if appropriate in an effort to add a psychotropic medication review to the IDT care plan.
To determine whether the electronic template improved documentation, a retrospective chart review of CPRS was conducted February 2014. The study included veterans in the CLC who were prescribed psychotropic medications since January 2014. The subjects who screened positive for psychotropic medication use were further evaluated by chart review. Before and after implementation, information was collected on the number and percentage of patients who had documentation in their CPRS records of a psychotropic medication review and on whether a GDR evaluation was recorded in CPRS. Additionally, a clinical pharmacist specialist attending the IDT meeting monitored the incidence of psychotropic medication review or discussion that took place if the veteran was on an included agent.
Results
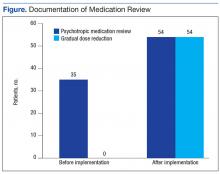
A total of 67 patients on psychotropic medication on the CLC wards since January 2014 were included in the program. Before implementation of the program, 35 patients had documentation of psychotropic medication review. Following implementation, this increased to 54 patients. Hence, 80% of the patients had appropriate documentation.
Before implementation, no patients had documentation regarding evaluation of a GDR in CPRS. After the program implementation, 54 patients had documentation of GDR. Of the 54 patients, 50 had documentation that a GDR could not be completed, and 4 patients had undergone a trial of GDR with appropriate documentation in CPRS (Figure).
The secondary objective evaluated how often a review of patient’s psychotropic medication occurred during IDT meetings. During March 2014, 21 patients with scheduled IDT meetings were prescribed psychotropic medications. For 14 of the 21 patients (67%) appropriateness of psychotropic medications was reviewed with the team and the resident’s families.
Discussion
Documentation is fundamental to improving quality of care, patient outcomes, and reimbursement for the facility. Effective since July 1, 2014, the Joint Commission standards for Nursing Care Center accreditation require the physician and consulting pharmacist to review the patients’ or residents’ medication list. The review should verify the following:
- Clinical indication for the antipsychotic medication;
- Necessity for ongoing use of the antipsychotic medication;
- Consideration of GDR of the antipsychotic medication;
- Consideration of an alternative to antipsychotic medication use.8
When used correctly, the psychotropic medication review and GDR template ensures veterans’ psychotropic medications are continually reviewed. Furthermore, better psychotropic medication management allows veterans to be adequately treated while minimizing the risk for potential AEs.
At CVVAMC, the electronic template helped improve documentation and evaluation of GDR. The CVVAMC success can be attributed partly to physicians who were willing to use the template.
New standards from the Joint Commission provided an additional incentive to use the template. The Joint Commission also required that patients and their family be involved in decisions about placing the resident on antipsychotic medication. The optimal time for this discussion is during IDT meetings. At CVVAMC, discussion of psychotropic medications took place in IDT meetings for 67% of the patients prescribed psychotropic medication.
It is important to note that tools, such as this template, are successful only if they are used by HCPs. The CPRS does not have any clinical reminders to prompt physicians to complete the task. Instead, HCPs had to develop a routine of entering information into the template. Additionally, if the resident assessment coordinator was not at the IDT meeting, discussion of psychotropic medication was not always completed. After completion of this project, psychotropic medication review has become a permanent component of the IDT care plan.
Conclusion
This project demonstrated that template development and use have the potential to improve documentation, patient care, and survey scores. The electronic template could potentially benefit any long-term care facility that uses an electronic recording system. Further research should focus on ease of use and on ensuring that a psychotropic medication review is added to all CLC IDT care plans.
Acknowledgments
The authors acknowledge Brooke Butler, clinical pharmacy specialist and project mentor for her assistance and guidance; and Deborah Hobbs, residency director, both at the Carl Vinson VA Medical Center for her input and leadership, and Freddie Miles, for her work on development of the electronic order set.
1. Medco Health Solutions. America’s state of mind. 2011. http://apps.who.int/medicinedocs/documents/s19032en/s19032en.pdf. Accessed August 31, 2016.
2. Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS. Behavioral symptoms and the administration of psychotropic drugs to aged patients with dementia in nursing homes and in acute geriatric wards. Int Psychogeriatr. 2004;16(1):61-74.
3. Rehnquist J. Psychotropic drug use in nursing homes. Office of Inspector General. https://oig.hhs.gov/oei/reports/oei-02-00-00491.pdf. Published November 2001. Accessed June 24, 2016.
4. Centers for Medicare and Medicaid Services. CMS Manual System. Pub 100-07 State Operations Provider Certification. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs-Items/CMS1201984.html. Published December 15, 2006. Accessed August 29, 2016.
5. Rose EA, Deshikachar AM, Schwartz KL, Severson RK. Use of a template to improve documentation and coding. Fam Med. 2001;33(7):516-521.
6. Fielstein EM, Brown SH, McBrine CS, Clark TK, Hardenbrook SP, Speroff T. The effect of standardized, computer-guided templates on quality of VA disability exams. AMIA Annu Symp Proc. 2006:249-253.
7. Edwards LB, Powers JB. Electronic medication reconciliation: a pilot demonstration on an inpatient geriatrics unit. Fed Pract. 2007;24(9):49-50, 53, 62.
8. Approved: memory care requirements for nursing center accreditation. http://www.jointcommission.org/ASSETS/1/18/JCP0114_MEMORY_CARE_NCC.PDF. Published January 2014. Accessed August 24, 2016.
One in 5 Americans are taking at least 1 psychotropic medication.1 Elderly dementia patients in extended care facilities are the most likely population to be prescribed psychotropic medication: 87% of these patients are on at least 1 psychotropic medication, 66% on at least 2, 36% on at least 3, and 11% on 4 or more.2 Psychotropic medications alleviate the symptoms of mental illness, such as depression, anxiety, and psychosis. Unfortunately, these medications often have adverse effects (AEs), including, but not limited to, excessive sedation, cardiac abnormalities, and tardive dyskinesia.
In 1987, Congress passed the Nursing Home Reform Act (NHRA) as part of the Omnibus Budget Reconciliation Act. The NHRA mandated that residents must remain free of “physical or chemical restraints imposed for the purpose of discipline or convenience.”3
In 1991, in order to meet the NHRA requirements, the Centers for Medicare and Medicaid Services (CMS) issued a guideline that nursing homes should use antipsychotic drug therapy only to treat a specific condition as diagnosed and documented in the clinical record. In 2006, CMS published guidance on the reduction of psychotropic medication usage. These CMS guidelines are the community-accepted standards for prescribing psychotropic medications, and accrediting bodies expect compliance with the guidelines. The guidelines also recommend that antipsychotics should be prescribed at the lowest possible dose, used for the shortest period, and continually undergo gradual dose reduction (GDR).4 To ensure these standards are met, a review of the use of psychotropic medications should be performed regularly.
The purpose of this project was to improve documentation of GDR and review of psychotropic medication based on CMS guidelines in the community living centers (CLCs) at the Carl Vinson Veterans Affairs Medical Center (CVVAMC) in Dublin, Georgia.
Background
The CVVAMC provides long-term care to veterans living in CLCs in a comfortable, homelike environment with a person-centered, nursing-home level of care. During a patient’s stay, clinical pharmacy services reviews patient medication use regularly. Additionally, an interdisciplinary team (IDT) of physicians, pharmacists, nurses, recreation therapists, dieticians, and chaplains meet to discuss the medical, psychosocial, and spiritual needs of the residents at the time of admission and quarterly thereafter. Family and caregivers are invited to attend as well.
During the meeting, the IDT care plan is updated and reviewed with the veteran. Currently at the CVVAMC, care plans include discussion of pain management; however, the plan does not address psychotropic medication use. To be compliant with CMS guidelines, during IDT meetings discussion of psychotropic medication use would be advantageous, because physicians could receive input and feedback from other health care team members in determining whether psychotropic medication changes are needed.
Methods
All documentation and chart notes are recorded and stored in the Computerized Patient Record System (CPRS). The purpose of an electronic template within CPRS is to provide standardized, guided documentation in an easy-to-use format that can be integrated smoothly into the existing system. Template utilization has been shown to improve documentation of medication reconciliation, health records, and coding in the VA health system.5-7 A review of CPRS showed that no template existed for documenting psychotropic medication initiation and GDR.
Objectives
The authors’ primary objective was to improve patient record documentation in CPRS through implementation of staff education and an electronic template for physicians to document the management of psychotropic medications and patients’ GDRs. The secondary objective was to implement a discussion involving the physician and other members of the team during IDT meetings about patient safety and effectiveness of psychotropic medications.
Template
To improve the suboptimal compliance with the CMS guidelines, an electronic template was created to evaluate GDR attempts and facilitate review of prescribed psychotropic medications. The pharmacy and therapeutics committee approved the template, and the clinical applications coordinator implemented the electronic template. The template was then revised as necessary for ease of use, based on health care provider (HCP) and pharmacist feedback.
The use of the electronic template was phased in over a 1-month period. Complete implementation was achieved in January 2014. An in-service was provided for nurses, HCPs, and directors on the importance of psychotropic medication review and GDRs. A second in-service was conducted for the HCPs to learn how to properly complete the electronic template. Providers were instructed to use the template during IDT meetings when completing monthly chart reviews and changing psychotropic medications.
Additionally, resident assessment coordinators who attend all IDT meetings were requested to remind the team to discuss psychotropic medication use if appropriate in an effort to add a psychotropic medication review to the IDT care plan.
To determine whether the electronic template improved documentation, a retrospective chart review of CPRS was conducted February 2014. The study included veterans in the CLC who were prescribed psychotropic medications since January 2014. The subjects who screened positive for psychotropic medication use were further evaluated by chart review. Before and after implementation, information was collected on the number and percentage of patients who had documentation in their CPRS records of a psychotropic medication review and on whether a GDR evaluation was recorded in CPRS. Additionally, a clinical pharmacist specialist attending the IDT meeting monitored the incidence of psychotropic medication review or discussion that took place if the veteran was on an included agent.
Results
A total of 67 patients on psychotropic medication on the CLC wards since January 2014 were included in the program. Before implementation of the program, 35 patients had documentation of psychotropic medication review. Following implementation, this increased to 54 patients. Hence, 80% of the patients had appropriate documentation.
Before implementation, no patients had documentation regarding evaluation of a GDR in CPRS. After the program implementation, 54 patients had documentation of GDR. Of the 54 patients, 50 had documentation that a GDR could not be completed, and 4 patients had undergone a trial of GDR with appropriate documentation in CPRS (Figure).
The secondary objective evaluated how often a review of patient’s psychotropic medication occurred during IDT meetings. During March 2014, 21 patients with scheduled IDT meetings were prescribed psychotropic medications. For 14 of the 21 patients (67%) appropriateness of psychotropic medications was reviewed with the team and the resident’s families.
Discussion
Documentation is fundamental to improving quality of care, patient outcomes, and reimbursement for the facility. Effective since July 1, 2014, the Joint Commission standards for Nursing Care Center accreditation require the physician and consulting pharmacist to review the patients’ or residents’ medication list. The review should verify the following:
- Clinical indication for the antipsychotic medication;
- Necessity for ongoing use of the antipsychotic medication;
- Consideration of GDR of the antipsychotic medication;
- Consideration of an alternative to antipsychotic medication use.8
When used correctly, the psychotropic medication review and GDR template ensures veterans’ psychotropic medications are continually reviewed. Furthermore, better psychotropic medication management allows veterans to be adequately treated while minimizing the risk for potential AEs.
At CVVAMC, the electronic template helped improve documentation and evaluation of GDR. The CVVAMC success can be attributed partly to physicians who were willing to use the template.
New standards from the Joint Commission provided an additional incentive to use the template. The Joint Commission also required that patients and their family be involved in decisions about placing the resident on antipsychotic medication. The optimal time for this discussion is during IDT meetings. At CVVAMC, discussion of psychotropic medications took place in IDT meetings for 67% of the patients prescribed psychotropic medication.
It is important to note that tools, such as this template, are successful only if they are used by HCPs. The CPRS does not have any clinical reminders to prompt physicians to complete the task. Instead, HCPs had to develop a routine of entering information into the template. Additionally, if the resident assessment coordinator was not at the IDT meeting, discussion of psychotropic medication was not always completed. After completion of this project, psychotropic medication review has become a permanent component of the IDT care plan.
Conclusion
This project demonstrated that template development and use have the potential to improve documentation, patient care, and survey scores. The electronic template could potentially benefit any long-term care facility that uses an electronic recording system. Further research should focus on ease of use and on ensuring that a psychotropic medication review is added to all CLC IDT care plans.
Acknowledgments
The authors acknowledge Brooke Butler, clinical pharmacy specialist and project mentor for her assistance and guidance; and Deborah Hobbs, residency director, both at the Carl Vinson VA Medical Center for her input and leadership, and Freddie Miles, for her work on development of the electronic order set.
One in 5 Americans are taking at least 1 psychotropic medication.1 Elderly dementia patients in extended care facilities are the most likely population to be prescribed psychotropic medication: 87% of these patients are on at least 1 psychotropic medication, 66% on at least 2, 36% on at least 3, and 11% on 4 or more.2 Psychotropic medications alleviate the symptoms of mental illness, such as depression, anxiety, and psychosis. Unfortunately, these medications often have adverse effects (AEs), including, but not limited to, excessive sedation, cardiac abnormalities, and tardive dyskinesia.
In 1987, Congress passed the Nursing Home Reform Act (NHRA) as part of the Omnibus Budget Reconciliation Act. The NHRA mandated that residents must remain free of “physical or chemical restraints imposed for the purpose of discipline or convenience.”3
In 1991, in order to meet the NHRA requirements, the Centers for Medicare and Medicaid Services (CMS) issued a guideline that nursing homes should use antipsychotic drug therapy only to treat a specific condition as diagnosed and documented in the clinical record. In 2006, CMS published guidance on the reduction of psychotropic medication usage. These CMS guidelines are the community-accepted standards for prescribing psychotropic medications, and accrediting bodies expect compliance with the guidelines. The guidelines also recommend that antipsychotics should be prescribed at the lowest possible dose, used for the shortest period, and continually undergo gradual dose reduction (GDR).4 To ensure these standards are met, a review of the use of psychotropic medications should be performed regularly.
The purpose of this project was to improve documentation of GDR and review of psychotropic medication based on CMS guidelines in the community living centers (CLCs) at the Carl Vinson Veterans Affairs Medical Center (CVVAMC) in Dublin, Georgia.
Background
The CVVAMC provides long-term care to veterans living in CLCs in a comfortable, homelike environment with a person-centered, nursing-home level of care. During a patient’s stay, clinical pharmacy services reviews patient medication use regularly. Additionally, an interdisciplinary team (IDT) of physicians, pharmacists, nurses, recreation therapists, dieticians, and chaplains meet to discuss the medical, psychosocial, and spiritual needs of the residents at the time of admission and quarterly thereafter. Family and caregivers are invited to attend as well.
During the meeting, the IDT care plan is updated and reviewed with the veteran. Currently at the CVVAMC, care plans include discussion of pain management; however, the plan does not address psychotropic medication use. To be compliant with CMS guidelines, during IDT meetings discussion of psychotropic medication use would be advantageous, because physicians could receive input and feedback from other health care team members in determining whether psychotropic medication changes are needed.
Methods
All documentation and chart notes are recorded and stored in the Computerized Patient Record System (CPRS). The purpose of an electronic template within CPRS is to provide standardized, guided documentation in an easy-to-use format that can be integrated smoothly into the existing system. Template utilization has been shown to improve documentation of medication reconciliation, health records, and coding in the VA health system.5-7 A review of CPRS showed that no template existed for documenting psychotropic medication initiation and GDR.
Objectives
The authors’ primary objective was to improve patient record documentation in CPRS through implementation of staff education and an electronic template for physicians to document the management of psychotropic medications and patients’ GDRs. The secondary objective was to implement a discussion involving the physician and other members of the team during IDT meetings about patient safety and effectiveness of psychotropic medications.
Template
To improve the suboptimal compliance with the CMS guidelines, an electronic template was created to evaluate GDR attempts and facilitate review of prescribed psychotropic medications. The pharmacy and therapeutics committee approved the template, and the clinical applications coordinator implemented the electronic template. The template was then revised as necessary for ease of use, based on health care provider (HCP) and pharmacist feedback.
The use of the electronic template was phased in over a 1-month period. Complete implementation was achieved in January 2014. An in-service was provided for nurses, HCPs, and directors on the importance of psychotropic medication review and GDRs. A second in-service was conducted for the HCPs to learn how to properly complete the electronic template. Providers were instructed to use the template during IDT meetings when completing monthly chart reviews and changing psychotropic medications.
Additionally, resident assessment coordinators who attend all IDT meetings were requested to remind the team to discuss psychotropic medication use if appropriate in an effort to add a psychotropic medication review to the IDT care plan.
To determine whether the electronic template improved documentation, a retrospective chart review of CPRS was conducted February 2014. The study included veterans in the CLC who were prescribed psychotropic medications since January 2014. The subjects who screened positive for psychotropic medication use were further evaluated by chart review. Before and after implementation, information was collected on the number and percentage of patients who had documentation in their CPRS records of a psychotropic medication review and on whether a GDR evaluation was recorded in CPRS. Additionally, a clinical pharmacist specialist attending the IDT meeting monitored the incidence of psychotropic medication review or discussion that took place if the veteran was on an included agent.
Results
A total of 67 patients on psychotropic medication on the CLC wards since January 2014 were included in the program. Before implementation of the program, 35 patients had documentation of psychotropic medication review. Following implementation, this increased to 54 patients. Hence, 80% of the patients had appropriate documentation.
Before implementation, no patients had documentation regarding evaluation of a GDR in CPRS. After the program implementation, 54 patients had documentation of GDR. Of the 54 patients, 50 had documentation that a GDR could not be completed, and 4 patients had undergone a trial of GDR with appropriate documentation in CPRS (Figure).
The secondary objective evaluated how often a review of patient’s psychotropic medication occurred during IDT meetings. During March 2014, 21 patients with scheduled IDT meetings were prescribed psychotropic medications. For 14 of the 21 patients (67%) appropriateness of psychotropic medications was reviewed with the team and the resident’s families.
Discussion
Documentation is fundamental to improving quality of care, patient outcomes, and reimbursement for the facility. Effective since July 1, 2014, the Joint Commission standards for Nursing Care Center accreditation require the physician and consulting pharmacist to review the patients’ or residents’ medication list. The review should verify the following:
- Clinical indication for the antipsychotic medication;
- Necessity for ongoing use of the antipsychotic medication;
- Consideration of GDR of the antipsychotic medication;
- Consideration of an alternative to antipsychotic medication use.8
When used correctly, the psychotropic medication review and GDR template ensures veterans’ psychotropic medications are continually reviewed. Furthermore, better psychotropic medication management allows veterans to be adequately treated while minimizing the risk for potential AEs.
At CVVAMC, the electronic template helped improve documentation and evaluation of GDR. The CVVAMC success can be attributed partly to physicians who were willing to use the template.
New standards from the Joint Commission provided an additional incentive to use the template. The Joint Commission also required that patients and their family be involved in decisions about placing the resident on antipsychotic medication. The optimal time for this discussion is during IDT meetings. At CVVAMC, discussion of psychotropic medications took place in IDT meetings for 67% of the patients prescribed psychotropic medication.
It is important to note that tools, such as this template, are successful only if they are used by HCPs. The CPRS does not have any clinical reminders to prompt physicians to complete the task. Instead, HCPs had to develop a routine of entering information into the template. Additionally, if the resident assessment coordinator was not at the IDT meeting, discussion of psychotropic medication was not always completed. After completion of this project, psychotropic medication review has become a permanent component of the IDT care plan.
Conclusion
This project demonstrated that template development and use have the potential to improve documentation, patient care, and survey scores. The electronic template could potentially benefit any long-term care facility that uses an electronic recording system. Further research should focus on ease of use and on ensuring that a psychotropic medication review is added to all CLC IDT care plans.
Acknowledgments
The authors acknowledge Brooke Butler, clinical pharmacy specialist and project mentor for her assistance and guidance; and Deborah Hobbs, residency director, both at the Carl Vinson VA Medical Center for her input and leadership, and Freddie Miles, for her work on development of the electronic order set.
1. Medco Health Solutions. America’s state of mind. 2011. http://apps.who.int/medicinedocs/documents/s19032en/s19032en.pdf. Accessed August 31, 2016.
2. Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS. Behavioral symptoms and the administration of psychotropic drugs to aged patients with dementia in nursing homes and in acute geriatric wards. Int Psychogeriatr. 2004;16(1):61-74.
3. Rehnquist J. Psychotropic drug use in nursing homes. Office of Inspector General. https://oig.hhs.gov/oei/reports/oei-02-00-00491.pdf. Published November 2001. Accessed June 24, 2016.
4. Centers for Medicare and Medicaid Services. CMS Manual System. Pub 100-07 State Operations Provider Certification. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs-Items/CMS1201984.html. Published December 15, 2006. Accessed August 29, 2016.
5. Rose EA, Deshikachar AM, Schwartz KL, Severson RK. Use of a template to improve documentation and coding. Fam Med. 2001;33(7):516-521.
6. Fielstein EM, Brown SH, McBrine CS, Clark TK, Hardenbrook SP, Speroff T. The effect of standardized, computer-guided templates on quality of VA disability exams. AMIA Annu Symp Proc. 2006:249-253.
7. Edwards LB, Powers JB. Electronic medication reconciliation: a pilot demonstration on an inpatient geriatrics unit. Fed Pract. 2007;24(9):49-50, 53, 62.
8. Approved: memory care requirements for nursing center accreditation. http://www.jointcommission.org/ASSETS/1/18/JCP0114_MEMORY_CARE_NCC.PDF. Published January 2014. Accessed August 24, 2016.
1. Medco Health Solutions. America’s state of mind. 2011. http://apps.who.int/medicinedocs/documents/s19032en/s19032en.pdf. Accessed August 31, 2016.
2. Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS. Behavioral symptoms and the administration of psychotropic drugs to aged patients with dementia in nursing homes and in acute geriatric wards. Int Psychogeriatr. 2004;16(1):61-74.
3. Rehnquist J. Psychotropic drug use in nursing homes. Office of Inspector General. https://oig.hhs.gov/oei/reports/oei-02-00-00491.pdf. Published November 2001. Accessed June 24, 2016.
4. Centers for Medicare and Medicaid Services. CMS Manual System. Pub 100-07 State Operations Provider Certification. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs-Items/CMS1201984.html. Published December 15, 2006. Accessed August 29, 2016.
5. Rose EA, Deshikachar AM, Schwartz KL, Severson RK. Use of a template to improve documentation and coding. Fam Med. 2001;33(7):516-521.
6. Fielstein EM, Brown SH, McBrine CS, Clark TK, Hardenbrook SP, Speroff T. The effect of standardized, computer-guided templates on quality of VA disability exams. AMIA Annu Symp Proc. 2006:249-253.
7. Edwards LB, Powers JB. Electronic medication reconciliation: a pilot demonstration on an inpatient geriatrics unit. Fed Pract. 2007;24(9):49-50, 53, 62.
8. Approved: memory care requirements for nursing center accreditation. http://www.jointcommission.org/ASSETS/1/18/JCP0114_MEMORY_CARE_NCC.PDF. Published January 2014. Accessed August 24, 2016.