User login
Total Brain Diagnostics: Advancing Precision Brain and Mental Health at the Department of Veterans Affairs
Total Brain Diagnostics: Advancing Precision Brain and Mental Health at the Department of Veterans Affairs
In leveraging existing, readily available evidence-based health care information (eg, systematic reviews, clinical practice guidelines), clinicians have historically made recommendations based on treatment responses of the average patient.1 Recently, this approach has been expanded into data-driven, evidence-based precision medical care for individuals across a wide range of disciplines and care settings. These precision medicine approaches use information related to an individual’s genes, environment, and lifestyle to tailor recommendations regarding prevention, diagnosis, and treatment.
Applying precision medicine approaches to the unique exposures and experiences of service members and veterans—particularly those who served in combat environments—through the incorporation of biopsychosocial factors into medical decision-making may be even more pertinent. This sentiment is reflected in Section 305 of the Commander John Scott Hannon Veterans Mental Health Care Improvement Act of 2019, which outlines the Precision Medicine Initiative of the US Department of Veterans Affairs (VA) to identify and validate brain and mental health biomarkers.2 Despite widespread consensus regarding the promise of precision medicine, large, rich datasets with elements pertaining to common military exposures such as traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD) are limited.
Existing datasets, most of which are relatively small or focus on specific cohorts (eg, older veterans, transitioning veterans), continue to create barriers to advancing precision medicine. For example, in classically designed clinical trials, analyses are generally conducted in a manner that may obfuscate efficacy among subcohorts of individuals, thereby underscoring the need to explore alternative strategies to unify existing datasets capable of revealing such heterogeneity.3 The evidence base for precision medical care is limited, drawing from published trials with relatively small sample sizes and even larger cohort studies have limited biomarker data. Additionally, these models are often exploratory during development, and to avoid statistical overfitting of an exploratory model, validation in similar datasets is needed—an added burden when data sources are small or underpowered to begin with.
A promising approach is to combine and harmonize the largest, most deeply characterized data sources from similar samples. Although combining such datasets may appear to require minimal time and effort, harmonizing similar variables in an evidence-based and replicable manner requires time and expertise, even when participant characteristics and outcomes are similar.4-7
Challenges related to harmonization are related to the wide range of strategies (eg, self-report questionnaires, clinical interviews, electronic health record review) used to measure common brain and mental health constructs, such as depression. Even when similar methods (eg, self-report measures) are implemented, challenges persist. For example, if a study used a depression measure that focused primarily on cognitive symptoms (eg, pessimism, self-dislike, suicidal ideation) and another study used a depression measure composed of items more heavily weighted towards somatic symptoms (eg, insomnia, loss of appetite, weight loss, decreased libido), combining their data could be challenging, particularly if researchers, clinicians, or administrators are interested in more than dichotomous outcomes (eg, depression vs no depression).8,9
To address this knowledge gap and harmonize multimodal data from varied sources, well-planned and reproducible curation is needed. Longitudinal cohort studies of service members and veterans with military combat and training exposure histories provide researchers and other stakeholders access to extant biopsychosocial data shown to affect risk for adverse health outcomes; however, efforts to facilitate individually tailored treatment or other precision medicine approaches would benefit from the synthesis of such datasets.10
Members of the VA Total Brain Diagnostics (TBD) team are engaged in harmonizing variables from the Long-Term Impact of Military-Relevant Brain Injury Consortium–Chronic Effects of Neurotrauma Consortium (LIMBIC-CENC)11 and the Translational Research Center for TBI and Stress Disorders (TRACTS).12-21 While there is overlap across LIMBIC-CENC and TRACTS with respect to data domains, considerable data harmonization is needed to allow for future valid and meaningful analyses, particularly those involving multivariable predictors.
Data Sources
Both data sources for the TBD harmonization project, LIMBIC-CENC and TRACTS, include extensive, longitudinal data collected from relatively large cohorts of veterans and service members with combat exposure. Both studies collect detailed data related to potential brain injury history and include participants with and without a history of TBI. Similarly, both include extensive collection of fluid biomarkers and imaging data, as well as measures of biopsychosocial functioning.
Data collection sites for LIMBIC-CENC include 16 recruitment sites, 9 at VA medical centers (Richmond, Houston, Tampa, San Antonio, Portland, Minneapolis, Boston, Salisbury, San Diego) and 7 at military treatment sites (Alexandria, San Diego, Tampa, Tacoma, Columbia, Coronado, Hinesville), in addition to 11 assessment sites (Richmond, Houston, Tampa, San Antonio, Portland, Minneapolis, Boston, Salisbury, San Diego, Alexandria, Augusta). Data for TRACTS are collected at sites in Boston and Houston.
LIMBIC-CENC is a 12-year, 17-site cohort of service members and veteran participants with combat exposure who are well characterized at baseline and undergo annual reassessments. As of December 2025, > 3100 participants have been recruited, and nearly 90% remain in follow-up. Data collection includes > 6200 annual follow-up evaluations and > 1550 5-year re-evaluations, with 400 enrolled participants followed up annually.
TRACTS is a 16-year, 2-site cohort of veterans with combat exposure who complete comprehensive assessments at enrollment, undergo annual reassessments, and complete comprehensive reassessment every 5 years thereafter. As of December 2025, > 1075 participants have completed baseline (Time 1) assessments, > 600 have completed the 2-year re-evaluation (Time 2), > 175 have completed the 5-year re-evaluation (Time 3), and > 35 have completed 10-year evaluations (Time 4), with about 50 new participants added and 100 enrolled participants followed up annually. More data on participant characteristics are available for both LIMBIC-CENC and TRACTS in previous publications.11,22These 2 ongoing, prospective, longitudinal cohorts of service members and veterans offer access to a wide range of potential risk factors that can affect response to care and outcomes, including demographics (eg, age, sex), injury characteristics (eg, pre-exposure factors, exposure factors), biomarkers (eg, serum, saliva, brain imaging, evoked potentials), and functional measures (eg, computerized posturography, computerized eye tracking, sensory testing, clinical examination, neuropsychological assessments, symptom questionnaires).
Harmonization Strategy
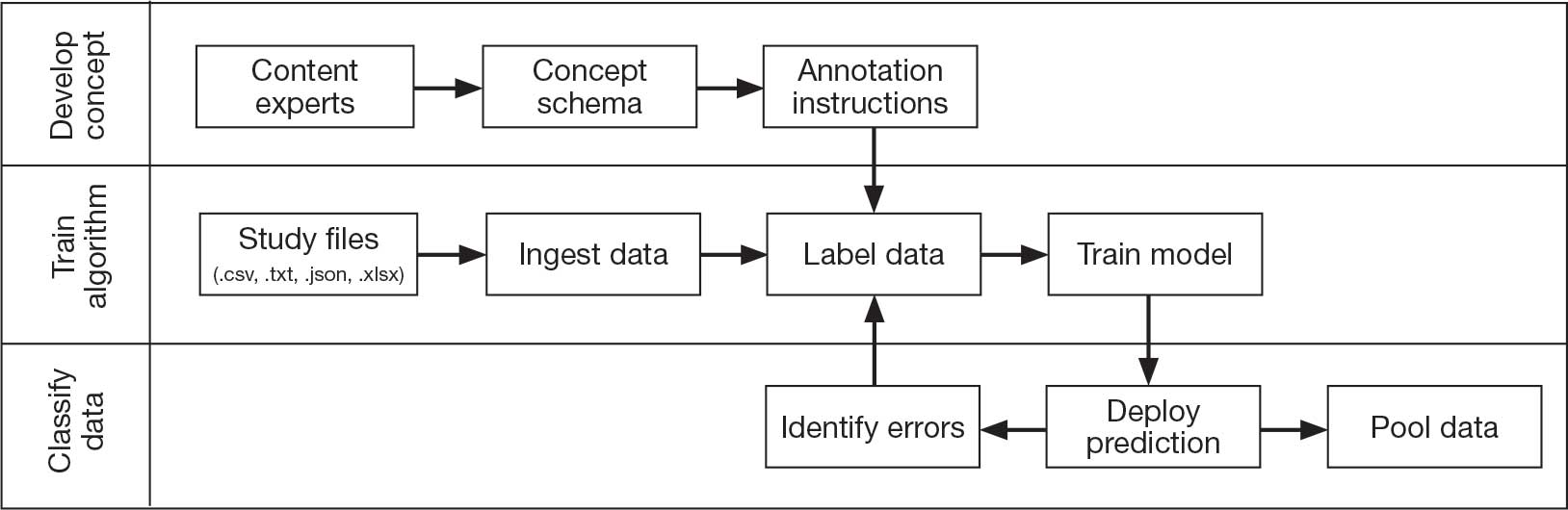
Pooling and harmonizing data from large studies evaluating similar participant cohorts and conditions involves numerous steps to appropriately handle a variety of measurements and disparate variable names. The TBD team adapted a model data harmonization system developed by O’Neil et al through initial work harmonizing the Federal Interagency Traumatic Brain Injury Research Informatics System (FITBIR).4-7 This process was expanded and generalized by the research team to combine data from LIMBIC-CENC and TRACTS to create a single pooled dataset for analysis (Figure).
Injury Research database.
This approach was selected because it accommodates heterogeneous study designs (eg, cross-sectional, longitudinal, case-control), data collection methods (eg, clinical assessment, self-reported, objective blood, and imaging biomarkers), and various assessments of the same construct (ie, different measures of brain injury). While exact matches for data collection methods and measures may be easily harmonized, the timing of assessment, number of assessments, assessment tool version, and other factors must be considered. The goal was to harmonize data from LIMBIC-CENC and TRACTS to allow additional data sources to be harmonized and incorporated in the future.
Original data files from each study were reshaped to represent participant-level observations with 1 unique measurement per row. The measurement represents what information was collected and the value recorded represents the unique observation. These data are linked to metadata from the original study, which includes the study’s definition of each measurement, how it was collected, and any available information regarding when it was collected in reference to study enrollment or injury. Additional information on the file source, row, and column position of each data point was added to enable recreation of the original data as needed.
The resulting dataset was used to harmonize measurements from LIMBIC-CENC and TRACTS into a priori-defined schemas for brain- and mental health-relevant concepts, including TBI severity, PTSD, substance use, depression, suicidal ideation, and functioning (including cognitive, physical, and social functioning). This process was facilitated using natural language processing (NLP). Each study uniquely defines all measurements and provides written definitions with the data. Measurement definitions serve as records describing what was collected, how it was collected, and how the study may have uniquely defined information for its purposes. For example, definitions of exposure to brain injury and severity of brain injury may differ between studies, and the study-provided definition defines these differences.
Definitions were converted into numeric vectors through sentence embedding, a process that preserves the semantic meaning of the definition.23 Cosine similarity was used as the primary metric to compare the semantic textual similarity between pairs of measurement definitions. Cosine similarity ranges from 0 to 1, where 0 indicates no meaningful similarity and 1 indicates they have identical meanings.24 This approach leverages the relationship between the definitions of each measurement provided by a study and enables quick comparison of all pairwise combinations of measurement definitions between studies.
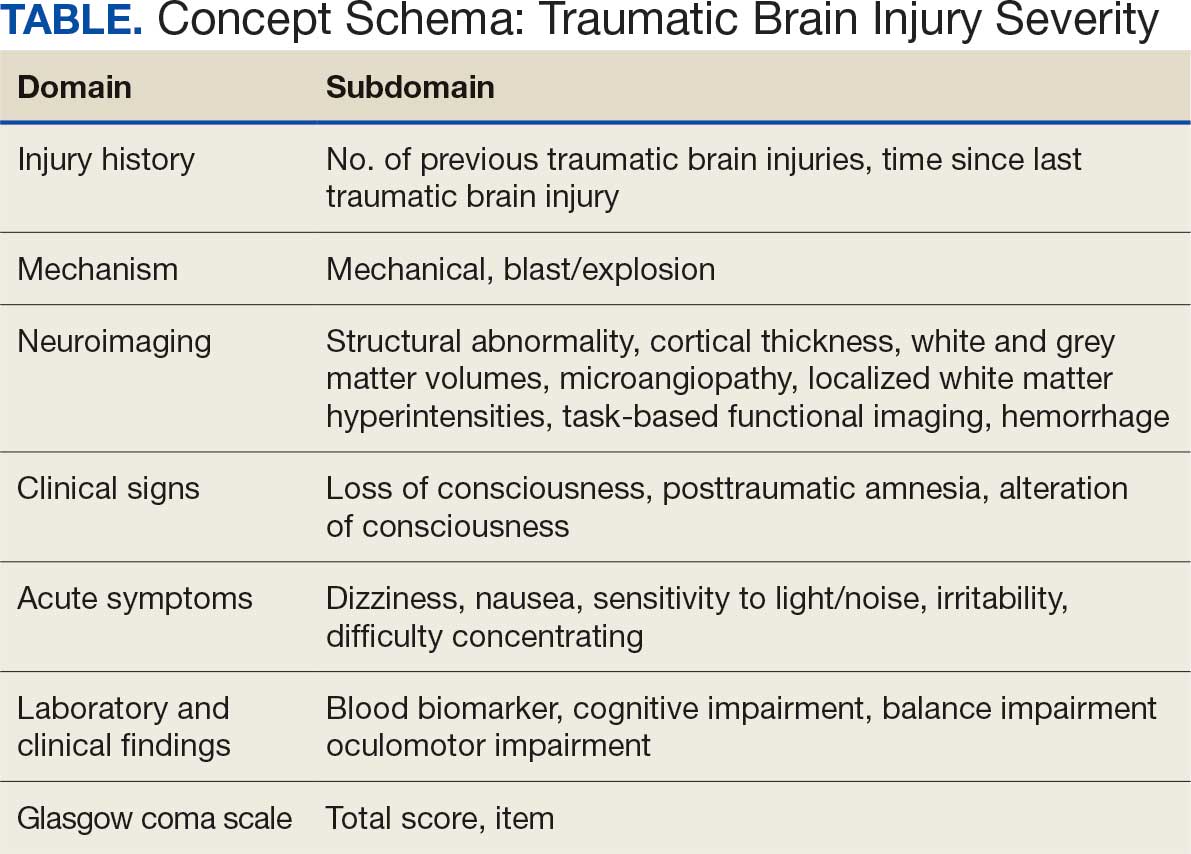
Subsets of similar measurements across studies were organized into a priori-defined schema. Clinical experts then reviewed each schema and further refined them into domains, (eg, mechanism of injury, clinical signs, acute symptoms) and subdomains (children), such as loss of consciousness, amnesia, and alteration of consciousness. This approach allows efficient handling of 2 specific cases that commonly occur when pooling and harmonizing datasets: (1) identifying the same measurement with differing names; and (2) identifying different measurements with definitions that each relate to the same domain.
The Table provides a general example of the schema for TBI severity. This was an iterative process in which clinical experts reviewed study-defined measurement definitions to develop general harmonized domains, and NLP techniques facilitated and accelerated identification and organization of measurements within these domains.
Expected Impact
Harmonization combining LIMBIC-CENC and TRACTS datasets is ongoing. Preliminary descriptive analyses of baseline cohort data indicate that harmonization across data sources is appropriate, given the lack of significant heterogeneity across sites and studies for most domains. Work by members of the TBD team is expected to lay the foundation for the use of existing and ongoing prospective, longitudinal datasets (eg, LIMBIC-CENC, TRACTS) and linked large datasets (eg, VA Informatics and Computing Infrastructure including electronic health records, VA Million Veteran Program, DaVINCI [US Department of Defense and VA Infrastructure for Clinical Intelligence]) to generate generalizable, clinically relevant information to advance precision brain and mental health care among service members and veterans.
By enhancing existing practice, this synthesized dataset has the potential to inform tailored and personalized medicine approaches designed to meet the needs of veterans and service members. These data will serve as the starting point for multivariable models examining the intersection of physiologic, behavioral, and environmental factors. The goal of this data harmonization effort is to better elucidate how clinicians and researchers can select optimal approaches for veterans and service members with TBI histories by accounting for a comprehensive set of physiologic, behavioral, and environmental factors in an individually tailored manner. These data may further extend existing clinical practice guideline approaches, inform shared decision-making, and enhance functional outcomes beyond those currently available.
Conclusions
Individuals who have served in the military have unique biopsychosocial exposures that are associated with brain and mental health disorders. To address these needs, the nationwide TBD team has initiated the creation of a unified, longitudinal dataset that includes harmonized measures from existing LIMBIC-CENC and TRACTS protocols. Initial data harmonization efforts are required to facilitate precision prognostics, diagnostics, and tailored interventions, with the goal of improving veterans’ brain and mental health and psychosocial functioning and enabling tailored and evidence-informed, individualized clinical care.
- The Promise of Precision Medicine. National Institutes of Health (NIH). Updated January 21, 2025. Accessed January 5, 2026. https://www.nih.gov/about-nih/nih-turning-discovery-into-health/promise-precision-medicine.
- Commander John Scott Hannon Veterans Mental Health Care Improvement Act of 2019, S 785, 116th Cong (2019-2020) Accessed January 5, 2026. https://www.congress.gov/bill/116th-congress/senate-bill/785
- Cheng C, Messerschmidt L, Bravo I, et al. A general primer for data harmonization. Sci Data. 2024;11:152. doi:10.1038/s41597-024-02956-3
- Neil M, Cameron D, Clauss K, et al. A proof-of-concept study demonstrating how FITBIR datasets can be harmonized to examine posttraumatic stress disorder-traumatic brain injury associations. J Behav Data Sci. 2024;4:45-62. doi:10.35566/jbds/oneil
- O’Neil ME, Cameron D, Krushnic D, et al. Using harmonized FITBIR datasets to examine associations between TBI history and cognitive functioning. Appl Neuropsychol Adult. doi:10.1080/23279095.2024.2401974
- O’Neil ME, Krushnic D, Clauss K, et al. Harmonizing federal interagency traumatic brain injury research data to examine depression and suicide-related outcomes. Rehabil Psychol. 2024;69:159-170. doi:10.1037/rep0000547
- O’Neil ME, Krushnic D, Walker WC, et al. Increased risk for clinically significant sleep disturbances in mild traumatic brain injury: an approach to leveraging the federal interagency traumatic brain injury research database. Brain Sci. 2024;14:921. doi:10.3390/brainsci14090921
- Uher R, Perlis RH, Placentino A, et al. Self-report and clinician-rated measures of depression severity: can one replace the other? Depress Anxiety. 2012;29:1043-1049. doi:10.1002/da.21993
- Hung CI, Weng LJ, Su YJ, et al. Depression and somatic symptoms scale: a new scale with both depression and somatic symptoms emphasized. Psychiatry Clin Neurosci. 2006;60:700-708. doi:10.1111/j.1440-1819.2006.01585.x
- Stewart IJ, Howard JT, Amuan ME, et al. Traumatic brain injury is associated with the subsequent risk of atrial fibrillation or atrial flutter. Heart Rhythm. 2025;22:661-667. doi:10.1016/j.hrthm.2024.09.019
- Cifu DX. Clinical research findings from the long-term impact of military-relevant brain injury consortium-chronic effects of neurotrauma consortium (LIMBIC-CENC) 2013-2021. Brain Inj. 2022;36:587-597.doi:10.1080/02699052.2022.2033843
- Fonda JR, Fredman L, Brogly SB, et al. Traumatic brain injury and attempted suicide among veterans of the wars in Iraq and Afghanistan. Am J Epidemiol. 2017;186:220-226. doi:10.1093/aje/kwx044
- Fortier CB, Amick MM, Kenna A, et al. Correspondence of the Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) clinical interview and the VA TBI screen. J Head Trauma Rehabil. 2015;30:E1-7. doi:10.1097/htr.0000000000000008
- Grande LJ, Robinson ME, Radigan LJ, et al. Verbal memory deficits in OEF/OIF/OND veterans exposed to blasts at close range. J Int Neuropsychol Soc. 2018;24:466-475. doi:10.1017/S1355617717001242
- Hayes JP, Logue MW, Sadeh N, et al. Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain. 2017;140:813-825. doi:10.1093/brain/aww344
- Lippa SM, Fonda JR, Fortier CB, et al. Deployment-related psychiatric and behavioral conditions and their association with functional disability in OEF/OIF/OND veterans. J Trauma Stress. 2015;28:25-33. doi:10.1002/jts.21979
- McGlinchey RE, Milberg WP, Fonda JR, et al. A methodology for assessing deployment trauma and its consequences in OEF/OIF/OND veterans: the TRACTS longitudinal prospective cohort study. Int J Methods Psychiatr Res. 2017;26:e1556. doi:10.1002/mpr.1556
- Radigan LJ, McGlinchey RE, Milberg WP, et al. Correspondence of the Boston Assessment of Traumatic Brain Injury-Lifetime and the VA Comprehensive TBI Evaluation. J Head Trauma Rehabil. 2018;33:E51-E55. doi:10.1097/htr.0000000000000361
- Sydnor VJ, Bouix S, Pasternak O, et al. Mild traumatic brain injury impacts associations between limbic system microstructure and post-traumatic stress disorder symptomatology. Neuroimage Clin. 2020;26:102190. doi:10.1016/j.nicl.2020.102190
- Van Etten EJ, Knight AR, Colaizzi TA, et al. Peritraumatic context and long-term outcomes of concussion. JAMA Netw Open. 2025;8:e2455622. doi:10.1001/jamanetworkopen.2024.55622
- Andrews RJ, Fonda JR, Levin LK, et al. Comprehensive analysis of the predictors of neurobehavioral symptom reporting in veterans. Neurology. 2018;91:e732-e745. doi:10.1212/wnl.0000000000006034
- McGlinchey RE, Milberg WP, Fonda JR, Fortier CB. A methodology for assessing deployment trauma and its consequences in OEF/OIF/OND veterans: the TRACTS longitudional prospective cohort study. Int J Methods Psychiatr Res. 2017;26:e1556. doi:10.1002/mpr.1556
- Reimers N, Gurevych I. Sentence-BERT: Sentence embeddings using Siamese BERT-Networks. 2019. Conference on Empirical Methods in Natural Language Processing.
- Singhal A. Modern information retrieval: a brief overview. IEEE Data Eng Bull. 2001;24:34-43.
In leveraging existing, readily available evidence-based health care information (eg, systematic reviews, clinical practice guidelines), clinicians have historically made recommendations based on treatment responses of the average patient.1 Recently, this approach has been expanded into data-driven, evidence-based precision medical care for individuals across a wide range of disciplines and care settings. These precision medicine approaches use information related to an individual’s genes, environment, and lifestyle to tailor recommendations regarding prevention, diagnosis, and treatment.
Applying precision medicine approaches to the unique exposures and experiences of service members and veterans—particularly those who served in combat environments—through the incorporation of biopsychosocial factors into medical decision-making may be even more pertinent. This sentiment is reflected in Section 305 of the Commander John Scott Hannon Veterans Mental Health Care Improvement Act of 2019, which outlines the Precision Medicine Initiative of the US Department of Veterans Affairs (VA) to identify and validate brain and mental health biomarkers.2 Despite widespread consensus regarding the promise of precision medicine, large, rich datasets with elements pertaining to common military exposures such as traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD) are limited.
Existing datasets, most of which are relatively small or focus on specific cohorts (eg, older veterans, transitioning veterans), continue to create barriers to advancing precision medicine. For example, in classically designed clinical trials, analyses are generally conducted in a manner that may obfuscate efficacy among subcohorts of individuals, thereby underscoring the need to explore alternative strategies to unify existing datasets capable of revealing such heterogeneity.3 The evidence base for precision medical care is limited, drawing from published trials with relatively small sample sizes and even larger cohort studies have limited biomarker data. Additionally, these models are often exploratory during development, and to avoid statistical overfitting of an exploratory model, validation in similar datasets is needed—an added burden when data sources are small or underpowered to begin with.
A promising approach is to combine and harmonize the largest, most deeply characterized data sources from similar samples. Although combining such datasets may appear to require minimal time and effort, harmonizing similar variables in an evidence-based and replicable manner requires time and expertise, even when participant characteristics and outcomes are similar.4-7
Challenges related to harmonization are related to the wide range of strategies (eg, self-report questionnaires, clinical interviews, electronic health record review) used to measure common brain and mental health constructs, such as depression. Even when similar methods (eg, self-report measures) are implemented, challenges persist. For example, if a study used a depression measure that focused primarily on cognitive symptoms (eg, pessimism, self-dislike, suicidal ideation) and another study used a depression measure composed of items more heavily weighted towards somatic symptoms (eg, insomnia, loss of appetite, weight loss, decreased libido), combining their data could be challenging, particularly if researchers, clinicians, or administrators are interested in more than dichotomous outcomes (eg, depression vs no depression).8,9
To address this knowledge gap and harmonize multimodal data from varied sources, well-planned and reproducible curation is needed. Longitudinal cohort studies of service members and veterans with military combat and training exposure histories provide researchers and other stakeholders access to extant biopsychosocial data shown to affect risk for adverse health outcomes; however, efforts to facilitate individually tailored treatment or other precision medicine approaches would benefit from the synthesis of such datasets.10
Members of the VA Total Brain Diagnostics (TBD) team are engaged in harmonizing variables from the Long-Term Impact of Military-Relevant Brain Injury Consortium–Chronic Effects of Neurotrauma Consortium (LIMBIC-CENC)11 and the Translational Research Center for TBI and Stress Disorders (TRACTS).12-21 While there is overlap across LIMBIC-CENC and TRACTS with respect to data domains, considerable data harmonization is needed to allow for future valid and meaningful analyses, particularly those involving multivariable predictors.
Data Sources
Both data sources for the TBD harmonization project, LIMBIC-CENC and TRACTS, include extensive, longitudinal data collected from relatively large cohorts of veterans and service members with combat exposure. Both studies collect detailed data related to potential brain injury history and include participants with and without a history of TBI. Similarly, both include extensive collection of fluid biomarkers and imaging data, as well as measures of biopsychosocial functioning.
Data collection sites for LIMBIC-CENC include 16 recruitment sites, 9 at VA medical centers (Richmond, Houston, Tampa, San Antonio, Portland, Minneapolis, Boston, Salisbury, San Diego) and 7 at military treatment sites (Alexandria, San Diego, Tampa, Tacoma, Columbia, Coronado, Hinesville), in addition to 11 assessment sites (Richmond, Houston, Tampa, San Antonio, Portland, Minneapolis, Boston, Salisbury, San Diego, Alexandria, Augusta). Data for TRACTS are collected at sites in Boston and Houston.
LIMBIC-CENC is a 12-year, 17-site cohort of service members and veteran participants with combat exposure who are well characterized at baseline and undergo annual reassessments. As of December 2025, > 3100 participants have been recruited, and nearly 90% remain in follow-up. Data collection includes > 6200 annual follow-up evaluations and > 1550 5-year re-evaluations, with 400 enrolled participants followed up annually.
TRACTS is a 16-year, 2-site cohort of veterans with combat exposure who complete comprehensive assessments at enrollment, undergo annual reassessments, and complete comprehensive reassessment every 5 years thereafter. As of December 2025, > 1075 participants have completed baseline (Time 1) assessments, > 600 have completed the 2-year re-evaluation (Time 2), > 175 have completed the 5-year re-evaluation (Time 3), and > 35 have completed 10-year evaluations (Time 4), with about 50 new participants added and 100 enrolled participants followed up annually. More data on participant characteristics are available for both LIMBIC-CENC and TRACTS in previous publications.11,22These 2 ongoing, prospective, longitudinal cohorts of service members and veterans offer access to a wide range of potential risk factors that can affect response to care and outcomes, including demographics (eg, age, sex), injury characteristics (eg, pre-exposure factors, exposure factors), biomarkers (eg, serum, saliva, brain imaging, evoked potentials), and functional measures (eg, computerized posturography, computerized eye tracking, sensory testing, clinical examination, neuropsychological assessments, symptom questionnaires).
Harmonization Strategy
Pooling and harmonizing data from large studies evaluating similar participant cohorts and conditions involves numerous steps to appropriately handle a variety of measurements and disparate variable names. The TBD team adapted a model data harmonization system developed by O’Neil et al through initial work harmonizing the Federal Interagency Traumatic Brain Injury Research Informatics System (FITBIR).4-7 This process was expanded and generalized by the research team to combine data from LIMBIC-CENC and TRACTS to create a single pooled dataset for analysis (Figure).

Injury Research database.
This approach was selected because it accommodates heterogeneous study designs (eg, cross-sectional, longitudinal, case-control), data collection methods (eg, clinical assessment, self-reported, objective blood, and imaging biomarkers), and various assessments of the same construct (ie, different measures of brain injury). While exact matches for data collection methods and measures may be easily harmonized, the timing of assessment, number of assessments, assessment tool version, and other factors must be considered. The goal was to harmonize data from LIMBIC-CENC and TRACTS to allow additional data sources to be harmonized and incorporated in the future.
Original data files from each study were reshaped to represent participant-level observations with 1 unique measurement per row. The measurement represents what information was collected and the value recorded represents the unique observation. These data are linked to metadata from the original study, which includes the study’s definition of each measurement, how it was collected, and any available information regarding when it was collected in reference to study enrollment or injury. Additional information on the file source, row, and column position of each data point was added to enable recreation of the original data as needed.
The resulting dataset was used to harmonize measurements from LIMBIC-CENC and TRACTS into a priori-defined schemas for brain- and mental health-relevant concepts, including TBI severity, PTSD, substance use, depression, suicidal ideation, and functioning (including cognitive, physical, and social functioning). This process was facilitated using natural language processing (NLP). Each study uniquely defines all measurements and provides written definitions with the data. Measurement definitions serve as records describing what was collected, how it was collected, and how the study may have uniquely defined information for its purposes. For example, definitions of exposure to brain injury and severity of brain injury may differ between studies, and the study-provided definition defines these differences.
Definitions were converted into numeric vectors through sentence embedding, a process that preserves the semantic meaning of the definition.23 Cosine similarity was used as the primary metric to compare the semantic textual similarity between pairs of measurement definitions. Cosine similarity ranges from 0 to 1, where 0 indicates no meaningful similarity and 1 indicates they have identical meanings.24 This approach leverages the relationship between the definitions of each measurement provided by a study and enables quick comparison of all pairwise combinations of measurement definitions between studies.
Subsets of similar measurements across studies were organized into a priori-defined schema. Clinical experts then reviewed each schema and further refined them into domains, (eg, mechanism of injury, clinical signs, acute symptoms) and subdomains (children), such as loss of consciousness, amnesia, and alteration of consciousness. This approach allows efficient handling of 2 specific cases that commonly occur when pooling and harmonizing datasets: (1) identifying the same measurement with differing names; and (2) identifying different measurements with definitions that each relate to the same domain.
The Table provides a general example of the schema for TBI severity. This was an iterative process in which clinical experts reviewed study-defined measurement definitions to develop general harmonized domains, and NLP techniques facilitated and accelerated identification and organization of measurements within these domains.

Expected Impact
Harmonization combining LIMBIC-CENC and TRACTS datasets is ongoing. Preliminary descriptive analyses of baseline cohort data indicate that harmonization across data sources is appropriate, given the lack of significant heterogeneity across sites and studies for most domains. Work by members of the TBD team is expected to lay the foundation for the use of existing and ongoing prospective, longitudinal datasets (eg, LIMBIC-CENC, TRACTS) and linked large datasets (eg, VA Informatics and Computing Infrastructure including electronic health records, VA Million Veteran Program, DaVINCI [US Department of Defense and VA Infrastructure for Clinical Intelligence]) to generate generalizable, clinically relevant information to advance precision brain and mental health care among service members and veterans.
By enhancing existing practice, this synthesized dataset has the potential to inform tailored and personalized medicine approaches designed to meet the needs of veterans and service members. These data will serve as the starting point for multivariable models examining the intersection of physiologic, behavioral, and environmental factors. The goal of this data harmonization effort is to better elucidate how clinicians and researchers can select optimal approaches for veterans and service members with TBI histories by accounting for a comprehensive set of physiologic, behavioral, and environmental factors in an individually tailored manner. These data may further extend existing clinical practice guideline approaches, inform shared decision-making, and enhance functional outcomes beyond those currently available.
Conclusions
Individuals who have served in the military have unique biopsychosocial exposures that are associated with brain and mental health disorders. To address these needs, the nationwide TBD team has initiated the creation of a unified, longitudinal dataset that includes harmonized measures from existing LIMBIC-CENC and TRACTS protocols. Initial data harmonization efforts are required to facilitate precision prognostics, diagnostics, and tailored interventions, with the goal of improving veterans’ brain and mental health and psychosocial functioning and enabling tailored and evidence-informed, individualized clinical care.
In leveraging existing, readily available evidence-based health care information (eg, systematic reviews, clinical practice guidelines), clinicians have historically made recommendations based on treatment responses of the average patient.1 Recently, this approach has been expanded into data-driven, evidence-based precision medical care for individuals across a wide range of disciplines and care settings. These precision medicine approaches use information related to an individual’s genes, environment, and lifestyle to tailor recommendations regarding prevention, diagnosis, and treatment.
Applying precision medicine approaches to the unique exposures and experiences of service members and veterans—particularly those who served in combat environments—through the incorporation of biopsychosocial factors into medical decision-making may be even more pertinent. This sentiment is reflected in Section 305 of the Commander John Scott Hannon Veterans Mental Health Care Improvement Act of 2019, which outlines the Precision Medicine Initiative of the US Department of Veterans Affairs (VA) to identify and validate brain and mental health biomarkers.2 Despite widespread consensus regarding the promise of precision medicine, large, rich datasets with elements pertaining to common military exposures such as traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD) are limited.
Existing datasets, most of which are relatively small or focus on specific cohorts (eg, older veterans, transitioning veterans), continue to create barriers to advancing precision medicine. For example, in classically designed clinical trials, analyses are generally conducted in a manner that may obfuscate efficacy among subcohorts of individuals, thereby underscoring the need to explore alternative strategies to unify existing datasets capable of revealing such heterogeneity.3 The evidence base for precision medical care is limited, drawing from published trials with relatively small sample sizes and even larger cohort studies have limited biomarker data. Additionally, these models are often exploratory during development, and to avoid statistical overfitting of an exploratory model, validation in similar datasets is needed—an added burden when data sources are small or underpowered to begin with.
A promising approach is to combine and harmonize the largest, most deeply characterized data sources from similar samples. Although combining such datasets may appear to require minimal time and effort, harmonizing similar variables in an evidence-based and replicable manner requires time and expertise, even when participant characteristics and outcomes are similar.4-7
Challenges related to harmonization are related to the wide range of strategies (eg, self-report questionnaires, clinical interviews, electronic health record review) used to measure common brain and mental health constructs, such as depression. Even when similar methods (eg, self-report measures) are implemented, challenges persist. For example, if a study used a depression measure that focused primarily on cognitive symptoms (eg, pessimism, self-dislike, suicidal ideation) and another study used a depression measure composed of items more heavily weighted towards somatic symptoms (eg, insomnia, loss of appetite, weight loss, decreased libido), combining their data could be challenging, particularly if researchers, clinicians, or administrators are interested in more than dichotomous outcomes (eg, depression vs no depression).8,9
To address this knowledge gap and harmonize multimodal data from varied sources, well-planned and reproducible curation is needed. Longitudinal cohort studies of service members and veterans with military combat and training exposure histories provide researchers and other stakeholders access to extant biopsychosocial data shown to affect risk for adverse health outcomes; however, efforts to facilitate individually tailored treatment or other precision medicine approaches would benefit from the synthesis of such datasets.10
Members of the VA Total Brain Diagnostics (TBD) team are engaged in harmonizing variables from the Long-Term Impact of Military-Relevant Brain Injury Consortium–Chronic Effects of Neurotrauma Consortium (LIMBIC-CENC)11 and the Translational Research Center for TBI and Stress Disorders (TRACTS).12-21 While there is overlap across LIMBIC-CENC and TRACTS with respect to data domains, considerable data harmonization is needed to allow for future valid and meaningful analyses, particularly those involving multivariable predictors.
Data Sources
Both data sources for the TBD harmonization project, LIMBIC-CENC and TRACTS, include extensive, longitudinal data collected from relatively large cohorts of veterans and service members with combat exposure. Both studies collect detailed data related to potential brain injury history and include participants with and without a history of TBI. Similarly, both include extensive collection of fluid biomarkers and imaging data, as well as measures of biopsychosocial functioning.
Data collection sites for LIMBIC-CENC include 16 recruitment sites, 9 at VA medical centers (Richmond, Houston, Tampa, San Antonio, Portland, Minneapolis, Boston, Salisbury, San Diego) and 7 at military treatment sites (Alexandria, San Diego, Tampa, Tacoma, Columbia, Coronado, Hinesville), in addition to 11 assessment sites (Richmond, Houston, Tampa, San Antonio, Portland, Minneapolis, Boston, Salisbury, San Diego, Alexandria, Augusta). Data for TRACTS are collected at sites in Boston and Houston.
LIMBIC-CENC is a 12-year, 17-site cohort of service members and veteran participants with combat exposure who are well characterized at baseline and undergo annual reassessments. As of December 2025, > 3100 participants have been recruited, and nearly 90% remain in follow-up. Data collection includes > 6200 annual follow-up evaluations and > 1550 5-year re-evaluations, with 400 enrolled participants followed up annually.
TRACTS is a 16-year, 2-site cohort of veterans with combat exposure who complete comprehensive assessments at enrollment, undergo annual reassessments, and complete comprehensive reassessment every 5 years thereafter. As of December 2025, > 1075 participants have completed baseline (Time 1) assessments, > 600 have completed the 2-year re-evaluation (Time 2), > 175 have completed the 5-year re-evaluation (Time 3), and > 35 have completed 10-year evaluations (Time 4), with about 50 new participants added and 100 enrolled participants followed up annually. More data on participant characteristics are available for both LIMBIC-CENC and TRACTS in previous publications.11,22These 2 ongoing, prospective, longitudinal cohorts of service members and veterans offer access to a wide range of potential risk factors that can affect response to care and outcomes, including demographics (eg, age, sex), injury characteristics (eg, pre-exposure factors, exposure factors), biomarkers (eg, serum, saliva, brain imaging, evoked potentials), and functional measures (eg, computerized posturography, computerized eye tracking, sensory testing, clinical examination, neuropsychological assessments, symptom questionnaires).
Harmonization Strategy
Pooling and harmonizing data from large studies evaluating similar participant cohorts and conditions involves numerous steps to appropriately handle a variety of measurements and disparate variable names. The TBD team adapted a model data harmonization system developed by O’Neil et al through initial work harmonizing the Federal Interagency Traumatic Brain Injury Research Informatics System (FITBIR).4-7 This process was expanded and generalized by the research team to combine data from LIMBIC-CENC and TRACTS to create a single pooled dataset for analysis (Figure).

Injury Research database.
This approach was selected because it accommodates heterogeneous study designs (eg, cross-sectional, longitudinal, case-control), data collection methods (eg, clinical assessment, self-reported, objective blood, and imaging biomarkers), and various assessments of the same construct (ie, different measures of brain injury). While exact matches for data collection methods and measures may be easily harmonized, the timing of assessment, number of assessments, assessment tool version, and other factors must be considered. The goal was to harmonize data from LIMBIC-CENC and TRACTS to allow additional data sources to be harmonized and incorporated in the future.
Original data files from each study were reshaped to represent participant-level observations with 1 unique measurement per row. The measurement represents what information was collected and the value recorded represents the unique observation. These data are linked to metadata from the original study, which includes the study’s definition of each measurement, how it was collected, and any available information regarding when it was collected in reference to study enrollment or injury. Additional information on the file source, row, and column position of each data point was added to enable recreation of the original data as needed.
The resulting dataset was used to harmonize measurements from LIMBIC-CENC and TRACTS into a priori-defined schemas for brain- and mental health-relevant concepts, including TBI severity, PTSD, substance use, depression, suicidal ideation, and functioning (including cognitive, physical, and social functioning). This process was facilitated using natural language processing (NLP). Each study uniquely defines all measurements and provides written definitions with the data. Measurement definitions serve as records describing what was collected, how it was collected, and how the study may have uniquely defined information for its purposes. For example, definitions of exposure to brain injury and severity of brain injury may differ between studies, and the study-provided definition defines these differences.
Definitions were converted into numeric vectors through sentence embedding, a process that preserves the semantic meaning of the definition.23 Cosine similarity was used as the primary metric to compare the semantic textual similarity between pairs of measurement definitions. Cosine similarity ranges from 0 to 1, where 0 indicates no meaningful similarity and 1 indicates they have identical meanings.24 This approach leverages the relationship between the definitions of each measurement provided by a study and enables quick comparison of all pairwise combinations of measurement definitions between studies.
Subsets of similar measurements across studies were organized into a priori-defined schema. Clinical experts then reviewed each schema and further refined them into domains, (eg, mechanism of injury, clinical signs, acute symptoms) and subdomains (children), such as loss of consciousness, amnesia, and alteration of consciousness. This approach allows efficient handling of 2 specific cases that commonly occur when pooling and harmonizing datasets: (1) identifying the same measurement with differing names; and (2) identifying different measurements with definitions that each relate to the same domain.
The Table provides a general example of the schema for TBI severity. This was an iterative process in which clinical experts reviewed study-defined measurement definitions to develop general harmonized domains, and NLP techniques facilitated and accelerated identification and organization of measurements within these domains.

Expected Impact
Harmonization combining LIMBIC-CENC and TRACTS datasets is ongoing. Preliminary descriptive analyses of baseline cohort data indicate that harmonization across data sources is appropriate, given the lack of significant heterogeneity across sites and studies for most domains. Work by members of the TBD team is expected to lay the foundation for the use of existing and ongoing prospective, longitudinal datasets (eg, LIMBIC-CENC, TRACTS) and linked large datasets (eg, VA Informatics and Computing Infrastructure including electronic health records, VA Million Veteran Program, DaVINCI [US Department of Defense and VA Infrastructure for Clinical Intelligence]) to generate generalizable, clinically relevant information to advance precision brain and mental health care among service members and veterans.
By enhancing existing practice, this synthesized dataset has the potential to inform tailored and personalized medicine approaches designed to meet the needs of veterans and service members. These data will serve as the starting point for multivariable models examining the intersection of physiologic, behavioral, and environmental factors. The goal of this data harmonization effort is to better elucidate how clinicians and researchers can select optimal approaches for veterans and service members with TBI histories by accounting for a comprehensive set of physiologic, behavioral, and environmental factors in an individually tailored manner. These data may further extend existing clinical practice guideline approaches, inform shared decision-making, and enhance functional outcomes beyond those currently available.
Conclusions
Individuals who have served in the military have unique biopsychosocial exposures that are associated with brain and mental health disorders. To address these needs, the nationwide TBD team has initiated the creation of a unified, longitudinal dataset that includes harmonized measures from existing LIMBIC-CENC and TRACTS protocols. Initial data harmonization efforts are required to facilitate precision prognostics, diagnostics, and tailored interventions, with the goal of improving veterans’ brain and mental health and psychosocial functioning and enabling tailored and evidence-informed, individualized clinical care.
- The Promise of Precision Medicine. National Institutes of Health (NIH). Updated January 21, 2025. Accessed January 5, 2026. https://www.nih.gov/about-nih/nih-turning-discovery-into-health/promise-precision-medicine.
- Commander John Scott Hannon Veterans Mental Health Care Improvement Act of 2019, S 785, 116th Cong (2019-2020) Accessed January 5, 2026. https://www.congress.gov/bill/116th-congress/senate-bill/785
- Cheng C, Messerschmidt L, Bravo I, et al. A general primer for data harmonization. Sci Data. 2024;11:152. doi:10.1038/s41597-024-02956-3
- Neil M, Cameron D, Clauss K, et al. A proof-of-concept study demonstrating how FITBIR datasets can be harmonized to examine posttraumatic stress disorder-traumatic brain injury associations. J Behav Data Sci. 2024;4:45-62. doi:10.35566/jbds/oneil
- O’Neil ME, Cameron D, Krushnic D, et al. Using harmonized FITBIR datasets to examine associations between TBI history and cognitive functioning. Appl Neuropsychol Adult. doi:10.1080/23279095.2024.2401974
- O’Neil ME, Krushnic D, Clauss K, et al. Harmonizing federal interagency traumatic brain injury research data to examine depression and suicide-related outcomes. Rehabil Psychol. 2024;69:159-170. doi:10.1037/rep0000547
- O’Neil ME, Krushnic D, Walker WC, et al. Increased risk for clinically significant sleep disturbances in mild traumatic brain injury: an approach to leveraging the federal interagency traumatic brain injury research database. Brain Sci. 2024;14:921. doi:10.3390/brainsci14090921
- Uher R, Perlis RH, Placentino A, et al. Self-report and clinician-rated measures of depression severity: can one replace the other? Depress Anxiety. 2012;29:1043-1049. doi:10.1002/da.21993
- Hung CI, Weng LJ, Su YJ, et al. Depression and somatic symptoms scale: a new scale with both depression and somatic symptoms emphasized. Psychiatry Clin Neurosci. 2006;60:700-708. doi:10.1111/j.1440-1819.2006.01585.x
- Stewart IJ, Howard JT, Amuan ME, et al. Traumatic brain injury is associated with the subsequent risk of atrial fibrillation or atrial flutter. Heart Rhythm. 2025;22:661-667. doi:10.1016/j.hrthm.2024.09.019
- Cifu DX. Clinical research findings from the long-term impact of military-relevant brain injury consortium-chronic effects of neurotrauma consortium (LIMBIC-CENC) 2013-2021. Brain Inj. 2022;36:587-597.doi:10.1080/02699052.2022.2033843
- Fonda JR, Fredman L, Brogly SB, et al. Traumatic brain injury and attempted suicide among veterans of the wars in Iraq and Afghanistan. Am J Epidemiol. 2017;186:220-226. doi:10.1093/aje/kwx044
- Fortier CB, Amick MM, Kenna A, et al. Correspondence of the Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) clinical interview and the VA TBI screen. J Head Trauma Rehabil. 2015;30:E1-7. doi:10.1097/htr.0000000000000008
- Grande LJ, Robinson ME, Radigan LJ, et al. Verbal memory deficits in OEF/OIF/OND veterans exposed to blasts at close range. J Int Neuropsychol Soc. 2018;24:466-475. doi:10.1017/S1355617717001242
- Hayes JP, Logue MW, Sadeh N, et al. Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain. 2017;140:813-825. doi:10.1093/brain/aww344
- Lippa SM, Fonda JR, Fortier CB, et al. Deployment-related psychiatric and behavioral conditions and their association with functional disability in OEF/OIF/OND veterans. J Trauma Stress. 2015;28:25-33. doi:10.1002/jts.21979
- McGlinchey RE, Milberg WP, Fonda JR, et al. A methodology for assessing deployment trauma and its consequences in OEF/OIF/OND veterans: the TRACTS longitudinal prospective cohort study. Int J Methods Psychiatr Res. 2017;26:e1556. doi:10.1002/mpr.1556
- Radigan LJ, McGlinchey RE, Milberg WP, et al. Correspondence of the Boston Assessment of Traumatic Brain Injury-Lifetime and the VA Comprehensive TBI Evaluation. J Head Trauma Rehabil. 2018;33:E51-E55. doi:10.1097/htr.0000000000000361
- Sydnor VJ, Bouix S, Pasternak O, et al. Mild traumatic brain injury impacts associations between limbic system microstructure and post-traumatic stress disorder symptomatology. Neuroimage Clin. 2020;26:102190. doi:10.1016/j.nicl.2020.102190
- Van Etten EJ, Knight AR, Colaizzi TA, et al. Peritraumatic context and long-term outcomes of concussion. JAMA Netw Open. 2025;8:e2455622. doi:10.1001/jamanetworkopen.2024.55622
- Andrews RJ, Fonda JR, Levin LK, et al. Comprehensive analysis of the predictors of neurobehavioral symptom reporting in veterans. Neurology. 2018;91:e732-e745. doi:10.1212/wnl.0000000000006034
- McGlinchey RE, Milberg WP, Fonda JR, Fortier CB. A methodology for assessing deployment trauma and its consequences in OEF/OIF/OND veterans: the TRACTS longitudional prospective cohort study. Int J Methods Psychiatr Res. 2017;26:e1556. doi:10.1002/mpr.1556
- Reimers N, Gurevych I. Sentence-BERT: Sentence embeddings using Siamese BERT-Networks. 2019. Conference on Empirical Methods in Natural Language Processing.
- Singhal A. Modern information retrieval: a brief overview. IEEE Data Eng Bull. 2001;24:34-43.
- The Promise of Precision Medicine. National Institutes of Health (NIH). Updated January 21, 2025. Accessed January 5, 2026. https://www.nih.gov/about-nih/nih-turning-discovery-into-health/promise-precision-medicine.
- Commander John Scott Hannon Veterans Mental Health Care Improvement Act of 2019, S 785, 116th Cong (2019-2020) Accessed January 5, 2026. https://www.congress.gov/bill/116th-congress/senate-bill/785
- Cheng C, Messerschmidt L, Bravo I, et al. A general primer for data harmonization. Sci Data. 2024;11:152. doi:10.1038/s41597-024-02956-3
- Neil M, Cameron D, Clauss K, et al. A proof-of-concept study demonstrating how FITBIR datasets can be harmonized to examine posttraumatic stress disorder-traumatic brain injury associations. J Behav Data Sci. 2024;4:45-62. doi:10.35566/jbds/oneil
- O’Neil ME, Cameron D, Krushnic D, et al. Using harmonized FITBIR datasets to examine associations between TBI history and cognitive functioning. Appl Neuropsychol Adult. doi:10.1080/23279095.2024.2401974
- O’Neil ME, Krushnic D, Clauss K, et al. Harmonizing federal interagency traumatic brain injury research data to examine depression and suicide-related outcomes. Rehabil Psychol. 2024;69:159-170. doi:10.1037/rep0000547
- O’Neil ME, Krushnic D, Walker WC, et al. Increased risk for clinically significant sleep disturbances in mild traumatic brain injury: an approach to leveraging the federal interagency traumatic brain injury research database. Brain Sci. 2024;14:921. doi:10.3390/brainsci14090921
- Uher R, Perlis RH, Placentino A, et al. Self-report and clinician-rated measures of depression severity: can one replace the other? Depress Anxiety. 2012;29:1043-1049. doi:10.1002/da.21993
- Hung CI, Weng LJ, Su YJ, et al. Depression and somatic symptoms scale: a new scale with both depression and somatic symptoms emphasized. Psychiatry Clin Neurosci. 2006;60:700-708. doi:10.1111/j.1440-1819.2006.01585.x
- Stewart IJ, Howard JT, Amuan ME, et al. Traumatic brain injury is associated with the subsequent risk of atrial fibrillation or atrial flutter. Heart Rhythm. 2025;22:661-667. doi:10.1016/j.hrthm.2024.09.019
- Cifu DX. Clinical research findings from the long-term impact of military-relevant brain injury consortium-chronic effects of neurotrauma consortium (LIMBIC-CENC) 2013-2021. Brain Inj. 2022;36:587-597.doi:10.1080/02699052.2022.2033843
- Fonda JR, Fredman L, Brogly SB, et al. Traumatic brain injury and attempted suicide among veterans of the wars in Iraq and Afghanistan. Am J Epidemiol. 2017;186:220-226. doi:10.1093/aje/kwx044
- Fortier CB, Amick MM, Kenna A, et al. Correspondence of the Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) clinical interview and the VA TBI screen. J Head Trauma Rehabil. 2015;30:E1-7. doi:10.1097/htr.0000000000000008
- Grande LJ, Robinson ME, Radigan LJ, et al. Verbal memory deficits in OEF/OIF/OND veterans exposed to blasts at close range. J Int Neuropsychol Soc. 2018;24:466-475. doi:10.1017/S1355617717001242
- Hayes JP, Logue MW, Sadeh N, et al. Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain. 2017;140:813-825. doi:10.1093/brain/aww344
- Lippa SM, Fonda JR, Fortier CB, et al. Deployment-related psychiatric and behavioral conditions and their association with functional disability in OEF/OIF/OND veterans. J Trauma Stress. 2015;28:25-33. doi:10.1002/jts.21979
- McGlinchey RE, Milberg WP, Fonda JR, et al. A methodology for assessing deployment trauma and its consequences in OEF/OIF/OND veterans: the TRACTS longitudinal prospective cohort study. Int J Methods Psychiatr Res. 2017;26:e1556. doi:10.1002/mpr.1556
- Radigan LJ, McGlinchey RE, Milberg WP, et al. Correspondence of the Boston Assessment of Traumatic Brain Injury-Lifetime and the VA Comprehensive TBI Evaluation. J Head Trauma Rehabil. 2018;33:E51-E55. doi:10.1097/htr.0000000000000361
- Sydnor VJ, Bouix S, Pasternak O, et al. Mild traumatic brain injury impacts associations between limbic system microstructure and post-traumatic stress disorder symptomatology. Neuroimage Clin. 2020;26:102190. doi:10.1016/j.nicl.2020.102190
- Van Etten EJ, Knight AR, Colaizzi TA, et al. Peritraumatic context and long-term outcomes of concussion. JAMA Netw Open. 2025;8:e2455622. doi:10.1001/jamanetworkopen.2024.55622
- Andrews RJ, Fonda JR, Levin LK, et al. Comprehensive analysis of the predictors of neurobehavioral symptom reporting in veterans. Neurology. 2018;91:e732-e745. doi:10.1212/wnl.0000000000006034
- McGlinchey RE, Milberg WP, Fonda JR, Fortier CB. A methodology for assessing deployment trauma and its consequences in OEF/OIF/OND veterans: the TRACTS longitudional prospective cohort study. Int J Methods Psychiatr Res. 2017;26:e1556. doi:10.1002/mpr.1556
- Reimers N, Gurevych I. Sentence-BERT: Sentence embeddings using Siamese BERT-Networks. 2019. Conference on Empirical Methods in Natural Language Processing.
- Singhal A. Modern information retrieval: a brief overview. IEEE Data Eng Bull. 2001;24:34-43.
Total Brain Diagnostics: Advancing Precision Brain and Mental Health at the Department of Veterans Affairs
Total Brain Diagnostics: Advancing Precision Brain and Mental Health at the Department of Veterans Affairs
Trauma, Military Fitness, and Eating Disorders
Military culture may hold 2 salient risk factors for eating disorders: exposure to trauma and body condition standards. A recent study from the US Department of Veteran Affairs (VA) Salisbury Health Care System (VASHCS) found that veterans with posttraumatic stress disorder (PTSD) are more likely to report eating disturbances—particularly issues related to body dissatisfaction and dissatisfaction with eating habits. A 2019 study found that one-third of veterans who were overweight or obese screened positive for engaging in “making weight” behaviors during military service, or unhealthy weight control strategies. Frequently reported weight management behavior was excessive exercise, fasting/skipping meals, sitting in a sauna/wearing a latex suit, laxatives, diuretics, and vomiting.
Service members who are “normal” weight by civilian standards may be labeled “overweight” by the military. In a March 12 memo, Secretary of Defense Pete Hegseth ordered a US Department of Defense review of existing standards for physical fitness, body composition, and grooming. “Our troops will be fit — not fat. Our troops will look sharp — not sloppy. We seek only quality — not quotas. BOTTOM LINE: our @DeptofDefense will make standards HIGH & GREAT again — across the entire force,” he posted on X.
The desire to control weight to fit military standards, however, isn’t the only risk factor. Researchers at VASHCS surveyed 527 post-9/11 veterans (80.7% male) who typically deployed 1 or 2 times. All participants completed the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; the Neuro-Quality of Life in Neurological Disorders Positive Affect and Well-Being Scale (PAWB); and the Eating Disturbances Scale.
Nearly half (46%) of the sample met diagnostic criteria for a lifetime PTSD diagnosis. The study also reported significantly greater eating disturbances in veterans with a lifetime PTSD diagnosis than those without. Women reported significantly greater eating disturbances than men.
Most participants (80%) reported some level of dissatisfaction with their eating disturbances and 74% of participants reported feeling as if they were too fat.
Eating disturbances include refusing food, overexercising, overeating, and misusing laxatives or diuretic pills. Previous research that suggest that 10% to 15% of female veterans and 4% to 8% of male veterans report clinically significant disordered eating behaviors, especially binge eating. One study found that 78% of 45,477 overweight or obese veterans receiving care in VA facilities reported clinically significant binge eating. In a 2021 study, 254 veterans presenting for routine clinical care completed self‐report questionnaires assessing eating disorders, PTSD, depression, and shame, and 31% met probable criteria for bulimia nervosa, binge‐eating disorder, or purging disorder.
According to a 2023 study, eating disturbances that do not meet diagnostic criteria for a formal disorder can be problematic and may function as coping strategies for some facets of military life. The VASHCS researchers found that interventions focused on PAWB, such as acceptance and commitment therapy or compassion-focused therapy, may have potential as a protective factor. Including components that foster hope, optimism, and personal strength may positively mitigate the relationship between PTSD and eating disturbances. PAWB was significantly correlated with eating disturbances; individuals with a lifetime PTSD diagnosis reported significantly lower PAWB than those without.
Interventions grounded in positive psychology have shown promise. A group-based program found “noticeable” (although nonsignificant) improvements in optimistic thinking and treatment engagement. The study also cites that clinicians are beginning to incorporate positive psychology strategies (eg, gratitude journaling, goal setting, and “best possible self” visualization) as adjuncts to traditional treatments. Positive psychology, they write, holds “significant promise as a complementary approach to enhance recovery outcomes in both PTSD and eating disorders.”
Military culture may hold 2 salient risk factors for eating disorders: exposure to trauma and body condition standards. A recent study from the US Department of Veteran Affairs (VA) Salisbury Health Care System (VASHCS) found that veterans with posttraumatic stress disorder (PTSD) are more likely to report eating disturbances—particularly issues related to body dissatisfaction and dissatisfaction with eating habits. A 2019 study found that one-third of veterans who were overweight or obese screened positive for engaging in “making weight” behaviors during military service, or unhealthy weight control strategies. Frequently reported weight management behavior was excessive exercise, fasting/skipping meals, sitting in a sauna/wearing a latex suit, laxatives, diuretics, and vomiting.
Service members who are “normal” weight by civilian standards may be labeled “overweight” by the military. In a March 12 memo, Secretary of Defense Pete Hegseth ordered a US Department of Defense review of existing standards for physical fitness, body composition, and grooming. “Our troops will be fit — not fat. Our troops will look sharp — not sloppy. We seek only quality — not quotas. BOTTOM LINE: our @DeptofDefense will make standards HIGH & GREAT again — across the entire force,” he posted on X.
The desire to control weight to fit military standards, however, isn’t the only risk factor. Researchers at VASHCS surveyed 527 post-9/11 veterans (80.7% male) who typically deployed 1 or 2 times. All participants completed the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; the Neuro-Quality of Life in Neurological Disorders Positive Affect and Well-Being Scale (PAWB); and the Eating Disturbances Scale.
Nearly half (46%) of the sample met diagnostic criteria for a lifetime PTSD diagnosis. The study also reported significantly greater eating disturbances in veterans with a lifetime PTSD diagnosis than those without. Women reported significantly greater eating disturbances than men.
Most participants (80%) reported some level of dissatisfaction with their eating disturbances and 74% of participants reported feeling as if they were too fat.
Eating disturbances include refusing food, overexercising, overeating, and misusing laxatives or diuretic pills. Previous research that suggest that 10% to 15% of female veterans and 4% to 8% of male veterans report clinically significant disordered eating behaviors, especially binge eating. One study found that 78% of 45,477 overweight or obese veterans receiving care in VA facilities reported clinically significant binge eating. In a 2021 study, 254 veterans presenting for routine clinical care completed self‐report questionnaires assessing eating disorders, PTSD, depression, and shame, and 31% met probable criteria for bulimia nervosa, binge‐eating disorder, or purging disorder.
According to a 2023 study, eating disturbances that do not meet diagnostic criteria for a formal disorder can be problematic and may function as coping strategies for some facets of military life. The VASHCS researchers found that interventions focused on PAWB, such as acceptance and commitment therapy or compassion-focused therapy, may have potential as a protective factor. Including components that foster hope, optimism, and personal strength may positively mitigate the relationship between PTSD and eating disturbances. PAWB was significantly correlated with eating disturbances; individuals with a lifetime PTSD diagnosis reported significantly lower PAWB than those without.
Interventions grounded in positive psychology have shown promise. A group-based program found “noticeable” (although nonsignificant) improvements in optimistic thinking and treatment engagement. The study also cites that clinicians are beginning to incorporate positive psychology strategies (eg, gratitude journaling, goal setting, and “best possible self” visualization) as adjuncts to traditional treatments. Positive psychology, they write, holds “significant promise as a complementary approach to enhance recovery outcomes in both PTSD and eating disorders.”
Military culture may hold 2 salient risk factors for eating disorders: exposure to trauma and body condition standards. A recent study from the US Department of Veteran Affairs (VA) Salisbury Health Care System (VASHCS) found that veterans with posttraumatic stress disorder (PTSD) are more likely to report eating disturbances—particularly issues related to body dissatisfaction and dissatisfaction with eating habits. A 2019 study found that one-third of veterans who were overweight or obese screened positive for engaging in “making weight” behaviors during military service, or unhealthy weight control strategies. Frequently reported weight management behavior was excessive exercise, fasting/skipping meals, sitting in a sauna/wearing a latex suit, laxatives, diuretics, and vomiting.
Service members who are “normal” weight by civilian standards may be labeled “overweight” by the military. In a March 12 memo, Secretary of Defense Pete Hegseth ordered a US Department of Defense review of existing standards for physical fitness, body composition, and grooming. “Our troops will be fit — not fat. Our troops will look sharp — not sloppy. We seek only quality — not quotas. BOTTOM LINE: our @DeptofDefense will make standards HIGH & GREAT again — across the entire force,” he posted on X.
The desire to control weight to fit military standards, however, isn’t the only risk factor. Researchers at VASHCS surveyed 527 post-9/11 veterans (80.7% male) who typically deployed 1 or 2 times. All participants completed the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; the Neuro-Quality of Life in Neurological Disorders Positive Affect and Well-Being Scale (PAWB); and the Eating Disturbances Scale.
Nearly half (46%) of the sample met diagnostic criteria for a lifetime PTSD diagnosis. The study also reported significantly greater eating disturbances in veterans with a lifetime PTSD diagnosis than those without. Women reported significantly greater eating disturbances than men.
Most participants (80%) reported some level of dissatisfaction with their eating disturbances and 74% of participants reported feeling as if they were too fat.
Eating disturbances include refusing food, overexercising, overeating, and misusing laxatives or diuretic pills. Previous research that suggest that 10% to 15% of female veterans and 4% to 8% of male veterans report clinically significant disordered eating behaviors, especially binge eating. One study found that 78% of 45,477 overweight or obese veterans receiving care in VA facilities reported clinically significant binge eating. In a 2021 study, 254 veterans presenting for routine clinical care completed self‐report questionnaires assessing eating disorders, PTSD, depression, and shame, and 31% met probable criteria for bulimia nervosa, binge‐eating disorder, or purging disorder.
According to a 2023 study, eating disturbances that do not meet diagnostic criteria for a formal disorder can be problematic and may function as coping strategies for some facets of military life. The VASHCS researchers found that interventions focused on PAWB, such as acceptance and commitment therapy or compassion-focused therapy, may have potential as a protective factor. Including components that foster hope, optimism, and personal strength may positively mitigate the relationship between PTSD and eating disturbances. PAWB was significantly correlated with eating disturbances; individuals with a lifetime PTSD diagnosis reported significantly lower PAWB than those without.
Interventions grounded in positive psychology have shown promise. A group-based program found “noticeable” (although nonsignificant) improvements in optimistic thinking and treatment engagement. The study also cites that clinicians are beginning to incorporate positive psychology strategies (eg, gratitude journaling, goal setting, and “best possible self” visualization) as adjuncts to traditional treatments. Positive psychology, they write, holds “significant promise as a complementary approach to enhance recovery outcomes in both PTSD and eating disorders.”
'Distress is the Norm': How Oncologists Can Open the Door to Patient Mental Health
'Distress is the Norm': How Oncologists Can Open the Door to Patient Mental Health
For patients with cancer, the determining factor in whether they pursue mental health services is often whether their oncologist explicitly says it is a good idea, a psychologist said during the July Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, on treating veterans with renal cell carcinoma (RCC).
Kysa Christie, PhD, of the West Los Angeles Veterans Affairs Medical Center, presented findings from a 2018 study in which researchers asked Swiss patients with cancer whether their oncologist discussed their emotional health with them.
In terms of boosting intake, it did not matter if oncologists acknowledged distress or pointed out that psychosocial services existed. Instead, a direct recommendation made a difference, increasing the likelihood of using the services over a 4-month period after initial assessment (odds ratio, 6.27).
“What it took was, ‘I really recommend this. This is something that I would want you to try,’” Christie said.
Oncologists are crucial links between patients and mental health services, Christie said: “If people don’t ask about [distress], you’re not going to see it, but it’s there. Distress is the norm, right? It is not a weakness. It is something that we expect to see.”
Christie noted that an estimated 20% of cancer patients have major depressive disorder, and 35% to 40% have a diagnosable psychiatric condition. RCC shows disproportionately high rates of mental strain. According to Christie, research suggests that about three-fourths of the population report elevated levels of distress as evidenced by patients who scored ≥ 5 on the NCCN Distress Thermometer. Patients with cancer have an estimated 20% higher risk of suicide, especially during the first 12 months after diagnosis and at end of life, she added.
“Early during a diagnosis phase, where you’re having a lot of tests being done, you know something is happening. But you don’t know what,” Christie said. “It could be very serious. That’s just a lot of stress to hold and not know how to plan for.”
After diagnosis, routine could set in and lower distress, she said. Then terminal illness may spike it back up again. Does mental health treatment work in patients with cancer?
“There’s a really strong body of evidence-based treatments for depression, anxiety, adjustment disorders, and coping with different cancers,” Christie said. But it is a step too far to expect patients to ask for help while they are juggling appointments, tests, infusions, and more. “It’s a big ask, right? It’s setting people up for failure.”
To help, Christie said she is embedded with a medical oncology team and routinely talks with the staff about which patients may need help. “One thing I like to do is try to have brief visits with veterans and introduce myself when they come to clinic. I treat it like an opt-out rather than an opt-in program: I’ll just pop into the exam room. They don’t have to ask to see me.”
Christie focuses on open-ended questions and talks about resources ranging from support groups and brief appointments to extensive individual therapy.
Another approach is a strategy known as the “warm handoff,” when an oncologist directly introduces a patient to a mental health professional. “It’s a transfer of care in front of the veteran: It’s much more time-efficient than putting in a referral.”
Christie explained how this can work. A clinician will ask her to meet with a patient during an appointment, perhaps in a couple minutes.
“Then I pop into the room, and the oncologist says, ‘Thanks for joining us. This is Mr. Jones. He has been experiencing feelings of anxiety and sadness, and we’d appreciate your help in exploring some options that might help.’ I turn to the patient and ask, ‘What more would you add?’ Then I either take Mr. Jones back to my office or stay in clinic, and we’re off to the races.”
Christie reported no disclosures.
For patients with cancer, the determining factor in whether they pursue mental health services is often whether their oncologist explicitly says it is a good idea, a psychologist said during the July Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, on treating veterans with renal cell carcinoma (RCC).
Kysa Christie, PhD, of the West Los Angeles Veterans Affairs Medical Center, presented findings from a 2018 study in which researchers asked Swiss patients with cancer whether their oncologist discussed their emotional health with them.
In terms of boosting intake, it did not matter if oncologists acknowledged distress or pointed out that psychosocial services existed. Instead, a direct recommendation made a difference, increasing the likelihood of using the services over a 4-month period after initial assessment (odds ratio, 6.27).
“What it took was, ‘I really recommend this. This is something that I would want you to try,’” Christie said.
Oncologists are crucial links between patients and mental health services, Christie said: “If people don’t ask about [distress], you’re not going to see it, but it’s there. Distress is the norm, right? It is not a weakness. It is something that we expect to see.”
Christie noted that an estimated 20% of cancer patients have major depressive disorder, and 35% to 40% have a diagnosable psychiatric condition. RCC shows disproportionately high rates of mental strain. According to Christie, research suggests that about three-fourths of the population report elevated levels of distress as evidenced by patients who scored ≥ 5 on the NCCN Distress Thermometer. Patients with cancer have an estimated 20% higher risk of suicide, especially during the first 12 months after diagnosis and at end of life, she added.
“Early during a diagnosis phase, where you’re having a lot of tests being done, you know something is happening. But you don’t know what,” Christie said. “It could be very serious. That’s just a lot of stress to hold and not know how to plan for.”
After diagnosis, routine could set in and lower distress, she said. Then terminal illness may spike it back up again. Does mental health treatment work in patients with cancer?
“There’s a really strong body of evidence-based treatments for depression, anxiety, adjustment disorders, and coping with different cancers,” Christie said. But it is a step too far to expect patients to ask for help while they are juggling appointments, tests, infusions, and more. “It’s a big ask, right? It’s setting people up for failure.”
To help, Christie said she is embedded with a medical oncology team and routinely talks with the staff about which patients may need help. “One thing I like to do is try to have brief visits with veterans and introduce myself when they come to clinic. I treat it like an opt-out rather than an opt-in program: I’ll just pop into the exam room. They don’t have to ask to see me.”
Christie focuses on open-ended questions and talks about resources ranging from support groups and brief appointments to extensive individual therapy.
Another approach is a strategy known as the “warm handoff,” when an oncologist directly introduces a patient to a mental health professional. “It’s a transfer of care in front of the veteran: It’s much more time-efficient than putting in a referral.”
Christie explained how this can work. A clinician will ask her to meet with a patient during an appointment, perhaps in a couple minutes.
“Then I pop into the room, and the oncologist says, ‘Thanks for joining us. This is Mr. Jones. He has been experiencing feelings of anxiety and sadness, and we’d appreciate your help in exploring some options that might help.’ I turn to the patient and ask, ‘What more would you add?’ Then I either take Mr. Jones back to my office or stay in clinic, and we’re off to the races.”
Christie reported no disclosures.
For patients with cancer, the determining factor in whether they pursue mental health services is often whether their oncologist explicitly says it is a good idea, a psychologist said during the July Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, on treating veterans with renal cell carcinoma (RCC).
Kysa Christie, PhD, of the West Los Angeles Veterans Affairs Medical Center, presented findings from a 2018 study in which researchers asked Swiss patients with cancer whether their oncologist discussed their emotional health with them.
In terms of boosting intake, it did not matter if oncologists acknowledged distress or pointed out that psychosocial services existed. Instead, a direct recommendation made a difference, increasing the likelihood of using the services over a 4-month period after initial assessment (odds ratio, 6.27).
“What it took was, ‘I really recommend this. This is something that I would want you to try,’” Christie said.
Oncologists are crucial links between patients and mental health services, Christie said: “If people don’t ask about [distress], you’re not going to see it, but it’s there. Distress is the norm, right? It is not a weakness. It is something that we expect to see.”
Christie noted that an estimated 20% of cancer patients have major depressive disorder, and 35% to 40% have a diagnosable psychiatric condition. RCC shows disproportionately high rates of mental strain. According to Christie, research suggests that about three-fourths of the population report elevated levels of distress as evidenced by patients who scored ≥ 5 on the NCCN Distress Thermometer. Patients with cancer have an estimated 20% higher risk of suicide, especially during the first 12 months after diagnosis and at end of life, she added.
“Early during a diagnosis phase, where you’re having a lot of tests being done, you know something is happening. But you don’t know what,” Christie said. “It could be very serious. That’s just a lot of stress to hold and not know how to plan for.”
After diagnosis, routine could set in and lower distress, she said. Then terminal illness may spike it back up again. Does mental health treatment work in patients with cancer?
“There’s a really strong body of evidence-based treatments for depression, anxiety, adjustment disorders, and coping with different cancers,” Christie said. But it is a step too far to expect patients to ask for help while they are juggling appointments, tests, infusions, and more. “It’s a big ask, right? It’s setting people up for failure.”
To help, Christie said she is embedded with a medical oncology team and routinely talks with the staff about which patients may need help. “One thing I like to do is try to have brief visits with veterans and introduce myself when they come to clinic. I treat it like an opt-out rather than an opt-in program: I’ll just pop into the exam room. They don’t have to ask to see me.”
Christie focuses on open-ended questions and talks about resources ranging from support groups and brief appointments to extensive individual therapy.
Another approach is a strategy known as the “warm handoff,” when an oncologist directly introduces a patient to a mental health professional. “It’s a transfer of care in front of the veteran: It’s much more time-efficient than putting in a referral.”
Christie explained how this can work. A clinician will ask her to meet with a patient during an appointment, perhaps in a couple minutes.
“Then I pop into the room, and the oncologist says, ‘Thanks for joining us. This is Mr. Jones. He has been experiencing feelings of anxiety and sadness, and we’d appreciate your help in exploring some options that might help.’ I turn to the patient and ask, ‘What more would you add?’ Then I either take Mr. Jones back to my office or stay in clinic, and we’re off to the races.”
Christie reported no disclosures.
'Distress is the Norm': How Oncologists Can Open the Door to Patient Mental Health
'Distress is the Norm': How Oncologists Can Open the Door to Patient Mental Health
Fibromyalgia-PTSD Link Shows Bidirectional Relationship With Exposure to Combat Environments
Fibromyalgia-PTSD Link Shows Bidirectional Relationship With Exposure to Combat Environments
Spending time in a war zone can lead to chronic mental and physical pain. Now, research points to a link between two common disorders that can leave service members struggling.
Published in the journal Arthritis Care & Research, a longitudinal cohort study of 1761 US military service members found that those who had posttraumatic stress disorder (PTSD) before deployment were nearly 3times more likely to develop fibromyalgia after returning home (odds ratio, 2.96; 95% CI, 2.08-4.22). Those with fibromyalgia before deployment had more than threefold greater likelihood of developing PTSD after deployment (odds ratio, 3.12; 95% CI, 1.63-5.95).
This is the largest prospective study to date linking the stress of combat deployment to the onset of fibromyalgia.
“We had the advantage of observing a large population before and after exposure to an environment that often involves significant stress,” said lead study author Jay Higgs, MD, a retired rheumatologist with Brooke Army Medical Center and the University of Texas Health Science Center at San Antonio.
Here’s what the team found and why it matters.
Significant Increase in Fibromyalgia After Development
Service members were checked for fibromyalgia using the 2011 questionnaire modification of the 2010 American College of Rheumatology preliminary diagnostic criteria for fibromyalgia. They were assessed for PTSD using the PTSD Checklist Stressor-Specific Version.
Before deployment, service members had similar rates of fibromyalgia as the general population: 2.2% in men and 2.0% in women. After deployment, fibromyalgia rates increased significantly to 8.0% in men and 11.1% in women.
While fibromyalgia tends to be underreported in men, the findings suggest it should not be overlooked in this population. “Our results are consistent with the notion that there should be no gender bias when considering the possibility of fibromyalgia in an individual patient,” Higgs said.
Before deployment, 20.7% of men and 18.3% of women had PTSD symptoms. After deployment, the PTSD rate increased slightly to 22.7% in men and 25.5% in women.
The Link Between Fibromyalgia and PTSD
The researchers said the results suggest that PTSD and fibromyalgia might be linked through central nervous system mechanisms such as central sensitization, elevated hypothalamic-pituitary-adrenal axis activity, elevated cortisol, and proinflammatory cytokines. However, shared causation, associated risk factors, selection bias, or alternative mechanisms within the central and peripheral neuroendocrine and cytokine systems could also be part of the story.
“What we do not know is how much of what we see clinically represents central nervous system pathology, peripheral problems, or a combination of the 2,” Higgs said. “Neurotransmission in the central nervous system is highly complex, and may not only involve specific structures, but a web of communications between them.”
Loci in the midbrain appear especially important, he said.
Elizabeth Hoge, MD, professor and director of the Anxiety Disorders Research Program at Georgetown University School of Medicine, Washington, DC, said that patients with PTSD often have pain, headaches, sleep disturbances, and other symptoms that are part of the picture of fibromyalgia. It’s plausible that pain syndromes could be manifestations of PTSD or groupings of symptoms that suggest a subtype.
“Pain is one way that people experience distress, and we know that in PTSD, sometimes the trauma memories are encoded too strongly, more stressful and more alarming to the body system,” she said.
When patients have symptoms such as chronic pain, headaches, fatigue, or cognitive brain fog, clinicians should remember to ask about trauma exposure, Hoge said. You might be the first to broach the subject.
“I’ve certainly seen patients in clinic who never get asked about the exposure to trauma, including sexual trauma, so sometimes that can be the first pathway to helping people feel better is just to have their trauma recognized,” Hoge said.
If a patient has experienced or witnessed violence, consider a referral to a psychiatrist or psychologist to evaluate them for PTSD. Higgs said he collaborated closely with a psychologist to complement his treatment plans for active duty and retired military service members and families.
The US Department of Veterans Affairs and the Department of Defense (DoD) recommend trauma-focused psychotherapy as the first line of treatment for PTSD. This form of therapy deliberately focuses on bringing trauma memories into the open, Hoge said.
“When a person talks about their trauma, and it comes into direct consciousness, somehow it’s malleable, and so when it goes back down into the memory banks, it’s changed somewhat,” she said.
This study was supported by the DoD through awards from the US Army Medical Research and Materiel Command, Congressionally Directed Medical Research Programs, and Psychological Health and Traumatic Brain Injury Research Program. The funding organizations played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Higgs’s comments are his own and do not necessarily reflect the official policy or position of the Defense Health Agency, Brooke Army Medical Center, Carl R. Darnall Army Medical Center, the DoD, the Department of Veterans Affairs, or any agencies under the US government. Hoge had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Spending time in a war zone can lead to chronic mental and physical pain. Now, research points to a link between two common disorders that can leave service members struggling.
Published in the journal Arthritis Care & Research, a longitudinal cohort study of 1761 US military service members found that those who had posttraumatic stress disorder (PTSD) before deployment were nearly 3times more likely to develop fibromyalgia after returning home (odds ratio, 2.96; 95% CI, 2.08-4.22). Those with fibromyalgia before deployment had more than threefold greater likelihood of developing PTSD after deployment (odds ratio, 3.12; 95% CI, 1.63-5.95).
This is the largest prospective study to date linking the stress of combat deployment to the onset of fibromyalgia.
“We had the advantage of observing a large population before and after exposure to an environment that often involves significant stress,” said lead study author Jay Higgs, MD, a retired rheumatologist with Brooke Army Medical Center and the University of Texas Health Science Center at San Antonio.
Here’s what the team found and why it matters.
Significant Increase in Fibromyalgia After Development
Service members were checked for fibromyalgia using the 2011 questionnaire modification of the 2010 American College of Rheumatology preliminary diagnostic criteria for fibromyalgia. They were assessed for PTSD using the PTSD Checklist Stressor-Specific Version.
Before deployment, service members had similar rates of fibromyalgia as the general population: 2.2% in men and 2.0% in women. After deployment, fibromyalgia rates increased significantly to 8.0% in men and 11.1% in women.
While fibromyalgia tends to be underreported in men, the findings suggest it should not be overlooked in this population. “Our results are consistent with the notion that there should be no gender bias when considering the possibility of fibromyalgia in an individual patient,” Higgs said.
Before deployment, 20.7% of men and 18.3% of women had PTSD symptoms. After deployment, the PTSD rate increased slightly to 22.7% in men and 25.5% in women.
The Link Between Fibromyalgia and PTSD
The researchers said the results suggest that PTSD and fibromyalgia might be linked through central nervous system mechanisms such as central sensitization, elevated hypothalamic-pituitary-adrenal axis activity, elevated cortisol, and proinflammatory cytokines. However, shared causation, associated risk factors, selection bias, or alternative mechanisms within the central and peripheral neuroendocrine and cytokine systems could also be part of the story.
“What we do not know is how much of what we see clinically represents central nervous system pathology, peripheral problems, or a combination of the 2,” Higgs said. “Neurotransmission in the central nervous system is highly complex, and may not only involve specific structures, but a web of communications between them.”
Loci in the midbrain appear especially important, he said.
Elizabeth Hoge, MD, professor and director of the Anxiety Disorders Research Program at Georgetown University School of Medicine, Washington, DC, said that patients with PTSD often have pain, headaches, sleep disturbances, and other symptoms that are part of the picture of fibromyalgia. It’s plausible that pain syndromes could be manifestations of PTSD or groupings of symptoms that suggest a subtype.
“Pain is one way that people experience distress, and we know that in PTSD, sometimes the trauma memories are encoded too strongly, more stressful and more alarming to the body system,” she said.
When patients have symptoms such as chronic pain, headaches, fatigue, or cognitive brain fog, clinicians should remember to ask about trauma exposure, Hoge said. You might be the first to broach the subject.
“I’ve certainly seen patients in clinic who never get asked about the exposure to trauma, including sexual trauma, so sometimes that can be the first pathway to helping people feel better is just to have their trauma recognized,” Hoge said.
If a patient has experienced or witnessed violence, consider a referral to a psychiatrist or psychologist to evaluate them for PTSD. Higgs said he collaborated closely with a psychologist to complement his treatment plans for active duty and retired military service members and families.
The US Department of Veterans Affairs and the Department of Defense (DoD) recommend trauma-focused psychotherapy as the first line of treatment for PTSD. This form of therapy deliberately focuses on bringing trauma memories into the open, Hoge said.
“When a person talks about their trauma, and it comes into direct consciousness, somehow it’s malleable, and so when it goes back down into the memory banks, it’s changed somewhat,” she said.
This study was supported by the DoD through awards from the US Army Medical Research and Materiel Command, Congressionally Directed Medical Research Programs, and Psychological Health and Traumatic Brain Injury Research Program. The funding organizations played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Higgs’s comments are his own and do not necessarily reflect the official policy or position of the Defense Health Agency, Brooke Army Medical Center, Carl R. Darnall Army Medical Center, the DoD, the Department of Veterans Affairs, or any agencies under the US government. Hoge had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Spending time in a war zone can lead to chronic mental and physical pain. Now, research points to a link between two common disorders that can leave service members struggling.
Published in the journal Arthritis Care & Research, a longitudinal cohort study of 1761 US military service members found that those who had posttraumatic stress disorder (PTSD) before deployment were nearly 3times more likely to develop fibromyalgia after returning home (odds ratio, 2.96; 95% CI, 2.08-4.22). Those with fibromyalgia before deployment had more than threefold greater likelihood of developing PTSD after deployment (odds ratio, 3.12; 95% CI, 1.63-5.95).
This is the largest prospective study to date linking the stress of combat deployment to the onset of fibromyalgia.
“We had the advantage of observing a large population before and after exposure to an environment that often involves significant stress,” said lead study author Jay Higgs, MD, a retired rheumatologist with Brooke Army Medical Center and the University of Texas Health Science Center at San Antonio.
Here’s what the team found and why it matters.
Significant Increase in Fibromyalgia After Development
Service members were checked for fibromyalgia using the 2011 questionnaire modification of the 2010 American College of Rheumatology preliminary diagnostic criteria for fibromyalgia. They were assessed for PTSD using the PTSD Checklist Stressor-Specific Version.
Before deployment, service members had similar rates of fibromyalgia as the general population: 2.2% in men and 2.0% in women. After deployment, fibromyalgia rates increased significantly to 8.0% in men and 11.1% in women.
While fibromyalgia tends to be underreported in men, the findings suggest it should not be overlooked in this population. “Our results are consistent with the notion that there should be no gender bias when considering the possibility of fibromyalgia in an individual patient,” Higgs said.
Before deployment, 20.7% of men and 18.3% of women had PTSD symptoms. After deployment, the PTSD rate increased slightly to 22.7% in men and 25.5% in women.
The Link Between Fibromyalgia and PTSD
The researchers said the results suggest that PTSD and fibromyalgia might be linked through central nervous system mechanisms such as central sensitization, elevated hypothalamic-pituitary-adrenal axis activity, elevated cortisol, and proinflammatory cytokines. However, shared causation, associated risk factors, selection bias, or alternative mechanisms within the central and peripheral neuroendocrine and cytokine systems could also be part of the story.
“What we do not know is how much of what we see clinically represents central nervous system pathology, peripheral problems, or a combination of the 2,” Higgs said. “Neurotransmission in the central nervous system is highly complex, and may not only involve specific structures, but a web of communications between them.”
Loci in the midbrain appear especially important, he said.
Elizabeth Hoge, MD, professor and director of the Anxiety Disorders Research Program at Georgetown University School of Medicine, Washington, DC, said that patients with PTSD often have pain, headaches, sleep disturbances, and other symptoms that are part of the picture of fibromyalgia. It’s plausible that pain syndromes could be manifestations of PTSD or groupings of symptoms that suggest a subtype.
“Pain is one way that people experience distress, and we know that in PTSD, sometimes the trauma memories are encoded too strongly, more stressful and more alarming to the body system,” she said.
When patients have symptoms such as chronic pain, headaches, fatigue, or cognitive brain fog, clinicians should remember to ask about trauma exposure, Hoge said. You might be the first to broach the subject.
“I’ve certainly seen patients in clinic who never get asked about the exposure to trauma, including sexual trauma, so sometimes that can be the first pathway to helping people feel better is just to have their trauma recognized,” Hoge said.
If a patient has experienced or witnessed violence, consider a referral to a psychiatrist or psychologist to evaluate them for PTSD. Higgs said he collaborated closely with a psychologist to complement his treatment plans for active duty and retired military service members and families.
The US Department of Veterans Affairs and the Department of Defense (DoD) recommend trauma-focused psychotherapy as the first line of treatment for PTSD. This form of therapy deliberately focuses on bringing trauma memories into the open, Hoge said.
“When a person talks about their trauma, and it comes into direct consciousness, somehow it’s malleable, and so when it goes back down into the memory banks, it’s changed somewhat,” she said.
This study was supported by the DoD through awards from the US Army Medical Research and Materiel Command, Congressionally Directed Medical Research Programs, and Psychological Health and Traumatic Brain Injury Research Program. The funding organizations played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Higgs’s comments are his own and do not necessarily reflect the official policy or position of the Defense Health Agency, Brooke Army Medical Center, Carl R. Darnall Army Medical Center, the DoD, the Department of Veterans Affairs, or any agencies under the US government. Hoge had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Fibromyalgia-PTSD Link Shows Bidirectional Relationship With Exposure to Combat Environments
Fibromyalgia-PTSD Link Shows Bidirectional Relationship With Exposure to Combat Environments
Social Challenges Linked to More Suicidality in Vets
Veterans experiencing unstable housing, financial strain, and with poor access to health care have a higher risk of suicidal thoughts and behaviors, according to findings in a new study, leading researchers to call for additional screening to identify those in jeopardy.
Each incremental increase in social disadvantage was tied to increases in the likelihood of recent suicidal thoughts (odds ratio [OR], 2.14), future suicidal intent (OR, 2.21), and lifetime suicide attempt (OR, 1.78) in a weighted analysis. The self-reported data was published as a cross-sectional study by Pietrzak et al in the December 2025 issue of JAMA Psychiatry.
Veterans whose social plights ranked in the worst 5% were > 20 times more likely to report suicidal thoughts and behaviors than those in the top 5%. Especially striking were the magnitudes of the associations and their persistence after adjustment for psychiatric conditions and other suicide risk factors, lead author Robert H. Pietrzak, PhD, MPH said in an interview with Federal Practitioner.
“This finding highlights how extreme cumulative disadvantage can be overwhelming,” Pietrzak said. “It suggests that suicide risk among veterans increases dramatically when multiple social stressors cluster together. Rather than any single hardship driving risk, it is the cumulative impact of social disadvantage that appears most strongly linked to elevated suicide risk.”
As Pietrzak explained, veterans account for < 7% of total US adults but about 14% of suicide deaths. “Several factors may contribute to this difference, including higher exposure to trauma, elevated rates of psychiatric conditions, challenges with reintegration into civilian life, and structural barriers to care,” Pietrzak said. “Increasingly, social and economic stressors are also recognized by experts and researchers as critical contributors to suicide risk.”
Social determinants of health (SDOH) such as unemployment and lack of access to health care have also been linked to suicide risk, he said.
“Less well understood is how multiple adverse social conditions interact and accumulate to compound suicide risk,” Pietrzak said.
The new study sought to determine the impact of SDOH as a whole, not just in isolation. The study analyzed SDOH in 5 areas—education access and quality, economic conditions, health care access and quality, neighborhood and built environment, and social and community context—via the National Health and Resilience in Veterans Study, which surveyed 4069 veterans. The participants had weighted demographics of mean age 62.2 years; 90.2% were male; and 78.1% White, 11.2% Black, 6.6% Hispanic, 4.2% other.
Past-year suicidal ideation was most highly linked to psychosocial difficulties (OR, 1.58; 95% CI, 1.43-1.75). Future suicidal intent was most highly linked to residing in a mobile home, recreational vehicle, or van (OR, 1.60; 95% CI, 1.24-2.07) in addition to psychosocial difficulties (OR, 1.45; 95% CI, 1.18-1.80). Lifetime suicidal attempt was most highly linked to history of homelessness (OR, 1.37; 95% CI, 1.22-1.55; all P < .001).
“The results of our study underscore the importance of routine, standardized screening for cumulative social disadvantage within VA and community care settings that serve veterans,” Pietrzak said.
He added that findings make it clear that “suicide prevention extends beyond mental health care. Improving the social conditions in which veterans live, work, and age is not only good public policy. It may save lives.”
Mark S. Kaplan, DrPH, a research professor of Social Welfare at the University of California at Los Angeles Luskin School of Public Affairs is familiar with the study findings and said they highlight the need to “approach the question of suicide in much wider terms as opposed to reducing it to psychiatric traits.”
J. John Mann, MD, a professor of translational neuroscience in psychiatry and radiology who studies suicide at Columbia University, New York City, said the study’s findings illustrate that clinicians must do more to understand the lives of patients outside the examination room. He predicted that more screening for social determinants of health will “enrich the amount of information that the clinician will have and lead to a more comprehensive clinical care plan.”
The US Department of Veterans Affairs supported the study. Pietrzak has no disclosures. Other study authors report various disclosures.
Veterans experiencing unstable housing, financial strain, and with poor access to health care have a higher risk of suicidal thoughts and behaviors, according to findings in a new study, leading researchers to call for additional screening to identify those in jeopardy.
Each incremental increase in social disadvantage was tied to increases in the likelihood of recent suicidal thoughts (odds ratio [OR], 2.14), future suicidal intent (OR, 2.21), and lifetime suicide attempt (OR, 1.78) in a weighted analysis. The self-reported data was published as a cross-sectional study by Pietrzak et al in the December 2025 issue of JAMA Psychiatry.
Veterans whose social plights ranked in the worst 5% were > 20 times more likely to report suicidal thoughts and behaviors than those in the top 5%. Especially striking were the magnitudes of the associations and their persistence after adjustment for psychiatric conditions and other suicide risk factors, lead author Robert H. Pietrzak, PhD, MPH said in an interview with Federal Practitioner.
“This finding highlights how extreme cumulative disadvantage can be overwhelming,” Pietrzak said. “It suggests that suicide risk among veterans increases dramatically when multiple social stressors cluster together. Rather than any single hardship driving risk, it is the cumulative impact of social disadvantage that appears most strongly linked to elevated suicide risk.”
As Pietrzak explained, veterans account for < 7% of total US adults but about 14% of suicide deaths. “Several factors may contribute to this difference, including higher exposure to trauma, elevated rates of psychiatric conditions, challenges with reintegration into civilian life, and structural barriers to care,” Pietrzak said. “Increasingly, social and economic stressors are also recognized by experts and researchers as critical contributors to suicide risk.”
Social determinants of health (SDOH) such as unemployment and lack of access to health care have also been linked to suicide risk, he said.
“Less well understood is how multiple adverse social conditions interact and accumulate to compound suicide risk,” Pietrzak said.
The new study sought to determine the impact of SDOH as a whole, not just in isolation. The study analyzed SDOH in 5 areas—education access and quality, economic conditions, health care access and quality, neighborhood and built environment, and social and community context—via the National Health and Resilience in Veterans Study, which surveyed 4069 veterans. The participants had weighted demographics of mean age 62.2 years; 90.2% were male; and 78.1% White, 11.2% Black, 6.6% Hispanic, 4.2% other.
Past-year suicidal ideation was most highly linked to psychosocial difficulties (OR, 1.58; 95% CI, 1.43-1.75). Future suicidal intent was most highly linked to residing in a mobile home, recreational vehicle, or van (OR, 1.60; 95% CI, 1.24-2.07) in addition to psychosocial difficulties (OR, 1.45; 95% CI, 1.18-1.80). Lifetime suicidal attempt was most highly linked to history of homelessness (OR, 1.37; 95% CI, 1.22-1.55; all P < .001).
“The results of our study underscore the importance of routine, standardized screening for cumulative social disadvantage within VA and community care settings that serve veterans,” Pietrzak said.
He added that findings make it clear that “suicide prevention extends beyond mental health care. Improving the social conditions in which veterans live, work, and age is not only good public policy. It may save lives.”
Mark S. Kaplan, DrPH, a research professor of Social Welfare at the University of California at Los Angeles Luskin School of Public Affairs is familiar with the study findings and said they highlight the need to “approach the question of suicide in much wider terms as opposed to reducing it to psychiatric traits.”
J. John Mann, MD, a professor of translational neuroscience in psychiatry and radiology who studies suicide at Columbia University, New York City, said the study’s findings illustrate that clinicians must do more to understand the lives of patients outside the examination room. He predicted that more screening for social determinants of health will “enrich the amount of information that the clinician will have and lead to a more comprehensive clinical care plan.”
The US Department of Veterans Affairs supported the study. Pietrzak has no disclosures. Other study authors report various disclosures.
Veterans experiencing unstable housing, financial strain, and with poor access to health care have a higher risk of suicidal thoughts and behaviors, according to findings in a new study, leading researchers to call for additional screening to identify those in jeopardy.
Each incremental increase in social disadvantage was tied to increases in the likelihood of recent suicidal thoughts (odds ratio [OR], 2.14), future suicidal intent (OR, 2.21), and lifetime suicide attempt (OR, 1.78) in a weighted analysis. The self-reported data was published as a cross-sectional study by Pietrzak et al in the December 2025 issue of JAMA Psychiatry.
Veterans whose social plights ranked in the worst 5% were > 20 times more likely to report suicidal thoughts and behaviors than those in the top 5%. Especially striking were the magnitudes of the associations and their persistence after adjustment for psychiatric conditions and other suicide risk factors, lead author Robert H. Pietrzak, PhD, MPH said in an interview with Federal Practitioner.
“This finding highlights how extreme cumulative disadvantage can be overwhelming,” Pietrzak said. “It suggests that suicide risk among veterans increases dramatically when multiple social stressors cluster together. Rather than any single hardship driving risk, it is the cumulative impact of social disadvantage that appears most strongly linked to elevated suicide risk.”
As Pietrzak explained, veterans account for < 7% of total US adults but about 14% of suicide deaths. “Several factors may contribute to this difference, including higher exposure to trauma, elevated rates of psychiatric conditions, challenges with reintegration into civilian life, and structural barriers to care,” Pietrzak said. “Increasingly, social and economic stressors are also recognized by experts and researchers as critical contributors to suicide risk.”
Social determinants of health (SDOH) such as unemployment and lack of access to health care have also been linked to suicide risk, he said.
“Less well understood is how multiple adverse social conditions interact and accumulate to compound suicide risk,” Pietrzak said.
The new study sought to determine the impact of SDOH as a whole, not just in isolation. The study analyzed SDOH in 5 areas—education access and quality, economic conditions, health care access and quality, neighborhood and built environment, and social and community context—via the National Health and Resilience in Veterans Study, which surveyed 4069 veterans. The participants had weighted demographics of mean age 62.2 years; 90.2% were male; and 78.1% White, 11.2% Black, 6.6% Hispanic, 4.2% other.
Past-year suicidal ideation was most highly linked to psychosocial difficulties (OR, 1.58; 95% CI, 1.43-1.75). Future suicidal intent was most highly linked to residing in a mobile home, recreational vehicle, or van (OR, 1.60; 95% CI, 1.24-2.07) in addition to psychosocial difficulties (OR, 1.45; 95% CI, 1.18-1.80). Lifetime suicidal attempt was most highly linked to history of homelessness (OR, 1.37; 95% CI, 1.22-1.55; all P < .001).
“The results of our study underscore the importance of routine, standardized screening for cumulative social disadvantage within VA and community care settings that serve veterans,” Pietrzak said.
He added that findings make it clear that “suicide prevention extends beyond mental health care. Improving the social conditions in which veterans live, work, and age is not only good public policy. It may save lives.”
Mark S. Kaplan, DrPH, a research professor of Social Welfare at the University of California at Los Angeles Luskin School of Public Affairs is familiar with the study findings and said they highlight the need to “approach the question of suicide in much wider terms as opposed to reducing it to psychiatric traits.”
J. John Mann, MD, a professor of translational neuroscience in psychiatry and radiology who studies suicide at Columbia University, New York City, said the study’s findings illustrate that clinicians must do more to understand the lives of patients outside the examination room. He predicted that more screening for social determinants of health will “enrich the amount of information that the clinician will have and lead to a more comprehensive clinical care plan.”
The US Department of Veterans Affairs supported the study. Pietrzak has no disclosures. Other study authors report various disclosures.
PTSD Boosts Risk of Violence, Legal and Financial Problems, and More
PTSD Boosts Risk of Violence, Legal and Financial Problems, and More
Veterans with posttraumatic stress disorder (PTSD) were much more likely than their counterparts to be a perpetrator or victim of violence and suffer from social, legal, and financial problems, a new retrospective analysis finds.
An analysis of 62,298 matched veterans found that those newly diagnosed with PTSD were more likely to be linked to violence (adjusted odds ratio [aOR], 3.98), social problems (aOR, 2.87) legal problems (aOR, 1.75), and financial problems (aOR, 2.01), reported Ouyang et al in the November 2025 issue of the Journal of Affective Disorders.
A separate analysis of 11,758 propensity-matched veterans found that those with PTSD were more likely to experience violence (50.15% vs 11.26%), social problems (64.44% vs 25.32%), legal problems (24.84% vs 8.07%), and financial problems (48.60% vs 19.21%).
The study does not prove that PTSD is directly linked to these problems. However, Ouyang told Federal Practitioner that the findings suggest "PTSD extends beyond psychiatric symptoms: It significantly impacts economic stability, housing security, and legal safety."
Clinicians should screen for various problems in patients with PTSD, Ouyang said, “particularly given that the risk is highest during the first year.” The study also sought to better understand the effects of PTSD over time.
“While it is established that PTSD creates serious challenges regarding employment, family dynamics, and substance use, most previous studies provided only a cross-sectional snapshot,” Ouyang said. “We aimed to understand the progression over a 10-year period.”
In addition, “previous studies relied heavily on standard diagnosis codes and missed a significant amount of unstructured data,” she said. The new study uses natural language processing, an artificial intelligence field that parses the words people use, to gain insight from clinical notes.
In the cross-sectional analysis of 62,298 veterans, including 31,149 diagnosed with PTSD in the 2011-2012 fiscal year and 31,149 without PTSD (average age 60, 91.49% male, 71.50% White and 19.27% Black), PTSD was linked to higher rates of housing instability (aOR, 1.65), barriers to care (aOR, 1.45), transitions of care (aOR, 1.58), food insecurity (aOR, 1.37), and nonspecific psychosocial needs (aOR, 1.31).
Why might PTSD be linked to violence, which was defined as perpetrated by or against the veteran?
“The primary theory centers on hyperarousal, a symptom of PTSD characterized by a state of constant high alert and anxiety,” Ouyang said. “This state creates difficulties in emotional regulation and impulse control, which can lead to aggressive reactions.”
Patients are also at risk of revictimization, Ouyang added, “where the erosion of social support networks leaves veterans more vulnerable to harm from others.”
Aspects of PTSD are also thought to contribute to problems other than violence, Ouyang said. For example, mental health struggles can make it hard to keep a job and stay financially stable “and veterans may be hesitant to seek help due to stigma until the situation becomes critical, potentially leading to housing loss.”
In terms of solutions, “clinical treatment alone is insufficient,” she said. “We recommend an integrated health care model that combines mental health treatment with referrals to social work and economic support services to address the broader determinants of well-being.”
Brian Klassen, PhD, an associate professor with the Department of Psychiatry and Behavioral Sciences at Rush University Medical Center, reviewed the study for Federal Practitioner.
The research “underscores how problematic the diagnosis of PTSD is for folks,” said Klassen, the director of Strategic Partnership for the Road Home Program/Center for Veterans and Their Families. “It plays out in lives in trouble with relationships, work, and housing, things like that.”
How PTSD cultivates a veteran’s everyday life is important for clinicians to understand, he said. “A lot of our treatments directly target symptoms: how to help people sleep better, manage their mood. This encourages practitioners to look at the whole person,” Klassen said. “What other kind of resource needs might this person have that are related to—or maybe caused by—their PTSD diagnosis?”
These resources can “include things like job training and housing and financial assistance, maybe help to get out in the community and form relationships with people.”
The US Department of Veterans Affairs and National Institutes of Health funded the study. The study authors and Klassen have no disclosures.
Veterans with posttraumatic stress disorder (PTSD) were much more likely than their counterparts to be a perpetrator or victim of violence and suffer from social, legal, and financial problems, a new retrospective analysis finds.
An analysis of 62,298 matched veterans found that those newly diagnosed with PTSD were more likely to be linked to violence (adjusted odds ratio [aOR], 3.98), social problems (aOR, 2.87) legal problems (aOR, 1.75), and financial problems (aOR, 2.01), reported Ouyang et al in the November 2025 issue of the Journal of Affective Disorders.
A separate analysis of 11,758 propensity-matched veterans found that those with PTSD were more likely to experience violence (50.15% vs 11.26%), social problems (64.44% vs 25.32%), legal problems (24.84% vs 8.07%), and financial problems (48.60% vs 19.21%).
The study does not prove that PTSD is directly linked to these problems. However, Ouyang told Federal Practitioner that the findings suggest "PTSD extends beyond psychiatric symptoms: It significantly impacts economic stability, housing security, and legal safety."
Clinicians should screen for various problems in patients with PTSD, Ouyang said, “particularly given that the risk is highest during the first year.” The study also sought to better understand the effects of PTSD over time.
“While it is established that PTSD creates serious challenges regarding employment, family dynamics, and substance use, most previous studies provided only a cross-sectional snapshot,” Ouyang said. “We aimed to understand the progression over a 10-year period.”
In addition, “previous studies relied heavily on standard diagnosis codes and missed a significant amount of unstructured data,” she said. The new study uses natural language processing, an artificial intelligence field that parses the words people use, to gain insight from clinical notes.
In the cross-sectional analysis of 62,298 veterans, including 31,149 diagnosed with PTSD in the 2011-2012 fiscal year and 31,149 without PTSD (average age 60, 91.49% male, 71.50% White and 19.27% Black), PTSD was linked to higher rates of housing instability (aOR, 1.65), barriers to care (aOR, 1.45), transitions of care (aOR, 1.58), food insecurity (aOR, 1.37), and nonspecific psychosocial needs (aOR, 1.31).
Why might PTSD be linked to violence, which was defined as perpetrated by or against the veteran?
“The primary theory centers on hyperarousal, a symptom of PTSD characterized by a state of constant high alert and anxiety,” Ouyang said. “This state creates difficulties in emotional regulation and impulse control, which can lead to aggressive reactions.”
Patients are also at risk of revictimization, Ouyang added, “where the erosion of social support networks leaves veterans more vulnerable to harm from others.”
Aspects of PTSD are also thought to contribute to problems other than violence, Ouyang said. For example, mental health struggles can make it hard to keep a job and stay financially stable “and veterans may be hesitant to seek help due to stigma until the situation becomes critical, potentially leading to housing loss.”
In terms of solutions, “clinical treatment alone is insufficient,” she said. “We recommend an integrated health care model that combines mental health treatment with referrals to social work and economic support services to address the broader determinants of well-being.”
Brian Klassen, PhD, an associate professor with the Department of Psychiatry and Behavioral Sciences at Rush University Medical Center, reviewed the study for Federal Practitioner.
The research “underscores how problematic the diagnosis of PTSD is for folks,” said Klassen, the director of Strategic Partnership for the Road Home Program/Center for Veterans and Their Families. “It plays out in lives in trouble with relationships, work, and housing, things like that.”
How PTSD cultivates a veteran’s everyday life is important for clinicians to understand, he said. “A lot of our treatments directly target symptoms: how to help people sleep better, manage their mood. This encourages practitioners to look at the whole person,” Klassen said. “What other kind of resource needs might this person have that are related to—or maybe caused by—their PTSD diagnosis?”
These resources can “include things like job training and housing and financial assistance, maybe help to get out in the community and form relationships with people.”
The US Department of Veterans Affairs and National Institutes of Health funded the study. The study authors and Klassen have no disclosures.
Veterans with posttraumatic stress disorder (PTSD) were much more likely than their counterparts to be a perpetrator or victim of violence and suffer from social, legal, and financial problems, a new retrospective analysis finds.
An analysis of 62,298 matched veterans found that those newly diagnosed with PTSD were more likely to be linked to violence (adjusted odds ratio [aOR], 3.98), social problems (aOR, 2.87) legal problems (aOR, 1.75), and financial problems (aOR, 2.01), reported Ouyang et al in the November 2025 issue of the Journal of Affective Disorders.
A separate analysis of 11,758 propensity-matched veterans found that those with PTSD were more likely to experience violence (50.15% vs 11.26%), social problems (64.44% vs 25.32%), legal problems (24.84% vs 8.07%), and financial problems (48.60% vs 19.21%).
The study does not prove that PTSD is directly linked to these problems. However, Ouyang told Federal Practitioner that the findings suggest "PTSD extends beyond psychiatric symptoms: It significantly impacts economic stability, housing security, and legal safety."
Clinicians should screen for various problems in patients with PTSD, Ouyang said, “particularly given that the risk is highest during the first year.” The study also sought to better understand the effects of PTSD over time.
“While it is established that PTSD creates serious challenges regarding employment, family dynamics, and substance use, most previous studies provided only a cross-sectional snapshot,” Ouyang said. “We aimed to understand the progression over a 10-year period.”
In addition, “previous studies relied heavily on standard diagnosis codes and missed a significant amount of unstructured data,” she said. The new study uses natural language processing, an artificial intelligence field that parses the words people use, to gain insight from clinical notes.
In the cross-sectional analysis of 62,298 veterans, including 31,149 diagnosed with PTSD in the 2011-2012 fiscal year and 31,149 without PTSD (average age 60, 91.49% male, 71.50% White and 19.27% Black), PTSD was linked to higher rates of housing instability (aOR, 1.65), barriers to care (aOR, 1.45), transitions of care (aOR, 1.58), food insecurity (aOR, 1.37), and nonspecific psychosocial needs (aOR, 1.31).
Why might PTSD be linked to violence, which was defined as perpetrated by or against the veteran?
“The primary theory centers on hyperarousal, a symptom of PTSD characterized by a state of constant high alert and anxiety,” Ouyang said. “This state creates difficulties in emotional regulation and impulse control, which can lead to aggressive reactions.”
Patients are also at risk of revictimization, Ouyang added, “where the erosion of social support networks leaves veterans more vulnerable to harm from others.”
Aspects of PTSD are also thought to contribute to problems other than violence, Ouyang said. For example, mental health struggles can make it hard to keep a job and stay financially stable “and veterans may be hesitant to seek help due to stigma until the situation becomes critical, potentially leading to housing loss.”
In terms of solutions, “clinical treatment alone is insufficient,” she said. “We recommend an integrated health care model that combines mental health treatment with referrals to social work and economic support services to address the broader determinants of well-being.”
Brian Klassen, PhD, an associate professor with the Department of Psychiatry and Behavioral Sciences at Rush University Medical Center, reviewed the study for Federal Practitioner.
The research “underscores how problematic the diagnosis of PTSD is for folks,” said Klassen, the director of Strategic Partnership for the Road Home Program/Center for Veterans and Their Families. “It plays out in lives in trouble with relationships, work, and housing, things like that.”
How PTSD cultivates a veteran’s everyday life is important for clinicians to understand, he said. “A lot of our treatments directly target symptoms: how to help people sleep better, manage their mood. This encourages practitioners to look at the whole person,” Klassen said. “What other kind of resource needs might this person have that are related to—or maybe caused by—their PTSD diagnosis?”
These resources can “include things like job training and housing and financial assistance, maybe help to get out in the community and form relationships with people.”
The US Department of Veterans Affairs and National Institutes of Health funded the study. The study authors and Klassen have no disclosures.
PTSD Boosts Risk of Violence, Legal and Financial Problems, and More
PTSD Boosts Risk of Violence, Legal and Financial Problems, and More
Female Veterans' Telemental Health Use: Rural-Urban Shift
Female Veterans' Telemental Health Use: Rural-Urban Shift
TOPLINE:
The use of services for mental health involving video chats with medical professionals increased in female veterans from 2019 to 2022, with women living in urban areas more likely to use the services than their rural counterparts. Black and Hispanic women showed the largest increases.
METHODOLOGY:
- Researchers analyzed trends in video telemental health utilization among female veterans within an observational cohort of Veterans Health Administration (VHA) mental health outpatient visits, by rurality and race, from 2019 to 2022.
- The study included 470,863 female veterans (mean age, 43 years; 51% White individuals) who had ≥ 1 outpatient mental health visit; a subsample of 141,349 veterans with mental health visits in both 2019 and 2022 was analyzed for changes in telemental health use.
- Video telemental health encounters were identified using specific codes for synchronous, video-based mental health care and included both visits at clinics and those at home through the VA Video Connect system.
- The researchers categorized race into 5 groups and classified veterans’ residences as rural and urban using commuting area codes.
TAKEAWAY:
- The use of synchronous video telemental health services among female veterans increased from < 7% to 32% from 2019 to 2022, with stable in-person care rates.
- In 2019, female veterans living in rural areas had an increased likelihood of using video telemental health. However, by 2022, this difference decreased, and female veterans living in urban areas showed equivalent or higher usage. Female veterans living in urban areas had a greater increase in the number of visits in 2022 than their peers living in rural areas.
- Black and Hispanic female veterans showed greater increases in video tele-mental health usage in both urban and rural areas. No significant change in telemental health visits was noted for American Indian and Alaska Native female veterans between 2019 and 2022.
- In the analysis of the subsample, female veterans living in urban areas were 21-35 times more likely to use video telemental health in 2022 vs 2019, whereas female veterans living in rural areas were 7-11 times more likely.
IN PRACTICE:
“The rapid changes observed in SVT-MH [synchronous video telehealth for mental health] use over a relatively short time period underscore the potential for achieving equity through intentional system-level efforts. However, our findings also highlight the risk of overgeneralizing telehealth utilization patterns,” the authors wrote. “Our findings underscore the need for targeted digital care strategies — especially for rural and AIAN [American Indian and Alaska Native] women veterans — to ensure that all veterans benefit equally from virtual care options,” they added.
SOURCE:
This study was led by Michelle A. Mengeling, PhD, MS, of the VHA Office of Rural Health at the Veterans Rural Health Resource Center in Iowa City, Iowa. It was published online on December 10, 2025, in The Journal of Rural Health.
LIMITATIONS:
Female veterans older than 60 years were excluded to avoid confounding with Medicare service usage. Rurality was classified as urban or rural, which may overlook variations in highly rural or isolated areas. The focus on VHA-delivered mental health care might not fully capture the use of video-based telemental health services.
DISCLOSURES:
This study was supported by grants from the US Department of Veterans Affairs, VHA, Office of Rural Health, Veterans Rural Health Resource Center - Iowa City. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this story first appeared on Medscape.com.
TOPLINE:
The use of services for mental health involving video chats with medical professionals increased in female veterans from 2019 to 2022, with women living in urban areas more likely to use the services than their rural counterparts. Black and Hispanic women showed the largest increases.
METHODOLOGY:
- Researchers analyzed trends in video telemental health utilization among female veterans within an observational cohort of Veterans Health Administration (VHA) mental health outpatient visits, by rurality and race, from 2019 to 2022.
- The study included 470,863 female veterans (mean age, 43 years; 51% White individuals) who had ≥ 1 outpatient mental health visit; a subsample of 141,349 veterans with mental health visits in both 2019 and 2022 was analyzed for changes in telemental health use.
- Video telemental health encounters were identified using specific codes for synchronous, video-based mental health care and included both visits at clinics and those at home through the VA Video Connect system.
- The researchers categorized race into 5 groups and classified veterans’ residences as rural and urban using commuting area codes.
TAKEAWAY:
- The use of synchronous video telemental health services among female veterans increased from < 7% to 32% from 2019 to 2022, with stable in-person care rates.
- In 2019, female veterans living in rural areas had an increased likelihood of using video telemental health. However, by 2022, this difference decreased, and female veterans living in urban areas showed equivalent or higher usage. Female veterans living in urban areas had a greater increase in the number of visits in 2022 than their peers living in rural areas.
- Black and Hispanic female veterans showed greater increases in video tele-mental health usage in both urban and rural areas. No significant change in telemental health visits was noted for American Indian and Alaska Native female veterans between 2019 and 2022.
- In the analysis of the subsample, female veterans living in urban areas were 21-35 times more likely to use video telemental health in 2022 vs 2019, whereas female veterans living in rural areas were 7-11 times more likely.
IN PRACTICE:
“The rapid changes observed in SVT-MH [synchronous video telehealth for mental health] use over a relatively short time period underscore the potential for achieving equity through intentional system-level efforts. However, our findings also highlight the risk of overgeneralizing telehealth utilization patterns,” the authors wrote. “Our findings underscore the need for targeted digital care strategies — especially for rural and AIAN [American Indian and Alaska Native] women veterans — to ensure that all veterans benefit equally from virtual care options,” they added.
SOURCE:
This study was led by Michelle A. Mengeling, PhD, MS, of the VHA Office of Rural Health at the Veterans Rural Health Resource Center in Iowa City, Iowa. It was published online on December 10, 2025, in The Journal of Rural Health.
LIMITATIONS:
Female veterans older than 60 years were excluded to avoid confounding with Medicare service usage. Rurality was classified as urban or rural, which may overlook variations in highly rural or isolated areas. The focus on VHA-delivered mental health care might not fully capture the use of video-based telemental health services.
DISCLOSURES:
This study was supported by grants from the US Department of Veterans Affairs, VHA, Office of Rural Health, Veterans Rural Health Resource Center - Iowa City. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this story first appeared on Medscape.com.
TOPLINE:
The use of services for mental health involving video chats with medical professionals increased in female veterans from 2019 to 2022, with women living in urban areas more likely to use the services than their rural counterparts. Black and Hispanic women showed the largest increases.
METHODOLOGY:
- Researchers analyzed trends in video telemental health utilization among female veterans within an observational cohort of Veterans Health Administration (VHA) mental health outpatient visits, by rurality and race, from 2019 to 2022.
- The study included 470,863 female veterans (mean age, 43 years; 51% White individuals) who had ≥ 1 outpatient mental health visit; a subsample of 141,349 veterans with mental health visits in both 2019 and 2022 was analyzed for changes in telemental health use.
- Video telemental health encounters were identified using specific codes for synchronous, video-based mental health care and included both visits at clinics and those at home through the VA Video Connect system.
- The researchers categorized race into 5 groups and classified veterans’ residences as rural and urban using commuting area codes.
TAKEAWAY:
- The use of synchronous video telemental health services among female veterans increased from < 7% to 32% from 2019 to 2022, with stable in-person care rates.
- In 2019, female veterans living in rural areas had an increased likelihood of using video telemental health. However, by 2022, this difference decreased, and female veterans living in urban areas showed equivalent or higher usage. Female veterans living in urban areas had a greater increase in the number of visits in 2022 than their peers living in rural areas.
- Black and Hispanic female veterans showed greater increases in video tele-mental health usage in both urban and rural areas. No significant change in telemental health visits was noted for American Indian and Alaska Native female veterans between 2019 and 2022.
- In the analysis of the subsample, female veterans living in urban areas were 21-35 times more likely to use video telemental health in 2022 vs 2019, whereas female veterans living in rural areas were 7-11 times more likely.
IN PRACTICE:
“The rapid changes observed in SVT-MH [synchronous video telehealth for mental health] use over a relatively short time period underscore the potential for achieving equity through intentional system-level efforts. However, our findings also highlight the risk of overgeneralizing telehealth utilization patterns,” the authors wrote. “Our findings underscore the need for targeted digital care strategies — especially for rural and AIAN [American Indian and Alaska Native] women veterans — to ensure that all veterans benefit equally from virtual care options,” they added.
SOURCE:
This study was led by Michelle A. Mengeling, PhD, MS, of the VHA Office of Rural Health at the Veterans Rural Health Resource Center in Iowa City, Iowa. It was published online on December 10, 2025, in The Journal of Rural Health.
LIMITATIONS:
Female veterans older than 60 years were excluded to avoid confounding with Medicare service usage. Rurality was classified as urban or rural, which may overlook variations in highly rural or isolated areas. The focus on VHA-delivered mental health care might not fully capture the use of video-based telemental health services.
DISCLOSURES:
This study was supported by grants from the US Department of Veterans Affairs, VHA, Office of Rural Health, Veterans Rural Health Resource Center - Iowa City. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this story first appeared on Medscape.com.
Female Veterans' Telemental Health Use: Rural-Urban Shift
Female Veterans' Telemental Health Use: Rural-Urban Shift
Research Focuses on Mental Health Needs of Women Veterans
The more than 2 million women US veterans are the fastest-growing military population. While research into women veterans has traditionally lagged, more recently studies have begun to focus on their needs impacts of combat and service on women. These studies have found that women veterans preferred tailored solutions focused on women veterans.
A November 2025 study is one of the first to examine the impact of combat on women veterans. It found that those in combat roles had higher levels of depression, posttraumatic stress disorder (PTSD), dissociation, and overall poorer health compared with civilians and noncombat women military personnel. Previous research had found that women veterans had higher rates of lifetime and past-year PTSD (13.4%) compared with female civilians (8.0%), male veterans (7.7%), and male civilians (3.4%). A 2020 US Department of Veterans (VA) study of 4,928,638 men and 448,455 women similarly found that women had nearly twice the rates of depression and anxiety compared with men.
For many veterans, mental health issues may develop or be exacerbated in their return to civilian life. That transition can be especially confusing and isolating for women veterans, according to a 2024 study: “They neither fit in the military due to gendered relations centered on masculinity, or civilian life where they are largely misunderstood as ‘veterans.’ This ‘no woman’s land’ is poorly understood.” Few programs for transitioning veterans have been found effective for women veterans because they’ve been developed for a largely male veteran population. That includes mental health support programs.
Some women may prefer women-only groups, and even that choice may be dependent on their background, service history, socioeconomic level, and other factors. They may feel more comfortable in women-only groups if they’ve experienced MST. Others who have served in combat may choose mixed-gender programs. One study found that some women benefited from being in a mixed-gender group because it enabled them to work on difficulties with men in a safe environment. Other research has found that women veterans with substance use disorders are reluctant to seek help alongside men in the same facilities.
Accessing care may be especially challenging for rural women veterans. However, separate facilities and women-only groups are not always available, particularly in rural areas where there may be very few women veterans. And even if they are available, rural women are often up against barriers that urban women do not face, such as having to travel long distances to get care. Clinicians also may be hard to find in rural areas. Some participants in a 2025 study were hampered not only by a lack of female practitioners, but practitioners who were well trained to understand and treat the unique needs of female veterans: “[It’s] incredibly difficult to find a mental health practitioner that understands a veteran’s unique experience as a woman,” a participant said.
The more than 2 million women US veterans are the fastest-growing military population. While research into women veterans has traditionally lagged, more recently studies have begun to focus on their needs impacts of combat and service on women. These studies have found that women veterans preferred tailored solutions focused on women veterans.
A November 2025 study is one of the first to examine the impact of combat on women veterans. It found that those in combat roles had higher levels of depression, posttraumatic stress disorder (PTSD), dissociation, and overall poorer health compared with civilians and noncombat women military personnel. Previous research had found that women veterans had higher rates of lifetime and past-year PTSD (13.4%) compared with female civilians (8.0%), male veterans (7.7%), and male civilians (3.4%). A 2020 US Department of Veterans (VA) study of 4,928,638 men and 448,455 women similarly found that women had nearly twice the rates of depression and anxiety compared with men.
For many veterans, mental health issues may develop or be exacerbated in their return to civilian life. That transition can be especially confusing and isolating for women veterans, according to a 2024 study: “They neither fit in the military due to gendered relations centered on masculinity, or civilian life where they are largely misunderstood as ‘veterans.’ This ‘no woman’s land’ is poorly understood.” Few programs for transitioning veterans have been found effective for women veterans because they’ve been developed for a largely male veteran population. That includes mental health support programs.
Some women may prefer women-only groups, and even that choice may be dependent on their background, service history, socioeconomic level, and other factors. They may feel more comfortable in women-only groups if they’ve experienced MST. Others who have served in combat may choose mixed-gender programs. One study found that some women benefited from being in a mixed-gender group because it enabled them to work on difficulties with men in a safe environment. Other research has found that women veterans with substance use disorders are reluctant to seek help alongside men in the same facilities.
Accessing care may be especially challenging for rural women veterans. However, separate facilities and women-only groups are not always available, particularly in rural areas where there may be very few women veterans. And even if they are available, rural women are often up against barriers that urban women do not face, such as having to travel long distances to get care. Clinicians also may be hard to find in rural areas. Some participants in a 2025 study were hampered not only by a lack of female practitioners, but practitioners who were well trained to understand and treat the unique needs of female veterans: “[It’s] incredibly difficult to find a mental health practitioner that understands a veteran’s unique experience as a woman,” a participant said.
The more than 2 million women US veterans are the fastest-growing military population. While research into women veterans has traditionally lagged, more recently studies have begun to focus on their needs impacts of combat and service on women. These studies have found that women veterans preferred tailored solutions focused on women veterans.
A November 2025 study is one of the first to examine the impact of combat on women veterans. It found that those in combat roles had higher levels of depression, posttraumatic stress disorder (PTSD), dissociation, and overall poorer health compared with civilians and noncombat women military personnel. Previous research had found that women veterans had higher rates of lifetime and past-year PTSD (13.4%) compared with female civilians (8.0%), male veterans (7.7%), and male civilians (3.4%). A 2020 US Department of Veterans (VA) study of 4,928,638 men and 448,455 women similarly found that women had nearly twice the rates of depression and anxiety compared with men.
For many veterans, mental health issues may develop or be exacerbated in their return to civilian life. That transition can be especially confusing and isolating for women veterans, according to a 2024 study: “They neither fit in the military due to gendered relations centered on masculinity, or civilian life where they are largely misunderstood as ‘veterans.’ This ‘no woman’s land’ is poorly understood.” Few programs for transitioning veterans have been found effective for women veterans because they’ve been developed for a largely male veteran population. That includes mental health support programs.
Some women may prefer women-only groups, and even that choice may be dependent on their background, service history, socioeconomic level, and other factors. They may feel more comfortable in women-only groups if they’ve experienced MST. Others who have served in combat may choose mixed-gender programs. One study found that some women benefited from being in a mixed-gender group because it enabled them to work on difficulties with men in a safe environment. Other research has found that women veterans with substance use disorders are reluctant to seek help alongside men in the same facilities.
Accessing care may be especially challenging for rural women veterans. However, separate facilities and women-only groups are not always available, particularly in rural areas where there may be very few women veterans. And even if they are available, rural women are often up against barriers that urban women do not face, such as having to travel long distances to get care. Clinicians also may be hard to find in rural areas. Some participants in a 2025 study were hampered not only by a lack of female practitioners, but practitioners who were well trained to understand and treat the unique needs of female veterans: “[It’s] incredibly difficult to find a mental health practitioner that understands a veteran’s unique experience as a woman,” a participant said.
Text vs Video Psychotherapy: Which Is Better for Depression?
Text vs Video Psychotherapy: Which Is Better for Depression?
TOPLINE:
Message-based psychotherapy (MBP), which uses asynchronous emails or texts, showed effectiveness comparable with that of video-based psychotherapy (VBP) for the treatment of depression on a commercial digital mental health platform, a new study showed.
METHODOLOGY:
- Investigators conducted a pragmatic sequential multiple-assignment randomized clinical trial from 2022 to 2024 involving 850 adult patients with a diagnosis of depression (mean age, 34 years; 66% women; 60% White, 22% Black and 14% Hispanic).
- Patients were initially randomly assigned to receive weekly MBP (n = 423) or VBP (n = 427), with nonresponders randomly assigned at week 6 to receive combination therapy of MBP plus weekly or monthly VBP. All patients received treatment for up to 12 weeks.
- Primary outcomes included depression severity measured by the 9-item Patient Health Questionnaire (PHQ-9), social functioning measured by the Quality of Life in Neurological Disorders 8-item tool, response to treatment (≥ 50% reduction in PHQ-9 total score or Clinical Global Impressions-Improvement score ≤ 2), and remissions (PHQ-9 score < 5).
- Secondary outcomes were treating disengagement, therapeutic alliance measured on the Working Alliance Inventory-Short Revised, quality of care in the past 4 weeks, and treatment satisfaction.
TAKEAWAY:
- Rates of response (47.5% and 47.2%, respectively) and remission (31.4% and 30.3%, respectively) were not significantly different at week 12 between the MBP and VBP groups or for nonresponders rerandomized to either group.
- There were also no significant differences in depression change scores between the MBP and VBP groups or for nonresponders rerandomized to either group.
- Treatment disengagement by week 5 was significantly higher in the VBP vs MBP group (21.3% vs 13.2%; P = .003); VBP responders had stronger initial therapeutic alliance at week 4 than MBP responders (P < .001).
- No significant differences were observed in the quality of care among those who responded only after the second randomization to MBP or VBP.
IN PRACTICE:
"Findings reinforced MBP as viable alternative to VBP. Broader insurance reimbursement for MBP could improve access to evidence-based care," the investigators wrote.
SOURCE:
The study was led by Michael D. Pullmann, PhD, School of Medicine, University of Washington, Seattle. It was published online on October 30 in JAMA Network Open.
LIMITATIONS:
The absence of a waiting list or a no-treatment control group made it difficult to rule out regression to the mean as an explanation for improvements. Additionally, missing data may have affected the robustness of some findings.
DISCLOSURES:
The research was funded by the National Institute of Mental Health. Several investigators reported having financial ties with various sources. Details are provided in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Message-based psychotherapy (MBP), which uses asynchronous emails or texts, showed effectiveness comparable with that of video-based psychotherapy (VBP) for the treatment of depression on a commercial digital mental health platform, a new study showed.
METHODOLOGY:
- Investigators conducted a pragmatic sequential multiple-assignment randomized clinical trial from 2022 to 2024 involving 850 adult patients with a diagnosis of depression (mean age, 34 years; 66% women; 60% White, 22% Black and 14% Hispanic).
- Patients were initially randomly assigned to receive weekly MBP (n = 423) or VBP (n = 427), with nonresponders randomly assigned at week 6 to receive combination therapy of MBP plus weekly or monthly VBP. All patients received treatment for up to 12 weeks.
- Primary outcomes included depression severity measured by the 9-item Patient Health Questionnaire (PHQ-9), social functioning measured by the Quality of Life in Neurological Disorders 8-item tool, response to treatment (≥ 50% reduction in PHQ-9 total score or Clinical Global Impressions-Improvement score ≤ 2), and remissions (PHQ-9 score < 5).
- Secondary outcomes were treating disengagement, therapeutic alliance measured on the Working Alliance Inventory-Short Revised, quality of care in the past 4 weeks, and treatment satisfaction.
TAKEAWAY:
- Rates of response (47.5% and 47.2%, respectively) and remission (31.4% and 30.3%, respectively) were not significantly different at week 12 between the MBP and VBP groups or for nonresponders rerandomized to either group.
- There were also no significant differences in depression change scores between the MBP and VBP groups or for nonresponders rerandomized to either group.
- Treatment disengagement by week 5 was significantly higher in the VBP vs MBP group (21.3% vs 13.2%; P = .003); VBP responders had stronger initial therapeutic alliance at week 4 than MBP responders (P < .001).
- No significant differences were observed in the quality of care among those who responded only after the second randomization to MBP or VBP.
IN PRACTICE:
"Findings reinforced MBP as viable alternative to VBP. Broader insurance reimbursement for MBP could improve access to evidence-based care," the investigators wrote.
SOURCE:
The study was led by Michael D. Pullmann, PhD, School of Medicine, University of Washington, Seattle. It was published online on October 30 in JAMA Network Open.
LIMITATIONS:
The absence of a waiting list or a no-treatment control group made it difficult to rule out regression to the mean as an explanation for improvements. Additionally, missing data may have affected the robustness of some findings.
DISCLOSURES:
The research was funded by the National Institute of Mental Health. Several investigators reported having financial ties with various sources. Details are provided in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Message-based psychotherapy (MBP), which uses asynchronous emails or texts, showed effectiveness comparable with that of video-based psychotherapy (VBP) for the treatment of depression on a commercial digital mental health platform, a new study showed.
METHODOLOGY:
- Investigators conducted a pragmatic sequential multiple-assignment randomized clinical trial from 2022 to 2024 involving 850 adult patients with a diagnosis of depression (mean age, 34 years; 66% women; 60% White, 22% Black and 14% Hispanic).
- Patients were initially randomly assigned to receive weekly MBP (n = 423) or VBP (n = 427), with nonresponders randomly assigned at week 6 to receive combination therapy of MBP plus weekly or monthly VBP. All patients received treatment for up to 12 weeks.
- Primary outcomes included depression severity measured by the 9-item Patient Health Questionnaire (PHQ-9), social functioning measured by the Quality of Life in Neurological Disorders 8-item tool, response to treatment (≥ 50% reduction in PHQ-9 total score or Clinical Global Impressions-Improvement score ≤ 2), and remissions (PHQ-9 score < 5).
- Secondary outcomes were treating disengagement, therapeutic alliance measured on the Working Alliance Inventory-Short Revised, quality of care in the past 4 weeks, and treatment satisfaction.
TAKEAWAY:
- Rates of response (47.5% and 47.2%, respectively) and remission (31.4% and 30.3%, respectively) were not significantly different at week 12 between the MBP and VBP groups or for nonresponders rerandomized to either group.
- There were also no significant differences in depression change scores between the MBP and VBP groups or for nonresponders rerandomized to either group.
- Treatment disengagement by week 5 was significantly higher in the VBP vs MBP group (21.3% vs 13.2%; P = .003); VBP responders had stronger initial therapeutic alliance at week 4 than MBP responders (P < .001).
- No significant differences were observed in the quality of care among those who responded only after the second randomization to MBP or VBP.
IN PRACTICE:
"Findings reinforced MBP as viable alternative to VBP. Broader insurance reimbursement for MBP could improve access to evidence-based care," the investigators wrote.
SOURCE:
The study was led by Michael D. Pullmann, PhD, School of Medicine, University of Washington, Seattle. It was published online on October 30 in JAMA Network Open.
LIMITATIONS:
The absence of a waiting list or a no-treatment control group made it difficult to rule out regression to the mean as an explanation for improvements. Additionally, missing data may have affected the robustness of some findings.
DISCLOSURES:
The research was funded by the National Institute of Mental Health. Several investigators reported having financial ties with various sources. Details are provided in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Text vs Video Psychotherapy: Which Is Better for Depression?
Text vs Video Psychotherapy: Which Is Better for Depression?
Can Clinical Resource Hubs Address Mental Health Staffing Gaps?
TOPLINE: The Veterans Health Administration implemented 18 regional Clinical Resource Hubs (CRHs), where remote clinicians deliver virtual mental health care, addressing staffing gaps amid increasing demand and workforce shortages. Early implementation showed promise in improving access, with program benefits extending beyond temporary staffing solutions.
METHODOLOGY:
Semistructured interviews were conducted with 36 CRH mental health leaders across all 18 regions.
A rapid qualitative approach was used, incorporating templated summaries and matrix analysis.
Participants included leads responsible for implementation and coordination, as well as Chief Mental Health Officers overseeing facility-based services.
Regional leaders collaborated through executive meetings to ensure appropriate mental health practitioner assignments and effective service delivery to facilities in need.
TAKEAWAY:
The CRH program demonstrated 3 key values: enhanced integration compared with community care, expanded specialty mental health services in rural areas, and improved provider recruitment and satisfaction.
Leaders argued that the program could prevent unnecessary delays for veterans who might experience longer wait times for mental health services in the community.
Mental health practitioners can work virtually across multiple health care systems, with hybrid schedules combining on-site and virtual care delivery.
The program attracted numerous qualified applicants for virtual care.
IN PRACTICE: “Mental health leaders’ perspectives on CRH value suggest the program is more than a contingency staffing solution for mental health care access challenges, but also potentially offers additional benefits that could be leveraged to improve mental health care services more generally," wrote the authors of the study.
SOURCE: The study was led by the Center for the Study of Healthcare Innovation in Los Angeles. It was published online in Administration and Policy in Mental Health and Mental Health Services Research.
LIMITATIONS: The researchers identified lower productivity among CRH staff compared with facility staff, indicating unused capacity. The program's rapid national implementation may have contributed to challenges, as hubs were established quickly, potentially before fully determining regional demand. Some facilities requiring services may have lacked the necessary infrastructure for timely implementation.
DISCLOSURES: This work received support from the Veterans Health Administration Primary Care Analytics Team, funded by the Veterans Health Administration Office of Primary Care. The views expressed do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. Government.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: The Veterans Health Administration implemented 18 regional Clinical Resource Hubs (CRHs), where remote clinicians deliver virtual mental health care, addressing staffing gaps amid increasing demand and workforce shortages. Early implementation showed promise in improving access, with program benefits extending beyond temporary staffing solutions.
METHODOLOGY:
Semistructured interviews were conducted with 36 CRH mental health leaders across all 18 regions.
A rapid qualitative approach was used, incorporating templated summaries and matrix analysis.
Participants included leads responsible for implementation and coordination, as well as Chief Mental Health Officers overseeing facility-based services.
Regional leaders collaborated through executive meetings to ensure appropriate mental health practitioner assignments and effective service delivery to facilities in need.
TAKEAWAY:
The CRH program demonstrated 3 key values: enhanced integration compared with community care, expanded specialty mental health services in rural areas, and improved provider recruitment and satisfaction.
Leaders argued that the program could prevent unnecessary delays for veterans who might experience longer wait times for mental health services in the community.
Mental health practitioners can work virtually across multiple health care systems, with hybrid schedules combining on-site and virtual care delivery.
The program attracted numerous qualified applicants for virtual care.
IN PRACTICE: “Mental health leaders’ perspectives on CRH value suggest the program is more than a contingency staffing solution for mental health care access challenges, but also potentially offers additional benefits that could be leveraged to improve mental health care services more generally," wrote the authors of the study.
SOURCE: The study was led by the Center for the Study of Healthcare Innovation in Los Angeles. It was published online in Administration and Policy in Mental Health and Mental Health Services Research.
LIMITATIONS: The researchers identified lower productivity among CRH staff compared with facility staff, indicating unused capacity. The program's rapid national implementation may have contributed to challenges, as hubs were established quickly, potentially before fully determining regional demand. Some facilities requiring services may have lacked the necessary infrastructure for timely implementation.
DISCLOSURES: This work received support from the Veterans Health Administration Primary Care Analytics Team, funded by the Veterans Health Administration Office of Primary Care. The views expressed do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. Government.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: The Veterans Health Administration implemented 18 regional Clinical Resource Hubs (CRHs), where remote clinicians deliver virtual mental health care, addressing staffing gaps amid increasing demand and workforce shortages. Early implementation showed promise in improving access, with program benefits extending beyond temporary staffing solutions.
METHODOLOGY:
Semistructured interviews were conducted with 36 CRH mental health leaders across all 18 regions.
A rapid qualitative approach was used, incorporating templated summaries and matrix analysis.
Participants included leads responsible for implementation and coordination, as well as Chief Mental Health Officers overseeing facility-based services.
Regional leaders collaborated through executive meetings to ensure appropriate mental health practitioner assignments and effective service delivery to facilities in need.
TAKEAWAY:
The CRH program demonstrated 3 key values: enhanced integration compared with community care, expanded specialty mental health services in rural areas, and improved provider recruitment and satisfaction.
Leaders argued that the program could prevent unnecessary delays for veterans who might experience longer wait times for mental health services in the community.
Mental health practitioners can work virtually across multiple health care systems, with hybrid schedules combining on-site and virtual care delivery.
The program attracted numerous qualified applicants for virtual care.
IN PRACTICE: “Mental health leaders’ perspectives on CRH value suggest the program is more than a contingency staffing solution for mental health care access challenges, but also potentially offers additional benefits that could be leveraged to improve mental health care services more generally," wrote the authors of the study.
SOURCE: The study was led by the Center for the Study of Healthcare Innovation in Los Angeles. It was published online in Administration and Policy in Mental Health and Mental Health Services Research.
LIMITATIONS: The researchers identified lower productivity among CRH staff compared with facility staff, indicating unused capacity. The program's rapid national implementation may have contributed to challenges, as hubs were established quickly, potentially before fully determining regional demand. Some facilities requiring services may have lacked the necessary infrastructure for timely implementation.
DISCLOSURES: This work received support from the Veterans Health Administration Primary Care Analytics Team, funded by the Veterans Health Administration Office of Primary Care. The views expressed do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. Government.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.