User login
SAN DIEGO – A novel oral Janus kinase 3 inhibitor known for now as VX-509 readily hit both of its coprimary endpoints in a phase IIb study in rheumatoid arthritis patients presented at the annual meeting of the American College of Rheumatology.
The 24-week, double-blind, international study included 358 patients with active rheumatoid arthritis on stable doses of background methotrexate who were randomized to one of four VX-509 dosing regimens or placebo.
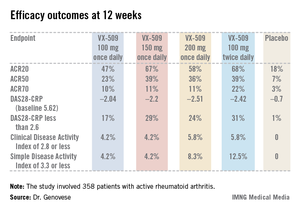
An ACR20 response at 12 weeks occurred in 47%-68% of subjects on various doses of the oral JAK 3 inhibitor, compared with 18% of controls. Robust improvements in the Disease Activity Score 28 C-reactive protein (DAS28-CRP) were also noted in the VX-509-treated patients. The improvement in these two primary endpoints was rapid, with the oral JAK 3 inhibitor’s advantage over placebo becoming significant during the first week, reported Dr. Mark C. Genovese, professor of medicine at Stanford (Calif.) University.
Infections, the most common type of adverse events, occurred in 22% of patients on VX-509 and 15.5% on placebo. Serious infections occurred in 2.8% of patients on the JAK 3 inhibitor, twice the rate observed in controls. Bronchitis, pneumonia, cellulitis, and one severe case of herpes zoster accounted for most of the serious infections in the VX-509 group.
Modest elevations in transaminase levels and reductions in median neutrophil and lymphocyte counts occurred in the VX-509 groups. In addition, dose-dependent increases were observed in low-density lipoprotein (LDL) cholesterol and fasting triglycerides. The LDL increases ranged from 3.1 to 11.6 mg/dL, while triglyceride levels in VX-509–treated patients climbed by 25.7-39 mg/dL.
JAK 3 is exclusively involved in immune function and prevents signaling by inflammatory cytokines, including interleukin-2, -4, -7, -9, -15, and -21. VX-509’s high degree of selectivity for JAK 3 is desirable, Dr. Genovese explained, because the drug doesn’t target JAK 2, which is involved growth factor and hematopoietic signaling and whose inhibition could thereby lead to unwanted effects.
Vertex Pharmaceuticals, which sponsored the phase IIb study, subsequently announced its strong interest in further developing VX-509 for the marketplace. Dr. Genovese disclosed having received research grants from and serving as a consultant to Vertex.
SAN DIEGO – A novel oral Janus kinase 3 inhibitor known for now as VX-509 readily hit both of its coprimary endpoints in a phase IIb study in rheumatoid arthritis patients presented at the annual meeting of the American College of Rheumatology.
The 24-week, double-blind, international study included 358 patients with active rheumatoid arthritis on stable doses of background methotrexate who were randomized to one of four VX-509 dosing regimens or placebo.

An ACR20 response at 12 weeks occurred in 47%-68% of subjects on various doses of the oral JAK 3 inhibitor, compared with 18% of controls. Robust improvements in the Disease Activity Score 28 C-reactive protein (DAS28-CRP) were also noted in the VX-509-treated patients. The improvement in these two primary endpoints was rapid, with the oral JAK 3 inhibitor’s advantage over placebo becoming significant during the first week, reported Dr. Mark C. Genovese, professor of medicine at Stanford (Calif.) University.
Infections, the most common type of adverse events, occurred in 22% of patients on VX-509 and 15.5% on placebo. Serious infections occurred in 2.8% of patients on the JAK 3 inhibitor, twice the rate observed in controls. Bronchitis, pneumonia, cellulitis, and one severe case of herpes zoster accounted for most of the serious infections in the VX-509 group.
Modest elevations in transaminase levels and reductions in median neutrophil and lymphocyte counts occurred in the VX-509 groups. In addition, dose-dependent increases were observed in low-density lipoprotein (LDL) cholesterol and fasting triglycerides. The LDL increases ranged from 3.1 to 11.6 mg/dL, while triglyceride levels in VX-509–treated patients climbed by 25.7-39 mg/dL.
JAK 3 is exclusively involved in immune function and prevents signaling by inflammatory cytokines, including interleukin-2, -4, -7, -9, -15, and -21. VX-509’s high degree of selectivity for JAK 3 is desirable, Dr. Genovese explained, because the drug doesn’t target JAK 2, which is involved growth factor and hematopoietic signaling and whose inhibition could thereby lead to unwanted effects.
Vertex Pharmaceuticals, which sponsored the phase IIb study, subsequently announced its strong interest in further developing VX-509 for the marketplace. Dr. Genovese disclosed having received research grants from and serving as a consultant to Vertex.
SAN DIEGO – A novel oral Janus kinase 3 inhibitor known for now as VX-509 readily hit both of its coprimary endpoints in a phase IIb study in rheumatoid arthritis patients presented at the annual meeting of the American College of Rheumatology.
The 24-week, double-blind, international study included 358 patients with active rheumatoid arthritis on stable doses of background methotrexate who were randomized to one of four VX-509 dosing regimens or placebo.

An ACR20 response at 12 weeks occurred in 47%-68% of subjects on various doses of the oral JAK 3 inhibitor, compared with 18% of controls. Robust improvements in the Disease Activity Score 28 C-reactive protein (DAS28-CRP) were also noted in the VX-509-treated patients. The improvement in these two primary endpoints was rapid, with the oral JAK 3 inhibitor’s advantage over placebo becoming significant during the first week, reported Dr. Mark C. Genovese, professor of medicine at Stanford (Calif.) University.
Infections, the most common type of adverse events, occurred in 22% of patients on VX-509 and 15.5% on placebo. Serious infections occurred in 2.8% of patients on the JAK 3 inhibitor, twice the rate observed in controls. Bronchitis, pneumonia, cellulitis, and one severe case of herpes zoster accounted for most of the serious infections in the VX-509 group.
Modest elevations in transaminase levels and reductions in median neutrophil and lymphocyte counts occurred in the VX-509 groups. In addition, dose-dependent increases were observed in low-density lipoprotein (LDL) cholesterol and fasting triglycerides. The LDL increases ranged from 3.1 to 11.6 mg/dL, while triglyceride levels in VX-509–treated patients climbed by 25.7-39 mg/dL.
JAK 3 is exclusively involved in immune function and prevents signaling by inflammatory cytokines, including interleukin-2, -4, -7, -9, -15, and -21. VX-509’s high degree of selectivity for JAK 3 is desirable, Dr. Genovese explained, because the drug doesn’t target JAK 2, which is involved growth factor and hematopoietic signaling and whose inhibition could thereby lead to unwanted effects.
Vertex Pharmaceuticals, which sponsored the phase IIb study, subsequently announced its strong interest in further developing VX-509 for the marketplace. Dr. Genovese disclosed having received research grants from and serving as a consultant to Vertex.
AT THE ACR ANNUAL MEETING
Major finding: Rheumatoid arthritis patients had ACR20 responses of 47%-68% to various dosing regimens of the novel oral Janus kinase 3 inhibitor VX-509 at 12 weeks, compared with 18% in placebo-treated controls.
Data source: This was a phase IIb, double-blind, placebo-controlled, international study involving 358 patients with rheumatoid arthritis on background stable doses of methotrexate, continued during the trial.
Disclosures: The study was sponsored by Vertex Pharmaceuticals. The presenter disclosed receiving research grants from and serving as a consultant to Vertex.