User login
Endometriosis has always posed a treatment challenge. Take the early 19th Century, for example, before the widespread advent of surgery, when the disease was managed by applying leeches to the cervix. In fact, as Nezhat and colleagues note in their comprehensive survey of the 4,000-year history of endometriosis, “leeches were considered a mainstay in treating any condition associated with menstruation.”1
Fast forward to the 21st Century, and the picture is a lot clearer, though still not crystal clear. The optimal approach to endometriosis depends on numerous factors, foremost among them the chief complaint of the patient—pain or infertility (or both).
In this article—Part 2 of a 3-part series on endometriosis—the focus is on medical and surgical management of pain. Six experts address such questions as when is laparoscopy indicated, who is best qualified to treat endometriosis, is excision or ablation of lesions preferred, what is the role of hysterectomy in eliminating pain, and what to do about the problem of recurrence.
In Part 3, to be published in the June 2015 issue of OBG Management, endometriosis-associated infertility will be the topic of discussion.
In Part 1, 7 experts answer crucial questions on the diagnosis of endometriosis.
For a detailed look at the pathophysiology of endometriosis-associated pain, see “Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain,” by Kenneth A. Levey, MD, MPH, in this issue.
1. What are the options for empiric therapy?
One reason for the diagnostic delay for endometriosis, which still averages about 6 years, is that definitive diagnosis is achieved only through laparoscopic investigation and histologic confirmation. For many women who experience pain thought to be associated with endometriosis, however, clinicians begin empiric treatment with medical agents as a way to avert the need for surgery, if at all possible.
“There is no cure for endometriosis,” says John R. Lue, MD, MPH, “but there are many ways that endometriosis can be treated” and the impact of the disease reduced in a patient’s life. Dr. Lue is Associate Professor and Chief of the Section of General Obstetrics and Gynecology and Medical Director of Women’s Ambulatory Services at the Medical College of Georgia and Georgia Regents University in Augusta, Georgia.
Among the medical and hormonal management options:
- Nonsteroidal anti-inflammatory drugs (NSAIDs), often used with combined oral contraceptives (OCs). NSAIDs are not a long-term treatment option because of their effect on cyclo-oxygenase (COX) 1 and 2 enzymes, says Dr. Lue. COX-1 protects the gastrointestinal (GI) system, and prolonged use of NSAIDs can cause adverse GI effects.
- Cyclic combined OCs “are recommended as first-line therapy in the absence of contraindications,” says Dr. Lue, and are often used in combination with NSAIDs. However, the failure rate may be as high as 20% to 25%.2 “If pain persists after a trial of 3 to 6 months of cyclic OCs, one can consider switching to continuous low-dose combined OCs for an additional 6 months,” says Dr. Lue. When combined OCs were compared with placebo in the treatment of dysmenorrhea, they reduced baseline pain scores by 45% to 52%, compared with 14% to 17% for placebo (P<.001).2 They also reduced the volume of endometriomas by 48%, compared with 32% for placebo (P = .04). According to Linda C. Giudice, MD, PhD, “In women with severe dysmenorrhea who have been treated with cyclic combined OCs, a switch to continuous combined OCs reduced pain scores by 58% within 6 months and by 75% at 2 years” (P<.001).2 Dr. Giudice is the Robert B. Jaffe, MD, Endowed Professor in the Reproductive Sciences and Chair of Obstetrics, Gynecology, and Reproductive Sciences at the University of California, San Francisco.
- Depot medroxyprogesterone acetate (DMPA) or the levonorgestrel-releasing intrauterine system (LNG-IUS). These agents suppress the hypothalamic-pituitary-ovarian (HPO) axis to different degrees. DMPA suppresses the HPO completely, preventing ovulation. The LNG-IUS does not fully suppress the HPO but acts directly on endometrial tissue, with antiproliferative effects on eutopic and endometriotic implants, says Dr. Lue. The LNG-IUS also is effective at suppressing disease after surgical treatment, says Dr. Giudice.2
- Gonadotropin-releasing hormone (GnRH) agonist therapy, with estrogen and/or progestin add-back therapy to temper the associated loss in bone mineral density, “may be effective—if only temporarily—as it inhibits the HPO axis and blocks ovarian function, thereby greatly reducing systemic estrogen levels and inducing artificial menopause,” says Dr. Lue.
- Norethindrone acetate, a synthetic progestational agent, is occasionally used as empiric therapy for endometriosis because of its ability to inhibit ovulation. It has antiandrogenic and antiestrogenic effects.
- Aromatase inhibitors. Dr. Lue points to considerable evidence that endometriotic implants are an autocrine source of estrogen.3 “This locally produced estrogen results from overexpression of the enzyme P450 aromatase by endometriotic tissue,” he says. Consequently, in postmenopausal women, “aromatase inhibitors may be used orally in a daily pill form to curtail endometriotic implant production of estrogen and subsequent implant growth.”4 In women of reproductive age, aromatase inhibitors are combined with an HPO-suppressive therapy, such as norethindrone acetate. These strategies represent off-label use of aromatase inhibitors.
- Danazol, a synthetic androgen, has been used in the past to treat dysmenorrhea and dyspareunia. Because of its severe androgenic effects, however, it is not widely used today.
“For those using medical approaches, endometriosis-related pain may be reduced by using hormonal treatments to modify reproductive tract events, thereby decreasing local peritoneal inflammation and cytokine production,” says Pamela Stratton, MD. Because endometriosis is a “central sensitivity syndrome,” multidisciplinary approaches may be beneficial to treat myofascial dysfunction and sensitization, such as physical therapy. “Chronic pain conditions that overlap with endometriosis-associated pain, such as migraines, irritable bowel syndrome, or painful bladder syndrome should be identified and treated. Mood changes of depression and anxiety common to women with endometriosis-associated pain also warrant treatment,” she says.
Dr. Stratton is Chief of the Gynecology Consult Service, Program in Reproductive and Adult Endocrinology, at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
2. When is laparoscopy indicated?
When medical and hormonal treatments fail to control a patient’s pain, laparoscopy is indicated to confirm the diagnosis of endometriosis. During that procedure, it is also advisable to treat any endometriosis that is present, provided the surgeon is highly experienced in such treatment.
Proper treatment is preferable—even if it requires expert consultation. “No treatment and referral to a more experienced surgeon are better than incomplete treatment by an inexperienced surgeon,” says Ceana Nezhat, MD. “Not all GYN surgeons have the expertise to treat advanced endometriosis.” Dr. Nezhat is Director of the Nezhat Medical Center and Medical Director of Training and Education at Northside Hospital, both in Atlanta, Georgia.
Dr. Stratton agrees about the importance of thorough treatment of endometriosis at the time of diagnostic laparoscopy. “At the laparoscopy, the patient benefits if all potential sources of pain are investigated and addressed.” At surgery, the surgeon should look for and treat any lesions suspicious for endometriosis, as well as any other finding that might contribute to pain, she says. “For example, routinely inspecting the appendix for endometriosis or other lesions, and removing affected appendices is reasonable; also, lysis and, where possible, excision of adhesions is an important strategy.”
If a medical approach fails for a patient, “then surgery is indicated to confirm the diagnosis and treat the disease,” agrees Tommaso Falcone, MD.
“Surgery is very effective in treating the pain associated with endometriosis,” Dr. Falcone continues. Randomized clinical trials have shown that up to 90% of patients who obtain pain relief from surgery will have an effect lasting 1 year.6 If patients do not get relief, then the association of the pain with endometriosis should be questioned and other causes searched.” Dr. Falcone is Professor and Chair of Obstetrics and Gynecology at the Cleveland Clinic in Cleveland, Ohio.
The most common anatomic sites of implants
“The most common accepted theory for pathogenesis of endometriosis suggests that implants develop when debris from retrograde menstruation attaches to the pelvic peritoneum,” says Dr. Stratton.7 “Thus, the vast majority of lesions occur in the dependent portions of the pelvis, which include the ovarian fossae (posterior broad ligament under the ovaries), cul de sac, and the uterosacral ligaments.8 The bladder peritoneum, ovarian surface, uterine peritoneal surface, fallopian tube, and pelvic sidewall are also frequent sites. The colon and appendix are less common sites, and small bowel lesions are rare.”
“However, pain location does not correlate with lesion location,” Dr. Stratton notes. “For this reason, the goal at surgery is to treat all lesions, even ones that are not in sites of pain.”
3. How should disease be staged?
Most surgeons with expertise in treating endometriosis attempt to stage the disease at the time of initial laparoscopy, even though a patient’s pain does not always correlate with the stage of disease.
“The staging system for endometriosis is a means to systematically catalogue where lesions are located,” says Dr. Stratton.
The most commonly used classification system was developed by the American Society for Reproductive Medicine (ASRM). It takes into account such characteristics as how deep an implant lies, the extent to which it obliterates the posterior cul de sac, and the presence and extent of adhesions. Although the classification system is broken down into 4 stages ranging from minimal to severe disease, it is fairly complex. For example, it assigns a score for each lesion as well as the size and location of that lesion, notes Dr. Stratton. The presence of an endometrioma automatically renders the disease as stage III or IV, and an obliterated cul de sac means the endometriosis is graded as stage IV.
“This system enables us to communicate with each other about patients and may guide future surgeries for assessment of lesion recurrence or the planning of treatment for lesions the surgeon was unable to treat at an initial surgery,” says Dr. Stratton.
“Women with uterosacral nodularity, fixed pelvic organs, or severe pain with endometriomas may have deep infiltrating lesions. These lesions, in particular, are not captured well with the current staging system,” says Dr. Stratton. Because they appear to be innervated, “the greatest benefit to the patient is achieved by completely excising these lesions.” Preoperative imaging may help confirm the existence, location, and extent of these deep lesions and help the surgeon plan her approach “based on clinical and imaging findings.”
“Severity of pain or duration of surgical effect does not correlate with stage or extent of disease,” Dr. Stratton says.9 “In fact, patients with the least amount of disease noted at surgery experience pain sooner, suggesting that the central nervous system may have been remodeled prior to surgery or that the pain is in part due to some other cause.10 This observation underscores the principle that, while endometriosis may initiate pain, the pain experience is determined by engagement of the central nervous system.”
For more information on the ASRM revised classification of endometriosis, go to http://www.fertstert.org/article/S0015-0282(97)81391-X/pdf.
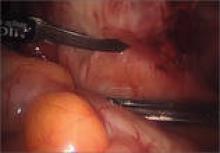
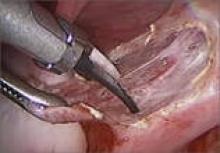
4. Which is preferable—excision or ablation?
In a prospective, randomized, double-blind study, Healey and colleagues compared pain levels following laparoscopic treatment of endometriosis with either excision or ablation. Preoperatively, women in the study completed a questionnaire rating various types of pain using visual analogue scales. They then were randomly assigned to treatment of endometriosis via excision or ablation. Postoperatively, they again completed a questionnaire about pain levels at 3, 6, 9, and 12 months. Investigators found no significant difference in pain scores at 12 months.11
Five-year follow-up of the same population yielded slightly different findings, however. Although there was a reduction in all pain scores at 5 years in both the excision and ablation groups, a significantly greater reduction in dyspareunia was observed in the excision group at 5 years.12
In an editorial accompanying the 5-year follow-up data, Dr. Falcone and a coauthor called excision versus ablation of ovarian, bowel, and peritoneal endometriosis one of the “great debates” in the surgical management of endometriosis.13
“When there is deep involvement of adjacent organs, there is general consensus that excision is best for optimal surgical outcome,” they write. “However, for disease involving the peritoneum alone, there are proponents for either option.”13
“This is a very controversial issue,” says Dr. Falcone, “and the debate can sometimes be somewhat inflammatory…. It is hard to understand how a comparative trial could even be accomplished between excision and ablation,” he adds. “In my experience, deep disease typically occurs on the pelvic sidewall over the ureter or in the cul de sac on the bowel or infiltrating the bladder peritoneum. Therefore, ablation would increase the risk of damaging any of these structures. With superficial disease away from critical structures, it should be fine to ablate. Everywhere else and with deep disease you need to excise or leave disease behind.”
“Endometriomas are a special situation,” Dr. Falcone says. “Excision of the cyst has been shown in randomized controlled trials (RCTs) to be associated with less risk of recurrence.14 Therefore, it should be the treatment of choice. However, in patients interested in future fertility, we must take into consideration the potential damage to ovarian reserve associated with excision.”
Endometriosis of the ovaries has unique manifestations. “My approach to ovarian cysts depends on their classification,” says Dr. Nezhat.15 In general, primary endometriomas (Type 1) are small, superficial cysts that contain dark “chocolate” fluid. They tend to be firmly adherent to the ovarian tissue and difficult to remove surgically.
Secondary endometriomas (Type 2) are follicular or luteal cysts that have been involved or invaded by cortical endometriotic implants or by primary endometrioma. Secondary endometriomas are further classified by the relationship between cortical endometriosis and the cyst wall. Type 2A endometriomas are usually large, with a capsule that is easily separated from ovarian tissue. Type 2B endometriomas have some features of functional cysts but show deep involvement with surface endometriosis. Type 2C endometriomas are similar, showing extensive surface endometrial implants but with deep penetration of the endometriosis into the cyst wall.15
“For Type 1 endometriomas, I biopsy the cyst to ensure the lesion is benign, then vaporize the endometrioma,” Dr. Nezhat says. “In cases of type 2A and 2B endometriomas, the cyst capsule is easily enucleated and removed. Type 2C endometriomas are biopsied as well and then I proceed with vaporizing the fibrotic area with a low-power energy source, such as neutral argon plasma, avoiding excessive coagulation and thermal injury.” Recent literature supports the idea of evaluation and biopsy of fibrotic endometriomas to confirm benign conditions, followed by ablation without compromising ovarian function.16
“Excision and ablation both have indications,” Dr. Nezhat asserts. “It depends on the location and depth of penetration of implants, as well as the patient’s ultimate goal. For example, if the patient desires future fertility and has endometriosis on the ovary, removal by excision could damage ovarian function. The same holds true for endometriosis on the fallopian tubes. It’s better in such cases to ablate.”
“Ablation is different from coagulation, which is not recommended,” Dr. Nezhat explains. “Ablation vaporizes the diseased area layer by layer, like peeling an onion, until the disease is eradicated. It is similar to dermatological skin resurfacing. Vaporization is preferable for endometriosis on the tubes and ovaries in patients who desire pregnancy. The choice between excision and ablation depends on the location, depth of penetration, and the patient’s desire for fertility.”
Either way, and regardless of the primary indication for surgery—pain versus infertility—a minimally invasive gynecologic surgeon is expected to have ability in performing both techniques, Dr. Nezhat says.
The following videos have been provided by AAGL SurgeryU to compliment the content of this article regarding endometriosis. You can watch these videos, and more than 1,500 others, at AAGL.org/surgeryu. Laparoscopic excision of stage IV endometriosis Einarsson JI This case, originally presented as a SurgeryU live event, features a 41-year-old woman (G3P1) with a 3-year history of left-sided pelvic pain, deep dyspareunia, constipation, and dysmenorrhea. She also has infertility and is planning an IVF treatment shortly. On examination she was noted to have significant rectovaginal tenderness and nodularity. A pelvic MRI demonstrated a 3-cm irregular mass extending from the cervix into the cul-de-sac up to the left lateral pelvic sidewall. Abdominal wall endometriosis Hawkins E, Patzkowsky K, Lopez J This video demonstrates a typical presentation of abdominal wall endometriosis (AWE), also known as subcutaneous endometriosis or scar endometriosis. It is important for gynecologists to be familiar with this more uncommon form of the disease and its management. This video also demonstrates surgical management of advanced AWE involving the subcutaneous tissue, fascia, and rectus muscle. Laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain Pendergrass M This video depicts the laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain. The patient underwent menarche at age 11 and developed cyclic pelvic pain 6 months later. Due to the severity of the pain she has been unable to attend school for the past 2 months, and has stopped participating in sports. A diagnostic laparoscopy revealed red/brown superficial endometriosis lesions on the peritoneum in the posterior cul de sac, bilateral uterosacral ligaments, and bilateral broad ligaments. |
5. Is hysterectomy definitive treatment for pain?
“Not necessarily,” says Dr. Nezhat. “Hysterectomy by itself doesn’t take care of endometriosis unless the patient has adenomyosis. If a patient has endometriosis, the first step is complete treatment of the disease to restore the anatomy. Then the next step might be hysterectomy to give a better long-term result, especially in cases of adenomyosis. Removal of the ovaries at the time of hysterectomy has to be individualized.”
“The implication that hysterectomy ‘cures’ endometriosis is false yet is stated in some textbooks,” says Dr. Nezhat. “Even at the time of hysterectomy, the first step should be complete treatment of endometriosis and restoration of anatomy, followed by the hysterectomy. Leaving endometriosis behind, believing it will go away by itself or not cause future issues, is a gross misperception.”
Removal of the ovaries at hysterectomy?
“There are few comparative studies on the long-term follow-up of patients who have undergone hysterectomy with or without removal of both ovaries,” says Dr. Falcone. “The conventional dogma has been that, in women undergoing definitive surgery for endometriosis, both ovaries should be removed, even if they are normal. I personally believe that this was the case because hysterectomy was often performed without excision of the endometriosis. So the uterus was removed and disease was left behind. In these cases, recurrent symptoms were due to persistent disease.”
“We reported our experience at the Cleveland Clinic with a 7-year follow-up,” Dr. Falcone continues. “Hysterectomy was performed with excision of all visible disease. Ovaries were conserved if normal and removed if they had disease. We looked at the reoperation-free frequency over time. In women undergoing hysterectomy with excision of visible disease but ovarian preservation, the reoperation-free percentages at 2, 5, and 7 years were 95%, 86%, and 77%, respectively, versus 96%, 91%, and 91% in those without ovarian preservation. So, overall, there was an advantage over time for removal of the ovaries. However, in the subset of women between ages 30 and 39 years, there was no difference in the long-term recurrence rate if the ovaries were left in. For this reason, in women under 40, we recommend keeping normal ovaries if all disease is removed.”17
6. Can the risk of postoperative recurrence be reduced?
“The main problem with surgery is the recurrence rate,” Dr. Falcone says. “Studies have shown that the recurrence rate of pain at 7 years may be as high as 50%.”17 Furthermore, “the recurrence of pain may not be associated with visualized endometriosis at laparoscopy.”
“Incomplete removal of lesions may be associated with an increase in pain after surgery,” says Dr. Stratton.18 “Incomplete removal of lesions may occur because of varying technical skill or specific lesion characteristics. The lesions may be difficult to remove because of their location. Lesions may not be recognized because their appearance can vary from subtle (red or clear or white) to classic (blue-black). The depth of the lesion may not be appreciated until surgery is under way and a surgeon may not be adequately prepared to treat deep lesions when they are identified.”
Another reason pain may persist or recur after surgery for endometriosis: Adenomyosis.19 “Adenomyosis appears as either diffuse or focal thickening of the junctional zone between the endometrium and myometrium of the uterus on T2 weighted magnetic resonance imaging (MRI),” says Dr. Stratton. “After excision of endometriosis, chronic pelvic pain is significantly more likely to persist in women who have a junctional zone thickness of more than 11 mm on MRI,” she says.
The frequent recurrence of pain after surgery for endometriosis means that the disease is a long-term challenge.
“Pelvic pain caused by endometriosis is a chronic problem that requires a multiyear management plan, involving both surgery and hormonal therapy,” says Robert L. Barbieri, MD. “To reduce the number of surgical procedures in the lifetime of a woman with endometriosis and pain, I suggest hormonal medical therapy following conservative surgery for endometriosis.”
“Definitive surgery, such as hysterectomy or hysterectomy plus bilateral salpingo-oophorectomy (BSO) typicallyresults in prolonged symptom relief,” Dr. Barbieri says. “Following hysterectomy, hormonal therapy may not be needed. Following BSO, low-dose hormonal therapy is often needed to reduce the severity of menopausal symptoms.”
Dr. Barbieri is Editor in Chief of OBG Management; Chair of Obstetrics and Gynecology at Brigham and Women’s Hospital in Boston, Massachusetts; and the Kate Macy Ladd Professor of Obstetrics, Gynecology, and Reproductive Biology at Harvard Medical School in Boston.
After surgical treatment of endometriosis associated with pain, Dr. Barbieri presents the patient with the following menu of hormonal options:
- no hormonal therapy
- estrogen-progestin contraceptives, either cyclic or continuous
- the LNG-IUS
- norethindrone acetate 5 mg daily
- DMPA 150 mg every 3 months
- leuprolide acetate depot 3.75 mg intramuscularly monthly
- nafarelin nasal spray 200 µg twice a day
- danazol 200 mg twice a day.
“I explain the side effects common with each approach and have the patient select what she determines to be her best option,” says Dr. Barbieri. “In my experience, conservative surgery followed by hormonal therapy is effective in more than 75% of women.”
“The evidence to support postoperative hormonal therapy is modest,” Dr. Barbieri notes. “The best evidence is available for use of the LNG-IUS, estrogen-progestin contraceptives, and GnRH agonists.”20–22
In addition, “major professional societies have highlighted the option of postoperative hormonal therapy to reduce the risk of recurrent pain and repetitive surgical procedures in the future,” Dr. Barbieri says.23,24
When pain recurs after surgery for endometriosis, it pays to consider what type of pain it is, says Dr. Barbieri.
“There are 2 major types of pain—nociceptive and neuropathic,” he says. “Nociceptive pain is caused by an injury, acute or chronic. Neuropathic pain is caused by ‘activation’ of neural circuits, sometimes in the absence of an ongoing injury. Many women with endometriosis and chronic pain have both nociceptive and neuropathic pain. Consequently, it is important to consider the use of a multidisciplinary pain practice in the management of chronic pain syndromes. Multidisciplinary pain practices have special expertise in the management of neuropathic pain. Standard conservative surgical intervention is unlikely to improve pain caused by neuropathic mechanisms. Likewise, opioid analgesics are not recommended for the treatment of neuropathic pain.”
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6S):S1–S62.
2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398.
3. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis: a review. Fertil Steril. 2012;98(6):1370–1379.
4. Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;9:87.
5. Chwalisz K, Garg R, Brenner RM, Schubert G, Elger W. Selective progesterone receptor modulators (SPRMs): a novel therapeutic concept in endometriosis. Ann N Y Acad Sci. 2002;955:373–393, 396–406.
6. Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031.
7. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279.
8. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632–1636.
9. Hsu AL, Sinaii N, Segars J, Nieman LK, Stratton P. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011;118(2 pt 1):223–230.
10. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346.
11. Healey M, Ang WC, Cheng C. Surgical treatment of endometriosis: a prospective randomized double-blinded trial comparing excision and ablation. Fertil Steril. 2010;94(7):2536–2540.
12. Healey M, Chang C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. JMIG. 2014;21(6):999–1004.
13. Falcone T, Wilson JR. Surgical management of endometriosis: excision or ablation. JMIG. 2014;21(6):969.
14. Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
15. Nezhat C, Nezhat F, Nezhat CH, Seidman D. Classification of endometriosis: improving the classification of endometriotic ovarian cysts. Hum Reprod 1994;9(12):2212–2216.
16. Roman H, Auber M, Mokdad C, et al. Ovarian endometrioma ablation using plasma energy versus cystectomy: a step toward better preservation of the ovarian parenchyma in women wishing to conceive. Fertil Steril. 2011;96(6):1396–1400.
17. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285–1292.
18. McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147(1–3):255–264.
19. Parker JD, Leondires M, Sinaii N, Premkumar A, Nieman LK, Stratton P. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86(3):711–715.
20. Abou-Setta AM, Al-Inany HG, Farquar CM. Levonorgestrel-releasing intrauterine device for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;(1):CD005072.
21. Seracchioli R, Mabrouk M, Manuzzi L, et al. Postoperative use of oral contraceptive pills for prevention of anatomic relapse or symptom recurrence following surgery. Hum Reprod. 2009;24(11):2729–2735.
22. Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertil Steril. 1997;68(5):860–864.
23. Practice Committee of the American Society for Reproductive Medicine. Treatment of pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927–935.
24. Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
Endometriosis has always posed a treatment challenge. Take the early 19th Century, for example, before the widespread advent of surgery, when the disease was managed by applying leeches to the cervix. In fact, as Nezhat and colleagues note in their comprehensive survey of the 4,000-year history of endometriosis, “leeches were considered a mainstay in treating any condition associated with menstruation.”1
Fast forward to the 21st Century, and the picture is a lot clearer, though still not crystal clear. The optimal approach to endometriosis depends on numerous factors, foremost among them the chief complaint of the patient—pain or infertility (or both).
In this article—Part 2 of a 3-part series on endometriosis—the focus is on medical and surgical management of pain. Six experts address such questions as when is laparoscopy indicated, who is best qualified to treat endometriosis, is excision or ablation of lesions preferred, what is the role of hysterectomy in eliminating pain, and what to do about the problem of recurrence.
In Part 3, to be published in the June 2015 issue of OBG Management, endometriosis-associated infertility will be the topic of discussion.
In Part 1, 7 experts answer crucial questions on the diagnosis of endometriosis.
For a detailed look at the pathophysiology of endometriosis-associated pain, see “Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain,” by Kenneth A. Levey, MD, MPH, in this issue.
1. What are the options for empiric therapy?
One reason for the diagnostic delay for endometriosis, which still averages about 6 years, is that definitive diagnosis is achieved only through laparoscopic investigation and histologic confirmation. For many women who experience pain thought to be associated with endometriosis, however, clinicians begin empiric treatment with medical agents as a way to avert the need for surgery, if at all possible.
“There is no cure for endometriosis,” says John R. Lue, MD, MPH, “but there are many ways that endometriosis can be treated” and the impact of the disease reduced in a patient’s life. Dr. Lue is Associate Professor and Chief of the Section of General Obstetrics and Gynecology and Medical Director of Women’s Ambulatory Services at the Medical College of Georgia and Georgia Regents University in Augusta, Georgia.
Among the medical and hormonal management options:
- Nonsteroidal anti-inflammatory drugs (NSAIDs), often used with combined oral contraceptives (OCs). NSAIDs are not a long-term treatment option because of their effect on cyclo-oxygenase (COX) 1 and 2 enzymes, says Dr. Lue. COX-1 protects the gastrointestinal (GI) system, and prolonged use of NSAIDs can cause adverse GI effects.
- Cyclic combined OCs “are recommended as first-line therapy in the absence of contraindications,” says Dr. Lue, and are often used in combination with NSAIDs. However, the failure rate may be as high as 20% to 25%.2 “If pain persists after a trial of 3 to 6 months of cyclic OCs, one can consider switching to continuous low-dose combined OCs for an additional 6 months,” says Dr. Lue. When combined OCs were compared with placebo in the treatment of dysmenorrhea, they reduced baseline pain scores by 45% to 52%, compared with 14% to 17% for placebo (P<.001).2 They also reduced the volume of endometriomas by 48%, compared with 32% for placebo (P = .04). According to Linda C. Giudice, MD, PhD, “In women with severe dysmenorrhea who have been treated with cyclic combined OCs, a switch to continuous combined OCs reduced pain scores by 58% within 6 months and by 75% at 2 years” (P<.001).2 Dr. Giudice is the Robert B. Jaffe, MD, Endowed Professor in the Reproductive Sciences and Chair of Obstetrics, Gynecology, and Reproductive Sciences at the University of California, San Francisco.
- Depot medroxyprogesterone acetate (DMPA) or the levonorgestrel-releasing intrauterine system (LNG-IUS). These agents suppress the hypothalamic-pituitary-ovarian (HPO) axis to different degrees. DMPA suppresses the HPO completely, preventing ovulation. The LNG-IUS does not fully suppress the HPO but acts directly on endometrial tissue, with antiproliferative effects on eutopic and endometriotic implants, says Dr. Lue. The LNG-IUS also is effective at suppressing disease after surgical treatment, says Dr. Giudice.2
- Gonadotropin-releasing hormone (GnRH) agonist therapy, with estrogen and/or progestin add-back therapy to temper the associated loss in bone mineral density, “may be effective—if only temporarily—as it inhibits the HPO axis and blocks ovarian function, thereby greatly reducing systemic estrogen levels and inducing artificial menopause,” says Dr. Lue.
- Norethindrone acetate, a synthetic progestational agent, is occasionally used as empiric therapy for endometriosis because of its ability to inhibit ovulation. It has antiandrogenic and antiestrogenic effects.
- Aromatase inhibitors. Dr. Lue points to considerable evidence that endometriotic implants are an autocrine source of estrogen.3 “This locally produced estrogen results from overexpression of the enzyme P450 aromatase by endometriotic tissue,” he says. Consequently, in postmenopausal women, “aromatase inhibitors may be used orally in a daily pill form to curtail endometriotic implant production of estrogen and subsequent implant growth.”4 In women of reproductive age, aromatase inhibitors are combined with an HPO-suppressive therapy, such as norethindrone acetate. These strategies represent off-label use of aromatase inhibitors.
- Danazol, a synthetic androgen, has been used in the past to treat dysmenorrhea and dyspareunia. Because of its severe androgenic effects, however, it is not widely used today.
“For those using medical approaches, endometriosis-related pain may be reduced by using hormonal treatments to modify reproductive tract events, thereby decreasing local peritoneal inflammation and cytokine production,” says Pamela Stratton, MD. Because endometriosis is a “central sensitivity syndrome,” multidisciplinary approaches may be beneficial to treat myofascial dysfunction and sensitization, such as physical therapy. “Chronic pain conditions that overlap with endometriosis-associated pain, such as migraines, irritable bowel syndrome, or painful bladder syndrome should be identified and treated. Mood changes of depression and anxiety common to women with endometriosis-associated pain also warrant treatment,” she says.
Dr. Stratton is Chief of the Gynecology Consult Service, Program in Reproductive and Adult Endocrinology, at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
2. When is laparoscopy indicated?
When medical and hormonal treatments fail to control a patient’s pain, laparoscopy is indicated to confirm the diagnosis of endometriosis. During that procedure, it is also advisable to treat any endometriosis that is present, provided the surgeon is highly experienced in such treatment.
Proper treatment is preferable—even if it requires expert consultation. “No treatment and referral to a more experienced surgeon are better than incomplete treatment by an inexperienced surgeon,” says Ceana Nezhat, MD. “Not all GYN surgeons have the expertise to treat advanced endometriosis.” Dr. Nezhat is Director of the Nezhat Medical Center and Medical Director of Training and Education at Northside Hospital, both in Atlanta, Georgia.
Dr. Stratton agrees about the importance of thorough treatment of endometriosis at the time of diagnostic laparoscopy. “At the laparoscopy, the patient benefits if all potential sources of pain are investigated and addressed.” At surgery, the surgeon should look for and treat any lesions suspicious for endometriosis, as well as any other finding that might contribute to pain, she says. “For example, routinely inspecting the appendix for endometriosis or other lesions, and removing affected appendices is reasonable; also, lysis and, where possible, excision of adhesions is an important strategy.”
If a medical approach fails for a patient, “then surgery is indicated to confirm the diagnosis and treat the disease,” agrees Tommaso Falcone, MD.
“Surgery is very effective in treating the pain associated with endometriosis,” Dr. Falcone continues. Randomized clinical trials have shown that up to 90% of patients who obtain pain relief from surgery will have an effect lasting 1 year.6 If patients do not get relief, then the association of the pain with endometriosis should be questioned and other causes searched.” Dr. Falcone is Professor and Chair of Obstetrics and Gynecology at the Cleveland Clinic in Cleveland, Ohio.
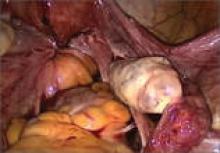
The most common anatomic sites of implants
“The most common accepted theory for pathogenesis of endometriosis suggests that implants develop when debris from retrograde menstruation attaches to the pelvic peritoneum,” says Dr. Stratton.7 “Thus, the vast majority of lesions occur in the dependent portions of the pelvis, which include the ovarian fossae (posterior broad ligament under the ovaries), cul de sac, and the uterosacral ligaments.8 The bladder peritoneum, ovarian surface, uterine peritoneal surface, fallopian tube, and pelvic sidewall are also frequent sites. The colon and appendix are less common sites, and small bowel lesions are rare.”
“However, pain location does not correlate with lesion location,” Dr. Stratton notes. “For this reason, the goal at surgery is to treat all lesions, even ones that are not in sites of pain.”
3. How should disease be staged?
Most surgeons with expertise in treating endometriosis attempt to stage the disease at the time of initial laparoscopy, even though a patient’s pain does not always correlate with the stage of disease.
“The staging system for endometriosis is a means to systematically catalogue where lesions are located,” says Dr. Stratton.
The most commonly used classification system was developed by the American Society for Reproductive Medicine (ASRM). It takes into account such characteristics as how deep an implant lies, the extent to which it obliterates the posterior cul de sac, and the presence and extent of adhesions. Although the classification system is broken down into 4 stages ranging from minimal to severe disease, it is fairly complex. For example, it assigns a score for each lesion as well as the size and location of that lesion, notes Dr. Stratton. The presence of an endometrioma automatically renders the disease as stage III or IV, and an obliterated cul de sac means the endometriosis is graded as stage IV.
“This system enables us to communicate with each other about patients and may guide future surgeries for assessment of lesion recurrence or the planning of treatment for lesions the surgeon was unable to treat at an initial surgery,” says Dr. Stratton.
“Women with uterosacral nodularity, fixed pelvic organs, or severe pain with endometriomas may have deep infiltrating lesions. These lesions, in particular, are not captured well with the current staging system,” says Dr. Stratton. Because they appear to be innervated, “the greatest benefit to the patient is achieved by completely excising these lesions.” Preoperative imaging may help confirm the existence, location, and extent of these deep lesions and help the surgeon plan her approach “based on clinical and imaging findings.”
“Severity of pain or duration of surgical effect does not correlate with stage or extent of disease,” Dr. Stratton says.9 “In fact, patients with the least amount of disease noted at surgery experience pain sooner, suggesting that the central nervous system may have been remodeled prior to surgery or that the pain is in part due to some other cause.10 This observation underscores the principle that, while endometriosis may initiate pain, the pain experience is determined by engagement of the central nervous system.”
For more information on the ASRM revised classification of endometriosis, go to http://www.fertstert.org/article/S0015-0282(97)81391-X/pdf.
4. Which is preferable—excision or ablation?
In a prospective, randomized, double-blind study, Healey and colleagues compared pain levels following laparoscopic treatment of endometriosis with either excision or ablation. Preoperatively, women in the study completed a questionnaire rating various types of pain using visual analogue scales. They then were randomly assigned to treatment of endometriosis via excision or ablation. Postoperatively, they again completed a questionnaire about pain levels at 3, 6, 9, and 12 months. Investigators found no significant difference in pain scores at 12 months.11
Five-year follow-up of the same population yielded slightly different findings, however. Although there was a reduction in all pain scores at 5 years in both the excision and ablation groups, a significantly greater reduction in dyspareunia was observed in the excision group at 5 years.12
In an editorial accompanying the 5-year follow-up data, Dr. Falcone and a coauthor called excision versus ablation of ovarian, bowel, and peritoneal endometriosis one of the “great debates” in the surgical management of endometriosis.13
“When there is deep involvement of adjacent organs, there is general consensus that excision is best for optimal surgical outcome,” they write. “However, for disease involving the peritoneum alone, there are proponents for either option.”13
“This is a very controversial issue,” says Dr. Falcone, “and the debate can sometimes be somewhat inflammatory…. It is hard to understand how a comparative trial could even be accomplished between excision and ablation,” he adds. “In my experience, deep disease typically occurs on the pelvic sidewall over the ureter or in the cul de sac on the bowel or infiltrating the bladder peritoneum. Therefore, ablation would increase the risk of damaging any of these structures. With superficial disease away from critical structures, it should be fine to ablate. Everywhere else and with deep disease you need to excise or leave disease behind.”
“Endometriomas are a special situation,” Dr. Falcone says. “Excision of the cyst has been shown in randomized controlled trials (RCTs) to be associated with less risk of recurrence.14 Therefore, it should be the treatment of choice. However, in patients interested in future fertility, we must take into consideration the potential damage to ovarian reserve associated with excision.”
Endometriosis of the ovaries has unique manifestations. “My approach to ovarian cysts depends on their classification,” says Dr. Nezhat.15 In general, primary endometriomas (Type 1) are small, superficial cysts that contain dark “chocolate” fluid. They tend to be firmly adherent to the ovarian tissue and difficult to remove surgically.
Secondary endometriomas (Type 2) are follicular or luteal cysts that have been involved or invaded by cortical endometriotic implants or by primary endometrioma. Secondary endometriomas are further classified by the relationship between cortical endometriosis and the cyst wall. Type 2A endometriomas are usually large, with a capsule that is easily separated from ovarian tissue. Type 2B endometriomas have some features of functional cysts but show deep involvement with surface endometriosis. Type 2C endometriomas are similar, showing extensive surface endometrial implants but with deep penetration of the endometriosis into the cyst wall.15
“For Type 1 endometriomas, I biopsy the cyst to ensure the lesion is benign, then vaporize the endometrioma,” Dr. Nezhat says. “In cases of type 2A and 2B endometriomas, the cyst capsule is easily enucleated and removed. Type 2C endometriomas are biopsied as well and then I proceed with vaporizing the fibrotic area with a low-power energy source, such as neutral argon plasma, avoiding excessive coagulation and thermal injury.” Recent literature supports the idea of evaluation and biopsy of fibrotic endometriomas to confirm benign conditions, followed by ablation without compromising ovarian function.16
“Excision and ablation both have indications,” Dr. Nezhat asserts. “It depends on the location and depth of penetration of implants, as well as the patient’s ultimate goal. For example, if the patient desires future fertility and has endometriosis on the ovary, removal by excision could damage ovarian function. The same holds true for endometriosis on the fallopian tubes. It’s better in such cases to ablate.”
“Ablation is different from coagulation, which is not recommended,” Dr. Nezhat explains. “Ablation vaporizes the diseased area layer by layer, like peeling an onion, until the disease is eradicated. It is similar to dermatological skin resurfacing. Vaporization is preferable for endometriosis on the tubes and ovaries in patients who desire pregnancy. The choice between excision and ablation depends on the location, depth of penetration, and the patient’s desire for fertility.”
Either way, and regardless of the primary indication for surgery—pain versus infertility—a minimally invasive gynecologic surgeon is expected to have ability in performing both techniques, Dr. Nezhat says.
The following videos have been provided by AAGL SurgeryU to compliment the content of this article regarding endometriosis. You can watch these videos, and more than 1,500 others, at AAGL.org/surgeryu. Laparoscopic excision of stage IV endometriosis Einarsson JI This case, originally presented as a SurgeryU live event, features a 41-year-old woman (G3P1) with a 3-year history of left-sided pelvic pain, deep dyspareunia, constipation, and dysmenorrhea. She also has infertility and is planning an IVF treatment shortly. On examination she was noted to have significant rectovaginal tenderness and nodularity. A pelvic MRI demonstrated a 3-cm irregular mass extending from the cervix into the cul-de-sac up to the left lateral pelvic sidewall. Abdominal wall endometriosis Hawkins E, Patzkowsky K, Lopez J This video demonstrates a typical presentation of abdominal wall endometriosis (AWE), also known as subcutaneous endometriosis or scar endometriosis. It is important for gynecologists to be familiar with this more uncommon form of the disease and its management. This video also demonstrates surgical management of advanced AWE involving the subcutaneous tissue, fascia, and rectus muscle. Laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain Pendergrass M This video depicts the laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain. The patient underwent menarche at age 11 and developed cyclic pelvic pain 6 months later. Due to the severity of the pain she has been unable to attend school for the past 2 months, and has stopped participating in sports. A diagnostic laparoscopy revealed red/brown superficial endometriosis lesions on the peritoneum in the posterior cul de sac, bilateral uterosacral ligaments, and bilateral broad ligaments. |
5. Is hysterectomy definitive treatment for pain?
“Not necessarily,” says Dr. Nezhat. “Hysterectomy by itself doesn’t take care of endometriosis unless the patient has adenomyosis. If a patient has endometriosis, the first step is complete treatment of the disease to restore the anatomy. Then the next step might be hysterectomy to give a better long-term result, especially in cases of adenomyosis. Removal of the ovaries at the time of hysterectomy has to be individualized.”
“The implication that hysterectomy ‘cures’ endometriosis is false yet is stated in some textbooks,” says Dr. Nezhat. “Even at the time of hysterectomy, the first step should be complete treatment of endometriosis and restoration of anatomy, followed by the hysterectomy. Leaving endometriosis behind, believing it will go away by itself or not cause future issues, is a gross misperception.”
Removal of the ovaries at hysterectomy?
“There are few comparative studies on the long-term follow-up of patients who have undergone hysterectomy with or without removal of both ovaries,” says Dr. Falcone. “The conventional dogma has been that, in women undergoing definitive surgery for endometriosis, both ovaries should be removed, even if they are normal. I personally believe that this was the case because hysterectomy was often performed without excision of the endometriosis. So the uterus was removed and disease was left behind. In these cases, recurrent symptoms were due to persistent disease.”
“We reported our experience at the Cleveland Clinic with a 7-year follow-up,” Dr. Falcone continues. “Hysterectomy was performed with excision of all visible disease. Ovaries were conserved if normal and removed if they had disease. We looked at the reoperation-free frequency over time. In women undergoing hysterectomy with excision of visible disease but ovarian preservation, the reoperation-free percentages at 2, 5, and 7 years were 95%, 86%, and 77%, respectively, versus 96%, 91%, and 91% in those without ovarian preservation. So, overall, there was an advantage over time for removal of the ovaries. However, in the subset of women between ages 30 and 39 years, there was no difference in the long-term recurrence rate if the ovaries were left in. For this reason, in women under 40, we recommend keeping normal ovaries if all disease is removed.”17
6. Can the risk of postoperative recurrence be reduced?
“The main problem with surgery is the recurrence rate,” Dr. Falcone says. “Studies have shown that the recurrence rate of pain at 7 years may be as high as 50%.”17 Furthermore, “the recurrence of pain may not be associated with visualized endometriosis at laparoscopy.”
“Incomplete removal of lesions may be associated with an increase in pain after surgery,” says Dr. Stratton.18 “Incomplete removal of lesions may occur because of varying technical skill or specific lesion characteristics. The lesions may be difficult to remove because of their location. Lesions may not be recognized because their appearance can vary from subtle (red or clear or white) to classic (blue-black). The depth of the lesion may not be appreciated until surgery is under way and a surgeon may not be adequately prepared to treat deep lesions when they are identified.”
Another reason pain may persist or recur after surgery for endometriosis: Adenomyosis.19 “Adenomyosis appears as either diffuse or focal thickening of the junctional zone between the endometrium and myometrium of the uterus on T2 weighted magnetic resonance imaging (MRI),” says Dr. Stratton. “After excision of endometriosis, chronic pelvic pain is significantly more likely to persist in women who have a junctional zone thickness of more than 11 mm on MRI,” she says.
The frequent recurrence of pain after surgery for endometriosis means that the disease is a long-term challenge.
“Pelvic pain caused by endometriosis is a chronic problem that requires a multiyear management plan, involving both surgery and hormonal therapy,” says Robert L. Barbieri, MD. “To reduce the number of surgical procedures in the lifetime of a woman with endometriosis and pain, I suggest hormonal medical therapy following conservative surgery for endometriosis.”
“Definitive surgery, such as hysterectomy or hysterectomy plus bilateral salpingo-oophorectomy (BSO) typicallyresults in prolonged symptom relief,” Dr. Barbieri says. “Following hysterectomy, hormonal therapy may not be needed. Following BSO, low-dose hormonal therapy is often needed to reduce the severity of menopausal symptoms.”
Dr. Barbieri is Editor in Chief of OBG Management; Chair of Obstetrics and Gynecology at Brigham and Women’s Hospital in Boston, Massachusetts; and the Kate Macy Ladd Professor of Obstetrics, Gynecology, and Reproductive Biology at Harvard Medical School in Boston.
After surgical treatment of endometriosis associated with pain, Dr. Barbieri presents the patient with the following menu of hormonal options:
- no hormonal therapy
- estrogen-progestin contraceptives, either cyclic or continuous
- the LNG-IUS
- norethindrone acetate 5 mg daily
- DMPA 150 mg every 3 months
- leuprolide acetate depot 3.75 mg intramuscularly monthly
- nafarelin nasal spray 200 µg twice a day
- danazol 200 mg twice a day.
“I explain the side effects common with each approach and have the patient select what she determines to be her best option,” says Dr. Barbieri. “In my experience, conservative surgery followed by hormonal therapy is effective in more than 75% of women.”
“The evidence to support postoperative hormonal therapy is modest,” Dr. Barbieri notes. “The best evidence is available for use of the LNG-IUS, estrogen-progestin contraceptives, and GnRH agonists.”20–22
In addition, “major professional societies have highlighted the option of postoperative hormonal therapy to reduce the risk of recurrent pain and repetitive surgical procedures in the future,” Dr. Barbieri says.23,24
When pain recurs after surgery for endometriosis, it pays to consider what type of pain it is, says Dr. Barbieri.
“There are 2 major types of pain—nociceptive and neuropathic,” he says. “Nociceptive pain is caused by an injury, acute or chronic. Neuropathic pain is caused by ‘activation’ of neural circuits, sometimes in the absence of an ongoing injury. Many women with endometriosis and chronic pain have both nociceptive and neuropathic pain. Consequently, it is important to consider the use of a multidisciplinary pain practice in the management of chronic pain syndromes. Multidisciplinary pain practices have special expertise in the management of neuropathic pain. Standard conservative surgical intervention is unlikely to improve pain caused by neuropathic mechanisms. Likewise, opioid analgesics are not recommended for the treatment of neuropathic pain.”
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endometriosis has always posed a treatment challenge. Take the early 19th Century, for example, before the widespread advent of surgery, when the disease was managed by applying leeches to the cervix. In fact, as Nezhat and colleagues note in their comprehensive survey of the 4,000-year history of endometriosis, “leeches were considered a mainstay in treating any condition associated with menstruation.”1
Fast forward to the 21st Century, and the picture is a lot clearer, though still not crystal clear. The optimal approach to endometriosis depends on numerous factors, foremost among them the chief complaint of the patient—pain or infertility (or both).
In this article—Part 2 of a 3-part series on endometriosis—the focus is on medical and surgical management of pain. Six experts address such questions as when is laparoscopy indicated, who is best qualified to treat endometriosis, is excision or ablation of lesions preferred, what is the role of hysterectomy in eliminating pain, and what to do about the problem of recurrence.
In Part 3, to be published in the June 2015 issue of OBG Management, endometriosis-associated infertility will be the topic of discussion.
In Part 1, 7 experts answer crucial questions on the diagnosis of endometriosis.
For a detailed look at the pathophysiology of endometriosis-associated pain, see “Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain,” by Kenneth A. Levey, MD, MPH, in this issue.
1. What are the options for empiric therapy?
One reason for the diagnostic delay for endometriosis, which still averages about 6 years, is that definitive diagnosis is achieved only through laparoscopic investigation and histologic confirmation. For many women who experience pain thought to be associated with endometriosis, however, clinicians begin empiric treatment with medical agents as a way to avert the need for surgery, if at all possible.
“There is no cure for endometriosis,” says John R. Lue, MD, MPH, “but there are many ways that endometriosis can be treated” and the impact of the disease reduced in a patient’s life. Dr. Lue is Associate Professor and Chief of the Section of General Obstetrics and Gynecology and Medical Director of Women’s Ambulatory Services at the Medical College of Georgia and Georgia Regents University in Augusta, Georgia.
Among the medical and hormonal management options:
- Nonsteroidal anti-inflammatory drugs (NSAIDs), often used with combined oral contraceptives (OCs). NSAIDs are not a long-term treatment option because of their effect on cyclo-oxygenase (COX) 1 and 2 enzymes, says Dr. Lue. COX-1 protects the gastrointestinal (GI) system, and prolonged use of NSAIDs can cause adverse GI effects.
- Cyclic combined OCs “are recommended as first-line therapy in the absence of contraindications,” says Dr. Lue, and are often used in combination with NSAIDs. However, the failure rate may be as high as 20% to 25%.2 “If pain persists after a trial of 3 to 6 months of cyclic OCs, one can consider switching to continuous low-dose combined OCs for an additional 6 months,” says Dr. Lue. When combined OCs were compared with placebo in the treatment of dysmenorrhea, they reduced baseline pain scores by 45% to 52%, compared with 14% to 17% for placebo (P<.001).2 They also reduced the volume of endometriomas by 48%, compared with 32% for placebo (P = .04). According to Linda C. Giudice, MD, PhD, “In women with severe dysmenorrhea who have been treated with cyclic combined OCs, a switch to continuous combined OCs reduced pain scores by 58% within 6 months and by 75% at 2 years” (P<.001).2 Dr. Giudice is the Robert B. Jaffe, MD, Endowed Professor in the Reproductive Sciences and Chair of Obstetrics, Gynecology, and Reproductive Sciences at the University of California, San Francisco.
- Depot medroxyprogesterone acetate (DMPA) or the levonorgestrel-releasing intrauterine system (LNG-IUS). These agents suppress the hypothalamic-pituitary-ovarian (HPO) axis to different degrees. DMPA suppresses the HPO completely, preventing ovulation. The LNG-IUS does not fully suppress the HPO but acts directly on endometrial tissue, with antiproliferative effects on eutopic and endometriotic implants, says Dr. Lue. The LNG-IUS also is effective at suppressing disease after surgical treatment, says Dr. Giudice.2
- Gonadotropin-releasing hormone (GnRH) agonist therapy, with estrogen and/or progestin add-back therapy to temper the associated loss in bone mineral density, “may be effective—if only temporarily—as it inhibits the HPO axis and blocks ovarian function, thereby greatly reducing systemic estrogen levels and inducing artificial menopause,” says Dr. Lue.
- Norethindrone acetate, a synthetic progestational agent, is occasionally used as empiric therapy for endometriosis because of its ability to inhibit ovulation. It has antiandrogenic and antiestrogenic effects.
- Aromatase inhibitors. Dr. Lue points to considerable evidence that endometriotic implants are an autocrine source of estrogen.3 “This locally produced estrogen results from overexpression of the enzyme P450 aromatase by endometriotic tissue,” he says. Consequently, in postmenopausal women, “aromatase inhibitors may be used orally in a daily pill form to curtail endometriotic implant production of estrogen and subsequent implant growth.”4 In women of reproductive age, aromatase inhibitors are combined with an HPO-suppressive therapy, such as norethindrone acetate. These strategies represent off-label use of aromatase inhibitors.
- Danazol, a synthetic androgen, has been used in the past to treat dysmenorrhea and dyspareunia. Because of its severe androgenic effects, however, it is not widely used today.
“For those using medical approaches, endometriosis-related pain may be reduced by using hormonal treatments to modify reproductive tract events, thereby decreasing local peritoneal inflammation and cytokine production,” says Pamela Stratton, MD. Because endometriosis is a “central sensitivity syndrome,” multidisciplinary approaches may be beneficial to treat myofascial dysfunction and sensitization, such as physical therapy. “Chronic pain conditions that overlap with endometriosis-associated pain, such as migraines, irritable bowel syndrome, or painful bladder syndrome should be identified and treated. Mood changes of depression and anxiety common to women with endometriosis-associated pain also warrant treatment,” she says.
Dr. Stratton is Chief of the Gynecology Consult Service, Program in Reproductive and Adult Endocrinology, at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
2. When is laparoscopy indicated?
When medical and hormonal treatments fail to control a patient’s pain, laparoscopy is indicated to confirm the diagnosis of endometriosis. During that procedure, it is also advisable to treat any endometriosis that is present, provided the surgeon is highly experienced in such treatment.
Proper treatment is preferable—even if it requires expert consultation. “No treatment and referral to a more experienced surgeon are better than incomplete treatment by an inexperienced surgeon,” says Ceana Nezhat, MD. “Not all GYN surgeons have the expertise to treat advanced endometriosis.” Dr. Nezhat is Director of the Nezhat Medical Center and Medical Director of Training and Education at Northside Hospital, both in Atlanta, Georgia.
Dr. Stratton agrees about the importance of thorough treatment of endometriosis at the time of diagnostic laparoscopy. “At the laparoscopy, the patient benefits if all potential sources of pain are investigated and addressed.” At surgery, the surgeon should look for and treat any lesions suspicious for endometriosis, as well as any other finding that might contribute to pain, she says. “For example, routinely inspecting the appendix for endometriosis or other lesions, and removing affected appendices is reasonable; also, lysis and, where possible, excision of adhesions is an important strategy.”
If a medical approach fails for a patient, “then surgery is indicated to confirm the diagnosis and treat the disease,” agrees Tommaso Falcone, MD.
“Surgery is very effective in treating the pain associated with endometriosis,” Dr. Falcone continues. Randomized clinical trials have shown that up to 90% of patients who obtain pain relief from surgery will have an effect lasting 1 year.6 If patients do not get relief, then the association of the pain with endometriosis should be questioned and other causes searched.” Dr. Falcone is Professor and Chair of Obstetrics and Gynecology at the Cleveland Clinic in Cleveland, Ohio.
The most common anatomic sites of implants
“The most common accepted theory for pathogenesis of endometriosis suggests that implants develop when debris from retrograde menstruation attaches to the pelvic peritoneum,” says Dr. Stratton.7 “Thus, the vast majority of lesions occur in the dependent portions of the pelvis, which include the ovarian fossae (posterior broad ligament under the ovaries), cul de sac, and the uterosacral ligaments.8 The bladder peritoneum, ovarian surface, uterine peritoneal surface, fallopian tube, and pelvic sidewall are also frequent sites. The colon and appendix are less common sites, and small bowel lesions are rare.”
“However, pain location does not correlate with lesion location,” Dr. Stratton notes. “For this reason, the goal at surgery is to treat all lesions, even ones that are not in sites of pain.”
3. How should disease be staged?
Most surgeons with expertise in treating endometriosis attempt to stage the disease at the time of initial laparoscopy, even though a patient’s pain does not always correlate with the stage of disease.
“The staging system for endometriosis is a means to systematically catalogue where lesions are located,” says Dr. Stratton.
The most commonly used classification system was developed by the American Society for Reproductive Medicine (ASRM). It takes into account such characteristics as how deep an implant lies, the extent to which it obliterates the posterior cul de sac, and the presence and extent of adhesions. Although the classification system is broken down into 4 stages ranging from minimal to severe disease, it is fairly complex. For example, it assigns a score for each lesion as well as the size and location of that lesion, notes Dr. Stratton. The presence of an endometrioma automatically renders the disease as stage III or IV, and an obliterated cul de sac means the endometriosis is graded as stage IV.
“This system enables us to communicate with each other about patients and may guide future surgeries for assessment of lesion recurrence or the planning of treatment for lesions the surgeon was unable to treat at an initial surgery,” says Dr. Stratton.
“Women with uterosacral nodularity, fixed pelvic organs, or severe pain with endometriomas may have deep infiltrating lesions. These lesions, in particular, are not captured well with the current staging system,” says Dr. Stratton. Because they appear to be innervated, “the greatest benefit to the patient is achieved by completely excising these lesions.” Preoperative imaging may help confirm the existence, location, and extent of these deep lesions and help the surgeon plan her approach “based on clinical and imaging findings.”
“Severity of pain or duration of surgical effect does not correlate with stage or extent of disease,” Dr. Stratton says.9 “In fact, patients with the least amount of disease noted at surgery experience pain sooner, suggesting that the central nervous system may have been remodeled prior to surgery or that the pain is in part due to some other cause.10 This observation underscores the principle that, while endometriosis may initiate pain, the pain experience is determined by engagement of the central nervous system.”
For more information on the ASRM revised classification of endometriosis, go to http://www.fertstert.org/article/S0015-0282(97)81391-X/pdf.
4. Which is preferable—excision or ablation?
In a prospective, randomized, double-blind study, Healey and colleagues compared pain levels following laparoscopic treatment of endometriosis with either excision or ablation. Preoperatively, women in the study completed a questionnaire rating various types of pain using visual analogue scales. They then were randomly assigned to treatment of endometriosis via excision or ablation. Postoperatively, they again completed a questionnaire about pain levels at 3, 6, 9, and 12 months. Investigators found no significant difference in pain scores at 12 months.11
Five-year follow-up of the same population yielded slightly different findings, however. Although there was a reduction in all pain scores at 5 years in both the excision and ablation groups, a significantly greater reduction in dyspareunia was observed in the excision group at 5 years.12
In an editorial accompanying the 5-year follow-up data, Dr. Falcone and a coauthor called excision versus ablation of ovarian, bowel, and peritoneal endometriosis one of the “great debates” in the surgical management of endometriosis.13
“When there is deep involvement of adjacent organs, there is general consensus that excision is best for optimal surgical outcome,” they write. “However, for disease involving the peritoneum alone, there are proponents for either option.”13
“This is a very controversial issue,” says Dr. Falcone, “and the debate can sometimes be somewhat inflammatory…. It is hard to understand how a comparative trial could even be accomplished between excision and ablation,” he adds. “In my experience, deep disease typically occurs on the pelvic sidewall over the ureter or in the cul de sac on the bowel or infiltrating the bladder peritoneum. Therefore, ablation would increase the risk of damaging any of these structures. With superficial disease away from critical structures, it should be fine to ablate. Everywhere else and with deep disease you need to excise or leave disease behind.”
“Endometriomas are a special situation,” Dr. Falcone says. “Excision of the cyst has been shown in randomized controlled trials (RCTs) to be associated with less risk of recurrence.14 Therefore, it should be the treatment of choice. However, in patients interested in future fertility, we must take into consideration the potential damage to ovarian reserve associated with excision.”
Endometriosis of the ovaries has unique manifestations. “My approach to ovarian cysts depends on their classification,” says Dr. Nezhat.15 In general, primary endometriomas (Type 1) are small, superficial cysts that contain dark “chocolate” fluid. They tend to be firmly adherent to the ovarian tissue and difficult to remove surgically.
Secondary endometriomas (Type 2) are follicular or luteal cysts that have been involved or invaded by cortical endometriotic implants or by primary endometrioma. Secondary endometriomas are further classified by the relationship between cortical endometriosis and the cyst wall. Type 2A endometriomas are usually large, with a capsule that is easily separated from ovarian tissue. Type 2B endometriomas have some features of functional cysts but show deep involvement with surface endometriosis. Type 2C endometriomas are similar, showing extensive surface endometrial implants but with deep penetration of the endometriosis into the cyst wall.15
“For Type 1 endometriomas, I biopsy the cyst to ensure the lesion is benign, then vaporize the endometrioma,” Dr. Nezhat says. “In cases of type 2A and 2B endometriomas, the cyst capsule is easily enucleated and removed. Type 2C endometriomas are biopsied as well and then I proceed with vaporizing the fibrotic area with a low-power energy source, such as neutral argon plasma, avoiding excessive coagulation and thermal injury.” Recent literature supports the idea of evaluation and biopsy of fibrotic endometriomas to confirm benign conditions, followed by ablation without compromising ovarian function.16
“Excision and ablation both have indications,” Dr. Nezhat asserts. “It depends on the location and depth of penetration of implants, as well as the patient’s ultimate goal. For example, if the patient desires future fertility and has endometriosis on the ovary, removal by excision could damage ovarian function. The same holds true for endometriosis on the fallopian tubes. It’s better in such cases to ablate.”
“Ablation is different from coagulation, which is not recommended,” Dr. Nezhat explains. “Ablation vaporizes the diseased area layer by layer, like peeling an onion, until the disease is eradicated. It is similar to dermatological skin resurfacing. Vaporization is preferable for endometriosis on the tubes and ovaries in patients who desire pregnancy. The choice between excision and ablation depends on the location, depth of penetration, and the patient’s desire for fertility.”
Either way, and regardless of the primary indication for surgery—pain versus infertility—a minimally invasive gynecologic surgeon is expected to have ability in performing both techniques, Dr. Nezhat says.
The following videos have been provided by AAGL SurgeryU to compliment the content of this article regarding endometriosis. You can watch these videos, and more than 1,500 others, at AAGL.org/surgeryu. Laparoscopic excision of stage IV endometriosis Einarsson JI This case, originally presented as a SurgeryU live event, features a 41-year-old woman (G3P1) with a 3-year history of left-sided pelvic pain, deep dyspareunia, constipation, and dysmenorrhea. She also has infertility and is planning an IVF treatment shortly. On examination she was noted to have significant rectovaginal tenderness and nodularity. A pelvic MRI demonstrated a 3-cm irregular mass extending from the cervix into the cul-de-sac up to the left lateral pelvic sidewall. Abdominal wall endometriosis Hawkins E, Patzkowsky K, Lopez J This video demonstrates a typical presentation of abdominal wall endometriosis (AWE), also known as subcutaneous endometriosis or scar endometriosis. It is important for gynecologists to be familiar with this more uncommon form of the disease and its management. This video also demonstrates surgical management of advanced AWE involving the subcutaneous tissue, fascia, and rectus muscle. Laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain Pendergrass M This video depicts the laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain. The patient underwent menarche at age 11 and developed cyclic pelvic pain 6 months later. Due to the severity of the pain she has been unable to attend school for the past 2 months, and has stopped participating in sports. A diagnostic laparoscopy revealed red/brown superficial endometriosis lesions on the peritoneum in the posterior cul de sac, bilateral uterosacral ligaments, and bilateral broad ligaments. |
5. Is hysterectomy definitive treatment for pain?
“Not necessarily,” says Dr. Nezhat. “Hysterectomy by itself doesn’t take care of endometriosis unless the patient has adenomyosis. If a patient has endometriosis, the first step is complete treatment of the disease to restore the anatomy. Then the next step might be hysterectomy to give a better long-term result, especially in cases of adenomyosis. Removal of the ovaries at the time of hysterectomy has to be individualized.”
“The implication that hysterectomy ‘cures’ endometriosis is false yet is stated in some textbooks,” says Dr. Nezhat. “Even at the time of hysterectomy, the first step should be complete treatment of endometriosis and restoration of anatomy, followed by the hysterectomy. Leaving endometriosis behind, believing it will go away by itself or not cause future issues, is a gross misperception.”
Removal of the ovaries at hysterectomy?
“There are few comparative studies on the long-term follow-up of patients who have undergone hysterectomy with or without removal of both ovaries,” says Dr. Falcone. “The conventional dogma has been that, in women undergoing definitive surgery for endometriosis, both ovaries should be removed, even if they are normal. I personally believe that this was the case because hysterectomy was often performed without excision of the endometriosis. So the uterus was removed and disease was left behind. In these cases, recurrent symptoms were due to persistent disease.”
“We reported our experience at the Cleveland Clinic with a 7-year follow-up,” Dr. Falcone continues. “Hysterectomy was performed with excision of all visible disease. Ovaries were conserved if normal and removed if they had disease. We looked at the reoperation-free frequency over time. In women undergoing hysterectomy with excision of visible disease but ovarian preservation, the reoperation-free percentages at 2, 5, and 7 years were 95%, 86%, and 77%, respectively, versus 96%, 91%, and 91% in those without ovarian preservation. So, overall, there was an advantage over time for removal of the ovaries. However, in the subset of women between ages 30 and 39 years, there was no difference in the long-term recurrence rate if the ovaries were left in. For this reason, in women under 40, we recommend keeping normal ovaries if all disease is removed.”17
6. Can the risk of postoperative recurrence be reduced?
“The main problem with surgery is the recurrence rate,” Dr. Falcone says. “Studies have shown that the recurrence rate of pain at 7 years may be as high as 50%.”17 Furthermore, “the recurrence of pain may not be associated with visualized endometriosis at laparoscopy.”
“Incomplete removal of lesions may be associated with an increase in pain after surgery,” says Dr. Stratton.18 “Incomplete removal of lesions may occur because of varying technical skill or specific lesion characteristics. The lesions may be difficult to remove because of their location. Lesions may not be recognized because their appearance can vary from subtle (red or clear or white) to classic (blue-black). The depth of the lesion may not be appreciated until surgery is under way and a surgeon may not be adequately prepared to treat deep lesions when they are identified.”
Another reason pain may persist or recur after surgery for endometriosis: Adenomyosis.19 “Adenomyosis appears as either diffuse or focal thickening of the junctional zone between the endometrium and myometrium of the uterus on T2 weighted magnetic resonance imaging (MRI),” says Dr. Stratton. “After excision of endometriosis, chronic pelvic pain is significantly more likely to persist in women who have a junctional zone thickness of more than 11 mm on MRI,” she says.
The frequent recurrence of pain after surgery for endometriosis means that the disease is a long-term challenge.
“Pelvic pain caused by endometriosis is a chronic problem that requires a multiyear management plan, involving both surgery and hormonal therapy,” says Robert L. Barbieri, MD. “To reduce the number of surgical procedures in the lifetime of a woman with endometriosis and pain, I suggest hormonal medical therapy following conservative surgery for endometriosis.”
“Definitive surgery, such as hysterectomy or hysterectomy plus bilateral salpingo-oophorectomy (BSO) typicallyresults in prolonged symptom relief,” Dr. Barbieri says. “Following hysterectomy, hormonal therapy may not be needed. Following BSO, low-dose hormonal therapy is often needed to reduce the severity of menopausal symptoms.”
Dr. Barbieri is Editor in Chief of OBG Management; Chair of Obstetrics and Gynecology at Brigham and Women’s Hospital in Boston, Massachusetts; and the Kate Macy Ladd Professor of Obstetrics, Gynecology, and Reproductive Biology at Harvard Medical School in Boston.
After surgical treatment of endometriosis associated with pain, Dr. Barbieri presents the patient with the following menu of hormonal options:
- no hormonal therapy
- estrogen-progestin contraceptives, either cyclic or continuous
- the LNG-IUS
- norethindrone acetate 5 mg daily
- DMPA 150 mg every 3 months
- leuprolide acetate depot 3.75 mg intramuscularly monthly
- nafarelin nasal spray 200 µg twice a day
- danazol 200 mg twice a day.
“I explain the side effects common with each approach and have the patient select what she determines to be her best option,” says Dr. Barbieri. “In my experience, conservative surgery followed by hormonal therapy is effective in more than 75% of women.”
“The evidence to support postoperative hormonal therapy is modest,” Dr. Barbieri notes. “The best evidence is available for use of the LNG-IUS, estrogen-progestin contraceptives, and GnRH agonists.”20–22
In addition, “major professional societies have highlighted the option of postoperative hormonal therapy to reduce the risk of recurrent pain and repetitive surgical procedures in the future,” Dr. Barbieri says.23,24
When pain recurs after surgery for endometriosis, it pays to consider what type of pain it is, says Dr. Barbieri.
“There are 2 major types of pain—nociceptive and neuropathic,” he says. “Nociceptive pain is caused by an injury, acute or chronic. Neuropathic pain is caused by ‘activation’ of neural circuits, sometimes in the absence of an ongoing injury. Many women with endometriosis and chronic pain have both nociceptive and neuropathic pain. Consequently, it is important to consider the use of a multidisciplinary pain practice in the management of chronic pain syndromes. Multidisciplinary pain practices have special expertise in the management of neuropathic pain. Standard conservative surgical intervention is unlikely to improve pain caused by neuropathic mechanisms. Likewise, opioid analgesics are not recommended for the treatment of neuropathic pain.”
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6S):S1–S62.
2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398.
3. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis: a review. Fertil Steril. 2012;98(6):1370–1379.
4. Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;9:87.
5. Chwalisz K, Garg R, Brenner RM, Schubert G, Elger W. Selective progesterone receptor modulators (SPRMs): a novel therapeutic concept in endometriosis. Ann N Y Acad Sci. 2002;955:373–393, 396–406.
6. Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031.
7. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279.
8. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632–1636.
9. Hsu AL, Sinaii N, Segars J, Nieman LK, Stratton P. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011;118(2 pt 1):223–230.
10. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346.
11. Healey M, Ang WC, Cheng C. Surgical treatment of endometriosis: a prospective randomized double-blinded trial comparing excision and ablation. Fertil Steril. 2010;94(7):2536–2540.
12. Healey M, Chang C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. JMIG. 2014;21(6):999–1004.
13. Falcone T, Wilson JR. Surgical management of endometriosis: excision or ablation. JMIG. 2014;21(6):969.
14. Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
15. Nezhat C, Nezhat F, Nezhat CH, Seidman D. Classification of endometriosis: improving the classification of endometriotic ovarian cysts. Hum Reprod 1994;9(12):2212–2216.
16. Roman H, Auber M, Mokdad C, et al. Ovarian endometrioma ablation using plasma energy versus cystectomy: a step toward better preservation of the ovarian parenchyma in women wishing to conceive. Fertil Steril. 2011;96(6):1396–1400.
17. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285–1292.
18. McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147(1–3):255–264.
19. Parker JD, Leondires M, Sinaii N, Premkumar A, Nieman LK, Stratton P. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86(3):711–715.
20. Abou-Setta AM, Al-Inany HG, Farquar CM. Levonorgestrel-releasing intrauterine device for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;(1):CD005072.
21. Seracchioli R, Mabrouk M, Manuzzi L, et al. Postoperative use of oral contraceptive pills for prevention of anatomic relapse or symptom recurrence following surgery. Hum Reprod. 2009;24(11):2729–2735.
22. Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertil Steril. 1997;68(5):860–864.
23. Practice Committee of the American Society for Reproductive Medicine. Treatment of pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927–935.
24. Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
1. Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6S):S1–S62.
2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398.
3. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis: a review. Fertil Steril. 2012;98(6):1370–1379.
4. Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;9:87.
5. Chwalisz K, Garg R, Brenner RM, Schubert G, Elger W. Selective progesterone receptor modulators (SPRMs): a novel therapeutic concept in endometriosis. Ann N Y Acad Sci. 2002;955:373–393, 396–406.
6. Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031.
7. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279.
8. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632–1636.
9. Hsu AL, Sinaii N, Segars J, Nieman LK, Stratton P. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011;118(2 pt 1):223–230.
10. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346.
11. Healey M, Ang WC, Cheng C. Surgical treatment of endometriosis: a prospective randomized double-blinded trial comparing excision and ablation. Fertil Steril. 2010;94(7):2536–2540.
12. Healey M, Chang C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. JMIG. 2014;21(6):999–1004.
13. Falcone T, Wilson JR. Surgical management of endometriosis: excision or ablation. JMIG. 2014;21(6):969.
14. Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
15. Nezhat C, Nezhat F, Nezhat CH, Seidman D. Classification of endometriosis: improving the classification of endometriotic ovarian cysts. Hum Reprod 1994;9(12):2212–2216.
16. Roman H, Auber M, Mokdad C, et al. Ovarian endometrioma ablation using plasma energy versus cystectomy: a step toward better preservation of the ovarian parenchyma in women wishing to conceive. Fertil Steril. 2011;96(6):1396–1400.
17. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285–1292.
18. McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147(1–3):255–264.
19. Parker JD, Leondires M, Sinaii N, Premkumar A, Nieman LK, Stratton P. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86(3):711–715.
20. Abou-Setta AM, Al-Inany HG, Farquar CM. Levonorgestrel-releasing intrauterine device for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;(1):CD005072.
21. Seracchioli R, Mabrouk M, Manuzzi L, et al. Postoperative use of oral contraceptive pills for prevention of anatomic relapse or symptom recurrence following surgery. Hum Reprod. 2009;24(11):2729–2735.
22. Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertil Steril. 1997;68(5):860–864.
23. Practice Committee of the American Society for Reproductive Medicine. Treatment of pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927–935.
24. Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
Endometriosis: 3 intraoperative videos
The following videos have been provided by AAGL SurgeryU to compliment the content of this article regarding endometriosis. You can watch these videos, and more than 1,500 others, at AAGL.org/surgeryu.