User login
Endoscopic therapy has become an alternative to surgery for some patients with acute recurrent pancreatitis, ie, those whose disease is caused by gallstones or other mechanical processes that can obstruct the outflow from the pancreas.
In this paper, we review the specific situations in which endoscopic therapy might be useful in patients with acute recurrent pancreatitis.
ACUTE PANCREATITIS IS MANAGED DIFFERENTLY IF IT RECURS
Recurrent acute pancreatitis is defined as more than one episode of acute pancreatitis.1 In clinical practice, it is important to distinguish between the first and recurrent episodes of acute pancreatitis.
Most patients who have one episode of acute pancreatitis never have another one.2,3 Therefore, for patients having an initial attack, we recommend a limited workup that includes a detailed history, laboratory evaluation, and a noninvasive imaging study such as transcutaneous ultrasonography or computed tomography.
On the other hand, people who have a second attack are at higher risk of more recurrences. Therefore, patients having recurrent attacks need a more extensive workup to determine the underlying cause. We recommend referring them to a gastroenterologist for further evaluation.
WHICH CAUSES CAN BE MANAGED ENDOSCOPICALLY?
In the Western world, 70% to 80% of cases of recurrent pancreatitis are due to either alcohol abuse or gallstone disease.2,4 The rest are related to:
- Autoimmune disorders
- Cancer, including occult malignancies and premalignant conditions such as intraductal papillary mucinous neoplasm
- Chronic pancreatitis
- Drugs
- Heredity
- Metabolic abnormalities (hypertriglyceridemia, hypercalcemia)
- Sphincter of Oddi dysfunction
- Structural or congenital abnormalities (pancreas divisum)
- Trauma.
- Gallstone disease, including biliary microlithiasis and sludge (in patients with or without a gallbladder)
- Sphincter of Oddi dysfunction
- Pancreas divisum
- Obstruction to flow of pancreatic juice.
Endoscopy is not completely benign
Although endoscopic procedures are less invasive than surgery, they are not completely benign. They can cause anxiety and are associated with risks such as bleeding, perforation, and pancreatitis.5 The risks, benefits, and alternatives to these procedures should be discussed with the patient, and informed consent should be obtained before any endoscopic procedure.6
STONES (LARGE OR SMALL) OR SLUDGE IN PATIENTS WITH A GALLBLADDER
Gallstones can be large, but small stones (microlithiasis) and sludge are more common and therefore account for more cases of pancreatitis.
Strictly defined, microlithiasis refers to stones smaller than 2 mm in diameter in the biliary tract, whereas sludge is a suspension of biliary crystals, mucin, and cellular debris in the gallbladder or bile ducts.7 The terms are often used interchangeably, since the conditions often coexist and their treatment is similar.
Theories differ as to how microlithiasis or sludge can cause recurrent pancreatitis. According to one theory, the debris blocks the common channel, increasing the pancreatic intraductal pressure and leading to pancreatitis.8 A second theory is that small stones or biliary crystals passing through the sphincter of Oddi cause inflammation, and that repeated inflammation eventually leads to stenosis or dyskinesia of the sphincter, both of which have been associated with pancreatitis.9
Studies suggest that microlithiasis and sludge are common causes of recurrent pancreatitis, accounting for about two-thirds of cases according to estimates by Ros et al10 and Lee et al.11
Detecting small stones and sludge
The diagnosis of microlithiasis and biliary sludge in patients with a gallbladder is based on imaging studies and bile microscopy.12
Transabdominal ultrasonography is the imaging study most often used for diagnosing microlithiasis. The technology and expertise for this test are widely available, and it is relatively inexpensive.
Endoscopic ultrasonography is more sensitive for detecting microlithiasis and can examine the distal common bile duct.
Bile microscopy involves obtaining bile from the second portion of the duodenum (via an endoscope or a duodenal tube) or from the bile ducts (by cannulating the common bile duct and stimulating the gallbladder with cholecystokinin). The bile sample is centrifuged and inspected microscopically under plain light and polarized light (which aids the visualization of biliary crystals). The crystals can be cholesterol monohydrate, calcium bilirubinate, or calcium carbonate.7,13,14
Removing the gallbladder is the treatment of choice for small stones and sludge
Treatments to prevent recurrent attacks of acute pancreatitis due to microlithiasis and sludge include cholecystectomy, biliary sphincterotomy, and ursodioxycholic acid.10,11,15
In prospective observational studies by Ros et al10 and Lee et al,11 about half of the patients with recurrent pancreatitis were treated with cholecystectomy, endoscopic sphincterotomy, or ursodioxycholic acid in a nonrandomized fashion. The choice of therapy was based on the patient’s medical status and the preferences of the patient and the physician. Half the patients received no treatment. In both studies the median follow-up was 4 years. Treated patients had a significantly lower rate of recurrent attacks of pancreatitis during follow-up: less than 20% with therapy compared with more than 60% without therapy. Unfortunately, no published study has compared these three treatments head to head.
Cholecystectomy, however, is the most definitive therapy and is generally considered the treatment of choice.
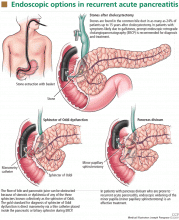
Biliary sphincterotomy is an endoscopic procedure that involves cutting the sphincter of Oddi to allow the stones and sludge to pass more freely. It is as effective as cholecystectomy in preventing recurrent attacks but does not eliminate the risk of cholecystitis and cholangitis (Figure 1). For this reason, it is usually reserved for patients who cannot tolerate surgery due to comorbidities, those who refuse surgery, or those who are pregnant.16
Ursodeoxycholic acid is a reasonable alternative in patients who cannot tolerate surgical or endoscopic biliary sphincterotomy.1,17–20 The dosage is 10 mg/kg/day, which can be in two or three divided doses. The optimal duration of treatment is not known; however, since this drug works slowly, it may need to be taken for 2 years or more. Ursodeoxycholic acid is more effective in patients with cholesterol-based stones and crystals. It is not effective for large stones (> 1 cm in diameter) or calcified stones.
STONES AFTER CHOLECYSTECTOMY
Bile duct stones can be classified as primary or secondary. A primary stone is one that remains where it was formed, whereas a secondary stone is one that has migrated from its site of formation.21
Some suggest that bile duct stones that are detected within 2 years of cholecystectomy originated in the gallbladder and were missed when the gallbladder was removed (and therefore are considered secondary stones), and that stones that present more than 2 years after cholecystectomy are de novo (ie, primary) stones.22,23
In any event, stones have been found in the common bile duct in 4% to 24% of patients up to 15 years after cholecystectomy.24–26 A fair number of these patients have no symptoms.27 Risk factors for stone recurrence are lithogenic bile (ie, high concentration of cholesterol, low concentration of bile salts), biliary stasis, strictures, dilated bile ducts, and advanced age.28–30
No role for crystal analysis after cholecystectomy
Biliary crystal analysis does not seem to have diagnostic value in patients with recurrent acute pancreatitis after cholecystectomy,31 because removing the gallbladder eliminates the crystals and sludge. Imaging studies are therefore the cornerstone of diagnosis.
Transabdominal ultrasonography is the most commonly used initial imaging test. However, abdominal fat and gas in the duodenum can obscure the distal common bile duct and decrease the sensitivity of this test.32
Endoscopic ultrasonography involves positioning the transducer in the second part of the duodenum, where it can show the adjacent biliary tree without interference from digestive gas or abdominal fat.
Magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasonography are both highly sensitive for detecting common bile duct stones and are recommended if they can be done without delay.
Endoscopic retrograde cholangiopancreatography (ERCP). As a rule, patients who are very likely to have gallstones are best served by proceeding directly to ERCP, a procedure that enables both imaging and treatment. However, ERCP exposes the patient to radiation and the risk of pancreatitis, so in some patients (eg, pregnant women, people who recently had acute pancreatitis), one may want to do ultrasonography first.
ERCP is the treatment of choice after cholecystectomy
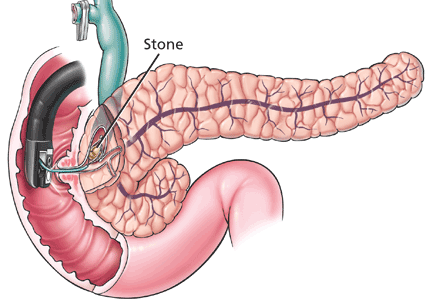
The treatment of choice in patients with choledocholithiasis is ERCP with biliary sphincterotomy and stone extraction. Success at clearing the biliary tree of all stones depends on the size, number, and location of the stones, the anatomy of the digestive tract and the bile duct, and the experience of the endoscopist. At specialized centers, the rate of successful clearance with subsequent procedures is close to 100%. Large stones may require fragmentation inside the bile duct to aid their removal.33
SPHINCTER OF ODDI DYSFUNCTION
The sphincter of Oddi, located where the bile and pancreatic ducts penetrate the wall of the duodenum, actually consists of three sphincters: the common, the biliary, and the pancreatic. Its physiologic role is to regulate the flow of bile and pancreatic juice into the duodenum and to prevent reflux into the ducts from the duodenum.34 Its basal pressure is the main regulating mechanism for pancreatic and biliary secretions into the intestine, and its phasic contractile activity is closely associated with duodenal motility.
Sphincter dysfunction: Stenosis, dyskinesia
The sphincter of Oddi can obstruct the flow of bile and pancreatic juice owing either to stenosis or to dyskinesia.35,36 Stenosis refers to structural alteration of the sphincter, probably from inflammation and subsequent fibrosis. In contrast, dyskinesia refers to a motor abnormality of the sphincter that makes it hypertonic.
Stenosis or dyskinesia can occur in the biliary sphincter, the pancreatic sphincter, the common sphincter, or any combination of the three. For example, dysfunction of the biliary sphincter can cause abnormalities in liver-associated enzyme levels and biliary-type pain, whereas pancreatic sphincter dysfunction can cause recurrent attacks of pancreatitis and pancreatic-type pain.37 Elevated pancreatic sphincter pressure has been shown to correlate with increased pancreatic ductal pressure, suggesting that the sphincter plays a role in the pathogenesis of acute pancreatitis.23,38
Sphincter pressure can be measured during ERCP, but ERCP is risky
The gold standard for the diagnosis of sphincter of Oddi dysfunction is manometry,23,35 ie, direct measurement of sphincter pressure via a thin catheter placed inside the pancreatic or biliary sphincter during ERCP (Figure 1).
However, in patients with suspected sphincter of Oddi dysfunction, ERCP with or without manometry is associated with a high rate of complications, with pancreatitis occurring in up to 25% of cases.39–41 Therefore, several noninvasive and provocative tests have been designed in an attempt to identify patients with this disorder. Unfortunately, none of them seems to be as sensitive and specific as manometry for diagnosing sphincter of Oddi dysfunction, and so they have not gained widespread use.
Opening the sphincter of Oddi with drugs, endoscopy, or surgery
Drug treatment of sphincter of Oddi dysfunction is based on drugs that relax smooth muscle, such as calcium channel blockers and nitrates. The treatment must be lifelong. Also, it does not improve sphincter stenosis, and only half of patients with sphincter dyskinesia respond to it. For these reasons, drug treatment of sphincter of Oddi dysfunction has not gained widespread acceptance.36,42
Endoscopic sphincterotomy is the current standard endoscopic therapy for sphincter of Oddi dysfunction. This procedure is performed during ERCP and involves cutting the sphincter with electrocautery.
Endoscopic pancreatic sphincterotomy prevents recurrent attacks of pancreatitis in patients with pancreatic sphincter dysfunction in more than 60% of cases.23,43–46 A potential complication is pancreatitis, which occurs more often in patients with pancreatic sphincter dyskinesia. Placing a stent in the pancreatic duct after pancreatic sphincterotomy reduces the risk of pancreatitis after ERCP.37,47,48
Surgery. Pancreatic sphincterotomy can also be done surgically, most commonly via transduodenal pancreatic sphincteroplasty. Surgical sphincteroplasty is as effective as endoscopic sphincterotomy for preventing recurrent attacks of pancreatitis in patients with pancreatic sphincter dysfunction.49 However, endoscopic therapy is much less invasive and remains the preferred treatment for sphincter of Oddi dysfunction in most centers with experience in this technique.50
PANCREAS DIVISUM
Pancreas divisum is the most common congenital anomaly of the pancreatic duct. Autopsy studies show it occurs in 5% to 10% of the population.51–53
At approximately the 5th week of gestation, there are two pancreatic buds: a ventral and a dorsal bud. The ventral bud eventually gives rise to part of the pancreatic head and uncinate process of the pancreas in the adult. The dorsal bud eventually gives rise to the rest of the pancreatic head, the pancreatic body, and the pancreatic tail. At 6 to 7 weeks of gestation, the ventral bud rotates clockwise and lies posterior to the dorsal bud. At this stage, both the dorsal and ventral pancreata have their own ducts, which do not communicate with each other. Normally, the ventral and dorsal pancreas and their ducts fuse together at 8 weeks of gestation; in people with pancreas divisum, this ductal fusion does not occur.51
The pancreas secretes 1.5 L of fluid per day. Normally, 90% to 95% of this volume drains through the major papilla. In people with pancreas divisum, 90% to 95% of the fluid drains through the minor papilla.
People with pancreas divisum are a heterogeneous group. Most have no symptoms, and their ductal anatomy is diagnosed only incidentally. However, a subgroup is prone to develop acute pancreatitis. The cause is thought to be the small diameter of the minor papilla, which poses a relative obstruction to the flow of pancreatic juice.54 Direct support for this theory comes from a study in which investigators measured pancreatic ductal pressures in eight people with normal anatomy and six people with pancreas divisum. The pressure in the main pancreatic duct in those with pancreas divisum was significantly higher than in those with normal anatomy.55 Additional evidence in favor of this theory is the effectiveness of treatment, which involves widening the minor papillary opening (minor papillary sphincterotomy).
Diagnosis of pancreas divisum
The diagnosis of pancreas divisum is based on imaging studies, and ERCP remains the gold standard for patients with equivocal results on noninvasive imaging. However, MRCP, especially secretin-enhanced MRCP, is as accurate as ERCP. In most cases, MRCP has replaced ERCP for the diagnosis of this condition, although a recent study suggests that MRCP is inferior to ERCP in the diagnosis of pancreas divisum.56 We recommend secretin-enhanced MRCP for this purpose.
Computed tomography and endoscopic ultrasonography can also diagnose pancreas divisum, but their diagnostic accuracy is lower than that of ERCP and MRCP.
Minor papillary sphincterotomy
Treating recurrent pancreatitis due to pancreas divisum involves relieving the relative obstruction of the minor papilla by minor papillary sphincterotomy. This can be done surgically or endoscopically (Figure 1).
Surgery. No randomized, controlled study has yet assessed the efficacy of surgical sphincteroplasty for recurrent pancreatitis in patients with pancreas divisum. However, retrospective studies and one prospective study have been published.57,58
In the retrospective study with the largest number of patients, Warshaw et al57 reported their experience in 49 patients who had recurrent pancreatitis due to pancreas divisum. After surgical sphincteroplasty, the patients were followed for a mean of 53 months; 40 (82%) of the 49 patients had no further episodes of acute pancreatitis during this time.
Bradley and Stephan58 studied 37 patients with pancreas divisum and recurrent pancreatitis.58 After surgical sphincteroplasty, the patients were followed for a mean of 60 months; 31 of the 37 patients had no further attacks, a success rate of 84%.
Endoscopic therapy. As with surgical therapy trials, most trials of endoscopic therapy of recurrent pancreatitis in patients with pancreas divisum are small case series. In a retrospective study with one of the largest number of patients, Heyries et al59 reported their experience with 24 patients with pancreas divisum and recurrent pancreatitis. After undergoing endoscopic minor papillary sphincterotomy, all patients were followed for a mean of 39 months, during which 22 (92%) did not have further episodes of acute pancreatitis.
In the only randomized controlled trial available, 19 patients with recurrent pancreatitis and pancreas divisum underwent either no treatment or endoscopic minor papillary sphincterotomy.60 In the treatment group, 9 of 10 patients had no further episodes of acute pancreatitis during the 3 years of follow-up, while 6 of 9 patients who were randomized to no treatment had at least one episode.60
Although surgical and endoscopic minor papillary sphincterotomy are equally effective, endoscopic therapy is preferred since it is less invasive, is associated with less morbidity, and costs less. It is also more convenient for patients, since it is an outpatient procedure. Surgical treatment is usually reserved for those in whom endoscopic treatment has failed or is not technically possible.
OTHER PROCESSES OBSTRUCTING THE FLOW OF PANCREATIC JUICE
Any process preventing free flow of pancreatic juice can lead to acute pancreatitis. The cause of the blockage can be around the ampulla, in the ampulla, or in the duct.61
Periampullary lesions, tumors, or polyps can press on the ampulla and cause complete or relative obstruction of the pancreatic duct with a subsequent increase in intraductal pressure and, thus, acute pancreatitis.62 Tumors or polyps of the ampulla, such as ampullary adenoma or carcinoma, can cause pancreatitis by directly obstructing the pancreatic duct where it opens into the duodenum.63–66 Intraductal processes such as ductal adenocarcinoma, intraductal papillary mucinous tumor, pancreatic duct stone, and intraductal stricture due to cancer, chronic pancreatitis, or trauma can also cause pancreatitis by preventing free flow of pancreatic juice.67–71
Although it is well known that sequelae of severe chronic pancreatitis such as ductal strictures or intraductal stones can lead to recurrent attacks of acute pancreatitis by preventing the free flow of pancreatic juice, a relationship also seems to exist between early chronic pancreatitis and recurrent acute pancreatitis.72 Several studies have shown that up to 50% of patients with idiopathic recurrent pancreatitis have evidence of chronic pancreatitis.72–74 However, it is still unclear whether early chronic pancreatitis is the underlying cause of the recurrent attacks of acute pancreatitis or whether recurrent attacks of acute pancreatitis might have led to the development of chronic pancreatitis.
Diagnosis
Ampullary and periampullary neoplasms can be diagnosed endoscopically. Intraductal lesions such as strictures can be diagnosed by MRCP, especially secretin-enhanced MRCP, or by ERCP. ERCP has the additional advantage of being able to deliver treatment, ie, balloon dilation and stenting. In the case of ductal strictures, upsizing of the stents or placement of multiple stents during subsequent procedures is usually needed. Pancreatic ductal calcifications associated with chronic pancreatitis are usually radiopaque and are easily visible on plain films or computed tomography of the abdomen. Parenchymal and ductal changes of chronic pancreatitis can be diagnosed by endoscopic ultrasonography.
Treatment
The treatment is to relieve the obstruction and re-establish the free flow of pancreatic juice.
Periampullary tumors or polyps can be resected surgically or, if they involve only the mucosa, by endoscopic mucosal resection. Ampullary adenomas can be resected endoscopically. Ampullary carcinomas usually require surgical resection.
Small, nonobstructive stones in the pancreatic duct can be removed during ERCP.75 Larger stones may need to be fragmented by extracorporeal shock wave lithotripsy to facilitate removal by ERCP.75
Intraductal strictures should raise the suspicion of pancreatic adenocarcinoma, especially in older patients.61 In these cases, relief of the obstruction by placement of a pancreatic stent can prevent further attacks of pancreatitis until a diagnosis can be established and a more definitive treatment can be offered.
- Levy MJ, Geenen JE. Idiopathic acute recurrent pancreatitis. Am J Gastroenterol 2001; 96:2540–2555.
- Gullo L, Migliori M, Pezzilli R, et al. An update on recurrent acute pancreatitis: data from five European countries. Am J Gastroenterol 2002; 97:1959–1962.
- Gao YJ, Li YQ, Wang Q, et al. Analysis of the clinical features of recurrent acute pancreatitis in China. J Gastroenterol 2006; 41:681–685.
- Somogyi L, Martin SP, Venkatesan T, Ulrich CD. Recurrent acute pancreatitis: an algorithmic approach to identification and elimination of inciting factors. Gastroenterology 2001; 120:708–717.
- Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007; 102:1781–1788.
- Standards of Practice Committee,Zuckerman MJ, Shen B, Harrison ME, et al. Informed consent for GI endoscopy. Gastrointest Endosc 2007; 66:213–218.
- Lee SP, Hayashi A, Kim YS. Biliary sludge: curiosity or culprit? Hepatology 1994; 20:523–525.
- Opie E. The etiology of acute hemorrhagic pancreatitis. Bull Johns Hopkins Hosp 1901; 12:182–188.
- Hernandez CA, Lerch MM. Sphincter stenosis and gallstone migration through the biliary tract. Lancet 1993; 341:1371–1373.
- Ros E, Navarro S, Bru C, Garcia-Puges A, Valderrama R. Occult microlithiasis in 'idiopathic' acute pancreatitis: prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology 1991; 101:1701–1709.
- Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med 1992; 326:589–593.
- Levy MJ. The hunt for microlithiasis in idiopathic acute recurrent pancreatitis: should we abandon the search or intensify our efforts? Gastrointest Endosc 2002; 55:286–293.
- Delchier JC, Benfredj P, Preaux AM, Metreau JM, Dhumeaux D. The usefulness of microscopic bile examination in patients with suspected microlithiasis: a prospective evaluation. Hepatology 1986; 6:118–122.
- Lee SP, Nicholls JF. Nature and composition of biliary sludge. Gastroenterology 1986; 90:677–686.
- Testoni PA, Caporuscio S, Bagnolo F, Lella F. Idiopathic recurrent pancreatitis: long-term results after ERCP, endoscopic sphincterotomy, or ursodeoxycholic acid treatment. Am J Gastroenterol 2000; 95:1702–1707.
- Siddiqui AA, Mitroo P, Kowalski T, Loren D. Endoscopic sphincterotomy with or without cholecystectomy for choledocholithiasis in high-risk surgical patients: a decision analysis. Aliment Pharmacol Ther 2006; 24:1059–1066.
- Steinberg WM, Chari ST, Forsmark CE, et al. Controversies in clinical pancreatology: management of acute idiopathic recurrent pancreatitis. Pancreas 2003; 27:103–117.
- Khalid A, Slivka A. Approach to idiopathic recurrent pancreatitis. Gastrointest Endosc Clin North Am 2003; 13:695–716.
- Adler DG, Baron TH, Davila RE, et al; Standards of Practice Committee of American Society for Gastrointestinal Endoscopy. ASGE guideline: the role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc 2005; 62:1–8.
- Draganov P, Forsmark CE. “Idiopathic” pancreatitis. Gastroenterology 2005; 128:756–763.
- Chung EJ, Kim MH, Lee SS, Lee SK. Primary vs. secondary common bile duct stones: apples and oranges. Endoscopy 2003; 35:92.
- Saharia PC, Zuidema GD, Cameron JL. Primary common duct stones. Ann Surg 1977; 185:598–604.
- Elta GH. Sphincter of Oddi dysfunction and bile duct microlithiasis in acute idiopathic pancreatitis. World J Gastroenterol 2008; 14:1023–1026.
- Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med 1996; 335:909–918.
- Prat F, Malak NA, Pelletier G, et al. Biliary symptoms and complications more than 8 years after endoscopic sphincterotomy for choledocholithiasis. Gastroenterology 1996; 110:894–899.
- Hawes RH, Cotton PB, Vallon AG. Follow-up 6 to 11 years after duo-denoscopic sphincterotomy for stones in patients with prior cholecystectomy. Gastroenterology 1990; 98:1008–1012.
- Lai KH, Lo GH, Lin CK, et al. Do patients with recurrent choledocholithiasis after endoscopic sphincterotomy benefit from regular follow-up? Gastrointest Endosc 2002; 55:523–526.
- Kim DI, Kim MH, Lee SK, et al. Risk factors for recurrence of primary bile duct stones after endoscopic biliary sphincterotomy. Gastrointest Endosc 2001; 54:42–48.
- Costamagna G, Tringali A, Shah SK, Mutignani M, Zuccala G, Perri V. Long-term follow-up of patients after endoscopic sphincterotomy for choledocholithiasis, and risk factors for recurrence. Endoscopy 2002; 34:273–279.
- Keizman D, Ish Shalom M, Konikoff FM. Recurrent symptomatic common bile duct stones after endoscopic stone extraction in elderly patients. Gastrointest Endosc 2006; 64:60–65.
- Kaw M, Brodmerkel GJ. ERCP, biliary crystal analysis, and sphincter of Oddi manometry in idiopathic recurrent pancreatitis. Gastrointest Endosc 2002; 55:157–162.
- Chak A, Hawes RH, Cooper GS, et al. Prospective assessment of the utility of EUS in the evaluation of gallstone pancreatitis. Gastrointest Endosc 1999; 49:599–604.
- Parsi MA, Neuhaus H, Pleskow D, et al. Peroral cholangioscopy guided stone therapy—report of an international multicenter registry [abstract]. Gastrointest Endosc 2008; 67:AB102.
- Woods CM, Mawe GM, Toouli J, Saccone GT. The sphincter of Oddi: understanding its control and function. Neurogastroenterol Motil 2005; 17 suppl 1:31–40.
- McLoughlin MT, Mitchell RM. Sphincter of Oddi dysfunction and pancreatitis. World J Gastroenterol 2007; 13:6333–6343.
- Bosch A, Pena LR. The sphincter of Oddi. Dig Dis Sci 2007; 52:1211–1218.
- Devereaux BM, Sherman S, Lehman GA. Sphincter of Oddi (pancreatic) hypertension and recurrent pancreatitis. Curr Gastroenterol Rep 2002; 4:153–159.
- Fazel A, Geenen JE, MoezArdalan K, Catalano MF. Intrapancreatic ductal pressure in sphincter of Oddi dysfunction. Pancreas 2005; 30:359–362.
- Freeman ML. Role of pancreatic stents in prevention of post-ERCP pancreatitis. JOP 2004; 5:322–327.
- Singh P, Gurudu SR, Davidoff S, et al. Sphincter of Oddi manometry does not predispose to post-ERCP acute pancreatitis. Gastrointest Endosc 2004; 59:499–505.
- Guda NM, Freeman ML. True culprit or guilt by association? Is sphincter of Oddi manometry the cause of post-ERCP pancreatitis in patients with suspected sphincter of Oddi dysfunction, or is it the patients' susceptibility? Rev Gastroenterol Disord 2004; 4:211–213.
- Craig A, Toouli J. Sphincter of Oddi dysfunction: is there a role for medical therapy? Curr Gastroenterol Rep 2002; 4:172–176.
- Freeman ML, Gill M, Overby C, Cen YY. Predictors of outcomes after biliary and pancreatic sphincterotomy for sphincter of Oddi dysfunction. J Clin Gastroenterol 2007; 41:94–102.
- Sgouros SN, Pereira SP. Systematic review: sphincter of Oddi dysfunction—non-invasive diagnostic methods and long-term outcome after endoscopic sphincterotomy. Aliment Pharmacol Ther 2006; 24:237–246.
- Venu RP, Geenen JE, Hogan W, Stone J, Johnson GK, Soergel K. Idiopathic recurrent pancreatitis. An approach to diagnosis and treatment. Dig Dis Sci 1989; 34:56–60.
- Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989; 320:82–87.
- Fogel EL, Eversman D, Jamidar P, Sherman S, Lehman GA. Sphincter of Oddi dysfunction: pancreaticobiliary sphincterotomy with pancreatic stent placement has a lower rate of pancreatitis than biliary sphincterotomy alone. Endoscopy 2002; 34:280–285.
- Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol 2007; 5:1354–1365.
- Toouli J. The sphincter of Oddi and acute pancreatitis - revisited. HPB (Oxford) 2003; 5:142–145.
- Sherman S, Lehman GA. Sphincter of Oddi dysfunction: diagnosis and treatment. JOP 2001; 2:382–400.
- Klein SD, Affronti JP. Pancreas divisum, an evidence-based review: part I, pathophysiology. Gastrointest Endosc 2004; 60:419–425.
- Fogel EL, Toth TG, Lehman GA, DiMagno MJ, DiMagno EP. Does endoscopic therapy favorably affect the outcome of patients who have recurrent acute pancreatitis and pancreas divisum? Pancreas 2007; 34:21–45.
- Lehman GA. Acute recurrent pancreatitis. Can J Gastroenterol 2003; 17:381–383.
- Lehman GA, Sherman S. Pancreas divisum. Diagnosis, clinical significance, and management alternatives. Gastrointest Endosc Clin N Am 1995; 5:145–170.
- Staritz M, Meyer zum Buschenfelde KH. Elevated pressure in the dorsal part of pancreas divisum: the cause of chronic pancreatitis? Pancreas 1988; 3:108–110.
- Carnes M, Romagnuolo J, Cotton P. Miss rate of pancreas divisum by magnetic resonance cholangiopancreatography in clinical practice. Pancreas 2008; 37:151–153.
- Warshaw AL, Simeone JF, Schapiro RH, Flavin-Warshaw B. Evaluation and treatment of the dominant dorsal duct syndrome (pancreas divisum redefined). Am J Surg 1990; 159:59–64.
- Bradley EL, Stephan RN. Accessory duct sphincteroplasty is preferred for long-term prevention of recurrent acute pancreatitis in patients with pancreas divisum. J Am Coll Surg 1996; 183:65–70.
- Heyries L, Barthet M, Delvasto C, Zamora C, Bernard JP, Sahel J. Long-term results of endoscopic management of pancreas divisum with recurrent acute pancreatitis. Gastrointest Endosc 2002; 55:376–381.
- Lans JI, Geenen JE, Johanson JF, Hogan WJ. Endoscopic therapy in patients with pancreas divisum and acute pancreatitis: a prospective, randomized, controlled clinical trial. Gastrointest Endosc 1992; 38:430–434.
- Delhaye M, Matos C, Arvanitakis M, Deviere J. Pancreatic ductal system obstruction and acute recurrent pancreatitis. World J Gastroenterol 2008; 14:1027–1033.
- Finnie IA, Ghosh P, Garvey C, Poston GJ, Rhodes JM. Intraluminal duodenal diverticulum causing recurrent pancreatitis: treatment by endoscopic incision. Gut 1994; 35:557–559.
- Guzzardo G, Kleinman MS, Krackov JH, Schwartz SI. Recurrent acute pancreatitis caused by ampullary villous adenoma. J Clin Gastroenterol 1990; 12:200–202.
- Wright BE, Kozarek RA, Traverso LW, Wechter D, Thirlby R, Raltz SL. Recurrent pancreatitis in Gardner variant familial polyposis: etiology, diagnostic approach, and interventional results. Arch Surg 1999; 134:311–315.
- Tanasijtchouk T, Vaisbein E, Lachter J, Nassar F. Carcinoma of Papilla Vateri presenting as recurrent acute pancreatitis. Acta Gastroenterol Belg 2004; 67:309–310.
- Kwon TH, Park do H, Shim KY, et al. Ampullary adenomyoma presenting as acute recurrent pancreatitis. World J Gastroenterol 2007; 13:2892–2894.
- Lorente JA, Ruiz del Arbol L, Moreira VF, Garcia-Plaza A. Recurrent pancreatitis in a young patient associated with a solitary nonopaque concretion in the main pancreatic duct. Gastrointest Endosc 1990; 36:63–65.
- Chung JP, Chi SW, Park YN, et al. A case of minute intraductal papillary mucinous tumor of the pancreas presenting with recurrent acute pancreatitis. Yonsei Med J 2000; 41:528–532.
- Tikhomirov V, Tikhomirova S, Sieber S, Schiffman MK. A pancreatic intraductal papillary mucinous tumor causing recurrent acute pancreatitis at the onset of menstrual periods. J Clin Gastroenterol 2000; 31:172–174.
- Mosca S, Bottino V, Molino C. Hepatobiliary and pancreatic: a woman with recurrent idiopathic acute pancreatitis. Intraductal papillary mucinous tumor of the pancreas. J Gastroenterol Hepatol 2001; 16:1070,1075.
- Howard TJ, Moore SA, Saxena R, Matthews DE, Schmidt CM, Wiebke EA. Pancreatic duct strictures are a common cause of recurrent pancreatitis after successful management of pancreatic necrosis. Surgery 2004; 136:909–916.
- Garg PK, Tandon RK, Madan K. Is biliary microlithiasis a significant cause of idiopathic recurrent acute pancreatitis? A long-term follow-up study. Clin Gastroenterol Hepatol 2007; 5:75–79.
- Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol 2001; 96:705–709.
- Yusoff IF, Raymond G, Sahai AV. A prospective comparison of the yield of EUS in primary vs. recurrent idiopathic acute pancreatitis. Gastrointest Endosc 2004; 60:673–678.
- Cahen DL, Gouma DJ, Nio Y, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med 2007; 356:676–684.
Endoscopic therapy has become an alternative to surgery for some patients with acute recurrent pancreatitis, ie, those whose disease is caused by gallstones or other mechanical processes that can obstruct the outflow from the pancreas.
In this paper, we review the specific situations in which endoscopic therapy might be useful in patients with acute recurrent pancreatitis.
ACUTE PANCREATITIS IS MANAGED DIFFERENTLY IF IT RECURS
Recurrent acute pancreatitis is defined as more than one episode of acute pancreatitis.1 In clinical practice, it is important to distinguish between the first and recurrent episodes of acute pancreatitis.
Most patients who have one episode of acute pancreatitis never have another one.2,3 Therefore, for patients having an initial attack, we recommend a limited workup that includes a detailed history, laboratory evaluation, and a noninvasive imaging study such as transcutaneous ultrasonography or computed tomography.
On the other hand, people who have a second attack are at higher risk of more recurrences. Therefore, patients having recurrent attacks need a more extensive workup to determine the underlying cause. We recommend referring them to a gastroenterologist for further evaluation.
WHICH CAUSES CAN BE MANAGED ENDOSCOPICALLY?
In the Western world, 70% to 80% of cases of recurrent pancreatitis are due to either alcohol abuse or gallstone disease.2,4 The rest are related to:
- Autoimmune disorders
- Cancer, including occult malignancies and premalignant conditions such as intraductal papillary mucinous neoplasm
- Chronic pancreatitis
- Drugs
- Heredity
- Metabolic abnormalities (hypertriglyceridemia, hypercalcemia)
- Sphincter of Oddi dysfunction
- Structural or congenital abnormalities (pancreas divisum)
- Trauma.
- Gallstone disease, including biliary microlithiasis and sludge (in patients with or without a gallbladder)
- Sphincter of Oddi dysfunction
- Pancreas divisum
- Obstruction to flow of pancreatic juice.
Endoscopy is not completely benign
Although endoscopic procedures are less invasive than surgery, they are not completely benign. They can cause anxiety and are associated with risks such as bleeding, perforation, and pancreatitis.5 The risks, benefits, and alternatives to these procedures should be discussed with the patient, and informed consent should be obtained before any endoscopic procedure.6
STONES (LARGE OR SMALL) OR SLUDGE IN PATIENTS WITH A GALLBLADDER
Gallstones can be large, but small stones (microlithiasis) and sludge are more common and therefore account for more cases of pancreatitis.
Strictly defined, microlithiasis refers to stones smaller than 2 mm in diameter in the biliary tract, whereas sludge is a suspension of biliary crystals, mucin, and cellular debris in the gallbladder or bile ducts.7 The terms are often used interchangeably, since the conditions often coexist and their treatment is similar.
Theories differ as to how microlithiasis or sludge can cause recurrent pancreatitis. According to one theory, the debris blocks the common channel, increasing the pancreatic intraductal pressure and leading to pancreatitis.8 A second theory is that small stones or biliary crystals passing through the sphincter of Oddi cause inflammation, and that repeated inflammation eventually leads to stenosis or dyskinesia of the sphincter, both of which have been associated with pancreatitis.9
Studies suggest that microlithiasis and sludge are common causes of recurrent pancreatitis, accounting for about two-thirds of cases according to estimates by Ros et al10 and Lee et al.11
Detecting small stones and sludge
The diagnosis of microlithiasis and biliary sludge in patients with a gallbladder is based on imaging studies and bile microscopy.12
Transabdominal ultrasonography is the imaging study most often used for diagnosing microlithiasis. The technology and expertise for this test are widely available, and it is relatively inexpensive.
Endoscopic ultrasonography is more sensitive for detecting microlithiasis and can examine the distal common bile duct.
Bile microscopy involves obtaining bile from the second portion of the duodenum (via an endoscope or a duodenal tube) or from the bile ducts (by cannulating the common bile duct and stimulating the gallbladder with cholecystokinin). The bile sample is centrifuged and inspected microscopically under plain light and polarized light (which aids the visualization of biliary crystals). The crystals can be cholesterol monohydrate, calcium bilirubinate, or calcium carbonate.7,13,14
Removing the gallbladder is the treatment of choice for small stones and sludge
Treatments to prevent recurrent attacks of acute pancreatitis due to microlithiasis and sludge include cholecystectomy, biliary sphincterotomy, and ursodioxycholic acid.10,11,15
In prospective observational studies by Ros et al10 and Lee et al,11 about half of the patients with recurrent pancreatitis were treated with cholecystectomy, endoscopic sphincterotomy, or ursodioxycholic acid in a nonrandomized fashion. The choice of therapy was based on the patient’s medical status and the preferences of the patient and the physician. Half the patients received no treatment. In both studies the median follow-up was 4 years. Treated patients had a significantly lower rate of recurrent attacks of pancreatitis during follow-up: less than 20% with therapy compared with more than 60% without therapy. Unfortunately, no published study has compared these three treatments head to head.
Cholecystectomy, however, is the most definitive therapy and is generally considered the treatment of choice.
Biliary sphincterotomy is an endoscopic procedure that involves cutting the sphincter of Oddi to allow the stones and sludge to pass more freely. It is as effective as cholecystectomy in preventing recurrent attacks but does not eliminate the risk of cholecystitis and cholangitis (Figure 1). For this reason, it is usually reserved for patients who cannot tolerate surgery due to comorbidities, those who refuse surgery, or those who are pregnant.16
Ursodeoxycholic acid is a reasonable alternative in patients who cannot tolerate surgical or endoscopic biliary sphincterotomy.1,17–20 The dosage is 10 mg/kg/day, which can be in two or three divided doses. The optimal duration of treatment is not known; however, since this drug works slowly, it may need to be taken for 2 years or more. Ursodeoxycholic acid is more effective in patients with cholesterol-based stones and crystals. It is not effective for large stones (> 1 cm in diameter) or calcified stones.
STONES AFTER CHOLECYSTECTOMY
Bile duct stones can be classified as primary or secondary. A primary stone is one that remains where it was formed, whereas a secondary stone is one that has migrated from its site of formation.21
Some suggest that bile duct stones that are detected within 2 years of cholecystectomy originated in the gallbladder and were missed when the gallbladder was removed (and therefore are considered secondary stones), and that stones that present more than 2 years after cholecystectomy are de novo (ie, primary) stones.22,23
In any event, stones have been found in the common bile duct in 4% to 24% of patients up to 15 years after cholecystectomy.24–26 A fair number of these patients have no symptoms.27 Risk factors for stone recurrence are lithogenic bile (ie, high concentration of cholesterol, low concentration of bile salts), biliary stasis, strictures, dilated bile ducts, and advanced age.28–30
No role for crystal analysis after cholecystectomy
Biliary crystal analysis does not seem to have diagnostic value in patients with recurrent acute pancreatitis after cholecystectomy,31 because removing the gallbladder eliminates the crystals and sludge. Imaging studies are therefore the cornerstone of diagnosis.
Transabdominal ultrasonography is the most commonly used initial imaging test. However, abdominal fat and gas in the duodenum can obscure the distal common bile duct and decrease the sensitivity of this test.32
Endoscopic ultrasonography involves positioning the transducer in the second part of the duodenum, where it can show the adjacent biliary tree without interference from digestive gas or abdominal fat.
Magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasonography are both highly sensitive for detecting common bile duct stones and are recommended if they can be done without delay.
Endoscopic retrograde cholangiopancreatography (ERCP). As a rule, patients who are very likely to have gallstones are best served by proceeding directly to ERCP, a procedure that enables both imaging and treatment. However, ERCP exposes the patient to radiation and the risk of pancreatitis, so in some patients (eg, pregnant women, people who recently had acute pancreatitis), one may want to do ultrasonography first.
ERCP is the treatment of choice after cholecystectomy
The treatment of choice in patients with choledocholithiasis is ERCP with biliary sphincterotomy and stone extraction. Success at clearing the biliary tree of all stones depends on the size, number, and location of the stones, the anatomy of the digestive tract and the bile duct, and the experience of the endoscopist. At specialized centers, the rate of successful clearance with subsequent procedures is close to 100%. Large stones may require fragmentation inside the bile duct to aid their removal.33
SPHINCTER OF ODDI DYSFUNCTION
The sphincter of Oddi, located where the bile and pancreatic ducts penetrate the wall of the duodenum, actually consists of three sphincters: the common, the biliary, and the pancreatic. Its physiologic role is to regulate the flow of bile and pancreatic juice into the duodenum and to prevent reflux into the ducts from the duodenum.34 Its basal pressure is the main regulating mechanism for pancreatic and biliary secretions into the intestine, and its phasic contractile activity is closely associated with duodenal motility.
Sphincter dysfunction: Stenosis, dyskinesia
The sphincter of Oddi can obstruct the flow of bile and pancreatic juice owing either to stenosis or to dyskinesia.35,36 Stenosis refers to structural alteration of the sphincter, probably from inflammation and subsequent fibrosis. In contrast, dyskinesia refers to a motor abnormality of the sphincter that makes it hypertonic.
Stenosis or dyskinesia can occur in the biliary sphincter, the pancreatic sphincter, the common sphincter, or any combination of the three. For example, dysfunction of the biliary sphincter can cause abnormalities in liver-associated enzyme levels and biliary-type pain, whereas pancreatic sphincter dysfunction can cause recurrent attacks of pancreatitis and pancreatic-type pain.37 Elevated pancreatic sphincter pressure has been shown to correlate with increased pancreatic ductal pressure, suggesting that the sphincter plays a role in the pathogenesis of acute pancreatitis.23,38
Sphincter pressure can be measured during ERCP, but ERCP is risky
The gold standard for the diagnosis of sphincter of Oddi dysfunction is manometry,23,35 ie, direct measurement of sphincter pressure via a thin catheter placed inside the pancreatic or biliary sphincter during ERCP (Figure 1).
However, in patients with suspected sphincter of Oddi dysfunction, ERCP with or without manometry is associated with a high rate of complications, with pancreatitis occurring in up to 25% of cases.39–41 Therefore, several noninvasive and provocative tests have been designed in an attempt to identify patients with this disorder. Unfortunately, none of them seems to be as sensitive and specific as manometry for diagnosing sphincter of Oddi dysfunction, and so they have not gained widespread use.
Opening the sphincter of Oddi with drugs, endoscopy, or surgery
Drug treatment of sphincter of Oddi dysfunction is based on drugs that relax smooth muscle, such as calcium channel blockers and nitrates. The treatment must be lifelong. Also, it does not improve sphincter stenosis, and only half of patients with sphincter dyskinesia respond to it. For these reasons, drug treatment of sphincter of Oddi dysfunction has not gained widespread acceptance.36,42
Endoscopic sphincterotomy is the current standard endoscopic therapy for sphincter of Oddi dysfunction. This procedure is performed during ERCP and involves cutting the sphincter with electrocautery.
Endoscopic pancreatic sphincterotomy prevents recurrent attacks of pancreatitis in patients with pancreatic sphincter dysfunction in more than 60% of cases.23,43–46 A potential complication is pancreatitis, which occurs more often in patients with pancreatic sphincter dyskinesia. Placing a stent in the pancreatic duct after pancreatic sphincterotomy reduces the risk of pancreatitis after ERCP.37,47,48
Surgery. Pancreatic sphincterotomy can also be done surgically, most commonly via transduodenal pancreatic sphincteroplasty. Surgical sphincteroplasty is as effective as endoscopic sphincterotomy for preventing recurrent attacks of pancreatitis in patients with pancreatic sphincter dysfunction.49 However, endoscopic therapy is much less invasive and remains the preferred treatment for sphincter of Oddi dysfunction in most centers with experience in this technique.50
PANCREAS DIVISUM
Pancreas divisum is the most common congenital anomaly of the pancreatic duct. Autopsy studies show it occurs in 5% to 10% of the population.51–53
At approximately the 5th week of gestation, there are two pancreatic buds: a ventral and a dorsal bud. The ventral bud eventually gives rise to part of the pancreatic head and uncinate process of the pancreas in the adult. The dorsal bud eventually gives rise to the rest of the pancreatic head, the pancreatic body, and the pancreatic tail. At 6 to 7 weeks of gestation, the ventral bud rotates clockwise and lies posterior to the dorsal bud. At this stage, both the dorsal and ventral pancreata have their own ducts, which do not communicate with each other. Normally, the ventral and dorsal pancreas and their ducts fuse together at 8 weeks of gestation; in people with pancreas divisum, this ductal fusion does not occur.51
The pancreas secretes 1.5 L of fluid per day. Normally, 90% to 95% of this volume drains through the major papilla. In people with pancreas divisum, 90% to 95% of the fluid drains through the minor papilla.
People with pancreas divisum are a heterogeneous group. Most have no symptoms, and their ductal anatomy is diagnosed only incidentally. However, a subgroup is prone to develop acute pancreatitis. The cause is thought to be the small diameter of the minor papilla, which poses a relative obstruction to the flow of pancreatic juice.54 Direct support for this theory comes from a study in which investigators measured pancreatic ductal pressures in eight people with normal anatomy and six people with pancreas divisum. The pressure in the main pancreatic duct in those with pancreas divisum was significantly higher than in those with normal anatomy.55 Additional evidence in favor of this theory is the effectiveness of treatment, which involves widening the minor papillary opening (minor papillary sphincterotomy).
Diagnosis of pancreas divisum
The diagnosis of pancreas divisum is based on imaging studies, and ERCP remains the gold standard for patients with equivocal results on noninvasive imaging. However, MRCP, especially secretin-enhanced MRCP, is as accurate as ERCP. In most cases, MRCP has replaced ERCP for the diagnosis of this condition, although a recent study suggests that MRCP is inferior to ERCP in the diagnosis of pancreas divisum.56 We recommend secretin-enhanced MRCP for this purpose.
Computed tomography and endoscopic ultrasonography can also diagnose pancreas divisum, but their diagnostic accuracy is lower than that of ERCP and MRCP.
Minor papillary sphincterotomy
Treating recurrent pancreatitis due to pancreas divisum involves relieving the relative obstruction of the minor papilla by minor papillary sphincterotomy. This can be done surgically or endoscopically (Figure 1).
Surgery. No randomized, controlled study has yet assessed the efficacy of surgical sphincteroplasty for recurrent pancreatitis in patients with pancreas divisum. However, retrospective studies and one prospective study have been published.57,58
In the retrospective study with the largest number of patients, Warshaw et al57 reported their experience in 49 patients who had recurrent pancreatitis due to pancreas divisum. After surgical sphincteroplasty, the patients were followed for a mean of 53 months; 40 (82%) of the 49 patients had no further episodes of acute pancreatitis during this time.
Bradley and Stephan58 studied 37 patients with pancreas divisum and recurrent pancreatitis.58 After surgical sphincteroplasty, the patients were followed for a mean of 60 months; 31 of the 37 patients had no further attacks, a success rate of 84%.
Endoscopic therapy. As with surgical therapy trials, most trials of endoscopic therapy of recurrent pancreatitis in patients with pancreas divisum are small case series. In a retrospective study with one of the largest number of patients, Heyries et al59 reported their experience with 24 patients with pancreas divisum and recurrent pancreatitis. After undergoing endoscopic minor papillary sphincterotomy, all patients were followed for a mean of 39 months, during which 22 (92%) did not have further episodes of acute pancreatitis.
In the only randomized controlled trial available, 19 patients with recurrent pancreatitis and pancreas divisum underwent either no treatment or endoscopic minor papillary sphincterotomy.60 In the treatment group, 9 of 10 patients had no further episodes of acute pancreatitis during the 3 years of follow-up, while 6 of 9 patients who were randomized to no treatment had at least one episode.60
Although surgical and endoscopic minor papillary sphincterotomy are equally effective, endoscopic therapy is preferred since it is less invasive, is associated with less morbidity, and costs less. It is also more convenient for patients, since it is an outpatient procedure. Surgical treatment is usually reserved for those in whom endoscopic treatment has failed or is not technically possible.
OTHER PROCESSES OBSTRUCTING THE FLOW OF PANCREATIC JUICE
Any process preventing free flow of pancreatic juice can lead to acute pancreatitis. The cause of the blockage can be around the ampulla, in the ampulla, or in the duct.61
Periampullary lesions, tumors, or polyps can press on the ampulla and cause complete or relative obstruction of the pancreatic duct with a subsequent increase in intraductal pressure and, thus, acute pancreatitis.62 Tumors or polyps of the ampulla, such as ampullary adenoma or carcinoma, can cause pancreatitis by directly obstructing the pancreatic duct where it opens into the duodenum.63–66 Intraductal processes such as ductal adenocarcinoma, intraductal papillary mucinous tumor, pancreatic duct stone, and intraductal stricture due to cancer, chronic pancreatitis, or trauma can also cause pancreatitis by preventing free flow of pancreatic juice.67–71
Although it is well known that sequelae of severe chronic pancreatitis such as ductal strictures or intraductal stones can lead to recurrent attacks of acute pancreatitis by preventing the free flow of pancreatic juice, a relationship also seems to exist between early chronic pancreatitis and recurrent acute pancreatitis.72 Several studies have shown that up to 50% of patients with idiopathic recurrent pancreatitis have evidence of chronic pancreatitis.72–74 However, it is still unclear whether early chronic pancreatitis is the underlying cause of the recurrent attacks of acute pancreatitis or whether recurrent attacks of acute pancreatitis might have led to the development of chronic pancreatitis.
Diagnosis
Ampullary and periampullary neoplasms can be diagnosed endoscopically. Intraductal lesions such as strictures can be diagnosed by MRCP, especially secretin-enhanced MRCP, or by ERCP. ERCP has the additional advantage of being able to deliver treatment, ie, balloon dilation and stenting. In the case of ductal strictures, upsizing of the stents or placement of multiple stents during subsequent procedures is usually needed. Pancreatic ductal calcifications associated with chronic pancreatitis are usually radiopaque and are easily visible on plain films or computed tomography of the abdomen. Parenchymal and ductal changes of chronic pancreatitis can be diagnosed by endoscopic ultrasonography.
Treatment
The treatment is to relieve the obstruction and re-establish the free flow of pancreatic juice.
Periampullary tumors or polyps can be resected surgically or, if they involve only the mucosa, by endoscopic mucosal resection. Ampullary adenomas can be resected endoscopically. Ampullary carcinomas usually require surgical resection.
Small, nonobstructive stones in the pancreatic duct can be removed during ERCP.75 Larger stones may need to be fragmented by extracorporeal shock wave lithotripsy to facilitate removal by ERCP.75
Intraductal strictures should raise the suspicion of pancreatic adenocarcinoma, especially in older patients.61 In these cases, relief of the obstruction by placement of a pancreatic stent can prevent further attacks of pancreatitis until a diagnosis can be established and a more definitive treatment can be offered.
Endoscopic therapy has become an alternative to surgery for some patients with acute recurrent pancreatitis, ie, those whose disease is caused by gallstones or other mechanical processes that can obstruct the outflow from the pancreas.
In this paper, we review the specific situations in which endoscopic therapy might be useful in patients with acute recurrent pancreatitis.
ACUTE PANCREATITIS IS MANAGED DIFFERENTLY IF IT RECURS
Recurrent acute pancreatitis is defined as more than one episode of acute pancreatitis.1 In clinical practice, it is important to distinguish between the first and recurrent episodes of acute pancreatitis.
Most patients who have one episode of acute pancreatitis never have another one.2,3 Therefore, for patients having an initial attack, we recommend a limited workup that includes a detailed history, laboratory evaluation, and a noninvasive imaging study such as transcutaneous ultrasonography or computed tomography.
On the other hand, people who have a second attack are at higher risk of more recurrences. Therefore, patients having recurrent attacks need a more extensive workup to determine the underlying cause. We recommend referring them to a gastroenterologist for further evaluation.
WHICH CAUSES CAN BE MANAGED ENDOSCOPICALLY?
In the Western world, 70% to 80% of cases of recurrent pancreatitis are due to either alcohol abuse or gallstone disease.2,4 The rest are related to:
- Autoimmune disorders
- Cancer, including occult malignancies and premalignant conditions such as intraductal papillary mucinous neoplasm
- Chronic pancreatitis
- Drugs
- Heredity
- Metabolic abnormalities (hypertriglyceridemia, hypercalcemia)
- Sphincter of Oddi dysfunction
- Structural or congenital abnormalities (pancreas divisum)
- Trauma.
- Gallstone disease, including biliary microlithiasis and sludge (in patients with or without a gallbladder)
- Sphincter of Oddi dysfunction
- Pancreas divisum
- Obstruction to flow of pancreatic juice.
Endoscopy is not completely benign
Although endoscopic procedures are less invasive than surgery, they are not completely benign. They can cause anxiety and are associated with risks such as bleeding, perforation, and pancreatitis.5 The risks, benefits, and alternatives to these procedures should be discussed with the patient, and informed consent should be obtained before any endoscopic procedure.6
STONES (LARGE OR SMALL) OR SLUDGE IN PATIENTS WITH A GALLBLADDER
Gallstones can be large, but small stones (microlithiasis) and sludge are more common and therefore account for more cases of pancreatitis.
Strictly defined, microlithiasis refers to stones smaller than 2 mm in diameter in the biliary tract, whereas sludge is a suspension of biliary crystals, mucin, and cellular debris in the gallbladder or bile ducts.7 The terms are often used interchangeably, since the conditions often coexist and their treatment is similar.
Theories differ as to how microlithiasis or sludge can cause recurrent pancreatitis. According to one theory, the debris blocks the common channel, increasing the pancreatic intraductal pressure and leading to pancreatitis.8 A second theory is that small stones or biliary crystals passing through the sphincter of Oddi cause inflammation, and that repeated inflammation eventually leads to stenosis or dyskinesia of the sphincter, both of which have been associated with pancreatitis.9
Studies suggest that microlithiasis and sludge are common causes of recurrent pancreatitis, accounting for about two-thirds of cases according to estimates by Ros et al10 and Lee et al.11
Detecting small stones and sludge
The diagnosis of microlithiasis and biliary sludge in patients with a gallbladder is based on imaging studies and bile microscopy.12
Transabdominal ultrasonography is the imaging study most often used for diagnosing microlithiasis. The technology and expertise for this test are widely available, and it is relatively inexpensive.
Endoscopic ultrasonography is more sensitive for detecting microlithiasis and can examine the distal common bile duct.
Bile microscopy involves obtaining bile from the second portion of the duodenum (via an endoscope or a duodenal tube) or from the bile ducts (by cannulating the common bile duct and stimulating the gallbladder with cholecystokinin). The bile sample is centrifuged and inspected microscopically under plain light and polarized light (which aids the visualization of biliary crystals). The crystals can be cholesterol monohydrate, calcium bilirubinate, or calcium carbonate.7,13,14
Removing the gallbladder is the treatment of choice for small stones and sludge
Treatments to prevent recurrent attacks of acute pancreatitis due to microlithiasis and sludge include cholecystectomy, biliary sphincterotomy, and ursodioxycholic acid.10,11,15
In prospective observational studies by Ros et al10 and Lee et al,11 about half of the patients with recurrent pancreatitis were treated with cholecystectomy, endoscopic sphincterotomy, or ursodioxycholic acid in a nonrandomized fashion. The choice of therapy was based on the patient’s medical status and the preferences of the patient and the physician. Half the patients received no treatment. In both studies the median follow-up was 4 years. Treated patients had a significantly lower rate of recurrent attacks of pancreatitis during follow-up: less than 20% with therapy compared with more than 60% without therapy. Unfortunately, no published study has compared these three treatments head to head.
Cholecystectomy, however, is the most definitive therapy and is generally considered the treatment of choice.
Biliary sphincterotomy is an endoscopic procedure that involves cutting the sphincter of Oddi to allow the stones and sludge to pass more freely. It is as effective as cholecystectomy in preventing recurrent attacks but does not eliminate the risk of cholecystitis and cholangitis (Figure 1). For this reason, it is usually reserved for patients who cannot tolerate surgery due to comorbidities, those who refuse surgery, or those who are pregnant.16
Ursodeoxycholic acid is a reasonable alternative in patients who cannot tolerate surgical or endoscopic biliary sphincterotomy.1,17–20 The dosage is 10 mg/kg/day, which can be in two or three divided doses. The optimal duration of treatment is not known; however, since this drug works slowly, it may need to be taken for 2 years or more. Ursodeoxycholic acid is more effective in patients with cholesterol-based stones and crystals. It is not effective for large stones (> 1 cm in diameter) or calcified stones.
STONES AFTER CHOLECYSTECTOMY
Bile duct stones can be classified as primary or secondary. A primary stone is one that remains where it was formed, whereas a secondary stone is one that has migrated from its site of formation.21
Some suggest that bile duct stones that are detected within 2 years of cholecystectomy originated in the gallbladder and were missed when the gallbladder was removed (and therefore are considered secondary stones), and that stones that present more than 2 years after cholecystectomy are de novo (ie, primary) stones.22,23
In any event, stones have been found in the common bile duct in 4% to 24% of patients up to 15 years after cholecystectomy.24–26 A fair number of these patients have no symptoms.27 Risk factors for stone recurrence are lithogenic bile (ie, high concentration of cholesterol, low concentration of bile salts), biliary stasis, strictures, dilated bile ducts, and advanced age.28–30
No role for crystal analysis after cholecystectomy
Biliary crystal analysis does not seem to have diagnostic value in patients with recurrent acute pancreatitis after cholecystectomy,31 because removing the gallbladder eliminates the crystals and sludge. Imaging studies are therefore the cornerstone of diagnosis.
Transabdominal ultrasonography is the most commonly used initial imaging test. However, abdominal fat and gas in the duodenum can obscure the distal common bile duct and decrease the sensitivity of this test.32
Endoscopic ultrasonography involves positioning the transducer in the second part of the duodenum, where it can show the adjacent biliary tree without interference from digestive gas or abdominal fat.
Magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasonography are both highly sensitive for detecting common bile duct stones and are recommended if they can be done without delay.
Endoscopic retrograde cholangiopancreatography (ERCP). As a rule, patients who are very likely to have gallstones are best served by proceeding directly to ERCP, a procedure that enables both imaging and treatment. However, ERCP exposes the patient to radiation and the risk of pancreatitis, so in some patients (eg, pregnant women, people who recently had acute pancreatitis), one may want to do ultrasonography first.
ERCP is the treatment of choice after cholecystectomy
The treatment of choice in patients with choledocholithiasis is ERCP with biliary sphincterotomy and stone extraction. Success at clearing the biliary tree of all stones depends on the size, number, and location of the stones, the anatomy of the digestive tract and the bile duct, and the experience of the endoscopist. At specialized centers, the rate of successful clearance with subsequent procedures is close to 100%. Large stones may require fragmentation inside the bile duct to aid their removal.33
SPHINCTER OF ODDI DYSFUNCTION
The sphincter of Oddi, located where the bile and pancreatic ducts penetrate the wall of the duodenum, actually consists of three sphincters: the common, the biliary, and the pancreatic. Its physiologic role is to regulate the flow of bile and pancreatic juice into the duodenum and to prevent reflux into the ducts from the duodenum.34 Its basal pressure is the main regulating mechanism for pancreatic and biliary secretions into the intestine, and its phasic contractile activity is closely associated with duodenal motility.
Sphincter dysfunction: Stenosis, dyskinesia
The sphincter of Oddi can obstruct the flow of bile and pancreatic juice owing either to stenosis or to dyskinesia.35,36 Stenosis refers to structural alteration of the sphincter, probably from inflammation and subsequent fibrosis. In contrast, dyskinesia refers to a motor abnormality of the sphincter that makes it hypertonic.
Stenosis or dyskinesia can occur in the biliary sphincter, the pancreatic sphincter, the common sphincter, or any combination of the three. For example, dysfunction of the biliary sphincter can cause abnormalities in liver-associated enzyme levels and biliary-type pain, whereas pancreatic sphincter dysfunction can cause recurrent attacks of pancreatitis and pancreatic-type pain.37 Elevated pancreatic sphincter pressure has been shown to correlate with increased pancreatic ductal pressure, suggesting that the sphincter plays a role in the pathogenesis of acute pancreatitis.23,38
Sphincter pressure can be measured during ERCP, but ERCP is risky
The gold standard for the diagnosis of sphincter of Oddi dysfunction is manometry,23,35 ie, direct measurement of sphincter pressure via a thin catheter placed inside the pancreatic or biliary sphincter during ERCP (Figure 1).
However, in patients with suspected sphincter of Oddi dysfunction, ERCP with or without manometry is associated with a high rate of complications, with pancreatitis occurring in up to 25% of cases.39–41 Therefore, several noninvasive and provocative tests have been designed in an attempt to identify patients with this disorder. Unfortunately, none of them seems to be as sensitive and specific as manometry for diagnosing sphincter of Oddi dysfunction, and so they have not gained widespread use.
Opening the sphincter of Oddi with drugs, endoscopy, or surgery
Drug treatment of sphincter of Oddi dysfunction is based on drugs that relax smooth muscle, such as calcium channel blockers and nitrates. The treatment must be lifelong. Also, it does not improve sphincter stenosis, and only half of patients with sphincter dyskinesia respond to it. For these reasons, drug treatment of sphincter of Oddi dysfunction has not gained widespread acceptance.36,42
Endoscopic sphincterotomy is the current standard endoscopic therapy for sphincter of Oddi dysfunction. This procedure is performed during ERCP and involves cutting the sphincter with electrocautery.
Endoscopic pancreatic sphincterotomy prevents recurrent attacks of pancreatitis in patients with pancreatic sphincter dysfunction in more than 60% of cases.23,43–46 A potential complication is pancreatitis, which occurs more often in patients with pancreatic sphincter dyskinesia. Placing a stent in the pancreatic duct after pancreatic sphincterotomy reduces the risk of pancreatitis after ERCP.37,47,48
Surgery. Pancreatic sphincterotomy can also be done surgically, most commonly via transduodenal pancreatic sphincteroplasty. Surgical sphincteroplasty is as effective as endoscopic sphincterotomy for preventing recurrent attacks of pancreatitis in patients with pancreatic sphincter dysfunction.49 However, endoscopic therapy is much less invasive and remains the preferred treatment for sphincter of Oddi dysfunction in most centers with experience in this technique.50
PANCREAS DIVISUM
Pancreas divisum is the most common congenital anomaly of the pancreatic duct. Autopsy studies show it occurs in 5% to 10% of the population.51–53
At approximately the 5th week of gestation, there are two pancreatic buds: a ventral and a dorsal bud. The ventral bud eventually gives rise to part of the pancreatic head and uncinate process of the pancreas in the adult. The dorsal bud eventually gives rise to the rest of the pancreatic head, the pancreatic body, and the pancreatic tail. At 6 to 7 weeks of gestation, the ventral bud rotates clockwise and lies posterior to the dorsal bud. At this stage, both the dorsal and ventral pancreata have their own ducts, which do not communicate with each other. Normally, the ventral and dorsal pancreas and their ducts fuse together at 8 weeks of gestation; in people with pancreas divisum, this ductal fusion does not occur.51
The pancreas secretes 1.5 L of fluid per day. Normally, 90% to 95% of this volume drains through the major papilla. In people with pancreas divisum, 90% to 95% of the fluid drains through the minor papilla.
People with pancreas divisum are a heterogeneous group. Most have no symptoms, and their ductal anatomy is diagnosed only incidentally. However, a subgroup is prone to develop acute pancreatitis. The cause is thought to be the small diameter of the minor papilla, which poses a relative obstruction to the flow of pancreatic juice.54 Direct support for this theory comes from a study in which investigators measured pancreatic ductal pressures in eight people with normal anatomy and six people with pancreas divisum. The pressure in the main pancreatic duct in those with pancreas divisum was significantly higher than in those with normal anatomy.55 Additional evidence in favor of this theory is the effectiveness of treatment, which involves widening the minor papillary opening (minor papillary sphincterotomy).
Diagnosis of pancreas divisum
The diagnosis of pancreas divisum is based on imaging studies, and ERCP remains the gold standard for patients with equivocal results on noninvasive imaging. However, MRCP, especially secretin-enhanced MRCP, is as accurate as ERCP. In most cases, MRCP has replaced ERCP for the diagnosis of this condition, although a recent study suggests that MRCP is inferior to ERCP in the diagnosis of pancreas divisum.56 We recommend secretin-enhanced MRCP for this purpose.
Computed tomography and endoscopic ultrasonography can also diagnose pancreas divisum, but their diagnostic accuracy is lower than that of ERCP and MRCP.
Minor papillary sphincterotomy
Treating recurrent pancreatitis due to pancreas divisum involves relieving the relative obstruction of the minor papilla by minor papillary sphincterotomy. This can be done surgically or endoscopically (Figure 1).
Surgery. No randomized, controlled study has yet assessed the efficacy of surgical sphincteroplasty for recurrent pancreatitis in patients with pancreas divisum. However, retrospective studies and one prospective study have been published.57,58
In the retrospective study with the largest number of patients, Warshaw et al57 reported their experience in 49 patients who had recurrent pancreatitis due to pancreas divisum. After surgical sphincteroplasty, the patients were followed for a mean of 53 months; 40 (82%) of the 49 patients had no further episodes of acute pancreatitis during this time.
Bradley and Stephan58 studied 37 patients with pancreas divisum and recurrent pancreatitis.58 After surgical sphincteroplasty, the patients were followed for a mean of 60 months; 31 of the 37 patients had no further attacks, a success rate of 84%.
Endoscopic therapy. As with surgical therapy trials, most trials of endoscopic therapy of recurrent pancreatitis in patients with pancreas divisum are small case series. In a retrospective study with one of the largest number of patients, Heyries et al59 reported their experience with 24 patients with pancreas divisum and recurrent pancreatitis. After undergoing endoscopic minor papillary sphincterotomy, all patients were followed for a mean of 39 months, during which 22 (92%) did not have further episodes of acute pancreatitis.
In the only randomized controlled trial available, 19 patients with recurrent pancreatitis and pancreas divisum underwent either no treatment or endoscopic minor papillary sphincterotomy.60 In the treatment group, 9 of 10 patients had no further episodes of acute pancreatitis during the 3 years of follow-up, while 6 of 9 patients who were randomized to no treatment had at least one episode.60
Although surgical and endoscopic minor papillary sphincterotomy are equally effective, endoscopic therapy is preferred since it is less invasive, is associated with less morbidity, and costs less. It is also more convenient for patients, since it is an outpatient procedure. Surgical treatment is usually reserved for those in whom endoscopic treatment has failed or is not technically possible.
OTHER PROCESSES OBSTRUCTING THE FLOW OF PANCREATIC JUICE
Any process preventing free flow of pancreatic juice can lead to acute pancreatitis. The cause of the blockage can be around the ampulla, in the ampulla, or in the duct.61
Periampullary lesions, tumors, or polyps can press on the ampulla and cause complete or relative obstruction of the pancreatic duct with a subsequent increase in intraductal pressure and, thus, acute pancreatitis.62 Tumors or polyps of the ampulla, such as ampullary adenoma or carcinoma, can cause pancreatitis by directly obstructing the pancreatic duct where it opens into the duodenum.63–66 Intraductal processes such as ductal adenocarcinoma, intraductal papillary mucinous tumor, pancreatic duct stone, and intraductal stricture due to cancer, chronic pancreatitis, or trauma can also cause pancreatitis by preventing free flow of pancreatic juice.67–71
Although it is well known that sequelae of severe chronic pancreatitis such as ductal strictures or intraductal stones can lead to recurrent attacks of acute pancreatitis by preventing the free flow of pancreatic juice, a relationship also seems to exist between early chronic pancreatitis and recurrent acute pancreatitis.72 Several studies have shown that up to 50% of patients with idiopathic recurrent pancreatitis have evidence of chronic pancreatitis.72–74 However, it is still unclear whether early chronic pancreatitis is the underlying cause of the recurrent attacks of acute pancreatitis or whether recurrent attacks of acute pancreatitis might have led to the development of chronic pancreatitis.
Diagnosis
Ampullary and periampullary neoplasms can be diagnosed endoscopically. Intraductal lesions such as strictures can be diagnosed by MRCP, especially secretin-enhanced MRCP, or by ERCP. ERCP has the additional advantage of being able to deliver treatment, ie, balloon dilation and stenting. In the case of ductal strictures, upsizing of the stents or placement of multiple stents during subsequent procedures is usually needed. Pancreatic ductal calcifications associated with chronic pancreatitis are usually radiopaque and are easily visible on plain films or computed tomography of the abdomen. Parenchymal and ductal changes of chronic pancreatitis can be diagnosed by endoscopic ultrasonography.
Treatment
The treatment is to relieve the obstruction and re-establish the free flow of pancreatic juice.
Periampullary tumors or polyps can be resected surgically or, if they involve only the mucosa, by endoscopic mucosal resection. Ampullary adenomas can be resected endoscopically. Ampullary carcinomas usually require surgical resection.
Small, nonobstructive stones in the pancreatic duct can be removed during ERCP.75 Larger stones may need to be fragmented by extracorporeal shock wave lithotripsy to facilitate removal by ERCP.75
Intraductal strictures should raise the suspicion of pancreatic adenocarcinoma, especially in older patients.61 In these cases, relief of the obstruction by placement of a pancreatic stent can prevent further attacks of pancreatitis until a diagnosis can be established and a more definitive treatment can be offered.
- Levy MJ, Geenen JE. Idiopathic acute recurrent pancreatitis. Am J Gastroenterol 2001; 96:2540–2555.
- Gullo L, Migliori M, Pezzilli R, et al. An update on recurrent acute pancreatitis: data from five European countries. Am J Gastroenterol 2002; 97:1959–1962.
- Gao YJ, Li YQ, Wang Q, et al. Analysis of the clinical features of recurrent acute pancreatitis in China. J Gastroenterol 2006; 41:681–685.
- Somogyi L, Martin SP, Venkatesan T, Ulrich CD. Recurrent acute pancreatitis: an algorithmic approach to identification and elimination of inciting factors. Gastroenterology 2001; 120:708–717.
- Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007; 102:1781–1788.
- Standards of Practice Committee,Zuckerman MJ, Shen B, Harrison ME, et al. Informed consent for GI endoscopy. Gastrointest Endosc 2007; 66:213–218.
- Lee SP, Hayashi A, Kim YS. Biliary sludge: curiosity or culprit? Hepatology 1994; 20:523–525.
- Opie E. The etiology of acute hemorrhagic pancreatitis. Bull Johns Hopkins Hosp 1901; 12:182–188.
- Hernandez CA, Lerch MM. Sphincter stenosis and gallstone migration through the biliary tract. Lancet 1993; 341:1371–1373.
- Ros E, Navarro S, Bru C, Garcia-Puges A, Valderrama R. Occult microlithiasis in 'idiopathic' acute pancreatitis: prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology 1991; 101:1701–1709.
- Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med 1992; 326:589–593.
- Levy MJ. The hunt for microlithiasis in idiopathic acute recurrent pancreatitis: should we abandon the search or intensify our efforts? Gastrointest Endosc 2002; 55:286–293.
- Delchier JC, Benfredj P, Preaux AM, Metreau JM, Dhumeaux D. The usefulness of microscopic bile examination in patients with suspected microlithiasis: a prospective evaluation. Hepatology 1986; 6:118–122.
- Lee SP, Nicholls JF. Nature and composition of biliary sludge. Gastroenterology 1986; 90:677–686.
- Testoni PA, Caporuscio S, Bagnolo F, Lella F. Idiopathic recurrent pancreatitis: long-term results after ERCP, endoscopic sphincterotomy, or ursodeoxycholic acid treatment. Am J Gastroenterol 2000; 95:1702–1707.
- Siddiqui AA, Mitroo P, Kowalski T, Loren D. Endoscopic sphincterotomy with or without cholecystectomy for choledocholithiasis in high-risk surgical patients: a decision analysis. Aliment Pharmacol Ther 2006; 24:1059–1066.
- Steinberg WM, Chari ST, Forsmark CE, et al. Controversies in clinical pancreatology: management of acute idiopathic recurrent pancreatitis. Pancreas 2003; 27:103–117.
- Khalid A, Slivka A. Approach to idiopathic recurrent pancreatitis. Gastrointest Endosc Clin North Am 2003; 13:695–716.
- Adler DG, Baron TH, Davila RE, et al; Standards of Practice Committee of American Society for Gastrointestinal Endoscopy. ASGE guideline: the role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc 2005; 62:1–8.
- Draganov P, Forsmark CE. “Idiopathic” pancreatitis. Gastroenterology 2005; 128:756–763.
- Chung EJ, Kim MH, Lee SS, Lee SK. Primary vs. secondary common bile duct stones: apples and oranges. Endoscopy 2003; 35:92.
- Saharia PC, Zuidema GD, Cameron JL. Primary common duct stones. Ann Surg 1977; 185:598–604.
- Elta GH. Sphincter of Oddi dysfunction and bile duct microlithiasis in acute idiopathic pancreatitis. World J Gastroenterol 2008; 14:1023–1026.
- Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med 1996; 335:909–918.
- Prat F, Malak NA, Pelletier G, et al. Biliary symptoms and complications more than 8 years after endoscopic sphincterotomy for choledocholithiasis. Gastroenterology 1996; 110:894–899.
- Hawes RH, Cotton PB, Vallon AG. Follow-up 6 to 11 years after duo-denoscopic sphincterotomy for stones in patients with prior cholecystectomy. Gastroenterology 1990; 98:1008–1012.
- Lai KH, Lo GH, Lin CK, et al. Do patients with recurrent choledocholithiasis after endoscopic sphincterotomy benefit from regular follow-up? Gastrointest Endosc 2002; 55:523–526.
- Kim DI, Kim MH, Lee SK, et al. Risk factors for recurrence of primary bile duct stones after endoscopic biliary sphincterotomy. Gastrointest Endosc 2001; 54:42–48.
- Costamagna G, Tringali A, Shah SK, Mutignani M, Zuccala G, Perri V. Long-term follow-up of patients after endoscopic sphincterotomy for choledocholithiasis, and risk factors for recurrence. Endoscopy 2002; 34:273–279.
- Keizman D, Ish Shalom M, Konikoff FM. Recurrent symptomatic common bile duct stones after endoscopic stone extraction in elderly patients. Gastrointest Endosc 2006; 64:60–65.
- Kaw M, Brodmerkel GJ. ERCP, biliary crystal analysis, and sphincter of Oddi manometry in idiopathic recurrent pancreatitis. Gastrointest Endosc 2002; 55:157–162.
- Chak A, Hawes RH, Cooper GS, et al. Prospective assessment of the utility of EUS in the evaluation of gallstone pancreatitis. Gastrointest Endosc 1999; 49:599–604.
- Parsi MA, Neuhaus H, Pleskow D, et al. Peroral cholangioscopy guided stone therapy—report of an international multicenter registry [abstract]. Gastrointest Endosc 2008; 67:AB102.
- Woods CM, Mawe GM, Toouli J, Saccone GT. The sphincter of Oddi: understanding its control and function. Neurogastroenterol Motil 2005; 17 suppl 1:31–40.
- McLoughlin MT, Mitchell RM. Sphincter of Oddi dysfunction and pancreatitis. World J Gastroenterol 2007; 13:6333–6343.
- Bosch A, Pena LR. The sphincter of Oddi. Dig Dis Sci 2007; 52:1211–1218.
- Devereaux BM, Sherman S, Lehman GA. Sphincter of Oddi (pancreatic) hypertension and recurrent pancreatitis. Curr Gastroenterol Rep 2002; 4:153–159.
- Fazel A, Geenen JE, MoezArdalan K, Catalano MF. Intrapancreatic ductal pressure in sphincter of Oddi dysfunction. Pancreas 2005; 30:359–362.
- Freeman ML. Role of pancreatic stents in prevention of post-ERCP pancreatitis. JOP 2004; 5:322–327.
- Singh P, Gurudu SR, Davidoff S, et al. Sphincter of Oddi manometry does not predispose to post-ERCP acute pancreatitis. Gastrointest Endosc 2004; 59:499–505.
- Guda NM, Freeman ML. True culprit or guilt by association? Is sphincter of Oddi manometry the cause of post-ERCP pancreatitis in patients with suspected sphincter of Oddi dysfunction, or is it the patients' susceptibility? Rev Gastroenterol Disord 2004; 4:211–213.
- Craig A, Toouli J. Sphincter of Oddi dysfunction: is there a role for medical therapy? Curr Gastroenterol Rep 2002; 4:172–176.
- Freeman ML, Gill M, Overby C, Cen YY. Predictors of outcomes after biliary and pancreatic sphincterotomy for sphincter of Oddi dysfunction. J Clin Gastroenterol 2007; 41:94–102.
- Sgouros SN, Pereira SP. Systematic review: sphincter of Oddi dysfunction—non-invasive diagnostic methods and long-term outcome after endoscopic sphincterotomy. Aliment Pharmacol Ther 2006; 24:237–246.
- Venu RP, Geenen JE, Hogan W, Stone J, Johnson GK, Soergel K. Idiopathic recurrent pancreatitis. An approach to diagnosis and treatment. Dig Dis Sci 1989; 34:56–60.
- Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989; 320:82–87.
- Fogel EL, Eversman D, Jamidar P, Sherman S, Lehman GA. Sphincter of Oddi dysfunction: pancreaticobiliary sphincterotomy with pancreatic stent placement has a lower rate of pancreatitis than biliary sphincterotomy alone. Endoscopy 2002; 34:280–285.
- Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol 2007; 5:1354–1365.
- Toouli J. The sphincter of Oddi and acute pancreatitis - revisited. HPB (Oxford) 2003; 5:142–145.
- Sherman S, Lehman GA. Sphincter of Oddi dysfunction: diagnosis and treatment. JOP 2001; 2:382–400.
- Klein SD, Affronti JP. Pancreas divisum, an evidence-based review: part I, pathophysiology. Gastrointest Endosc 2004; 60:419–425.
- Fogel EL, Toth TG, Lehman GA, DiMagno MJ, DiMagno EP. Does endoscopic therapy favorably affect the outcome of patients who have recurrent acute pancreatitis and pancreas divisum? Pancreas 2007; 34:21–45.
- Lehman GA. Acute recurrent pancreatitis. Can J Gastroenterol 2003; 17:381–383.
- Lehman GA, Sherman S. Pancreas divisum. Diagnosis, clinical significance, and management alternatives. Gastrointest Endosc Clin N Am 1995; 5:145–170.
- Staritz M, Meyer zum Buschenfelde KH. Elevated pressure in the dorsal part of pancreas divisum: the cause of chronic pancreatitis? Pancreas 1988; 3:108–110.
- Carnes M, Romagnuolo J, Cotton P. Miss rate of pancreas divisum by magnetic resonance cholangiopancreatography in clinical practice. Pancreas 2008; 37:151–153.
- Warshaw AL, Simeone JF, Schapiro RH, Flavin-Warshaw B. Evaluation and treatment of the dominant dorsal duct syndrome (pancreas divisum redefined). Am J Surg 1990; 159:59–64.
- Bradley EL, Stephan RN. Accessory duct sphincteroplasty is preferred for long-term prevention of recurrent acute pancreatitis in patients with pancreas divisum. J Am Coll Surg 1996; 183:65–70.
- Heyries L, Barthet M, Delvasto C, Zamora C, Bernard JP, Sahel J. Long-term results of endoscopic management of pancreas divisum with recurrent acute pancreatitis. Gastrointest Endosc 2002; 55:376–381.
- Lans JI, Geenen JE, Johanson JF, Hogan WJ. Endoscopic therapy in patients with pancreas divisum and acute pancreatitis: a prospective, randomized, controlled clinical trial. Gastrointest Endosc 1992; 38:430–434.
- Delhaye M, Matos C, Arvanitakis M, Deviere J. Pancreatic ductal system obstruction and acute recurrent pancreatitis. World J Gastroenterol 2008; 14:1027–1033.
- Finnie IA, Ghosh P, Garvey C, Poston GJ, Rhodes JM. Intraluminal duodenal diverticulum causing recurrent pancreatitis: treatment by endoscopic incision. Gut 1994; 35:557–559.
- Guzzardo G, Kleinman MS, Krackov JH, Schwartz SI. Recurrent acute pancreatitis caused by ampullary villous adenoma. J Clin Gastroenterol 1990; 12:200–202.
- Wright BE, Kozarek RA, Traverso LW, Wechter D, Thirlby R, Raltz SL. Recurrent pancreatitis in Gardner variant familial polyposis: etiology, diagnostic approach, and interventional results. Arch Surg 1999; 134:311–315.
- Tanasijtchouk T, Vaisbein E, Lachter J, Nassar F. Carcinoma of Papilla Vateri presenting as recurrent acute pancreatitis. Acta Gastroenterol Belg 2004; 67:309–310.
- Kwon TH, Park do H, Shim KY, et al. Ampullary adenomyoma presenting as acute recurrent pancreatitis. World J Gastroenterol 2007; 13:2892–2894.
- Lorente JA, Ruiz del Arbol L, Moreira VF, Garcia-Plaza A. Recurrent pancreatitis in a young patient associated with a solitary nonopaque concretion in the main pancreatic duct. Gastrointest Endosc 1990; 36:63–65.
- Chung JP, Chi SW, Park YN, et al. A case of minute intraductal papillary mucinous tumor of the pancreas presenting with recurrent acute pancreatitis. Yonsei Med J 2000; 41:528–532.
- Tikhomirov V, Tikhomirova S, Sieber S, Schiffman MK. A pancreatic intraductal papillary mucinous tumor causing recurrent acute pancreatitis at the onset of menstrual periods. J Clin Gastroenterol 2000; 31:172–174.
- Mosca S, Bottino V, Molino C. Hepatobiliary and pancreatic: a woman with recurrent idiopathic acute pancreatitis. Intraductal papillary mucinous tumor of the pancreas. J Gastroenterol Hepatol 2001; 16:1070,1075.
- Howard TJ, Moore SA, Saxena R, Matthews DE, Schmidt CM, Wiebke EA. Pancreatic duct strictures are a common cause of recurrent pancreatitis after successful management of pancreatic necrosis. Surgery 2004; 136:909–916.
- Garg PK, Tandon RK, Madan K. Is biliary microlithiasis a significant cause of idiopathic recurrent acute pancreatitis? A long-term follow-up study. Clin Gastroenterol Hepatol 2007; 5:75–79.
- Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol 2001; 96:705–709.
- Yusoff IF, Raymond G, Sahai AV. A prospective comparison of the yield of EUS in primary vs. recurrent idiopathic acute pancreatitis. Gastrointest Endosc 2004; 60:673–678.
- Cahen DL, Gouma DJ, Nio Y, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med 2007; 356:676–684.
- Levy MJ, Geenen JE. Idiopathic acute recurrent pancreatitis. Am J Gastroenterol 2001; 96:2540–2555.
- Gullo L, Migliori M, Pezzilli R, et al. An update on recurrent acute pancreatitis: data from five European countries. Am J Gastroenterol 2002; 97:1959–1962.
- Gao YJ, Li YQ, Wang Q, et al. Analysis of the clinical features of recurrent acute pancreatitis in China. J Gastroenterol 2006; 41:681–685.
- Somogyi L, Martin SP, Venkatesan T, Ulrich CD. Recurrent acute pancreatitis: an algorithmic approach to identification and elimination of inciting factors. Gastroenterology 2001; 120:708–717.
- Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007; 102:1781–1788.
- Standards of Practice Committee,Zuckerman MJ, Shen B, Harrison ME, et al. Informed consent for GI endoscopy. Gastrointest Endosc 2007; 66:213–218.
- Lee SP, Hayashi A, Kim YS. Biliary sludge: curiosity or culprit? Hepatology 1994; 20:523–525.
- Opie E. The etiology of acute hemorrhagic pancreatitis. Bull Johns Hopkins Hosp 1901; 12:182–188.
- Hernandez CA, Lerch MM. Sphincter stenosis and gallstone migration through the biliary tract. Lancet 1993; 341:1371–1373.
- Ros E, Navarro S, Bru C, Garcia-Puges A, Valderrama R. Occult microlithiasis in 'idiopathic' acute pancreatitis: prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology 1991; 101:1701–1709.
- Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med 1992; 326:589–593.
- Levy MJ. The hunt for microlithiasis in idiopathic acute recurrent pancreatitis: should we abandon the search or intensify our efforts? Gastrointest Endosc 2002; 55:286–293.
- Delchier JC, Benfredj P, Preaux AM, Metreau JM, Dhumeaux D. The usefulness of microscopic bile examination in patients with suspected microlithiasis: a prospective evaluation. Hepatology 1986; 6:118–122.
- Lee SP, Nicholls JF. Nature and composition of biliary sludge. Gastroenterology 1986; 90:677–686.
- Testoni PA, Caporuscio S, Bagnolo F, Lella F. Idiopathic recurrent pancreatitis: long-term results after ERCP, endoscopic sphincterotomy, or ursodeoxycholic acid treatment. Am J Gastroenterol 2000; 95:1702–1707.
- Siddiqui AA, Mitroo P, Kowalski T, Loren D. Endoscopic sphincterotomy with or without cholecystectomy for choledocholithiasis in high-risk surgical patients: a decision analysis. Aliment Pharmacol Ther 2006; 24:1059–1066.
- Steinberg WM, Chari ST, Forsmark CE, et al. Controversies in clinical pancreatology: management of acute idiopathic recurrent pancreatitis. Pancreas 2003; 27:103–117.
- Khalid A, Slivka A. Approach to idiopathic recurrent pancreatitis. Gastrointest Endosc Clin North Am 2003; 13:695–716.
- Adler DG, Baron TH, Davila RE, et al; Standards of Practice Committee of American Society for Gastrointestinal Endoscopy. ASGE guideline: the role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc 2005; 62:1–8.
- Draganov P, Forsmark CE. “Idiopathic” pancreatitis. Gastroenterology 2005; 128:756–763.
- Chung EJ, Kim MH, Lee SS, Lee SK. Primary vs. secondary common bile duct stones: apples and oranges. Endoscopy 2003; 35:92.
- Saharia PC, Zuidema GD, Cameron JL. Primary common duct stones. Ann Surg 1977; 185:598–604.
- Elta GH. Sphincter of Oddi dysfunction and bile duct microlithiasis in acute idiopathic pancreatitis. World J Gastroenterol 2008; 14:1023–1026.
- Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med 1996; 335:909–918.
- Prat F, Malak NA, Pelletier G, et al. Biliary symptoms and complications more than 8 years after endoscopic sphincterotomy for choledocholithiasis. Gastroenterology 1996; 110:894–899.
- Hawes RH, Cotton PB, Vallon AG. Follow-up 6 to 11 years after duo-denoscopic sphincterotomy for stones in patients with prior cholecystectomy. Gastroenterology 1990; 98:1008–1012.
- Lai KH, Lo GH, Lin CK, et al. Do patients with recurrent choledocholithiasis after endoscopic sphincterotomy benefit from regular follow-up? Gastrointest Endosc 2002; 55:523–526.
- Kim DI, Kim MH, Lee SK, et al. Risk factors for recurrence of primary bile duct stones after endoscopic biliary sphincterotomy. Gastrointest Endosc 2001; 54:42–48.
- Costamagna G, Tringali A, Shah SK, Mutignani M, Zuccala G, Perri V. Long-term follow-up of patients after endoscopic sphincterotomy for choledocholithiasis, and risk factors for recurrence. Endoscopy 2002; 34:273–279.
- Keizman D, Ish Shalom M, Konikoff FM. Recurrent symptomatic common bile duct stones after endoscopic stone extraction in elderly patients. Gastrointest Endosc 2006; 64:60–65.
- Kaw M, Brodmerkel GJ. ERCP, biliary crystal analysis, and sphincter of Oddi manometry in idiopathic recurrent pancreatitis. Gastrointest Endosc 2002; 55:157–162.
- Chak A, Hawes RH, Cooper GS, et al. Prospective assessment of the utility of EUS in the evaluation of gallstone pancreatitis. Gastrointest Endosc 1999; 49:599–604.
- Parsi MA, Neuhaus H, Pleskow D, et al. Peroral cholangioscopy guided stone therapy—report of an international multicenter registry [abstract]. Gastrointest Endosc 2008; 67:AB102.
- Woods CM, Mawe GM, Toouli J, Saccone GT. The sphincter of Oddi: understanding its control and function. Neurogastroenterol Motil 2005; 17 suppl 1:31–40.
- McLoughlin MT, Mitchell RM. Sphincter of Oddi dysfunction and pancreatitis. World J Gastroenterol 2007; 13:6333–6343.
- Bosch A, Pena LR. The sphincter of Oddi. Dig Dis Sci 2007; 52:1211–1218.
- Devereaux BM, Sherman S, Lehman GA. Sphincter of Oddi (pancreatic) hypertension and recurrent pancreatitis. Curr Gastroenterol Rep 2002; 4:153–159.
- Fazel A, Geenen JE, MoezArdalan K, Catalano MF. Intrapancreatic ductal pressure in sphincter of Oddi dysfunction. Pancreas 2005; 30:359–362.
- Freeman ML. Role of pancreatic stents in prevention of post-ERCP pancreatitis. JOP 2004; 5:322–327.
- Singh P, Gurudu SR, Davidoff S, et al. Sphincter of Oddi manometry does not predispose to post-ERCP acute pancreatitis. Gastrointest Endosc 2004; 59:499–505.
- Guda NM, Freeman ML. True culprit or guilt by association? Is sphincter of Oddi manometry the cause of post-ERCP pancreatitis in patients with suspected sphincter of Oddi dysfunction, or is it the patients' susceptibility? Rev Gastroenterol Disord 2004; 4:211–213.
- Craig A, Toouli J. Sphincter of Oddi dysfunction: is there a role for medical therapy? Curr Gastroenterol Rep 2002; 4:172–176.
- Freeman ML, Gill M, Overby C, Cen YY. Predictors of outcomes after biliary and pancreatic sphincterotomy for sphincter of Oddi dysfunction. J Clin Gastroenterol 2007; 41:94–102.
- Sgouros SN, Pereira SP. Systematic review: sphincter of Oddi dysfunction—non-invasive diagnostic methods and long-term outcome after endoscopic sphincterotomy. Aliment Pharmacol Ther 2006; 24:237–246.
- Venu RP, Geenen JE, Hogan W, Stone J, Johnson GK, Soergel K. Idiopathic recurrent pancreatitis. An approach to diagnosis and treatment. Dig Dis Sci 1989; 34:56–60.
- Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989; 320:82–87.
- Fogel EL, Eversman D, Jamidar P, Sherman S, Lehman GA. Sphincter of Oddi dysfunction: pancreaticobiliary sphincterotomy with pancreatic stent placement has a lower rate of pancreatitis than biliary sphincterotomy alone. Endoscopy 2002; 34:280–285.
- Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol 2007; 5:1354–1365.
- Toouli J. The sphincter of Oddi and acute pancreatitis - revisited. HPB (Oxford) 2003; 5:142–145.
- Sherman S, Lehman GA. Sphincter of Oddi dysfunction: diagnosis and treatment. JOP 2001; 2:382–400.
- Klein SD, Affronti JP. Pancreas divisum, an evidence-based review: part I, pathophysiology. Gastrointest Endosc 2004; 60:419–425.
- Fogel EL, Toth TG, Lehman GA, DiMagno MJ, DiMagno EP. Does endoscopic therapy favorably affect the outcome of patients who have recurrent acute pancreatitis and pancreas divisum? Pancreas 2007; 34:21–45.
- Lehman GA. Acute recurrent pancreatitis. Can J Gastroenterol 2003; 17:381–383.
- Lehman GA, Sherman S. Pancreas divisum. Diagnosis, clinical significance, and management alternatives. Gastrointest Endosc Clin N Am 1995; 5:145–170.
- Staritz M, Meyer zum Buschenfelde KH. Elevated pressure in the dorsal part of pancreas divisum: the cause of chronic pancreatitis? Pancreas 1988; 3:108–110.
- Carnes M, Romagnuolo J, Cotton P. Miss rate of pancreas divisum by magnetic resonance cholangiopancreatography in clinical practice. Pancreas 2008; 37:151–153.
- Warshaw AL, Simeone JF, Schapiro RH, Flavin-Warshaw B. Evaluation and treatment of the dominant dorsal duct syndrome (pancreas divisum redefined). Am J Surg 1990; 159:59–64.
- Bradley EL, Stephan RN. Accessory duct sphincteroplasty is preferred for long-term prevention of recurrent acute pancreatitis in patients with pancreas divisum. J Am Coll Surg 1996; 183:65–70.
- Heyries L, Barthet M, Delvasto C, Zamora C, Bernard JP, Sahel J. Long-term results of endoscopic management of pancreas divisum with recurrent acute pancreatitis. Gastrointest Endosc 2002; 55:376–381.
- Lans JI, Geenen JE, Johanson JF, Hogan WJ. Endoscopic therapy in patients with pancreas divisum and acute pancreatitis: a prospective, randomized, controlled clinical trial. Gastrointest Endosc 1992; 38:430–434.
- Delhaye M, Matos C, Arvanitakis M, Deviere J. Pancreatic ductal system obstruction and acute recurrent pancreatitis. World J Gastroenterol 2008; 14:1027–1033.
- Finnie IA, Ghosh P, Garvey C, Poston GJ, Rhodes JM. Intraluminal duodenal diverticulum causing recurrent pancreatitis: treatment by endoscopic incision. Gut 1994; 35:557–559.
- Guzzardo G, Kleinman MS, Krackov JH, Schwartz SI. Recurrent acute pancreatitis caused by ampullary villous adenoma. J Clin Gastroenterol 1990; 12:200–202.
- Wright BE, Kozarek RA, Traverso LW, Wechter D, Thirlby R, Raltz SL. Recurrent pancreatitis in Gardner variant familial polyposis: etiology, diagnostic approach, and interventional results. Arch Surg 1999; 134:311–315.
- Tanasijtchouk T, Vaisbein E, Lachter J, Nassar F. Carcinoma of Papilla Vateri presenting as recurrent acute pancreatitis. Acta Gastroenterol Belg 2004; 67:309–310.
- Kwon TH, Park do H, Shim KY, et al. Ampullary adenomyoma presenting as acute recurrent pancreatitis. World J Gastroenterol 2007; 13:2892–2894.
- Lorente JA, Ruiz del Arbol L, Moreira VF, Garcia-Plaza A. Recurrent pancreatitis in a young patient associated with a solitary nonopaque concretion in the main pancreatic duct. Gastrointest Endosc 1990; 36:63–65.
- Chung JP, Chi SW, Park YN, et al. A case of minute intraductal papillary mucinous tumor of the pancreas presenting with recurrent acute pancreatitis. Yonsei Med J 2000; 41:528–532.
- Tikhomirov V, Tikhomirova S, Sieber S, Schiffman MK. A pancreatic intraductal papillary mucinous tumor causing recurrent acute pancreatitis at the onset of menstrual periods. J Clin Gastroenterol 2000; 31:172–174.
- Mosca S, Bottino V, Molino C. Hepatobiliary and pancreatic: a woman with recurrent idiopathic acute pancreatitis. Intraductal papillary mucinous tumor of the pancreas. J Gastroenterol Hepatol 2001; 16:1070,1075.
- Howard TJ, Moore SA, Saxena R, Matthews DE, Schmidt CM, Wiebke EA. Pancreatic duct strictures are a common cause of recurrent pancreatitis after successful management of pancreatic necrosis. Surgery 2004; 136:909–916.
- Garg PK, Tandon RK, Madan K. Is biliary microlithiasis a significant cause of idiopathic recurrent acute pancreatitis? A long-term follow-up study. Clin Gastroenterol Hepatol 2007; 5:75–79.
- Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol 2001; 96:705–709.
- Yusoff IF, Raymond G, Sahai AV. A prospective comparison of the yield of EUS in primary vs. recurrent idiopathic acute pancreatitis. Gastrointest Endosc 2004; 60:673–678.
- Cahen DL, Gouma DJ, Nio Y, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med 2007; 356:676–684.
KEY POINTS
- Recurrent attacks of acute pancreatitis can be prevented only by determining and treating the underlying cause.
- Endoscopic procedures can cause anxiety and carry a risk of bleeding, perforation, and pancreatitis. The risks, benefits, and other treatment options should be discussed with the patient.
- Endoscopic therapy is now the preferred treatment of sphincter of Oddi dysfunction at centers that have experience with this technique.
- In patients with pancreas divisum and recurrent acute pancreatitis, surgical and endoscopic minor sphincterotomy are equally effective.