User login
According to the American Diabetes Association (ADA), about 29 million Americans have diabetes mellitus (DM). Uncontrolled DM causes various microvascular and macrovascular complications and leads to significant mortality. In 2011, DM was the seventh leading cause of death.1 The ADA recommends setting a hemoglobin A1c (HbA1c) goal of < 7% to prevent microvascular and macrovascular complications.1
The treatment cost of DM continues to rise and accounts for about $245 billion annually.1 Given its effectiveness, low cost, and low adverse-event (AE) profile, metformin has been the cornerstone of therapy in DM over the past 20 years. The ADA recommends metformin as first-line therapy in type 2 DM (T2DM). In 2014, 14.4 million Americans were dispensed a metformin-containing product.2 Metformin exerts its effect mainly by decreasing hepatic glucose production and increasing insulin sensitivity. Study results suggest gluconeogenesis may be decreased up to 75% in these patients.3 Metformin is effective in reducing the level of HbA1c by an average of 1.5%.3
Background
Metformin-induced lactic acidosis is a rare concern in patients with renal impairment (0.03 case/1,000 patient-years).4 Much of this concern stems from the high incidence of lactic acidosis associated with the medication phenformin, which was approved in the 1950s but taken off the market because of its high incidence of lactic acidosis in patients with a serum creatinine (SCr) level > 1.4 mg/dL.
Although phenformin and metformin are both biguanide class medications, they vastly differ. Increased phenformin levels in the blood are correlated with decreased glucose oxidation and increased lactate production. Conversely, metformin may enhance glucose oxidation, and there seems to be no correlation between metformin levels with lactate levels. Lactic acidosis occurred 10 to 20 times more often with phenformin than it does with metformin.5 In studies in which patients with an estimated glomerular filtration rate (eGFR) of 30 to 60 mL/min/1.73 m2 continued to use metformin, lactic acidosis was rare, even in the presence of comorbid conditions that may promote lactic acidosis, such as chronic obstructive pulmonary disease, congestive heart failure, and liver disease.6 In 2012, the National Kidney Foundation (NKF) suggested an eGFR cutoff be considered when prescribing metformin.7
When the present study was initiated, metformin was contraindicated in patients with renal dysfunction (SCr levels ≥ 1.5 mg/dL in males≥ 1.4 mg/dL in females).5 The estimated incidence of renal dysfunction in patients with T2DM is 12%. Under this labeling, metformin use is prohibited in at least 2.5 million people. Study results have shown that, when package insert guidelines were disregarded and metformin was given against renal recommendations, the rate of AEs was not increased, and patients benefited clinically.8 Data suggest that the rate of lactic acidosis may be increased in patients with advanced kidney disease.8
In April 2016, the FDA started requiring that manufacturers update their labeling to indicate metformin may be used safely in cases of mild-to-moderate renal impairment. The FDA also changed a recommendation: now, before starting metformin, health care professionals should obtain the patient’s eGFR, which provides a more accurate determination of kidney function by taking into account age, sex, and race. Metformin is contraindicated in patients with an eGFR < 30 mL/min/1.73 m2 and is not recommended to be initiated in patients with an eGFR of 30 to 45 mL/min/1.73 m2. The suggestion for patients already using metformin is to obtain eGFR at least annually. In addition, when eGFR drops to between 30 and 45 mL/min/1.73 m2, the risks and benefits of continuing metformin should be weighed on a patient-specific basis.2,4
Methods
The authors retrospectively reviewed the charts of 199 randomly selected patients at Huntington VAMC in West Virginia who had metformin discontinued because of elevated SCr (defined as ≥ 1.5 mg/dL) between September 1, 2009 and September 1, 2014. Clinician notes written at time of discontinuation were assessed for other reasons for discontinuation, and patients thus identified were excluded. Change in glycemic control was assessed by comparing first HbA1c level 60 to 365 days after discontinuation of metformin with the most recent HbA1c level before discontinuation. Other data analyzed included age, time to next recorded SCr level, reinitiation of metformin (yes or no), and change in diabetic medication regimen. Class of medication initiated was recorded but not dose or insulin type. Subgroup analysis was performed on patients initiated on insulin after discontinuation of metformin. Evaluations were made of most recent HbA1c level at time of discontinuation of metformin, first HbA1c level after discontinuation, and HbA1c level 1 year after discontinuation in patients on insulin.
The primary endpoint of the study was change in HbA1c after discontinuation of metformin. This was studied to justify the value of metformin in T2DM and to evaluate whether patients could remain on metformin with mild-to-moderate renal impairment without AEs. Secondary endpoints were time to next recorded SCr level after discontinuation of metformin, reinitiation of metformin (yes or no), when next recorded SCr level was < 1.5 mg/dL, change in medication regimen after discontinuation of metformin, and incidence of lactic acidosis. Study inclusion criteria were male sex, age between 18 and 89 years, discontinuation of metformin because of elevated SCr, and documented repeat HbA1c level 60 to 365 days after discontinuation of metformin. Exclusion criteria were insulin therapy at time of discontinuation of metformin and type 1 DM diagnosis. A 2-sided t test was used to compare change in HbA1c level.
Results
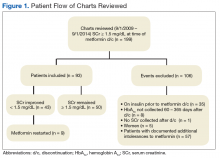
Of the 199 patients who had metformin discontinued because of elevated SCr (> 1.5 mg/dL), 106 were excluded for reasons listed in Figure 1; the other 93 met the study inclusion criteria and had their cases analyzed for change in glycemic control after discontinuation of metformin.
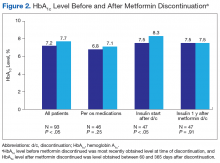
The mean age was 68.2 years and mean weight was 97.36 kg for the included patients. Four were African American, and 89 were white. At time of discontinuation of metformin, mean SCr level was 1.65 mg/dL, and mean eGFR was 43.25 mL/min/1.73 m2 (Table). Mean (SD) HbA1c level was 7.2% (1.1%) before discontinuation of metformin and 7.7% (1.5%) after discontinuation (P < .05) (Figure 2). Subgroup analysis of patients initiated on insulin after discontinuation of metformin (n = 47) revealed mean (SD) HbA1c levels of 7.5% (1.1%) before discontinuation and 8.3% (1.3%) after discontinuation (P < .05). One year or more after discontinuation of metformin in patients in whom insulin was initiated, mean HbA1c level decreased to the prediscontinuation (baseline) level of 7.5% (P = .91).
Twenty of these patients initiated on insulin had improved renal function and would have met the criteria for restarting metformin. After discontinuation of metformin, mean (SD) time to next recorded SCr level was 95.7 (89.9) days. Of the 93 study patients, 43 met the criterion for reinitiating metformin (rechecked SCr level, < 1.5 mg/dL), but in only 9 (21%) of these patients was metformin restarted.
Discussion
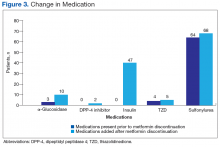
Overall, a decline in glycemic control was found in patients who had metformin discontinued. This anticipated decline prompted clinicians to replace metformin with other oral medications as well as insulin. Despite DM medication regimen changes, mean HbA1c level increased significantly after discontinuation of metformin. The initial decline in glycemic control after starting insulin could be attributable either to clinician preference in insulin initiation—starting at lower doses to avoid hypoglycemia—or to a delay in initiating insulin, as opposed to initiating insulin at time of discontinuation of metformin. Subgroup analysis of the large number of patients who started on insulin after discontinuation of metformin (51%) revealed the same HbA1c levels before and 1 year after discontinuation. This finding is clinically relevant because many patients showed a decline in glycemic control for a year, despite initiation of insulin therapy.
In 43 (46%) of the 93 patients studied, SCr level improved to < 1.5 mg/dL after discontinuation of metformin. Of the patients in the subgroup started on insulin, 20 had improved renal function. This finding suggests that many of the patients who were initiated on insulin showed an improvement in renal function and potentially could have had metformin reinitiated. If these patients had continued or restarted metformin, insulin therapy may have been avoided or delayed. Overall, many opportunities to resume metformin were missed; only 9 of the 43 patients with improved SCr levels (< 1.5 mg/dL) on recheck were restarted on metformin. Many clinicians seemed hesitant to restart metformin even after kidney function improved. In addition, mean time to next recorded SCr level after discontinuation of metformin was 95.7 days. If SCr levels are more closely monitored after discontinuation of metformin, metformin possibly could be restarted sooner, leading to improved glycemic control and prevention of both microvascular and macrovascular complications.
In its 2012 update, the NKF suggested that it may be reasonable to consider using an eGFR cutoff when prescribing metformin. Clearance of metformin is reduced by 75% when eGFR is under 60 mL/min/1.73m2 but declines no further until eGFR is < 30 mL/min/1.73 m2.7 A systematic review of 65 articles found that, overall, levels of metformin remained in the therapeutic range, and lactate concentrations did not increase significantly in mild-to-moderate renal impairment (eGFR, 30-60 mL/min/1.73 m2).8 This finding corresponds to the updated 2016 FDA recommendations regarding use of metformin in mild-to-moderate renal impairment.
In the present study, patients who had metformin discontinued earlier, under strict package labeling, may have been able to continue metformin with use of eGFR under the revised labeling. Thirty-seven patients had an eGFR > 45 mL/min/1.73 m2 at the time of discontinuation of metformin, 49 had an eGFR between 45 and 30 mL/min/1.73 m2, and 7 had an eGFR < 30 mL/min/1.73 m2. Only 7 (8%) of the 93 patients would have had a contraindication to continuing metformin on the basis of current FDA recommendations. Forty-nine patients (53%) could have continued metformin if the benefit outweighed the risk, and 37 (39%) could have continued metformin given an eGFR > 45 mL/min/1.73 m2. The earlier labeling required initial discontinuation of metformin in these patients, but new FDA recommendations would allow more of them with mild-to-moderate renal impairment to benefit from treatment with metformin.
Limitations
This study had a few limitations. Its design was retrospective, and its narrow demographics may not permit generalizability to other patient populations. In addition, the study evaluated initiation of new medications at time of discontinuation of metformin but not dosage adjustments of current medications. Insulin type and dosage were not evaluated—only whether insulin was initiated. Further, follow-up time was limited; change in long-term glycemic control requires more study. Another limitation was that adherence could not be assessed.
Conclusion
After discontinuation of metformin, there was a statistically significant increase in HbA1c level. Insulin was initiated in 51% of patients after discontinuation of metformin. Subgroup analysis of the patients who started insulin after discontinuation of metformin revealed the same HbA1c levels before and 1 year after discontinuation with a loss of glycemic control throughout the year. Of the 47 patients who were initiated on insulin, 20 had their SCr level decrease to < 1.5 mg/dL and could have been restarted on metformin. This finding indicates that many patients may have been able to delay time to insulin initiation and maintain the same glycemic control if metformin could have been continued. With more study, long-term change in glycemic control after discontinuation of metformin can be determined. In many patients, metformin is needed for adequate glycemic control. The revised FDA labeling allows many patients with mild-to-moderate kidney disease to benefit from treatment with metformin.
Click here to read the digital edition.
1. American Diabetes Association. Statistics about diabetes. http://www.diabetes.org/diabetesbasics/statistics/#sthash.3vJD53aO.dpuf. Accessed August 31, 2017.
2. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. https://www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Published April 8, 2016. Updated April 5, 2017. Accessed August 31, 2017.
3. Fowler MJ. Diabetes treatment, part 2: oral agents for glycemic management. Clin Diabetes. 2007;25(4):131-134.
4. Metformin [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2015.
5. Lipska KJ, Baily CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34(6):1431-1437.
6. Triplitt CL, Reasner CA. Chapter 83. Diabetes mellitus. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York, NY: McGraw-Hill; 2011:chap 83.
7. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850-886.
8. Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease. JAMA. 2014;312(24):2668-2675.
According to the American Diabetes Association (ADA), about 29 million Americans have diabetes mellitus (DM). Uncontrolled DM causes various microvascular and macrovascular complications and leads to significant mortality. In 2011, DM was the seventh leading cause of death.1 The ADA recommends setting a hemoglobin A1c (HbA1c) goal of < 7% to prevent microvascular and macrovascular complications.1
The treatment cost of DM continues to rise and accounts for about $245 billion annually.1 Given its effectiveness, low cost, and low adverse-event (AE) profile, metformin has been the cornerstone of therapy in DM over the past 20 years. The ADA recommends metformin as first-line therapy in type 2 DM (T2DM). In 2014, 14.4 million Americans were dispensed a metformin-containing product.2 Metformin exerts its effect mainly by decreasing hepatic glucose production and increasing insulin sensitivity. Study results suggest gluconeogenesis may be decreased up to 75% in these patients.3 Metformin is effective in reducing the level of HbA1c by an average of 1.5%.3
Background
Metformin-induced lactic acidosis is a rare concern in patients with renal impairment (0.03 case/1,000 patient-years).4 Much of this concern stems from the high incidence of lactic acidosis associated with the medication phenformin, which was approved in the 1950s but taken off the market because of its high incidence of lactic acidosis in patients with a serum creatinine (SCr) level > 1.4 mg/dL.
Although phenformin and metformin are both biguanide class medications, they vastly differ. Increased phenformin levels in the blood are correlated with decreased glucose oxidation and increased lactate production. Conversely, metformin may enhance glucose oxidation, and there seems to be no correlation between metformin levels with lactate levels. Lactic acidosis occurred 10 to 20 times more often with phenformin than it does with metformin.5 In studies in which patients with an estimated glomerular filtration rate (eGFR) of 30 to 60 mL/min/1.73 m2 continued to use metformin, lactic acidosis was rare, even in the presence of comorbid conditions that may promote lactic acidosis, such as chronic obstructive pulmonary disease, congestive heart failure, and liver disease.6 In 2012, the National Kidney Foundation (NKF) suggested an eGFR cutoff be considered when prescribing metformin.7
When the present study was initiated, metformin was contraindicated in patients with renal dysfunction (SCr levels ≥ 1.5 mg/dL in males≥ 1.4 mg/dL in females).5 The estimated incidence of renal dysfunction in patients with T2DM is 12%. Under this labeling, metformin use is prohibited in at least 2.5 million people. Study results have shown that, when package insert guidelines were disregarded and metformin was given against renal recommendations, the rate of AEs was not increased, and patients benefited clinically.8 Data suggest that the rate of lactic acidosis may be increased in patients with advanced kidney disease.8
In April 2016, the FDA started requiring that manufacturers update their labeling to indicate metformin may be used safely in cases of mild-to-moderate renal impairment. The FDA also changed a recommendation: now, before starting metformin, health care professionals should obtain the patient’s eGFR, which provides a more accurate determination of kidney function by taking into account age, sex, and race. Metformin is contraindicated in patients with an eGFR < 30 mL/min/1.73 m2 and is not recommended to be initiated in patients with an eGFR of 30 to 45 mL/min/1.73 m2. The suggestion for patients already using metformin is to obtain eGFR at least annually. In addition, when eGFR drops to between 30 and 45 mL/min/1.73 m2, the risks and benefits of continuing metformin should be weighed on a patient-specific basis.2,4
Methods
The authors retrospectively reviewed the charts of 199 randomly selected patients at Huntington VAMC in West Virginia who had metformin discontinued because of elevated SCr (defined as ≥ 1.5 mg/dL) between September 1, 2009 and September 1, 2014. Clinician notes written at time of discontinuation were assessed for other reasons for discontinuation, and patients thus identified were excluded. Change in glycemic control was assessed by comparing first HbA1c level 60 to 365 days after discontinuation of metformin with the most recent HbA1c level before discontinuation. Other data analyzed included age, time to next recorded SCr level, reinitiation of metformin (yes or no), and change in diabetic medication regimen. Class of medication initiated was recorded but not dose or insulin type. Subgroup analysis was performed on patients initiated on insulin after discontinuation of metformin. Evaluations were made of most recent HbA1c level at time of discontinuation of metformin, first HbA1c level after discontinuation, and HbA1c level 1 year after discontinuation in patients on insulin.
The primary endpoint of the study was change in HbA1c after discontinuation of metformin. This was studied to justify the value of metformin in T2DM and to evaluate whether patients could remain on metformin with mild-to-moderate renal impairment without AEs. Secondary endpoints were time to next recorded SCr level after discontinuation of metformin, reinitiation of metformin (yes or no), when next recorded SCr level was < 1.5 mg/dL, change in medication regimen after discontinuation of metformin, and incidence of lactic acidosis. Study inclusion criteria were male sex, age between 18 and 89 years, discontinuation of metformin because of elevated SCr, and documented repeat HbA1c level 60 to 365 days after discontinuation of metformin. Exclusion criteria were insulin therapy at time of discontinuation of metformin and type 1 DM diagnosis. A 2-sided t test was used to compare change in HbA1c level.
Results
Of the 199 patients who had metformin discontinued because of elevated SCr (> 1.5 mg/dL), 106 were excluded for reasons listed in Figure 1; the other 93 met the study inclusion criteria and had their cases analyzed for change in glycemic control after discontinuation of metformin.
The mean age was 68.2 years and mean weight was 97.36 kg for the included patients. Four were African American, and 89 were white. At time of discontinuation of metformin, mean SCr level was 1.65 mg/dL, and mean eGFR was 43.25 mL/min/1.73 m2 (Table). Mean (SD) HbA1c level was 7.2% (1.1%) before discontinuation of metformin and 7.7% (1.5%) after discontinuation (P < .05) (Figure 2). Subgroup analysis of patients initiated on insulin after discontinuation of metformin (n = 47) revealed mean (SD) HbA1c levels of 7.5% (1.1%) before discontinuation and 8.3% (1.3%) after discontinuation (P < .05). One year or more after discontinuation of metformin in patients in whom insulin was initiated, mean HbA1c level decreased to the prediscontinuation (baseline) level of 7.5% (P = .91).
Twenty of these patients initiated on insulin had improved renal function and would have met the criteria for restarting metformin. After discontinuation of metformin, mean (SD) time to next recorded SCr level was 95.7 (89.9) days. Of the 93 study patients, 43 met the criterion for reinitiating metformin (rechecked SCr level, < 1.5 mg/dL), but in only 9 (21%) of these patients was metformin restarted.
Discussion
Overall, a decline in glycemic control was found in patients who had metformin discontinued. This anticipated decline prompted clinicians to replace metformin with other oral medications as well as insulin. Despite DM medication regimen changes, mean HbA1c level increased significantly after discontinuation of metformin. The initial decline in glycemic control after starting insulin could be attributable either to clinician preference in insulin initiation—starting at lower doses to avoid hypoglycemia—or to a delay in initiating insulin, as opposed to initiating insulin at time of discontinuation of metformin. Subgroup analysis of the large number of patients who started on insulin after discontinuation of metformin (51%) revealed the same HbA1c levels before and 1 year after discontinuation. This finding is clinically relevant because many patients showed a decline in glycemic control for a year, despite initiation of insulin therapy.
In 43 (46%) of the 93 patients studied, SCr level improved to < 1.5 mg/dL after discontinuation of metformin. Of the patients in the subgroup started on insulin, 20 had improved renal function. This finding suggests that many of the patients who were initiated on insulin showed an improvement in renal function and potentially could have had metformin reinitiated. If these patients had continued or restarted metformin, insulin therapy may have been avoided or delayed. Overall, many opportunities to resume metformin were missed; only 9 of the 43 patients with improved SCr levels (< 1.5 mg/dL) on recheck were restarted on metformin. Many clinicians seemed hesitant to restart metformin even after kidney function improved. In addition, mean time to next recorded SCr level after discontinuation of metformin was 95.7 days. If SCr levels are more closely monitored after discontinuation of metformin, metformin possibly could be restarted sooner, leading to improved glycemic control and prevention of both microvascular and macrovascular complications.
In its 2012 update, the NKF suggested that it may be reasonable to consider using an eGFR cutoff when prescribing metformin. Clearance of metformin is reduced by 75% when eGFR is under 60 mL/min/1.73m2 but declines no further until eGFR is < 30 mL/min/1.73 m2.7 A systematic review of 65 articles found that, overall, levels of metformin remained in the therapeutic range, and lactate concentrations did not increase significantly in mild-to-moderate renal impairment (eGFR, 30-60 mL/min/1.73 m2).8 This finding corresponds to the updated 2016 FDA recommendations regarding use of metformin in mild-to-moderate renal impairment.
In the present study, patients who had metformin discontinued earlier, under strict package labeling, may have been able to continue metformin with use of eGFR under the revised labeling. Thirty-seven patients had an eGFR > 45 mL/min/1.73 m2 at the time of discontinuation of metformin, 49 had an eGFR between 45 and 30 mL/min/1.73 m2, and 7 had an eGFR < 30 mL/min/1.73 m2. Only 7 (8%) of the 93 patients would have had a contraindication to continuing metformin on the basis of current FDA recommendations. Forty-nine patients (53%) could have continued metformin if the benefit outweighed the risk, and 37 (39%) could have continued metformin given an eGFR > 45 mL/min/1.73 m2. The earlier labeling required initial discontinuation of metformin in these patients, but new FDA recommendations would allow more of them with mild-to-moderate renal impairment to benefit from treatment with metformin.
Limitations
This study had a few limitations. Its design was retrospective, and its narrow demographics may not permit generalizability to other patient populations. In addition, the study evaluated initiation of new medications at time of discontinuation of metformin but not dosage adjustments of current medications. Insulin type and dosage were not evaluated—only whether insulin was initiated. Further, follow-up time was limited; change in long-term glycemic control requires more study. Another limitation was that adherence could not be assessed.
Conclusion
After discontinuation of metformin, there was a statistically significant increase in HbA1c level. Insulin was initiated in 51% of patients after discontinuation of metformin. Subgroup analysis of the patients who started insulin after discontinuation of metformin revealed the same HbA1c levels before and 1 year after discontinuation with a loss of glycemic control throughout the year. Of the 47 patients who were initiated on insulin, 20 had their SCr level decrease to < 1.5 mg/dL and could have been restarted on metformin. This finding indicates that many patients may have been able to delay time to insulin initiation and maintain the same glycemic control if metformin could have been continued. With more study, long-term change in glycemic control after discontinuation of metformin can be determined. In many patients, metformin is needed for adequate glycemic control. The revised FDA labeling allows many patients with mild-to-moderate kidney disease to benefit from treatment with metformin.
Click here to read the digital edition.
According to the American Diabetes Association (ADA), about 29 million Americans have diabetes mellitus (DM). Uncontrolled DM causes various microvascular and macrovascular complications and leads to significant mortality. In 2011, DM was the seventh leading cause of death.1 The ADA recommends setting a hemoglobin A1c (HbA1c) goal of < 7% to prevent microvascular and macrovascular complications.1
The treatment cost of DM continues to rise and accounts for about $245 billion annually.1 Given its effectiveness, low cost, and low adverse-event (AE) profile, metformin has been the cornerstone of therapy in DM over the past 20 years. The ADA recommends metformin as first-line therapy in type 2 DM (T2DM). In 2014, 14.4 million Americans were dispensed a metformin-containing product.2 Metformin exerts its effect mainly by decreasing hepatic glucose production and increasing insulin sensitivity. Study results suggest gluconeogenesis may be decreased up to 75% in these patients.3 Metformin is effective in reducing the level of HbA1c by an average of 1.5%.3
Background
Metformin-induced lactic acidosis is a rare concern in patients with renal impairment (0.03 case/1,000 patient-years).4 Much of this concern stems from the high incidence of lactic acidosis associated with the medication phenformin, which was approved in the 1950s but taken off the market because of its high incidence of lactic acidosis in patients with a serum creatinine (SCr) level > 1.4 mg/dL.
Although phenformin and metformin are both biguanide class medications, they vastly differ. Increased phenformin levels in the blood are correlated with decreased glucose oxidation and increased lactate production. Conversely, metformin may enhance glucose oxidation, and there seems to be no correlation between metformin levels with lactate levels. Lactic acidosis occurred 10 to 20 times more often with phenformin than it does with metformin.5 In studies in which patients with an estimated glomerular filtration rate (eGFR) of 30 to 60 mL/min/1.73 m2 continued to use metformin, lactic acidosis was rare, even in the presence of comorbid conditions that may promote lactic acidosis, such as chronic obstructive pulmonary disease, congestive heart failure, and liver disease.6 In 2012, the National Kidney Foundation (NKF) suggested an eGFR cutoff be considered when prescribing metformin.7
When the present study was initiated, metformin was contraindicated in patients with renal dysfunction (SCr levels ≥ 1.5 mg/dL in males≥ 1.4 mg/dL in females).5 The estimated incidence of renal dysfunction in patients with T2DM is 12%. Under this labeling, metformin use is prohibited in at least 2.5 million people. Study results have shown that, when package insert guidelines were disregarded and metformin was given against renal recommendations, the rate of AEs was not increased, and patients benefited clinically.8 Data suggest that the rate of lactic acidosis may be increased in patients with advanced kidney disease.8
In April 2016, the FDA started requiring that manufacturers update their labeling to indicate metformin may be used safely in cases of mild-to-moderate renal impairment. The FDA also changed a recommendation: now, before starting metformin, health care professionals should obtain the patient’s eGFR, which provides a more accurate determination of kidney function by taking into account age, sex, and race. Metformin is contraindicated in patients with an eGFR < 30 mL/min/1.73 m2 and is not recommended to be initiated in patients with an eGFR of 30 to 45 mL/min/1.73 m2. The suggestion for patients already using metformin is to obtain eGFR at least annually. In addition, when eGFR drops to between 30 and 45 mL/min/1.73 m2, the risks and benefits of continuing metformin should be weighed on a patient-specific basis.2,4
Methods
The authors retrospectively reviewed the charts of 199 randomly selected patients at Huntington VAMC in West Virginia who had metformin discontinued because of elevated SCr (defined as ≥ 1.5 mg/dL) between September 1, 2009 and September 1, 2014. Clinician notes written at time of discontinuation were assessed for other reasons for discontinuation, and patients thus identified were excluded. Change in glycemic control was assessed by comparing first HbA1c level 60 to 365 days after discontinuation of metformin with the most recent HbA1c level before discontinuation. Other data analyzed included age, time to next recorded SCr level, reinitiation of metformin (yes or no), and change in diabetic medication regimen. Class of medication initiated was recorded but not dose or insulin type. Subgroup analysis was performed on patients initiated on insulin after discontinuation of metformin. Evaluations were made of most recent HbA1c level at time of discontinuation of metformin, first HbA1c level after discontinuation, and HbA1c level 1 year after discontinuation in patients on insulin.
The primary endpoint of the study was change in HbA1c after discontinuation of metformin. This was studied to justify the value of metformin in T2DM and to evaluate whether patients could remain on metformin with mild-to-moderate renal impairment without AEs. Secondary endpoints were time to next recorded SCr level after discontinuation of metformin, reinitiation of metformin (yes or no), when next recorded SCr level was < 1.5 mg/dL, change in medication regimen after discontinuation of metformin, and incidence of lactic acidosis. Study inclusion criteria were male sex, age between 18 and 89 years, discontinuation of metformin because of elevated SCr, and documented repeat HbA1c level 60 to 365 days after discontinuation of metformin. Exclusion criteria were insulin therapy at time of discontinuation of metformin and type 1 DM diagnosis. A 2-sided t test was used to compare change in HbA1c level.
Results
Of the 199 patients who had metformin discontinued because of elevated SCr (> 1.5 mg/dL), 106 were excluded for reasons listed in Figure 1; the other 93 met the study inclusion criteria and had their cases analyzed for change in glycemic control after discontinuation of metformin.
The mean age was 68.2 years and mean weight was 97.36 kg for the included patients. Four were African American, and 89 were white. At time of discontinuation of metformin, mean SCr level was 1.65 mg/dL, and mean eGFR was 43.25 mL/min/1.73 m2 (Table). Mean (SD) HbA1c level was 7.2% (1.1%) before discontinuation of metformin and 7.7% (1.5%) after discontinuation (P < .05) (Figure 2). Subgroup analysis of patients initiated on insulin after discontinuation of metformin (n = 47) revealed mean (SD) HbA1c levels of 7.5% (1.1%) before discontinuation and 8.3% (1.3%) after discontinuation (P < .05). One year or more after discontinuation of metformin in patients in whom insulin was initiated, mean HbA1c level decreased to the prediscontinuation (baseline) level of 7.5% (P = .91).
Twenty of these patients initiated on insulin had improved renal function and would have met the criteria for restarting metformin. After discontinuation of metformin, mean (SD) time to next recorded SCr level was 95.7 (89.9) days. Of the 93 study patients, 43 met the criterion for reinitiating metformin (rechecked SCr level, < 1.5 mg/dL), but in only 9 (21%) of these patients was metformin restarted.
Discussion
Overall, a decline in glycemic control was found in patients who had metformin discontinued. This anticipated decline prompted clinicians to replace metformin with other oral medications as well as insulin. Despite DM medication regimen changes, mean HbA1c level increased significantly after discontinuation of metformin. The initial decline in glycemic control after starting insulin could be attributable either to clinician preference in insulin initiation—starting at lower doses to avoid hypoglycemia—or to a delay in initiating insulin, as opposed to initiating insulin at time of discontinuation of metformin. Subgroup analysis of the large number of patients who started on insulin after discontinuation of metformin (51%) revealed the same HbA1c levels before and 1 year after discontinuation. This finding is clinically relevant because many patients showed a decline in glycemic control for a year, despite initiation of insulin therapy.
In 43 (46%) of the 93 patients studied, SCr level improved to < 1.5 mg/dL after discontinuation of metformin. Of the patients in the subgroup started on insulin, 20 had improved renal function. This finding suggests that many of the patients who were initiated on insulin showed an improvement in renal function and potentially could have had metformin reinitiated. If these patients had continued or restarted metformin, insulin therapy may have been avoided or delayed. Overall, many opportunities to resume metformin were missed; only 9 of the 43 patients with improved SCr levels (< 1.5 mg/dL) on recheck were restarted on metformin. Many clinicians seemed hesitant to restart metformin even after kidney function improved. In addition, mean time to next recorded SCr level after discontinuation of metformin was 95.7 days. If SCr levels are more closely monitored after discontinuation of metformin, metformin possibly could be restarted sooner, leading to improved glycemic control and prevention of both microvascular and macrovascular complications.
In its 2012 update, the NKF suggested that it may be reasonable to consider using an eGFR cutoff when prescribing metformin. Clearance of metformin is reduced by 75% when eGFR is under 60 mL/min/1.73m2 but declines no further until eGFR is < 30 mL/min/1.73 m2.7 A systematic review of 65 articles found that, overall, levels of metformin remained in the therapeutic range, and lactate concentrations did not increase significantly in mild-to-moderate renal impairment (eGFR, 30-60 mL/min/1.73 m2).8 This finding corresponds to the updated 2016 FDA recommendations regarding use of metformin in mild-to-moderate renal impairment.
In the present study, patients who had metformin discontinued earlier, under strict package labeling, may have been able to continue metformin with use of eGFR under the revised labeling. Thirty-seven patients had an eGFR > 45 mL/min/1.73 m2 at the time of discontinuation of metformin, 49 had an eGFR between 45 and 30 mL/min/1.73 m2, and 7 had an eGFR < 30 mL/min/1.73 m2. Only 7 (8%) of the 93 patients would have had a contraindication to continuing metformin on the basis of current FDA recommendations. Forty-nine patients (53%) could have continued metformin if the benefit outweighed the risk, and 37 (39%) could have continued metformin given an eGFR > 45 mL/min/1.73 m2. The earlier labeling required initial discontinuation of metformin in these patients, but new FDA recommendations would allow more of them with mild-to-moderate renal impairment to benefit from treatment with metformin.
Limitations
This study had a few limitations. Its design was retrospective, and its narrow demographics may not permit generalizability to other patient populations. In addition, the study evaluated initiation of new medications at time of discontinuation of metformin but not dosage adjustments of current medications. Insulin type and dosage were not evaluated—only whether insulin was initiated. Further, follow-up time was limited; change in long-term glycemic control requires more study. Another limitation was that adherence could not be assessed.
Conclusion
After discontinuation of metformin, there was a statistically significant increase in HbA1c level. Insulin was initiated in 51% of patients after discontinuation of metformin. Subgroup analysis of the patients who started insulin after discontinuation of metformin revealed the same HbA1c levels before and 1 year after discontinuation with a loss of glycemic control throughout the year. Of the 47 patients who were initiated on insulin, 20 had their SCr level decrease to < 1.5 mg/dL and could have been restarted on metformin. This finding indicates that many patients may have been able to delay time to insulin initiation and maintain the same glycemic control if metformin could have been continued. With more study, long-term change in glycemic control after discontinuation of metformin can be determined. In many patients, metformin is needed for adequate glycemic control. The revised FDA labeling allows many patients with mild-to-moderate kidney disease to benefit from treatment with metformin.
Click here to read the digital edition.
1. American Diabetes Association. Statistics about diabetes. http://www.diabetes.org/diabetesbasics/statistics/#sthash.3vJD53aO.dpuf. Accessed August 31, 2017.
2. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. https://www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Published April 8, 2016. Updated April 5, 2017. Accessed August 31, 2017.
3. Fowler MJ. Diabetes treatment, part 2: oral agents for glycemic management. Clin Diabetes. 2007;25(4):131-134.
4. Metformin [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2015.
5. Lipska KJ, Baily CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34(6):1431-1437.
6. Triplitt CL, Reasner CA. Chapter 83. Diabetes mellitus. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York, NY: McGraw-Hill; 2011:chap 83.
7. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850-886.
8. Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease. JAMA. 2014;312(24):2668-2675.
1. American Diabetes Association. Statistics about diabetes. http://www.diabetes.org/diabetesbasics/statistics/#sthash.3vJD53aO.dpuf. Accessed August 31, 2017.
2. U.S. Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. https://www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Published April 8, 2016. Updated April 5, 2017. Accessed August 31, 2017.
3. Fowler MJ. Diabetes treatment, part 2: oral agents for glycemic management. Clin Diabetes. 2007;25(4):131-134.
4. Metformin [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2015.
5. Lipska KJ, Baily CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34(6):1431-1437.
6. Triplitt CL, Reasner CA. Chapter 83. Diabetes mellitus. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York, NY: McGraw-Hill; 2011:chap 83.
7. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850-886.
8. Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease. JAMA. 2014;312(24):2668-2675.