User login
An adaptive autoimmune response against myelin that occurs late after an initial loss of oligodendrocytes in a new mouse model offers evidence in support of the “inside-out” hypothesis for multiple sclerosis pathogenesis in which “certain forms of MS may be caused by primary oligodendrocyte loss or myelin damage that secondarily leads to activation of the permissive adaptive immune system and CNS inflammation,” according to Maria Traka, Ph.D., of the University of Chicago. The report was published Dec. 14 in Nature Neuroscience.
“To our knowledge, this is the first experimental evidence to support the hypothesis that oligodendrocyte loss or myelin degeneration triggers myelin autoimmunity and initiates inflammation and tissue damage in the CNS during MS,” wrote Dr. Traka and her colleagues.
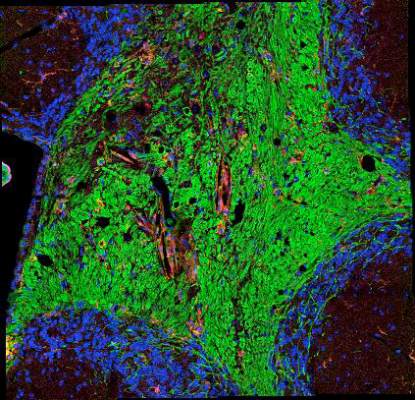
The new mouse model that the investigators used to support the hypothesis undergoes a genetically induced loss of oligodendrocytes and demyelination from which it recovers, but then later in life develops an infiltration of CD4-positive T cells into the CNS that causes a secondary, fatal demyelinating disease that occurs with axonal loss, CNS inflammation, and the presence of myelin oligodendrocyte glycoprotein (MOG)–specific T cells in peripheral lymphoid organs. The investigators found that the transfer of these MOG-specific T cells could induce mild neurological symptoms and inflammatory CNS white matter lesions in recipient, naive mice and that they could block the progression of late-onset disease symptoms and induce immune tolerance to MOG by injecting MOG-specific T cells coupled to nanoparticles at just the right point in time prior to late-onset disease. The investigators concluded that this disease process is “likely to be a T-cell mediated autoimmune response,” consistent with MS.
The researchers suggested that the “development of CNS autoimmunity after oligodendrocyte loss could be a slow process ... [which] might explain the difficulty in observing a correlation between oligodendrocyte loss, whether induced by viral cytotoxicity or other environmental factors, and a myelin immune response in patients with MS.”
A news release from Northwestern University, one of the institutions involved in the research, said that the nanoparticle technology used in the experiments has been licensed to Cour Pharmaceutical Development Company to develop it for human trials in autoimmune disease.
Read the full paper at Nat Neurosci. 2015 Dec 14. doi: 10.1038/nn.4193.
An adaptive autoimmune response against myelin that occurs late after an initial loss of oligodendrocytes in a new mouse model offers evidence in support of the “inside-out” hypothesis for multiple sclerosis pathogenesis in which “certain forms of MS may be caused by primary oligodendrocyte loss or myelin damage that secondarily leads to activation of the permissive adaptive immune system and CNS inflammation,” according to Maria Traka, Ph.D., of the University of Chicago. The report was published Dec. 14 in Nature Neuroscience.
“To our knowledge, this is the first experimental evidence to support the hypothesis that oligodendrocyte loss or myelin degeneration triggers myelin autoimmunity and initiates inflammation and tissue damage in the CNS during MS,” wrote Dr. Traka and her colleagues.
The new mouse model that the investigators used to support the hypothesis undergoes a genetically induced loss of oligodendrocytes and demyelination from which it recovers, but then later in life develops an infiltration of CD4-positive T cells into the CNS that causes a secondary, fatal demyelinating disease that occurs with axonal loss, CNS inflammation, and the presence of myelin oligodendrocyte glycoprotein (MOG)–specific T cells in peripheral lymphoid organs. The investigators found that the transfer of these MOG-specific T cells could induce mild neurological symptoms and inflammatory CNS white matter lesions in recipient, naive mice and that they could block the progression of late-onset disease symptoms and induce immune tolerance to MOG by injecting MOG-specific T cells coupled to nanoparticles at just the right point in time prior to late-onset disease. The investigators concluded that this disease process is “likely to be a T-cell mediated autoimmune response,” consistent with MS.
The researchers suggested that the “development of CNS autoimmunity after oligodendrocyte loss could be a slow process ... [which] might explain the difficulty in observing a correlation between oligodendrocyte loss, whether induced by viral cytotoxicity or other environmental factors, and a myelin immune response in patients with MS.”
A news release from Northwestern University, one of the institutions involved in the research, said that the nanoparticle technology used in the experiments has been licensed to Cour Pharmaceutical Development Company to develop it for human trials in autoimmune disease.
Read the full paper at Nat Neurosci. 2015 Dec 14. doi: 10.1038/nn.4193.
An adaptive autoimmune response against myelin that occurs late after an initial loss of oligodendrocytes in a new mouse model offers evidence in support of the “inside-out” hypothesis for multiple sclerosis pathogenesis in which “certain forms of MS may be caused by primary oligodendrocyte loss or myelin damage that secondarily leads to activation of the permissive adaptive immune system and CNS inflammation,” according to Maria Traka, Ph.D., of the University of Chicago. The report was published Dec. 14 in Nature Neuroscience.
“To our knowledge, this is the first experimental evidence to support the hypothesis that oligodendrocyte loss or myelin degeneration triggers myelin autoimmunity and initiates inflammation and tissue damage in the CNS during MS,” wrote Dr. Traka and her colleagues.
The new mouse model that the investigators used to support the hypothesis undergoes a genetically induced loss of oligodendrocytes and demyelination from which it recovers, but then later in life develops an infiltration of CD4-positive T cells into the CNS that causes a secondary, fatal demyelinating disease that occurs with axonal loss, CNS inflammation, and the presence of myelin oligodendrocyte glycoprotein (MOG)–specific T cells in peripheral lymphoid organs. The investigators found that the transfer of these MOG-specific T cells could induce mild neurological symptoms and inflammatory CNS white matter lesions in recipient, naive mice and that they could block the progression of late-onset disease symptoms and induce immune tolerance to MOG by injecting MOG-specific T cells coupled to nanoparticles at just the right point in time prior to late-onset disease. The investigators concluded that this disease process is “likely to be a T-cell mediated autoimmune response,” consistent with MS.
The researchers suggested that the “development of CNS autoimmunity after oligodendrocyte loss could be a slow process ... [which] might explain the difficulty in observing a correlation between oligodendrocyte loss, whether induced by viral cytotoxicity or other environmental factors, and a myelin immune response in patients with MS.”
A news release from Northwestern University, one of the institutions involved in the research, said that the nanoparticle technology used in the experiments has been licensed to Cour Pharmaceutical Development Company to develop it for human trials in autoimmune disease.
Read the full paper at Nat Neurosci. 2015 Dec 14. doi: 10.1038/nn.4193.
FROM NATURE NEUROSCIENCE