User login
Updates in Multiple Sclerosis Imaging
Updates in Multiple Sclerosis Imaging
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
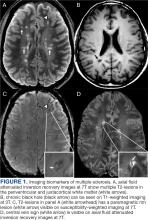
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
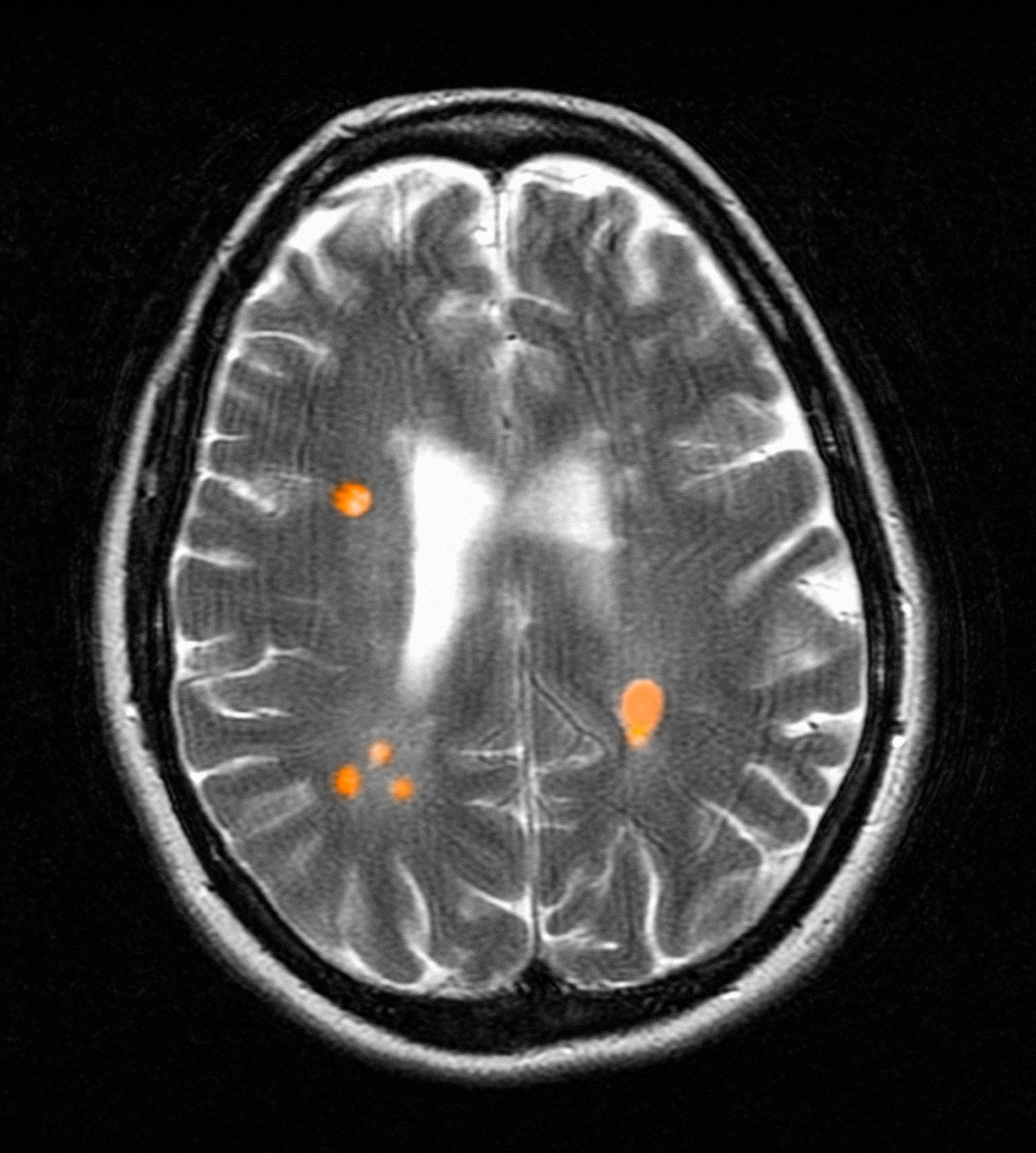
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
Updates in Multiple Sclerosis Imaging
Updates in Multiple Sclerosis Imaging
Around 5% of US Population Diagnosed With Autoimmune Disease
TOPLINE:
In 2022, autoimmune diseases affected over 15 million individuals in the United States, with women nearly twice as likely to be affected as men and more than one third of affected individuals having more than one autoimmune condition.
METHODOLOGY:
- Researchers used electronic health record (EHR) data from six healthcare systems in the United States between 2011 and 2022 to estimate the prevalence of autoimmune diseases according to sex and age.
- They selected 105 autoimmune diseases from the textbook The Autoimmune Diseases and estimated their prevalence in more than 10 million individuals from these healthcare systems; these statistics were subsequently extrapolated to an estimated US population of 333.3 million.
- An individual was considered to have a diagnosis of an autoimmune disease if they had at least two diagnosis codes for the condition, with the codes being at least 30 days apart.
- A software program was developed to compute the prevalence of autoimmune diseases alone and in aggregate, enabling other researchers to replicate or modify the analysis over time.
TAKEAWAY:
- More than 15 million people, accounting for 4.6% of the US population, were diagnosed with at least one autoimmune disease from January 2011 to June 2022; 34% were diagnosed with more than one autoimmune disease.
- Sex-stratified analysis revealed that 63% of patients diagnosed with autoimmune disease were women, and only 37% were men, establishing a female-to-male ratio of 1.7:1; age-stratified analysis revealed increasing prevalence of autoimmune conditions with age, peaking in individuals aged ≥ 65 years.
- Among individuals with autoimmune diseases, 65% of patients had one condition, whereas 24% had two, 8% had three, and 2% had four or more autoimmune diseases (does not add to 100% due to rounding).
- Rheumatoid arthritis emerged as the most prevalent autoimmune disease, followed by psoriasis, type 1 diabetes, Grave’s disease, and autoimmune thyroiditis; 19 of the top 20 most prevalent autoimmune diseases occurred more frequently in women.
IN PRACTICE:
“Accurate data on the prevalence of autoimmune diseases as a category of disease and for individual autoimmune diseases are needed to further clinical and basic research to improve diagnosis, biomarkers, and therapies for these diseases, which significantly impact the US population,” the authors wrote.
SOURCE:
The study was led by Aaron H. Abend, Autoimmune Registry, Guilford, Connecticut, and was published online in The Journal of Clinical Investigation.
LIMITATIONS:
The use of EHR data presented several challenges, including potential inaccuracies in diagnosis codes and the possibility of missing patients with single diagnosis codes because of the two-code requirement. Certain autoimmune diseases evolve over time and involve nonspecific clinical signs and symptoms that can mimic other diseases, potentially resulting in underdiagnosis. Moreover, rare diseases lacking specific diagnosis codes may have been underrepresented.
DISCLOSURES:
The study received support from Autoimmune Registry; the National Institutes of Health National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and other sources. Information on potential conflicts of interest was not disclosed.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In 2022, autoimmune diseases affected over 15 million individuals in the United States, with women nearly twice as likely to be affected as men and more than one third of affected individuals having more than one autoimmune condition.
METHODOLOGY:
- Researchers used electronic health record (EHR) data from six healthcare systems in the United States between 2011 and 2022 to estimate the prevalence of autoimmune diseases according to sex and age.
- They selected 105 autoimmune diseases from the textbook The Autoimmune Diseases and estimated their prevalence in more than 10 million individuals from these healthcare systems; these statistics were subsequently extrapolated to an estimated US population of 333.3 million.
- An individual was considered to have a diagnosis of an autoimmune disease if they had at least two diagnosis codes for the condition, with the codes being at least 30 days apart.
- A software program was developed to compute the prevalence of autoimmune diseases alone and in aggregate, enabling other researchers to replicate or modify the analysis over time.
TAKEAWAY:
- More than 15 million people, accounting for 4.6% of the US population, were diagnosed with at least one autoimmune disease from January 2011 to June 2022; 34% were diagnosed with more than one autoimmune disease.
- Sex-stratified analysis revealed that 63% of patients diagnosed with autoimmune disease were women, and only 37% were men, establishing a female-to-male ratio of 1.7:1; age-stratified analysis revealed increasing prevalence of autoimmune conditions with age, peaking in individuals aged ≥ 65 years.
- Among individuals with autoimmune diseases, 65% of patients had one condition, whereas 24% had two, 8% had three, and 2% had four or more autoimmune diseases (does not add to 100% due to rounding).
- Rheumatoid arthritis emerged as the most prevalent autoimmune disease, followed by psoriasis, type 1 diabetes, Grave’s disease, and autoimmune thyroiditis; 19 of the top 20 most prevalent autoimmune diseases occurred more frequently in women.
IN PRACTICE:
“Accurate data on the prevalence of autoimmune diseases as a category of disease and for individual autoimmune diseases are needed to further clinical and basic research to improve diagnosis, biomarkers, and therapies for these diseases, which significantly impact the US population,” the authors wrote.
SOURCE:
The study was led by Aaron H. Abend, Autoimmune Registry, Guilford, Connecticut, and was published online in The Journal of Clinical Investigation.
LIMITATIONS:
The use of EHR data presented several challenges, including potential inaccuracies in diagnosis codes and the possibility of missing patients with single diagnosis codes because of the two-code requirement. Certain autoimmune diseases evolve over time and involve nonspecific clinical signs and symptoms that can mimic other diseases, potentially resulting in underdiagnosis. Moreover, rare diseases lacking specific diagnosis codes may have been underrepresented.
DISCLOSURES:
The study received support from Autoimmune Registry; the National Institutes of Health National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and other sources. Information on potential conflicts of interest was not disclosed.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
In 2022, autoimmune diseases affected over 15 million individuals in the United States, with women nearly twice as likely to be affected as men and more than one third of affected individuals having more than one autoimmune condition.
METHODOLOGY:
- Researchers used electronic health record (EHR) data from six healthcare systems in the United States between 2011 and 2022 to estimate the prevalence of autoimmune diseases according to sex and age.
- They selected 105 autoimmune diseases from the textbook The Autoimmune Diseases and estimated their prevalence in more than 10 million individuals from these healthcare systems; these statistics were subsequently extrapolated to an estimated US population of 333.3 million.
- An individual was considered to have a diagnosis of an autoimmune disease if they had at least two diagnosis codes for the condition, with the codes being at least 30 days apart.
- A software program was developed to compute the prevalence of autoimmune diseases alone and in aggregate, enabling other researchers to replicate or modify the analysis over time.
TAKEAWAY:
- More than 15 million people, accounting for 4.6% of the US population, were diagnosed with at least one autoimmune disease from January 2011 to June 2022; 34% were diagnosed with more than one autoimmune disease.
- Sex-stratified analysis revealed that 63% of patients diagnosed with autoimmune disease were women, and only 37% were men, establishing a female-to-male ratio of 1.7:1; age-stratified analysis revealed increasing prevalence of autoimmune conditions with age, peaking in individuals aged ≥ 65 years.
- Among individuals with autoimmune diseases, 65% of patients had one condition, whereas 24% had two, 8% had three, and 2% had four or more autoimmune diseases (does not add to 100% due to rounding).
- Rheumatoid arthritis emerged as the most prevalent autoimmune disease, followed by psoriasis, type 1 diabetes, Grave’s disease, and autoimmune thyroiditis; 19 of the top 20 most prevalent autoimmune diseases occurred more frequently in women.
IN PRACTICE:
“Accurate data on the prevalence of autoimmune diseases as a category of disease and for individual autoimmune diseases are needed to further clinical and basic research to improve diagnosis, biomarkers, and therapies for these diseases, which significantly impact the US population,” the authors wrote.
SOURCE:
The study was led by Aaron H. Abend, Autoimmune Registry, Guilford, Connecticut, and was published online in The Journal of Clinical Investigation.
LIMITATIONS:
The use of EHR data presented several challenges, including potential inaccuracies in diagnosis codes and the possibility of missing patients with single diagnosis codes because of the two-code requirement. Certain autoimmune diseases evolve over time and involve nonspecific clinical signs and symptoms that can mimic other diseases, potentially resulting in underdiagnosis. Moreover, rare diseases lacking specific diagnosis codes may have been underrepresented.
DISCLOSURES:
The study received support from Autoimmune Registry; the National Institutes of Health National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; and other sources. Information on potential conflicts of interest was not disclosed.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Cancer Mortality Not Higher for Patients With Autoimmune Disease on Checkpoint Inhibitors
WASHINGTON — Immune checkpoint inhibitor (ICI) therapy does not increase mortality in people with preexisting autoimmune diseases, new research has found.
Results from a large database analysis of patients with and without autoimmune diseases suggest it is safe to treat them with ICI if they develop a cancer for which it is indicated, Greg Challener, MD, a postdoctoral fellow at the Rheumatology and Allergy Clinical Epidemiology Research Center, Massachusetts General Hospital, Boston, said at the American College of Rheumatology 2024 Annual Meeting.
“One message is that, when rheumatologists are asked by oncologists about patients with rheumatoid arthritis or vasculitis or other autoimmune diseases and whether it’s safe to treat them with immune checkpoint inhibitors, this result provides some evidence that it probably is safe…. Checkpoint inhibitors are really incredible drugs, and they’ve improved mortality for a lot of cancers, particularly melanoma, and so I think there should be a pretty high threshold for us to say a patient shouldn’t receive them because of an autoimmune condition,” he told this news organization.
Another implication, Challener said, is that people with autoimmune diseases shouldn’t routinely be excluded from clinical trials of ICIs. Currently they are excluded because of concerns about exacerbation of underlying autoimmunity, possible interference between the ICI and the immunosuppressive drugs used to treat the autoimmune condition, and a theoretical risk for serious adverse events.
“Clinical trials are continuing to exclude these patients, and they paint with a very broad brush anyone with underlying autoimmunity ... I’m hoping that that changes. I don’t think there’s a great evidence base to support that practice, and it’s unfortunate that patients with underlying autoimmune diseases are excluded from important studies,” Challener said.
Asked to comment, session moderator Matlock Jeffries, MD, director of the Arthritis Research Unit at the Oklahoma Medical Research Foundation, Oklahoma City, told this news organization that he agrees the data are generally reassuring. “If one of our patients gets cancer and their oncologist wants to use a checkpoint inhibitor, we’d obviously still monitor them for complications, but we wouldn’t automatically assume the combination of a checkpoint inhibitor and autoimmune disease would increase their mortality.”
No Difference in Mortality for Those With and Without Autoimmune Disease
Challener and colleagues used administrative health data from the TriNetX Diamond network of 92 US healthcare sites with 212 million patients. All patients included in the study were receiving anti-programmed death protein 1/programmed death ligand 1 to treat malignancies involving the skin, lung/bronchus, digestive organs, or urinary tract. The study population also had at least one rheumatologic, gastrointestinal, neurologic, dermatologic, or endocrine autoimmune disease.
Propensity score matching between those with and without autoimmune disease was performed for about 100 covariates. Prior to the matching, the autoimmune disease group had significantly higher rates of cardiovascular and other comorbidities. The matching yielded 23,714 individuals with autoimmune disease and the same number without who had similar demographics and comorbidity rates, as well as malignancy type, alcohol/tobacco use, and medication use.
At a median follow-up of 250 days, the risk for mortality prior to propensity matching was 40.0% in the autoimmune disease group and 38.1% for those without, a significant difference with hazard ratio 1.07 (95% CI, 1.05-1.10). But after the matching, the difference was no longer significant: 39.8% vs 40.2%, respectively (0.97, 0.94-1.00).
The Kaplan-Meier curves for survival probability for those with or without autoimmune disease were nearly superimposed, showing no difference up to 1600 days. An analysis of just the patients with rheumatic diseases yielded similar results, Challener said.
Some Caveats About the Data
Jeffries, who is also an associate professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, and the Oklahoma VA, said he would like to see additional data on outcomes, both for the autoimmune conditions and the cancers. Challener said there are plans to look at other hard endpoints such as myocardial infarction and end-stage renal disease, but that the database is limited.
Both Challener and Jeffries also cautioned that the reassurance may not apply to patients with active disease.
“One thing this research doesn’t address is whether active autoimmune disease might have a different outcome compared to more kind of quiet disease…. If you have a patient who has extremely active rheumatoid arthritis or extremely active giant cell arthritis, for instance, I think that could be more challenging. I would be frightened to put a patient with really active GCA on pembrolizumab or say that it’s safe without their disease being controlled. But for someone who has well-controlled disease or minimally active disease, this is very reassuring,” Challener told this news organization.
“I think this may also be important in that it’s a good argument to tell the drug companies to include autoimmune patients in these trials so we can get better data,” Jeffries said.
Challener and Jeffries had no relevant disclosures.
A version of this article appeared on Medscape.com.
WASHINGTON — Immune checkpoint inhibitor (ICI) therapy does not increase mortality in people with preexisting autoimmune diseases, new research has found.
Results from a large database analysis of patients with and without autoimmune diseases suggest it is safe to treat them with ICI if they develop a cancer for which it is indicated, Greg Challener, MD, a postdoctoral fellow at the Rheumatology and Allergy Clinical Epidemiology Research Center, Massachusetts General Hospital, Boston, said at the American College of Rheumatology 2024 Annual Meeting.
“One message is that, when rheumatologists are asked by oncologists about patients with rheumatoid arthritis or vasculitis or other autoimmune diseases and whether it’s safe to treat them with immune checkpoint inhibitors, this result provides some evidence that it probably is safe…. Checkpoint inhibitors are really incredible drugs, and they’ve improved mortality for a lot of cancers, particularly melanoma, and so I think there should be a pretty high threshold for us to say a patient shouldn’t receive them because of an autoimmune condition,” he told this news organization.
Another implication, Challener said, is that people with autoimmune diseases shouldn’t routinely be excluded from clinical trials of ICIs. Currently they are excluded because of concerns about exacerbation of underlying autoimmunity, possible interference between the ICI and the immunosuppressive drugs used to treat the autoimmune condition, and a theoretical risk for serious adverse events.
“Clinical trials are continuing to exclude these patients, and they paint with a very broad brush anyone with underlying autoimmunity ... I’m hoping that that changes. I don’t think there’s a great evidence base to support that practice, and it’s unfortunate that patients with underlying autoimmune diseases are excluded from important studies,” Challener said.
Asked to comment, session moderator Matlock Jeffries, MD, director of the Arthritis Research Unit at the Oklahoma Medical Research Foundation, Oklahoma City, told this news organization that he agrees the data are generally reassuring. “If one of our patients gets cancer and their oncologist wants to use a checkpoint inhibitor, we’d obviously still monitor them for complications, but we wouldn’t automatically assume the combination of a checkpoint inhibitor and autoimmune disease would increase their mortality.”
No Difference in Mortality for Those With and Without Autoimmune Disease
Challener and colleagues used administrative health data from the TriNetX Diamond network of 92 US healthcare sites with 212 million patients. All patients included in the study were receiving anti-programmed death protein 1/programmed death ligand 1 to treat malignancies involving the skin, lung/bronchus, digestive organs, or urinary tract. The study population also had at least one rheumatologic, gastrointestinal, neurologic, dermatologic, or endocrine autoimmune disease.
Propensity score matching between those with and without autoimmune disease was performed for about 100 covariates. Prior to the matching, the autoimmune disease group had significantly higher rates of cardiovascular and other comorbidities. The matching yielded 23,714 individuals with autoimmune disease and the same number without who had similar demographics and comorbidity rates, as well as malignancy type, alcohol/tobacco use, and medication use.
At a median follow-up of 250 days, the risk for mortality prior to propensity matching was 40.0% in the autoimmune disease group and 38.1% for those without, a significant difference with hazard ratio 1.07 (95% CI, 1.05-1.10). But after the matching, the difference was no longer significant: 39.8% vs 40.2%, respectively (0.97, 0.94-1.00).
The Kaplan-Meier curves for survival probability for those with or without autoimmune disease were nearly superimposed, showing no difference up to 1600 days. An analysis of just the patients with rheumatic diseases yielded similar results, Challener said.
Some Caveats About the Data
Jeffries, who is also an associate professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, and the Oklahoma VA, said he would like to see additional data on outcomes, both for the autoimmune conditions and the cancers. Challener said there are plans to look at other hard endpoints such as myocardial infarction and end-stage renal disease, but that the database is limited.
Both Challener and Jeffries also cautioned that the reassurance may not apply to patients with active disease.
“One thing this research doesn’t address is whether active autoimmune disease might have a different outcome compared to more kind of quiet disease…. If you have a patient who has extremely active rheumatoid arthritis or extremely active giant cell arthritis, for instance, I think that could be more challenging. I would be frightened to put a patient with really active GCA on pembrolizumab or say that it’s safe without their disease being controlled. But for someone who has well-controlled disease or minimally active disease, this is very reassuring,” Challener told this news organization.
“I think this may also be important in that it’s a good argument to tell the drug companies to include autoimmune patients in these trials so we can get better data,” Jeffries said.
Challener and Jeffries had no relevant disclosures.
A version of this article appeared on Medscape.com.
WASHINGTON — Immune checkpoint inhibitor (ICI) therapy does not increase mortality in people with preexisting autoimmune diseases, new research has found.
Results from a large database analysis of patients with and without autoimmune diseases suggest it is safe to treat them with ICI if they develop a cancer for which it is indicated, Greg Challener, MD, a postdoctoral fellow at the Rheumatology and Allergy Clinical Epidemiology Research Center, Massachusetts General Hospital, Boston, said at the American College of Rheumatology 2024 Annual Meeting.
“One message is that, when rheumatologists are asked by oncologists about patients with rheumatoid arthritis or vasculitis or other autoimmune diseases and whether it’s safe to treat them with immune checkpoint inhibitors, this result provides some evidence that it probably is safe…. Checkpoint inhibitors are really incredible drugs, and they’ve improved mortality for a lot of cancers, particularly melanoma, and so I think there should be a pretty high threshold for us to say a patient shouldn’t receive them because of an autoimmune condition,” he told this news organization.
Another implication, Challener said, is that people with autoimmune diseases shouldn’t routinely be excluded from clinical trials of ICIs. Currently they are excluded because of concerns about exacerbation of underlying autoimmunity, possible interference between the ICI and the immunosuppressive drugs used to treat the autoimmune condition, and a theoretical risk for serious adverse events.
“Clinical trials are continuing to exclude these patients, and they paint with a very broad brush anyone with underlying autoimmunity ... I’m hoping that that changes. I don’t think there’s a great evidence base to support that practice, and it’s unfortunate that patients with underlying autoimmune diseases are excluded from important studies,” Challener said.
Asked to comment, session moderator Matlock Jeffries, MD, director of the Arthritis Research Unit at the Oklahoma Medical Research Foundation, Oklahoma City, told this news organization that he agrees the data are generally reassuring. “If one of our patients gets cancer and their oncologist wants to use a checkpoint inhibitor, we’d obviously still monitor them for complications, but we wouldn’t automatically assume the combination of a checkpoint inhibitor and autoimmune disease would increase their mortality.”
No Difference in Mortality for Those With and Without Autoimmune Disease
Challener and colleagues used administrative health data from the TriNetX Diamond network of 92 US healthcare sites with 212 million patients. All patients included in the study were receiving anti-programmed death protein 1/programmed death ligand 1 to treat malignancies involving the skin, lung/bronchus, digestive organs, or urinary tract. The study population also had at least one rheumatologic, gastrointestinal, neurologic, dermatologic, or endocrine autoimmune disease.
Propensity score matching between those with and without autoimmune disease was performed for about 100 covariates. Prior to the matching, the autoimmune disease group had significantly higher rates of cardiovascular and other comorbidities. The matching yielded 23,714 individuals with autoimmune disease and the same number without who had similar demographics and comorbidity rates, as well as malignancy type, alcohol/tobacco use, and medication use.
At a median follow-up of 250 days, the risk for mortality prior to propensity matching was 40.0% in the autoimmune disease group and 38.1% for those without, a significant difference with hazard ratio 1.07 (95% CI, 1.05-1.10). But after the matching, the difference was no longer significant: 39.8% vs 40.2%, respectively (0.97, 0.94-1.00).
The Kaplan-Meier curves for survival probability for those with or without autoimmune disease were nearly superimposed, showing no difference up to 1600 days. An analysis of just the patients with rheumatic diseases yielded similar results, Challener said.
Some Caveats About the Data
Jeffries, who is also an associate professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, and the Oklahoma VA, said he would like to see additional data on outcomes, both for the autoimmune conditions and the cancers. Challener said there are plans to look at other hard endpoints such as myocardial infarction and end-stage renal disease, but that the database is limited.
Both Challener and Jeffries also cautioned that the reassurance may not apply to patients with active disease.
“One thing this research doesn’t address is whether active autoimmune disease might have a different outcome compared to more kind of quiet disease…. If you have a patient who has extremely active rheumatoid arthritis or extremely active giant cell arthritis, for instance, I think that could be more challenging. I would be frightened to put a patient with really active GCA on pembrolizumab or say that it’s safe without their disease being controlled. But for someone who has well-controlled disease or minimally active disease, this is very reassuring,” Challener told this news organization.
“I think this may also be important in that it’s a good argument to tell the drug companies to include autoimmune patients in these trials so we can get better data,” Jeffries said.
Challener and Jeffries had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ACR 2024
Smoldering MS May Warrant Unique Diagnosis, Treatment, and Research Strategies
Smoldering-associated worsening (SAW) of multiple sclerosis (MS) deserves a broader, more comprehensive approach to diagnosis, treatment, and research that goes beyond neurologists’ understanding of progression independent of relapse activity (PIRA), according to a recently published international consensus. However, an outside expert said that promulgating the “smoldering” concept may stoke patient and provider confusion.
Although current disease-modifying therapies (DMTs) for MS exclusively target focal white matter (WM) inflammation, wrote authors lead by Antonio Scalfari, MD, PhD, of Charing Cross Hospital, Imperial College London in England, many people with MS experience worsening disability in a more indolent fashion — despite stable inflammatory markers.
“The gradual accumulation of physical and cognitive disability is driven by smoldering pathological processes via biological substrates, which are different from those of acute focal damage, remain an important unmet therapeutic target,” they wrote.
The same research team first described smoldering MS in a 2022 publication. In the present paper, Scalfari and colleagues reviewed emerging clinical, radiological, and pathological evidence and presented 29 consensus statements in areas ranging from the definition, pathology, and clinical manifestations of smoldering MS to appropriate biomarkers and best clinical practices.
Definition
By definition, the authors wrote, SAW encompasses PIRA but also includes a range of gradually worsening, relapse-independent symptoms that remain undetectable on standard assessments, including the Expanded Disability Status Scale (EDSS) or EDSS-Plus, especially in early disease. To capture symptoms such as subtle motor impairment, cognitive slowing, and fatigue, Scalfari and colleagues recommend tools such as neurological stress tests, fatigue/mood scales, wearable devices, and patient reported outcomes.
Disease Mechanisms
Pathologically, the authors wrote, smoldering MS may stem from intrinsic central nervous system processes that likely incorporate various glial, immune, and neural cells. Smoldering MS also could contribute to aging, and vice versa, the latter possibly through dynamics such as age-related exhaustion of compensatory mechanisms, reduction in remyelination efficiency, and telomere shortening, they added.
Clinical Implementation
Current MS management rests on crude estimates of physical disability and overemphasizes identifying relapses and new MRI lesions as the principal markers of disease activity, wrote Scalfari and colleagues. Instead, they suggested combining motor-associated assessments such as EDSS-Plus with cognitive gauges such as the Brief International Cognitive Assessment for Multiple Sclerosis.
Providers are uncomfortable identifying and discussing smoldering MS, authors allowed, because no licensed treatments target SAW. However, the authors wrote, a principal reason for discussing smoldering MS with patients is to help manage their expectations of current DMTs, which may have little effect on SAW.
‘More Than Lesions’
Bruce Cree, MD, PhD, MAS, professor of neurology at the University of California, San Francisco, said that it is extremely important to raise awareness of physicians’ emerging understanding that “there is more going on in MS than lesions and relapses,” a concept that has been a work in progress for several years. He was not involved with the study but was asked to comment.
A 2019 report on the EPIC cohort coauthored by Cree labeled the disconnect between disability accumulation and relapse occurrence “silent progression.” The observation that disability accumulates in early relapsing MS independent of relapsing activity has been replicated in virtually every dataset worldwide, he added.
“What I don’t like about this article is the reliance on the term ‘smoldering’ and the acceptance that this is an actual phenomenon supported by data.” And authors’ leveraging “smoldering” into additional acronyms such as SAW likely will confuse rather than clarify physicians’ and patients’ understanding of the situation, Cree added. “Clinicians don’t need yet another snappy acronym.” Many are still trying to grasp the PIRA concept in relapsing MS, he said.
“One of the reasons this topic has become so important is that we recognize that even when we have very good control of relapsing disease activity — clinical relapses as well as radiographic large lesion formation on MRI — some patients still develop insidious worsening of disability. And the reasons for that are not well understood,” said Cree.
Accumulating disability absent relapse activity could stem from any number of microscopic inflammatory processes, possibly involving abnormal microglial activation, fibrinogen deposition, microscopic inflammatory infiltrates of CD8-positive T cells, or mitochondrial damage from iron deposition, he said. Or the processes driving PIRA may not even involve inflammation, he added. “We still don’t have a unifying way of understanding how these processes work.”
Cree suspects that, despite investigators’ good intentions, the study’s sponsor, Sanofi, may have influenced the resultant messaging. The company’s tolebrutinib recently completed phase 3 trials in secondary progressive MS and relapsing MS, and a phase 3 trial in primary progressive MS is scheduled for completion in 2025. “A hallmark of Sanofi’s messaging has been this idea that there is smoldering inflammation occurring in MS that tolebrutinib is going to address,” he said.
If clinicians really knew what drove progressive MS, said Cree, “we would be keen on developing therapies targeting that fundamental process. But because we don’t know what’s driving it, we don’t know what to go after.”
The study was supported by Sanofi. Cree is a coauthor of the GEMINI 1 and GEMINI 2 tolebrutinib studies.
A version of this article first appeared on Medscape.com.
Smoldering-associated worsening (SAW) of multiple sclerosis (MS) deserves a broader, more comprehensive approach to diagnosis, treatment, and research that goes beyond neurologists’ understanding of progression independent of relapse activity (PIRA), according to a recently published international consensus. However, an outside expert said that promulgating the “smoldering” concept may stoke patient and provider confusion.
Although current disease-modifying therapies (DMTs) for MS exclusively target focal white matter (WM) inflammation, wrote authors lead by Antonio Scalfari, MD, PhD, of Charing Cross Hospital, Imperial College London in England, many people with MS experience worsening disability in a more indolent fashion — despite stable inflammatory markers.
“The gradual accumulation of physical and cognitive disability is driven by smoldering pathological processes via biological substrates, which are different from those of acute focal damage, remain an important unmet therapeutic target,” they wrote.
The same research team first described smoldering MS in a 2022 publication. In the present paper, Scalfari and colleagues reviewed emerging clinical, radiological, and pathological evidence and presented 29 consensus statements in areas ranging from the definition, pathology, and clinical manifestations of smoldering MS to appropriate biomarkers and best clinical practices.
Definition
By definition, the authors wrote, SAW encompasses PIRA but also includes a range of gradually worsening, relapse-independent symptoms that remain undetectable on standard assessments, including the Expanded Disability Status Scale (EDSS) or EDSS-Plus, especially in early disease. To capture symptoms such as subtle motor impairment, cognitive slowing, and fatigue, Scalfari and colleagues recommend tools such as neurological stress tests, fatigue/mood scales, wearable devices, and patient reported outcomes.
Disease Mechanisms
Pathologically, the authors wrote, smoldering MS may stem from intrinsic central nervous system processes that likely incorporate various glial, immune, and neural cells. Smoldering MS also could contribute to aging, and vice versa, the latter possibly through dynamics such as age-related exhaustion of compensatory mechanisms, reduction in remyelination efficiency, and telomere shortening, they added.
Clinical Implementation
Current MS management rests on crude estimates of physical disability and overemphasizes identifying relapses and new MRI lesions as the principal markers of disease activity, wrote Scalfari and colleagues. Instead, they suggested combining motor-associated assessments such as EDSS-Plus with cognitive gauges such as the Brief International Cognitive Assessment for Multiple Sclerosis.
Providers are uncomfortable identifying and discussing smoldering MS, authors allowed, because no licensed treatments target SAW. However, the authors wrote, a principal reason for discussing smoldering MS with patients is to help manage their expectations of current DMTs, which may have little effect on SAW.
‘More Than Lesions’
Bruce Cree, MD, PhD, MAS, professor of neurology at the University of California, San Francisco, said that it is extremely important to raise awareness of physicians’ emerging understanding that “there is more going on in MS than lesions and relapses,” a concept that has been a work in progress for several years. He was not involved with the study but was asked to comment.
A 2019 report on the EPIC cohort coauthored by Cree labeled the disconnect between disability accumulation and relapse occurrence “silent progression.” The observation that disability accumulates in early relapsing MS independent of relapsing activity has been replicated in virtually every dataset worldwide, he added.
“What I don’t like about this article is the reliance on the term ‘smoldering’ and the acceptance that this is an actual phenomenon supported by data.” And authors’ leveraging “smoldering” into additional acronyms such as SAW likely will confuse rather than clarify physicians’ and patients’ understanding of the situation, Cree added. “Clinicians don’t need yet another snappy acronym.” Many are still trying to grasp the PIRA concept in relapsing MS, he said.
“One of the reasons this topic has become so important is that we recognize that even when we have very good control of relapsing disease activity — clinical relapses as well as radiographic large lesion formation on MRI — some patients still develop insidious worsening of disability. And the reasons for that are not well understood,” said Cree.
Accumulating disability absent relapse activity could stem from any number of microscopic inflammatory processes, possibly involving abnormal microglial activation, fibrinogen deposition, microscopic inflammatory infiltrates of CD8-positive T cells, or mitochondrial damage from iron deposition, he said. Or the processes driving PIRA may not even involve inflammation, he added. “We still don’t have a unifying way of understanding how these processes work.”
Cree suspects that, despite investigators’ good intentions, the study’s sponsor, Sanofi, may have influenced the resultant messaging. The company’s tolebrutinib recently completed phase 3 trials in secondary progressive MS and relapsing MS, and a phase 3 trial in primary progressive MS is scheduled for completion in 2025. “A hallmark of Sanofi’s messaging has been this idea that there is smoldering inflammation occurring in MS that tolebrutinib is going to address,” he said.
If clinicians really knew what drove progressive MS, said Cree, “we would be keen on developing therapies targeting that fundamental process. But because we don’t know what’s driving it, we don’t know what to go after.”
The study was supported by Sanofi. Cree is a coauthor of the GEMINI 1 and GEMINI 2 tolebrutinib studies.
A version of this article first appeared on Medscape.com.
Smoldering-associated worsening (SAW) of multiple sclerosis (MS) deserves a broader, more comprehensive approach to diagnosis, treatment, and research that goes beyond neurologists’ understanding of progression independent of relapse activity (PIRA), according to a recently published international consensus. However, an outside expert said that promulgating the “smoldering” concept may stoke patient and provider confusion.
Although current disease-modifying therapies (DMTs) for MS exclusively target focal white matter (WM) inflammation, wrote authors lead by Antonio Scalfari, MD, PhD, of Charing Cross Hospital, Imperial College London in England, many people with MS experience worsening disability in a more indolent fashion — despite stable inflammatory markers.
“The gradual accumulation of physical and cognitive disability is driven by smoldering pathological processes via biological substrates, which are different from those of acute focal damage, remain an important unmet therapeutic target,” they wrote.
The same research team first described smoldering MS in a 2022 publication. In the present paper, Scalfari and colleagues reviewed emerging clinical, radiological, and pathological evidence and presented 29 consensus statements in areas ranging from the definition, pathology, and clinical manifestations of smoldering MS to appropriate biomarkers and best clinical practices.
Definition
By definition, the authors wrote, SAW encompasses PIRA but also includes a range of gradually worsening, relapse-independent symptoms that remain undetectable on standard assessments, including the Expanded Disability Status Scale (EDSS) or EDSS-Plus, especially in early disease. To capture symptoms such as subtle motor impairment, cognitive slowing, and fatigue, Scalfari and colleagues recommend tools such as neurological stress tests, fatigue/mood scales, wearable devices, and patient reported outcomes.
Disease Mechanisms
Pathologically, the authors wrote, smoldering MS may stem from intrinsic central nervous system processes that likely incorporate various glial, immune, and neural cells. Smoldering MS also could contribute to aging, and vice versa, the latter possibly through dynamics such as age-related exhaustion of compensatory mechanisms, reduction in remyelination efficiency, and telomere shortening, they added.
Clinical Implementation
Current MS management rests on crude estimates of physical disability and overemphasizes identifying relapses and new MRI lesions as the principal markers of disease activity, wrote Scalfari and colleagues. Instead, they suggested combining motor-associated assessments such as EDSS-Plus with cognitive gauges such as the Brief International Cognitive Assessment for Multiple Sclerosis.
Providers are uncomfortable identifying and discussing smoldering MS, authors allowed, because no licensed treatments target SAW. However, the authors wrote, a principal reason for discussing smoldering MS with patients is to help manage their expectations of current DMTs, which may have little effect on SAW.
‘More Than Lesions’
Bruce Cree, MD, PhD, MAS, professor of neurology at the University of California, San Francisco, said that it is extremely important to raise awareness of physicians’ emerging understanding that “there is more going on in MS than lesions and relapses,” a concept that has been a work in progress for several years. He was not involved with the study but was asked to comment.
A 2019 report on the EPIC cohort coauthored by Cree labeled the disconnect between disability accumulation and relapse occurrence “silent progression.” The observation that disability accumulates in early relapsing MS independent of relapsing activity has been replicated in virtually every dataset worldwide, he added.
“What I don’t like about this article is the reliance on the term ‘smoldering’ and the acceptance that this is an actual phenomenon supported by data.” And authors’ leveraging “smoldering” into additional acronyms such as SAW likely will confuse rather than clarify physicians’ and patients’ understanding of the situation, Cree added. “Clinicians don’t need yet another snappy acronym.” Many are still trying to grasp the PIRA concept in relapsing MS, he said.
“One of the reasons this topic has become so important is that we recognize that even when we have very good control of relapsing disease activity — clinical relapses as well as radiographic large lesion formation on MRI — some patients still develop insidious worsening of disability. And the reasons for that are not well understood,” said Cree.
Accumulating disability absent relapse activity could stem from any number of microscopic inflammatory processes, possibly involving abnormal microglial activation, fibrinogen deposition, microscopic inflammatory infiltrates of CD8-positive T cells, or mitochondrial damage from iron deposition, he said. Or the processes driving PIRA may not even involve inflammation, he added. “We still don’t have a unifying way of understanding how these processes work.”
Cree suspects that, despite investigators’ good intentions, the study’s sponsor, Sanofi, may have influenced the resultant messaging. The company’s tolebrutinib recently completed phase 3 trials in secondary progressive MS and relapsing MS, and a phase 3 trial in primary progressive MS is scheduled for completion in 2025. “A hallmark of Sanofi’s messaging has been this idea that there is smoldering inflammation occurring in MS that tolebrutinib is going to address,” he said.
If clinicians really knew what drove progressive MS, said Cree, “we would be keen on developing therapies targeting that fundamental process. But because we don’t know what’s driving it, we don’t know what to go after.”
The study was supported by Sanofi. Cree is a coauthor of the GEMINI 1 and GEMINI 2 tolebrutinib studies.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF NEUROLOGY
Multiple Sclerosis Highlights From ECTRIMS 2024
The latest research on therapeutic management of patients with relapsing-remitting multiple sclerosis (MS) presented at the European Committee for Treatment and Research in Multiple Sclerosis 2024 Congress is reported by Dr Patricia Coyle from Stony Brook University Hospital, in Stony Brook, New York.
Dr Coyle first discusses a registry study looking at initiation of monoclonal antibody therapy for patients with pediatric-onset MS. Results showed a significant reduction in disability at age 23 and beyond when therapy was initiated in childhood.
Next, Dr Coyle discusses a trial examining the safety and efficacy of frexalimab, a second-generation anti-CD40L antibody. In an open-label extension trial through 72 weeks, frexalimab provided a sustained reduction of disease activity, as measured by MRI, and was well tolerated.
She then details a study looking at the effects of disease-modifying therapies (DMTs) on pregnancy outcomes in patients with MS. Using a German MS registry, researchers looked at 3722 pregnancies, 2885 with DMT exposure, and concluded that most pregnancy outcomes are unaffected by DMT exposure; however, the data showed the potential risk for reduced birth rates.
Finally, Dr Coyle examines the efficacy of the Bruton tyrosine kinase (BTK) inhibitor tolebrutinib, as evidenced by the HERCULES trial and the two GEMINI trials. In HERCULES, the BTK inhibitor reduced 6-month disability progression by a significant 31% compared with placebo.
--
Patricia K. Coyle, MD, Professor and Interim Chair, Department of Neurology; Director, MS Comprehensive Care Center, Stony Brook University Hospital, Stony Brook, New York
Patricia K. Coyle, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Accordant; Amgen; Biogen; Bristol Myers Squibb; Eli Lilly & Company; EMD Serono; GSK; Genentech; Horizon; LabCorp; Mylan; Novartis; Sanofi Genzyme; Viatris
Received research grant from: Celgene; CorEvitas LLC; Genentech/Roche; National Institute of Neurological Disorders and Stroke; Sanofi Genzyme
The latest research on therapeutic management of patients with relapsing-remitting multiple sclerosis (MS) presented at the European Committee for Treatment and Research in Multiple Sclerosis 2024 Congress is reported by Dr Patricia Coyle from Stony Brook University Hospital, in Stony Brook, New York.
Dr Coyle first discusses a registry study looking at initiation of monoclonal antibody therapy for patients with pediatric-onset MS. Results showed a significant reduction in disability at age 23 and beyond when therapy was initiated in childhood.
Next, Dr Coyle discusses a trial examining the safety and efficacy of frexalimab, a second-generation anti-CD40L antibody. In an open-label extension trial through 72 weeks, frexalimab provided a sustained reduction of disease activity, as measured by MRI, and was well tolerated.
She then details a study looking at the effects of disease-modifying therapies (DMTs) on pregnancy outcomes in patients with MS. Using a German MS registry, researchers looked at 3722 pregnancies, 2885 with DMT exposure, and concluded that most pregnancy outcomes are unaffected by DMT exposure; however, the data showed the potential risk for reduced birth rates.
Finally, Dr Coyle examines the efficacy of the Bruton tyrosine kinase (BTK) inhibitor tolebrutinib, as evidenced by the HERCULES trial and the two GEMINI trials. In HERCULES, the BTK inhibitor reduced 6-month disability progression by a significant 31% compared with placebo.
--
Patricia K. Coyle, MD, Professor and Interim Chair, Department of Neurology; Director, MS Comprehensive Care Center, Stony Brook University Hospital, Stony Brook, New York
Patricia K. Coyle, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Accordant; Amgen; Biogen; Bristol Myers Squibb; Eli Lilly & Company; EMD Serono; GSK; Genentech; Horizon; LabCorp; Mylan; Novartis; Sanofi Genzyme; Viatris
Received research grant from: Celgene; CorEvitas LLC; Genentech/Roche; National Institute of Neurological Disorders and Stroke; Sanofi Genzyme
The latest research on therapeutic management of patients with relapsing-remitting multiple sclerosis (MS) presented at the European Committee for Treatment and Research in Multiple Sclerosis 2024 Congress is reported by Dr Patricia Coyle from Stony Brook University Hospital, in Stony Brook, New York.
Dr Coyle first discusses a registry study looking at initiation of monoclonal antibody therapy for patients with pediatric-onset MS. Results showed a significant reduction in disability at age 23 and beyond when therapy was initiated in childhood.
Next, Dr Coyle discusses a trial examining the safety and efficacy of frexalimab, a second-generation anti-CD40L antibody. In an open-label extension trial through 72 weeks, frexalimab provided a sustained reduction of disease activity, as measured by MRI, and was well tolerated.
She then details a study looking at the effects of disease-modifying therapies (DMTs) on pregnancy outcomes in patients with MS. Using a German MS registry, researchers looked at 3722 pregnancies, 2885 with DMT exposure, and concluded that most pregnancy outcomes are unaffected by DMT exposure; however, the data showed the potential risk for reduced birth rates.
Finally, Dr Coyle examines the efficacy of the Bruton tyrosine kinase (BTK) inhibitor tolebrutinib, as evidenced by the HERCULES trial and the two GEMINI trials. In HERCULES, the BTK inhibitor reduced 6-month disability progression by a significant 31% compared with placebo.
--
Patricia K. Coyle, MD, Professor and Interim Chair, Department of Neurology; Director, MS Comprehensive Care Center, Stony Brook University Hospital, Stony Brook, New York
Patricia K. Coyle, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Accordant; Amgen; Biogen; Bristol Myers Squibb; Eli Lilly & Company; EMD Serono; GSK; Genentech; Horizon; LabCorp; Mylan; Novartis; Sanofi Genzyme; Viatris
Received research grant from: Celgene; CorEvitas LLC; Genentech/Roche; National Institute of Neurological Disorders and Stroke; Sanofi Genzyme

Statins for MS (Not)
Hidden behind all of the new drugs and breakthroughs reported at the 2024 ECTRIMS meetings was one paper that caught my attention.
It was that, after several years of study, simvastatin had no benefit for multiple sclerosis.
Statins for MS (and for Alzheimer’s disease) have been bandied about for some time, with arguments based on theoretical ideas, and small studies, that they’d have a beneficial effect on the disease – maybe from anti-inflammatory and other properties. In addition, they offered the benefit of being widely available and comparatively inexpensive.
Because of those studies, 15-20 years ago I used them off label for MS in a handful of patients – sometimes as an adjunct to their current treatment (limited at that point to interferons and Copaxone), or in patients who couldn’t afford the FDA-approved drugs. Although not without their drawbacks, the statins are relatively well understood and tolerated.
At some point, for reasons I’ve long forgotten, they all came off of them (at least for MS purposes). Maybe for side effects, or lack of benefit, or because new medications, with much clearer efficacies, were rolling out.
Now it seems pretty clear that statins don’t work for MS.
So was it a bad idea to try? No. Without asking questions we don’t find answers. If they’d worked out it would have been great, another tool on the neurology workbench to reach for in the right situation. It might also have led us to new avenues in MS treatment.
But it didn’t, and that’s fine. Although they don’t get the attention, we learn as much (sometimes more) from negative studies as we do from positive ones. If we put people on every drug that initially showed promise for their conditions, my patients would have a pretty huge medication list. For Alzheimer’s disease alone I remember studies that once suggested ibuprofen, statins, estrogen, nicotine, and several vitamins might be effective (“might” being the key word). Today we’re looking at the PDE5 inhibitors and semaglutide. The jury is still out on them, but whichever way it goes we’ll still learn something.
The statins are good drugs. Their benefits in cardiac and cerebrovascular disease can’t be disputed (I’m sure someone would, but that’s not the point of this piece). But, like all drugs, they don’t work for everything.
We learn from both and keep moving forward.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Hidden behind all of the new drugs and breakthroughs reported at the 2024 ECTRIMS meetings was one paper that caught my attention.
It was that, after several years of study, simvastatin had no benefit for multiple sclerosis.
Statins for MS (and for Alzheimer’s disease) have been bandied about for some time, with arguments based on theoretical ideas, and small studies, that they’d have a beneficial effect on the disease – maybe from anti-inflammatory and other properties. In addition, they offered the benefit of being widely available and comparatively inexpensive.
Because of those studies, 15-20 years ago I used them off label for MS in a handful of patients – sometimes as an adjunct to their current treatment (limited at that point to interferons and Copaxone), or in patients who couldn’t afford the FDA-approved drugs. Although not without their drawbacks, the statins are relatively well understood and tolerated.
At some point, for reasons I’ve long forgotten, they all came off of them (at least for MS purposes). Maybe for side effects, or lack of benefit, or because new medications, with much clearer efficacies, were rolling out.
Now it seems pretty clear that statins don’t work for MS.
So was it a bad idea to try? No. Without asking questions we don’t find answers. If they’d worked out it would have been great, another tool on the neurology workbench to reach for in the right situation. It might also have led us to new avenues in MS treatment.
But it didn’t, and that’s fine. Although they don’t get the attention, we learn as much (sometimes more) from negative studies as we do from positive ones. If we put people on every drug that initially showed promise for their conditions, my patients would have a pretty huge medication list. For Alzheimer’s disease alone I remember studies that once suggested ibuprofen, statins, estrogen, nicotine, and several vitamins might be effective (“might” being the key word). Today we’re looking at the PDE5 inhibitors and semaglutide. The jury is still out on them, but whichever way it goes we’ll still learn something.
The statins are good drugs. Their benefits in cardiac and cerebrovascular disease can’t be disputed (I’m sure someone would, but that’s not the point of this piece). But, like all drugs, they don’t work for everything.
We learn from both and keep moving forward.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Hidden behind all of the new drugs and breakthroughs reported at the 2024 ECTRIMS meetings was one paper that caught my attention.
It was that, after several years of study, simvastatin had no benefit for multiple sclerosis.
Statins for MS (and for Alzheimer’s disease) have been bandied about for some time, with arguments based on theoretical ideas, and small studies, that they’d have a beneficial effect on the disease – maybe from anti-inflammatory and other properties. In addition, they offered the benefit of being widely available and comparatively inexpensive.
Because of those studies, 15-20 years ago I used them off label for MS in a handful of patients – sometimes as an adjunct to their current treatment (limited at that point to interferons and Copaxone), or in patients who couldn’t afford the FDA-approved drugs. Although not without their drawbacks, the statins are relatively well understood and tolerated.
At some point, for reasons I’ve long forgotten, they all came off of them (at least for MS purposes). Maybe for side effects, or lack of benefit, or because new medications, with much clearer efficacies, were rolling out.
Now it seems pretty clear that statins don’t work for MS.
So was it a bad idea to try? No. Without asking questions we don’t find answers. If they’d worked out it would have been great, another tool on the neurology workbench to reach for in the right situation. It might also have led us to new avenues in MS treatment.
But it didn’t, and that’s fine. Although they don’t get the attention, we learn as much (sometimes more) from negative studies as we do from positive ones. If we put people on every drug that initially showed promise for their conditions, my patients would have a pretty huge medication list. For Alzheimer’s disease alone I remember studies that once suggested ibuprofen, statins, estrogen, nicotine, and several vitamins might be effective (“might” being the key word). Today we’re looking at the PDE5 inhibitors and semaglutide. The jury is still out on them, but whichever way it goes we’ll still learn something.
The statins are good drugs. Their benefits in cardiac and cerebrovascular disease can’t be disputed (I’m sure someone would, but that’s not the point of this piece). But, like all drugs, they don’t work for everything.
We learn from both and keep moving forward.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
sNFl and sGFAP Predict MS Disability in Unique Ways
COPENHAGEN — , according to multiple independent studies.
The basic consensus is that “elevated sNFl levels predict inflammatory-associated worsening, while sGFAP values correlate with progression independent of inflammation,” said Enric Monreal, MD, Immunology Department, Ramón y Cajal University Hospital, Madrid, Spain.
This key message was repeated by several researchers presenting data at the 2024 ECTRIMS 2004 meeting, including one delivered as a latebreaker. There was also general agreement that sGFAP will eventually be a routine prognostic tool even if more data are needed to validate how it will be used in routine MS management.
A New Biomarker for MS Disability Progression
Although apparently reliable for predicting MS disability, “sGFAP is about 5 years behind where we are with sNFl,” said Evan Madill, MD, a clinical research fellow at the Brigham Multiple Sclerosis Research Center, Harvard Medical School, Boston. He does think, however, that it is coming to clinical practice.
In the study he presented, 744 patients from the Brigham MS Research Center database were evaluated retrospectively for sGFAP levels and subsequent disability progression. Among this cohort, for which sGFAP levels were collected at baseline and over time, 46.5% had 6-month confirmed disability progression (CDP) over follow-up.
On univariate analysis, sGFAP levels correlated with and predicted CDP, need for a new ambulatory aid, and conversion to secondary progressive MS (SPMS). For patients less than 60 years of age, all of these correlations were highly significant (P ≤ .002). On multivariate analysis, the significance was preserved for CDP (P = .032) and for need of a new ambulatory aid (P = .007), but it was lost for SPMS conversion.
Notably, his data suggest that a one-time baseline measurement of sGFAP was more useful than change in sGFAP as a predictor.
It is unclear why sGFAP is less predictive in older individuals, but Dr. Madill speculated that non-MS phenomena might play a role at older ages. Treatment did not influence sGFAP levels in this study, but Dr. Madill said most of the data were collected before anti-CD20 monoclonal antibodies were widely available.
The observational study data presented by Dr. Monreal involved 725 patients drawn from 13 European hospitals. sGFAP and sNFl levels were evaluated from blood drawn within 12 months of MS onset. Over time these biomarkers had overlapping but different predictive strengths.
Consistent with previously published studies, which link elevations in sNFl to neuronal damage and elevations in sGFAP to astrogliosis, sGFAP was found to be more useful for predicting progression independent of relapse activity (PIRA), particularly in patients with low sNFl levels.
Increases in sNFl were associated with an increased risk of both PIRA and relapse-associated worsening (RAW), but sNFl was more closely associated with RAW in untreated patients. The risk of PIRA and RAW were similar across GFAP and sNFl levels in those patients treated with high-efficacy disease-modifying therapies (DMT).
Overall, when stratifying the cohort into three groups, those with both low sNFl and low GFAP, those with high sNFl with low GFAP, and those with high GFAP and low sNFl, the relative risks of disability associated with PIRA and RAW diverged, suggesting these biomarkers correlate with different processes of progression.
Comparing sGFAP and sNFl
This same principle was explored further in the latebreaking presentation by Ahmed Abdelhak, MD, a clinical instructor, Weill Institute for Neurosciences, University of California, San Francisco. The objective of his study was to compare sGFAP and sNFl for predicting PIRA in patients on treatment.
The study included 212 patients from the Swiss Multiple Sclerosis Cohort who were started on fingolimod or on B-cell depleting therapies like rituximab. After correcting for sex, age at onset, baseline Expanded Disability Status Scale (EDSS) scores, and other variables, Dr. Abdelhak also reported that the predictive values for PIRA were different for sGFAP relative to sNFl at least on the group level.
However, in this study, unlike the analysis of the Brigham MS Research Center data, changes in sGFAP over time when on treatment did have prognostic value, and there was a relationship between sGFAP levels and treatment. Although reductions in GFAP predicted less disability progression whether patients were treated with fingolimod B-cell depleting therapies, that patterns were different. Dr. Abdelhak, like the other investigators speaking at ECTRIMS, also said the data so far favor sGFAP over sNFl for predicting PIRA.
Each z-score unit change in sGFAP corresponded to a 47% lower risk of PIRA in follow-up over 6.8 years, Dr. Abdelhak reported, adding that the predictive value of sGFAP was “numerically stronger than the corresponding relation for sNFl.”
So far, clinical utility of sGFAP remains speculative. Most of the correlations he presented were on a group rather than the individual level. Moreover, Dr. Abdelhak cautioned that these correlations, based on observational data, do not necessarily reflect causation.
Nonetheless, remarking on the parallels of his data on sGFAP and sNFl with other studies presented at the ECTRIMS meeting, Dr. Abdelhak foresees a time when GFAP will be a prognostic tool, offering relative simplicity and lower cost than the current standard of imaging. He also sees a role in clinical research.
“Monitoring of sGFAP dynamics following DMT initiation could be used to prognosticate long-term PIRA risk and provide insights valuable for design and interpretation of trial outcomes,” he said.
Dr. Monreal reported financial relationships with Almirall, Biogen, Bristol-Myers Squibb, Janssen, Merck, Novartis, Roche, and Sanofi. Dr. Madill and Dr. Abdelhak reported no potential conflicts of interest.
COPENHAGEN — , according to multiple independent studies.
The basic consensus is that “elevated sNFl levels predict inflammatory-associated worsening, while sGFAP values correlate with progression independent of inflammation,” said Enric Monreal, MD, Immunology Department, Ramón y Cajal University Hospital, Madrid, Spain.
This key message was repeated by several researchers presenting data at the 2024 ECTRIMS 2004 meeting, including one delivered as a latebreaker. There was also general agreement that sGFAP will eventually be a routine prognostic tool even if more data are needed to validate how it will be used in routine MS management.
A New Biomarker for MS Disability Progression
Although apparently reliable for predicting MS disability, “sGFAP is about 5 years behind where we are with sNFl,” said Evan Madill, MD, a clinical research fellow at the Brigham Multiple Sclerosis Research Center, Harvard Medical School, Boston. He does think, however, that it is coming to clinical practice.
In the study he presented, 744 patients from the Brigham MS Research Center database were evaluated retrospectively for sGFAP levels and subsequent disability progression. Among this cohort, for which sGFAP levels were collected at baseline and over time, 46.5% had 6-month confirmed disability progression (CDP) over follow-up.
On univariate analysis, sGFAP levels correlated with and predicted CDP, need for a new ambulatory aid, and conversion to secondary progressive MS (SPMS). For patients less than 60 years of age, all of these correlations were highly significant (P ≤ .002). On multivariate analysis, the significance was preserved for CDP (P = .032) and for need of a new ambulatory aid (P = .007), but it was lost for SPMS conversion.
Notably, his data suggest that a one-time baseline measurement of sGFAP was more useful than change in sGFAP as a predictor.
It is unclear why sGFAP is less predictive in older individuals, but Dr. Madill speculated that non-MS phenomena might play a role at older ages. Treatment did not influence sGFAP levels in this study, but Dr. Madill said most of the data were collected before anti-CD20 monoclonal antibodies were widely available.
The observational study data presented by Dr. Monreal involved 725 patients drawn from 13 European hospitals. sGFAP and sNFl levels were evaluated from blood drawn within 12 months of MS onset. Over time these biomarkers had overlapping but different predictive strengths.
Consistent with previously published studies, which link elevations in sNFl to neuronal damage and elevations in sGFAP to astrogliosis, sGFAP was found to be more useful for predicting progression independent of relapse activity (PIRA), particularly in patients with low sNFl levels.
Increases in sNFl were associated with an increased risk of both PIRA and relapse-associated worsening (RAW), but sNFl was more closely associated with RAW in untreated patients. The risk of PIRA and RAW were similar across GFAP and sNFl levels in those patients treated with high-efficacy disease-modifying therapies (DMT).
Overall, when stratifying the cohort into three groups, those with both low sNFl and low GFAP, those with high sNFl with low GFAP, and those with high GFAP and low sNFl, the relative risks of disability associated with PIRA and RAW diverged, suggesting these biomarkers correlate with different processes of progression.
Comparing sGFAP and sNFl
This same principle was explored further in the latebreaking presentation by Ahmed Abdelhak, MD, a clinical instructor, Weill Institute for Neurosciences, University of California, San Francisco. The objective of his study was to compare sGFAP and sNFl for predicting PIRA in patients on treatment.
The study included 212 patients from the Swiss Multiple Sclerosis Cohort who were started on fingolimod or on B-cell depleting therapies like rituximab. After correcting for sex, age at onset, baseline Expanded Disability Status Scale (EDSS) scores, and other variables, Dr. Abdelhak also reported that the predictive values for PIRA were different for sGFAP relative to sNFl at least on the group level.
However, in this study, unlike the analysis of the Brigham MS Research Center data, changes in sGFAP over time when on treatment did have prognostic value, and there was a relationship between sGFAP levels and treatment. Although reductions in GFAP predicted less disability progression whether patients were treated with fingolimod B-cell depleting therapies, that patterns were different. Dr. Abdelhak, like the other investigators speaking at ECTRIMS, also said the data so far favor sGFAP over sNFl for predicting PIRA.
Each z-score unit change in sGFAP corresponded to a 47% lower risk of PIRA in follow-up over 6.8 years, Dr. Abdelhak reported, adding that the predictive value of sGFAP was “numerically stronger than the corresponding relation for sNFl.”
So far, clinical utility of sGFAP remains speculative. Most of the correlations he presented were on a group rather than the individual level. Moreover, Dr. Abdelhak cautioned that these correlations, based on observational data, do not necessarily reflect causation.
Nonetheless, remarking on the parallels of his data on sGFAP and sNFl with other studies presented at the ECTRIMS meeting, Dr. Abdelhak foresees a time when GFAP will be a prognostic tool, offering relative simplicity and lower cost than the current standard of imaging. He also sees a role in clinical research.
“Monitoring of sGFAP dynamics following DMT initiation could be used to prognosticate long-term PIRA risk and provide insights valuable for design and interpretation of trial outcomes,” he said.
Dr. Monreal reported financial relationships with Almirall, Biogen, Bristol-Myers Squibb, Janssen, Merck, Novartis, Roche, and Sanofi. Dr. Madill and Dr. Abdelhak reported no potential conflicts of interest.
COPENHAGEN — , according to multiple independent studies.
The basic consensus is that “elevated sNFl levels predict inflammatory-associated worsening, while sGFAP values correlate with progression independent of inflammation,” said Enric Monreal, MD, Immunology Department, Ramón y Cajal University Hospital, Madrid, Spain.
This key message was repeated by several researchers presenting data at the 2024 ECTRIMS 2004 meeting, including one delivered as a latebreaker. There was also general agreement that sGFAP will eventually be a routine prognostic tool even if more data are needed to validate how it will be used in routine MS management.
A New Biomarker for MS Disability Progression
Although apparently reliable for predicting MS disability, “sGFAP is about 5 years behind where we are with sNFl,” said Evan Madill, MD, a clinical research fellow at the Brigham Multiple Sclerosis Research Center, Harvard Medical School, Boston. He does think, however, that it is coming to clinical practice.
In the study he presented, 744 patients from the Brigham MS Research Center database were evaluated retrospectively for sGFAP levels and subsequent disability progression. Among this cohort, for which sGFAP levels were collected at baseline and over time, 46.5% had 6-month confirmed disability progression (CDP) over follow-up.
On univariate analysis, sGFAP levels correlated with and predicted CDP, need for a new ambulatory aid, and conversion to secondary progressive MS (SPMS). For patients less than 60 years of age, all of these correlations were highly significant (P ≤ .002). On multivariate analysis, the significance was preserved for CDP (P = .032) and for need of a new ambulatory aid (P = .007), but it was lost for SPMS conversion.
Notably, his data suggest that a one-time baseline measurement of sGFAP was more useful than change in sGFAP as a predictor.
It is unclear why sGFAP is less predictive in older individuals, but Dr. Madill speculated that non-MS phenomena might play a role at older ages. Treatment did not influence sGFAP levels in this study, but Dr. Madill said most of the data were collected before anti-CD20 monoclonal antibodies were widely available.
The observational study data presented by Dr. Monreal involved 725 patients drawn from 13 European hospitals. sGFAP and sNFl levels were evaluated from blood drawn within 12 months of MS onset. Over time these biomarkers had overlapping but different predictive strengths.
Consistent with previously published studies, which link elevations in sNFl to neuronal damage and elevations in sGFAP to astrogliosis, sGFAP was found to be more useful for predicting progression independent of relapse activity (PIRA), particularly in patients with low sNFl levels.
Increases in sNFl were associated with an increased risk of both PIRA and relapse-associated worsening (RAW), but sNFl was more closely associated with RAW in untreated patients. The risk of PIRA and RAW were similar across GFAP and sNFl levels in those patients treated with high-efficacy disease-modifying therapies (DMT).
Overall, when stratifying the cohort into three groups, those with both low sNFl and low GFAP, those with high sNFl with low GFAP, and those with high GFAP and low sNFl, the relative risks of disability associated with PIRA and RAW diverged, suggesting these biomarkers correlate with different processes of progression.
Comparing sGFAP and sNFl
This same principle was explored further in the latebreaking presentation by Ahmed Abdelhak, MD, a clinical instructor, Weill Institute for Neurosciences, University of California, San Francisco. The objective of his study was to compare sGFAP and sNFl for predicting PIRA in patients on treatment.
The study included 212 patients from the Swiss Multiple Sclerosis Cohort who were started on fingolimod or on B-cell depleting therapies like rituximab. After correcting for sex, age at onset, baseline Expanded Disability Status Scale (EDSS) scores, and other variables, Dr. Abdelhak also reported that the predictive values for PIRA were different for sGFAP relative to sNFl at least on the group level.
However, in this study, unlike the analysis of the Brigham MS Research Center data, changes in sGFAP over time when on treatment did have prognostic value, and there was a relationship between sGFAP levels and treatment. Although reductions in GFAP predicted less disability progression whether patients were treated with fingolimod B-cell depleting therapies, that patterns were different. Dr. Abdelhak, like the other investigators speaking at ECTRIMS, also said the data so far favor sGFAP over sNFl for predicting PIRA.
Each z-score unit change in sGFAP corresponded to a 47% lower risk of PIRA in follow-up over 6.8 years, Dr. Abdelhak reported, adding that the predictive value of sGFAP was “numerically stronger than the corresponding relation for sNFl.”
So far, clinical utility of sGFAP remains speculative. Most of the correlations he presented were on a group rather than the individual level. Moreover, Dr. Abdelhak cautioned that these correlations, based on observational data, do not necessarily reflect causation.
Nonetheless, remarking on the parallels of his data on sGFAP and sNFl with other studies presented at the ECTRIMS meeting, Dr. Abdelhak foresees a time when GFAP will be a prognostic tool, offering relative simplicity and lower cost than the current standard of imaging. He also sees a role in clinical research.
“Monitoring of sGFAP dynamics following DMT initiation could be used to prognosticate long-term PIRA risk and provide insights valuable for design and interpretation of trial outcomes,” he said.
Dr. Monreal reported financial relationships with Almirall, Biogen, Bristol-Myers Squibb, Janssen, Merck, Novartis, Roche, and Sanofi. Dr. Madill and Dr. Abdelhak reported no potential conflicts of interest.
FROM ECTRIMS 2024
Time to Revisit the Standard Treatment Approach in Children With MS?
COPENHAGEN — However, only few of these medications are licensed for pediatric use, indicating it may be time to reconsider the standard treatment approach for this patient population.
Treatments for pediatric-onset MS have mostly been used off-label until the recent approvals of fingolimod, dimethyl fumarate, and teriflunomide. Typically, children with MS start with moderately effective therapies, while more potent options are reserved for those who don’t respond.
However, recent research suggests this may not be the most effective treatment strategy for this patient population. Several studies suggesting impressive treatment responses to highly effective therapies (HETs) in children were presented at the 2024 ECTRIMS annual meeting.
In one study, initiating monoclonal antibody treatment during childhood was associated with reduced disability into early adulthood and beyond.
“Our findings are a strong argument for rethinking current treatment guidelines,” said study investigator Sifat Sharmin, PhD, The University of Melbourne, Australia.
“By allowing earlier access to highly effective treatments, we can significantly enhance the quality of life for children with MS and reduce the burden of long-term disability,” she added.
In another presentation, Yael Hacohen, MD, Great Ormond Street Hospital, London, England, noted that the use of these more effective monoclonal antibody therapies in children with MS has been associated with some improvements in Expanded Disability Status Scale (EDSS) scores after 2 or 3 years of treatment.
Maybe this is a sign that “this is a population that can repair, in contrast to adult patients,” she wondered.
MS is primarily a disease of adults, but pediatric MS accounts for up to 5% of all cases. Children with MS tend to have much more active disease than adults, Dr. Hacohen explained. However, they also tend to recover from attacks more quickly with little disability, which sometimes causes diagnostic delays.
A pediatrician or family doctor will often dismiss pins and needles or blurred vision that only lasts a couple of days and won’t send the patient for an MRI, she said. But on MRI, pediatric patients with MS often have multiple lesions, even though they may have had very few symptoms. The EDSS may not change very much, but there can still be significant brain atrophy.
Over the past 20 years, there’s been an explosion of new disease-modifying treatments for MS, but these high-efficacy treatments, such as antibody therapies, are often not prescribed until the patient reaches the age of 18 years, both Dr. Sharmin and Dr. Hacohen pointed out.
“We need to get some of these medications approved for use in children,” Dr. Hacohen said.
Slowed Disability
In her presentation, Dr. Sharmin reported an observational study that included 282 patients younger than 18 years at MS onset identified from the French MS Registry, the Italian MS Register, and the Global MSBase Registry.
Of these, 110 (39%) had initiated therapy with ocrelizumab, rituximab, or natalizumab early in the disease course between ages 12 and 17 years and 172 (61%) had initiated treatment with one of these agents at ages 20-22 years.
The primary outcome was the difference in EDSS scores from baseline (at age 18 years) to ages 23-27 years between those who had started treatment with one of these agents early and those who had started late.
At the baseline of age 18 years, the median EDSS score was 1.5 in the early group and 1.3 in the late group. Median follow-up time was 10.8 years.
The data were adjusted for baseline differences in factors such as sex, age at symptom onset, time from onset to clinically definite MS, and the number of relapses (using inverse probability treatment weighting based on propensity scores).
Results showed that between ages 23 and 27 years, disability was a 0.57 step lower in the early group than in the late group. The mean absolute differences in EDSS from baseline were 0.40 in the early group and 0.95 in the late group. This benefit of early treatment persisted throughout the rest of the follow-up period.
The substantially lower risk of progressing to higher disability levels in the early treatment group was particularly evident in the moderate disability range, where further progression was reduced by up to 97%, Dr. Sharmin noted.
“Starting these highly effective therapies, before the onset of significant neurological impairments, appears crucial for preserving neurological function in children with relapsing-remitting MS over the long term,” she said.
These findings highlight the critical importance of early intervention in pediatric-onset MS, she concluded.
The researchers are planning further work to generate more evidence to support the proactive treatment of pediatric-onset MS, with a particular focus on assessing the long-term risks for immunosuppressive therapies in this population.
Ocrelizumab Experience in Children
Dr. Hacohen reported on a UK cohort of children with MS treated with ocrelizumab, with 66 patients having more than 12 months of follow-up. Of these, only four patients had relapses, and there was no evidence of disease activity in 94% patients.
“We’ve stopped doing relapse clinic because they really don’t relapse,” Dr. Hacohen reported.
“This has completely changed our practice in pediatric MS,” she said. Twice a year, patients come in to have pre-infusion bloods and clinical assessments and then return a month later for treatment.
“They only have to come to the hospital for 4 days a year, and the rest of the time, they can forget they have MS,” said Dr. Hacohen.
In terms of complications, one patient in the UK cohort developed enterovirus meningitis but recovered completely, and two patients had hypogammaglobulinemia and were changed to an extended interval or to a different agent.
Dr. Hacohen cautioned that hypogammaglobulinemia — a condition in which immunoglobulin levels are below normal — is “something that hypothetically we should maybe be more worried about in the pediatric population, particularly as these patients are more likely to be on anti-CD20 therapies for a much longer time.”
She said this complication tends to happen after about 4 or 5 years of treatment. “If we start seeing IgG levels dropping, we need to come up with a plan about extending the dosing interval. We need clinical trials to look at this.”
Dr. Hacohen also drew attention to the issue of vaccinations not being effective in patients on anti-CD20 antibody therapy, which could be a particular problem in children.
However, given that vaccinations do seem to be effective in patients taking natalizumab, pediatric patients with highly active disease could receive the drug for 3-6 months while receiving vaccines and then switched over to ocrelizumab, she said.
Giving natalizumab for such a short period is not believed to have a high risk of developing JCV antibodies, she added.
In another presentation, Brenda Banwell, MD, Johns Hopkins Children’s Center, Baltimore, reported new data from an early study (OPERETTA 1) with ocrelizumab in pediatric relapsing-remitting MS showing a safety profile similar to that observed in adults. The suggested dose is 300 mg for children under 35 kg and 600 mg for adults over 35 kg, administered every 24 weeks. These doses will be further investigated in the ongoing phase III OPERETTA 2 trial.
Dr. Sharmin received a postdoctoral fellowship from MS Australia. The OPERETTA studies were sponsored by F. Hoffmann-La Roche. Dr. Banwell served as a consultant to Roche. Dr. Hacohen reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
COPENHAGEN — However, only few of these medications are licensed for pediatric use, indicating it may be time to reconsider the standard treatment approach for this patient population.
Treatments for pediatric-onset MS have mostly been used off-label until the recent approvals of fingolimod, dimethyl fumarate, and teriflunomide. Typically, children with MS start with moderately effective therapies, while more potent options are reserved for those who don’t respond.
However, recent research suggests this may not be the most effective treatment strategy for this patient population. Several studies suggesting impressive treatment responses to highly effective therapies (HETs) in children were presented at the 2024 ECTRIMS annual meeting.
In one study, initiating monoclonal antibody treatment during childhood was associated with reduced disability into early adulthood and beyond.
“Our findings are a strong argument for rethinking current treatment guidelines,” said study investigator Sifat Sharmin, PhD, The University of Melbourne, Australia.
“By allowing earlier access to highly effective treatments, we can significantly enhance the quality of life for children with MS and reduce the burden of long-term disability,” she added.
In another presentation, Yael Hacohen, MD, Great Ormond Street Hospital, London, England, noted that the use of these more effective monoclonal antibody therapies in children with MS has been associated with some improvements in Expanded Disability Status Scale (EDSS) scores after 2 or 3 years of treatment.
Maybe this is a sign that “this is a population that can repair, in contrast to adult patients,” she wondered.
MS is primarily a disease of adults, but pediatric MS accounts for up to 5% of all cases. Children with MS tend to have much more active disease than adults, Dr. Hacohen explained. However, they also tend to recover from attacks more quickly with little disability, which sometimes causes diagnostic delays.
A pediatrician or family doctor will often dismiss pins and needles or blurred vision that only lasts a couple of days and won’t send the patient for an MRI, she said. But on MRI, pediatric patients with MS often have multiple lesions, even though they may have had very few symptoms. The EDSS may not change very much, but there can still be significant brain atrophy.
Over the past 20 years, there’s been an explosion of new disease-modifying treatments for MS, but these high-efficacy treatments, such as antibody therapies, are often not prescribed until the patient reaches the age of 18 years, both Dr. Sharmin and Dr. Hacohen pointed out.
“We need to get some of these medications approved for use in children,” Dr. Hacohen said.
Slowed Disability
In her presentation, Dr. Sharmin reported an observational study that included 282 patients younger than 18 years at MS onset identified from the French MS Registry, the Italian MS Register, and the Global MSBase Registry.
Of these, 110 (39%) had initiated therapy with ocrelizumab, rituximab, or natalizumab early in the disease course between ages 12 and 17 years and 172 (61%) had initiated treatment with one of these agents at ages 20-22 years.
The primary outcome was the difference in EDSS scores from baseline (at age 18 years) to ages 23-27 years between those who had started treatment with one of these agents early and those who had started late.
At the baseline of age 18 years, the median EDSS score was 1.5 in the early group and 1.3 in the late group. Median follow-up time was 10.8 years.
The data were adjusted for baseline differences in factors such as sex, age at symptom onset, time from onset to clinically definite MS, and the number of relapses (using inverse probability treatment weighting based on propensity scores).
Results showed that between ages 23 and 27 years, disability was a 0.57 step lower in the early group than in the late group. The mean absolute differences in EDSS from baseline were 0.40 in the early group and 0.95 in the late group. This benefit of early treatment persisted throughout the rest of the follow-up period.
The substantially lower risk of progressing to higher disability levels in the early treatment group was particularly evident in the moderate disability range, where further progression was reduced by up to 97%, Dr. Sharmin noted.
“Starting these highly effective therapies, before the onset of significant neurological impairments, appears crucial for preserving neurological function in children with relapsing-remitting MS over the long term,” she said.
These findings highlight the critical importance of early intervention in pediatric-onset MS, she concluded.
The researchers are planning further work to generate more evidence to support the proactive treatment of pediatric-onset MS, with a particular focus on assessing the long-term risks for immunosuppressive therapies in this population.
Ocrelizumab Experience in Children
Dr. Hacohen reported on a UK cohort of children with MS treated with ocrelizumab, with 66 patients having more than 12 months of follow-up. Of these, only four patients had relapses, and there was no evidence of disease activity in 94% patients.
“We’ve stopped doing relapse clinic because they really don’t relapse,” Dr. Hacohen reported.
“This has completely changed our practice in pediatric MS,” she said. Twice a year, patients come in to have pre-infusion bloods and clinical assessments and then return a month later for treatment.
“They only have to come to the hospital for 4 days a year, and the rest of the time, they can forget they have MS,” said Dr. Hacohen.
In terms of complications, one patient in the UK cohort developed enterovirus meningitis but recovered completely, and two patients had hypogammaglobulinemia and were changed to an extended interval or to a different agent.
Dr. Hacohen cautioned that hypogammaglobulinemia — a condition in which immunoglobulin levels are below normal — is “something that hypothetically we should maybe be more worried about in the pediatric population, particularly as these patients are more likely to be on anti-CD20 therapies for a much longer time.”
She said this complication tends to happen after about 4 or 5 years of treatment. “If we start seeing IgG levels dropping, we need to come up with a plan about extending the dosing interval. We need clinical trials to look at this.”
Dr. Hacohen also drew attention to the issue of vaccinations not being effective in patients on anti-CD20 antibody therapy, which could be a particular problem in children.
However, given that vaccinations do seem to be effective in patients taking natalizumab, pediatric patients with highly active disease could receive the drug for 3-6 months while receiving vaccines and then switched over to ocrelizumab, she said.
Giving natalizumab for such a short period is not believed to have a high risk of developing JCV antibodies, she added.
In another presentation, Brenda Banwell, MD, Johns Hopkins Children’s Center, Baltimore, reported new data from an early study (OPERETTA 1) with ocrelizumab in pediatric relapsing-remitting MS showing a safety profile similar to that observed in adults. The suggested dose is 300 mg for children under 35 kg and 600 mg for adults over 35 kg, administered every 24 weeks. These doses will be further investigated in the ongoing phase III OPERETTA 2 trial.
Dr. Sharmin received a postdoctoral fellowship from MS Australia. The OPERETTA studies were sponsored by F. Hoffmann-La Roche. Dr. Banwell served as a consultant to Roche. Dr. Hacohen reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
COPENHAGEN — However, only few of these medications are licensed for pediatric use, indicating it may be time to reconsider the standard treatment approach for this patient population.
Treatments for pediatric-onset MS have mostly been used off-label until the recent approvals of fingolimod, dimethyl fumarate, and teriflunomide. Typically, children with MS start with moderately effective therapies, while more potent options are reserved for those who don’t respond.
However, recent research suggests this may not be the most effective treatment strategy for this patient population. Several studies suggesting impressive treatment responses to highly effective therapies (HETs) in children were presented at the 2024 ECTRIMS annual meeting.
In one study, initiating monoclonal antibody treatment during childhood was associated with reduced disability into early adulthood and beyond.
“Our findings are a strong argument for rethinking current treatment guidelines,” said study investigator Sifat Sharmin, PhD, The University of Melbourne, Australia.
“By allowing earlier access to highly effective treatments, we can significantly enhance the quality of life for children with MS and reduce the burden of long-term disability,” she added.
In another presentation, Yael Hacohen, MD, Great Ormond Street Hospital, London, England, noted that the use of these more effective monoclonal antibody therapies in children with MS has been associated with some improvements in Expanded Disability Status Scale (EDSS) scores after 2 or 3 years of treatment.
Maybe this is a sign that “this is a population that can repair, in contrast to adult patients,” she wondered.
MS is primarily a disease of adults, but pediatric MS accounts for up to 5% of all cases. Children with MS tend to have much more active disease than adults, Dr. Hacohen explained. However, they also tend to recover from attacks more quickly with little disability, which sometimes causes diagnostic delays.
A pediatrician or family doctor will often dismiss pins and needles or blurred vision that only lasts a couple of days and won’t send the patient for an MRI, she said. But on MRI, pediatric patients with MS often have multiple lesions, even though they may have had very few symptoms. The EDSS may not change very much, but there can still be significant brain atrophy.
Over the past 20 years, there’s been an explosion of new disease-modifying treatments for MS, but these high-efficacy treatments, such as antibody therapies, are often not prescribed until the patient reaches the age of 18 years, both Dr. Sharmin and Dr. Hacohen pointed out.
“We need to get some of these medications approved for use in children,” Dr. Hacohen said.
Slowed Disability
In her presentation, Dr. Sharmin reported an observational study that included 282 patients younger than 18 years at MS onset identified from the French MS Registry, the Italian MS Register, and the Global MSBase Registry.
Of these, 110 (39%) had initiated therapy with ocrelizumab, rituximab, or natalizumab early in the disease course between ages 12 and 17 years and 172 (61%) had initiated treatment with one of these agents at ages 20-22 years.
The primary outcome was the difference in EDSS scores from baseline (at age 18 years) to ages 23-27 years between those who had started treatment with one of these agents early and those who had started late.
At the baseline of age 18 years, the median EDSS score was 1.5 in the early group and 1.3 in the late group. Median follow-up time was 10.8 years.
The data were adjusted for baseline differences in factors such as sex, age at symptom onset, time from onset to clinically definite MS, and the number of relapses (using inverse probability treatment weighting based on propensity scores).
Results showed that between ages 23 and 27 years, disability was a 0.57 step lower in the early group than in the late group. The mean absolute differences in EDSS from baseline were 0.40 in the early group and 0.95 in the late group. This benefit of early treatment persisted throughout the rest of the follow-up period.
The substantially lower risk of progressing to higher disability levels in the early treatment group was particularly evident in the moderate disability range, where further progression was reduced by up to 97%, Dr. Sharmin noted.
“Starting these highly effective therapies, before the onset of significant neurological impairments, appears crucial for preserving neurological function in children with relapsing-remitting MS over the long term,” she said.
These findings highlight the critical importance of early intervention in pediatric-onset MS, she concluded.
The researchers are planning further work to generate more evidence to support the proactive treatment of pediatric-onset MS, with a particular focus on assessing the long-term risks for immunosuppressive therapies in this population.
Ocrelizumab Experience in Children
Dr. Hacohen reported on a UK cohort of children with MS treated with ocrelizumab, with 66 patients having more than 12 months of follow-up. Of these, only four patients had relapses, and there was no evidence of disease activity in 94% patients.
“We’ve stopped doing relapse clinic because they really don’t relapse,” Dr. Hacohen reported.
“This has completely changed our practice in pediatric MS,” she said. Twice a year, patients come in to have pre-infusion bloods and clinical assessments and then return a month later for treatment.
“They only have to come to the hospital for 4 days a year, and the rest of the time, they can forget they have MS,” said Dr. Hacohen.
In terms of complications, one patient in the UK cohort developed enterovirus meningitis but recovered completely, and two patients had hypogammaglobulinemia and were changed to an extended interval or to a different agent.
Dr. Hacohen cautioned that hypogammaglobulinemia — a condition in which immunoglobulin levels are below normal — is “something that hypothetically we should maybe be more worried about in the pediatric population, particularly as these patients are more likely to be on anti-CD20 therapies for a much longer time.”
She said this complication tends to happen after about 4 or 5 years of treatment. “If we start seeing IgG levels dropping, we need to come up with a plan about extending the dosing interval. We need clinical trials to look at this.”
Dr. Hacohen also drew attention to the issue of vaccinations not being effective in patients on anti-CD20 antibody therapy, which could be a particular problem in children.
However, given that vaccinations do seem to be effective in patients taking natalizumab, pediatric patients with highly active disease could receive the drug for 3-6 months while receiving vaccines and then switched over to ocrelizumab, she said.
Giving natalizumab for such a short period is not believed to have a high risk of developing JCV antibodies, she added.
In another presentation, Brenda Banwell, MD, Johns Hopkins Children’s Center, Baltimore, reported new data from an early study (OPERETTA 1) with ocrelizumab in pediatric relapsing-remitting MS showing a safety profile similar to that observed in adults. The suggested dose is 300 mg for children under 35 kg and 600 mg for adults over 35 kg, administered every 24 weeks. These doses will be further investigated in the ongoing phase III OPERETTA 2 trial.
Dr. Sharmin received a postdoctoral fellowship from MS Australia. The OPERETTA studies were sponsored by F. Hoffmann-La Roche. Dr. Banwell served as a consultant to Roche. Dr. Hacohen reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ECTRIMS 2024
Undertreatment of Women With MS Unjustified
COPENHAGEN — , even after accounting for treatment discontinuations during pregnancy and the postpartum period, new research suggested.
“We believe that pregnancy-related considerations probably still explain the major part of this gap,” said Antoine Gavoille, MD, University of Lyon, France, who presented the study at the 2024 ECTRIMS annual meeting.
This is likely due to “factors such as anticipation of pregnancy long before it occurs and fear of exposing women of childbearing age to certain treatments even in the absence of planned pregnancy,” he added.
Caution is warranted when medications are first marketed because there are no data on safety in pregnancy. However, in 2024, “this lesser treatment in women is unacceptable,” said Dr. Gavoille. “We now have several highly effective treatment options which are compatible with pregnancy,” he noted.
The researchers analyzed the French MS registry of 22,657 patients with relapsing MS (74.2% women) between 1997 and 2022 for treatment differences between women and their male counterparts. The results were adjusted for multiple factors including educational level, disease activity, disability levels, and discontinuation of drugs during pregnancy.
They found that over a median follow-up of 11.6 years, women had a significantly lower probability of receiving any disease-modifying treatment (odds ratio [OR], 0.92; 95% CI, 0.87-0.97).
In addition, women were even less likely to receive high-efficacy treatments such as natalizumab, anti-CD20 antibodies, or S1P modulators such as fingolimod (OR, 0.80; 95% CI, 0.74-0.86).
The difference in disease-modifying treatment usage varied across different treatments and over time. Teriflunomide, fingolimod, and anti-CD20 therapies were significantly underused throughout their entire availability (OR, 0.87, 0.78, and 0.80, respectively).
Interferon and natalizumab were initially used less frequently in women, but the use of these medications equalized over time.
In contrast, glatiramer acetate and dimethyl fumarate were initially used equally between genders but eventually became more commonly prescribed to women (OR, 1.27 and 1.17, respectively).
The disparity in treatment emerged after 2 years of disease duration for disease-modifying treatments in general and as early as 1 year for highly effective treatments.
The gender-based treatment gap did not significantly vary with patient age, indicating that therapeutic inertia may persist regardless of a woman’s age.
“Women may not be receiving the most effective therapies at the optimal time, often due to concerns about pregnancy risks that may never materialize,” said the study’s lead investigator Sandra Vukusic, MD, Lyon University Hospital, France.
“The main impact of this therapeutic inertia in women is the less effective control of disease activity, leading to the accumulation of lesions and an increased risk of long-term disability. This represents a real loss of opportunity for women, especially in an era where disease-modifying treatments so effective when used early,” she added.
Dr. Gavoille said that recommendations in France allow the use of moderately active drugs, including interferon and glatiramer acetate, during pregnancy or in women planning a pregnancy. More recently there has been enough data to allow the use of natalizumab up until the second trimester.
In addition, although not in the guidelines, it is thought that the anti-CD20 monoclonal antibodies, such as rituximab or ocrelizumab, may be safe as they are very long acting. Women can be dosed before pregnancy and be covered for the whole pregnancy period without exposing the fetus to the drug, he explained.
“The message is that now we have both moderately and highly effective treatments that are compatible with a pregnancy plan,” Dr. Gavoille said.
First, clinicians have to select a level of treatment based on disease activity and then choose the best option, depending on the woman’s plans with respect to pregnancy.
Drugs that are contraindicated in pregnancy include teriflunomide and S1P modulators such as fingolimod, which have been shown to be harmful to the fetus.
“But they could still be used in women of childbearing years as long as they are not planning a pregnancy and understand the need for contraception,” Dr. Gavoille noted.
He believes both neurologists and patients are afraid of using drugs in pregnancy. “It is, of course, important to be cautious on this issue, but we should not let fear stop these women receiving the best treatments available.”
However, he added, clinical practice is changing, and confidence is gradually building around using highly effective treatments in women of childbearing age.
Dr. Gavoille also called for more research to collate data in pregnant women with MS who are exposed to various treatments, starting with case reports and then academic registries, which he described as “difficult but important work.”
Commenting on the study, Robert Hoepner, MD, University Hospital of Bern, Switzerland, agreed that this treatment disparity between men and women is “unacceptable.”
Dr. Hoepner noted that a recent study showed that women have different relapse symptoms than men, which may also affect treatment choice.
Dr. Gavoille responded that other research has shown that women are less likely to have treatment escalation post-relapse. “This could be because of a difference in symptoms. But this is something we haven’t looked at yet.”
Also commenting on the research, Frauke Zipp, MD, University Medical Center Mainz in Germany, said it would be interesting to follow this cohort over the long term to see if the women do less well several years down the line.
The study authors and commentators reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
COPENHAGEN — , even after accounting for treatment discontinuations during pregnancy and the postpartum period, new research suggested.
“We believe that pregnancy-related considerations probably still explain the major part of this gap,” said Antoine Gavoille, MD, University of Lyon, France, who presented the study at the 2024 ECTRIMS annual meeting.
This is likely due to “factors such as anticipation of pregnancy long before it occurs and fear of exposing women of childbearing age to certain treatments even in the absence of planned pregnancy,” he added.
Caution is warranted when medications are first marketed because there are no data on safety in pregnancy. However, in 2024, “this lesser treatment in women is unacceptable,” said Dr. Gavoille. “We now have several highly effective treatment options which are compatible with pregnancy,” he noted.
The researchers analyzed the French MS registry of 22,657 patients with relapsing MS (74.2% women) between 1997 and 2022 for treatment differences between women and their male counterparts. The results were adjusted for multiple factors including educational level, disease activity, disability levels, and discontinuation of drugs during pregnancy.
They found that over a median follow-up of 11.6 years, women had a significantly lower probability of receiving any disease-modifying treatment (odds ratio [OR], 0.92; 95% CI, 0.87-0.97).
In addition, women were even less likely to receive high-efficacy treatments such as natalizumab, anti-CD20 antibodies, or S1P modulators such as fingolimod (OR, 0.80; 95% CI, 0.74-0.86).
The difference in disease-modifying treatment usage varied across different treatments and over time. Teriflunomide, fingolimod, and anti-CD20 therapies were significantly underused throughout their entire availability (OR, 0.87, 0.78, and 0.80, respectively).
Interferon and natalizumab were initially used less frequently in women, but the use of these medications equalized over time.
In contrast, glatiramer acetate and dimethyl fumarate were initially used equally between genders but eventually became more commonly prescribed to women (OR, 1.27 and 1.17, respectively).
The disparity in treatment emerged after 2 years of disease duration for disease-modifying treatments in general and as early as 1 year for highly effective treatments.
The gender-based treatment gap did not significantly vary with patient age, indicating that therapeutic inertia may persist regardless of a woman’s age.
“Women may not be receiving the most effective therapies at the optimal time, often due to concerns about pregnancy risks that may never materialize,” said the study’s lead investigator Sandra Vukusic, MD, Lyon University Hospital, France.
“The main impact of this therapeutic inertia in women is the less effective control of disease activity, leading to the accumulation of lesions and an increased risk of long-term disability. This represents a real loss of opportunity for women, especially in an era where disease-modifying treatments so effective when used early,” she added.
Dr. Gavoille said that recommendations in France allow the use of moderately active drugs, including interferon and glatiramer acetate, during pregnancy or in women planning a pregnancy. More recently there has been enough data to allow the use of natalizumab up until the second trimester.
In addition, although not in the guidelines, it is thought that the anti-CD20 monoclonal antibodies, such as rituximab or ocrelizumab, may be safe as they are very long acting. Women can be dosed before pregnancy and be covered for the whole pregnancy period without exposing the fetus to the drug, he explained.
“The message is that now we have both moderately and highly effective treatments that are compatible with a pregnancy plan,” Dr. Gavoille said.
First, clinicians have to select a level of treatment based on disease activity and then choose the best option, depending on the woman’s plans with respect to pregnancy.
Drugs that are contraindicated in pregnancy include teriflunomide and S1P modulators such as fingolimod, which have been shown to be harmful to the fetus.
“But they could still be used in women of childbearing years as long as they are not planning a pregnancy and understand the need for contraception,” Dr. Gavoille noted.
He believes both neurologists and patients are afraid of using drugs in pregnancy. “It is, of course, important to be cautious on this issue, but we should not let fear stop these women receiving the best treatments available.”
However, he added, clinical practice is changing, and confidence is gradually building around using highly effective treatments in women of childbearing age.
Dr. Gavoille also called for more research to collate data in pregnant women with MS who are exposed to various treatments, starting with case reports and then academic registries, which he described as “difficult but important work.”
Commenting on the study, Robert Hoepner, MD, University Hospital of Bern, Switzerland, agreed that this treatment disparity between men and women is “unacceptable.”
Dr. Hoepner noted that a recent study showed that women have different relapse symptoms than men, which may also affect treatment choice.
Dr. Gavoille responded that other research has shown that women are less likely to have treatment escalation post-relapse. “This could be because of a difference in symptoms. But this is something we haven’t looked at yet.”
Also commenting on the research, Frauke Zipp, MD, University Medical Center Mainz in Germany, said it would be interesting to follow this cohort over the long term to see if the women do less well several years down the line.
The study authors and commentators reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
COPENHAGEN — , even after accounting for treatment discontinuations during pregnancy and the postpartum period, new research suggested.
“We believe that pregnancy-related considerations probably still explain the major part of this gap,” said Antoine Gavoille, MD, University of Lyon, France, who presented the study at the 2024 ECTRIMS annual meeting.
This is likely due to “factors such as anticipation of pregnancy long before it occurs and fear of exposing women of childbearing age to certain treatments even in the absence of planned pregnancy,” he added.
Caution is warranted when medications are first marketed because there are no data on safety in pregnancy. However, in 2024, “this lesser treatment in women is unacceptable,” said Dr. Gavoille. “We now have several highly effective treatment options which are compatible with pregnancy,” he noted.
The researchers analyzed the French MS registry of 22,657 patients with relapsing MS (74.2% women) between 1997 and 2022 for treatment differences between women and their male counterparts. The results were adjusted for multiple factors including educational level, disease activity, disability levels, and discontinuation of drugs during pregnancy.
They found that over a median follow-up of 11.6 years, women had a significantly lower probability of receiving any disease-modifying treatment (odds ratio [OR], 0.92; 95% CI, 0.87-0.97).
In addition, women were even less likely to receive high-efficacy treatments such as natalizumab, anti-CD20 antibodies, or S1P modulators such as fingolimod (OR, 0.80; 95% CI, 0.74-0.86).
The difference in disease-modifying treatment usage varied across different treatments and over time. Teriflunomide, fingolimod, and anti-CD20 therapies were significantly underused throughout their entire availability (OR, 0.87, 0.78, and 0.80, respectively).
Interferon and natalizumab were initially used less frequently in women, but the use of these medications equalized over time.
In contrast, glatiramer acetate and dimethyl fumarate were initially used equally between genders but eventually became more commonly prescribed to women (OR, 1.27 and 1.17, respectively).
The disparity in treatment emerged after 2 years of disease duration for disease-modifying treatments in general and as early as 1 year for highly effective treatments.
The gender-based treatment gap did not significantly vary with patient age, indicating that therapeutic inertia may persist regardless of a woman’s age.
“Women may not be receiving the most effective therapies at the optimal time, often due to concerns about pregnancy risks that may never materialize,” said the study’s lead investigator Sandra Vukusic, MD, Lyon University Hospital, France.
“The main impact of this therapeutic inertia in women is the less effective control of disease activity, leading to the accumulation of lesions and an increased risk of long-term disability. This represents a real loss of opportunity for women, especially in an era where disease-modifying treatments so effective when used early,” she added.
Dr. Gavoille said that recommendations in France allow the use of moderately active drugs, including interferon and glatiramer acetate, during pregnancy or in women planning a pregnancy. More recently there has been enough data to allow the use of natalizumab up until the second trimester.
In addition, although not in the guidelines, it is thought that the anti-CD20 monoclonal antibodies, such as rituximab or ocrelizumab, may be safe as they are very long acting. Women can be dosed before pregnancy and be covered for the whole pregnancy period without exposing the fetus to the drug, he explained.
“The message is that now we have both moderately and highly effective treatments that are compatible with a pregnancy plan,” Dr. Gavoille said.
First, clinicians have to select a level of treatment based on disease activity and then choose the best option, depending on the woman’s plans with respect to pregnancy.
Drugs that are contraindicated in pregnancy include teriflunomide and S1P modulators such as fingolimod, which have been shown to be harmful to the fetus.
“But they could still be used in women of childbearing years as long as they are not planning a pregnancy and understand the need for contraception,” Dr. Gavoille noted.
He believes both neurologists and patients are afraid of using drugs in pregnancy. “It is, of course, important to be cautious on this issue, but we should not let fear stop these women receiving the best treatments available.”
However, he added, clinical practice is changing, and confidence is gradually building around using highly effective treatments in women of childbearing age.
Dr. Gavoille also called for more research to collate data in pregnant women with MS who are exposed to various treatments, starting with case reports and then academic registries, which he described as “difficult but important work.”
Commenting on the study, Robert Hoepner, MD, University Hospital of Bern, Switzerland, agreed that this treatment disparity between men and women is “unacceptable.”
Dr. Hoepner noted that a recent study showed that women have different relapse symptoms than men, which may also affect treatment choice.
Dr. Gavoille responded that other research has shown that women are less likely to have treatment escalation post-relapse. “This could be because of a difference in symptoms. But this is something we haven’t looked at yet.”
Also commenting on the research, Frauke Zipp, MD, University Medical Center Mainz in Germany, said it would be interesting to follow this cohort over the long term to see if the women do less well several years down the line.
The study authors and commentators reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ECTRIMS 2024
McDonald Criteria Update Aims to Simplify, Speed MS Diagnosis
COPENHAGEN —
Among its recommendations, the expert panel advises incorporating optic nerve imaging for diagnosis and applying stricter criteria for older patients. In addition, it proposes that radiologically isolated syndrome (RIS) may be diagnosed as MS in certain cases and that disease dissemination in time (DIT) should no longer be required.
The proposed criteria changes were presented at the 2024 ECTRIMS annual meeting.
Committee member Xavier Montalban, MD, PhD, from the Department of Neurology and the MS Centre of Catalonia at Vall d’Hebron University Hospital in Barcelona, Spain, told conference attendees that MS is a diagnosis of exclusion.
Brain and spinal cord MRI remains the most useful paraclinical test to diagnose the disease, he said, and an abnormal MRI showing typical lesions is required.
Dr. Montalban noted that optic neuritis is the first manifestation of MS in 25%-35% of cases with clinically isolated syndrome (CIS) — one of the four MS disease courses.
Therefore, he said, the panel is recommending that the optic nerve serve as the “fifth topography” or a fifth anatomical location to demonstrate dissemination in space (DIS) if there’s no better explanation for optic nerve pathology, he said.
Considerable evidence supports the minimal threshold of at least one lesion in at least two of the five topographies after including the optic nerve, he added.
DIS Alone Sufficient?
The panel also concluded that demonstrating DIS alone, without the need for DIT or positive cerebrospinal fluid (CSF), may be sufficient for an MS diagnosis. Currently, both DIS and DIT are required.
The committee broached the topic of RIS, which is identified by the incidental discovery of central nervous system (CNS) white matter T2-weighted hyperintense foci on MRI. These hyperintense foci demonstrate morphological and spatial characteristics highly typical of MS but without clinical symptomatology related to inflammatory demyelination.
Dr. Montalban noted that most patients with RIS will develop MS within 10 years. For these individuals, the panel concluded that the following criteria are sufficient for an MS diagnosis: fulfilling both DIS and DIT; fulfilling DIS and the presence of oligoclonal bands (OCBs) in the cerebrospinal fluid; or fulfilling DIS along with six or more central vein signs (CVS).
The panel proposes the addition of CVS and paramagnetic rim lesions, which are MRI markers of chronic active lesions, as optional tools for MS diagnosis in certain situations. Demonstration of CVS by MRI can increase specificity, said Dr. Montalban.
Evidence also suggests that kappa free light chains (KFLCs) could serve as a valid, simpler, and rater-independent alternative to detecting OCBs, he added. Because KFLCs are interchangeable with OCBs, they can be used in place of OCBs for diagnosing MS through CSF analysis.
Stricter Criteria
The panel is also calling for stricter criteria for confirming an MS diagnosis in those over age 50 or individuals with headache or vascular disorders. In such patients, they strongly recommend additional features such as a spinal cord lesion, positive CSF, and CVS select 6 (six positive lesions).
The panel is also recommending laboratory tests (MOG-IgG Ab) to confirm a diagnosis in children and adolescents. Dr. Montalban noted the presence of CVS in about 50% of T2 lesions strongly suggests MS in this population.
Primary progressive MS (PPMS) requires evidence of clinical progression over at least 12 months. The committee determined that the same criteria for relapsing-remitting MS could be used for PPMS.
Having a single, unified framework of diagnostic criteria will be “very useful,” said Dr. Montalban.
During the same meeting session, Marcello Moccia, MD, PhD, University College London (UCL) Queen Square Institute of Neurology, Faculty of Brain Sciences, London, England, presented examples of patients for whom the revised criteria could be beneficial.
These examples help illustrate how using the new criteria, for example optic nerve imaging, could lead to earlier diagnoses, and, in some cases, easier diagnoses, possibly with less CSF, he said. It could also lead to fewer misdiagnoses, he added, thanks to high-specificity tools.
Implementing the new criteria could offer greater flexibility and reduce complexity, Dr. Moccia concluded, adding that not every patient with suspected MS requires exhaustive testing.
The committee’s next steps will include consulting with the wider MS community and preparing the information for publication, said Dr. Montalban.
Commenting on the proposals, Bruce Bebo, executive vice president of research, National MS Society, agreed the proposed changes to the McDonald Criteria will make diagnosing MS “faster and easier.”
“Importantly, we are providing guidance that is inclusive — how to confirm diagnoses in children, or in people over the age of 50,” said Dr. Bebo. “We’re bringing the latest research and imaging technology to the forefront, to help people with MS get treatment faster, so they can live their best lives.”
Dr. Montalban’s institution has received compensation for lecture honoraria and travel expenses, participation in scientific meetings, clinical trial steering committee membership, or clinical advisory board participation in recent years from AbbVie, Actelion, Alexion, Bial PD, Biogen, Bristol Myers Squibb/Celgene, EMD Serona, Genzyme, Hoffmann-La Roche, Immunic Therapeutics, Janssen Pharmaceuticals, MedDay, Merck, Mylan, Nervgen, Neuraxpharm, Novartis, PeerVoice, Samsung-Biosys Sandoz Sanofi-Genzyme, Teva Pharmaceuticals, TG Therapeutics, EXCEMED, ECTRIMS, MSIF, and NMSS or any of their affiliates. Dr. Moccia reports receiving a salary from University of Naples, Policlinico University Hospital (Naples) and Neurology (US); research grants from MUR PNRR Extended Partnership, ECTRIMS-MAGNIMS, UK MS Society, and Merck; honoraria from AbbVie, Biogen, BMS Celgene, Ipsen, Jansen, Merck, Novartis, Roche, and Sanofi-Genzyme.
A version of this article appeared on Medscape.com.
COPENHAGEN —
Among its recommendations, the expert panel advises incorporating optic nerve imaging for diagnosis and applying stricter criteria for older patients. In addition, it proposes that radiologically isolated syndrome (RIS) may be diagnosed as MS in certain cases and that disease dissemination in time (DIT) should no longer be required.
The proposed criteria changes were presented at the 2024 ECTRIMS annual meeting.
Committee member Xavier Montalban, MD, PhD, from the Department of Neurology and the MS Centre of Catalonia at Vall d’Hebron University Hospital in Barcelona, Spain, told conference attendees that MS is a diagnosis of exclusion.
Brain and spinal cord MRI remains the most useful paraclinical test to diagnose the disease, he said, and an abnormal MRI showing typical lesions is required.
Dr. Montalban noted that optic neuritis is the first manifestation of MS in 25%-35% of cases with clinically isolated syndrome (CIS) — one of the four MS disease courses.
Therefore, he said, the panel is recommending that the optic nerve serve as the “fifth topography” or a fifth anatomical location to demonstrate dissemination in space (DIS) if there’s no better explanation for optic nerve pathology, he said.
Considerable evidence supports the minimal threshold of at least one lesion in at least two of the five topographies after including the optic nerve, he added.
DIS Alone Sufficient?
The panel also concluded that demonstrating DIS alone, without the need for DIT or positive cerebrospinal fluid (CSF), may be sufficient for an MS diagnosis. Currently, both DIS and DIT are required.
The committee broached the topic of RIS, which is identified by the incidental discovery of central nervous system (CNS) white matter T2-weighted hyperintense foci on MRI. These hyperintense foci demonstrate morphological and spatial characteristics highly typical of MS but without clinical symptomatology related to inflammatory demyelination.
Dr. Montalban noted that most patients with RIS will develop MS within 10 years. For these individuals, the panel concluded that the following criteria are sufficient for an MS diagnosis: fulfilling both DIS and DIT; fulfilling DIS and the presence of oligoclonal bands (OCBs) in the cerebrospinal fluid; or fulfilling DIS along with six or more central vein signs (CVS).
The panel proposes the addition of CVS and paramagnetic rim lesions, which are MRI markers of chronic active lesions, as optional tools for MS diagnosis in certain situations. Demonstration of CVS by MRI can increase specificity, said Dr. Montalban.
Evidence also suggests that kappa free light chains (KFLCs) could serve as a valid, simpler, and rater-independent alternative to detecting OCBs, he added. Because KFLCs are interchangeable with OCBs, they can be used in place of OCBs for diagnosing MS through CSF analysis.
Stricter Criteria
The panel is also calling for stricter criteria for confirming an MS diagnosis in those over age 50 or individuals with headache or vascular disorders. In such patients, they strongly recommend additional features such as a spinal cord lesion, positive CSF, and CVS select 6 (six positive lesions).
The panel is also recommending laboratory tests (MOG-IgG Ab) to confirm a diagnosis in children and adolescents. Dr. Montalban noted the presence of CVS in about 50% of T2 lesions strongly suggests MS in this population.
Primary progressive MS (PPMS) requires evidence of clinical progression over at least 12 months. The committee determined that the same criteria for relapsing-remitting MS could be used for PPMS.
Having a single, unified framework of diagnostic criteria will be “very useful,” said Dr. Montalban.
During the same meeting session, Marcello Moccia, MD, PhD, University College London (UCL) Queen Square Institute of Neurology, Faculty of Brain Sciences, London, England, presented examples of patients for whom the revised criteria could be beneficial.
These examples help illustrate how using the new criteria, for example optic nerve imaging, could lead to earlier diagnoses, and, in some cases, easier diagnoses, possibly with less CSF, he said. It could also lead to fewer misdiagnoses, he added, thanks to high-specificity tools.
Implementing the new criteria could offer greater flexibility and reduce complexity, Dr. Moccia concluded, adding that not every patient with suspected MS requires exhaustive testing.
The committee’s next steps will include consulting with the wider MS community and preparing the information for publication, said Dr. Montalban.
Commenting on the proposals, Bruce Bebo, executive vice president of research, National MS Society, agreed the proposed changes to the McDonald Criteria will make diagnosing MS “faster and easier.”
“Importantly, we are providing guidance that is inclusive — how to confirm diagnoses in children, or in people over the age of 50,” said Dr. Bebo. “We’re bringing the latest research and imaging technology to the forefront, to help people with MS get treatment faster, so they can live their best lives.”
Dr. Montalban’s institution has received compensation for lecture honoraria and travel expenses, participation in scientific meetings, clinical trial steering committee membership, or clinical advisory board participation in recent years from AbbVie, Actelion, Alexion, Bial PD, Biogen, Bristol Myers Squibb/Celgene, EMD Serona, Genzyme, Hoffmann-La Roche, Immunic Therapeutics, Janssen Pharmaceuticals, MedDay, Merck, Mylan, Nervgen, Neuraxpharm, Novartis, PeerVoice, Samsung-Biosys Sandoz Sanofi-Genzyme, Teva Pharmaceuticals, TG Therapeutics, EXCEMED, ECTRIMS, MSIF, and NMSS or any of their affiliates. Dr. Moccia reports receiving a salary from University of Naples, Policlinico University Hospital (Naples) and Neurology (US); research grants from MUR PNRR Extended Partnership, ECTRIMS-MAGNIMS, UK MS Society, and Merck; honoraria from AbbVie, Biogen, BMS Celgene, Ipsen, Jansen, Merck, Novartis, Roche, and Sanofi-Genzyme.
A version of this article appeared on Medscape.com.
COPENHAGEN —
Among its recommendations, the expert panel advises incorporating optic nerve imaging for diagnosis and applying stricter criteria for older patients. In addition, it proposes that radiologically isolated syndrome (RIS) may be diagnosed as MS in certain cases and that disease dissemination in time (DIT) should no longer be required.
The proposed criteria changes were presented at the 2024 ECTRIMS annual meeting.
Committee member Xavier Montalban, MD, PhD, from the Department of Neurology and the MS Centre of Catalonia at Vall d’Hebron University Hospital in Barcelona, Spain, told conference attendees that MS is a diagnosis of exclusion.
Brain and spinal cord MRI remains the most useful paraclinical test to diagnose the disease, he said, and an abnormal MRI showing typical lesions is required.
Dr. Montalban noted that optic neuritis is the first manifestation of MS in 25%-35% of cases with clinically isolated syndrome (CIS) — one of the four MS disease courses.
Therefore, he said, the panel is recommending that the optic nerve serve as the “fifth topography” or a fifth anatomical location to demonstrate dissemination in space (DIS) if there’s no better explanation for optic nerve pathology, he said.
Considerable evidence supports the minimal threshold of at least one lesion in at least two of the five topographies after including the optic nerve, he added.
DIS Alone Sufficient?
The panel also concluded that demonstrating DIS alone, without the need for DIT or positive cerebrospinal fluid (CSF), may be sufficient for an MS diagnosis. Currently, both DIS and DIT are required.
The committee broached the topic of RIS, which is identified by the incidental discovery of central nervous system (CNS) white matter T2-weighted hyperintense foci on MRI. These hyperintense foci demonstrate morphological and spatial characteristics highly typical of MS but without clinical symptomatology related to inflammatory demyelination.
Dr. Montalban noted that most patients with RIS will develop MS within 10 years. For these individuals, the panel concluded that the following criteria are sufficient for an MS diagnosis: fulfilling both DIS and DIT; fulfilling DIS and the presence of oligoclonal bands (OCBs) in the cerebrospinal fluid; or fulfilling DIS along with six or more central vein signs (CVS).
The panel proposes the addition of CVS and paramagnetic rim lesions, which are MRI markers of chronic active lesions, as optional tools for MS diagnosis in certain situations. Demonstration of CVS by MRI can increase specificity, said Dr. Montalban.
Evidence also suggests that kappa free light chains (KFLCs) could serve as a valid, simpler, and rater-independent alternative to detecting OCBs, he added. Because KFLCs are interchangeable with OCBs, they can be used in place of OCBs for diagnosing MS through CSF analysis.
Stricter Criteria
The panel is also calling for stricter criteria for confirming an MS diagnosis in those over age 50 or individuals with headache or vascular disorders. In such patients, they strongly recommend additional features such as a spinal cord lesion, positive CSF, and CVS select 6 (six positive lesions).
The panel is also recommending laboratory tests (MOG-IgG Ab) to confirm a diagnosis in children and adolescents. Dr. Montalban noted the presence of CVS in about 50% of T2 lesions strongly suggests MS in this population.
Primary progressive MS (PPMS) requires evidence of clinical progression over at least 12 months. The committee determined that the same criteria for relapsing-remitting MS could be used for PPMS.
Having a single, unified framework of diagnostic criteria will be “very useful,” said Dr. Montalban.
During the same meeting session, Marcello Moccia, MD, PhD, University College London (UCL) Queen Square Institute of Neurology, Faculty of Brain Sciences, London, England, presented examples of patients for whom the revised criteria could be beneficial.
These examples help illustrate how using the new criteria, for example optic nerve imaging, could lead to earlier diagnoses, and, in some cases, easier diagnoses, possibly with less CSF, he said. It could also lead to fewer misdiagnoses, he added, thanks to high-specificity tools.
Implementing the new criteria could offer greater flexibility and reduce complexity, Dr. Moccia concluded, adding that not every patient with suspected MS requires exhaustive testing.
The committee’s next steps will include consulting with the wider MS community and preparing the information for publication, said Dr. Montalban.
Commenting on the proposals, Bruce Bebo, executive vice president of research, National MS Society, agreed the proposed changes to the McDonald Criteria will make diagnosing MS “faster and easier.”
“Importantly, we are providing guidance that is inclusive — how to confirm diagnoses in children, or in people over the age of 50,” said Dr. Bebo. “We’re bringing the latest research and imaging technology to the forefront, to help people with MS get treatment faster, so they can live their best lives.”
Dr. Montalban’s institution has received compensation for lecture honoraria and travel expenses, participation in scientific meetings, clinical trial steering committee membership, or clinical advisory board participation in recent years from AbbVie, Actelion, Alexion, Bial PD, Biogen, Bristol Myers Squibb/Celgene, EMD Serona, Genzyme, Hoffmann-La Roche, Immunic Therapeutics, Janssen Pharmaceuticals, MedDay, Merck, Mylan, Nervgen, Neuraxpharm, Novartis, PeerVoice, Samsung-Biosys Sandoz Sanofi-Genzyme, Teva Pharmaceuticals, TG Therapeutics, EXCEMED, ECTRIMS, MSIF, and NMSS or any of their affiliates. Dr. Moccia reports receiving a salary from University of Naples, Policlinico University Hospital (Naples) and Neurology (US); research grants from MUR PNRR Extended Partnership, ECTRIMS-MAGNIMS, UK MS Society, and Merck; honoraria from AbbVie, Biogen, BMS Celgene, Ipsen, Jansen, Merck, Novartis, Roche, and Sanofi-Genzyme.
A version of this article appeared on Medscape.com.
FROM ECTRIMS 2024