User login
The who are ineligible for autologous stem cell transplant (ASCT).
The drug is approved in combination with a standard VMP regimen – bortezomib (Velcade), melphalan, and prednisone. The FDA had granted priority review to the drug application in January 2018 based on the results of the phase 3 ALCYONE study (NCT02195479).
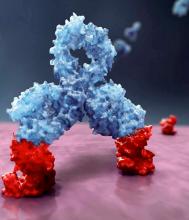
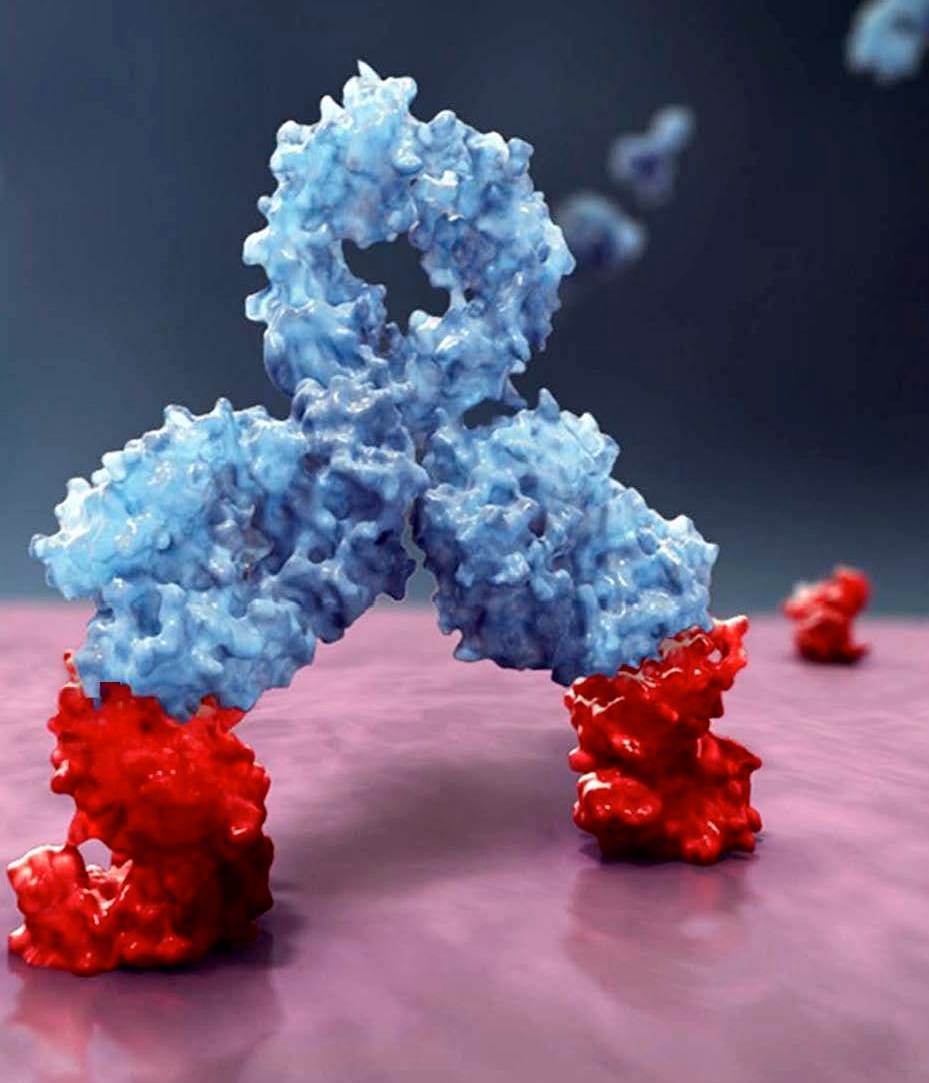
Daratumumab, an anti-CD38 monoclonal antibody, reduced the risk of disease progression or death by 50%, compared with VMP alone in the ALCYONE study. The median progression-free survival had not yet been reached in the daratumumab arm; the median progression-free survival was 18.1 months in the VMP-only arm (N Engl J Med. 2018;378:518-28).
Daratumumab is marketed by Janssen Biotech as Darzalex.
The who are ineligible for autologous stem cell transplant (ASCT).
The drug is approved in combination with a standard VMP regimen – bortezomib (Velcade), melphalan, and prednisone. The FDA had granted priority review to the drug application in January 2018 based on the results of the phase 3 ALCYONE study (NCT02195479).
Daratumumab, an anti-CD38 monoclonal antibody, reduced the risk of disease progression or death by 50%, compared with VMP alone in the ALCYONE study. The median progression-free survival had not yet been reached in the daratumumab arm; the median progression-free survival was 18.1 months in the VMP-only arm (N Engl J Med. 2018;378:518-28).
Daratumumab is marketed by Janssen Biotech as Darzalex.
The who are ineligible for autologous stem cell transplant (ASCT).
The drug is approved in combination with a standard VMP regimen – bortezomib (Velcade), melphalan, and prednisone. The FDA had granted priority review to the drug application in January 2018 based on the results of the phase 3 ALCYONE study (NCT02195479).
Daratumumab, an anti-CD38 monoclonal antibody, reduced the risk of disease progression or death by 50%, compared with VMP alone in the ALCYONE study. The median progression-free survival had not yet been reached in the daratumumab arm; the median progression-free survival was 18.1 months in the VMP-only arm (N Engl J Med. 2018;378:518-28).
Daratumumab is marketed by Janssen Biotech as Darzalex.