User login
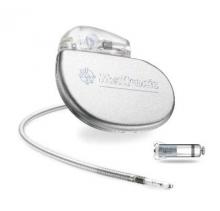
The Food and Drug Administration has approved the first leadless pacemaker, the Micra transcatheter pacing system, the FDA stated in a release accompanying its approval.
The Micra pacemaker eliminates the need for wired leads and the risk of associated complications. The single-chamber ventricular pacemaker is 93% smaller than traditional pacemakers, according to a summary document submitted to the FDA by Medtronic, which makes the device. Like other ventricular pacemakers, Micra provides rate-adaptive pacing, with automated pacing capture threshold management to maximize battery life, which the company estimates at about 10 years.
The pacemaker is inserted directly into the right ventricle through the femoral vein by means of a steerable catheter. Pressing a button on the distal end of the catheter releases four flexible, electrically inactive nitinol tines that hook into the myocardium to secure the device. Engagement by two tines exerts 15 times the amount of force needed to secure the device in place, according to Medtronic.
The device’s approval was based on a pivotal prospective, nonrandomized uncontrolled study of 719 patients at 56 investigational sites in North America, Europe, Asia, Australia, and Africa. The primary efficacy endpoint, low and stable pacing capture thresholds at 6 months (up to 2.0 V at a pulse width of 0.24 milliseconds and an increase of up to 1.5 V from the time of implantation) was achieved for 98% of patients (95% confidence interval, 96%-99.5%), reported Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center in Oklahoma City and his associates (N Engl J Med. 2016 Feb. 11. doi: 10.1056/NEJMoa1511643).
The researchers also compared safety outcomes among Micra recipients and 2,667 historical controls from six previously published studies. The Micra pacemaker was associated with significantly lower hospitalization and system revision rates, with “no systemic infections, no pneumothoraxes, and no radiographically visible dislodgements or device emboli,” they said. In all, 4% of Micra recipients had complications leading to death or requiring invasive revision, treatment cessation, or hospitalization, which resembled recent reports of transvenous systems and was significantly lower than the rate for historical controls, according to the investigators. However, the rate of cardiac perforation or effusion was 1.6%, slightly higher than the rate of 1.1% for historical controls. Other major complications included cardiac failure (0.9% of study patients), atrioventricular fistula or pseudoaneurysm at the groin puncture site (0.7%), and deep vein thrombosis or pulmonary thromboembolism (0.3%).
Medtronic funded the pivotal study on which approval of the Micra pacemaker was based. Dr. Reynolds had no relevant financial disclosures. Several coinvestigators reported financial relationships with Medtronic and several other cardiac device manufacturers.
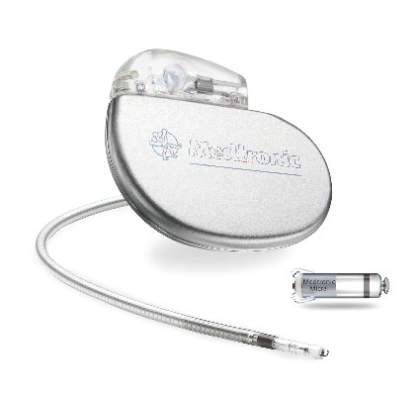
The Food and Drug Administration has approved the first leadless pacemaker, the Micra transcatheter pacing system, the FDA stated in a release accompanying its approval.
The Micra pacemaker eliminates the need for wired leads and the risk of associated complications. The single-chamber ventricular pacemaker is 93% smaller than traditional pacemakers, according to a summary document submitted to the FDA by Medtronic, which makes the device. Like other ventricular pacemakers, Micra provides rate-adaptive pacing, with automated pacing capture threshold management to maximize battery life, which the company estimates at about 10 years.
The pacemaker is inserted directly into the right ventricle through the femoral vein by means of a steerable catheter. Pressing a button on the distal end of the catheter releases four flexible, electrically inactive nitinol tines that hook into the myocardium to secure the device. Engagement by two tines exerts 15 times the amount of force needed to secure the device in place, according to Medtronic.
The device’s approval was based on a pivotal prospective, nonrandomized uncontrolled study of 719 patients at 56 investigational sites in North America, Europe, Asia, Australia, and Africa. The primary efficacy endpoint, low and stable pacing capture thresholds at 6 months (up to 2.0 V at a pulse width of 0.24 milliseconds and an increase of up to 1.5 V from the time of implantation) was achieved for 98% of patients (95% confidence interval, 96%-99.5%), reported Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center in Oklahoma City and his associates (N Engl J Med. 2016 Feb. 11. doi: 10.1056/NEJMoa1511643).
The researchers also compared safety outcomes among Micra recipients and 2,667 historical controls from six previously published studies. The Micra pacemaker was associated with significantly lower hospitalization and system revision rates, with “no systemic infections, no pneumothoraxes, and no radiographically visible dislodgements or device emboli,” they said. In all, 4% of Micra recipients had complications leading to death or requiring invasive revision, treatment cessation, or hospitalization, which resembled recent reports of transvenous systems and was significantly lower than the rate for historical controls, according to the investigators. However, the rate of cardiac perforation or effusion was 1.6%, slightly higher than the rate of 1.1% for historical controls. Other major complications included cardiac failure (0.9% of study patients), atrioventricular fistula or pseudoaneurysm at the groin puncture site (0.7%), and deep vein thrombosis or pulmonary thromboembolism (0.3%).
Medtronic funded the pivotal study on which approval of the Micra pacemaker was based. Dr. Reynolds had no relevant financial disclosures. Several coinvestigators reported financial relationships with Medtronic and several other cardiac device manufacturers.
The Food and Drug Administration has approved the first leadless pacemaker, the Micra transcatheter pacing system, the FDA stated in a release accompanying its approval.
The Micra pacemaker eliminates the need for wired leads and the risk of associated complications. The single-chamber ventricular pacemaker is 93% smaller than traditional pacemakers, according to a summary document submitted to the FDA by Medtronic, which makes the device. Like other ventricular pacemakers, Micra provides rate-adaptive pacing, with automated pacing capture threshold management to maximize battery life, which the company estimates at about 10 years.
The pacemaker is inserted directly into the right ventricle through the femoral vein by means of a steerable catheter. Pressing a button on the distal end of the catheter releases four flexible, electrically inactive nitinol tines that hook into the myocardium to secure the device. Engagement by two tines exerts 15 times the amount of force needed to secure the device in place, according to Medtronic.
The device’s approval was based on a pivotal prospective, nonrandomized uncontrolled study of 719 patients at 56 investigational sites in North America, Europe, Asia, Australia, and Africa. The primary efficacy endpoint, low and stable pacing capture thresholds at 6 months (up to 2.0 V at a pulse width of 0.24 milliseconds and an increase of up to 1.5 V from the time of implantation) was achieved for 98% of patients (95% confidence interval, 96%-99.5%), reported Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center in Oklahoma City and his associates (N Engl J Med. 2016 Feb. 11. doi: 10.1056/NEJMoa1511643).
The researchers also compared safety outcomes among Micra recipients and 2,667 historical controls from six previously published studies. The Micra pacemaker was associated with significantly lower hospitalization and system revision rates, with “no systemic infections, no pneumothoraxes, and no radiographically visible dislodgements or device emboli,” they said. In all, 4% of Micra recipients had complications leading to death or requiring invasive revision, treatment cessation, or hospitalization, which resembled recent reports of transvenous systems and was significantly lower than the rate for historical controls, according to the investigators. However, the rate of cardiac perforation or effusion was 1.6%, slightly higher than the rate of 1.1% for historical controls. Other major complications included cardiac failure (0.9% of study patients), atrioventricular fistula or pseudoaneurysm at the groin puncture site (0.7%), and deep vein thrombosis or pulmonary thromboembolism (0.3%).
Medtronic funded the pivotal study on which approval of the Micra pacemaker was based. Dr. Reynolds had no relevant financial disclosures. Several coinvestigators reported financial relationships with Medtronic and several other cardiac device manufacturers.