User login
The US Food and Drug Administration (FDA) has approved an intravenous (IV) formulation of rolapitant (VARUBI®) for the same indication as the oral formulation.
This means IV rolapitant is now FDA-approved for use in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with initial and repeat courses of emetogenic chemotherapy in adults with cancer.
TESARO Inc., makers of rolapitant, said the US commercial launch of IV rolapitant is planned for November.
Rolapitant is a selective and competitive antagonist of human substance P/neurokinin 1 receptors, which play an important role in the delayed phase of chemotherapy-induced nausea and vomiting (CINV).
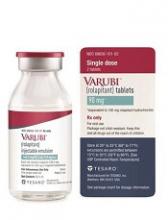
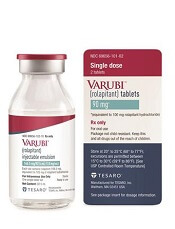
IV rolapitant features a ready-to-use, single-dose vial for administration. It does not require refrigerated storage or mixing.
The recommended dose of IV rolapitant is 166.5 mg, administered over 30 minutes. The drug is to be administered up to 2 hours before chemotherapy administration in combination with a 5-HT3 receptor antagonist and dexamethasone.
The full prescribing information for IV rolapitant is available at www.varubirx.com.
Bioequivalence trial
Results from a bioequivalence trial suggest the IV and oral formulations of rolapitant are comparable.
The study was conducted in healthy volunteers. Subjects were randomized to receive a single dose of IV rolapitant at 166.5 mg administered over 30 minutes (n=61) or oral rolapitant at 180 mg (n=62).
The primary endpoint was bioequivalence, and the 166.5 mg IV infusion of rolapitant met bioequivalence criteria.
Researchers said the safety profile of IV rolapitant was largely consistent with that of oral rolapitant, although infusion-site reactions were observed with the IV formulation. These included the sensation of warmth, abdominal pain, dizziness, and paresthesia.
These results were recently published in The Journal of Clinical Pharmacology.
Oral rolapitant trials
Two phase 3 trials showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after highly emetogenic chemotherapy.
Results from these trials (NCT01499849 and NCT01500213) were published in a single article in The Lancet Oncology.
A third phase 3 trial showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after moderately emetogenic chemotherapy.
Results from this trial (NCT01500226) were also published in The Lancet Oncology. ![]()
The US Food and Drug Administration (FDA) has approved an intravenous (IV) formulation of rolapitant (VARUBI®) for the same indication as the oral formulation.
This means IV rolapitant is now FDA-approved for use in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with initial and repeat courses of emetogenic chemotherapy in adults with cancer.
TESARO Inc., makers of rolapitant, said the US commercial launch of IV rolapitant is planned for November.
Rolapitant is a selective and competitive antagonist of human substance P/neurokinin 1 receptors, which play an important role in the delayed phase of chemotherapy-induced nausea and vomiting (CINV).
IV rolapitant features a ready-to-use, single-dose vial for administration. It does not require refrigerated storage or mixing.
The recommended dose of IV rolapitant is 166.5 mg, administered over 30 minutes. The drug is to be administered up to 2 hours before chemotherapy administration in combination with a 5-HT3 receptor antagonist and dexamethasone.
The full prescribing information for IV rolapitant is available at www.varubirx.com.
Bioequivalence trial
Results from a bioequivalence trial suggest the IV and oral formulations of rolapitant are comparable.
The study was conducted in healthy volunteers. Subjects were randomized to receive a single dose of IV rolapitant at 166.5 mg administered over 30 minutes (n=61) or oral rolapitant at 180 mg (n=62).
The primary endpoint was bioequivalence, and the 166.5 mg IV infusion of rolapitant met bioequivalence criteria.
Researchers said the safety profile of IV rolapitant was largely consistent with that of oral rolapitant, although infusion-site reactions were observed with the IV formulation. These included the sensation of warmth, abdominal pain, dizziness, and paresthesia.
These results were recently published in The Journal of Clinical Pharmacology.
Oral rolapitant trials
Two phase 3 trials showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after highly emetogenic chemotherapy.
Results from these trials (NCT01499849 and NCT01500213) were published in a single article in The Lancet Oncology.
A third phase 3 trial showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after moderately emetogenic chemotherapy.
Results from this trial (NCT01500226) were also published in The Lancet Oncology. ![]()
The US Food and Drug Administration (FDA) has approved an intravenous (IV) formulation of rolapitant (VARUBI®) for the same indication as the oral formulation.
This means IV rolapitant is now FDA-approved for use in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with initial and repeat courses of emetogenic chemotherapy in adults with cancer.
TESARO Inc., makers of rolapitant, said the US commercial launch of IV rolapitant is planned for November.
Rolapitant is a selective and competitive antagonist of human substance P/neurokinin 1 receptors, which play an important role in the delayed phase of chemotherapy-induced nausea and vomiting (CINV).
IV rolapitant features a ready-to-use, single-dose vial for administration. It does not require refrigerated storage or mixing.
The recommended dose of IV rolapitant is 166.5 mg, administered over 30 minutes. The drug is to be administered up to 2 hours before chemotherapy administration in combination with a 5-HT3 receptor antagonist and dexamethasone.
The full prescribing information for IV rolapitant is available at www.varubirx.com.
Bioequivalence trial
Results from a bioequivalence trial suggest the IV and oral formulations of rolapitant are comparable.
The study was conducted in healthy volunteers. Subjects were randomized to receive a single dose of IV rolapitant at 166.5 mg administered over 30 minutes (n=61) or oral rolapitant at 180 mg (n=62).
The primary endpoint was bioequivalence, and the 166.5 mg IV infusion of rolapitant met bioequivalence criteria.
Researchers said the safety profile of IV rolapitant was largely consistent with that of oral rolapitant, although infusion-site reactions were observed with the IV formulation. These included the sensation of warmth, abdominal pain, dizziness, and paresthesia.
These results were recently published in The Journal of Clinical Pharmacology.
Oral rolapitant trials
Two phase 3 trials showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after highly emetogenic chemotherapy.
Results from these trials (NCT01499849 and NCT01500213) were published in a single article in The Lancet Oncology.
A third phase 3 trial showed that oral rolapitant, in combination with a 5-HT3 receptor antagonist and dexamethasone, was well tolerated and more effective than active control in preventing CINV after moderately emetogenic chemotherapy.
Results from this trial (NCT01500226) were also published in The Lancet Oncology. ![]()