User login
The Food and Drug Administration has deemed MelaFind, a noninvasive melanoma detection device, approvable, according to the manufacturer.
The FDA still has a list of requirements the manufacturer, Mela Sciences, must fulfill before MelaFind is given final approval. But company CEO, Joseph V. Gulfo, M.D., said in a conference call with analysts that much of the work is already done and that he expects no significant delays to market.
The road to approval has been somewhat rocky. In November 2010, an FDA advisory panel split 8-7, with one abstention over whether MelaFind should be approved.
Then in May 2011, the company submitted a Citizen Petition to the FDA seeking action on its application for approval.
The company will have to work with the FDA on physician and patient labeling, a package insert and user’s guide, a training program for dermatologists, and the protocol for a postmarketing study.
Dr. Gulfo said that he expects MelaFind to be commercially available in the spring of 2012. Initially, it will only be sold to a handful of dermatologists in Connecticut, New Jersey, and New York. Mela Sciences will be working with dermatologists to fine tune the device before rolling it out to a larger number of practices, he said.
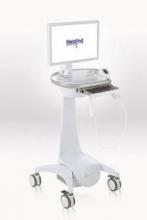
MelaFind is a multispectral computer vision system with a handheld imager that captures the image of a lesion; software uses algorithms to analyze the image, indicating within 2 minutes whether a biopsy should be done.
At the FDA panel meeting in November, some advisers expressed concern that MelaFind might be used by nondermatologists or that it would be used in place of clinical evaluation.
Mela Sciences and the FDA worked on clarifying the indications for use. Those indications now cover multiple paragraphs. According to a Mela Sciences press release, the device "is intended for use on clinically atypical cutaneous pigmented lesions with one or more clinical or historical characteristics of melanoma, excluding those with a clinical diagnosis of melanoma or likely melanoma."
Labeling will also state that the device is "only for use by physicians trained in the clinical diagnosis and management of skin cancer (i.e., dermatologists) who have also successfully completed a training program in the appropriate use of MelaFind."
Dr. Darrell S. Rigel, a consultant for Mela Sciences, said in the company statement that the device can help dermatologists decide which lesions to biopsy and will be an aid to clinical evaluation. "While there have been incremental improvements in imaging tools for melanoma detection, we still primarily rely on our judgment based on a visual examination to select the lesions to biopsy; data show that this is often not enough," said Dr. Rigel, a clinical professor of dermatology at New York University.
The pivotal study of the device included 1,383 patients. According to the company, MelaFind had a sensitivity of 98%. The device’s sensitivity rating for malignant melanoma was significantly better than that of dermatologists, who showed a wide range of variability about which lesions would have been recommended for biopsy and which relegated to observation.
MelaFind will also soon be available in Germany, which has some of the highest rates of melanoma in Europe, said Dr. Gulfo. The device received European Union approval in September. The company aims to have about 75 systems in Germany by next September, Dr. Gulfo said.
The Food and Drug Administration has deemed MelaFind, a noninvasive melanoma detection device, approvable, according to the manufacturer.
The FDA still has a list of requirements the manufacturer, Mela Sciences, must fulfill before MelaFind is given final approval. But company CEO, Joseph V. Gulfo, M.D., said in a conference call with analysts that much of the work is already done and that he expects no significant delays to market.
The road to approval has been somewhat rocky. In November 2010, an FDA advisory panel split 8-7, with one abstention over whether MelaFind should be approved.
Then in May 2011, the company submitted a Citizen Petition to the FDA seeking action on its application for approval.
The company will have to work with the FDA on physician and patient labeling, a package insert and user’s guide, a training program for dermatologists, and the protocol for a postmarketing study.
Dr. Gulfo said that he expects MelaFind to be commercially available in the spring of 2012. Initially, it will only be sold to a handful of dermatologists in Connecticut, New Jersey, and New York. Mela Sciences will be working with dermatologists to fine tune the device before rolling it out to a larger number of practices, he said.
MelaFind is a multispectral computer vision system with a handheld imager that captures the image of a lesion; software uses algorithms to analyze the image, indicating within 2 minutes whether a biopsy should be done.
At the FDA panel meeting in November, some advisers expressed concern that MelaFind might be used by nondermatologists or that it would be used in place of clinical evaluation.
Mela Sciences and the FDA worked on clarifying the indications for use. Those indications now cover multiple paragraphs. According to a Mela Sciences press release, the device "is intended for use on clinically atypical cutaneous pigmented lesions with one or more clinical or historical characteristics of melanoma, excluding those with a clinical diagnosis of melanoma or likely melanoma."
Labeling will also state that the device is "only for use by physicians trained in the clinical diagnosis and management of skin cancer (i.e., dermatologists) who have also successfully completed a training program in the appropriate use of MelaFind."
Dr. Darrell S. Rigel, a consultant for Mela Sciences, said in the company statement that the device can help dermatologists decide which lesions to biopsy and will be an aid to clinical evaluation. "While there have been incremental improvements in imaging tools for melanoma detection, we still primarily rely on our judgment based on a visual examination to select the lesions to biopsy; data show that this is often not enough," said Dr. Rigel, a clinical professor of dermatology at New York University.
The pivotal study of the device included 1,383 patients. According to the company, MelaFind had a sensitivity of 98%. The device’s sensitivity rating for malignant melanoma was significantly better than that of dermatologists, who showed a wide range of variability about which lesions would have been recommended for biopsy and which relegated to observation.
MelaFind will also soon be available in Germany, which has some of the highest rates of melanoma in Europe, said Dr. Gulfo. The device received European Union approval in September. The company aims to have about 75 systems in Germany by next September, Dr. Gulfo said.
The Food and Drug Administration has deemed MelaFind, a noninvasive melanoma detection device, approvable, according to the manufacturer.
The FDA still has a list of requirements the manufacturer, Mela Sciences, must fulfill before MelaFind is given final approval. But company CEO, Joseph V. Gulfo, M.D., said in a conference call with analysts that much of the work is already done and that he expects no significant delays to market.
The road to approval has been somewhat rocky. In November 2010, an FDA advisory panel split 8-7, with one abstention over whether MelaFind should be approved.
Then in May 2011, the company submitted a Citizen Petition to the FDA seeking action on its application for approval.
The company will have to work with the FDA on physician and patient labeling, a package insert and user’s guide, a training program for dermatologists, and the protocol for a postmarketing study.
Dr. Gulfo said that he expects MelaFind to be commercially available in the spring of 2012. Initially, it will only be sold to a handful of dermatologists in Connecticut, New Jersey, and New York. Mela Sciences will be working with dermatologists to fine tune the device before rolling it out to a larger number of practices, he said.
MelaFind is a multispectral computer vision system with a handheld imager that captures the image of a lesion; software uses algorithms to analyze the image, indicating within 2 minutes whether a biopsy should be done.
At the FDA panel meeting in November, some advisers expressed concern that MelaFind might be used by nondermatologists or that it would be used in place of clinical evaluation.
Mela Sciences and the FDA worked on clarifying the indications for use. Those indications now cover multiple paragraphs. According to a Mela Sciences press release, the device "is intended for use on clinically atypical cutaneous pigmented lesions with one or more clinical or historical characteristics of melanoma, excluding those with a clinical diagnosis of melanoma or likely melanoma."
Labeling will also state that the device is "only for use by physicians trained in the clinical diagnosis and management of skin cancer (i.e., dermatologists) who have also successfully completed a training program in the appropriate use of MelaFind."
Dr. Darrell S. Rigel, a consultant for Mela Sciences, said in the company statement that the device can help dermatologists decide which lesions to biopsy and will be an aid to clinical evaluation. "While there have been incremental improvements in imaging tools for melanoma detection, we still primarily rely on our judgment based on a visual examination to select the lesions to biopsy; data show that this is often not enough," said Dr. Rigel, a clinical professor of dermatology at New York University.
The pivotal study of the device included 1,383 patients. According to the company, MelaFind had a sensitivity of 98%. The device’s sensitivity rating for malignant melanoma was significantly better than that of dermatologists, who showed a wide range of variability about which lesions would have been recommended for biopsy and which relegated to observation.
MelaFind will also soon be available in Germany, which has some of the highest rates of melanoma in Europe, said Dr. Gulfo. The device received European Union approval in September. The company aims to have about 75 systems in Germany by next September, Dr. Gulfo said.