User login
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
The veteran population, with its unique and diverse types of exposure and military service experiences, faces distinct health factors compared with the general population. These factors can be categorized into exposures during military service and those occurring postservice. While the latter phase incorporates psychological issues that may arise while transitioning to civilian life, the service period is associated with major physical, chemical, and psychological exposures that can impact veterans’ health. Carcinogenesis related to military exposures is concerning, and different types of malignancies have been associated with military exposures.1 The 2022 introduction of the Cancer Moonshot initiative served as a breeding ground for multiple projects aimed at investigation of exposure-related carcinogenesis, prompting increased attention and efforts to linking specific exposures to specific malignancies.2
Melanoma is the deadliest skin cancer, accounting for 1.3% of all cancer deaths.3 Although it may only account for 1% to 5% of skin cancer diagnoses, its incidence in the United States’ population has been increasing.4,5 There were 97,610 estimated new cases of melanoma in 2023, according to the National Cancer Institute.6
The incidence of melanoma may be higher in the military population compared with the general population.7 Melanoma is the fourth-most common cancer diagnosed in veterans.8
Several demographic characteristics of the US military population are associated with higher melanoma incidence and poorer prognosis, including male sex, older age, and White race. Apart from sun exposure—a known risk factor for melanoma development—other factors, such as service branch, seem to contribute to risk, with the highest melanoma rates noted in the Air Force.9 According to a study by Chang et al, veterans have a higher risk of stage III (18%) or stage IV (13%) melanoma at initial diagnosis.8
Molecular testing of metastatic melanoma is currently the standard of care for guiding the use of US Food and Drug Administration-approved targeted therapies such as BRAF, MEK, and KIT inhibitors. This comparative analysis details the melanoma comprehensive genomic profiles observed at a large US Department of Veterans Affairs (VA) medical center (VAMC) and those reported in reference databases.
Methods
A query to select all metastatic melanomas sent for comprehensive genomic profiling from the Kansas City VAMC (KCVAMC), identified 35 cases from 2019 through 2023 as the study population. The health records of these patients were reviewed to collect demographic information, military service history, melanoma history, other medical, social, and family histories. The comprehensive genomic profiling reports were reviewed to collect the reported pathogenic variants, microsatellite instability (MSI) status, and tumor mutational burden (TMB) for each case.
The Catalogue of Somatic Mutations in Cancer (COSMIC) was used to identify the most commonly mutated genes in melanomas from The Cancer Genome Atlas for the general population.4,5 The literature was consulted to determine the MSI status and TMB in melanomas from The Cancer Genome Atlas for separate reference populations.6,7 The frequency of MSI-high (MSI-H) status, TMB ≥ 10 mutations/megabase (mut/Mb), and mutations in each of the 20 most commonly mutated genes was determined and compared between melanomas from The Cancer Genome Atlas and KCVAMC cases. Corresponding P values were calculated to identify significant differences. Values were calculated for the entire sample as well as a subgroup with Agent Orange (AO) exposure. The study was approved by the KCVAMC Institutional Review Board.
Results
The mean (SD) age of study participants was 72.9 (9.4) years (range, 39-90 years). The mean (SD) duration of military service was 1654 (1421) days (about 4 years, 6 months, and 10 days). Of the 35 patients included, 22 (63%) served during the Vietnam era (November 1, 1965, to April 30, 1975) and 2 (6%) served during the Persian Gulf War era (August 2, 1990, to February 28, 1991). Seventeen veterans (49%) served in the Army, 9 in the Navy (26%), 5 in the Air Force (14%), and 4 in the Marine Corps (11%). Definitive AO exposure was noted in 13 patients (37%) (Table 1).
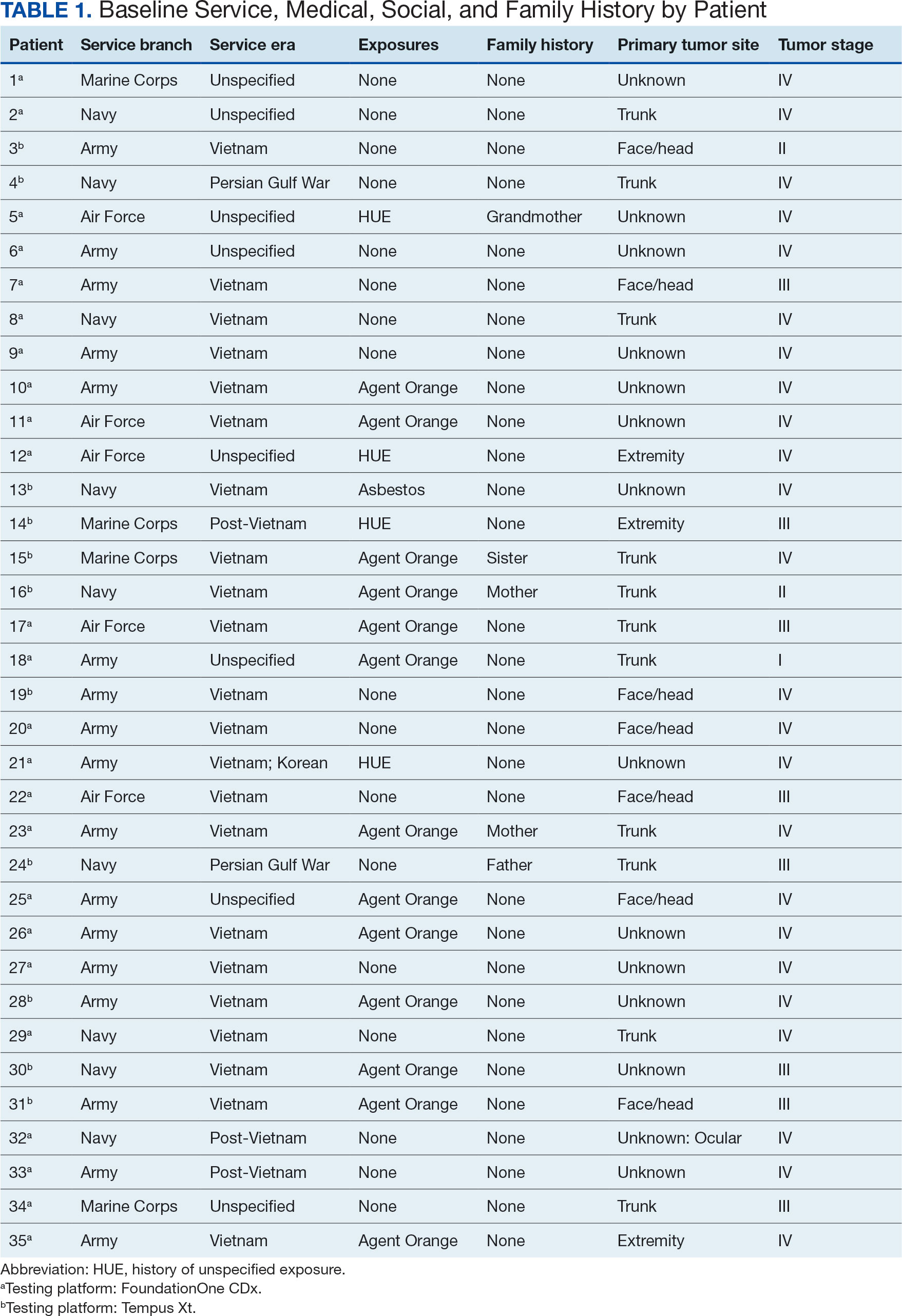
Of the 35 patients, 24 (69%) had metastatic disease and the primary site of melanoma was unknown in 14 patients (40%). One patient (Patient 32) had an intraocular melanoma. The primary site was the trunk for 11 patients (31%), the face/head for 7 patients (20%) and extremities for 3 patients (9%). Eight patients (23%) were pT3 stage (thickness > 2 mm but < 4 mm), 7 patients (20%) were pT4 stage (thickness > 4 mm), and 5 patients (14%) were pT1 (thickness ≥ 1 mm). One patient had a primary lesion at pT2 stage, and 1 had a Tis stage lesion. Three patients (9%) had a family history of melanoma in a first-degree relative.
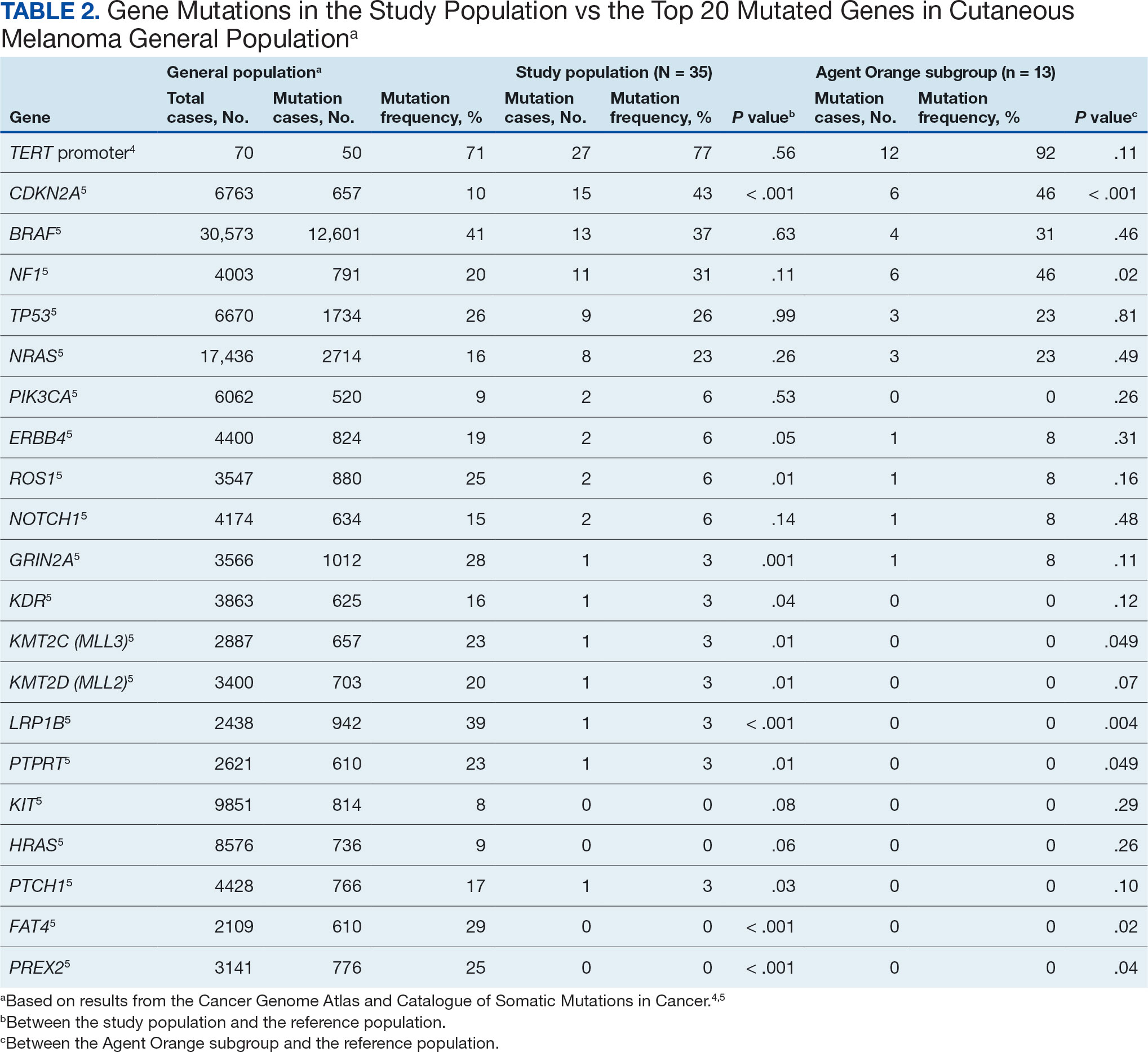
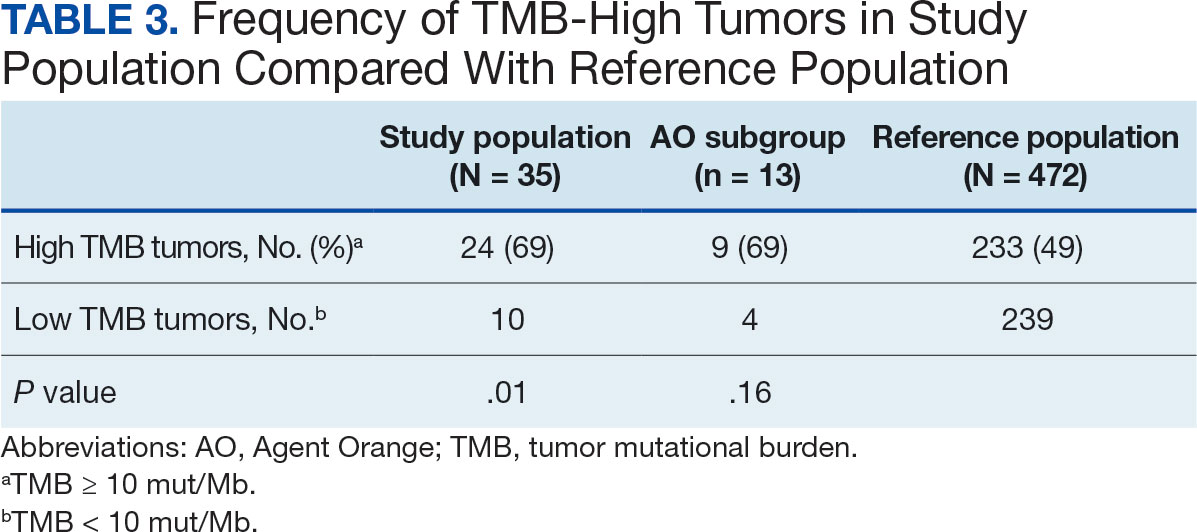
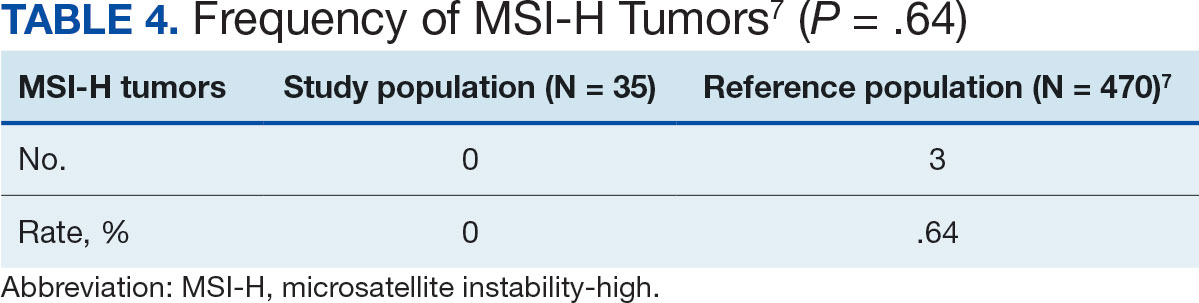
The list of genes mutated in melanoma cells in the study population is provided in the eAppendix.10,11 Twenty-seven patients (77%) had mutations in TERT promoter, 15 (43%) in CDKN2A/B, 13 (37%) in BRAF, 11 (31%) in NF1, 9 (26%) in TP53, and 8 (23%) in NRAS (Table 2). The majority of mutations in TERT promoter were c.- 146C>T (18 of 27 patients [67%]), whereas c.-124C>T was the second-most common (8 of 27 patients [30%]). The 2 observed mutations in the 13 patients with BRAF mutations were V600E and V600K, with almost equal distribution (54% and 46%, respectively). The mean (SD) TMB was 33.2 (39) mut/Mb (range, 1-203 mut/Mb). Ten patients (29%) had a TMB < 10 mut/Mb, whereas 24 (69%) had a TMB > 10 mut/Mb. The TMB could not be determined in 1 case. The frequency of TMB-high tumors in the study population compared with frequency in the reference population is shown in Table 3.12 Only 3 patients (0.64%) in the reference population had MSI-H tumors, and the microsatellite status could not be determined in those tumors (Table 4).13 Table 5 outlines statistically significant findings.
Agent Orange Subgroup
AO was a tactical herbicide used by the US military, named for the orange band around the storage barrels. Possible mutagenic properties of AO have been attributed to its byproduct, dioxin. Among the most common cancers known to be associated with AO exposure are bladder and prostate carcinoma and hematopoietic neoplasms. The association between genetic alterations and AO exposure was studied in veterans with prostate cancer.14 However, to our knowledge, insufficient information is available to determine whether an association exists between exposure to herbicides used in Vietnam or the contaminant dioxin and melanoma. Because a significant proportion of this study population had a well-documented history of AO exposure (37.1%), we were able to analyze them as a subgroup and to separately compare their mutation frequency with the general population.
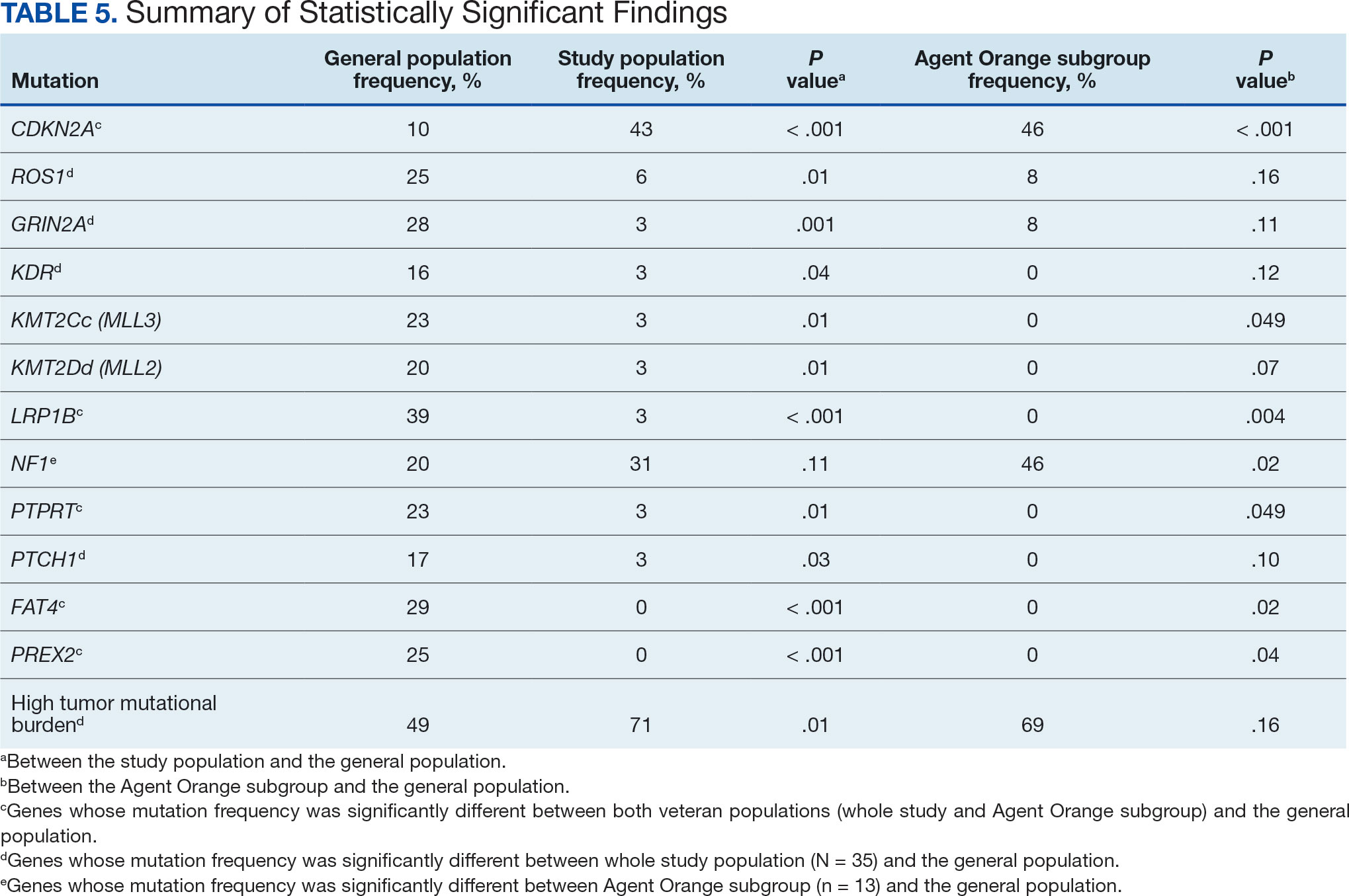
Results were notable for different distributions of the most frequently mutated genes in the AO subgroup compared with the whole study population. As such, TERT promoter remained the most frequently mutated gene (92%), followed by CDKN2A/B (46%); however, frequency of mutations in NF1 (46%) outnumbered those of BRAF (31%), the fourth-most common mutation. Moreover, when compared with the general melanoma population, a significantly higher frequency of mutations in the NF1 gene was observed in the AO subgroup—not the entire study population.
Discussion
Given that veterans constitute a distinct population, there is reasonable interest in investigating characteristic health issues related to military service. Skin cancer—melanoma in particular—has been researched recently in a veteran population. The differences in demographics, tumor characteristics, and melanoma- specific survival in veterans compared with the general population have already been assessed. According to Chang et al, compared with the general population, veterans are more likely to present with metastatic disease and have lower 5-year survival rates.8
Melanoma is one of the most highly mutated malignancies.15 Fortunately, the most common mutation in melanoma, BRAF V600E, is now considered therapeutically targetable. However, there are still many mutations that are less often discussed and not well understood. Regardless of therapeutic implications, all mutations observed in melanoma are worth investigating because a tumor’s genomic profile also can provide prognostic and etiologic information. Developing comprehensive descriptions of melanoma mutational profiles in specific populations is critical to advancing etiologic understanding and informing prevention strategies.
Our results demonstrate the high prevalence of TERT promoter mutations with characteristic ultraviolet signature (C>T) in the study population. This aligns with general evidence that TERT promoter mutations are common in cutaneous melanomas: 77% of this study sample and up to 86% of all mutations are TERT promoter mutations, according to Davis et al.15 TERT promoter mutations are positively associated with the initiation, invasion, and metastasis of melanoma. In certain subtypes, there is evidence that the presence of TERT promoter mutations is significantly associated with risk for extranodal metastasis and death.16 The second-most common mutated gene in the veteran study population was CDKN2A/B (43%), and the third-most mutated gene was BRAF (37%).
In chronically sun-exposed skin NF1, NRAS, and occasionally BRAF V600K mutations tend to predominate. BRAF V600E mutations, on the other hand, are rare in these melanomas.15 In our study population, the most prevalent melanoma site was the trunk (31%), which is considered a location with an intermittent pattern of sun exposure.17
This study population also had a higher frequency of CDKN2A/B mutations. High frequencies of CDKN2A/B mutations have been reported in familial melanomas, but only 1 patient with CDKN2A/B mutations had a known family history of melanoma.15 Tumors in the study population showed significantly lower frequency of mutations in ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 (P < .05).
In this study the subgroup of veterans with AO exposure differed from the whole study population. As such, CDKN2A/B mutations were observed with the same frequency as NF1 mutations (46% each); however, BRAF mutations constituted only 31% of the mutations. In addition, the frequency of NF1 mutations was significantly higher in the AO subgroup compared with the general population, but not in the whole study population.
Our sample also differed from the reference population by showing a significantly higher frequency of TMB-high (ie, ≥ 10 mut/Mb) tumors (71% vs 49%; P = .01).12 Interestingly, no significant difference in the frequency of TMB-high tumors was observed between the AO subgroup and the reference population (69% vs 49%; P = .16). There also was no statistically significant difference between the frequency of MSI-H tumors in our study population and the reference population (P = .64).13
One patient in the study population had uveal melanoma. Mutations encountered in this patient’s tumor differed from the general mutational profile of tumors. None of the 21 mutations depicted in Table 2 were present in this sample.10,11 On the other hand, those mutations frequently observed in intraocular melanomas, BAP1 and GNA11, were present in this patient.18 Additionally, this particular melanoma possessed mutations in genes RICTOR, RAD21, and PIK3R1.
Limitations
This study population consisted exclusively of male patients, introducing sex as a potential confounder in analyzing differences between the study population and the general population. As noted in a 2020 systematic review, there were no sex-based differences in the frequency of mutations in BRAF, NRAS, and KIT genes.19
Regarding NF1 mutations, only NF1-mutated acral and mucosal melanomas were more frequently observed in female patients, whereas nonacral NF1-mutated melanomas were more frequently observed in male patients.20 However, there is currently no clear evidence of whether the mutational landscapes of cutaneous melanoma differ by sex.21 Among the 11 cases with NF1-mutatation, site of origin was known in 6, 5 of which originated at nonacral sites. Although the AO subgroup also consisted entirely of male patients, this does not explain the observed increased frequency of NF1 mutations relative to the general population. No such difference was observed between the whole study population, which also consisted exclusively of male patients, and the general population. The similar frequencies of nonacral location in the whole study population (3 acral, 18 nonacral, 14 unknown site of origin) and AO subgroup (1 acral, 7 nonacral, 5 unknown site of origin) preclude location as an explanation.
The Cancer Genome Atlas Network proposed a framework for genomic classification of melanoma into 4 subtypes based on the pattern of the most prevalent significantly mutated genes: mutant BRAF, mutant RAS, mutant NF1, and triple–wild-type. According to that study, BRAF mutations were indeed associated with younger age, in contrast to the NF1-mutant genomic subtype, which was more prevalent in older individuals with higher TMB.22 This emphasizes the need to interpret the potential association of AO exposure and NF1 mutation in melanoma with caution, although additional studies are required to observe the difference between the veteran population and age-matched general population.
On the other hand, Yu et al reported no significant differences of TMB values between patients aged < 60 and ≥ 60 years with melanoma.23 In short, the observed differences we report in our limited study warrant additional investigation with larger sample sizes, sex-matched controlling, and age-matched controlling. The study was limited by its small sample size and the single location.
Conclusion
The genomic profile of melanomas in the veteran population appears to be similar to that of the general population with a few possible differences. Melanomas in the veteran study population showed a higher frequency of CDKN2A/B mutations; lower frequency of ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 mutations; and higher TMB. In addition, melanomas in the AO subgroup showed higher frequencies of NF1 mutations. The significance of such findings remains to be determined by further investigation.
- Bytnar JA, McGlynn KA, et al. Cancer incidence in the US military: An updated analysis. Cancer. 2024;130(1):96-106. doi:10.1002/cncr.34978
- Singer DS. A new phase of the Cancer Moonshot to end cancer as we know it. Nat Med. 2022;28(7):1345-1347. doi:10.1038/s41591-022-01881-5
- Koczkodaj P, Sulkowska U, Didkowska J, et al. Melanoma mortality trends in 28 European countries: a retrospective analysis for the years 1960-2020. Cancers (Basel). 2023;15(5):1514. Published 2023 Feb 28. doi:10.3390/cancers15051514
- Okobi OE, Abreo E, Sams NP, et al. Trends in melanoma incidence, prevalence, stage at diagnosis, and survival: an analysis of the United States Cancer Statistics (USCS) database. Cureus. 2024;16(10):e70697. doi:10.7759/cureus.70697
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:e2034-e2039. doi:10.7205/MILMED-D-17-00127
- American Cancer Society. Cancer facts & figures 2023. American Cancer Society; 2023. Accessed June 20, 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Rezaei SJ, Kim J, Onyeka S, et al. Skin cancer and other dermatologic conditions among US veterans. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol.2024.3043
- Chang MS, La J, Trepanowski N, et al. Increased relative proportions of advanced melanoma among veterans: a comparative analysis with the Surveillance, Epidemiology, and End Results registry. J Am Acad Dermatol. 2022;87:72-79. doi:10.1016/j.jaad.2022.02.063
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957-959. doi:10.1126/science.1229259
- Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47:D941-D947. doi:10.1093/nar/gky1015
- Li M, Gao X, Wang X. Identification of tumor mutation burden-associated molecular and clinical features in cancer by analyzing multi-omics data. Front Immunol. 2023;14:1090838. doi:10.3389/fimmu.2023.1090838
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073. doi:10.1200/PO.17.00073
- Lui AJ, Pagadala MS, Zhong AY, et al. Agent Orange exposure and prostate cancer risk in the Million Veteran Program. medRxiv [Preprint]. 2023:2023.06.14.23291413. doi:10.1101/2023.06.14.23291413
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499. doi:10.1002/cncr.31345
- Guo Y, Chen Y, Zhang L, et al. TERT promoter mutations and telomerase in melanoma. J Oncol. 2022;2022:6300329. doi:10.1155/2022/6300329
- Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24:3172-3177. doi:10.1200/JCO.2006.06.1325
- Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134:728-733. doi:10.1001/jamaophthalmol.2016.0903
- Gutiérrez-Castañeda LD, Nova JA, Tovar-Parra JD. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: a systemic review. Melanoma Res. 2020;30:62- 70. doi:10.1097/CMR.0000000000000628
- Thielmann CM, Chorti E, Matull J, et al. NF1-mutated melanomas reveal distinct clinical characteristics depending on tumour origin and respond favourably to immune checkpoint inhibitors. Eur J Cancer. 2021;159:113-124. doi:10.1016/j.ejca.2021.09.035
- D’Ecclesiis O, Caini S, Martinoli C, et al. Gender-dependent specificities in cutaneous melanoma predisposition, risk factors, somatic mutations, prognostic and predictive factors: a systematic review. Int J Environ Res Public Health. 2021;18:7945. doi:10.3390/ijerph18157945
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681-1696. doi:10.1016/j.cell.2015.05.044
- Yu Z, Wang J, Feng L, et al. Association of tumor mutational burden with age in solid tumors. J Clin Oncol. 2020;38:e13590-e13590. doi:10.1200/JCO.2020.38.15_suppl.e13590
The veteran population, with its unique and diverse types of exposure and military service experiences, faces distinct health factors compared with the general population. These factors can be categorized into exposures during military service and those occurring postservice. While the latter phase incorporates psychological issues that may arise while transitioning to civilian life, the service period is associated with major physical, chemical, and psychological exposures that can impact veterans’ health. Carcinogenesis related to military exposures is concerning, and different types of malignancies have been associated with military exposures.1 The 2022 introduction of the Cancer Moonshot initiative served as a breeding ground for multiple projects aimed at investigation of exposure-related carcinogenesis, prompting increased attention and efforts to linking specific exposures to specific malignancies.2
Melanoma is the deadliest skin cancer, accounting for 1.3% of all cancer deaths.3 Although it may only account for 1% to 5% of skin cancer diagnoses, its incidence in the United States’ population has been increasing.4,5 There were 97,610 estimated new cases of melanoma in 2023, according to the National Cancer Institute.6
The incidence of melanoma may be higher in the military population compared with the general population.7 Melanoma is the fourth-most common cancer diagnosed in veterans.8
Several demographic characteristics of the US military population are associated with higher melanoma incidence and poorer prognosis, including male sex, older age, and White race. Apart from sun exposure—a known risk factor for melanoma development—other factors, such as service branch, seem to contribute to risk, with the highest melanoma rates noted in the Air Force.9 According to a study by Chang et al, veterans have a higher risk of stage III (18%) or stage IV (13%) melanoma at initial diagnosis.8
Molecular testing of metastatic melanoma is currently the standard of care for guiding the use of US Food and Drug Administration-approved targeted therapies such as BRAF, MEK, and KIT inhibitors. This comparative analysis details the melanoma comprehensive genomic profiles observed at a large US Department of Veterans Affairs (VA) medical center (VAMC) and those reported in reference databases.
Methods
A query to select all metastatic melanomas sent for comprehensive genomic profiling from the Kansas City VAMC (KCVAMC), identified 35 cases from 2019 through 2023 as the study population. The health records of these patients were reviewed to collect demographic information, military service history, melanoma history, other medical, social, and family histories. The comprehensive genomic profiling reports were reviewed to collect the reported pathogenic variants, microsatellite instability (MSI) status, and tumor mutational burden (TMB) for each case.
The Catalogue of Somatic Mutations in Cancer (COSMIC) was used to identify the most commonly mutated genes in melanomas from The Cancer Genome Atlas for the general population.4,5 The literature was consulted to determine the MSI status and TMB in melanomas from The Cancer Genome Atlas for separate reference populations.6,7 The frequency of MSI-high (MSI-H) status, TMB ≥ 10 mutations/megabase (mut/Mb), and mutations in each of the 20 most commonly mutated genes was determined and compared between melanomas from The Cancer Genome Atlas and KCVAMC cases. Corresponding P values were calculated to identify significant differences. Values were calculated for the entire sample as well as a subgroup with Agent Orange (AO) exposure. The study was approved by the KCVAMC Institutional Review Board.
Results
The mean (SD) age of study participants was 72.9 (9.4) years (range, 39-90 years). The mean (SD) duration of military service was 1654 (1421) days (about 4 years, 6 months, and 10 days). Of the 35 patients included, 22 (63%) served during the Vietnam era (November 1, 1965, to April 30, 1975) and 2 (6%) served during the Persian Gulf War era (August 2, 1990, to February 28, 1991). Seventeen veterans (49%) served in the Army, 9 in the Navy (26%), 5 in the Air Force (14%), and 4 in the Marine Corps (11%). Definitive AO exposure was noted in 13 patients (37%) (Table 1).

Of the 35 patients, 24 (69%) had metastatic disease and the primary site of melanoma was unknown in 14 patients (40%). One patient (Patient 32) had an intraocular melanoma. The primary site was the trunk for 11 patients (31%), the face/head for 7 patients (20%) and extremities for 3 patients (9%). Eight patients (23%) were pT3 stage (thickness > 2 mm but < 4 mm), 7 patients (20%) were pT4 stage (thickness > 4 mm), and 5 patients (14%) were pT1 (thickness ≥ 1 mm). One patient had a primary lesion at pT2 stage, and 1 had a Tis stage lesion. Three patients (9%) had a family history of melanoma in a first-degree relative.
The list of genes mutated in melanoma cells in the study population is provided in the eAppendix.10,11 Twenty-seven patients (77%) had mutations in TERT promoter, 15 (43%) in CDKN2A/B, 13 (37%) in BRAF, 11 (31%) in NF1, 9 (26%) in TP53, and 8 (23%) in NRAS (Table 2). The majority of mutations in TERT promoter were c.- 146C>T (18 of 27 patients [67%]), whereas c.-124C>T was the second-most common (8 of 27 patients [30%]). The 2 observed mutations in the 13 patients with BRAF mutations were V600E and V600K, with almost equal distribution (54% and 46%, respectively). The mean (SD) TMB was 33.2 (39) mut/Mb (range, 1-203 mut/Mb). Ten patients (29%) had a TMB < 10 mut/Mb, whereas 24 (69%) had a TMB > 10 mut/Mb. The TMB could not be determined in 1 case. The frequency of TMB-high tumors in the study population compared with frequency in the reference population is shown in Table 3.12 Only 3 patients (0.64%) in the reference population had MSI-H tumors, and the microsatellite status could not be determined in those tumors (Table 4).13 Table 5 outlines statistically significant findings.




Agent Orange Subgroup
AO was a tactical herbicide used by the US military, named for the orange band around the storage barrels. Possible mutagenic properties of AO have been attributed to its byproduct, dioxin. Among the most common cancers known to be associated with AO exposure are bladder and prostate carcinoma and hematopoietic neoplasms. The association between genetic alterations and AO exposure was studied in veterans with prostate cancer.14 However, to our knowledge, insufficient information is available to determine whether an association exists between exposure to herbicides used in Vietnam or the contaminant dioxin and melanoma. Because a significant proportion of this study population had a well-documented history of AO exposure (37.1%), we were able to analyze them as a subgroup and to separately compare their mutation frequency with the general population.
Results were notable for different distributions of the most frequently mutated genes in the AO subgroup compared with the whole study population. As such, TERT promoter remained the most frequently mutated gene (92%), followed by CDKN2A/B (46%); however, frequency of mutations in NF1 (46%) outnumbered those of BRAF (31%), the fourth-most common mutation. Moreover, when compared with the general melanoma population, a significantly higher frequency of mutations in the NF1 gene was observed in the AO subgroup—not the entire study population.
Discussion
Given that veterans constitute a distinct population, there is reasonable interest in investigating characteristic health issues related to military service. Skin cancer—melanoma in particular—has been researched recently in a veteran population. The differences in demographics, tumor characteristics, and melanoma- specific survival in veterans compared with the general population have already been assessed. According to Chang et al, compared with the general population, veterans are more likely to present with metastatic disease and have lower 5-year survival rates.8
Melanoma is one of the most highly mutated malignancies.15 Fortunately, the most common mutation in melanoma, BRAF V600E, is now considered therapeutically targetable. However, there are still many mutations that are less often discussed and not well understood. Regardless of therapeutic implications, all mutations observed in melanoma are worth investigating because a tumor’s genomic profile also can provide prognostic and etiologic information. Developing comprehensive descriptions of melanoma mutational profiles in specific populations is critical to advancing etiologic understanding and informing prevention strategies.
Our results demonstrate the high prevalence of TERT promoter mutations with characteristic ultraviolet signature (C>T) in the study population. This aligns with general evidence that TERT promoter mutations are common in cutaneous melanomas: 77% of this study sample and up to 86% of all mutations are TERT promoter mutations, according to Davis et al.15 TERT promoter mutations are positively associated with the initiation, invasion, and metastasis of melanoma. In certain subtypes, there is evidence that the presence of TERT promoter mutations is significantly associated with risk for extranodal metastasis and death.16 The second-most common mutated gene in the veteran study population was CDKN2A/B (43%), and the third-most mutated gene was BRAF (37%).
In chronically sun-exposed skin NF1, NRAS, and occasionally BRAF V600K mutations tend to predominate. BRAF V600E mutations, on the other hand, are rare in these melanomas.15 In our study population, the most prevalent melanoma site was the trunk (31%), which is considered a location with an intermittent pattern of sun exposure.17
This study population also had a higher frequency of CDKN2A/B mutations. High frequencies of CDKN2A/B mutations have been reported in familial melanomas, but only 1 patient with CDKN2A/B mutations had a known family history of melanoma.15 Tumors in the study population showed significantly lower frequency of mutations in ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 (P < .05).
In this study the subgroup of veterans with AO exposure differed from the whole study population. As such, CDKN2A/B mutations were observed with the same frequency as NF1 mutations (46% each); however, BRAF mutations constituted only 31% of the mutations. In addition, the frequency of NF1 mutations was significantly higher in the AO subgroup compared with the general population, but not in the whole study population.
Our sample also differed from the reference population by showing a significantly higher frequency of TMB-high (ie, ≥ 10 mut/Mb) tumors (71% vs 49%; P = .01).12 Interestingly, no significant difference in the frequency of TMB-high tumors was observed between the AO subgroup and the reference population (69% vs 49%; P = .16). There also was no statistically significant difference between the frequency of MSI-H tumors in our study population and the reference population (P = .64).13
One patient in the study population had uveal melanoma. Mutations encountered in this patient’s tumor differed from the general mutational profile of tumors. None of the 21 mutations depicted in Table 2 were present in this sample.10,11 On the other hand, those mutations frequently observed in intraocular melanomas, BAP1 and GNA11, were present in this patient.18 Additionally, this particular melanoma possessed mutations in genes RICTOR, RAD21, and PIK3R1.
Limitations
This study population consisted exclusively of male patients, introducing sex as a potential confounder in analyzing differences between the study population and the general population. As noted in a 2020 systematic review, there were no sex-based differences in the frequency of mutations in BRAF, NRAS, and KIT genes.19
Regarding NF1 mutations, only NF1-mutated acral and mucosal melanomas were more frequently observed in female patients, whereas nonacral NF1-mutated melanomas were more frequently observed in male patients.20 However, there is currently no clear evidence of whether the mutational landscapes of cutaneous melanoma differ by sex.21 Among the 11 cases with NF1-mutatation, site of origin was known in 6, 5 of which originated at nonacral sites. Although the AO subgroup also consisted entirely of male patients, this does not explain the observed increased frequency of NF1 mutations relative to the general population. No such difference was observed between the whole study population, which also consisted exclusively of male patients, and the general population. The similar frequencies of nonacral location in the whole study population (3 acral, 18 nonacral, 14 unknown site of origin) and AO subgroup (1 acral, 7 nonacral, 5 unknown site of origin) preclude location as an explanation.
The Cancer Genome Atlas Network proposed a framework for genomic classification of melanoma into 4 subtypes based on the pattern of the most prevalent significantly mutated genes: mutant BRAF, mutant RAS, mutant NF1, and triple–wild-type. According to that study, BRAF mutations were indeed associated with younger age, in contrast to the NF1-mutant genomic subtype, which was more prevalent in older individuals with higher TMB.22 This emphasizes the need to interpret the potential association of AO exposure and NF1 mutation in melanoma with caution, although additional studies are required to observe the difference between the veteran population and age-matched general population.
On the other hand, Yu et al reported no significant differences of TMB values between patients aged < 60 and ≥ 60 years with melanoma.23 In short, the observed differences we report in our limited study warrant additional investigation with larger sample sizes, sex-matched controlling, and age-matched controlling. The study was limited by its small sample size and the single location.
Conclusion
The genomic profile of melanomas in the veteran population appears to be similar to that of the general population with a few possible differences. Melanomas in the veteran study population showed a higher frequency of CDKN2A/B mutations; lower frequency of ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 mutations; and higher TMB. In addition, melanomas in the AO subgroup showed higher frequencies of NF1 mutations. The significance of such findings remains to be determined by further investigation.
The veteran population, with its unique and diverse types of exposure and military service experiences, faces distinct health factors compared with the general population. These factors can be categorized into exposures during military service and those occurring postservice. While the latter phase incorporates psychological issues that may arise while transitioning to civilian life, the service period is associated with major physical, chemical, and psychological exposures that can impact veterans’ health. Carcinogenesis related to military exposures is concerning, and different types of malignancies have been associated with military exposures.1 The 2022 introduction of the Cancer Moonshot initiative served as a breeding ground for multiple projects aimed at investigation of exposure-related carcinogenesis, prompting increased attention and efforts to linking specific exposures to specific malignancies.2
Melanoma is the deadliest skin cancer, accounting for 1.3% of all cancer deaths.3 Although it may only account for 1% to 5% of skin cancer diagnoses, its incidence in the United States’ population has been increasing.4,5 There were 97,610 estimated new cases of melanoma in 2023, according to the National Cancer Institute.6
The incidence of melanoma may be higher in the military population compared with the general population.7 Melanoma is the fourth-most common cancer diagnosed in veterans.8
Several demographic characteristics of the US military population are associated with higher melanoma incidence and poorer prognosis, including male sex, older age, and White race. Apart from sun exposure—a known risk factor for melanoma development—other factors, such as service branch, seem to contribute to risk, with the highest melanoma rates noted in the Air Force.9 According to a study by Chang et al, veterans have a higher risk of stage III (18%) or stage IV (13%) melanoma at initial diagnosis.8
Molecular testing of metastatic melanoma is currently the standard of care for guiding the use of US Food and Drug Administration-approved targeted therapies such as BRAF, MEK, and KIT inhibitors. This comparative analysis details the melanoma comprehensive genomic profiles observed at a large US Department of Veterans Affairs (VA) medical center (VAMC) and those reported in reference databases.
Methods
A query to select all metastatic melanomas sent for comprehensive genomic profiling from the Kansas City VAMC (KCVAMC), identified 35 cases from 2019 through 2023 as the study population. The health records of these patients were reviewed to collect demographic information, military service history, melanoma history, other medical, social, and family histories. The comprehensive genomic profiling reports were reviewed to collect the reported pathogenic variants, microsatellite instability (MSI) status, and tumor mutational burden (TMB) for each case.
The Catalogue of Somatic Mutations in Cancer (COSMIC) was used to identify the most commonly mutated genes in melanomas from The Cancer Genome Atlas for the general population.4,5 The literature was consulted to determine the MSI status and TMB in melanomas from The Cancer Genome Atlas for separate reference populations.6,7 The frequency of MSI-high (MSI-H) status, TMB ≥ 10 mutations/megabase (mut/Mb), and mutations in each of the 20 most commonly mutated genes was determined and compared between melanomas from The Cancer Genome Atlas and KCVAMC cases. Corresponding P values were calculated to identify significant differences. Values were calculated for the entire sample as well as a subgroup with Agent Orange (AO) exposure. The study was approved by the KCVAMC Institutional Review Board.
Results
The mean (SD) age of study participants was 72.9 (9.4) years (range, 39-90 years). The mean (SD) duration of military service was 1654 (1421) days (about 4 years, 6 months, and 10 days). Of the 35 patients included, 22 (63%) served during the Vietnam era (November 1, 1965, to April 30, 1975) and 2 (6%) served during the Persian Gulf War era (August 2, 1990, to February 28, 1991). Seventeen veterans (49%) served in the Army, 9 in the Navy (26%), 5 in the Air Force (14%), and 4 in the Marine Corps (11%). Definitive AO exposure was noted in 13 patients (37%) (Table 1).

Of the 35 patients, 24 (69%) had metastatic disease and the primary site of melanoma was unknown in 14 patients (40%). One patient (Patient 32) had an intraocular melanoma. The primary site was the trunk for 11 patients (31%), the face/head for 7 patients (20%) and extremities for 3 patients (9%). Eight patients (23%) were pT3 stage (thickness > 2 mm but < 4 mm), 7 patients (20%) were pT4 stage (thickness > 4 mm), and 5 patients (14%) were pT1 (thickness ≥ 1 mm). One patient had a primary lesion at pT2 stage, and 1 had a Tis stage lesion. Three patients (9%) had a family history of melanoma in a first-degree relative.
The list of genes mutated in melanoma cells in the study population is provided in the eAppendix.10,11 Twenty-seven patients (77%) had mutations in TERT promoter, 15 (43%) in CDKN2A/B, 13 (37%) in BRAF, 11 (31%) in NF1, 9 (26%) in TP53, and 8 (23%) in NRAS (Table 2). The majority of mutations in TERT promoter were c.- 146C>T (18 of 27 patients [67%]), whereas c.-124C>T was the second-most common (8 of 27 patients [30%]). The 2 observed mutations in the 13 patients with BRAF mutations were V600E and V600K, with almost equal distribution (54% and 46%, respectively). The mean (SD) TMB was 33.2 (39) mut/Mb (range, 1-203 mut/Mb). Ten patients (29%) had a TMB < 10 mut/Mb, whereas 24 (69%) had a TMB > 10 mut/Mb. The TMB could not be determined in 1 case. The frequency of TMB-high tumors in the study population compared with frequency in the reference population is shown in Table 3.12 Only 3 patients (0.64%) in the reference population had MSI-H tumors, and the microsatellite status could not be determined in those tumors (Table 4).13 Table 5 outlines statistically significant findings.




Agent Orange Subgroup
AO was a tactical herbicide used by the US military, named for the orange band around the storage barrels. Possible mutagenic properties of AO have been attributed to its byproduct, dioxin. Among the most common cancers known to be associated with AO exposure are bladder and prostate carcinoma and hematopoietic neoplasms. The association between genetic alterations and AO exposure was studied in veterans with prostate cancer.14 However, to our knowledge, insufficient information is available to determine whether an association exists between exposure to herbicides used in Vietnam or the contaminant dioxin and melanoma. Because a significant proportion of this study population had a well-documented history of AO exposure (37.1%), we were able to analyze them as a subgroup and to separately compare their mutation frequency with the general population.
Results were notable for different distributions of the most frequently mutated genes in the AO subgroup compared with the whole study population. As such, TERT promoter remained the most frequently mutated gene (92%), followed by CDKN2A/B (46%); however, frequency of mutations in NF1 (46%) outnumbered those of BRAF (31%), the fourth-most common mutation. Moreover, when compared with the general melanoma population, a significantly higher frequency of mutations in the NF1 gene was observed in the AO subgroup—not the entire study population.
Discussion
Given that veterans constitute a distinct population, there is reasonable interest in investigating characteristic health issues related to military service. Skin cancer—melanoma in particular—has been researched recently in a veteran population. The differences in demographics, tumor characteristics, and melanoma- specific survival in veterans compared with the general population have already been assessed. According to Chang et al, compared with the general population, veterans are more likely to present with metastatic disease and have lower 5-year survival rates.8
Melanoma is one of the most highly mutated malignancies.15 Fortunately, the most common mutation in melanoma, BRAF V600E, is now considered therapeutically targetable. However, there are still many mutations that are less often discussed and not well understood. Regardless of therapeutic implications, all mutations observed in melanoma are worth investigating because a tumor’s genomic profile also can provide prognostic and etiologic information. Developing comprehensive descriptions of melanoma mutational profiles in specific populations is critical to advancing etiologic understanding and informing prevention strategies.
Our results demonstrate the high prevalence of TERT promoter mutations with characteristic ultraviolet signature (C>T) in the study population. This aligns with general evidence that TERT promoter mutations are common in cutaneous melanomas: 77% of this study sample and up to 86% of all mutations are TERT promoter mutations, according to Davis et al.15 TERT promoter mutations are positively associated with the initiation, invasion, and metastasis of melanoma. In certain subtypes, there is evidence that the presence of TERT promoter mutations is significantly associated with risk for extranodal metastasis and death.16 The second-most common mutated gene in the veteran study population was CDKN2A/B (43%), and the third-most mutated gene was BRAF (37%).
In chronically sun-exposed skin NF1, NRAS, and occasionally BRAF V600K mutations tend to predominate. BRAF V600E mutations, on the other hand, are rare in these melanomas.15 In our study population, the most prevalent melanoma site was the trunk (31%), which is considered a location with an intermittent pattern of sun exposure.17
This study population also had a higher frequency of CDKN2A/B mutations. High frequencies of CDKN2A/B mutations have been reported in familial melanomas, but only 1 patient with CDKN2A/B mutations had a known family history of melanoma.15 Tumors in the study population showed significantly lower frequency of mutations in ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 (P < .05).
In this study the subgroup of veterans with AO exposure differed from the whole study population. As such, CDKN2A/B mutations were observed with the same frequency as NF1 mutations (46% each); however, BRAF mutations constituted only 31% of the mutations. In addition, the frequency of NF1 mutations was significantly higher in the AO subgroup compared with the general population, but not in the whole study population.
Our sample also differed from the reference population by showing a significantly higher frequency of TMB-high (ie, ≥ 10 mut/Mb) tumors (71% vs 49%; P = .01).12 Interestingly, no significant difference in the frequency of TMB-high tumors was observed between the AO subgroup and the reference population (69% vs 49%; P = .16). There also was no statistically significant difference between the frequency of MSI-H tumors in our study population and the reference population (P = .64).13
One patient in the study population had uveal melanoma. Mutations encountered in this patient’s tumor differed from the general mutational profile of tumors. None of the 21 mutations depicted in Table 2 were present in this sample.10,11 On the other hand, those mutations frequently observed in intraocular melanomas, BAP1 and GNA11, were present in this patient.18 Additionally, this particular melanoma possessed mutations in genes RICTOR, RAD21, and PIK3R1.
Limitations
This study population consisted exclusively of male patients, introducing sex as a potential confounder in analyzing differences between the study population and the general population. As noted in a 2020 systematic review, there were no sex-based differences in the frequency of mutations in BRAF, NRAS, and KIT genes.19
Regarding NF1 mutations, only NF1-mutated acral and mucosal melanomas were more frequently observed in female patients, whereas nonacral NF1-mutated melanomas were more frequently observed in male patients.20 However, there is currently no clear evidence of whether the mutational landscapes of cutaneous melanoma differ by sex.21 Among the 11 cases with NF1-mutatation, site of origin was known in 6, 5 of which originated at nonacral sites. Although the AO subgroup also consisted entirely of male patients, this does not explain the observed increased frequency of NF1 mutations relative to the general population. No such difference was observed between the whole study population, which also consisted exclusively of male patients, and the general population. The similar frequencies of nonacral location in the whole study population (3 acral, 18 nonacral, 14 unknown site of origin) and AO subgroup (1 acral, 7 nonacral, 5 unknown site of origin) preclude location as an explanation.
The Cancer Genome Atlas Network proposed a framework for genomic classification of melanoma into 4 subtypes based on the pattern of the most prevalent significantly mutated genes: mutant BRAF, mutant RAS, mutant NF1, and triple–wild-type. According to that study, BRAF mutations were indeed associated with younger age, in contrast to the NF1-mutant genomic subtype, which was more prevalent in older individuals with higher TMB.22 This emphasizes the need to interpret the potential association of AO exposure and NF1 mutation in melanoma with caution, although additional studies are required to observe the difference between the veteran population and age-matched general population.
On the other hand, Yu et al reported no significant differences of TMB values between patients aged < 60 and ≥ 60 years with melanoma.23 In short, the observed differences we report in our limited study warrant additional investigation with larger sample sizes, sex-matched controlling, and age-matched controlling. The study was limited by its small sample size and the single location.
Conclusion
The genomic profile of melanomas in the veteran population appears to be similar to that of the general population with a few possible differences. Melanomas in the veteran study population showed a higher frequency of CDKN2A/B mutations; lower frequency of ROS1, GRIN2A, KDR, KMT2C (MLL3), KMT2D (MLL2), LRP1B, PTPRT, PTCH1, FAT4, and PREX2 mutations; and higher TMB. In addition, melanomas in the AO subgroup showed higher frequencies of NF1 mutations. The significance of such findings remains to be determined by further investigation.
- Bytnar JA, McGlynn KA, et al. Cancer incidence in the US military: An updated analysis. Cancer. 2024;130(1):96-106. doi:10.1002/cncr.34978
- Singer DS. A new phase of the Cancer Moonshot to end cancer as we know it. Nat Med. 2022;28(7):1345-1347. doi:10.1038/s41591-022-01881-5
- Koczkodaj P, Sulkowska U, Didkowska J, et al. Melanoma mortality trends in 28 European countries: a retrospective analysis for the years 1960-2020. Cancers (Basel). 2023;15(5):1514. Published 2023 Feb 28. doi:10.3390/cancers15051514
- Okobi OE, Abreo E, Sams NP, et al. Trends in melanoma incidence, prevalence, stage at diagnosis, and survival: an analysis of the United States Cancer Statistics (USCS) database. Cureus. 2024;16(10):e70697. doi:10.7759/cureus.70697
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:e2034-e2039. doi:10.7205/MILMED-D-17-00127
- American Cancer Society. Cancer facts & figures 2023. American Cancer Society; 2023. Accessed June 20, 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Rezaei SJ, Kim J, Onyeka S, et al. Skin cancer and other dermatologic conditions among US veterans. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol.2024.3043
- Chang MS, La J, Trepanowski N, et al. Increased relative proportions of advanced melanoma among veterans: a comparative analysis with the Surveillance, Epidemiology, and End Results registry. J Am Acad Dermatol. 2022;87:72-79. doi:10.1016/j.jaad.2022.02.063
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957-959. doi:10.1126/science.1229259
- Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47:D941-D947. doi:10.1093/nar/gky1015
- Li M, Gao X, Wang X. Identification of tumor mutation burden-associated molecular and clinical features in cancer by analyzing multi-omics data. Front Immunol. 2023;14:1090838. doi:10.3389/fimmu.2023.1090838
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073. doi:10.1200/PO.17.00073
- Lui AJ, Pagadala MS, Zhong AY, et al. Agent Orange exposure and prostate cancer risk in the Million Veteran Program. medRxiv [Preprint]. 2023:2023.06.14.23291413. doi:10.1101/2023.06.14.23291413
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499. doi:10.1002/cncr.31345
- Guo Y, Chen Y, Zhang L, et al. TERT promoter mutations and telomerase in melanoma. J Oncol. 2022;2022:6300329. doi:10.1155/2022/6300329
- Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24:3172-3177. doi:10.1200/JCO.2006.06.1325
- Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134:728-733. doi:10.1001/jamaophthalmol.2016.0903
- Gutiérrez-Castañeda LD, Nova JA, Tovar-Parra JD. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: a systemic review. Melanoma Res. 2020;30:62- 70. doi:10.1097/CMR.0000000000000628
- Thielmann CM, Chorti E, Matull J, et al. NF1-mutated melanomas reveal distinct clinical characteristics depending on tumour origin and respond favourably to immune checkpoint inhibitors. Eur J Cancer. 2021;159:113-124. doi:10.1016/j.ejca.2021.09.035
- D’Ecclesiis O, Caini S, Martinoli C, et al. Gender-dependent specificities in cutaneous melanoma predisposition, risk factors, somatic mutations, prognostic and predictive factors: a systematic review. Int J Environ Res Public Health. 2021;18:7945. doi:10.3390/ijerph18157945
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681-1696. doi:10.1016/j.cell.2015.05.044
- Yu Z, Wang J, Feng L, et al. Association of tumor mutational burden with age in solid tumors. J Clin Oncol. 2020;38:e13590-e13590. doi:10.1200/JCO.2020.38.15_suppl.e13590
- Bytnar JA, McGlynn KA, et al. Cancer incidence in the US military: An updated analysis. Cancer. 2024;130(1):96-106. doi:10.1002/cncr.34978
- Singer DS. A new phase of the Cancer Moonshot to end cancer as we know it. Nat Med. 2022;28(7):1345-1347. doi:10.1038/s41591-022-01881-5
- Koczkodaj P, Sulkowska U, Didkowska J, et al. Melanoma mortality trends in 28 European countries: a retrospective analysis for the years 1960-2020. Cancers (Basel). 2023;15(5):1514. Published 2023 Feb 28. doi:10.3390/cancers15051514
- Okobi OE, Abreo E, Sams NP, et al. Trends in melanoma incidence, prevalence, stage at diagnosis, and survival: an analysis of the United States Cancer Statistics (USCS) database. Cureus. 2024;16(10):e70697. doi:10.7759/cureus.70697
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:e2034-e2039. doi:10.7205/MILMED-D-17-00127
- American Cancer Society. Cancer facts & figures 2023. American Cancer Society; 2023. Accessed June 20, 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Rezaei SJ, Kim J, Onyeka S, et al. Skin cancer and other dermatologic conditions among US veterans. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol.2024.3043
- Chang MS, La J, Trepanowski N, et al. Increased relative proportions of advanced melanoma among veterans: a comparative analysis with the Surveillance, Epidemiology, and End Results registry. J Am Acad Dermatol. 2022;87:72-79. doi:10.1016/j.jaad.2022.02.063
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957-959. doi:10.1126/science.1229259
- Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47:D941-D947. doi:10.1093/nar/gky1015
- Li M, Gao X, Wang X. Identification of tumor mutation burden-associated molecular and clinical features in cancer by analyzing multi-omics data. Front Immunol. 2023;14:1090838. doi:10.3389/fimmu.2023.1090838
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017:PO.17.00073. doi:10.1200/PO.17.00073
- Lui AJ, Pagadala MS, Zhong AY, et al. Agent Orange exposure and prostate cancer risk in the Million Veteran Program. medRxiv [Preprint]. 2023:2023.06.14.23291413. doi:10.1101/2023.06.14.23291413
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499. doi:10.1002/cncr.31345
- Guo Y, Chen Y, Zhang L, et al. TERT promoter mutations and telomerase in melanoma. J Oncol. 2022;2022:6300329. doi:10.1155/2022/6300329
- Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24:3172-3177. doi:10.1200/JCO.2006.06.1325
- Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134:728-733. doi:10.1001/jamaophthalmol.2016.0903
- Gutiérrez-Castañeda LD, Nova JA, Tovar-Parra JD. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: a systemic review. Melanoma Res. 2020;30:62- 70. doi:10.1097/CMR.0000000000000628
- Thielmann CM, Chorti E, Matull J, et al. NF1-mutated melanomas reveal distinct clinical characteristics depending on tumour origin and respond favourably to immune checkpoint inhibitors. Eur J Cancer. 2021;159:113-124. doi:10.1016/j.ejca.2021.09.035
- D’Ecclesiis O, Caini S, Martinoli C, et al. Gender-dependent specificities in cutaneous melanoma predisposition, risk factors, somatic mutations, prognostic and predictive factors: a systematic review. Int J Environ Res Public Health. 2021;18:7945. doi:10.3390/ijerph18157945
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681-1696. doi:10.1016/j.cell.2015.05.044
- Yu Z, Wang J, Feng L, et al. Association of tumor mutational burden with age in solid tumors. J Clin Oncol. 2020;38:e13590-e13590. doi:10.1200/JCO.2020.38.15_suppl.e13590
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
Comprehensive Genomic Profiles of Melanoma in Veterans Compared to Reference Databases
US Vet Study Identifies Risk Factors for Acral Melanoma
US Vet Study Identifies Risk Factors for Acral Melanoma
TOPLINE:
Exposure to Agent Orange, the defoliant used by the US Air Force during the Vietnam War, was one of the factors associated with increased odds of acral melanoma (AM), a rare melanoma subtype affecting palms, soles, and nail units.
METHODOLOGY:
- Researchers conducted a nested case-control study in the Veterans Affairs healthcare system, and identified 1292 veterans (median age, 70.13 years; 94.0% men; 73.4% White, 14.6% Black) with AM through the Veterans Affairs Cancer Registry and a validated natural language processing pipeline from 2000 to 2024.
- Researchers matched each case of AM to 4 individuals with nonacral cutaneous melanoma (CM) and 4 control individuals without melanoma diagnoses, based on diagnosis year and outpatient visit frequency.
- Exposures included age, sex, race, ethnicity, rurality, region, military branch, comorbidities, smoking status, alcohol use, BMI, Agent Orange exposure, prior photosensitizing medications, nevi, and keratinocyte carcinoma.
TAKEAWAY:
- Veterans exposed to Agent Orange had higher odds of AM than individuals with CM (adjusted odds ratio [AOR], 1.31; 95% CI, 1.06-1.62) and control individuals without melanoma (AOR, 1.27; 95% CI, 1.04-1.56).
- Individuals with current smoking habit had lower odds of AM than those with CM (AOR, 0.65; 95% CI, 0.52-0.81) and control individuals without melanoma (AOR, 0.50; 95% CI, 0.40-0.62).
- Patients with prior keratinocyte carcinoma and actinic keratosis had higher odds of AM than control individuals without melanoma but lower odds than those with CM.
- History of nevus was associated with higher odds of acral melanoma compared with individuals without melanoma (AOR, 2.11; 95% CI, 1.49-2.98).
IN PRACTICE:
“Our results support the need for continued investigation of AM as a distinct entity from CM and may inform future evaluations of the associations between [Agent Orange exposure] in veteran populations, as well as those between other environmental exposures in different populations," the study authors wrote. Referring to the “continued search for a better understanding of a potential link” between Agent Orange and melanoma, as well as AM, and other possible etiologic factors for AM, this study “provides a strong impetus to further these research goals and contribute to the investigation of the legacy of the Vietnam War and honor a commitment to the veterans community,” Andrew F. Olshan, PhD, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, wrote in an accompanying editorial.
SOURCE:
The study was led by Jonathan C. Hwang, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, and was published online on February 4 in JAMA Dermatology.
LIMITATIONS:
The case-control design limits causal inference and the findings might not be generalized outside US veterans. Exposure misclassification could be present.
DISCLOSURES:
The study was supported by the Department of Defense and the Department of Veterans Affairs. Several authors reported receiving grants from CU Anschutz Medical Center, Department of Defense, CDMRP Melanoma Research Program, and Merck, Bayer, and Department of Veteran Affairs. They also reported receiving royalty from UpToDate, and being shareholder in many companies, including Apple. NVIDIA, Amazon, Gilead, AstraZeneca, BioNTech, and Moderna. Olshan declared being a member of the National Academies of Sciences, Engineering, and Medicine Veterans and Agent Orange review committee.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Exposure to Agent Orange, the defoliant used by the US Air Force during the Vietnam War, was one of the factors associated with increased odds of acral melanoma (AM), a rare melanoma subtype affecting palms, soles, and nail units.
METHODOLOGY:
- Researchers conducted a nested case-control study in the Veterans Affairs healthcare system, and identified 1292 veterans (median age, 70.13 years; 94.0% men; 73.4% White, 14.6% Black) with AM through the Veterans Affairs Cancer Registry and a validated natural language processing pipeline from 2000 to 2024.
- Researchers matched each case of AM to 4 individuals with nonacral cutaneous melanoma (CM) and 4 control individuals without melanoma diagnoses, based on diagnosis year and outpatient visit frequency.
- Exposures included age, sex, race, ethnicity, rurality, region, military branch, comorbidities, smoking status, alcohol use, BMI, Agent Orange exposure, prior photosensitizing medications, nevi, and keratinocyte carcinoma.
TAKEAWAY:
- Veterans exposed to Agent Orange had higher odds of AM than individuals with CM (adjusted odds ratio [AOR], 1.31; 95% CI, 1.06-1.62) and control individuals without melanoma (AOR, 1.27; 95% CI, 1.04-1.56).
- Individuals with current smoking habit had lower odds of AM than those with CM (AOR, 0.65; 95% CI, 0.52-0.81) and control individuals without melanoma (AOR, 0.50; 95% CI, 0.40-0.62).
- Patients with prior keratinocyte carcinoma and actinic keratosis had higher odds of AM than control individuals without melanoma but lower odds than those with CM.
- History of nevus was associated with higher odds of acral melanoma compared with individuals without melanoma (AOR, 2.11; 95% CI, 1.49-2.98).
IN PRACTICE:
“Our results support the need for continued investigation of AM as a distinct entity from CM and may inform future evaluations of the associations between [Agent Orange exposure] in veteran populations, as well as those between other environmental exposures in different populations," the study authors wrote. Referring to the “continued search for a better understanding of a potential link” between Agent Orange and melanoma, as well as AM, and other possible etiologic factors for AM, this study “provides a strong impetus to further these research goals and contribute to the investigation of the legacy of the Vietnam War and honor a commitment to the veterans community,” Andrew F. Olshan, PhD, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, wrote in an accompanying editorial.
SOURCE:
The study was led by Jonathan C. Hwang, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, and was published online on February 4 in JAMA Dermatology.
LIMITATIONS:
The case-control design limits causal inference and the findings might not be generalized outside US veterans. Exposure misclassification could be present.
DISCLOSURES:
The study was supported by the Department of Defense and the Department of Veterans Affairs. Several authors reported receiving grants from CU Anschutz Medical Center, Department of Defense, CDMRP Melanoma Research Program, and Merck, Bayer, and Department of Veteran Affairs. They also reported receiving royalty from UpToDate, and being shareholder in many companies, including Apple. NVIDIA, Amazon, Gilead, AstraZeneca, BioNTech, and Moderna. Olshan declared being a member of the National Academies of Sciences, Engineering, and Medicine Veterans and Agent Orange review committee.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Exposure to Agent Orange, the defoliant used by the US Air Force during the Vietnam War, was one of the factors associated with increased odds of acral melanoma (AM), a rare melanoma subtype affecting palms, soles, and nail units.
METHODOLOGY:
- Researchers conducted a nested case-control study in the Veterans Affairs healthcare system, and identified 1292 veterans (median age, 70.13 years; 94.0% men; 73.4% White, 14.6% Black) with AM through the Veterans Affairs Cancer Registry and a validated natural language processing pipeline from 2000 to 2024.
- Researchers matched each case of AM to 4 individuals with nonacral cutaneous melanoma (CM) and 4 control individuals without melanoma diagnoses, based on diagnosis year and outpatient visit frequency.
- Exposures included age, sex, race, ethnicity, rurality, region, military branch, comorbidities, smoking status, alcohol use, BMI, Agent Orange exposure, prior photosensitizing medications, nevi, and keratinocyte carcinoma.
TAKEAWAY:
- Veterans exposed to Agent Orange had higher odds of AM than individuals with CM (adjusted odds ratio [AOR], 1.31; 95% CI, 1.06-1.62) and control individuals without melanoma (AOR, 1.27; 95% CI, 1.04-1.56).
- Individuals with current smoking habit had lower odds of AM than those with CM (AOR, 0.65; 95% CI, 0.52-0.81) and control individuals without melanoma (AOR, 0.50; 95% CI, 0.40-0.62).
- Patients with prior keratinocyte carcinoma and actinic keratosis had higher odds of AM than control individuals without melanoma but lower odds than those with CM.
- History of nevus was associated with higher odds of acral melanoma compared with individuals without melanoma (AOR, 2.11; 95% CI, 1.49-2.98).
IN PRACTICE:
“Our results support the need for continued investigation of AM as a distinct entity from CM and may inform future evaluations of the associations between [Agent Orange exposure] in veteran populations, as well as those between other environmental exposures in different populations," the study authors wrote. Referring to the “continued search for a better understanding of a potential link” between Agent Orange and melanoma, as well as AM, and other possible etiologic factors for AM, this study “provides a strong impetus to further these research goals and contribute to the investigation of the legacy of the Vietnam War and honor a commitment to the veterans community,” Andrew F. Olshan, PhD, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, wrote in an accompanying editorial.
SOURCE:
The study was led by Jonathan C. Hwang, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, and was published online on February 4 in JAMA Dermatology.
LIMITATIONS:
The case-control design limits causal inference and the findings might not be generalized outside US veterans. Exposure misclassification could be present.
DISCLOSURES:
The study was supported by the Department of Defense and the Department of Veterans Affairs. Several authors reported receiving grants from CU Anschutz Medical Center, Department of Defense, CDMRP Melanoma Research Program, and Merck, Bayer, and Department of Veteran Affairs. They also reported receiving royalty from UpToDate, and being shareholder in many companies, including Apple. NVIDIA, Amazon, Gilead, AstraZeneca, BioNTech, and Moderna. Olshan declared being a member of the National Academies of Sciences, Engineering, and Medicine Veterans and Agent Orange review committee.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
US Vet Study Identifies Risk Factors for Acral Melanoma
US Vet Study Identifies Risk Factors for Acral Melanoma
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
When Skin Cancer Awareness Month rolls around every May, my social media feed is inundated with posts extolling the benefits of total body skin examinations and the life-saving potential of skin cancer screenings; however, time and again the US Preventive Services Task Force (USPSTF)—the leading authority on evidence-based public health recommendations in the United States—has found the evidence supporting skin cancer screenings to be insufficient. The USPSTF has cited a lack of high-quality studies and inadequate data to recommend screening for the general population, excluding those at elevated risk due to personal, family, or occupational history.1 A 2019 Cochrane review went further, concluding that current evidence refutes the utility of population-based screening for melanoma.2
Despite these findings, skin cancer screenings and total body skin examinations remain popular among patients both with and without a personal or family history of cutaneous malignancy. Indeed, the anecdotal experience of dermatologists worldwide suggests an intangible benefit to screening that persists, even if robust data to support it remain elusive.
Putting aside studies that suggest these screenings help identify melanomas at earlier stages and with reduced Breslow thicknesses,3 there is a crucial benefit from face-to-face interaction between medical professionals and the public during skin cancer screening events or health fairs. This interaction has become especially important in an era when misinformation thrives online and so-called skin care “experts” with no formal training can amass tens of thousands—or even millions—of followers on social media.
So, what are the intangible benefits of the face-to-face interactions that occur naturally during skin cancer screenings? The most obvious is education. While the USPSTF may not recommend routine screening for skin cancer in the general population, it does endorse education for children, adolescents, and adults on the importance of minimizing exposure to UV radiation, particularly those with lighter skin tones.4 Publicly advertised skin cancer screenings at health fairs or other community events may offer an opportunity to raise awareness about sun safety and protection, including the value of peak UV avoidance, sun-protective clothing, and proper sunscreen use; these settings also serve as platforms for health care providers to counter misinformation, including concerns about sunscreen safety both for the patient and the environment, overhyped risks for vitamin D deficiency from sun avoidance, and myths about low skin cancer risk in patients with skin of color.
While the benefits of skin self-examination (SSE) remain uncertain, especially in low-risk populations, screening events provide an opportunity to educate patients on who is most likely to benefit from SSE and in whom the practice may cause more harm than good.5 For higher-risk individuals such as melanoma survivors or those with a strong family history, screening fairs can serve as meaningful touchpoints that reinforce the importance of sun protection and regular examinations with a health care provider. For those eager to perform SSEs, these events offer the chance to teach best practices—how to conduct SSEs effectively, what features to look for (eg, the ABCDE method or the ugly duckling sign), and when to seek professional care.
Finally (and importantly), skin cancer screening events provide peace of mind for patients. Reassurance from a professional about a benign skin lesion can alleviate anxiety that might otherwise lead to emergency or urgent care visits. While cellulitis and other skin infections are the most common dermatologic conditions seen in emergency settings, benign neoplasms and similar nonurgent conditions still contribute a substantial burden to urgent care systems in the United States.6 Outside emergency care, systems-level data support what many of us observe in practice: two of the most common reasons for referral to dermatology are benign neoplasms and epidermoid cysts, accounting for millions of visits annually.7 In fact, recent claims data suggest that the most common diagnosis made in US dermatology clinics in 2023 was (you guessed it!) seborrheic keratosis.8
What if instead of requiring a patient to wait weeks for a primary care appointment and months for a dermatology referral—all while worrying about a rapidly growing pigmented lesion and incurring costs in copays, travel, lost wages, and time away from work—we offered a fast, trustworthy, and free evaluation that meets the patient where they live, work, or socialize? An evaluation that not only eases their fears but also provides meaningful education about skin cancer prevention and screening guidelines? While precautions must of course be taken to ensure that the quality and completeness of such an examination equals that of an in-clinic evaluation, if services of this quality can be provided, public screening events may offer a simple, accessible, and valuable solution that delivers peace of mind and helps reduce unnecessary strain on emergency, primary, and specialty care networks.
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Johansson M, Brodersen J, Gøtzsche PC. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352. doi:10.1002/14651858.CD012352.pub2
- Matsumoto M, Wack S, Weinstock MA, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol. 2022;158:504-512. doi:10.1001/jamadermatol.2022.0253
- Grossman DC, Curry SJ, Owens DK, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
- Ersser SJ, Effah A, Dyson J, et al. Effectiveness of interventions to support the early detection of skin cancer through skin self‐examination: a systematic review and meta‐analysis. Br J Dermatol. 2019;180:1339-1347. doi:10.1111/bjd.17529
- Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1266. doi:10.1016/j.jaad.2016.07.054
- Grada A, Muddasani S, Fleischer AB Jr. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15:E82-E86.
- Definitive Healthcare. What are the most common diagnoses by dermatologists? Published January 31, 2024. Accessed May 5, 2025. https://www.definitivehc.com/resources/healthcare-insights/top-dermatologist-diagnoses
When Skin Cancer Awareness Month rolls around every May, my social media feed is inundated with posts extolling the benefits of total body skin examinations and the life-saving potential of skin cancer screenings; however, time and again the US Preventive Services Task Force (USPSTF)—the leading authority on evidence-based public health recommendations in the United States—has found the evidence supporting skin cancer screenings to be insufficient. The USPSTF has cited a lack of high-quality studies and inadequate data to recommend screening for the general population, excluding those at elevated risk due to personal, family, or occupational history.1 A 2019 Cochrane review went further, concluding that current evidence refutes the utility of population-based screening for melanoma.2
Despite these findings, skin cancer screenings and total body skin examinations remain popular among patients both with and without a personal or family history of cutaneous malignancy. Indeed, the anecdotal experience of dermatologists worldwide suggests an intangible benefit to screening that persists, even if robust data to support it remain elusive.
Putting aside studies that suggest these screenings help identify melanomas at earlier stages and with reduced Breslow thicknesses,3 there is a crucial benefit from face-to-face interaction between medical professionals and the public during skin cancer screening events or health fairs. This interaction has become especially important in an era when misinformation thrives online and so-called skin care “experts” with no formal training can amass tens of thousands—or even millions—of followers on social media.
So, what are the intangible benefits of the face-to-face interactions that occur naturally during skin cancer screenings? The most obvious is education. While the USPSTF may not recommend routine screening for skin cancer in the general population, it does endorse education for children, adolescents, and adults on the importance of minimizing exposure to UV radiation, particularly those with lighter skin tones.4 Publicly advertised skin cancer screenings at health fairs or other community events may offer an opportunity to raise awareness about sun safety and protection, including the value of peak UV avoidance, sun-protective clothing, and proper sunscreen use; these settings also serve as platforms for health care providers to counter misinformation, including concerns about sunscreen safety both for the patient and the environment, overhyped risks for vitamin D deficiency from sun avoidance, and myths about low skin cancer risk in patients with skin of color.
While the benefits of skin self-examination (SSE) remain uncertain, especially in low-risk populations, screening events provide an opportunity to educate patients on who is most likely to benefit from SSE and in whom the practice may cause more harm than good.5 For higher-risk individuals such as melanoma survivors or those with a strong family history, screening fairs can serve as meaningful touchpoints that reinforce the importance of sun protection and regular examinations with a health care provider. For those eager to perform SSEs, these events offer the chance to teach best practices—how to conduct SSEs effectively, what features to look for (eg, the ABCDE method or the ugly duckling sign), and when to seek professional care.
Finally (and importantly), skin cancer screening events provide peace of mind for patients. Reassurance from a professional about a benign skin lesion can alleviate anxiety that might otherwise lead to emergency or urgent care visits. While cellulitis and other skin infections are the most common dermatologic conditions seen in emergency settings, benign neoplasms and similar nonurgent conditions still contribute a substantial burden to urgent care systems in the United States.6 Outside emergency care, systems-level data support what many of us observe in practice: two of the most common reasons for referral to dermatology are benign neoplasms and epidermoid cysts, accounting for millions of visits annually.7 In fact, recent claims data suggest that the most common diagnosis made in US dermatology clinics in 2023 was (you guessed it!) seborrheic keratosis.8
What if instead of requiring a patient to wait weeks for a primary care appointment and months for a dermatology referral—all while worrying about a rapidly growing pigmented lesion and incurring costs in copays, travel, lost wages, and time away from work—we offered a fast, trustworthy, and free evaluation that meets the patient where they live, work, or socialize? An evaluation that not only eases their fears but also provides meaningful education about skin cancer prevention and screening guidelines? While precautions must of course be taken to ensure that the quality and completeness of such an examination equals that of an in-clinic evaluation, if services of this quality can be provided, public screening events may offer a simple, accessible, and valuable solution that delivers peace of mind and helps reduce unnecessary strain on emergency, primary, and specialty care networks.
When Skin Cancer Awareness Month rolls around every May, my social media feed is inundated with posts extolling the benefits of total body skin examinations and the life-saving potential of skin cancer screenings; however, time and again the US Preventive Services Task Force (USPSTF)—the leading authority on evidence-based public health recommendations in the United States—has found the evidence supporting skin cancer screenings to be insufficient. The USPSTF has cited a lack of high-quality studies and inadequate data to recommend screening for the general population, excluding those at elevated risk due to personal, family, or occupational history.1 A 2019 Cochrane review went further, concluding that current evidence refutes the utility of population-based screening for melanoma.2
Despite these findings, skin cancer screenings and total body skin examinations remain popular among patients both with and without a personal or family history of cutaneous malignancy. Indeed, the anecdotal experience of dermatologists worldwide suggests an intangible benefit to screening that persists, even if robust data to support it remain elusive.
Putting aside studies that suggest these screenings help identify melanomas at earlier stages and with reduced Breslow thicknesses,3 there is a crucial benefit from face-to-face interaction between medical professionals and the public during skin cancer screening events or health fairs. This interaction has become especially important in an era when misinformation thrives online and so-called skin care “experts” with no formal training can amass tens of thousands—or even millions—of followers on social media.
So, what are the intangible benefits of the face-to-face interactions that occur naturally during skin cancer screenings? The most obvious is education. While the USPSTF may not recommend routine screening for skin cancer in the general population, it does endorse education for children, adolescents, and adults on the importance of minimizing exposure to UV radiation, particularly those with lighter skin tones.4 Publicly advertised skin cancer screenings at health fairs or other community events may offer an opportunity to raise awareness about sun safety and protection, including the value of peak UV avoidance, sun-protective clothing, and proper sunscreen use; these settings also serve as platforms for health care providers to counter misinformation, including concerns about sunscreen safety both for the patient and the environment, overhyped risks for vitamin D deficiency from sun avoidance, and myths about low skin cancer risk in patients with skin of color.
While the benefits of skin self-examination (SSE) remain uncertain, especially in low-risk populations, screening events provide an opportunity to educate patients on who is most likely to benefit from SSE and in whom the practice may cause more harm than good.5 For higher-risk individuals such as melanoma survivors or those with a strong family history, screening fairs can serve as meaningful touchpoints that reinforce the importance of sun protection and regular examinations with a health care provider. For those eager to perform SSEs, these events offer the chance to teach best practices—how to conduct SSEs effectively, what features to look for (eg, the ABCDE method or the ugly duckling sign), and when to seek professional care.
Finally (and importantly), skin cancer screening events provide peace of mind for patients. Reassurance from a professional about a benign skin lesion can alleviate anxiety that might otherwise lead to emergency or urgent care visits. While cellulitis and other skin infections are the most common dermatologic conditions seen in emergency settings, benign neoplasms and similar nonurgent conditions still contribute a substantial burden to urgent care systems in the United States.6 Outside emergency care, systems-level data support what many of us observe in practice: two of the most common reasons for referral to dermatology are benign neoplasms and epidermoid cysts, accounting for millions of visits annually.7 In fact, recent claims data suggest that the most common diagnosis made in US dermatology clinics in 2023 was (you guessed it!) seborrheic keratosis.8
What if instead of requiring a patient to wait weeks for a primary care appointment and months for a dermatology referral—all while worrying about a rapidly growing pigmented lesion and incurring costs in copays, travel, lost wages, and time away from work—we offered a fast, trustworthy, and free evaluation that meets the patient where they live, work, or socialize? An evaluation that not only eases their fears but also provides meaningful education about skin cancer prevention and screening guidelines? While precautions must of course be taken to ensure that the quality and completeness of such an examination equals that of an in-clinic evaluation, if services of this quality can be provided, public screening events may offer a simple, accessible, and valuable solution that delivers peace of mind and helps reduce unnecessary strain on emergency, primary, and specialty care networks.
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Johansson M, Brodersen J, Gøtzsche PC. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352. doi:10.1002/14651858.CD012352.pub2
- Matsumoto M, Wack S, Weinstock MA, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol. 2022;158:504-512. doi:10.1001/jamadermatol.2022.0253
- Grossman DC, Curry SJ, Owens DK, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
- Ersser SJ, Effah A, Dyson J, et al. Effectiveness of interventions to support the early detection of skin cancer through skin self‐examination: a systematic review and meta‐analysis. Br J Dermatol. 2019;180:1339-1347. doi:10.1111/bjd.17529
- Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1266. doi:10.1016/j.jaad.2016.07.054
- Grada A, Muddasani S, Fleischer AB Jr. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15:E82-E86.
- Definitive Healthcare. What are the most common diagnoses by dermatologists? Published January 31, 2024. Accessed May 5, 2025. https://www.definitivehc.com/resources/healthcare-insights/top-dermatologist-diagnoses
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Johansson M, Brodersen J, Gøtzsche PC. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352. doi:10.1002/14651858.CD012352.pub2
- Matsumoto M, Wack S, Weinstock MA, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol. 2022;158:504-512. doi:10.1001/jamadermatol.2022.0253
- Grossman DC, Curry SJ, Owens DK, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
- Ersser SJ, Effah A, Dyson J, et al. Effectiveness of interventions to support the early detection of skin cancer through skin self‐examination: a systematic review and meta‐analysis. Br J Dermatol. 2019;180:1339-1347. doi:10.1111/bjd.17529
- Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1266. doi:10.1016/j.jaad.2016.07.054
- Grada A, Muddasani S, Fleischer AB Jr. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15:E82-E86.
- Definitive Healthcare. What are the most common diagnoses by dermatologists? Published January 31, 2024. Accessed May 5, 2025. https://www.definitivehc.com/resources/healthcare-insights/top-dermatologist-diagnoses
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Practice Gap
Tissue processing and complete margin assessment in Mohs micrographic surgery (MMS) are challenging concepts for residents, yet they are essential components of the dermatology residency curriculum. We propose a hands-on active teaching method using craft foam blocks to help residents master these techniques. Prior educational tools have included instructional videos1 as well as the peanut butter–cup and cantaloupe analogies.2,3 Specifically, our method utilizes inexpensive, readily available supplies that allow for repeated practice in a low-stakes environment without limitation of resources. This method provides an immersive, hands-on experience that allows residents to perform multiple practice excisions and simulate positive peripheral or deep margins, unlike tools that offer only fixed-depth or purely visual representations. Additionally, our learning model uniquely enables residents to flatten the simulated tissue, providing a clearer understanding of how a 3-dimensional specimen is transformed on a slide during histologic preparation. This step is particularly important, as tissue architecture can shift during processing, making it one of the most difficult concepts to grasp without hands-on experience. Having a multitude of teaching methods is crucial to accommodate various learning styles, and active learning has been shown to enhance retention for dermatology residents.4
The Technique
Residents use simple art supplies (including craft foam blocks and ink) and inexpensive, readily available surgical tools to simulate MMS (Table)(Figure 1). If desired, the resident can follow along with the comprehensive, stepwise textbook description of MMS, outlined by Benedetto et al5 to contextualize this hands-on exercise within a standardized didactic framework.


The foam block, which represents patient tissue, serves as the specimen. The resident begins by freehand drawing a simulated cutaneous tumor directly onto the foam using a surgical marking pen. At this point, the instructor discusses the advantages and limitations of tumor debulking with a sharp blade or curette. Residents then mark appropriate margins (1-3 mm) of normal-appearing “epidermis” on the foam block and add hash marks for orientation. This is another opportunity for the instructor to discuss common methods for marking tissue in vivo and to review situations when larger or smaller margins might be appropriate.
Next, the resident removes the first layer of simulated tissue using a disposable #15 blade scalpel at a 45° angle circumferentially and deep around the representative tumor. The resident also may use scissors and tissue forceps to remove the representative tumor. Next, the excised foam layer (the simulated “specimen”) is transferred to gauze. To demonstrate a positive margin, the resident or instructor marks the deep or peripheral foam block with a surgical marking pen, indicating residual tumor (Figure 2). This allows for multiple sequential layers of foam to be removed, demonstrating successive stages of MMS.

An inkwell holds different colors of washable paint to simulate tissue inking. After excision, the resident uses cotton-tipped applicators to apply different paint colors to the edges of the excised foam specimen at designated orientation points (eg, 3 o'clock and 12 o'clock). The resident then records the location of the excised sample by hand-drawing it on a printable Mohs map, labeling the corresponding paint colors to indicate orientation (Figure 3).
The resident then places the specimen between 2 plastic page protectors mimicking a glass slide and cover slip. Clear tape can be used to help flatten the specimen (Figure 4). The tissue is compressed between the page protector so that the simulated epidermis, dermis, and subcutaneous fat are all in the same plane. At this stage, the instructor may discuss the use of relaxing incisions, especially for deeper tissue specimens or when excision at a 45° bevel is not achieved.5 The view from the underside of the page protector reveals 100% of the specimen’s margin and mimics the first cut off the tissue block. The resident can visualize the complete circumferential, peripheral, and deep margins and can easily identify any positive margins. At this point, the exercise can conclude, or the resident can explore further stages for positive margins, bisected specimens, or other tissue preparation variations.

Practice Implications
By individually designing and removing a representative tumor with margins, creating hash marks, and preparing a tissue specimen for histologic analysis, our interactive teaching method provides dermatology residents with a relatively simple, effective, and active learning experience for MMS outside the surgical setting. Using a piece of craft foam allows the representative tissue to be manipulated and flattened, similar to cutaneous tissue. This method was implemented and refined across 3 separate teaching sessions held by teaching faculty (E.I.P and E.B.W.) at the San Antonio Uniformed Services Health Education Consortium Dermatology Residency Program (San Antonio, Texas). This method has consistently generated strong resident engagement and prompted insightful questions and discussions. Program directors at other residency programs can readily incorporate this method in their surgical curriculum by allocating a brief didactic period to the exercise and facilitating the discussion with a dermatologic surgeon. Its simplicity, low cost, and effectiveness make the foam block model an easily adoptable teaching tool for dermatology residency programs seeking to provide a comprehensive, hands-on understanding of MMS.
- McNeil E, Reich H, Hurliman E. Educational video improves dermatology residents’ understanding of Mohs micrographic surgery: a surveybased matched cohort study. J Am Acad Dermatol. 2020;83:926-927. doi:10.1016/j.jaad.2020.01.013
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744. doi:10.1001 /jamadermatol.2017.0614
- Vassantachart JM, Guccione J, Seeburger J. Clinical pearl: Mohs cantaloupe analogy for the dermatology resident. Cutis. 2018; 102:65-66.
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425. doi:10.1080/01421590801946988
- Benedetto PX, Poblete-Lopez C. Mohs micrographic surgery technique. Dermatol Clinics. 2011;29:141-151. doi:10.1016/j.det.2011.02.002
Practice Gap
Tissue processing and complete margin assessment in Mohs micrographic surgery (MMS) are challenging concepts for residents, yet they are essential components of the dermatology residency curriculum. We propose a hands-on active teaching method using craft foam blocks to help residents master these techniques. Prior educational tools have included instructional videos1 as well as the peanut butter–cup and cantaloupe analogies.2,3 Specifically, our method utilizes inexpensive, readily available supplies that allow for repeated practice in a low-stakes environment without limitation of resources. This method provides an immersive, hands-on experience that allows residents to perform multiple practice excisions and simulate positive peripheral or deep margins, unlike tools that offer only fixed-depth or purely visual representations. Additionally, our learning model uniquely enables residents to flatten the simulated tissue, providing a clearer understanding of how a 3-dimensional specimen is transformed on a slide during histologic preparation. This step is particularly important, as tissue architecture can shift during processing, making it one of the most difficult concepts to grasp without hands-on experience. Having a multitude of teaching methods is crucial to accommodate various learning styles, and active learning has been shown to enhance retention for dermatology residents.4
The Technique
Residents use simple art supplies (including craft foam blocks and ink) and inexpensive, readily available surgical tools to simulate MMS (Table)(Figure 1). If desired, the resident can follow along with the comprehensive, stepwise textbook description of MMS, outlined by Benedetto et al5 to contextualize this hands-on exercise within a standardized didactic framework.


The foam block, which represents patient tissue, serves as the specimen. The resident begins by freehand drawing a simulated cutaneous tumor directly onto the foam using a surgical marking pen. At this point, the instructor discusses the advantages and limitations of tumor debulking with a sharp blade or curette. Residents then mark appropriate margins (1-3 mm) of normal-appearing “epidermis” on the foam block and add hash marks for orientation. This is another opportunity for the instructor to discuss common methods for marking tissue in vivo and to review situations when larger or smaller margins might be appropriate.
Next, the resident removes the first layer of simulated tissue using a disposable #15 blade scalpel at a 45° angle circumferentially and deep around the representative tumor. The resident also may use scissors and tissue forceps to remove the representative tumor. Next, the excised foam layer (the simulated “specimen”) is transferred to gauze. To demonstrate a positive margin, the resident or instructor marks the deep or peripheral foam block with a surgical marking pen, indicating residual tumor (Figure 2). This allows for multiple sequential layers of foam to be removed, demonstrating successive stages of MMS.

An inkwell holds different colors of washable paint to simulate tissue inking. After excision, the resident uses cotton-tipped applicators to apply different paint colors to the edges of the excised foam specimen at designated orientation points (eg, 3 o'clock and 12 o'clock). The resident then records the location of the excised sample by hand-drawing it on a printable Mohs map, labeling the corresponding paint colors to indicate orientation (Figure 3).

The resident then places the specimen between 2 plastic page protectors mimicking a glass slide and cover slip. Clear tape can be used to help flatten the specimen (Figure 4). The tissue is compressed between the page protector so that the simulated epidermis, dermis, and subcutaneous fat are all in the same plane. At this stage, the instructor may discuss the use of relaxing incisions, especially for deeper tissue specimens or when excision at a 45° bevel is not achieved.5 The view from the underside of the page protector reveals 100% of the specimen’s margin and mimics the first cut off the tissue block. The resident can visualize the complete circumferential, peripheral, and deep margins and can easily identify any positive margins. At this point, the exercise can conclude, or the resident can explore further stages for positive margins, bisected specimens, or other tissue preparation variations.

Practice Implications
By individually designing and removing a representative tumor with margins, creating hash marks, and preparing a tissue specimen for histologic analysis, our interactive teaching method provides dermatology residents with a relatively simple, effective, and active learning experience for MMS outside the surgical setting. Using a piece of craft foam allows the representative tissue to be manipulated and flattened, similar to cutaneous tissue. This method was implemented and refined across 3 separate teaching sessions held by teaching faculty (E.I.P and E.B.W.) at the San Antonio Uniformed Services Health Education Consortium Dermatology Residency Program (San Antonio, Texas). This method has consistently generated strong resident engagement and prompted insightful questions and discussions. Program directors at other residency programs can readily incorporate this method in their surgical curriculum by allocating a brief didactic period to the exercise and facilitating the discussion with a dermatologic surgeon. Its simplicity, low cost, and effectiveness make the foam block model an easily adoptable teaching tool for dermatology residency programs seeking to provide a comprehensive, hands-on understanding of MMS.
Practice Gap
Tissue processing and complete margin assessment in Mohs micrographic surgery (MMS) are challenging concepts for residents, yet they are essential components of the dermatology residency curriculum. We propose a hands-on active teaching method using craft foam blocks to help residents master these techniques. Prior educational tools have included instructional videos1 as well as the peanut butter–cup and cantaloupe analogies.2,3 Specifically, our method utilizes inexpensive, readily available supplies that allow for repeated practice in a low-stakes environment without limitation of resources. This method provides an immersive, hands-on experience that allows residents to perform multiple practice excisions and simulate positive peripheral or deep margins, unlike tools that offer only fixed-depth or purely visual representations. Additionally, our learning model uniquely enables residents to flatten the simulated tissue, providing a clearer understanding of how a 3-dimensional specimen is transformed on a slide during histologic preparation. This step is particularly important, as tissue architecture can shift during processing, making it one of the most difficult concepts to grasp without hands-on experience. Having a multitude of teaching methods is crucial to accommodate various learning styles, and active learning has been shown to enhance retention for dermatology residents.4
The Technique
Residents use simple art supplies (including craft foam blocks and ink) and inexpensive, readily available surgical tools to simulate MMS (Table)(Figure 1). If desired, the resident can follow along with the comprehensive, stepwise textbook description of MMS, outlined by Benedetto et al5 to contextualize this hands-on exercise within a standardized didactic framework.


The foam block, which represents patient tissue, serves as the specimen. The resident begins by freehand drawing a simulated cutaneous tumor directly onto the foam using a surgical marking pen. At this point, the instructor discusses the advantages and limitations of tumor debulking with a sharp blade or curette. Residents then mark appropriate margins (1-3 mm) of normal-appearing “epidermis” on the foam block and add hash marks for orientation. This is another opportunity for the instructor to discuss common methods for marking tissue in vivo and to review situations when larger or smaller margins might be appropriate.
Next, the resident removes the first layer of simulated tissue using a disposable #15 blade scalpel at a 45° angle circumferentially and deep around the representative tumor. The resident also may use scissors and tissue forceps to remove the representative tumor. Next, the excised foam layer (the simulated “specimen”) is transferred to gauze. To demonstrate a positive margin, the resident or instructor marks the deep or peripheral foam block with a surgical marking pen, indicating residual tumor (Figure 2). This allows for multiple sequential layers of foam to be removed, demonstrating successive stages of MMS.

An inkwell holds different colors of washable paint to simulate tissue inking. After excision, the resident uses cotton-tipped applicators to apply different paint colors to the edges of the excised foam specimen at designated orientation points (eg, 3 o'clock and 12 o'clock). The resident then records the location of the excised sample by hand-drawing it on a printable Mohs map, labeling the corresponding paint colors to indicate orientation (Figure 3).

The resident then places the specimen between 2 plastic page protectors mimicking a glass slide and cover slip. Clear tape can be used to help flatten the specimen (Figure 4). The tissue is compressed between the page protector so that the simulated epidermis, dermis, and subcutaneous fat are all in the same plane. At this stage, the instructor may discuss the use of relaxing incisions, especially for deeper tissue specimens or when excision at a 45° bevel is not achieved.5 The view from the underside of the page protector reveals 100% of the specimen’s margin and mimics the first cut off the tissue block. The resident can visualize the complete circumferential, peripheral, and deep margins and can easily identify any positive margins. At this point, the exercise can conclude, or the resident can explore further stages for positive margins, bisected specimens, or other tissue preparation variations.

Practice Implications
By individually designing and removing a representative tumor with margins, creating hash marks, and preparing a tissue specimen for histologic analysis, our interactive teaching method provides dermatology residents with a relatively simple, effective, and active learning experience for MMS outside the surgical setting. Using a piece of craft foam allows the representative tissue to be manipulated and flattened, similar to cutaneous tissue. This method was implemented and refined across 3 separate teaching sessions held by teaching faculty (E.I.P and E.B.W.) at the San Antonio Uniformed Services Health Education Consortium Dermatology Residency Program (San Antonio, Texas). This method has consistently generated strong resident engagement and prompted insightful questions and discussions. Program directors at other residency programs can readily incorporate this method in their surgical curriculum by allocating a brief didactic period to the exercise and facilitating the discussion with a dermatologic surgeon. Its simplicity, low cost, and effectiveness make the foam block model an easily adoptable teaching tool for dermatology residency programs seeking to provide a comprehensive, hands-on understanding of MMS.
- McNeil E, Reich H, Hurliman E. Educational video improves dermatology residents’ understanding of Mohs micrographic surgery: a surveybased matched cohort study. J Am Acad Dermatol. 2020;83:926-927. doi:10.1016/j.jaad.2020.01.013
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744. doi:10.1001 /jamadermatol.2017.0614
- Vassantachart JM, Guccione J, Seeburger J. Clinical pearl: Mohs cantaloupe analogy for the dermatology resident. Cutis. 2018; 102:65-66.
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425. doi:10.1080/01421590801946988
- Benedetto PX, Poblete-Lopez C. Mohs micrographic surgery technique. Dermatol Clinics. 2011;29:141-151. doi:10.1016/j.det.2011.02.002
- McNeil E, Reich H, Hurliman E. Educational video improves dermatology residents’ understanding of Mohs micrographic surgery: a surveybased matched cohort study. J Am Acad Dermatol. 2020;83:926-927. doi:10.1016/j.jaad.2020.01.013
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744. doi:10.1001 /jamadermatol.2017.0614
- Vassantachart JM, Guccione J, Seeburger J. Clinical pearl: Mohs cantaloupe analogy for the dermatology resident. Cutis. 2018; 102:65-66.
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425. doi:10.1080/01421590801946988
- Benedetto PX, Poblete-Lopez C. Mohs micrographic surgery technique. Dermatol Clinics. 2011;29:141-151. doi:10.1016/j.det.2011.02.002
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Does Ethnicity Affect Skin Cancer Risk?
Does Ethnicity Affect Skin Cancer Risk?
TOPLINE:
The incidence of skin cancer in England varied by ethnicity: White individuals had higher rates of melanoma, cutaneous squamous cell carcinoma, and basal cell carcinoma than Asian or Black individuals. In contrast, acral lentiginous melanoma was most common among Black individuals, whereas cutaneous T-cell lymphoma and Kaposi sarcoma were highest among those in the "Other" ethnic group.
METHODOLOGY:
- Researchers analysed all cases of cutaneous melanoma (melanoma and acral lentiginous melanoma), basal cell carcinoma, cutaneous squamous cell carcinoma, cutaneous T-cell lymphoma, and Kaposi sarcoma using data from the NHS National Disease Registration Service cancer registry between 2013 and 2020.
- Data collection incorporated ethnicity information from multiple health care datasets, including Clinical Outcomes and Services Dataset, Patient Administration System, Radiotherapy Dataset, Diagnostic Imaging Dataset, and Hospital Episode Statistics.
- A population analysis categorised patients into 7 standardised ethnic groups (on the basis of Office for National Statistics classifications): White, Asian, Chinese, Black, mixed, other, and unknown groups, with ethnicity data being self-reported by patients.
- Outcomes included European age-standardised rates calculated using the 2013 European Standard Population and reported per 100,000 person-years (PYs).
TAKEAWAY:
- White Individuals had 13-fold higher rates of cutaneous squamous cell carcinoma (61.75 per 100,000 PYs), 26-fold and 27-fold higher rates of basal cell carcinoma (153.69 per 100,000 PYs), and 33-fold and 16-fold higher rates of cutaneous melanoma (27.29 per 100,000 PYs) than Asian and Black individuals, respectively.
- Black individuals had the highest incidence of acral lentiginous melanoma (0.85 per 100,000 PYs), and those in the other ethnic group had the highest incidence of cutaneous T-cell lymphoma (1.74 per 100,000 PYs) and Kaposi sarcoma (1.57 per 100,000 PYs).
- The presentation of early-stage melanoma was low among Asian (53.5%), Black (62.4%), mixed (62.5%), and other (76.4%) ethnic groups compared to that among White ethnicities (79.8%).
- Acral lentiginous melanomas were less likely to get urgent suspected cancer pathway referrals than overall melanoma (40.1% vs 44.6%; P < .001) and more likely to be diagnosed late than overall melanoma (stage I/II at diagnosis; 72% vs 80%; P < .0001).
IN PRACTICE:
"The findings emphasise the need for better, targeted ethnicity data collection strategies to address incidence, outcomes and health care equity for not just skin cancer but all health conditions in underserved populations," the authors wrote. "While projects like the Global Burden of Disease have improved global health care reporting, continuous audit and improvement of collected data are essential to provide better care across people of all ethnicities."
SOURCE:
This study was led by Shehnaz Ahmed, British Association of Dermatologists, London, England. It was published online on September 10, 2025, in the British Journal of Dermatology.
LIMITATIONS:
Census data collection after every 10 years could have contributed to inaccurate population estimates and incidence rates. Small sample sizes in certain ethnic groups could have led to potential confounders, requiring a cautious interpretation of relative incidence. The NHS data included only self-reported ethnicity data with no available details of skin phototypes, skin tones, or racial ancestry. This study lacked granular ethnicity census data and stage data for basal cell carcinoma, cutaneous small cell carcinoma, and Kaposi sarcoma.
DISCLOSURES:
This research was supported through a partnership between the British Association of Dermatologists and NHS England's National Disease Registration Service. Two authors reported being employees of the British Association of Dermatologists.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
The incidence of skin cancer in England varied by ethnicity: White individuals had higher rates of melanoma, cutaneous squamous cell carcinoma, and basal cell carcinoma than Asian or Black individuals. In contrast, acral lentiginous melanoma was most common among Black individuals, whereas cutaneous T-cell lymphoma and Kaposi sarcoma were highest among those in the "Other" ethnic group.
METHODOLOGY:
- Researchers analysed all cases of cutaneous melanoma (melanoma and acral lentiginous melanoma), basal cell carcinoma, cutaneous squamous cell carcinoma, cutaneous T-cell lymphoma, and Kaposi sarcoma using data from the NHS National Disease Registration Service cancer registry between 2013 and 2020.
- Data collection incorporated ethnicity information from multiple health care datasets, including Clinical Outcomes and Services Dataset, Patient Administration System, Radiotherapy Dataset, Diagnostic Imaging Dataset, and Hospital Episode Statistics.
- A population analysis categorised patients into 7 standardised ethnic groups (on the basis of Office for National Statistics classifications): White, Asian, Chinese, Black, mixed, other, and unknown groups, with ethnicity data being self-reported by patients.
- Outcomes included European age-standardised rates calculated using the 2013 European Standard Population and reported per 100,000 person-years (PYs).
TAKEAWAY:
- White Individuals had 13-fold higher rates of cutaneous squamous cell carcinoma (61.75 per 100,000 PYs), 26-fold and 27-fold higher rates of basal cell carcinoma (153.69 per 100,000 PYs), and 33-fold and 16-fold higher rates of cutaneous melanoma (27.29 per 100,000 PYs) than Asian and Black individuals, respectively.
- Black individuals had the highest incidence of acral lentiginous melanoma (0.85 per 100,000 PYs), and those in the other ethnic group had the highest incidence of cutaneous T-cell lymphoma (1.74 per 100,000 PYs) and Kaposi sarcoma (1.57 per 100,000 PYs).
- The presentation of early-stage melanoma was low among Asian (53.5%), Black (62.4%), mixed (62.5%), and other (76.4%) ethnic groups compared to that among White ethnicities (79.8%).
- Acral lentiginous melanomas were less likely to get urgent suspected cancer pathway referrals than overall melanoma (40.1% vs 44.6%; P < .001) and more likely to be diagnosed late than overall melanoma (stage I/II at diagnosis; 72% vs 80%; P < .0001).
IN PRACTICE:
"The findings emphasise the need for better, targeted ethnicity data collection strategies to address incidence, outcomes and health care equity for not just skin cancer but all health conditions in underserved populations," the authors wrote. "While projects like the Global Burden of Disease have improved global health care reporting, continuous audit and improvement of collected data are essential to provide better care across people of all ethnicities."
SOURCE:
This study was led by Shehnaz Ahmed, British Association of Dermatologists, London, England. It was published online on September 10, 2025, in the British Journal of Dermatology.
LIMITATIONS:
Census data collection after every 10 years could have contributed to inaccurate population estimates and incidence rates. Small sample sizes in certain ethnic groups could have led to potential confounders, requiring a cautious interpretation of relative incidence. The NHS data included only self-reported ethnicity data with no available details of skin phototypes, skin tones, or racial ancestry. This study lacked granular ethnicity census data and stage data for basal cell carcinoma, cutaneous small cell carcinoma, and Kaposi sarcoma.
DISCLOSURES:
This research was supported through a partnership between the British Association of Dermatologists and NHS England's National Disease Registration Service. Two authors reported being employees of the British Association of Dermatologists.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
The incidence of skin cancer in England varied by ethnicity: White individuals had higher rates of melanoma, cutaneous squamous cell carcinoma, and basal cell carcinoma than Asian or Black individuals. In contrast, acral lentiginous melanoma was most common among Black individuals, whereas cutaneous T-cell lymphoma and Kaposi sarcoma were highest among those in the "Other" ethnic group.
METHODOLOGY:
- Researchers analysed all cases of cutaneous melanoma (melanoma and acral lentiginous melanoma), basal cell carcinoma, cutaneous squamous cell carcinoma, cutaneous T-cell lymphoma, and Kaposi sarcoma using data from the NHS National Disease Registration Service cancer registry between 2013 and 2020.
- Data collection incorporated ethnicity information from multiple health care datasets, including Clinical Outcomes and Services Dataset, Patient Administration System, Radiotherapy Dataset, Diagnostic Imaging Dataset, and Hospital Episode Statistics.
- A population analysis categorised patients into 7 standardised ethnic groups (on the basis of Office for National Statistics classifications): White, Asian, Chinese, Black, mixed, other, and unknown groups, with ethnicity data being self-reported by patients.
- Outcomes included European age-standardised rates calculated using the 2013 European Standard Population and reported per 100,000 person-years (PYs).
TAKEAWAY:
- White Individuals had 13-fold higher rates of cutaneous squamous cell carcinoma (61.75 per 100,000 PYs), 26-fold and 27-fold higher rates of basal cell carcinoma (153.69 per 100,000 PYs), and 33-fold and 16-fold higher rates of cutaneous melanoma (27.29 per 100,000 PYs) than Asian and Black individuals, respectively.
- Black individuals had the highest incidence of acral lentiginous melanoma (0.85 per 100,000 PYs), and those in the other ethnic group had the highest incidence of cutaneous T-cell lymphoma (1.74 per 100,000 PYs) and Kaposi sarcoma (1.57 per 100,000 PYs).
- The presentation of early-stage melanoma was low among Asian (53.5%), Black (62.4%), mixed (62.5%), and other (76.4%) ethnic groups compared to that among White ethnicities (79.8%).
- Acral lentiginous melanomas were less likely to get urgent suspected cancer pathway referrals than overall melanoma (40.1% vs 44.6%; P < .001) and more likely to be diagnosed late than overall melanoma (stage I/II at diagnosis; 72% vs 80%; P < .0001).
IN PRACTICE:
"The findings emphasise the need for better, targeted ethnicity data collection strategies to address incidence, outcomes and health care equity for not just skin cancer but all health conditions in underserved populations," the authors wrote. "While projects like the Global Burden of Disease have improved global health care reporting, continuous audit and improvement of collected data are essential to provide better care across people of all ethnicities."
SOURCE:
This study was led by Shehnaz Ahmed, British Association of Dermatologists, London, England. It was published online on September 10, 2025, in the British Journal of Dermatology.
LIMITATIONS:
Census data collection after every 10 years could have contributed to inaccurate population estimates and incidence rates. Small sample sizes in certain ethnic groups could have led to potential confounders, requiring a cautious interpretation of relative incidence. The NHS data included only self-reported ethnicity data with no available details of skin phototypes, skin tones, or racial ancestry. This study lacked granular ethnicity census data and stage data for basal cell carcinoma, cutaneous small cell carcinoma, and Kaposi sarcoma.
DISCLOSURES:
This research was supported through a partnership between the British Association of Dermatologists and NHS England's National Disease Registration Service. Two authors reported being employees of the British Association of Dermatologists.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Does Ethnicity Affect Skin Cancer Risk?
Does Ethnicity Affect Skin Cancer Risk?
Don’t Miss Those Blind Spots
Background
Choroidal malignant melanoma is a relatively rare condition, yet it remains the most common primary intraocular malignancy in adults, affecting approximately 5 individuals per million each year in the United States. Associated risk factors include fair skin, light-colored eyes, ocular melanocytosis, and BAP1 genetic mutations. While 13% of patients presenting with choroidal melanoma are asymptomatic, some symptoms can include photopsia, floaters, blurred vision, and progressive visual field loss.
Case Presentation
We present a case of choroidal melanoma in a 57-year-old male with a past medical history of hypertension, hyperlipidemia, major depressive disorder, and alcohol use disorder. This patient presented to the clinic following a detoxification admission, reporting one week of progressive vision loss in the left eye. Upon initial physical examination, the patient exhibited left superior quadrantanopia, with a visual acuity of 20/40 measured in the left eye. Initial imaging with CT head identified an intraocular hyperdensity within the left globe, raising concerns for potential retinal detachment. Urgent ophthalmologic evaluation revealed an afferent pupillary defect and a large choroidal lesion adjacent to the optic nerve head. Ultrasonography showed a low internal reflectivity mass (5.36 mm by 9.05 mm), and a subsequent dilated fundus examination confirmed a classic dome-shaped choroidal melanoma (11.5 mm by 16.5 mm). Gene expression profiling demonstrated a class 1b uveal melanoma with PRAME positivity and mutations in GNAQ and SF3B1. Comprehensive staging scans were negative for metastatic disease. The patient received four treatment sessions of proton beam therapy, which resulted in rapid improvements in his visual fields. For long-term management, he was scheduled for close ophthalmologic follow-up and regular imaging of the chest and abdomen every six months to monitor for recurrence.
Conclusions
This case highlights the challenges of diagnosing choroidal melanoma in the primary care setting and the importance of multidisciplinary involvement, multimodal imaging, and gene expression profiling in facilitating early diagnosis and treatment.
Background
Choroidal malignant melanoma is a relatively rare condition, yet it remains the most common primary intraocular malignancy in adults, affecting approximately 5 individuals per million each year in the United States. Associated risk factors include fair skin, light-colored eyes, ocular melanocytosis, and BAP1 genetic mutations. While 13% of patients presenting with choroidal melanoma are asymptomatic, some symptoms can include photopsia, floaters, blurred vision, and progressive visual field loss.
Case Presentation
We present a case of choroidal melanoma in a 57-year-old male with a past medical history of hypertension, hyperlipidemia, major depressive disorder, and alcohol use disorder. This patient presented to the clinic following a detoxification admission, reporting one week of progressive vision loss in the left eye. Upon initial physical examination, the patient exhibited left superior quadrantanopia, with a visual acuity of 20/40 measured in the left eye. Initial imaging with CT head identified an intraocular hyperdensity within the left globe, raising concerns for potential retinal detachment. Urgent ophthalmologic evaluation revealed an afferent pupillary defect and a large choroidal lesion adjacent to the optic nerve head. Ultrasonography showed a low internal reflectivity mass (5.36 mm by 9.05 mm), and a subsequent dilated fundus examination confirmed a classic dome-shaped choroidal melanoma (11.5 mm by 16.5 mm). Gene expression profiling demonstrated a class 1b uveal melanoma with PRAME positivity and mutations in GNAQ and SF3B1. Comprehensive staging scans were negative for metastatic disease. The patient received four treatment sessions of proton beam therapy, which resulted in rapid improvements in his visual fields. For long-term management, he was scheduled for close ophthalmologic follow-up and regular imaging of the chest and abdomen every six months to monitor for recurrence.
Conclusions
This case highlights the challenges of diagnosing choroidal melanoma in the primary care setting and the importance of multidisciplinary involvement, multimodal imaging, and gene expression profiling in facilitating early diagnosis and treatment.
Background
Choroidal malignant melanoma is a relatively rare condition, yet it remains the most common primary intraocular malignancy in adults, affecting approximately 5 individuals per million each year in the United States. Associated risk factors include fair skin, light-colored eyes, ocular melanocytosis, and BAP1 genetic mutations. While 13% of patients presenting with choroidal melanoma are asymptomatic, some symptoms can include photopsia, floaters, blurred vision, and progressive visual field loss.
Case Presentation
We present a case of choroidal melanoma in a 57-year-old male with a past medical history of hypertension, hyperlipidemia, major depressive disorder, and alcohol use disorder. This patient presented to the clinic following a detoxification admission, reporting one week of progressive vision loss in the left eye. Upon initial physical examination, the patient exhibited left superior quadrantanopia, with a visual acuity of 20/40 measured in the left eye. Initial imaging with CT head identified an intraocular hyperdensity within the left globe, raising concerns for potential retinal detachment. Urgent ophthalmologic evaluation revealed an afferent pupillary defect and a large choroidal lesion adjacent to the optic nerve head. Ultrasonography showed a low internal reflectivity mass (5.36 mm by 9.05 mm), and a subsequent dilated fundus examination confirmed a classic dome-shaped choroidal melanoma (11.5 mm by 16.5 mm). Gene expression profiling demonstrated a class 1b uveal melanoma with PRAME positivity and mutations in GNAQ and SF3B1. Comprehensive staging scans were negative for metastatic disease. The patient received four treatment sessions of proton beam therapy, which resulted in rapid improvements in his visual fields. For long-term management, he was scheduled for close ophthalmologic follow-up and regular imaging of the chest and abdomen every six months to monitor for recurrence.
Conclusions
This case highlights the challenges of diagnosing choroidal melanoma in the primary care setting and the importance of multidisciplinary involvement, multimodal imaging, and gene expression profiling in facilitating early diagnosis and treatment.
Pigmented Cystic Masses on the Scalp
THE DIAGNOSIS: Apocrine Hidrocystoma
Histology for all 3 lesions demonstrated similar cystic structures lined by a dual layer of epithelial cells, with the outermost layer composed of flattened myoepithelial cells and the inner layer composed of cells with apocrine features (Figure 1). Based on these findings, a diagnosis of apocrine hidrocystoma was made. The patient underwent successful surgical excision shortly thereafter without recurrence at follow-up 1 year later.
Apocrine hidrocystomas are rare benign cystic lesions that are considered to be adenomatous proliferations of apocrine glands. They typically manifest as solitary asymptomatic lesions measuring 3 to 15 mm.1 They tend to appear on the face, usually in the periorbital region, but also have been described on the neck, scalp, trunk, arms, and legs.2-4 Multiple apocrine hidrocystomas can be a marker of 2 rare inherited disorders: Gorlin-Goltz syndrome and Schopf-Schulz-Passarge syndrome.5 Apocrine hidrocystomas may be flesh colored or may have a blue, black, or brown appearance due to the Tyndall effect, in which light with shorter wavelengths is scattered by the contents of the lesions.2 Histologically, apocrine hidrocystomas are cysts lined by a dual layer of epithelial cells. The inner layer is composed of cells with apocrine features, and the outer layer is composed of flattened myoepithelial cells. Due to their range of colors and predilection for sun-exposed surfaces, apocrine hidrocystomas may be mistaken for various malignant neoplasms, including melanoma.6,7
The differential diagnosis for our patient included agminated blue nevi, melanoma, pigmented basal cell carcinoma (BCC), and seborrheic keratosis. A blue nevus is a dermal melanocytic lesion that manifests as a well-demarcated, blue to blue-black papule that typically appears on the face, scalp, arms, legs, lower back, and buttocks. Although there are several histologic subtypes, the common blue nevus usually manifests as a solitary lesion measuring less than 1 cm, often developing during childhood to young adulthood.8 Histologically, common blue nevi are characterized by a dermal proliferation of deeply pigmented bipolar spindled melanocytes embedded in thickened collagen bundles, often with scattered epithelioid melanophages, and no conspicuous mitotic activity (Figure 2).9 There are other types of blue nevi, including cellular blue nevi, which tend to be larger and manifest commonly on the buttocks and sacrococcygeal region in early adulthood.9 Histologically, cellular blue nevi contain oval to spindled melanocytes with scattered melanophages forming a well-demarcated nodule typically in the reticular dermis. There may be bulbous extension into the subcutaneous adipose tissue. Occasional mitoses may be seen.9,10 Melanoma can arise from common or cellular blue nevi, though it more frequently occurs with cellular blue nevi. Other subtypes of blue nevi have been described, including the sclerosing, plaque-type, combined, hypomelanotic/amelanotic, and pigmented epithelioid melanocytoma.11 However, they typically have features of the common blue nevus or cellular blue nevus, such as oval/spindle cell morphology, some degree of melanin, and biphasic architecture, but are classified according to their dominant histologic characteristics.
Given the location of our patient’s lesions on the scalp and his extensive history of sun exposure, malignancy was high in the differential. Multiple synchronous primary melanomas including nodular melanoma, blue nevus–like metastatic melanoma, and metastatic melanoma were considered. The leg and the scalp have the highest reported incidence of cutaneous metastases of melanoma, with many cases presenting as dermal or subcutaneous nodules and eruptive blue nevus–like papules, similar to our patient’s clinical presentation.12,13 Nodular melanoma (NM) is one of 4 major types of melanoma, accounting for approximately 15% to 30% of cases in the United States.14 Nodular melanoma typically manifests as a smooth, raised, symmetric, well-circumscribed lesion with variable pigmentation, from very dark to amelanotic. Histologically, NM is defined as a dermal mass, either in isolation or with an epidermal component, not to exceed 3 rete ridges beyond the dermal component.15 Tumor cells have a high cell density with pleomorphism, usually with atypical epithelioid cells with vesicular nuclei and irregular cytoplasm, and occasionally spindle cells (Figure 2).16 Mitoses and necrosis are frequent. Scalp location independently is responsible for worse survival, both overall and melanoma specific.17 Nodular melanoma tends to have greater Breslow thickness at diagnosis than other melanoma subtypes and often carries a worse prognosis.
Malignant melanomas that develop from or in conjunction with or bear histologic resemblance to blue nevi are termed blue nevus–like melanoma or blue nevus–associated melanoma. These malignancies are exceedingly rare, accounting for only 0.3% of melanomas in one Turkey-based multicenter study.18 The histologic criteria for diagnosing blue nevus–like melanoma are poorly defined, and terminology of these lesions has led to some debate in naming conventions.19 Nevertheless, unlike blue nevus, blue nevus–like melanoma demonstrates histologic features of malignancy, including pleomorphism, prominent nucleoli, mitotic activity, vascular invasion, and potential necrosis.10 The lack of an inflammatory infiltrate, surrounding fibrosis, junctional activity, and pre-existing nevus can help distinguish cutaneous melanoma metastases from primary nodular melanoma. Immunohistochemical stains such as S100, Melan-A/MART1, or SOX-10 can help confirm melanocytic lineage.12
Pigmented BCC is a clinical and histologic variant of BCC characterized by increased melanin pigmentation due to melanocytes admixed with tumor cells. Dermoscopically, the pigment can have a maple leaf–like appearance with spoke-wheel areas, in-focus dots, and concentric structures at the dermoepidermal junction, which is more characteristic of superficial and infiltrating BCC.20 In nodular BCC, the pigment occurs as blue-gray ovoid nests and globules in deeper layers of the dermis.20
Seborrheic keratoses (SKs) can vary widely in clinical appearance, with pigmentation ranging from flesh colored to yellow to brown to black. Melanoacanthomas are acanthotic SKs that are highly pigmented due to intermixed epidermal melanocytes and subepidermal melanophages.21 Dermoscopy can help distinguish cutaneous malignancies from SKs, which often demonstrate fissures and ridges, comedolike openings, and milialike cysts. Biopsy sometimes is required to assess for malignancy, as was the case in our patient. The classic histologic features of SKs include acanthosis, papillomatosis, and hyperkeratosis.22
This case highlights the need to consider apocrine hidrocystoma, along with malignancy, in the differential diagnosis of pigmented cystic masses of the face and scalp. Because apocrine hidrocystomas are benign, they do not need to be treated but often are surgically excised for cosmesis or complete histopathologic examination. Destruction via electrodessication, carbon dioxide ablation, trichloroacetic acid chemical ablation, botulinum toxin injection, and anticholinergic creams sometimes is used, especially for cosmetic treatment of multiple small lesions.5 Our patient was treated with surgical excision with no evidence of recurrence on follow-up 1 year later.
- Ioannidis DG, Drivas EI, Papadakis CE, et al. Hidrocystoma of the external auditory canal: a case report. Cases J. 2009;2:79. doi:10.1186/1757- 1626-2-79
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp. Dermatol Online J. 2020;26. doi:10.5070/D3268049895
- Mendoza-Cembranos MD, Haro R, Requena L, et al. Digital apocrine hidrocystoma: the exception confirms the rule. Am J Dermatopathol. 2019;41:79. doi:10.1097/DAD.0000000000001044
- May C, Chang O, Compton N. A giant apocrine hidrocystoma of the trunk. Dermatol Online J. 2017;23. doi:10.5070/D3239036497
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. Medscape Gen Med. 2006;8:57.
- Kruse ALD, Zwahlen R, Bredell MG, et al. Apocrine hidrocystoma of the cheek. J Craniofac Surg. 2010;21:594-596. doi:10.1097 /SCS.0b013e3181d08c77
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405. doi:10.1002 /1097-0142(196803)21:3<393::aid-cncr2820210309>3.0.co;2-k
- Murali R, McCarthy SW, Scolyer RA. Blue nevi and related lesions: a review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Adv Anat Pathol. 2009;16:365. doi:10.1097/PAP.0b013e3181bb6b53
- Borgenvik TL, Karlsvik TM, Ray S, et al. Blue nevus-like and blue nevusassociated melanoma: a comprehensive review of the literature. ANZ J Surg. 2017;87:345-349. doi:10.1111/ans.13946
- de la Fouchardiere A. Blue naevi and the blue tumour spectrum. Pathology. 2023;55:187-195. doi:10.1016/j.pathol.2022.12.342
- Lowe L. Metastatic melanoma and rare melanoma variants: a review. Pathology (Phila). 2023;55:236-244. doi:10.1016/j.pathol.2022.11.006
- Plaza JA, Torres-Cabala C, Evans H, et al. Cutaneous metastases of malignant melanoma: a clinicopathologic study of 192 cases with emphasis on the morphologic spectrum. Am J Dermatopathol. 2010;32:129-136. doi:10.1097/DAD.0b013e3181b34a19
- Shaikh WR, Xiong M, Weinstock MA. The contribution of nodular subtype to melanoma mortality in the United States, 1978 to 2007. Archives of Dermatology. 2012;148:30-36. doi:10.1001/archdermatol.2011.264
- Clark WH, From L, Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705-727.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321. doi:10.23736/S2784-8671.21.06958-3
- Ozao-Choy J, Nelson DW, Hiles J, et al. The prognostic importance of scalp location in primary head and neck melanoma. J Surg Oncol. 2017;116:337-343. doi:10.1002/jso.24679
- Gamsizkan M, Yilmaz I, Buyukbabani N, et al. A retrospective multicenter evaluation of cutaneous melanomas in Turkey. Asian Pac J Cancer Prev APJCP. 2014;15:10451-10456. doi:10.7314 /apjcp.2014.15.23.10451
- Mones JM, Ackerman AB. “Atypical” blue nevus, “malignant” blue nevus, and “metastasizing” blue nevus: a critique in historical perspective of three concepts flawed fatally. Am J Dermatopathol. 2004;26:407-430. doi:10.1097/00000372-200410000-00012
- Tanese K. Diagnosis and management of basal cell carcinoma Curr Treat Options Oncol. 2019;20:13. doi:10.1007/s11864 -019-0610-0
- Barthelmann S, Butsch F, Lang BM, et al. Seborrheic keratosis. JDDG J Dtsch Dermatol Ges. 2023;21:265-277. doi:10.1111/ddg.14984
- Taylor S. Advancing the understanding of seborrheic keratosis. J Drugs Dermatol. 2017;16:419-424.
THE DIAGNOSIS: Apocrine Hidrocystoma
Histology for all 3 lesions demonstrated similar cystic structures lined by a dual layer of epithelial cells, with the outermost layer composed of flattened myoepithelial cells and the inner layer composed of cells with apocrine features (Figure 1). Based on these findings, a diagnosis of apocrine hidrocystoma was made. The patient underwent successful surgical excision shortly thereafter without recurrence at follow-up 1 year later.
Apocrine hidrocystomas are rare benign cystic lesions that are considered to be adenomatous proliferations of apocrine glands. They typically manifest as solitary asymptomatic lesions measuring 3 to 15 mm.1 They tend to appear on the face, usually in the periorbital region, but also have been described on the neck, scalp, trunk, arms, and legs.2-4 Multiple apocrine hidrocystomas can be a marker of 2 rare inherited disorders: Gorlin-Goltz syndrome and Schopf-Schulz-Passarge syndrome.5 Apocrine hidrocystomas may be flesh colored or may have a blue, black, or brown appearance due to the Tyndall effect, in which light with shorter wavelengths is scattered by the contents of the lesions.2 Histologically, apocrine hidrocystomas are cysts lined by a dual layer of epithelial cells. The inner layer is composed of cells with apocrine features, and the outer layer is composed of flattened myoepithelial cells. Due to their range of colors and predilection for sun-exposed surfaces, apocrine hidrocystomas may be mistaken for various malignant neoplasms, including melanoma.6,7

The differential diagnosis for our patient included agminated blue nevi, melanoma, pigmented basal cell carcinoma (BCC), and seborrheic keratosis. A blue nevus is a dermal melanocytic lesion that manifests as a well-demarcated, blue to blue-black papule that typically appears on the face, scalp, arms, legs, lower back, and buttocks. Although there are several histologic subtypes, the common blue nevus usually manifests as a solitary lesion measuring less than 1 cm, often developing during childhood to young adulthood.8 Histologically, common blue nevi are characterized by a dermal proliferation of deeply pigmented bipolar spindled melanocytes embedded in thickened collagen bundles, often with scattered epithelioid melanophages, and no conspicuous mitotic activity (Figure 2).9 There are other types of blue nevi, including cellular blue nevi, which tend to be larger and manifest commonly on the buttocks and sacrococcygeal region in early adulthood.9 Histologically, cellular blue nevi contain oval to spindled melanocytes with scattered melanophages forming a well-demarcated nodule typically in the reticular dermis. There may be bulbous extension into the subcutaneous adipose tissue. Occasional mitoses may be seen.9,10 Melanoma can arise from common or cellular blue nevi, though it more frequently occurs with cellular blue nevi. Other subtypes of blue nevi have been described, including the sclerosing, plaque-type, combined, hypomelanotic/amelanotic, and pigmented epithelioid melanocytoma.11 However, they typically have features of the common blue nevus or cellular blue nevus, such as oval/spindle cell morphology, some degree of melanin, and biphasic architecture, but are classified according to their dominant histologic characteristics.

Given the location of our patient’s lesions on the scalp and his extensive history of sun exposure, malignancy was high in the differential. Multiple synchronous primary melanomas including nodular melanoma, blue nevus–like metastatic melanoma, and metastatic melanoma were considered. The leg and the scalp have the highest reported incidence of cutaneous metastases of melanoma, with many cases presenting as dermal or subcutaneous nodules and eruptive blue nevus–like papules, similar to our patient’s clinical presentation.12,13 Nodular melanoma (NM) is one of 4 major types of melanoma, accounting for approximately 15% to 30% of cases in the United States.14 Nodular melanoma typically manifests as a smooth, raised, symmetric, well-circumscribed lesion with variable pigmentation, from very dark to amelanotic. Histologically, NM is defined as a dermal mass, either in isolation or with an epidermal component, not to exceed 3 rete ridges beyond the dermal component.15 Tumor cells have a high cell density with pleomorphism, usually with atypical epithelioid cells with vesicular nuclei and irregular cytoplasm, and occasionally spindle cells (Figure 2).16 Mitoses and necrosis are frequent. Scalp location independently is responsible for worse survival, both overall and melanoma specific.17 Nodular melanoma tends to have greater Breslow thickness at diagnosis than other melanoma subtypes and often carries a worse prognosis.

Malignant melanomas that develop from or in conjunction with or bear histologic resemblance to blue nevi are termed blue nevus–like melanoma or blue nevus–associated melanoma. These malignancies are exceedingly rare, accounting for only 0.3% of melanomas in one Turkey-based multicenter study.18 The histologic criteria for diagnosing blue nevus–like melanoma are poorly defined, and terminology of these lesions has led to some debate in naming conventions.19 Nevertheless, unlike blue nevus, blue nevus–like melanoma demonstrates histologic features of malignancy, including pleomorphism, prominent nucleoli, mitotic activity, vascular invasion, and potential necrosis.10 The lack of an inflammatory infiltrate, surrounding fibrosis, junctional activity, and pre-existing nevus can help distinguish cutaneous melanoma metastases from primary nodular melanoma. Immunohistochemical stains such as S100, Melan-A/MART1, or SOX-10 can help confirm melanocytic lineage.12
Pigmented BCC is a clinical and histologic variant of BCC characterized by increased melanin pigmentation due to melanocytes admixed with tumor cells. Dermoscopically, the pigment can have a maple leaf–like appearance with spoke-wheel areas, in-focus dots, and concentric structures at the dermoepidermal junction, which is more characteristic of superficial and infiltrating BCC.20 In nodular BCC, the pigment occurs as blue-gray ovoid nests and globules in deeper layers of the dermis.20
Seborrheic keratoses (SKs) can vary widely in clinical appearance, with pigmentation ranging from flesh colored to yellow to brown to black. Melanoacanthomas are acanthotic SKs that are highly pigmented due to intermixed epidermal melanocytes and subepidermal melanophages.21 Dermoscopy can help distinguish cutaneous malignancies from SKs, which often demonstrate fissures and ridges, comedolike openings, and milialike cysts. Biopsy sometimes is required to assess for malignancy, as was the case in our patient. The classic histologic features of SKs include acanthosis, papillomatosis, and hyperkeratosis.22
This case highlights the need to consider apocrine hidrocystoma, along with malignancy, in the differential diagnosis of pigmented cystic masses of the face and scalp. Because apocrine hidrocystomas are benign, they do not need to be treated but often are surgically excised for cosmesis or complete histopathologic examination. Destruction via electrodessication, carbon dioxide ablation, trichloroacetic acid chemical ablation, botulinum toxin injection, and anticholinergic creams sometimes is used, especially for cosmetic treatment of multiple small lesions.5 Our patient was treated with surgical excision with no evidence of recurrence on follow-up 1 year later.
THE DIAGNOSIS: Apocrine Hidrocystoma
Histology for all 3 lesions demonstrated similar cystic structures lined by a dual layer of epithelial cells, with the outermost layer composed of flattened myoepithelial cells and the inner layer composed of cells with apocrine features (Figure 1). Based on these findings, a diagnosis of apocrine hidrocystoma was made. The patient underwent successful surgical excision shortly thereafter without recurrence at follow-up 1 year later.
Apocrine hidrocystomas are rare benign cystic lesions that are considered to be adenomatous proliferations of apocrine glands. They typically manifest as solitary asymptomatic lesions measuring 3 to 15 mm.1 They tend to appear on the face, usually in the periorbital region, but also have been described on the neck, scalp, trunk, arms, and legs.2-4 Multiple apocrine hidrocystomas can be a marker of 2 rare inherited disorders: Gorlin-Goltz syndrome and Schopf-Schulz-Passarge syndrome.5 Apocrine hidrocystomas may be flesh colored or may have a blue, black, or brown appearance due to the Tyndall effect, in which light with shorter wavelengths is scattered by the contents of the lesions.2 Histologically, apocrine hidrocystomas are cysts lined by a dual layer of epithelial cells. The inner layer is composed of cells with apocrine features, and the outer layer is composed of flattened myoepithelial cells. Due to their range of colors and predilection for sun-exposed surfaces, apocrine hidrocystomas may be mistaken for various malignant neoplasms, including melanoma.6,7

The differential diagnosis for our patient included agminated blue nevi, melanoma, pigmented basal cell carcinoma (BCC), and seborrheic keratosis. A blue nevus is a dermal melanocytic lesion that manifests as a well-demarcated, blue to blue-black papule that typically appears on the face, scalp, arms, legs, lower back, and buttocks. Although there are several histologic subtypes, the common blue nevus usually manifests as a solitary lesion measuring less than 1 cm, often developing during childhood to young adulthood.8 Histologically, common blue nevi are characterized by a dermal proliferation of deeply pigmented bipolar spindled melanocytes embedded in thickened collagen bundles, often with scattered epithelioid melanophages, and no conspicuous mitotic activity (Figure 2).9 There are other types of blue nevi, including cellular blue nevi, which tend to be larger and manifest commonly on the buttocks and sacrococcygeal region in early adulthood.9 Histologically, cellular blue nevi contain oval to spindled melanocytes with scattered melanophages forming a well-demarcated nodule typically in the reticular dermis. There may be bulbous extension into the subcutaneous adipose tissue. Occasional mitoses may be seen.9,10 Melanoma can arise from common or cellular blue nevi, though it more frequently occurs with cellular blue nevi. Other subtypes of blue nevi have been described, including the sclerosing, plaque-type, combined, hypomelanotic/amelanotic, and pigmented epithelioid melanocytoma.11 However, they typically have features of the common blue nevus or cellular blue nevus, such as oval/spindle cell morphology, some degree of melanin, and biphasic architecture, but are classified according to their dominant histologic characteristics.

Given the location of our patient’s lesions on the scalp and his extensive history of sun exposure, malignancy was high in the differential. Multiple synchronous primary melanomas including nodular melanoma, blue nevus–like metastatic melanoma, and metastatic melanoma were considered. The leg and the scalp have the highest reported incidence of cutaneous metastases of melanoma, with many cases presenting as dermal or subcutaneous nodules and eruptive blue nevus–like papules, similar to our patient’s clinical presentation.12,13 Nodular melanoma (NM) is one of 4 major types of melanoma, accounting for approximately 15% to 30% of cases in the United States.14 Nodular melanoma typically manifests as a smooth, raised, symmetric, well-circumscribed lesion with variable pigmentation, from very dark to amelanotic. Histologically, NM is defined as a dermal mass, either in isolation or with an epidermal component, not to exceed 3 rete ridges beyond the dermal component.15 Tumor cells have a high cell density with pleomorphism, usually with atypical epithelioid cells with vesicular nuclei and irregular cytoplasm, and occasionally spindle cells (Figure 2).16 Mitoses and necrosis are frequent. Scalp location independently is responsible for worse survival, both overall and melanoma specific.17 Nodular melanoma tends to have greater Breslow thickness at diagnosis than other melanoma subtypes and often carries a worse prognosis.

Malignant melanomas that develop from or in conjunction with or bear histologic resemblance to blue nevi are termed blue nevus–like melanoma or blue nevus–associated melanoma. These malignancies are exceedingly rare, accounting for only 0.3% of melanomas in one Turkey-based multicenter study.18 The histologic criteria for diagnosing blue nevus–like melanoma are poorly defined, and terminology of these lesions has led to some debate in naming conventions.19 Nevertheless, unlike blue nevus, blue nevus–like melanoma demonstrates histologic features of malignancy, including pleomorphism, prominent nucleoli, mitotic activity, vascular invasion, and potential necrosis.10 The lack of an inflammatory infiltrate, surrounding fibrosis, junctional activity, and pre-existing nevus can help distinguish cutaneous melanoma metastases from primary nodular melanoma. Immunohistochemical stains such as S100, Melan-A/MART1, or SOX-10 can help confirm melanocytic lineage.12
Pigmented BCC is a clinical and histologic variant of BCC characterized by increased melanin pigmentation due to melanocytes admixed with tumor cells. Dermoscopically, the pigment can have a maple leaf–like appearance with spoke-wheel areas, in-focus dots, and concentric structures at the dermoepidermal junction, which is more characteristic of superficial and infiltrating BCC.20 In nodular BCC, the pigment occurs as blue-gray ovoid nests and globules in deeper layers of the dermis.20
Seborrheic keratoses (SKs) can vary widely in clinical appearance, with pigmentation ranging from flesh colored to yellow to brown to black. Melanoacanthomas are acanthotic SKs that are highly pigmented due to intermixed epidermal melanocytes and subepidermal melanophages.21 Dermoscopy can help distinguish cutaneous malignancies from SKs, which often demonstrate fissures and ridges, comedolike openings, and milialike cysts. Biopsy sometimes is required to assess for malignancy, as was the case in our patient. The classic histologic features of SKs include acanthosis, papillomatosis, and hyperkeratosis.22
This case highlights the need to consider apocrine hidrocystoma, along with malignancy, in the differential diagnosis of pigmented cystic masses of the face and scalp. Because apocrine hidrocystomas are benign, they do not need to be treated but often are surgically excised for cosmesis or complete histopathologic examination. Destruction via electrodessication, carbon dioxide ablation, trichloroacetic acid chemical ablation, botulinum toxin injection, and anticholinergic creams sometimes is used, especially for cosmetic treatment of multiple small lesions.5 Our patient was treated with surgical excision with no evidence of recurrence on follow-up 1 year later.
- Ioannidis DG, Drivas EI, Papadakis CE, et al. Hidrocystoma of the external auditory canal: a case report. Cases J. 2009;2:79. doi:10.1186/1757- 1626-2-79
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp. Dermatol Online J. 2020;26. doi:10.5070/D3268049895
- Mendoza-Cembranos MD, Haro R, Requena L, et al. Digital apocrine hidrocystoma: the exception confirms the rule. Am J Dermatopathol. 2019;41:79. doi:10.1097/DAD.0000000000001044
- May C, Chang O, Compton N. A giant apocrine hidrocystoma of the trunk. Dermatol Online J. 2017;23. doi:10.5070/D3239036497
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. Medscape Gen Med. 2006;8:57.
- Kruse ALD, Zwahlen R, Bredell MG, et al. Apocrine hidrocystoma of the cheek. J Craniofac Surg. 2010;21:594-596. doi:10.1097 /SCS.0b013e3181d08c77
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405. doi:10.1002 /1097-0142(196803)21:3<393::aid-cncr2820210309>3.0.co;2-k
- Murali R, McCarthy SW, Scolyer RA. Blue nevi and related lesions: a review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Adv Anat Pathol. 2009;16:365. doi:10.1097/PAP.0b013e3181bb6b53
- Borgenvik TL, Karlsvik TM, Ray S, et al. Blue nevus-like and blue nevusassociated melanoma: a comprehensive review of the literature. ANZ J Surg. 2017;87:345-349. doi:10.1111/ans.13946
- de la Fouchardiere A. Blue naevi and the blue tumour spectrum. Pathology. 2023;55:187-195. doi:10.1016/j.pathol.2022.12.342
- Lowe L. Metastatic melanoma and rare melanoma variants: a review. Pathology (Phila). 2023;55:236-244. doi:10.1016/j.pathol.2022.11.006
- Plaza JA, Torres-Cabala C, Evans H, et al. Cutaneous metastases of malignant melanoma: a clinicopathologic study of 192 cases with emphasis on the morphologic spectrum. Am J Dermatopathol. 2010;32:129-136. doi:10.1097/DAD.0b013e3181b34a19
- Shaikh WR, Xiong M, Weinstock MA. The contribution of nodular subtype to melanoma mortality in the United States, 1978 to 2007. Archives of Dermatology. 2012;148:30-36. doi:10.1001/archdermatol.2011.264
- Clark WH, From L, Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705-727.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321. doi:10.23736/S2784-8671.21.06958-3
- Ozao-Choy J, Nelson DW, Hiles J, et al. The prognostic importance of scalp location in primary head and neck melanoma. J Surg Oncol. 2017;116:337-343. doi:10.1002/jso.24679
- Gamsizkan M, Yilmaz I, Buyukbabani N, et al. A retrospective multicenter evaluation of cutaneous melanomas in Turkey. Asian Pac J Cancer Prev APJCP. 2014;15:10451-10456. doi:10.7314 /apjcp.2014.15.23.10451
- Mones JM, Ackerman AB. “Atypical” blue nevus, “malignant” blue nevus, and “metastasizing” blue nevus: a critique in historical perspective of three concepts flawed fatally. Am J Dermatopathol. 2004;26:407-430. doi:10.1097/00000372-200410000-00012
- Tanese K. Diagnosis and management of basal cell carcinoma Curr Treat Options Oncol. 2019;20:13. doi:10.1007/s11864 -019-0610-0
- Barthelmann S, Butsch F, Lang BM, et al. Seborrheic keratosis. JDDG J Dtsch Dermatol Ges. 2023;21:265-277. doi:10.1111/ddg.14984
- Taylor S. Advancing the understanding of seborrheic keratosis. J Drugs Dermatol. 2017;16:419-424.
- Ioannidis DG, Drivas EI, Papadakis CE, et al. Hidrocystoma of the external auditory canal: a case report. Cases J. 2009;2:79. doi:10.1186/1757- 1626-2-79
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp. Dermatol Online J. 2020;26. doi:10.5070/D3268049895
- Mendoza-Cembranos MD, Haro R, Requena L, et al. Digital apocrine hidrocystoma: the exception confirms the rule. Am J Dermatopathol. 2019;41:79. doi:10.1097/DAD.0000000000001044
- May C, Chang O, Compton N. A giant apocrine hidrocystoma of the trunk. Dermatol Online J. 2017;23. doi:10.5070/D3239036497
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. Medscape Gen Med. 2006;8:57.
- Kruse ALD, Zwahlen R, Bredell MG, et al. Apocrine hidrocystoma of the cheek. J Craniofac Surg. 2010;21:594-596. doi:10.1097 /SCS.0b013e3181d08c77
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405. doi:10.1002 /1097-0142(196803)21:3<393::aid-cncr2820210309>3.0.co;2-k
- Murali R, McCarthy SW, Scolyer RA. Blue nevi and related lesions: a review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Adv Anat Pathol. 2009;16:365. doi:10.1097/PAP.0b013e3181bb6b53
- Borgenvik TL, Karlsvik TM, Ray S, et al. Blue nevus-like and blue nevusassociated melanoma: a comprehensive review of the literature. ANZ J Surg. 2017;87:345-349. doi:10.1111/ans.13946
- de la Fouchardiere A. Blue naevi and the blue tumour spectrum. Pathology. 2023;55:187-195. doi:10.1016/j.pathol.2022.12.342
- Lowe L. Metastatic melanoma and rare melanoma variants: a review. Pathology (Phila). 2023;55:236-244. doi:10.1016/j.pathol.2022.11.006
- Plaza JA, Torres-Cabala C, Evans H, et al. Cutaneous metastases of malignant melanoma: a clinicopathologic study of 192 cases with emphasis on the morphologic spectrum. Am J Dermatopathol. 2010;32:129-136. doi:10.1097/DAD.0b013e3181b34a19
- Shaikh WR, Xiong M, Weinstock MA. The contribution of nodular subtype to melanoma mortality in the United States, 1978 to 2007. Archives of Dermatology. 2012;148:30-36. doi:10.1001/archdermatol.2011.264
- Clark WH, From L, Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705-727.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321. doi:10.23736/S2784-8671.21.06958-3
- Ozao-Choy J, Nelson DW, Hiles J, et al. The prognostic importance of scalp location in primary head and neck melanoma. J Surg Oncol. 2017;116:337-343. doi:10.1002/jso.24679
- Gamsizkan M, Yilmaz I, Buyukbabani N, et al. A retrospective multicenter evaluation of cutaneous melanomas in Turkey. Asian Pac J Cancer Prev APJCP. 2014;15:10451-10456. doi:10.7314 /apjcp.2014.15.23.10451
- Mones JM, Ackerman AB. “Atypical” blue nevus, “malignant” blue nevus, and “metastasizing” blue nevus: a critique in historical perspective of three concepts flawed fatally. Am J Dermatopathol. 2004;26:407-430. doi:10.1097/00000372-200410000-00012
- Tanese K. Diagnosis and management of basal cell carcinoma Curr Treat Options Oncol. 2019;20:13. doi:10.1007/s11864 -019-0610-0
- Barthelmann S, Butsch F, Lang BM, et al. Seborrheic keratosis. JDDG J Dtsch Dermatol Ges. 2023;21:265-277. doi:10.1111/ddg.14984
- Taylor S. Advancing the understanding of seborrheic keratosis. J Drugs Dermatol. 2017;16:419-424.
A 67-year-old man presented to the dermatology clinic with 3 asymptomatic pigmented papules on the scalp. The patient reported that he was unaware of the lesions until they were pointed out weeks earlier by his primary care physician during a routine visit. He then was referred to dermatology for follow-up. Physical examination at the current presentation revealed clustered firm, smooth, well-circumscribed, pigmented papules on the scalp measuring 5 to 8 mm. The patient reported no personal or family history of skin cancer but stated that he spent a lot of time outdoors and had a history of 6 blistering sunburns in his life. A punch biopsy of each lesion was performed.
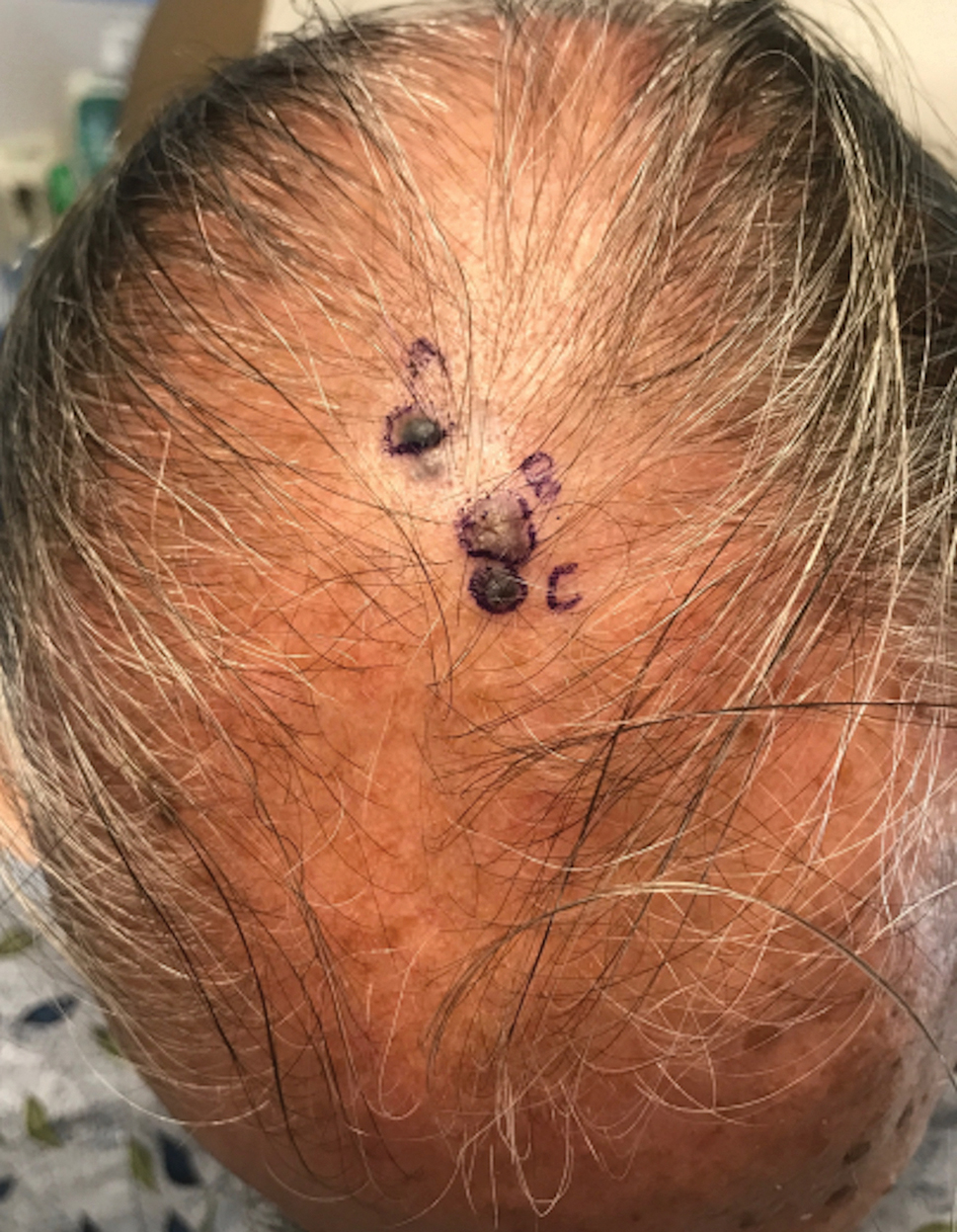
Handoff Delays in Teledermatology Lengthen Timeline of Care for Veterans With Melanoma
Handoff Delays in Teledermatology Lengthen Timeline of Care for Veterans With Melanoma
Store-and-forward teledermatology (SFT) allows clinical images and information to be sent to a dermatologist for evaluation. In fiscal year (FY) 2018, 117,780 SFT consultations were completed in the Veterans Health Administration. Continued growth is expected since SFT has proven to be an effective method for improving access to face-to-face (FTF) dermatology care.1 In the same period, the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS) completed 12,563 consultations in a mean 1.1 days from entry into episode of care (EEC), according to data reported by VA Teledermatology Program Administrator Chris Foster.
Obtaining a prompt consultation is reported to be an overwhelming advantage of using SFT.2-5 Rapid turnaround may appear to make SFT specialist care more accessible to veterans, yet this is an oversimplification. The process of delivering care (rather than consultation) through SFT is more complex than reading the images and reporting the findings. When a skin condition is identified by a primary care clinician and that person decides to request an SFT consultation, a complex set of tasks and handoffs is set into motion. A swim-lane diagram illustrates the numerous steps and handoffs that go into delivering care to a patient with a malignant melanoma on the SFT platform compared to FTF care, which requires fewer handoffs (Figure).
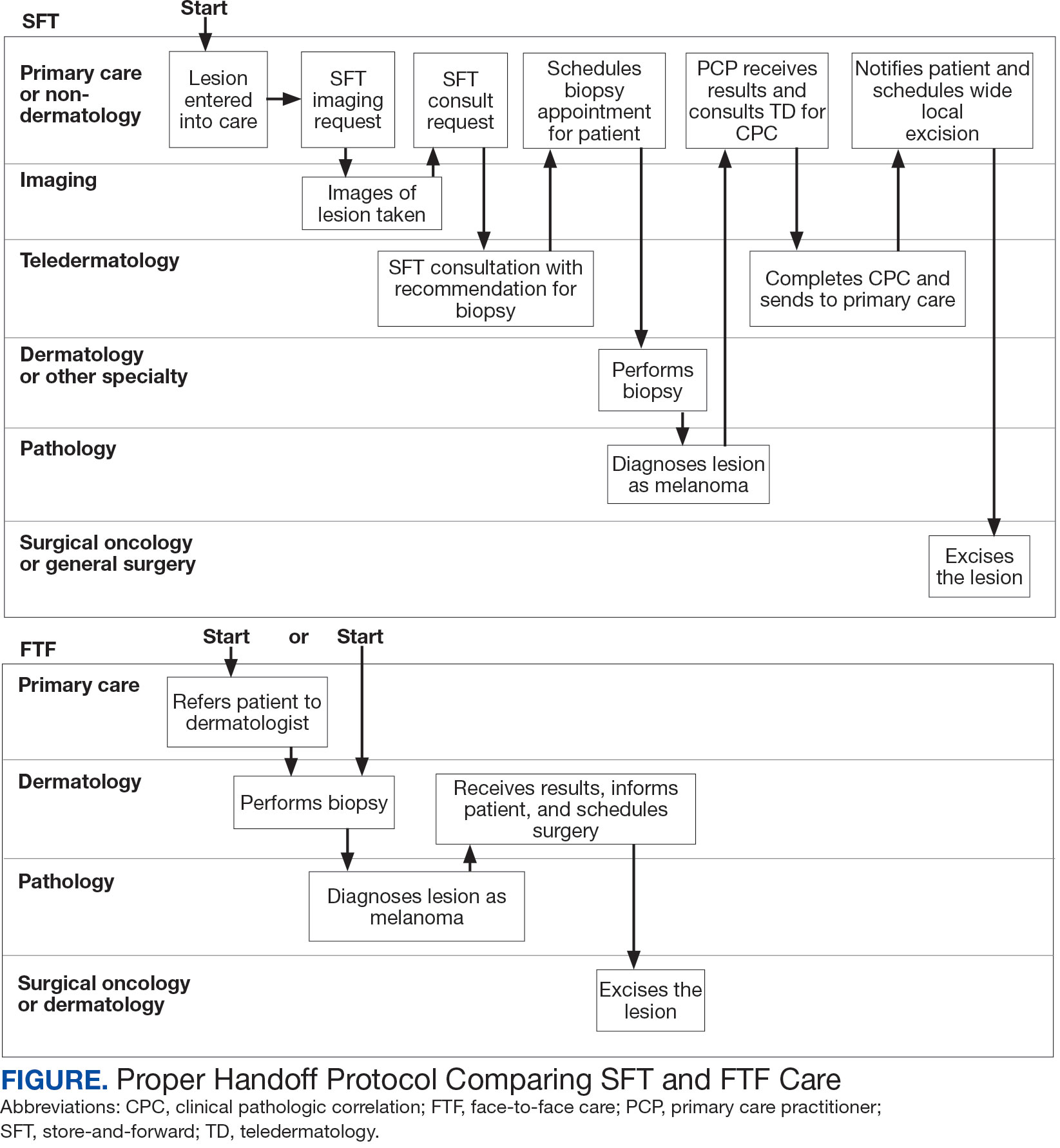
This process improvement project examined whether handoffs necessitated by SFT care lengthened the timeline of care for biopsy-proven primary cutaneous malignant melanoma. The stakes of delay in care are high. A 2018 study using the National Cancer Database found that a delay of > 30 days from biopsy to definitive excision (the date definitive surgical procedure for the condition is performed) resulted in a measurable increase in melanoma-related mortality. 6 This study sought to identify areas where the SFT timeline of care could be shortened.
Methods
This retrospective cohort study was approved by the VAPSHCS Institutional Review Board. The study drew from secondary data obtained from VistA, the VA Corporate Data Warehouse, the Veterans Integrated Service Network (VISN) 20 database, the American Academy of Dermatology Teledermatology Program database, and the VA Computerized Patient Record System.
Patients registered for ≥ 1 year at VAPSHCS with a diagnosis of primary cutaneous malignant melanoma by the Pathology service between January 1, 2006, and December 31, 2013, were included. Patients with metastatic or recurrent melanoma were excluded.
Cases were randomly selected from a melanoma database previously validated and used for another quality improvement project.7 There were initially 115 patient cases extracted from this database for both the FTF and SFT groups. Eighty-seven SFT and 107 FTF cases met inclusion criteria. To further analyze these groups, we split the FTF group into 2 subgroups: FTF dermatology (patients whose melanomas were entered into care in a dermatology clinic) and FTF primary care (patients whose melanomas were entered into care in primary care or a nondermatology setting).
The timeline of care was divided into 2 major time intervals: (1) entry into episode of care (EEC; the date a lesion was first documented in the electronic health record) to biopsy; and (2) biopsy to definitive excision. The SFT process was divided into the following intervals: EEC to imaging request (the date a clinician requested imaging); imaging request to imaging completion (the date an imager photographed a patient’s lesion); imaging completion to SFT consultation request (the date the SFT consultation was requested); SFT consultation request to consultation completion (the date an SFT reader completed the consultation request for a patient); and SFT consultation completion to biopsy. Mean and median interval lengths were compared between groups and additional analyses identified steps that may have contributed to delays in care.
To address potential bias based on access to care for rural veterans, SFT and FTF primary care cases were categorized into groups based on their location: (1) EEC and biopsy conducted at the same facility; (2) EEC and biopsy conducted at different facilities within the same health care system (main health care facility and its community-based outpatient clinics); and (3) EEC and biopsy conducted at different health care systems.
Statistics
Means, medians, and SDs were calculated in Excel. The Mann-Whitney U test was used to compare SFT medians to the FTF data and X2 test was used to compare proportions for secondary analyses.
Results
The median (mean) interval from EEC to definitive excision was 73 days (85) for SFT and 58 days (73) for FTF (P = .004) (Table). To understand this difference, the distribution of intervals from EEC to biopsy and biopsy to definitive excision were calculated. Only 38% of SFT cases were biopsied within 20 days compared to 65% of FTF cases (P < .001). The difference in time from biopsy to definitive excision distributions were not statistically significant, suggesting that the difference is actually a reflection of the differences seen in the period between EEC and biopsy.
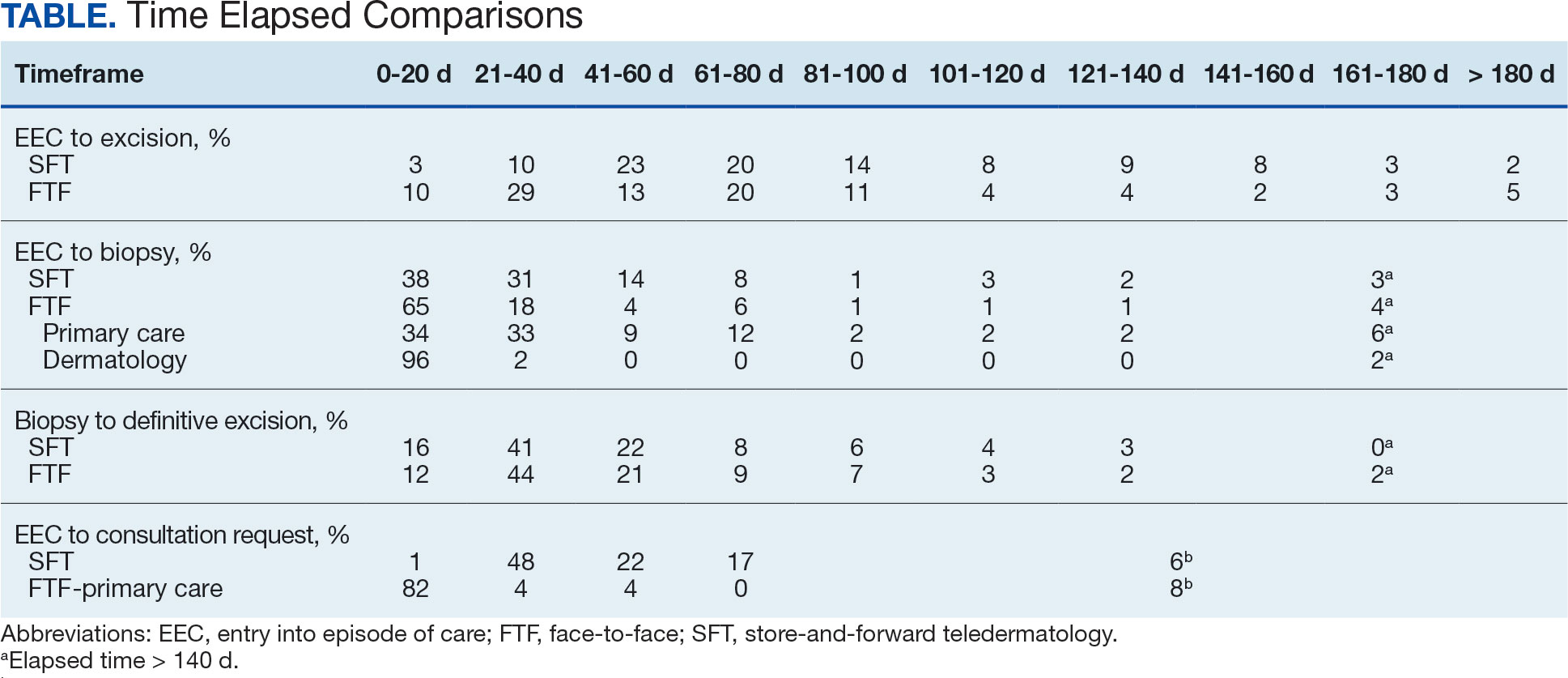
EEC and biopsy occurred at the same facility in 85% and 82% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different facilities within the same health care system in 15% and 16% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different health care systems in 0% and 2% of FTF primary care and SFT cases, respectively. Geographic bias did not impact results for either group of veterans.
The interval between EEC and biopsy was shorter for FTF dermatology cases than for FTF primary care cases. For FTF dermatology cases, 96% were biopsied within 20 days compared with 34% of FTF primary care cases (P < .001).
To further analyze the difference in the EEC to biopsy interval duration between SFT and FTF primary care the timeline was divided into smaller steps: EEC to imaging completion, imaging completion to SFT consult completion, and SFT consult completion to biopsy. From EEC to SFT consult completion, SFT cases took a median of 6.0 days and a mean of 12.3 days, reflecting the administrative handoffs that must occur in SFT. A total of 82% of FTF primary care cases were entered into care and consultation was requested on the same day, while this was true for only 1% of SFT cases.
Since mortality data were not collected, the frequency of in situ melanomas and invasive melanomas (pathologic stage pT1a or greater) was used as a proxy for comparing outcomes. No significant difference was found in the frequency of in situ vs invasive melanomas in the SFT and FTF dermatology groups; however, there was a much higher frequency of invasive melanomas in the FTF primary care group (P = .007).
Discussion
This study compared the time to treatment for SFT vs FTF and identified important differences. The episode of care for melanomas diagnosed by SFT was statistically significantly longer (15 days) than those diagnosed by FTF. The interval between biopsy and definitive excision was a median of 34 and 38 days, and a mean of 48 and 44 days for SFT and FTF, respectively, which were not statistically significant. The difference in the total duration of the interval between EEC and definitive excision was accounted for by the duration of the interval from EEC to biopsy. When excluding dermatology clinic cases from the FTF group, there was no difference in the interval between EEC and biopsy for SFT and FTF primary care. The handoffs in SFT accounted for a median of 6 days and mean of 12 days, a significant portion of the timeline, and is a target for process improvement. The delay necessitated by handoffs did not significantly affect the distribution of in situ and invasive melanomas in the SFT and FTF dermatology groups. This suggests that SFT may have better outcomes than FTF primary care.
There has been extensive research on the timeline from the patient initially noticing a lesion to the EEC.8-11 There is also a body of research on the timeline from biopsy to definitive excision. 6,12-16 However, there has been little research on the timeline between EEC and biopsy, which comprises a large portion of the overall timeline of both SFT care and FTF care. This study analyzed the delays that can occur in this interval. When patients first enter FTF dermatology care, this timeline is quite short because lesions are often biopsied on the same day. When patients enter into care with their primary or nondermatology clinician, there can be significant delays.
Since the stakes are high when it comes to treating melanoma, it is important to minimize the overall timeline. A 6-day median and 12-day mean were established as targets for teledermatology handoffs. Ideally, a lesion should be entered into an episode of care, imaged, and sent for consultation on the same day. To help further understand delays in administrative handoffs, we stratified the SFT cases by VISN 20 sites and spoke with an administrator at a top performing site. Between 2006 and 2013, this site had a dedicated full-time imager as well as a backup imager that ensured images were taken quickly, usually on the same day the lesion was entered into care. Unfortunately, this is not the standard at all VISN 20 sites and certainly contributes to the overall delay in care in SFT
Minimizing the timeline of care is possible, as shown by the Danish health system, which developed a fast-track referral system after recognizing the need to minimize delays between the presentation, diagnosis, and treatment of cutaneous melanomas. In Denmark, a patient who presents to a general practitioner with a suspicious lesion is referred to secondary care for excision biopsy within 6 days. Diagnosis is made within 2 weeks, and, if necessary, definitive excision is offered within 9 days of the diagnosis. This translates into a maximum 20-day EEC to biopsy timeline and maximum 29-day EEC to definitive excision timeline. Although an intervention such as this may be difficult to implement in the United States due to its size and decentralized health care system, it would, however, be more realistic within the VA due to its centralized structure. The Danish system shows that with appropriate resource allocation and strict timeframes for treatment referrals, the timeline can be minimized.17
Despite the delay in the SFT timeline, this study found no significant difference between the distribution of in situ vs invasive melanomas in FTF dermatology and SFT groups. One possible explanation for this is that SFT increases access to dermatologist care, meaning clinicians may be more willing to consult SFT for less advanced– appearing lesions.
The finding that SFT diagnosed a larger proportion of in situ melanomas than FTF primary care is consistent with the findings of Ferrándiz et al, who reported that the mean Breslow thickness was significantly lower among patients in an SFT group compared to patients in an FTF group consisting of general practitioners. 18 However, the study population was not randomized and the results may have been impacted by ascertainment bias. Ferrándiz et al hypothesized that clinicians may have a lower threshold for consulting teledermatology, resulting in lower mean Breslow thicknesses.18 Karavan et al found the opposite results, with a higher mean Breslow thickness in SFT compared to a primary care FTF group.19 The data presented here suggest that SFT has room for process improvement yet is essentially equivalent to FTF dermatology in terms of outcomes.
Limitations
The majority of patients in this study were aged > 50 years, White, and male. The results may not be representative for other populations. The study was relatively small compared to studies that looked at other aspects of the melanoma care timeline. The study was not powered to ascertain mortality, the most important metric for melanoma.
Conclusions
The episode of care was significantly longer for melanomas diagnosed by SFT than those diagnosed by FTF; however, timelines were not statistically different when FTF lesions entered into care in dermatology were excluded. A median 6-day and mean 12.3-day delay in administrative handoffs occurred at the beginning of the SFT process and is a target for process improvement. Considering the high stakes of melanoma, the SFT timeline could be reduced if EEC, imaging, and SFT consultation all happened in the same day.
- Raugi GJ, Nelson W, Miethke M, et al. Teledermatology implementation in a VHA secondary treatment facility improves access to face-to-face care. Telemed J E Health. 2016;22(1):12-17. doi:10.1089/tmj.2015.0036
- Moreno-Ramirez D, Ferrandiz L, Nieto-Garcia A, et al. Store-and-forward teledermatology in skin cancer triage: experience and evaluation of 2009 teleconsultations. Arch Dermatol. 2007;143(4):479-484. doi:10.1001/archderm.143.4.479
- Landow SM, Oh DH, Weinstock MA. Teledermatology within the Veterans Health Administration, 2002–2014. Telemed J E Health. 2015;21(10):769-773. doi:10.1089/tmj.2014.0225
- Whited JD, Hall RP, Foy ME, et al. Teledermatology’s impact on time to intervention among referrals to a dermatology consult service. Telemed J E Health. 2002;8(3):313-321. doi:10.1089/15305620260353207
- Hsiao JL, Oh DH. The impact of store-and-forward teledermatology on skin cancer diagnosis and treatment. J Am Acad Dermatol. 2008;59(2):260-267. doi:10.1016/j.jaad.2008.04.011
- Conic RZ, Cabrera CI, Khorana AA, Gastman BR. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78(1):40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Dougall B, Gendreau J, Das S, et al. Melanoma registry underreporting in the Veterans Health Administration. Fed Pract. 2016;33(suppl 5):55S-59S
- Xavier MHSB, Drummond-Lage AP, Baeta C, Rocha L, Almeida AM, Wainstein AJA. Delay in cutaneous melanoma diagnosis: sequence analyses from suspicion to diagnosis in 211 patients. Medicine (Baltimore). 2016;95(31):e4396. doi:10.1097/md.0000000000004396
- Schmid-Wendtner MH, Baumert J, Stange J, Volkenandt M. Delay in the diagnosis of cutaneous melanoma: an analysis of 233 patients. Melanoma Res. 2002;12(4):389-394. doi:10.1097/00008390-200208000-00012
- Betti, R, Vergani R, Tolomio E, Santambrogio R, Crosti C. Factors of delay in the diagnosis of melanoma. Eur J Dermatol. 2003;13(2):183-188.
- Blum A, Brand CU, Ellwanger U, et al. Awareness and early detection of cutaneous melanoma: An analysis of factors related to delay in treatment. Br J Dermatol. 1999;141(5):783-787. doi:10.1046/j.1365-2133.1999.03196.x
- Brian T, Adams B, Jameson M. Cutaneous melanoma: an audit of management timeliness against New Zealand guidelines. N Z Med J. 2017;130(1462):54-61. https://pubmed.ncbi.nlm.nih.gov/28934768
- Adamson AS, Zhou L, Baggett CD, Thomas NE, Meyer AM. Association of delays in surgery for melanoma with Insurance type. JAMA Dermatol. 2017;153(11):1106-1113. doi:https://doi.org/10.1001/jamadermatol.2017.3338
- Niehues NB, Evanson B, Smith WA, Fiore CT, Parekh P. Melanoma patient notification and treatment timelines. Dermatol Online J. 2019;25(4)13. doi:10.5070/d3254043588
- Lott JP, Narayan D, Soulos PR, Aminawung J, Gross CP. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151(7):731-741. doi:10.1001/jamadermatol.2015.119
- Baranowski MLH, Yeung H, Chen SC, Gillespie TW, Goodman M. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81(4):908-916. doi:10.1016/j.jaad.2019.05.079
- Jarjis RD, Hansen LB, Matzen SH. A fast-track referral system for skin lesions suspicious of melanoma: population-based cross-sectional study from a plastic surgery center. Plast Surg Int. 2016;2016:2908917. doi:10.1155/2016/2908917
- Ferrándiz L, Ruiz-de-Casas A, Martin-Gutierrez FJ, et al. Effect of teledermatology on the prognosis of patients with cutaneous melanoma. Arch Dermatol. 2012;148(9):1025-1028. doi:10.1001/archdermatol.2012.778
- Karavan M, Compton N, Knezevich S, et al. Teledermatology in the diagnosis of melanoma. J Telemed Telecare. 2014;20(1):18-23. doi:10.1177/1357633x13517354
Store-and-forward teledermatology (SFT) allows clinical images and information to be sent to a dermatologist for evaluation. In fiscal year (FY) 2018, 117,780 SFT consultations were completed in the Veterans Health Administration. Continued growth is expected since SFT has proven to be an effective method for improving access to face-to-face (FTF) dermatology care.1 In the same period, the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS) completed 12,563 consultations in a mean 1.1 days from entry into episode of care (EEC), according to data reported by VA Teledermatology Program Administrator Chris Foster.
Obtaining a prompt consultation is reported to be an overwhelming advantage of using SFT.2-5 Rapid turnaround may appear to make SFT specialist care more accessible to veterans, yet this is an oversimplification. The process of delivering care (rather than consultation) through SFT is more complex than reading the images and reporting the findings. When a skin condition is identified by a primary care clinician and that person decides to request an SFT consultation, a complex set of tasks and handoffs is set into motion. A swim-lane diagram illustrates the numerous steps and handoffs that go into delivering care to a patient with a malignant melanoma on the SFT platform compared to FTF care, which requires fewer handoffs (Figure).

This process improvement project examined whether handoffs necessitated by SFT care lengthened the timeline of care for biopsy-proven primary cutaneous malignant melanoma. The stakes of delay in care are high. A 2018 study using the National Cancer Database found that a delay of > 30 days from biopsy to definitive excision (the date definitive surgical procedure for the condition is performed) resulted in a measurable increase in melanoma-related mortality. 6 This study sought to identify areas where the SFT timeline of care could be shortened.
Methods
This retrospective cohort study was approved by the VAPSHCS Institutional Review Board. The study drew from secondary data obtained from VistA, the VA Corporate Data Warehouse, the Veterans Integrated Service Network (VISN) 20 database, the American Academy of Dermatology Teledermatology Program database, and the VA Computerized Patient Record System.
Patients registered for ≥ 1 year at VAPSHCS with a diagnosis of primary cutaneous malignant melanoma by the Pathology service between January 1, 2006, and December 31, 2013, were included. Patients with metastatic or recurrent melanoma were excluded.
Cases were randomly selected from a melanoma database previously validated and used for another quality improvement project.7 There were initially 115 patient cases extracted from this database for both the FTF and SFT groups. Eighty-seven SFT and 107 FTF cases met inclusion criteria. To further analyze these groups, we split the FTF group into 2 subgroups: FTF dermatology (patients whose melanomas were entered into care in a dermatology clinic) and FTF primary care (patients whose melanomas were entered into care in primary care or a nondermatology setting).
The timeline of care was divided into 2 major time intervals: (1) entry into episode of care (EEC; the date a lesion was first documented in the electronic health record) to biopsy; and (2) biopsy to definitive excision. The SFT process was divided into the following intervals: EEC to imaging request (the date a clinician requested imaging); imaging request to imaging completion (the date an imager photographed a patient’s lesion); imaging completion to SFT consultation request (the date the SFT consultation was requested); SFT consultation request to consultation completion (the date an SFT reader completed the consultation request for a patient); and SFT consultation completion to biopsy. Mean and median interval lengths were compared between groups and additional analyses identified steps that may have contributed to delays in care.
To address potential bias based on access to care for rural veterans, SFT and FTF primary care cases were categorized into groups based on their location: (1) EEC and biopsy conducted at the same facility; (2) EEC and biopsy conducted at different facilities within the same health care system (main health care facility and its community-based outpatient clinics); and (3) EEC and biopsy conducted at different health care systems.
Statistics
Means, medians, and SDs were calculated in Excel. The Mann-Whitney U test was used to compare SFT medians to the FTF data and X2 test was used to compare proportions for secondary analyses.
Results
The median (mean) interval from EEC to definitive excision was 73 days (85) for SFT and 58 days (73) for FTF (P = .004) (Table). To understand this difference, the distribution of intervals from EEC to biopsy and biopsy to definitive excision were calculated. Only 38% of SFT cases were biopsied within 20 days compared to 65% of FTF cases (P < .001). The difference in time from biopsy to definitive excision distributions were not statistically significant, suggesting that the difference is actually a reflection of the differences seen in the period between EEC and biopsy.

EEC and biopsy occurred at the same facility in 85% and 82% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different facilities within the same health care system in 15% and 16% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different health care systems in 0% and 2% of FTF primary care and SFT cases, respectively. Geographic bias did not impact results for either group of veterans.
The interval between EEC and biopsy was shorter for FTF dermatology cases than for FTF primary care cases. For FTF dermatology cases, 96% were biopsied within 20 days compared with 34% of FTF primary care cases (P < .001).
To further analyze the difference in the EEC to biopsy interval duration between SFT and FTF primary care the timeline was divided into smaller steps: EEC to imaging completion, imaging completion to SFT consult completion, and SFT consult completion to biopsy. From EEC to SFT consult completion, SFT cases took a median of 6.0 days and a mean of 12.3 days, reflecting the administrative handoffs that must occur in SFT. A total of 82% of FTF primary care cases were entered into care and consultation was requested on the same day, while this was true for only 1% of SFT cases.
Since mortality data were not collected, the frequency of in situ melanomas and invasive melanomas (pathologic stage pT1a or greater) was used as a proxy for comparing outcomes. No significant difference was found in the frequency of in situ vs invasive melanomas in the SFT and FTF dermatology groups; however, there was a much higher frequency of invasive melanomas in the FTF primary care group (P = .007).
Discussion
This study compared the time to treatment for SFT vs FTF and identified important differences. The episode of care for melanomas diagnosed by SFT was statistically significantly longer (15 days) than those diagnosed by FTF. The interval between biopsy and definitive excision was a median of 34 and 38 days, and a mean of 48 and 44 days for SFT and FTF, respectively, which were not statistically significant. The difference in the total duration of the interval between EEC and definitive excision was accounted for by the duration of the interval from EEC to biopsy. When excluding dermatology clinic cases from the FTF group, there was no difference in the interval between EEC and biopsy for SFT and FTF primary care. The handoffs in SFT accounted for a median of 6 days and mean of 12 days, a significant portion of the timeline, and is a target for process improvement. The delay necessitated by handoffs did not significantly affect the distribution of in situ and invasive melanomas in the SFT and FTF dermatology groups. This suggests that SFT may have better outcomes than FTF primary care.
There has been extensive research on the timeline from the patient initially noticing a lesion to the EEC.8-11 There is also a body of research on the timeline from biopsy to definitive excision. 6,12-16 However, there has been little research on the timeline between EEC and biopsy, which comprises a large portion of the overall timeline of both SFT care and FTF care. This study analyzed the delays that can occur in this interval. When patients first enter FTF dermatology care, this timeline is quite short because lesions are often biopsied on the same day. When patients enter into care with their primary or nondermatology clinician, there can be significant delays.
Since the stakes are high when it comes to treating melanoma, it is important to minimize the overall timeline. A 6-day median and 12-day mean were established as targets for teledermatology handoffs. Ideally, a lesion should be entered into an episode of care, imaged, and sent for consultation on the same day. To help further understand delays in administrative handoffs, we stratified the SFT cases by VISN 20 sites and spoke with an administrator at a top performing site. Between 2006 and 2013, this site had a dedicated full-time imager as well as a backup imager that ensured images were taken quickly, usually on the same day the lesion was entered into care. Unfortunately, this is not the standard at all VISN 20 sites and certainly contributes to the overall delay in care in SFT
Minimizing the timeline of care is possible, as shown by the Danish health system, which developed a fast-track referral system after recognizing the need to minimize delays between the presentation, diagnosis, and treatment of cutaneous melanomas. In Denmark, a patient who presents to a general practitioner with a suspicious lesion is referred to secondary care for excision biopsy within 6 days. Diagnosis is made within 2 weeks, and, if necessary, definitive excision is offered within 9 days of the diagnosis. This translates into a maximum 20-day EEC to biopsy timeline and maximum 29-day EEC to definitive excision timeline. Although an intervention such as this may be difficult to implement in the United States due to its size and decentralized health care system, it would, however, be more realistic within the VA due to its centralized structure. The Danish system shows that with appropriate resource allocation and strict timeframes for treatment referrals, the timeline can be minimized.17
Despite the delay in the SFT timeline, this study found no significant difference between the distribution of in situ vs invasive melanomas in FTF dermatology and SFT groups. One possible explanation for this is that SFT increases access to dermatologist care, meaning clinicians may be more willing to consult SFT for less advanced– appearing lesions.
The finding that SFT diagnosed a larger proportion of in situ melanomas than FTF primary care is consistent with the findings of Ferrándiz et al, who reported that the mean Breslow thickness was significantly lower among patients in an SFT group compared to patients in an FTF group consisting of general practitioners. 18 However, the study population was not randomized and the results may have been impacted by ascertainment bias. Ferrándiz et al hypothesized that clinicians may have a lower threshold for consulting teledermatology, resulting in lower mean Breslow thicknesses.18 Karavan et al found the opposite results, with a higher mean Breslow thickness in SFT compared to a primary care FTF group.19 The data presented here suggest that SFT has room for process improvement yet is essentially equivalent to FTF dermatology in terms of outcomes.
Limitations
The majority of patients in this study were aged > 50 years, White, and male. The results may not be representative for other populations. The study was relatively small compared to studies that looked at other aspects of the melanoma care timeline. The study was not powered to ascertain mortality, the most important metric for melanoma.
Conclusions
The episode of care was significantly longer for melanomas diagnosed by SFT than those diagnosed by FTF; however, timelines were not statistically different when FTF lesions entered into care in dermatology were excluded. A median 6-day and mean 12.3-day delay in administrative handoffs occurred at the beginning of the SFT process and is a target for process improvement. Considering the high stakes of melanoma, the SFT timeline could be reduced if EEC, imaging, and SFT consultation all happened in the same day.
Store-and-forward teledermatology (SFT) allows clinical images and information to be sent to a dermatologist for evaluation. In fiscal year (FY) 2018, 117,780 SFT consultations were completed in the Veterans Health Administration. Continued growth is expected since SFT has proven to be an effective method for improving access to face-to-face (FTF) dermatology care.1 In the same period, the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS) completed 12,563 consultations in a mean 1.1 days from entry into episode of care (EEC), according to data reported by VA Teledermatology Program Administrator Chris Foster.
Obtaining a prompt consultation is reported to be an overwhelming advantage of using SFT.2-5 Rapid turnaround may appear to make SFT specialist care more accessible to veterans, yet this is an oversimplification. The process of delivering care (rather than consultation) through SFT is more complex than reading the images and reporting the findings. When a skin condition is identified by a primary care clinician and that person decides to request an SFT consultation, a complex set of tasks and handoffs is set into motion. A swim-lane diagram illustrates the numerous steps and handoffs that go into delivering care to a patient with a malignant melanoma on the SFT platform compared to FTF care, which requires fewer handoffs (Figure).

This process improvement project examined whether handoffs necessitated by SFT care lengthened the timeline of care for biopsy-proven primary cutaneous malignant melanoma. The stakes of delay in care are high. A 2018 study using the National Cancer Database found that a delay of > 30 days from biopsy to definitive excision (the date definitive surgical procedure for the condition is performed) resulted in a measurable increase in melanoma-related mortality. 6 This study sought to identify areas where the SFT timeline of care could be shortened.
Methods
This retrospective cohort study was approved by the VAPSHCS Institutional Review Board. The study drew from secondary data obtained from VistA, the VA Corporate Data Warehouse, the Veterans Integrated Service Network (VISN) 20 database, the American Academy of Dermatology Teledermatology Program database, and the VA Computerized Patient Record System.
Patients registered for ≥ 1 year at VAPSHCS with a diagnosis of primary cutaneous malignant melanoma by the Pathology service between January 1, 2006, and December 31, 2013, were included. Patients with metastatic or recurrent melanoma were excluded.
Cases were randomly selected from a melanoma database previously validated and used for another quality improvement project.7 There were initially 115 patient cases extracted from this database for both the FTF and SFT groups. Eighty-seven SFT and 107 FTF cases met inclusion criteria. To further analyze these groups, we split the FTF group into 2 subgroups: FTF dermatology (patients whose melanomas were entered into care in a dermatology clinic) and FTF primary care (patients whose melanomas were entered into care in primary care or a nondermatology setting).
The timeline of care was divided into 2 major time intervals: (1) entry into episode of care (EEC; the date a lesion was first documented in the electronic health record) to biopsy; and (2) biopsy to definitive excision. The SFT process was divided into the following intervals: EEC to imaging request (the date a clinician requested imaging); imaging request to imaging completion (the date an imager photographed a patient’s lesion); imaging completion to SFT consultation request (the date the SFT consultation was requested); SFT consultation request to consultation completion (the date an SFT reader completed the consultation request for a patient); and SFT consultation completion to biopsy. Mean and median interval lengths were compared between groups and additional analyses identified steps that may have contributed to delays in care.
To address potential bias based on access to care for rural veterans, SFT and FTF primary care cases were categorized into groups based on their location: (1) EEC and biopsy conducted at the same facility; (2) EEC and biopsy conducted at different facilities within the same health care system (main health care facility and its community-based outpatient clinics); and (3) EEC and biopsy conducted at different health care systems.
Statistics
Means, medians, and SDs were calculated in Excel. The Mann-Whitney U test was used to compare SFT medians to the FTF data and X2 test was used to compare proportions for secondary analyses.
Results
The median (mean) interval from EEC to definitive excision was 73 days (85) for SFT and 58 days (73) for FTF (P = .004) (Table). To understand this difference, the distribution of intervals from EEC to biopsy and biopsy to definitive excision were calculated. Only 38% of SFT cases were biopsied within 20 days compared to 65% of FTF cases (P < .001). The difference in time from biopsy to definitive excision distributions were not statistically significant, suggesting that the difference is actually a reflection of the differences seen in the period between EEC and biopsy.

EEC and biopsy occurred at the same facility in 85% and 82% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different facilities within the same health care system in 15% and 16% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different health care systems in 0% and 2% of FTF primary care and SFT cases, respectively. Geographic bias did not impact results for either group of veterans.
The interval between EEC and biopsy was shorter for FTF dermatology cases than for FTF primary care cases. For FTF dermatology cases, 96% were biopsied within 20 days compared with 34% of FTF primary care cases (P < .001).
To further analyze the difference in the EEC to biopsy interval duration between SFT and FTF primary care the timeline was divided into smaller steps: EEC to imaging completion, imaging completion to SFT consult completion, and SFT consult completion to biopsy. From EEC to SFT consult completion, SFT cases took a median of 6.0 days and a mean of 12.3 days, reflecting the administrative handoffs that must occur in SFT. A total of 82% of FTF primary care cases were entered into care and consultation was requested on the same day, while this was true for only 1% of SFT cases.
Since mortality data were not collected, the frequency of in situ melanomas and invasive melanomas (pathologic stage pT1a or greater) was used as a proxy for comparing outcomes. No significant difference was found in the frequency of in situ vs invasive melanomas in the SFT and FTF dermatology groups; however, there was a much higher frequency of invasive melanomas in the FTF primary care group (P = .007).
Discussion
This study compared the time to treatment for SFT vs FTF and identified important differences. The episode of care for melanomas diagnosed by SFT was statistically significantly longer (15 days) than those diagnosed by FTF. The interval between biopsy and definitive excision was a median of 34 and 38 days, and a mean of 48 and 44 days for SFT and FTF, respectively, which were not statistically significant. The difference in the total duration of the interval between EEC and definitive excision was accounted for by the duration of the interval from EEC to biopsy. When excluding dermatology clinic cases from the FTF group, there was no difference in the interval between EEC and biopsy for SFT and FTF primary care. The handoffs in SFT accounted for a median of 6 days and mean of 12 days, a significant portion of the timeline, and is a target for process improvement. The delay necessitated by handoffs did not significantly affect the distribution of in situ and invasive melanomas in the SFT and FTF dermatology groups. This suggests that SFT may have better outcomes than FTF primary care.
There has been extensive research on the timeline from the patient initially noticing a lesion to the EEC.8-11 There is also a body of research on the timeline from biopsy to definitive excision. 6,12-16 However, there has been little research on the timeline between EEC and biopsy, which comprises a large portion of the overall timeline of both SFT care and FTF care. This study analyzed the delays that can occur in this interval. When patients first enter FTF dermatology care, this timeline is quite short because lesions are often biopsied on the same day. When patients enter into care with their primary or nondermatology clinician, there can be significant delays.
Since the stakes are high when it comes to treating melanoma, it is important to minimize the overall timeline. A 6-day median and 12-day mean were established as targets for teledermatology handoffs. Ideally, a lesion should be entered into an episode of care, imaged, and sent for consultation on the same day. To help further understand delays in administrative handoffs, we stratified the SFT cases by VISN 20 sites and spoke with an administrator at a top performing site. Between 2006 and 2013, this site had a dedicated full-time imager as well as a backup imager that ensured images were taken quickly, usually on the same day the lesion was entered into care. Unfortunately, this is not the standard at all VISN 20 sites and certainly contributes to the overall delay in care in SFT
Minimizing the timeline of care is possible, as shown by the Danish health system, which developed a fast-track referral system after recognizing the need to minimize delays between the presentation, diagnosis, and treatment of cutaneous melanomas. In Denmark, a patient who presents to a general practitioner with a suspicious lesion is referred to secondary care for excision biopsy within 6 days. Diagnosis is made within 2 weeks, and, if necessary, definitive excision is offered within 9 days of the diagnosis. This translates into a maximum 20-day EEC to biopsy timeline and maximum 29-day EEC to definitive excision timeline. Although an intervention such as this may be difficult to implement in the United States due to its size and decentralized health care system, it would, however, be more realistic within the VA due to its centralized structure. The Danish system shows that with appropriate resource allocation and strict timeframes for treatment referrals, the timeline can be minimized.17
Despite the delay in the SFT timeline, this study found no significant difference between the distribution of in situ vs invasive melanomas in FTF dermatology and SFT groups. One possible explanation for this is that SFT increases access to dermatologist care, meaning clinicians may be more willing to consult SFT for less advanced– appearing lesions.
The finding that SFT diagnosed a larger proportion of in situ melanomas than FTF primary care is consistent with the findings of Ferrándiz et al, who reported that the mean Breslow thickness was significantly lower among patients in an SFT group compared to patients in an FTF group consisting of general practitioners. 18 However, the study population was not randomized and the results may have been impacted by ascertainment bias. Ferrándiz et al hypothesized that clinicians may have a lower threshold for consulting teledermatology, resulting in lower mean Breslow thicknesses.18 Karavan et al found the opposite results, with a higher mean Breslow thickness in SFT compared to a primary care FTF group.19 The data presented here suggest that SFT has room for process improvement yet is essentially equivalent to FTF dermatology in terms of outcomes.
Limitations
The majority of patients in this study were aged > 50 years, White, and male. The results may not be representative for other populations. The study was relatively small compared to studies that looked at other aspects of the melanoma care timeline. The study was not powered to ascertain mortality, the most important metric for melanoma.
Conclusions
The episode of care was significantly longer for melanomas diagnosed by SFT than those diagnosed by FTF; however, timelines were not statistically different when FTF lesions entered into care in dermatology were excluded. A median 6-day and mean 12.3-day delay in administrative handoffs occurred at the beginning of the SFT process and is a target for process improvement. Considering the high stakes of melanoma, the SFT timeline could be reduced if EEC, imaging, and SFT consultation all happened in the same day.
- Raugi GJ, Nelson W, Miethke M, et al. Teledermatology implementation in a VHA secondary treatment facility improves access to face-to-face care. Telemed J E Health. 2016;22(1):12-17. doi:10.1089/tmj.2015.0036
- Moreno-Ramirez D, Ferrandiz L, Nieto-Garcia A, et al. Store-and-forward teledermatology in skin cancer triage: experience and evaluation of 2009 teleconsultations. Arch Dermatol. 2007;143(4):479-484. doi:10.1001/archderm.143.4.479
- Landow SM, Oh DH, Weinstock MA. Teledermatology within the Veterans Health Administration, 2002–2014. Telemed J E Health. 2015;21(10):769-773. doi:10.1089/tmj.2014.0225
- Whited JD, Hall RP, Foy ME, et al. Teledermatology’s impact on time to intervention among referrals to a dermatology consult service. Telemed J E Health. 2002;8(3):313-321. doi:10.1089/15305620260353207
- Hsiao JL, Oh DH. The impact of store-and-forward teledermatology on skin cancer diagnosis and treatment. J Am Acad Dermatol. 2008;59(2):260-267. doi:10.1016/j.jaad.2008.04.011
- Conic RZ, Cabrera CI, Khorana AA, Gastman BR. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78(1):40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Dougall B, Gendreau J, Das S, et al. Melanoma registry underreporting in the Veterans Health Administration. Fed Pract. 2016;33(suppl 5):55S-59S
- Xavier MHSB, Drummond-Lage AP, Baeta C, Rocha L, Almeida AM, Wainstein AJA. Delay in cutaneous melanoma diagnosis: sequence analyses from suspicion to diagnosis in 211 patients. Medicine (Baltimore). 2016;95(31):e4396. doi:10.1097/md.0000000000004396
- Schmid-Wendtner MH, Baumert J, Stange J, Volkenandt M. Delay in the diagnosis of cutaneous melanoma: an analysis of 233 patients. Melanoma Res. 2002;12(4):389-394. doi:10.1097/00008390-200208000-00012
- Betti, R, Vergani R, Tolomio E, Santambrogio R, Crosti C. Factors of delay in the diagnosis of melanoma. Eur J Dermatol. 2003;13(2):183-188.
- Blum A, Brand CU, Ellwanger U, et al. Awareness and early detection of cutaneous melanoma: An analysis of factors related to delay in treatment. Br J Dermatol. 1999;141(5):783-787. doi:10.1046/j.1365-2133.1999.03196.x
- Brian T, Adams B, Jameson M. Cutaneous melanoma: an audit of management timeliness against New Zealand guidelines. N Z Med J. 2017;130(1462):54-61. https://pubmed.ncbi.nlm.nih.gov/28934768
- Adamson AS, Zhou L, Baggett CD, Thomas NE, Meyer AM. Association of delays in surgery for melanoma with Insurance type. JAMA Dermatol. 2017;153(11):1106-1113. doi:https://doi.org/10.1001/jamadermatol.2017.3338
- Niehues NB, Evanson B, Smith WA, Fiore CT, Parekh P. Melanoma patient notification and treatment timelines. Dermatol Online J. 2019;25(4)13. doi:10.5070/d3254043588
- Lott JP, Narayan D, Soulos PR, Aminawung J, Gross CP. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151(7):731-741. doi:10.1001/jamadermatol.2015.119
- Baranowski MLH, Yeung H, Chen SC, Gillespie TW, Goodman M. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81(4):908-916. doi:10.1016/j.jaad.2019.05.079
- Jarjis RD, Hansen LB, Matzen SH. A fast-track referral system for skin lesions suspicious of melanoma: population-based cross-sectional study from a plastic surgery center. Plast Surg Int. 2016;2016:2908917. doi:10.1155/2016/2908917
- Ferrándiz L, Ruiz-de-Casas A, Martin-Gutierrez FJ, et al. Effect of teledermatology on the prognosis of patients with cutaneous melanoma. Arch Dermatol. 2012;148(9):1025-1028. doi:10.1001/archdermatol.2012.778
- Karavan M, Compton N, Knezevich S, et al. Teledermatology in the diagnosis of melanoma. J Telemed Telecare. 2014;20(1):18-23. doi:10.1177/1357633x13517354
- Raugi GJ, Nelson W, Miethke M, et al. Teledermatology implementation in a VHA secondary treatment facility improves access to face-to-face care. Telemed J E Health. 2016;22(1):12-17. doi:10.1089/tmj.2015.0036
- Moreno-Ramirez D, Ferrandiz L, Nieto-Garcia A, et al. Store-and-forward teledermatology in skin cancer triage: experience and evaluation of 2009 teleconsultations. Arch Dermatol. 2007;143(4):479-484. doi:10.1001/archderm.143.4.479
- Landow SM, Oh DH, Weinstock MA. Teledermatology within the Veterans Health Administration, 2002–2014. Telemed J E Health. 2015;21(10):769-773. doi:10.1089/tmj.2014.0225
- Whited JD, Hall RP, Foy ME, et al. Teledermatology’s impact on time to intervention among referrals to a dermatology consult service. Telemed J E Health. 2002;8(3):313-321. doi:10.1089/15305620260353207
- Hsiao JL, Oh DH. The impact of store-and-forward teledermatology on skin cancer diagnosis and treatment. J Am Acad Dermatol. 2008;59(2):260-267. doi:10.1016/j.jaad.2008.04.011
- Conic RZ, Cabrera CI, Khorana AA, Gastman BR. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78(1):40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Dougall B, Gendreau J, Das S, et al. Melanoma registry underreporting in the Veterans Health Administration. Fed Pract. 2016;33(suppl 5):55S-59S
- Xavier MHSB, Drummond-Lage AP, Baeta C, Rocha L, Almeida AM, Wainstein AJA. Delay in cutaneous melanoma diagnosis: sequence analyses from suspicion to diagnosis in 211 patients. Medicine (Baltimore). 2016;95(31):e4396. doi:10.1097/md.0000000000004396
- Schmid-Wendtner MH, Baumert J, Stange J, Volkenandt M. Delay in the diagnosis of cutaneous melanoma: an analysis of 233 patients. Melanoma Res. 2002;12(4):389-394. doi:10.1097/00008390-200208000-00012
- Betti, R, Vergani R, Tolomio E, Santambrogio R, Crosti C. Factors of delay in the diagnosis of melanoma. Eur J Dermatol. 2003;13(2):183-188.
- Blum A, Brand CU, Ellwanger U, et al. Awareness and early detection of cutaneous melanoma: An analysis of factors related to delay in treatment. Br J Dermatol. 1999;141(5):783-787. doi:10.1046/j.1365-2133.1999.03196.x
- Brian T, Adams B, Jameson M. Cutaneous melanoma: an audit of management timeliness against New Zealand guidelines. N Z Med J. 2017;130(1462):54-61. https://pubmed.ncbi.nlm.nih.gov/28934768
- Adamson AS, Zhou L, Baggett CD, Thomas NE, Meyer AM. Association of delays in surgery for melanoma with Insurance type. JAMA Dermatol. 2017;153(11):1106-1113. doi:https://doi.org/10.1001/jamadermatol.2017.3338
- Niehues NB, Evanson B, Smith WA, Fiore CT, Parekh P. Melanoma patient notification and treatment timelines. Dermatol Online J. 2019;25(4)13. doi:10.5070/d3254043588
- Lott JP, Narayan D, Soulos PR, Aminawung J, Gross CP. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151(7):731-741. doi:10.1001/jamadermatol.2015.119
- Baranowski MLH, Yeung H, Chen SC, Gillespie TW, Goodman M. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81(4):908-916. doi:10.1016/j.jaad.2019.05.079
- Jarjis RD, Hansen LB, Matzen SH. A fast-track referral system for skin lesions suspicious of melanoma: population-based cross-sectional study from a plastic surgery center. Plast Surg Int. 2016;2016:2908917. doi:10.1155/2016/2908917
- Ferrándiz L, Ruiz-de-Casas A, Martin-Gutierrez FJ, et al. Effect of teledermatology on the prognosis of patients with cutaneous melanoma. Arch Dermatol. 2012;148(9):1025-1028. doi:10.1001/archdermatol.2012.778
- Karavan M, Compton N, Knezevich S, et al. Teledermatology in the diagnosis of melanoma. J Telemed Telecare. 2014;20(1):18-23. doi:10.1177/1357633x13517354
Handoff Delays in Teledermatology Lengthen Timeline of Care for Veterans With Melanoma
Handoff Delays in Teledermatology Lengthen Timeline of Care for Veterans With Melanoma
Training Lifeguards to Assist in Skin Cancer Prevention
Training Lifeguards to Assist in Skin Cancer Prevention
Lifeguards play a crucial role in ensuring water safety, but they also are uniquely positioned to promote skin cancer prevention and proper sunscreen use.1,2 There are several benefits and challenges to offering skin cancer prevention training for lifeguards.3 We examine the advantages of training, highlight the role lifeguards can play in larger public skin cancer prevention efforts, and address practical techniques for developing lifeguardfocused skin cancer education programs. By providing this knowledge to lifeguards, we can improve community health outcomes and encourage sun-safe behaviors in high-risk outdoor locations.
Benefits of Skin Cancer Prevention Training for Lifeguards
Research has shown that lifeguards are at an elevated risk for basal cell carcinoma, squamous cell carcinoma, and melanoma due to frequent prolonged occupational sun exposure.1,2,4-6 Therefore, comprehensive education on skin cancer prevention—including instruction on proper sunscreen application techniques and the importance of regular reapplication as well as how to recognize suspicious skin lesions—should be incorporated into lifeguard certification programs. One study evaluating the effectiveness of a skin cancer prevention program for lifeguards found that many of the participants lacked a thorough understanding of the different types of skin cancer.5 Another study found that lifeguards at pools in areas where societal norms supporting sun safety are stronger exhibited noticeably more sun protection practices, with regression estimates of 0.22 (95% CI, 0.17-0.26).7 Empowering lifeguards with valuable health knowledge during their regular training could potentially reduce their risk for skin cancer,4 as they may be more inclined to use sunscreen appropriately and reach out to a dermatologist for regular skin checks and evaluation of suspicious lesions.
Role of Lifeguards in Public Skin Cancer Prevention Efforts
Once trained on skin cancer prevention, lifeguards also can play a pivotal role in promoting sunscreen use among the public. Despite the widespread availability of high-quality sunscreens, many swimmers and beachgoers neglect to regularly apply or reapply sunscreen, especially on commonly exposed areas such as the back, shoulders, and face.8 Educating lifeguards on skin cancer prevention could enhance health outcomes by increasing early detection rates and promoting sun-safe behaviors among the general public.9 However, additional training requirements might increase the cost and time commitment for lifeguard certification, potentially leading to staffing shortages.3,7 There also is a risk of lifeguards overstepping their role and providing inaccurate medical advice, which could cause distress or even lead to liability issues.7 Balancing these factors will be crucial in developing effective and sustainable skin cancer prevention programs for lifeguards.
Implementing Lifeguard Skin Cancer Training
Implementing skin cancer prevention training programs for lifeguards requires strategic collaboration between dermatologists, and lifeguard training organizations to ensure that the participants receive consistent and comprehensive training.10 Additionally, public health campaigns can support these efforts by raising awareness about the importance of sun safety and regular skin checks.6 Tailored training modules/materials, ongoing technical assistance, and active, multicomponent approaches that account for both individual and environmental factors can increase program implementation in a variety of community settings.
Final Thoughts
Through effective education, lifeguards can potentially have a substantial impact on skin cancer prevention, both among lifeguards themselves and the general public. By promoting proper sunscreen use, lifeguards can help reduce the incidence and mortality associated with skin cancers. Future studies should focus on developing and implementing targeted education initiatives for lifeguards, fostering collaboration between relevant stakeholders, and raising public awareness about the importance of sun safety and early skin cancer detection. These efforts ultimately could lead to improved public health outcomes and reduced skin cancer rates, particularly in high-risk populations that frequently are exposed to UV radiation.
- Enos CW, Rey S, Slocum J, et al. Sun-protection behaviors among active members of the United States Lifesaving Association. J Clin Aesthet Dermatol. 2021;14:14-20.
- Verma K, Lewis DJ, Siddiqui FS, et al. Mohs micrographic surgery management of melanoma and melanoma in situ. StatPearls. Updated August 28, 2024. Accessed April 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK606123/
- Verma KK, Joshi TP, Lewis DJ, et al. Nail technicians as partners in early melanoma detection: bridging the knowledge gap. Arch Dermatol Res. 2024;316:586. doi:10.1007/s00403-024-03342-0
- Geller AC, Glanz K, Shigaki D, et al. Impact of skin cancer prevention on outdoor aquatics staff: the Pool Cool program in Hawaii and Massachusetts. Prev Med. 2001;33:155-161. doi:10.1006/pmed.2001.0870
- Hiemstra M, Glanz K, Nehl E. Changes in sunburn and tanning attitudes among lifeguards over a summer season. J Am Acad Dermatol. 2012;66:430-437. doi:10.1016/j.jaad.2010.11.050
- Verma KK, Ahmad N, Friedmann DP, et al. Melanoma in tattooed skin: diagnostic challenges and the potential for tattoo artists in early detection. Arch Dermatol Res. 2024;316:690. doi:10.1007/s00403-024-03415-0
- Hall DM, McCarty F, Elliott T, et al. Lifeguards’ sun protection habits and sunburns: association with sun-safe environments and skin cancer prevention program participation. Arch Dermatol. 2009;145:139-144. doi:10.1001/archdermatol.2008.553
- Emmons KM, Geller AC, Puleo E, et al. Skin cancer education and early detection at the beach: a randomized trial of dermatologist examination and biometric feedback. J Am Acad Dermatol. 2011;64:282-289. doi:10.1016/j.jaad.2010.01.040
- Rabin BA, Nehl E, Elliott T, et al. Individual and setting level predictors of the implementation of a skin cancer prevention program: a multilevel analysis. Implement Sci. 2010;5:40. doi:10.1186/1748-5908-5-40
- Walkosz BJ, Buller D, Buller M, et al. Sun safe workplaces: effect of an occupational skin cancer prevention program on employee sun safety practices. J Occup Environ Med. 2018;60:900-997. doi:10.1097 /JOM.0000000000001427
Lifeguards play a crucial role in ensuring water safety, but they also are uniquely positioned to promote skin cancer prevention and proper sunscreen use.1,2 There are several benefits and challenges to offering skin cancer prevention training for lifeguards.3 We examine the advantages of training, highlight the role lifeguards can play in larger public skin cancer prevention efforts, and address practical techniques for developing lifeguardfocused skin cancer education programs. By providing this knowledge to lifeguards, we can improve community health outcomes and encourage sun-safe behaviors in high-risk outdoor locations.
Benefits of Skin Cancer Prevention Training for Lifeguards
Research has shown that lifeguards are at an elevated risk for basal cell carcinoma, squamous cell carcinoma, and melanoma due to frequent prolonged occupational sun exposure.1,2,4-6 Therefore, comprehensive education on skin cancer prevention—including instruction on proper sunscreen application techniques and the importance of regular reapplication as well as how to recognize suspicious skin lesions—should be incorporated into lifeguard certification programs. One study evaluating the effectiveness of a skin cancer prevention program for lifeguards found that many of the participants lacked a thorough understanding of the different types of skin cancer.5 Another study found that lifeguards at pools in areas where societal norms supporting sun safety are stronger exhibited noticeably more sun protection practices, with regression estimates of 0.22 (95% CI, 0.17-0.26).7 Empowering lifeguards with valuable health knowledge during their regular training could potentially reduce their risk for skin cancer,4 as they may be more inclined to use sunscreen appropriately and reach out to a dermatologist for regular skin checks and evaluation of suspicious lesions.
Role of Lifeguards in Public Skin Cancer Prevention Efforts
Once trained on skin cancer prevention, lifeguards also can play a pivotal role in promoting sunscreen use among the public. Despite the widespread availability of high-quality sunscreens, many swimmers and beachgoers neglect to regularly apply or reapply sunscreen, especially on commonly exposed areas such as the back, shoulders, and face.8 Educating lifeguards on skin cancer prevention could enhance health outcomes by increasing early detection rates and promoting sun-safe behaviors among the general public.9 However, additional training requirements might increase the cost and time commitment for lifeguard certification, potentially leading to staffing shortages.3,7 There also is a risk of lifeguards overstepping their role and providing inaccurate medical advice, which could cause distress or even lead to liability issues.7 Balancing these factors will be crucial in developing effective and sustainable skin cancer prevention programs for lifeguards.
Implementing Lifeguard Skin Cancer Training
Implementing skin cancer prevention training programs for lifeguards requires strategic collaboration between dermatologists, and lifeguard training organizations to ensure that the participants receive consistent and comprehensive training.10 Additionally, public health campaigns can support these efforts by raising awareness about the importance of sun safety and regular skin checks.6 Tailored training modules/materials, ongoing technical assistance, and active, multicomponent approaches that account for both individual and environmental factors can increase program implementation in a variety of community settings.
Final Thoughts
Through effective education, lifeguards can potentially have a substantial impact on skin cancer prevention, both among lifeguards themselves and the general public. By promoting proper sunscreen use, lifeguards can help reduce the incidence and mortality associated with skin cancers. Future studies should focus on developing and implementing targeted education initiatives for lifeguards, fostering collaboration between relevant stakeholders, and raising public awareness about the importance of sun safety and early skin cancer detection. These efforts ultimately could lead to improved public health outcomes and reduced skin cancer rates, particularly in high-risk populations that frequently are exposed to UV radiation.
Lifeguards play a crucial role in ensuring water safety, but they also are uniquely positioned to promote skin cancer prevention and proper sunscreen use.1,2 There are several benefits and challenges to offering skin cancer prevention training for lifeguards.3 We examine the advantages of training, highlight the role lifeguards can play in larger public skin cancer prevention efforts, and address practical techniques for developing lifeguardfocused skin cancer education programs. By providing this knowledge to lifeguards, we can improve community health outcomes and encourage sun-safe behaviors in high-risk outdoor locations.
Benefits of Skin Cancer Prevention Training for Lifeguards
Research has shown that lifeguards are at an elevated risk for basal cell carcinoma, squamous cell carcinoma, and melanoma due to frequent prolonged occupational sun exposure.1,2,4-6 Therefore, comprehensive education on skin cancer prevention—including instruction on proper sunscreen application techniques and the importance of regular reapplication as well as how to recognize suspicious skin lesions—should be incorporated into lifeguard certification programs. One study evaluating the effectiveness of a skin cancer prevention program for lifeguards found that many of the participants lacked a thorough understanding of the different types of skin cancer.5 Another study found that lifeguards at pools in areas where societal norms supporting sun safety are stronger exhibited noticeably more sun protection practices, with regression estimates of 0.22 (95% CI, 0.17-0.26).7 Empowering lifeguards with valuable health knowledge during their regular training could potentially reduce their risk for skin cancer,4 as they may be more inclined to use sunscreen appropriately and reach out to a dermatologist for regular skin checks and evaluation of suspicious lesions.
Role of Lifeguards in Public Skin Cancer Prevention Efforts
Once trained on skin cancer prevention, lifeguards also can play a pivotal role in promoting sunscreen use among the public. Despite the widespread availability of high-quality sunscreens, many swimmers and beachgoers neglect to regularly apply or reapply sunscreen, especially on commonly exposed areas such as the back, shoulders, and face.8 Educating lifeguards on skin cancer prevention could enhance health outcomes by increasing early detection rates and promoting sun-safe behaviors among the general public.9 However, additional training requirements might increase the cost and time commitment for lifeguard certification, potentially leading to staffing shortages.3,7 There also is a risk of lifeguards overstepping their role and providing inaccurate medical advice, which could cause distress or even lead to liability issues.7 Balancing these factors will be crucial in developing effective and sustainable skin cancer prevention programs for lifeguards.
Implementing Lifeguard Skin Cancer Training
Implementing skin cancer prevention training programs for lifeguards requires strategic collaboration between dermatologists, and lifeguard training organizations to ensure that the participants receive consistent and comprehensive training.10 Additionally, public health campaigns can support these efforts by raising awareness about the importance of sun safety and regular skin checks.6 Tailored training modules/materials, ongoing technical assistance, and active, multicomponent approaches that account for both individual and environmental factors can increase program implementation in a variety of community settings.
Final Thoughts
Through effective education, lifeguards can potentially have a substantial impact on skin cancer prevention, both among lifeguards themselves and the general public. By promoting proper sunscreen use, lifeguards can help reduce the incidence and mortality associated with skin cancers. Future studies should focus on developing and implementing targeted education initiatives for lifeguards, fostering collaboration between relevant stakeholders, and raising public awareness about the importance of sun safety and early skin cancer detection. These efforts ultimately could lead to improved public health outcomes and reduced skin cancer rates, particularly in high-risk populations that frequently are exposed to UV radiation.
- Enos CW, Rey S, Slocum J, et al. Sun-protection behaviors among active members of the United States Lifesaving Association. J Clin Aesthet Dermatol. 2021;14:14-20.
- Verma K, Lewis DJ, Siddiqui FS, et al. Mohs micrographic surgery management of melanoma and melanoma in situ. StatPearls. Updated August 28, 2024. Accessed April 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK606123/
- Verma KK, Joshi TP, Lewis DJ, et al. Nail technicians as partners in early melanoma detection: bridging the knowledge gap. Arch Dermatol Res. 2024;316:586. doi:10.1007/s00403-024-03342-0
- Geller AC, Glanz K, Shigaki D, et al. Impact of skin cancer prevention on outdoor aquatics staff: the Pool Cool program in Hawaii and Massachusetts. Prev Med. 2001;33:155-161. doi:10.1006/pmed.2001.0870
- Hiemstra M, Glanz K, Nehl E. Changes in sunburn and tanning attitudes among lifeguards over a summer season. J Am Acad Dermatol. 2012;66:430-437. doi:10.1016/j.jaad.2010.11.050
- Verma KK, Ahmad N, Friedmann DP, et al. Melanoma in tattooed skin: diagnostic challenges and the potential for tattoo artists in early detection. Arch Dermatol Res. 2024;316:690. doi:10.1007/s00403-024-03415-0
- Hall DM, McCarty F, Elliott T, et al. Lifeguards’ sun protection habits and sunburns: association with sun-safe environments and skin cancer prevention program participation. Arch Dermatol. 2009;145:139-144. doi:10.1001/archdermatol.2008.553
- Emmons KM, Geller AC, Puleo E, et al. Skin cancer education and early detection at the beach: a randomized trial of dermatologist examination and biometric feedback. J Am Acad Dermatol. 2011;64:282-289. doi:10.1016/j.jaad.2010.01.040
- Rabin BA, Nehl E, Elliott T, et al. Individual and setting level predictors of the implementation of a skin cancer prevention program: a multilevel analysis. Implement Sci. 2010;5:40. doi:10.1186/1748-5908-5-40
- Walkosz BJ, Buller D, Buller M, et al. Sun safe workplaces: effect of an occupational skin cancer prevention program on employee sun safety practices. J Occup Environ Med. 2018;60:900-997. doi:10.1097 /JOM.0000000000001427
- Enos CW, Rey S, Slocum J, et al. Sun-protection behaviors among active members of the United States Lifesaving Association. J Clin Aesthet Dermatol. 2021;14:14-20.
- Verma K, Lewis DJ, Siddiqui FS, et al. Mohs micrographic surgery management of melanoma and melanoma in situ. StatPearls. Updated August 28, 2024. Accessed April 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK606123/
- Verma KK, Joshi TP, Lewis DJ, et al. Nail technicians as partners in early melanoma detection: bridging the knowledge gap. Arch Dermatol Res. 2024;316:586. doi:10.1007/s00403-024-03342-0
- Geller AC, Glanz K, Shigaki D, et al. Impact of skin cancer prevention on outdoor aquatics staff: the Pool Cool program in Hawaii and Massachusetts. Prev Med. 2001;33:155-161. doi:10.1006/pmed.2001.0870
- Hiemstra M, Glanz K, Nehl E. Changes in sunburn and tanning attitudes among lifeguards over a summer season. J Am Acad Dermatol. 2012;66:430-437. doi:10.1016/j.jaad.2010.11.050
- Verma KK, Ahmad N, Friedmann DP, et al. Melanoma in tattooed skin: diagnostic challenges and the potential for tattoo artists in early detection. Arch Dermatol Res. 2024;316:690. doi:10.1007/s00403-024-03415-0
- Hall DM, McCarty F, Elliott T, et al. Lifeguards’ sun protection habits and sunburns: association with sun-safe environments and skin cancer prevention program participation. Arch Dermatol. 2009;145:139-144. doi:10.1001/archdermatol.2008.553
- Emmons KM, Geller AC, Puleo E, et al. Skin cancer education and early detection at the beach: a randomized trial of dermatologist examination and biometric feedback. J Am Acad Dermatol. 2011;64:282-289. doi:10.1016/j.jaad.2010.01.040
- Rabin BA, Nehl E, Elliott T, et al. Individual and setting level predictors of the implementation of a skin cancer prevention program: a multilevel analysis. Implement Sci. 2010;5:40. doi:10.1186/1748-5908-5-40
- Walkosz BJ, Buller D, Buller M, et al. Sun safe workplaces: effect of an occupational skin cancer prevention program on employee sun safety practices. J Occup Environ Med. 2018;60:900-997. doi:10.1097 /JOM.0000000000001427
Training Lifeguards to Assist in Skin Cancer Prevention
Training Lifeguards to Assist in Skin Cancer Prevention
Assay Shows Promise for Early-Stage Melanoma Risk Assessment Beyond SNLB
A noninvasive clinicopathologic and gene expression profiling (CP-GEP)–based tool, the Merlin assay, shows promise for identifying recurrence risks in patients with early-stage melanoma who do not undergo sentinel lymph node biopsy (SNLB).
This was the conclusion of a retrospective analysis of a large cohort of patients with stage I/II disease, reported by Teresa Amaral, MD, PhD, at the 11th World Congress of Melanoma and 21st European Association of Dermato-Oncology Congress 2025.
Of 930 patients included in the study, the assay identified 879 as having a low risk for recurrence and 51 as having high risk for recurrence. The overall 5-year recurrence-free survival (RFS), distant metastasis-free survival (DMFS) and melanoma-specific survival (MSS) rates were 90.9%, 96.9%, and 97.5%, respectively.
The corresponding rates among those stratified by the assay as having low vs high recurrence risk, respectively, were 94.6% and 26.6% for RFS (hazard ratio [HR], 25.08), 98.6% vs 62.1% for DMFS (HR, 35.39), and 99.4% vs 61.7% for MSS (HR, 71.05), said Amaral, during her presentation at the meeting.
Of 16 melanoma-specific deaths, 12 were stratified as high risk for recurrence by the CP-GEP assay, said Amaral, head of the Skin Cancer Clinical Trials Center at the University of Tübingen, Tübingen, Germany, and first author of the study.
Study participants had stages IA-IIC melanoma, 41% were women, and median age was 64 years. Median melanoma thickness was 0.5 mm, and 94% were not ulcerated.
No systemic treatment options currently exist for patients with this early-stage disease, the author said.
The CP-GEP model, initially developed by the Mayo Clinic and SkylineDx BV to predict the positivity of SNLB, has been validated in multiple studies.
Amaral and her colleagues previously demonstrated the ability of the CP-GEP model to stratify patients with stages I-II disease as having low or high risk for recurrence — including in a small number of patients without SNLB. Those findings are confirmed in this larger population of patients who did not undergo SNLB, she said.
SkylineDx announced the findings in a press release stating the results validate the prognostic power of the Merlin assay for “identifying tumors at high risk for relapse that would otherwise be missed by traditional clinical and pathological evaluation.”
SNLB is the gold standard for nodal assessment for staging cutaneous melanoma. More than 80% of patients who undergo SNLB are negative for nodal metastases, but most patients who relapse or die from their melanoma are initially stratified by SNLB as having low-risk early-stage disease, Amaral explained. This suggests “SNLB is not enough,” she noted.
The findings suggest that CP-GEP has the potential to risk stratify patients with early-stage melanoma that did not undergo SLNB and help select those who can forgo SLNB, she said.
Without another means of assessing risk, patients considered low risk based on SNLB are closely followed, the author explained.
“If we could identify the very low-risk patient and then allocate the resources to the very high-risk patients who really need a more detailed and more tailored approach…we would be doing a favor to our patients,” the author concluded.
Amaral disclosed personal financial relationships with Delcath, Philogen, Bristol Myers Squibb, NeraCare, Novartis, Pierre Fabre, CeCaVa, and MedTrix.
A version of this article first appeared on Medscape.com.
A noninvasive clinicopathologic and gene expression profiling (CP-GEP)–based tool, the Merlin assay, shows promise for identifying recurrence risks in patients with early-stage melanoma who do not undergo sentinel lymph node biopsy (SNLB).
This was the conclusion of a retrospective analysis of a large cohort of patients with stage I/II disease, reported by Teresa Amaral, MD, PhD, at the 11th World Congress of Melanoma and 21st European Association of Dermato-Oncology Congress 2025.
Of 930 patients included in the study, the assay identified 879 as having a low risk for recurrence and 51 as having high risk for recurrence. The overall 5-year recurrence-free survival (RFS), distant metastasis-free survival (DMFS) and melanoma-specific survival (MSS) rates were 90.9%, 96.9%, and 97.5%, respectively.
The corresponding rates among those stratified by the assay as having low vs high recurrence risk, respectively, were 94.6% and 26.6% for RFS (hazard ratio [HR], 25.08), 98.6% vs 62.1% for DMFS (HR, 35.39), and 99.4% vs 61.7% for MSS (HR, 71.05), said Amaral, during her presentation at the meeting.
Of 16 melanoma-specific deaths, 12 were stratified as high risk for recurrence by the CP-GEP assay, said Amaral, head of the Skin Cancer Clinical Trials Center at the University of Tübingen, Tübingen, Germany, and first author of the study.
Study participants had stages IA-IIC melanoma, 41% were women, and median age was 64 years. Median melanoma thickness was 0.5 mm, and 94% were not ulcerated.
No systemic treatment options currently exist for patients with this early-stage disease, the author said.
The CP-GEP model, initially developed by the Mayo Clinic and SkylineDx BV to predict the positivity of SNLB, has been validated in multiple studies.
Amaral and her colleagues previously demonstrated the ability of the CP-GEP model to stratify patients with stages I-II disease as having low or high risk for recurrence — including in a small number of patients without SNLB. Those findings are confirmed in this larger population of patients who did not undergo SNLB, she said.
SkylineDx announced the findings in a press release stating the results validate the prognostic power of the Merlin assay for “identifying tumors at high risk for relapse that would otherwise be missed by traditional clinical and pathological evaluation.”
SNLB is the gold standard for nodal assessment for staging cutaneous melanoma. More than 80% of patients who undergo SNLB are negative for nodal metastases, but most patients who relapse or die from their melanoma are initially stratified by SNLB as having low-risk early-stage disease, Amaral explained. This suggests “SNLB is not enough,” she noted.
The findings suggest that CP-GEP has the potential to risk stratify patients with early-stage melanoma that did not undergo SLNB and help select those who can forgo SLNB, she said.
Without another means of assessing risk, patients considered low risk based on SNLB are closely followed, the author explained.
“If we could identify the very low-risk patient and then allocate the resources to the very high-risk patients who really need a more detailed and more tailored approach…we would be doing a favor to our patients,” the author concluded.
Amaral disclosed personal financial relationships with Delcath, Philogen, Bristol Myers Squibb, NeraCare, Novartis, Pierre Fabre, CeCaVa, and MedTrix.
A version of this article first appeared on Medscape.com.
A noninvasive clinicopathologic and gene expression profiling (CP-GEP)–based tool, the Merlin assay, shows promise for identifying recurrence risks in patients with early-stage melanoma who do not undergo sentinel lymph node biopsy (SNLB).
This was the conclusion of a retrospective analysis of a large cohort of patients with stage I/II disease, reported by Teresa Amaral, MD, PhD, at the 11th World Congress of Melanoma and 21st European Association of Dermato-Oncology Congress 2025.
Of 930 patients included in the study, the assay identified 879 as having a low risk for recurrence and 51 as having high risk for recurrence. The overall 5-year recurrence-free survival (RFS), distant metastasis-free survival (DMFS) and melanoma-specific survival (MSS) rates were 90.9%, 96.9%, and 97.5%, respectively.
The corresponding rates among those stratified by the assay as having low vs high recurrence risk, respectively, were 94.6% and 26.6% for RFS (hazard ratio [HR], 25.08), 98.6% vs 62.1% for DMFS (HR, 35.39), and 99.4% vs 61.7% for MSS (HR, 71.05), said Amaral, during her presentation at the meeting.
Of 16 melanoma-specific deaths, 12 were stratified as high risk for recurrence by the CP-GEP assay, said Amaral, head of the Skin Cancer Clinical Trials Center at the University of Tübingen, Tübingen, Germany, and first author of the study.
Study participants had stages IA-IIC melanoma, 41% were women, and median age was 64 years. Median melanoma thickness was 0.5 mm, and 94% were not ulcerated.
No systemic treatment options currently exist for patients with this early-stage disease, the author said.
The CP-GEP model, initially developed by the Mayo Clinic and SkylineDx BV to predict the positivity of SNLB, has been validated in multiple studies.
Amaral and her colleagues previously demonstrated the ability of the CP-GEP model to stratify patients with stages I-II disease as having low or high risk for recurrence — including in a small number of patients without SNLB. Those findings are confirmed in this larger population of patients who did not undergo SNLB, she said.
SkylineDx announced the findings in a press release stating the results validate the prognostic power of the Merlin assay for “identifying tumors at high risk for relapse that would otherwise be missed by traditional clinical and pathological evaluation.”
SNLB is the gold standard for nodal assessment for staging cutaneous melanoma. More than 80% of patients who undergo SNLB are negative for nodal metastases, but most patients who relapse or die from their melanoma are initially stratified by SNLB as having low-risk early-stage disease, Amaral explained. This suggests “SNLB is not enough,” she noted.
The findings suggest that CP-GEP has the potential to risk stratify patients with early-stage melanoma that did not undergo SLNB and help select those who can forgo SLNB, she said.
Without another means of assessing risk, patients considered low risk based on SNLB are closely followed, the author explained.
“If we could identify the very low-risk patient and then allocate the resources to the very high-risk patients who really need a more detailed and more tailored approach…we would be doing a favor to our patients,” the author concluded.
Amaral disclosed personal financial relationships with Delcath, Philogen, Bristol Myers Squibb, NeraCare, Novartis, Pierre Fabre, CeCaVa, and MedTrix.
A version of this article first appeared on Medscape.com.
FROM WCM-EADO 2025