User login
The Food and Drug Administration has tracked counterfeit bevacizumab, the cancer drug marketed as Avastin, to at least one foreign supplier and identified 19 medical practices in the United States that purchased unapproved cancer medicines, possibly including counterfeit bevacizumab.
The FDA said the practices obtained the fake Avastin from Quality Specialty Products (QSP), a foreign supplier also known as Montana Health Care Solutions. QSP products are also distributed by Volunteer Distribution in Gainesboro, Tenn., the agency said.
The FDA has requested that medical practices stop using any remaining products purchased from these suppliers "or any other unapproved foreign source," because the agency cannot ensure the safety or efficacy of any of these unapproved products.
"The Agency is very concerned that these products may cause harm to patients because they are unsafe or ineffective. ... These products may be from unknown sources; have unknown ingredients; and may not have been manufactured, transported or stored under proper conditions required by U.S. law, regulations, and standards," the agency said.
Avastin’s manufacturers announced Feb. 14 that they learned of the distribution of the counterfeit in the United States. "The counterfeit product is not safe or effective and should not be used. Chemical analyses of the counterfeit vials tested to date have confirmed the product does not contain the active ingredients for Avastin," warned F. Hoffmann-La Roche and Genentech.
Avastin, an injectable medicine used to treat many cancers, is administered to patients in clinics, hospitals, and doctors’ offices. The counterfeit version does not contain the monoclonal antibody bevacizumab.
The FDA similarly warned health care professionals and patients about a counterfeit version of Avastin 400mg/16mL, which may have been purchased and used by some medical practices in the United States.
The FDA has since issued letters to 19 U.S. medical practices that purchased unapproved cancer medicines that may include the counterfeit Avastin. It is believed that some product in the United States labeled as Avastin 400 mg/16 mL with the following lot numbers on either the vials or packaging may be counterfeit: B86017, B6011, and B6010.
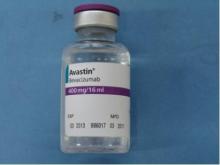
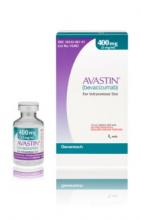
Importantly, the counterfeit product does not look like authentic Avastin. Authentic Avastin’s cartons and vials (approved for use in the United States) have "Genentech" or "Genentech, a member of the Roche Group" printed on the labels. The counterfeit version is labeled as Avastin, manufactured by Roche, which manufactures Avastin for marketing outside of the United States.
In addition, the lot number on the carton and vial should be six digits with no letters; the expiry date is formatted as a three-letter month and four-digit year (such as, JUL 2014); the date of manufacture is not printed on the carton or vial; and all the text on the vial labels, cartons, and package inserts is in English on products approved for sale in the United States.
The fake Avastin scandal comes at a time when many injectable cancer drugs are in short supply – a situation that "may present an opportunity for unscrupulous individuals to introduce non-FDA approved products into the drug supply," the FDA warned on Jan. 13.
At that time, it advised health care providers about the risks of purchasing unapproved injectable cancer medications that are not in short supply from unlicensed sources. The agency named Faslodex (fulvestrant), Neupogen (filgrastim), Rituxan (rituximab) and Herceptin (trastuzumab) – but not Avastin – in the January warning.
"In certain circumstances, the FDA may authorize limited importation of medications that are in short supply. Such medications are imported from approved international sources and distributed in the U.S. through a controlled network, and would not be sold in direct-to-clinic solicitations," the agency advised.
"If the FDA has arranged for limited importation of the foreign version of a medication, information on obtaining that medication will be available on the FDA drug shortages website, often in the form of a ‘Dear Healthcare Professional’ letter."
Based on information to date, the FDA has determined that none of the unapproved cancer medicines that medical practices are known to have received from Volunteer Distribution are in short supply in the United States. FDA-approved versions of these medicines are available in adequate supply to meet current demand.
Medical practices that have obtained unapproved products from foreign sources, in particular from Volunteer Distribution and/or QSP, should stop using them and contact the FDA. These products should be retained and securely stored.
To report suspect counterfeit products and other suspect unapproved products obtained from Volunteer Distribution or QSP/Montana Health Care Solutions or other sources: Call FDA’s Office of Criminal Investigations (OCI) at 800-551-3989, or visit OCI’s website.
For information about this counterfeit medicine, see Roche’s statement.
Click here for more information about counterfeit medicines found in the United States.
The Food and Drug Administration has tracked counterfeit bevacizumab, the cancer drug marketed as Avastin, to at least one foreign supplier and identified 19 medical practices in the United States that purchased unapproved cancer medicines, possibly including counterfeit bevacizumab.
The FDA said the practices obtained the fake Avastin from Quality Specialty Products (QSP), a foreign supplier also known as Montana Health Care Solutions. QSP products are also distributed by Volunteer Distribution in Gainesboro, Tenn., the agency said.
The FDA has requested that medical practices stop using any remaining products purchased from these suppliers "or any other unapproved foreign source," because the agency cannot ensure the safety or efficacy of any of these unapproved products.
"The Agency is very concerned that these products may cause harm to patients because they are unsafe or ineffective. ... These products may be from unknown sources; have unknown ingredients; and may not have been manufactured, transported or stored under proper conditions required by U.S. law, regulations, and standards," the agency said.
Avastin’s manufacturers announced Feb. 14 that they learned of the distribution of the counterfeit in the United States. "The counterfeit product is not safe or effective and should not be used. Chemical analyses of the counterfeit vials tested to date have confirmed the product does not contain the active ingredients for Avastin," warned F. Hoffmann-La Roche and Genentech.
Avastin, an injectable medicine used to treat many cancers, is administered to patients in clinics, hospitals, and doctors’ offices. The counterfeit version does not contain the monoclonal antibody bevacizumab.
The FDA similarly warned health care professionals and patients about a counterfeit version of Avastin 400mg/16mL, which may have been purchased and used by some medical practices in the United States.
The FDA has since issued letters to 19 U.S. medical practices that purchased unapproved cancer medicines that may include the counterfeit Avastin. It is believed that some product in the United States labeled as Avastin 400 mg/16 mL with the following lot numbers on either the vials or packaging may be counterfeit: B86017, B6011, and B6010.
Importantly, the counterfeit product does not look like authentic Avastin. Authentic Avastin’s cartons and vials (approved for use in the United States) have "Genentech" or "Genentech, a member of the Roche Group" printed on the labels. The counterfeit version is labeled as Avastin, manufactured by Roche, which manufactures Avastin for marketing outside of the United States.
In addition, the lot number on the carton and vial should be six digits with no letters; the expiry date is formatted as a three-letter month and four-digit year (such as, JUL 2014); the date of manufacture is not printed on the carton or vial; and all the text on the vial labels, cartons, and package inserts is in English on products approved for sale in the United States.
The fake Avastin scandal comes at a time when many injectable cancer drugs are in short supply – a situation that "may present an opportunity for unscrupulous individuals to introduce non-FDA approved products into the drug supply," the FDA warned on Jan. 13.
At that time, it advised health care providers about the risks of purchasing unapproved injectable cancer medications that are not in short supply from unlicensed sources. The agency named Faslodex (fulvestrant), Neupogen (filgrastim), Rituxan (rituximab) and Herceptin (trastuzumab) – but not Avastin – in the January warning.
"In certain circumstances, the FDA may authorize limited importation of medications that are in short supply. Such medications are imported from approved international sources and distributed in the U.S. through a controlled network, and would not be sold in direct-to-clinic solicitations," the agency advised.
"If the FDA has arranged for limited importation of the foreign version of a medication, information on obtaining that medication will be available on the FDA drug shortages website, often in the form of a ‘Dear Healthcare Professional’ letter."
Based on information to date, the FDA has determined that none of the unapproved cancer medicines that medical practices are known to have received from Volunteer Distribution are in short supply in the United States. FDA-approved versions of these medicines are available in adequate supply to meet current demand.
Medical practices that have obtained unapproved products from foreign sources, in particular from Volunteer Distribution and/or QSP, should stop using them and contact the FDA. These products should be retained and securely stored.
To report suspect counterfeit products and other suspect unapproved products obtained from Volunteer Distribution or QSP/Montana Health Care Solutions or other sources: Call FDA’s Office of Criminal Investigations (OCI) at 800-551-3989, or visit OCI’s website.
For information about this counterfeit medicine, see Roche’s statement.
Click here for more information about counterfeit medicines found in the United States.
The Food and Drug Administration has tracked counterfeit bevacizumab, the cancer drug marketed as Avastin, to at least one foreign supplier and identified 19 medical practices in the United States that purchased unapproved cancer medicines, possibly including counterfeit bevacizumab.
The FDA said the practices obtained the fake Avastin from Quality Specialty Products (QSP), a foreign supplier also known as Montana Health Care Solutions. QSP products are also distributed by Volunteer Distribution in Gainesboro, Tenn., the agency said.
The FDA has requested that medical practices stop using any remaining products purchased from these suppliers "or any other unapproved foreign source," because the agency cannot ensure the safety or efficacy of any of these unapproved products.
"The Agency is very concerned that these products may cause harm to patients because they are unsafe or ineffective. ... These products may be from unknown sources; have unknown ingredients; and may not have been manufactured, transported or stored under proper conditions required by U.S. law, regulations, and standards," the agency said.
Avastin’s manufacturers announced Feb. 14 that they learned of the distribution of the counterfeit in the United States. "The counterfeit product is not safe or effective and should not be used. Chemical analyses of the counterfeit vials tested to date have confirmed the product does not contain the active ingredients for Avastin," warned F. Hoffmann-La Roche and Genentech.
Avastin, an injectable medicine used to treat many cancers, is administered to patients in clinics, hospitals, and doctors’ offices. The counterfeit version does not contain the monoclonal antibody bevacizumab.
The FDA similarly warned health care professionals and patients about a counterfeit version of Avastin 400mg/16mL, which may have been purchased and used by some medical practices in the United States.
The FDA has since issued letters to 19 U.S. medical practices that purchased unapproved cancer medicines that may include the counterfeit Avastin. It is believed that some product in the United States labeled as Avastin 400 mg/16 mL with the following lot numbers on either the vials or packaging may be counterfeit: B86017, B6011, and B6010.
Importantly, the counterfeit product does not look like authentic Avastin. Authentic Avastin’s cartons and vials (approved for use in the United States) have "Genentech" or "Genentech, a member of the Roche Group" printed on the labels. The counterfeit version is labeled as Avastin, manufactured by Roche, which manufactures Avastin for marketing outside of the United States.
In addition, the lot number on the carton and vial should be six digits with no letters; the expiry date is formatted as a three-letter month and four-digit year (such as, JUL 2014); the date of manufacture is not printed on the carton or vial; and all the text on the vial labels, cartons, and package inserts is in English on products approved for sale in the United States.
The fake Avastin scandal comes at a time when many injectable cancer drugs are in short supply – a situation that "may present an opportunity for unscrupulous individuals to introduce non-FDA approved products into the drug supply," the FDA warned on Jan. 13.
At that time, it advised health care providers about the risks of purchasing unapproved injectable cancer medications that are not in short supply from unlicensed sources. The agency named Faslodex (fulvestrant), Neupogen (filgrastim), Rituxan (rituximab) and Herceptin (trastuzumab) – but not Avastin – in the January warning.
"In certain circumstances, the FDA may authorize limited importation of medications that are in short supply. Such medications are imported from approved international sources and distributed in the U.S. through a controlled network, and would not be sold in direct-to-clinic solicitations," the agency advised.
"If the FDA has arranged for limited importation of the foreign version of a medication, information on obtaining that medication will be available on the FDA drug shortages website, often in the form of a ‘Dear Healthcare Professional’ letter."
Based on information to date, the FDA has determined that none of the unapproved cancer medicines that medical practices are known to have received from Volunteer Distribution are in short supply in the United States. FDA-approved versions of these medicines are available in adequate supply to meet current demand.
Medical practices that have obtained unapproved products from foreign sources, in particular from Volunteer Distribution and/or QSP, should stop using them and contact the FDA. These products should be retained and securely stored.
To report suspect counterfeit products and other suspect unapproved products obtained from Volunteer Distribution or QSP/Montana Health Care Solutions or other sources: Call FDA’s Office of Criminal Investigations (OCI) at 800-551-3989, or visit OCI’s website.
For information about this counterfeit medicine, see Roche’s statement.
Click here for more information about counterfeit medicines found in the United States.