User login
From the VA Eastern Colorado Health Care System, University of Colorado School of Medicine, and the Colorado Cardiovascular Outcomes Research Group, Denver and Aurora, CO.
Abstract
- Objective: To review the use of elective percutaneous coronary intervention (PCI), evaluate what is currently known about elective PCI in the context of appropriate use criteria, and offer insight into next steps to optimize the use of elective PCI to achieve high-quality care.
- Methods: Review of the scientific literature, appropriate use criteria, and professional society guidelines relevant to elective PCI.
- Results: Recent studies have demonstrated as many as 1 in 6 elective PCIs are inappropriate as determined by appropriate use criteria. These inappropriate PCIs are not anticipated to benefit patients and result in unnecessary patient risk and cost. While these studies are consistent with regard to overuse of elective PCI, less is known about potential underuse of PCI for elective indications. We lack health status data on populations of ischemic heart disease patients to inform PCI underuse that may contribute to patient symptom burden, functional status, and quality of life. Optimal use of PCI will be attained with longitudinal capture of patient-reported health status, study of factors contributing to overuse and underuse, refinement of the appropriate use criteria with particular focus on patient-centered measures, and incorporation of patient preference and shared decision making into appropriateness evaluation tools.
- Conclusion: The use of elective PCI is less than optimal in current clinical practice. Continued effort is needed to ensure elective PCI is targeted to patients with anticipated benefit and use of the procedure is aligned with patient preferences.
Providing the right care to the right patient at the right time is essential to the practice of high-quality care. Reducing overuse of health care services is part of this equation, and initiatives to reduce inappropriate use and to encourage physicians and patients to “choose wisely” have been introduced [1]. One procedure that is being examined with a focus on appropriateness is percutaneous coronary intervention (PCI). This procedure is common (nearly 1 million inpatient PCI procedures performed in 2010), presents risks to the patient, and is expensive (attributable cost approximately $10 billion in 2010) [2,3]. While the clinical benefit of PCI in acute settings such as ST-segment elevation myocardial infarction is well established [4], the benefit of PCI in nonacute (elective) settings is less robust [5–7]. Prior studies have demonstrated PCI for stable ischemic heart disease does not result in mortality benefit [6]. Furthermore, PCI as an initial strategy for symptom relief of stable angina may offer little benefit relative to medications alone [5]. Given that PCI is common, costly, and associated with both short- and long-term risks [8,9], ensuring this therapy is provided to the right patient at the right time is important.
In 2009, appropriate use criteria (AUC) were developed by 6 professional organizations to support the rational and judicious use of PCI [10]; a focused update was published in 2012 [11]. In this review, we discuss the recommendations for appropriate use and their application and offer thoughts on next steps to optimize the use of elective PCI as part of high-quality care.
Variation in the Use of PCI
Additionally, significant public attention has been focused on the issue of overuse after lay press investigations into community practice patterns. In particular, a case study presented in the New York Times highlighted the community of Elyria, Ohio, which was found to have a PCI rate that was 4 times the national average [16]. This investigation sparked public debate and further focused attention on the issue of overuse of elective PCI. Conversely, others have pointed to data that suggest underuse of coronary procedural care, particularly among women and racial and ethnic minorities [17–22].
Appropriate Use Criteria
Development Methodology
AUC for PCI, which were developed through the collaborative efforts of 6 major cardiovascular professional organizations, are intended to support the effective, efficient, and equitable use of PCI [10,11]. They were developed in response to a growing need to support rational use of cardiovascular procedures as part of high-quality care. The methods of development for the AUC have been described in detail in the criteria publications [10,11]. We briefly review these methods here.
Panel members first individually assigned ratings to each clinical scenario that ranged from 1 (least appropriate) to 9 (most appropriate). This was followed by an in-person meeting in which technical panel members discussed scenarios for which there was wide variation in appropriateness assessment. After this meeting, technical panel members again assigned ratings for each scenario from 1 to 9. After this second round, the median values for the pooled ratings were used as the appropriateness classification for each scenario. Scenarios with median values of 1–3 were classified as “inappropriate,” 4–6 as “uncertain,” and 7–9 as “appropriate.” A rating of “appropriate” represented clinical scenarios in which the indication is considered generally acceptable and likely to improve health outcomes or survival. A rating of “uncertain” represented clinical scenarios where the indication may be reasonable but more research is necessary to further understand the relative benefits and risks of PCI in this setting. Finally, a rating of “inappropriate” represented clinical scenarios in which the indication is not generally acceptable as it is unlikely to improve health outcomes or survival.
The approach used for AUC development appears to be valid, as Class III indications for PCI in the ACC/AHA clinical guideline [24] (Class III = PCI should NOT be performed since it is not helpful and may be harmful) and AUC scenarios rated as inappropriate are in 100% agreement (personal communication, Ralph Brindis, past president of the American College of Cardiology).
Application
It is important to remember that the AUC are intended to aid in patient selection and are not absolute. Unique clinical factors and patient preference cannot feasibly be captured by the AUC scenarios. It should also be noted that the intent of the AUC is not to be punitive but rather to identify and assess variation in practice patterns. To reflect this intent, the terminology applied to appropriateness ratings has recently changed. Clinical scenarios previously classified as “inappropriate” are now termed “rarely appropriate” and clinical scenarios classified as “uncertain” are now termed “may be appropriate.”
Although the AUC were developed to help evaluate practice patterns of care delivery and serve as guides for clinical decision making, they were not intended to serve as mandates for or against treatment in individual patients or to be tied to reimbursement for individual patients. Despite this, health care organizations and payors have used other AUC documents for incentive pay and prior authorization programs, specifically for cardiovascular imaging [25]. Use of the AUC in this manner may still be reasonable if application and measurement is at the level of the practice, rather than the individual patient, but much remains to be understood about the implications of applying AUC in reimbursement
decisions.
Refinement
The AUC for PCI are designed to be dynamic and continually updated. As additional evidence becomes available regarding the efficacy of PCI in specific clinical scenarios, there will be ongoing efforts to update the AUC to reflect this new evidence. This is highlighted by the first update to the AUC occurring less than 3 years after the original publication date [11].
In addition to perpetual review of the data used to inform scenario ratings, there are opportunities to improve measurement of the clinical variables that are considered in rating PCI appropriateness (eg, clinical presentation, symptom severity, ischemia severity, extent of medical therapy, extent of anatomic disease). For example, in the current AUC, symptom severity is dependent on clinician assessment using the Canadian Cardiovascular Society Classification [25]. Moving toward a patient-centered assessment of symptom severity would ensure that the AUC more closely reflect the patient-perceived symptom burden. Further, the use of a patient-centered instrument would reduce the possibility of physician manipulation of symptom severity to influence the apparent appropriateness of PCI. There are similar opportunities to improve reporting of noninvasive stress test data, such as through standardized reporting of ischemic risk. Finally, the use of physiologic assessments of stenosis severity (eg, fractional flow reserve) and quantitative coronary angiography to standardize interpretations of diagnostic angiography may further optimize the assessment of PCI appropriateness.
Application of the Appropriate Use Criteria in Clinical Practice—Study Results
 Application of the AUC to clinical practice has highlighted potential overuse of PCI (Table). The first report came from applying the AUC to the National Cardiovascular Data Registry (NCDR) CathPCI Registry [26]. In this study of more than 500,000 PCIs from over 1000 facilities across the country, the authors found that PCIs performed in the acute setting (STEMI, NSTEMI, and high-risk unstable angina) were almost uniformly classified as appropriate. However, for nonacute (elective) PCI, application of the AUC resulted in the classification of 50% as appropriate, 38% as uncertain, and 12% as inappropriate. The majority of patients who received inappropriate PCI had a low-risk stress test (72%) or were asymptomatic (54%). Additionally, 96% of patients who received PCI classified as inappropriate had not been given a trial of adequate anti-anginal therapy. This analysis was supported by subsequent analyses of 2 other state-specific registries (New York and Washington), which found similar rates of PCI for nonacute indications rated as inappropriate [27,28]. Additionally, all 3 studies showed wide facility-level variation in the percentage of appropriate and inappropriate PCI for elective indications.
Application of the AUC to clinical practice has highlighted potential overuse of PCI (Table). The first report came from applying the AUC to the National Cardiovascular Data Registry (NCDR) CathPCI Registry [26]. In this study of more than 500,000 PCIs from over 1000 facilities across the country, the authors found that PCIs performed in the acute setting (STEMI, NSTEMI, and high-risk unstable angina) were almost uniformly classified as appropriate. However, for nonacute (elective) PCI, application of the AUC resulted in the classification of 50% as appropriate, 38% as uncertain, and 12% as inappropriate. The majority of patients who received inappropriate PCI had a low-risk stress test (72%) or were asymptomatic (54%). Additionally, 96% of patients who received PCI classified as inappropriate had not been given a trial of adequate anti-anginal therapy. This analysis was supported by subsequent analyses of 2 other state-specific registries (New York and Washington), which found similar rates of PCI for nonacute indications rated as inappropriate [27,28]. Additionally, all 3 studies showed wide facility-level variation in the percentage of appropriate and inappropriate PCI for elective indications.
These studies also highlight a gap in preprocedural care. The anticipated benefit of elective PCI is related to patient symptom burden, adequacy of anti-anginal therapy, and ischemic risk as determined by noninvasive stress testing. However, 30% to 50% of patients undergo elective PCI without evidence of preprocedural stress testing. Attempts are being made to address this gap with the recent release of PCI performance measures [29]. These performance measures, intended for cardiac catheterization labs, include comprehensive documentation of the indication for PCI, which is central to determination of appropriateness. This integration of procedural indication into a performance measure marks the first such occurrence in cardiology.
As documentation of procedural indication and appropriateness have become part and parcel of assessing quality of care, concerns about “gaming” have become more pertinent. Providers who perform PCI could potentially enhance the appearance of appropriateness by overstating the clinical symptom burden or stress test findings. The incorporation of validated, patient-centered health status questionnaires along with data audit programs have been proposed as measures to prevent this type of abuse. Addressing quality gaps in preprocedural assessment and documentation is critical to optimizing use of elective PCI [28].
The apparent overuse of PCI for elective indications may be a reflection of our fragmented, fee-for-service health care delivery system. However, recent studies challenge these assumptions. In a Canadian study, Ko et al found that 18% of elective PCIs were classified as inappropriate, a proportion similar to what had been found previously in the United States [30]. In a US study of Medicare beneficiaries, Matlock and colleagues observed a fourfold regional variation in use of elective coronary angiography and PCI in both Medicare fee-for-service and capitated Medicare Advantage beneficiaries [31]. Collectively, these studies suggest barriers to optimal patient selection for invasive coronary procedures in both capitated and fee-for-service health care systems. Without addressing factors that contribute to variation in the absence of fee-for-service incentives, efforts to improve integration and reduce fee-for-service reimbursement may be inadequate to optimize PCI use.
Evaluating Underuse
While potential underuse of PCI has been described for acute indications [17–22], study of underuse of PCI for elective indications is more challenging. Population data on the effect of underuse of elective PCI on patient symptom burden, functional status, and quality of life is lacking.
A population-based study from Australia highlights the potential importance of underuse in the care of patients with stable coronary disease. This study assessed symptom burden among patients with chronic stable angina using the Seattle Angina Questionnaire and included patients cared for by 207 primary care practitioners [32]. The authors noted that there was considerable variation in patient symptom burden between practices, with 14% of practices having no patients with more than 1 episode of angina per week and 18% of clinics having more than half of enrolled patients with at least 1 episode of angina per week. The authors postulate that this variability may be due to differences among providers in the identification and management of angina, including using PCI to minimize symptom burden.
In the Ko study mentioned earlier, the AUC was used to examine potential underuse of coronary revascularization procedures. In this study, they analyzed the association between AUC ratings and outcomes in patients undergoing diagnostic coronary angiography [30]. Of patients considered “appropriate” for revascularization following completion of diagnostic angiography, only 69% underwent revascularization. However, the clinical aspects that influence the decision to proceed with revascularization may not be fully captured in this study. Thus, the true degree of underuse of PCI remains elusive.
In summary, the relative lack of data that would allow for the assessment of underuse of elective PCI is an important quality concern. Health systems should work to systematically capture patient-reported health status, including symptom burden data, to identify inadequate symptom control and potential underuse of procedural care for CAD.
Facilitating Optimal Use
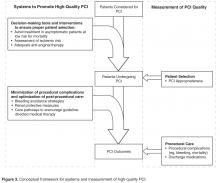
 In current practice, the AUC hold promise to minimize the overuse of elective PCI. This likely involves addressing processes occurring upstream of the cardiac catheterization lab, including employing systems to ensure that procedures are avoided in patients who are unlikely to benefit (eg, asymptomatic, low ischemic burden) (Figure 3) [33]. Studying hospitals that already have low rates of inappropriate PCI may inform the design and dissemination of strategies that will help improve patient selection at hospitals with higher rates. Although professional organizations have developed tools intended to facilitate appropriateness evaluation at the point-of-care [34], the use of these tools are likely to be sporadic without greater integration into the health care delivery system. Further, these applications are currently limited to determination of appropriateness of PCI after completion of the diagnostic coronary angiogram. Identifying processes prior to catheterization that contribute to PCI appropriateness may also streamline appropriate ad hoc PCI, as the need to reassess appropriateness after the diagnostic angiogram may be mitigated.
In current practice, the AUC hold promise to minimize the overuse of elective PCI. This likely involves addressing processes occurring upstream of the cardiac catheterization lab, including employing systems to ensure that procedures are avoided in patients who are unlikely to benefit (eg, asymptomatic, low ischemic burden) (Figure 3) [33]. Studying hospitals that already have low rates of inappropriate PCI may inform the design and dissemination of strategies that will help improve patient selection at hospitals with higher rates. Although professional organizations have developed tools intended to facilitate appropriateness evaluation at the point-of-care [34], the use of these tools are likely to be sporadic without greater integration into the health care delivery system. Further, these applications are currently limited to determination of appropriateness of PCI after completion of the diagnostic coronary angiogram. Identifying processes prior to catheterization that contribute to PCI appropriateness may also streamline appropriate ad hoc PCI, as the need to reassess appropriateness after the diagnostic angiogram may be mitigated.
Significant barriers exist to the application of the AUC for determination of procedural underuse. As described above, we lack adequate data to ascertain gaps in symptom management that could be mitigated by proper use of PCI. Further study of symptom burden in populations of patients with coronary artery disease is needed. This may help in the identification of patient populations whose symptom burden may warrant consideration of invasive coronary procedures, including coronary angiography and PCI.
Finally, it is important to note that the AUC are based on technical considerations, ie, practice guidelines and trial evidence. They do not take into consideration patient preferences. For example, PCI can be technically appropriate for the scenario but inappropriate for the individual if the procedure is not desired by the patient. Similarly, a procedure may be of uncertain benefit but appropriate if the patient desires more aggressive procedural care and has a full understanding of the risks and benefits. Currently, we fail to convey this information to patients, as evidenced by patients’ overestimation of the benefits of PCI [34]. As we continue to work toward optimal use of PCI, we must not only address the technical appropriateness of care, but move toward incorporating patient preferences through a robust process of shared decision-making.
Corresponding author: Preston M. Schneider, MD, VA Eastern Colorado Health Care System, Cardiology Section (111B), 1055 Clermont St., Denver, CO 80220, [email protected].
Funding/support: Dr. Schneider is supported by a T32 training grant from the National Institutes of Health (5T32HL00
7822-15). Dr. Bradley is supported by a Career Development Award (HSR&D-CDA2 10-199) from VA Health Services Research & Development.
Financial disclosures: None.
1. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
2. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013;127:e6–e245.
3. HCUPnet: A tool for identifying, tracking, and analyzing national hospital statistics. Accessed 22 Oct 2013 at http://hcupnet.ahrq.gov/HCUPnet.jsp?Parms=
H4sIAAAAAAAAABXBMQ6AIBAEwC9JAg.gsLAhRvjAnnuXgGihFb9XZwYe3EhLdpN2h2aIcsnQLCp9jQVbLDN3ksq
DnSeqVXzNfIAP9mtmLy0rZhdIAAAA83D0C2BCAE02DD1508408B2C5C094F1ADF6E788C&JS=Y.
4. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13–20.
5. Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–16.
6. Boden WE, O’Rourke RA, Teo KK, et al. Impact of optimal medical therapy with or without percutaneous coronary intervention on long-term cardiovascular end points in patients with stable coronary artery disease (from the COURAGE Trial). Am J Cardiol 2009;104:1–4.
7. Stergiopoulos K, Brown DL. Initial coronary stent implantation with medical therapy vs medical therapy alone for stable coronary artery disease: Meta-analysis of randomized controlled trials. Arch Intern Med 2012;172:312–9.
8. McCullough PA, Adam A, Becker CR, et al. Epidemiology and prognostic implications of contrast-induced nephropathy. Contrast-Induc Nephrop Clin Insights Pract Guid Rep CIN Consens Work Panel 2006;98:5–13.
9. Roe MT, Messenger JC, Weintraub WS, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol 2010;56:254–63.
10. Patel MR, Dehmer GJ, Hirshfeld JW, et al. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: A Report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2009;53:530–53.
11. Patel MR, Dehmer GJ, Hirshfeld JW, et al. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 Appropriate Use Criteria for Coronary Revascularization Focused Update: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2012;59:857–81.
12. Dartmouth Atlas of Health Care. Accessed 8 Jan 2014 at www.dartmouthatlas.org.
13. Dartmouth Atlas of Health Care: Studies of surgical variation. Cardiac surgery report. 2005. Accessed 8 Jan 2014 at www.dartmouthatlas.org/publications/reports.aspx.
14. Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in medicare spending. part 1: the content, quality, and accessibility of care. Ann Intern Med 2003;138:273–87.
15. Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in medicare spending. part 2: health outcomes and satisfaction with care. Ann Intern Med 2003;138:288–98.
16. Abelson R. Heart procedure is off the charts in an Ohio city. New York Times 2006. Accessed 23 Apr 2013 at www.nytimes.com/2006/08/18/business/18stent.html.
17. Akhter N, Milford-Beland S, Roe MT, et al. Gender differences among patients with acute coronary syndromes undergoing percutaneous coronary intervention in the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR). Am Heart J 2009;157:141–8.
18. Blomkalns AL, Chen AY, Hochman JS, et al. Gender disparities in the diagnosis and treatment of non–ST-segment elevation acute coronary syndromesLarge-scale observations from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines) National Quality Improvement Initiative. J Am Coll Cardiol 2005;45:832–7.
19. Daly C, Clemens F, Lopez Sendon JL, et al. Gender differences in the management and clinical outcome of stable angina. Circulation 2006;113:490–8.
20. Groeneveld PW, Heidenreich PA, Garber AM. Racial disparity in cardiac procedures and mortality among long-term survivors of cardiac arrest. Circulation 2003;108:286–91.
21. Hannan EL, Zhong Y, Walford G, et al. Underutilization of percutaneous coronary intervention for ST-elevation myocardial infarction in Medicaid patients relative to private insurance patients. J Intervent Cardiol 2013;26:470–81.
22. Sonel AF, Good CB, Mulgund J, et al. Racial variations in treatment and outcomes of black and white patients with high-risk non–ST-elevation acute coronary syndromes: insights From CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines?). Circulation 2005;111:1225–32.
23. Patel MR, Spertus JA, Brindis RG, et al. ACCF proposed method for evaluating the appropriateness of cardiovascular imaging. J Am Coll Cardiol 2005;46:1606–13.
24. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011;124:2574–609.
25. Campeau L. Letter: Grading of angina pectoris. Circulation 1976;54:522–3.
26. Chan PS, Patel MR, Klein LW, et al. Appropriateness of percutaneous coronary intervention. JAMA 2011;306:53–61.
27. Hannan EL, Cozzens K, Samadashvili Z, et al. Appropriateness of coronary revascularization for patients without acute coronary syndromes. J Am Coll Cardiol 2012;59:1870–6.
28. Bradley SM, Maynard C, Bryson CL. Appropriateness of percutaneous coronary interventions in Washington State. Circ Cardiovasc Qual Outcomes 2012;5:445–53.
29. Nallamothu BK, Tommaso CL, Anderson HV, et al. ACC/AHA/SCAI/AMA–Convened PCPI/NCQA 2013 Performance measures for adults undergoing percutaneous coronary intervention. A report of the American College of Cardiology/American Heart Association Task Force on Performance Measures, the Society for Cardiovascular Angiography and Interventions, the American Medical Association–Convened Physician Consortium for Performance Improvement, and the National Committee for Quality Assurance. J Am Coll Cardiol 2014;63:722–45.
30. Ko DT, Guo H, Wijeysundera HC, et al. Assessing the association of appropriateness of coronary revascularization and clinical outcomes for patients with stable coronary artery disease. J Am Coll Cardiol 2012;60:1876–84.
31. Matlock DD, Groeneveld PW, Sidney S, et al. Geographic variation in cardiovascular procedure use among medicare fee-for-service vs medicare advantage beneficiaries. JAMA 2013;310:155–62.
32. Beltrame JF, Weekes AJ, Morgan C, et al. The prevalence of weekly angina among patients with chronic stable angina in primary care practices: The coronary artery disease in general practice (cadence) study. Arch Intern Med 2009;169:1491–9.
33. Bradley SM, Spertus JA, Nallamothu BK, et al. The association between patient selection for diagnostic coronary angiography and hospital-level PCI appropriateness: Insights from the NCDR. Circ Cardiovasc Qual Outcomes 2013;6:A1. Accessed 20 Nov 2013 at http://circoutcomes.ahajournals.org/cgi/content/short/6/3_MeetingAbstracts/A1?rss=1.
34. Lee J, Chuu K, Spertus J, et al. Patients overestimate the potential benefits of elective percutaneous coronary intervention. Mo Med 2012;109:79.
From the VA Eastern Colorado Health Care System, University of Colorado School of Medicine, and the Colorado Cardiovascular Outcomes Research Group, Denver and Aurora, CO.
Abstract
- Objective: To review the use of elective percutaneous coronary intervention (PCI), evaluate what is currently known about elective PCI in the context of appropriate use criteria, and offer insight into next steps to optimize the use of elective PCI to achieve high-quality care.
- Methods: Review of the scientific literature, appropriate use criteria, and professional society guidelines relevant to elective PCI.
- Results: Recent studies have demonstrated as many as 1 in 6 elective PCIs are inappropriate as determined by appropriate use criteria. These inappropriate PCIs are not anticipated to benefit patients and result in unnecessary patient risk and cost. While these studies are consistent with regard to overuse of elective PCI, less is known about potential underuse of PCI for elective indications. We lack health status data on populations of ischemic heart disease patients to inform PCI underuse that may contribute to patient symptom burden, functional status, and quality of life. Optimal use of PCI will be attained with longitudinal capture of patient-reported health status, study of factors contributing to overuse and underuse, refinement of the appropriate use criteria with particular focus on patient-centered measures, and incorporation of patient preference and shared decision making into appropriateness evaluation tools.
- Conclusion: The use of elective PCI is less than optimal in current clinical practice. Continued effort is needed to ensure elective PCI is targeted to patients with anticipated benefit and use of the procedure is aligned with patient preferences.
Providing the right care to the right patient at the right time is essential to the practice of high-quality care. Reducing overuse of health care services is part of this equation, and initiatives to reduce inappropriate use and to encourage physicians and patients to “choose wisely” have been introduced [1]. One procedure that is being examined with a focus on appropriateness is percutaneous coronary intervention (PCI). This procedure is common (nearly 1 million inpatient PCI procedures performed in 2010), presents risks to the patient, and is expensive (attributable cost approximately $10 billion in 2010) [2,3]. While the clinical benefit of PCI in acute settings such as ST-segment elevation myocardial infarction is well established [4], the benefit of PCI in nonacute (elective) settings is less robust [5–7]. Prior studies have demonstrated PCI for stable ischemic heart disease does not result in mortality benefit [6]. Furthermore, PCI as an initial strategy for symptom relief of stable angina may offer little benefit relative to medications alone [5]. Given that PCI is common, costly, and associated with both short- and long-term risks [8,9], ensuring this therapy is provided to the right patient at the right time is important.
In 2009, appropriate use criteria (AUC) were developed by 6 professional organizations to support the rational and judicious use of PCI [10]; a focused update was published in 2012 [11]. In this review, we discuss the recommendations for appropriate use and their application and offer thoughts on next steps to optimize the use of elective PCI as part of high-quality care.
Variation in the Use of PCI
Additionally, significant public attention has been focused on the issue of overuse after lay press investigations into community practice patterns. In particular, a case study presented in the New York Times highlighted the community of Elyria, Ohio, which was found to have a PCI rate that was 4 times the national average [16]. This investigation sparked public debate and further focused attention on the issue of overuse of elective PCI. Conversely, others have pointed to data that suggest underuse of coronary procedural care, particularly among women and racial and ethnic minorities [17–22].
Appropriate Use Criteria
Development Methodology
AUC for PCI, which were developed through the collaborative efforts of 6 major cardiovascular professional organizations, are intended to support the effective, efficient, and equitable use of PCI [10,11]. They were developed in response to a growing need to support rational use of cardiovascular procedures as part of high-quality care. The methods of development for the AUC have been described in detail in the criteria publications [10,11]. We briefly review these methods here.
Panel members first individually assigned ratings to each clinical scenario that ranged from 1 (least appropriate) to 9 (most appropriate). This was followed by an in-person meeting in which technical panel members discussed scenarios for which there was wide variation in appropriateness assessment. After this meeting, technical panel members again assigned ratings for each scenario from 1 to 9. After this second round, the median values for the pooled ratings were used as the appropriateness classification for each scenario. Scenarios with median values of 1–3 were classified as “inappropriate,” 4–6 as “uncertain,” and 7–9 as “appropriate.” A rating of “appropriate” represented clinical scenarios in which the indication is considered generally acceptable and likely to improve health outcomes or survival. A rating of “uncertain” represented clinical scenarios where the indication may be reasonable but more research is necessary to further understand the relative benefits and risks of PCI in this setting. Finally, a rating of “inappropriate” represented clinical scenarios in which the indication is not generally acceptable as it is unlikely to improve health outcomes or survival.
The approach used for AUC development appears to be valid, as Class III indications for PCI in the ACC/AHA clinical guideline [24] (Class III = PCI should NOT be performed since it is not helpful and may be harmful) and AUC scenarios rated as inappropriate are in 100% agreement (personal communication, Ralph Brindis, past president of the American College of Cardiology).
Application
It is important to remember that the AUC are intended to aid in patient selection and are not absolute. Unique clinical factors and patient preference cannot feasibly be captured by the AUC scenarios. It should also be noted that the intent of the AUC is not to be punitive but rather to identify and assess variation in practice patterns. To reflect this intent, the terminology applied to appropriateness ratings has recently changed. Clinical scenarios previously classified as “inappropriate” are now termed “rarely appropriate” and clinical scenarios classified as “uncertain” are now termed “may be appropriate.”
Although the AUC were developed to help evaluate practice patterns of care delivery and serve as guides for clinical decision making, they were not intended to serve as mandates for or against treatment in individual patients or to be tied to reimbursement for individual patients. Despite this, health care organizations and payors have used other AUC documents for incentive pay and prior authorization programs, specifically for cardiovascular imaging [25]. Use of the AUC in this manner may still be reasonable if application and measurement is at the level of the practice, rather than the individual patient, but much remains to be understood about the implications of applying AUC in reimbursement
decisions.
Refinement
The AUC for PCI are designed to be dynamic and continually updated. As additional evidence becomes available regarding the efficacy of PCI in specific clinical scenarios, there will be ongoing efforts to update the AUC to reflect this new evidence. This is highlighted by the first update to the AUC occurring less than 3 years after the original publication date [11].
In addition to perpetual review of the data used to inform scenario ratings, there are opportunities to improve measurement of the clinical variables that are considered in rating PCI appropriateness (eg, clinical presentation, symptom severity, ischemia severity, extent of medical therapy, extent of anatomic disease). For example, in the current AUC, symptom severity is dependent on clinician assessment using the Canadian Cardiovascular Society Classification [25]. Moving toward a patient-centered assessment of symptom severity would ensure that the AUC more closely reflect the patient-perceived symptom burden. Further, the use of a patient-centered instrument would reduce the possibility of physician manipulation of symptom severity to influence the apparent appropriateness of PCI. There are similar opportunities to improve reporting of noninvasive stress test data, such as through standardized reporting of ischemic risk. Finally, the use of physiologic assessments of stenosis severity (eg, fractional flow reserve) and quantitative coronary angiography to standardize interpretations of diagnostic angiography may further optimize the assessment of PCI appropriateness.
Application of the Appropriate Use Criteria in Clinical Practice—Study Results
 Application of the AUC to clinical practice has highlighted potential overuse of PCI (Table). The first report came from applying the AUC to the National Cardiovascular Data Registry (NCDR) CathPCI Registry [26]. In this study of more than 500,000 PCIs from over 1000 facilities across the country, the authors found that PCIs performed in the acute setting (STEMI, NSTEMI, and high-risk unstable angina) were almost uniformly classified as appropriate. However, for nonacute (elective) PCI, application of the AUC resulted in the classification of 50% as appropriate, 38% as uncertain, and 12% as inappropriate. The majority of patients who received inappropriate PCI had a low-risk stress test (72%) or were asymptomatic (54%). Additionally, 96% of patients who received PCI classified as inappropriate had not been given a trial of adequate anti-anginal therapy. This analysis was supported by subsequent analyses of 2 other state-specific registries (New York and Washington), which found similar rates of PCI for nonacute indications rated as inappropriate [27,28]. Additionally, all 3 studies showed wide facility-level variation in the percentage of appropriate and inappropriate PCI for elective indications.
Application of the AUC to clinical practice has highlighted potential overuse of PCI (Table). The first report came from applying the AUC to the National Cardiovascular Data Registry (NCDR) CathPCI Registry [26]. In this study of more than 500,000 PCIs from over 1000 facilities across the country, the authors found that PCIs performed in the acute setting (STEMI, NSTEMI, and high-risk unstable angina) were almost uniformly classified as appropriate. However, for nonacute (elective) PCI, application of the AUC resulted in the classification of 50% as appropriate, 38% as uncertain, and 12% as inappropriate. The majority of patients who received inappropriate PCI had a low-risk stress test (72%) or were asymptomatic (54%). Additionally, 96% of patients who received PCI classified as inappropriate had not been given a trial of adequate anti-anginal therapy. This analysis was supported by subsequent analyses of 2 other state-specific registries (New York and Washington), which found similar rates of PCI for nonacute indications rated as inappropriate [27,28]. Additionally, all 3 studies showed wide facility-level variation in the percentage of appropriate and inappropriate PCI for elective indications.
These studies also highlight a gap in preprocedural care. The anticipated benefit of elective PCI is related to patient symptom burden, adequacy of anti-anginal therapy, and ischemic risk as determined by noninvasive stress testing. However, 30% to 50% of patients undergo elective PCI without evidence of preprocedural stress testing. Attempts are being made to address this gap with the recent release of PCI performance measures [29]. These performance measures, intended for cardiac catheterization labs, include comprehensive documentation of the indication for PCI, which is central to determination of appropriateness. This integration of procedural indication into a performance measure marks the first such occurrence in cardiology.
As documentation of procedural indication and appropriateness have become part and parcel of assessing quality of care, concerns about “gaming” have become more pertinent. Providers who perform PCI could potentially enhance the appearance of appropriateness by overstating the clinical symptom burden or stress test findings. The incorporation of validated, patient-centered health status questionnaires along with data audit programs have been proposed as measures to prevent this type of abuse. Addressing quality gaps in preprocedural assessment and documentation is critical to optimizing use of elective PCI [28].
The apparent overuse of PCI for elective indications may be a reflection of our fragmented, fee-for-service health care delivery system. However, recent studies challenge these assumptions. In a Canadian study, Ko et al found that 18% of elective PCIs were classified as inappropriate, a proportion similar to what had been found previously in the United States [30]. In a US study of Medicare beneficiaries, Matlock and colleagues observed a fourfold regional variation in use of elective coronary angiography and PCI in both Medicare fee-for-service and capitated Medicare Advantage beneficiaries [31]. Collectively, these studies suggest barriers to optimal patient selection for invasive coronary procedures in both capitated and fee-for-service health care systems. Without addressing factors that contribute to variation in the absence of fee-for-service incentives, efforts to improve integration and reduce fee-for-service reimbursement may be inadequate to optimize PCI use.
Evaluating Underuse
While potential underuse of PCI has been described for acute indications [17–22], study of underuse of PCI for elective indications is more challenging. Population data on the effect of underuse of elective PCI on patient symptom burden, functional status, and quality of life is lacking.
A population-based study from Australia highlights the potential importance of underuse in the care of patients with stable coronary disease. This study assessed symptom burden among patients with chronic stable angina using the Seattle Angina Questionnaire and included patients cared for by 207 primary care practitioners [32]. The authors noted that there was considerable variation in patient symptom burden between practices, with 14% of practices having no patients with more than 1 episode of angina per week and 18% of clinics having more than half of enrolled patients with at least 1 episode of angina per week. The authors postulate that this variability may be due to differences among providers in the identification and management of angina, including using PCI to minimize symptom burden.
In the Ko study mentioned earlier, the AUC was used to examine potential underuse of coronary revascularization procedures. In this study, they analyzed the association between AUC ratings and outcomes in patients undergoing diagnostic coronary angiography [30]. Of patients considered “appropriate” for revascularization following completion of diagnostic angiography, only 69% underwent revascularization. However, the clinical aspects that influence the decision to proceed with revascularization may not be fully captured in this study. Thus, the true degree of underuse of PCI remains elusive.
In summary, the relative lack of data that would allow for the assessment of underuse of elective PCI is an important quality concern. Health systems should work to systematically capture patient-reported health status, including symptom burden data, to identify inadequate symptom control and potential underuse of procedural care for CAD.
Facilitating Optimal Use
 In current practice, the AUC hold promise to minimize the overuse of elective PCI. This likely involves addressing processes occurring upstream of the cardiac catheterization lab, including employing systems to ensure that procedures are avoided in patients who are unlikely to benefit (eg, asymptomatic, low ischemic burden) (Figure 3) [33]. Studying hospitals that already have low rates of inappropriate PCI may inform the design and dissemination of strategies that will help improve patient selection at hospitals with higher rates. Although professional organizations have developed tools intended to facilitate appropriateness evaluation at the point-of-care [34], the use of these tools are likely to be sporadic without greater integration into the health care delivery system. Further, these applications are currently limited to determination of appropriateness of PCI after completion of the diagnostic coronary angiogram. Identifying processes prior to catheterization that contribute to PCI appropriateness may also streamline appropriate ad hoc PCI, as the need to reassess appropriateness after the diagnostic angiogram may be mitigated.
In current practice, the AUC hold promise to minimize the overuse of elective PCI. This likely involves addressing processes occurring upstream of the cardiac catheterization lab, including employing systems to ensure that procedures are avoided in patients who are unlikely to benefit (eg, asymptomatic, low ischemic burden) (Figure 3) [33]. Studying hospitals that already have low rates of inappropriate PCI may inform the design and dissemination of strategies that will help improve patient selection at hospitals with higher rates. Although professional organizations have developed tools intended to facilitate appropriateness evaluation at the point-of-care [34], the use of these tools are likely to be sporadic without greater integration into the health care delivery system. Further, these applications are currently limited to determination of appropriateness of PCI after completion of the diagnostic coronary angiogram. Identifying processes prior to catheterization that contribute to PCI appropriateness may also streamline appropriate ad hoc PCI, as the need to reassess appropriateness after the diagnostic angiogram may be mitigated.
Significant barriers exist to the application of the AUC for determination of procedural underuse. As described above, we lack adequate data to ascertain gaps in symptom management that could be mitigated by proper use of PCI. Further study of symptom burden in populations of patients with coronary artery disease is needed. This may help in the identification of patient populations whose symptom burden may warrant consideration of invasive coronary procedures, including coronary angiography and PCI.
Finally, it is important to note that the AUC are based on technical considerations, ie, practice guidelines and trial evidence. They do not take into consideration patient preferences. For example, PCI can be technically appropriate for the scenario but inappropriate for the individual if the procedure is not desired by the patient. Similarly, a procedure may be of uncertain benefit but appropriate if the patient desires more aggressive procedural care and has a full understanding of the risks and benefits. Currently, we fail to convey this information to patients, as evidenced by patients’ overestimation of the benefits of PCI [34]. As we continue to work toward optimal use of PCI, we must not only address the technical appropriateness of care, but move toward incorporating patient preferences through a robust process of shared decision-making.
Corresponding author: Preston M. Schneider, MD, VA Eastern Colorado Health Care System, Cardiology Section (111B), 1055 Clermont St., Denver, CO 80220, [email protected].
Funding/support: Dr. Schneider is supported by a T32 training grant from the National Institutes of Health (5T32HL00
7822-15). Dr. Bradley is supported by a Career Development Award (HSR&D-CDA2 10-199) from VA Health Services Research & Development.
Financial disclosures: None.
From the VA Eastern Colorado Health Care System, University of Colorado School of Medicine, and the Colorado Cardiovascular Outcomes Research Group, Denver and Aurora, CO.
Abstract
- Objective: To review the use of elective percutaneous coronary intervention (PCI), evaluate what is currently known about elective PCI in the context of appropriate use criteria, and offer insight into next steps to optimize the use of elective PCI to achieve high-quality care.
- Methods: Review of the scientific literature, appropriate use criteria, and professional society guidelines relevant to elective PCI.
- Results: Recent studies have demonstrated as many as 1 in 6 elective PCIs are inappropriate as determined by appropriate use criteria. These inappropriate PCIs are not anticipated to benefit patients and result in unnecessary patient risk and cost. While these studies are consistent with regard to overuse of elective PCI, less is known about potential underuse of PCI for elective indications. We lack health status data on populations of ischemic heart disease patients to inform PCI underuse that may contribute to patient symptom burden, functional status, and quality of life. Optimal use of PCI will be attained with longitudinal capture of patient-reported health status, study of factors contributing to overuse and underuse, refinement of the appropriate use criteria with particular focus on patient-centered measures, and incorporation of patient preference and shared decision making into appropriateness evaluation tools.
- Conclusion: The use of elective PCI is less than optimal in current clinical practice. Continued effort is needed to ensure elective PCI is targeted to patients with anticipated benefit and use of the procedure is aligned with patient preferences.
Providing the right care to the right patient at the right time is essential to the practice of high-quality care. Reducing overuse of health care services is part of this equation, and initiatives to reduce inappropriate use and to encourage physicians and patients to “choose wisely” have been introduced [1]. One procedure that is being examined with a focus on appropriateness is percutaneous coronary intervention (PCI). This procedure is common (nearly 1 million inpatient PCI procedures performed in 2010), presents risks to the patient, and is expensive (attributable cost approximately $10 billion in 2010) [2,3]. While the clinical benefit of PCI in acute settings such as ST-segment elevation myocardial infarction is well established [4], the benefit of PCI in nonacute (elective) settings is less robust [5–7]. Prior studies have demonstrated PCI for stable ischemic heart disease does not result in mortality benefit [6]. Furthermore, PCI as an initial strategy for symptom relief of stable angina may offer little benefit relative to medications alone [5]. Given that PCI is common, costly, and associated with both short- and long-term risks [8,9], ensuring this therapy is provided to the right patient at the right time is important.
In 2009, appropriate use criteria (AUC) were developed by 6 professional organizations to support the rational and judicious use of PCI [10]; a focused update was published in 2012 [11]. In this review, we discuss the recommendations for appropriate use and their application and offer thoughts on next steps to optimize the use of elective PCI as part of high-quality care.
Variation in the Use of PCI
Additionally, significant public attention has been focused on the issue of overuse after lay press investigations into community practice patterns. In particular, a case study presented in the New York Times highlighted the community of Elyria, Ohio, which was found to have a PCI rate that was 4 times the national average [16]. This investigation sparked public debate and further focused attention on the issue of overuse of elective PCI. Conversely, others have pointed to data that suggest underuse of coronary procedural care, particularly among women and racial and ethnic minorities [17–22].
Appropriate Use Criteria
Development Methodology
AUC for PCI, which were developed through the collaborative efforts of 6 major cardiovascular professional organizations, are intended to support the effective, efficient, and equitable use of PCI [10,11]. They were developed in response to a growing need to support rational use of cardiovascular procedures as part of high-quality care. The methods of development for the AUC have been described in detail in the criteria publications [10,11]. We briefly review these methods here.
Panel members first individually assigned ratings to each clinical scenario that ranged from 1 (least appropriate) to 9 (most appropriate). This was followed by an in-person meeting in which technical panel members discussed scenarios for which there was wide variation in appropriateness assessment. After this meeting, technical panel members again assigned ratings for each scenario from 1 to 9. After this second round, the median values for the pooled ratings were used as the appropriateness classification for each scenario. Scenarios with median values of 1–3 were classified as “inappropriate,” 4–6 as “uncertain,” and 7–9 as “appropriate.” A rating of “appropriate” represented clinical scenarios in which the indication is considered generally acceptable and likely to improve health outcomes or survival. A rating of “uncertain” represented clinical scenarios where the indication may be reasonable but more research is necessary to further understand the relative benefits and risks of PCI in this setting. Finally, a rating of “inappropriate” represented clinical scenarios in which the indication is not generally acceptable as it is unlikely to improve health outcomes or survival.
The approach used for AUC development appears to be valid, as Class III indications for PCI in the ACC/AHA clinical guideline [24] (Class III = PCI should NOT be performed since it is not helpful and may be harmful) and AUC scenarios rated as inappropriate are in 100% agreement (personal communication, Ralph Brindis, past president of the American College of Cardiology).
Application
It is important to remember that the AUC are intended to aid in patient selection and are not absolute. Unique clinical factors and patient preference cannot feasibly be captured by the AUC scenarios. It should also be noted that the intent of the AUC is not to be punitive but rather to identify and assess variation in practice patterns. To reflect this intent, the terminology applied to appropriateness ratings has recently changed. Clinical scenarios previously classified as “inappropriate” are now termed “rarely appropriate” and clinical scenarios classified as “uncertain” are now termed “may be appropriate.”
Although the AUC were developed to help evaluate practice patterns of care delivery and serve as guides for clinical decision making, they were not intended to serve as mandates for or against treatment in individual patients or to be tied to reimbursement for individual patients. Despite this, health care organizations and payors have used other AUC documents for incentive pay and prior authorization programs, specifically for cardiovascular imaging [25]. Use of the AUC in this manner may still be reasonable if application and measurement is at the level of the practice, rather than the individual patient, but much remains to be understood about the implications of applying AUC in reimbursement
decisions.
Refinement
The AUC for PCI are designed to be dynamic and continually updated. As additional evidence becomes available regarding the efficacy of PCI in specific clinical scenarios, there will be ongoing efforts to update the AUC to reflect this new evidence. This is highlighted by the first update to the AUC occurring less than 3 years after the original publication date [11].
In addition to perpetual review of the data used to inform scenario ratings, there are opportunities to improve measurement of the clinical variables that are considered in rating PCI appropriateness (eg, clinical presentation, symptom severity, ischemia severity, extent of medical therapy, extent of anatomic disease). For example, in the current AUC, symptom severity is dependent on clinician assessment using the Canadian Cardiovascular Society Classification [25]. Moving toward a patient-centered assessment of symptom severity would ensure that the AUC more closely reflect the patient-perceived symptom burden. Further, the use of a patient-centered instrument would reduce the possibility of physician manipulation of symptom severity to influence the apparent appropriateness of PCI. There are similar opportunities to improve reporting of noninvasive stress test data, such as through standardized reporting of ischemic risk. Finally, the use of physiologic assessments of stenosis severity (eg, fractional flow reserve) and quantitative coronary angiography to standardize interpretations of diagnostic angiography may further optimize the assessment of PCI appropriateness.
Application of the Appropriate Use Criteria in Clinical Practice—Study Results
 Application of the AUC to clinical practice has highlighted potential overuse of PCI (Table). The first report came from applying the AUC to the National Cardiovascular Data Registry (NCDR) CathPCI Registry [26]. In this study of more than 500,000 PCIs from over 1000 facilities across the country, the authors found that PCIs performed in the acute setting (STEMI, NSTEMI, and high-risk unstable angina) were almost uniformly classified as appropriate. However, for nonacute (elective) PCI, application of the AUC resulted in the classification of 50% as appropriate, 38% as uncertain, and 12% as inappropriate. The majority of patients who received inappropriate PCI had a low-risk stress test (72%) or were asymptomatic (54%). Additionally, 96% of patients who received PCI classified as inappropriate had not been given a trial of adequate anti-anginal therapy. This analysis was supported by subsequent analyses of 2 other state-specific registries (New York and Washington), which found similar rates of PCI for nonacute indications rated as inappropriate [27,28]. Additionally, all 3 studies showed wide facility-level variation in the percentage of appropriate and inappropriate PCI for elective indications.
Application of the AUC to clinical practice has highlighted potential overuse of PCI (Table). The first report came from applying the AUC to the National Cardiovascular Data Registry (NCDR) CathPCI Registry [26]. In this study of more than 500,000 PCIs from over 1000 facilities across the country, the authors found that PCIs performed in the acute setting (STEMI, NSTEMI, and high-risk unstable angina) were almost uniformly classified as appropriate. However, for nonacute (elective) PCI, application of the AUC resulted in the classification of 50% as appropriate, 38% as uncertain, and 12% as inappropriate. The majority of patients who received inappropriate PCI had a low-risk stress test (72%) or were asymptomatic (54%). Additionally, 96% of patients who received PCI classified as inappropriate had not been given a trial of adequate anti-anginal therapy. This analysis was supported by subsequent analyses of 2 other state-specific registries (New York and Washington), which found similar rates of PCI for nonacute indications rated as inappropriate [27,28]. Additionally, all 3 studies showed wide facility-level variation in the percentage of appropriate and inappropriate PCI for elective indications.
These studies also highlight a gap in preprocedural care. The anticipated benefit of elective PCI is related to patient symptom burden, adequacy of anti-anginal therapy, and ischemic risk as determined by noninvasive stress testing. However, 30% to 50% of patients undergo elective PCI without evidence of preprocedural stress testing. Attempts are being made to address this gap with the recent release of PCI performance measures [29]. These performance measures, intended for cardiac catheterization labs, include comprehensive documentation of the indication for PCI, which is central to determination of appropriateness. This integration of procedural indication into a performance measure marks the first such occurrence in cardiology.
As documentation of procedural indication and appropriateness have become part and parcel of assessing quality of care, concerns about “gaming” have become more pertinent. Providers who perform PCI could potentially enhance the appearance of appropriateness by overstating the clinical symptom burden or stress test findings. The incorporation of validated, patient-centered health status questionnaires along with data audit programs have been proposed as measures to prevent this type of abuse. Addressing quality gaps in preprocedural assessment and documentation is critical to optimizing use of elective PCI [28].
The apparent overuse of PCI for elective indications may be a reflection of our fragmented, fee-for-service health care delivery system. However, recent studies challenge these assumptions. In a Canadian study, Ko et al found that 18% of elective PCIs were classified as inappropriate, a proportion similar to what had been found previously in the United States [30]. In a US study of Medicare beneficiaries, Matlock and colleagues observed a fourfold regional variation in use of elective coronary angiography and PCI in both Medicare fee-for-service and capitated Medicare Advantage beneficiaries [31]. Collectively, these studies suggest barriers to optimal patient selection for invasive coronary procedures in both capitated and fee-for-service health care systems. Without addressing factors that contribute to variation in the absence of fee-for-service incentives, efforts to improve integration and reduce fee-for-service reimbursement may be inadequate to optimize PCI use.
Evaluating Underuse
While potential underuse of PCI has been described for acute indications [17–22], study of underuse of PCI for elective indications is more challenging. Population data on the effect of underuse of elective PCI on patient symptom burden, functional status, and quality of life is lacking.
A population-based study from Australia highlights the potential importance of underuse in the care of patients with stable coronary disease. This study assessed symptom burden among patients with chronic stable angina using the Seattle Angina Questionnaire and included patients cared for by 207 primary care practitioners [32]. The authors noted that there was considerable variation in patient symptom burden between practices, with 14% of practices having no patients with more than 1 episode of angina per week and 18% of clinics having more than half of enrolled patients with at least 1 episode of angina per week. The authors postulate that this variability may be due to differences among providers in the identification and management of angina, including using PCI to minimize symptom burden.
In the Ko study mentioned earlier, the AUC was used to examine potential underuse of coronary revascularization procedures. In this study, they analyzed the association between AUC ratings and outcomes in patients undergoing diagnostic coronary angiography [30]. Of patients considered “appropriate” for revascularization following completion of diagnostic angiography, only 69% underwent revascularization. However, the clinical aspects that influence the decision to proceed with revascularization may not be fully captured in this study. Thus, the true degree of underuse of PCI remains elusive.
In summary, the relative lack of data that would allow for the assessment of underuse of elective PCI is an important quality concern. Health systems should work to systematically capture patient-reported health status, including symptom burden data, to identify inadequate symptom control and potential underuse of procedural care for CAD.
Facilitating Optimal Use
 In current practice, the AUC hold promise to minimize the overuse of elective PCI. This likely involves addressing processes occurring upstream of the cardiac catheterization lab, including employing systems to ensure that procedures are avoided in patients who are unlikely to benefit (eg, asymptomatic, low ischemic burden) (Figure 3) [33]. Studying hospitals that already have low rates of inappropriate PCI may inform the design and dissemination of strategies that will help improve patient selection at hospitals with higher rates. Although professional organizations have developed tools intended to facilitate appropriateness evaluation at the point-of-care [34], the use of these tools are likely to be sporadic without greater integration into the health care delivery system. Further, these applications are currently limited to determination of appropriateness of PCI after completion of the diagnostic coronary angiogram. Identifying processes prior to catheterization that contribute to PCI appropriateness may also streamline appropriate ad hoc PCI, as the need to reassess appropriateness after the diagnostic angiogram may be mitigated.
In current practice, the AUC hold promise to minimize the overuse of elective PCI. This likely involves addressing processes occurring upstream of the cardiac catheterization lab, including employing systems to ensure that procedures are avoided in patients who are unlikely to benefit (eg, asymptomatic, low ischemic burden) (Figure 3) [33]. Studying hospitals that already have low rates of inappropriate PCI may inform the design and dissemination of strategies that will help improve patient selection at hospitals with higher rates. Although professional organizations have developed tools intended to facilitate appropriateness evaluation at the point-of-care [34], the use of these tools are likely to be sporadic without greater integration into the health care delivery system. Further, these applications are currently limited to determination of appropriateness of PCI after completion of the diagnostic coronary angiogram. Identifying processes prior to catheterization that contribute to PCI appropriateness may also streamline appropriate ad hoc PCI, as the need to reassess appropriateness after the diagnostic angiogram may be mitigated.
Significant barriers exist to the application of the AUC for determination of procedural underuse. As described above, we lack adequate data to ascertain gaps in symptom management that could be mitigated by proper use of PCI. Further study of symptom burden in populations of patients with coronary artery disease is needed. This may help in the identification of patient populations whose symptom burden may warrant consideration of invasive coronary procedures, including coronary angiography and PCI.
Finally, it is important to note that the AUC are based on technical considerations, ie, practice guidelines and trial evidence. They do not take into consideration patient preferences. For example, PCI can be technically appropriate for the scenario but inappropriate for the individual if the procedure is not desired by the patient. Similarly, a procedure may be of uncertain benefit but appropriate if the patient desires more aggressive procedural care and has a full understanding of the risks and benefits. Currently, we fail to convey this information to patients, as evidenced by patients’ overestimation of the benefits of PCI [34]. As we continue to work toward optimal use of PCI, we must not only address the technical appropriateness of care, but move toward incorporating patient preferences through a robust process of shared decision-making.
Corresponding author: Preston M. Schneider, MD, VA Eastern Colorado Health Care System, Cardiology Section (111B), 1055 Clermont St., Denver, CO 80220, [email protected].
Funding/support: Dr. Schneider is supported by a T32 training grant from the National Institutes of Health (5T32HL00
7822-15). Dr. Bradley is supported by a Career Development Award (HSR&D-CDA2 10-199) from VA Health Services Research & Development.
Financial disclosures: None.
1. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
2. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013;127:e6–e245.
3. HCUPnet: A tool for identifying, tracking, and analyzing national hospital statistics. Accessed 22 Oct 2013 at http://hcupnet.ahrq.gov/HCUPnet.jsp?Parms=
H4sIAAAAAAAAABXBMQ6AIBAEwC9JAg.gsLAhRvjAnnuXgGihFb9XZwYe3EhLdpN2h2aIcsnQLCp9jQVbLDN3ksq
DnSeqVXzNfIAP9mtmLy0rZhdIAAAA83D0C2BCAE02DD1508408B2C5C094F1ADF6E788C&JS=Y.
4. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13–20.
5. Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–16.
6. Boden WE, O’Rourke RA, Teo KK, et al. Impact of optimal medical therapy with or without percutaneous coronary intervention on long-term cardiovascular end points in patients with stable coronary artery disease (from the COURAGE Trial). Am J Cardiol 2009;104:1–4.
7. Stergiopoulos K, Brown DL. Initial coronary stent implantation with medical therapy vs medical therapy alone for stable coronary artery disease: Meta-analysis of randomized controlled trials. Arch Intern Med 2012;172:312–9.
8. McCullough PA, Adam A, Becker CR, et al. Epidemiology and prognostic implications of contrast-induced nephropathy. Contrast-Induc Nephrop Clin Insights Pract Guid Rep CIN Consens Work Panel 2006;98:5–13.
9. Roe MT, Messenger JC, Weintraub WS, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol 2010;56:254–63.
10. Patel MR, Dehmer GJ, Hirshfeld JW, et al. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: A Report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2009;53:530–53.
11. Patel MR, Dehmer GJ, Hirshfeld JW, et al. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 Appropriate Use Criteria for Coronary Revascularization Focused Update: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2012;59:857–81.
12. Dartmouth Atlas of Health Care. Accessed 8 Jan 2014 at www.dartmouthatlas.org.
13. Dartmouth Atlas of Health Care: Studies of surgical variation. Cardiac surgery report. 2005. Accessed 8 Jan 2014 at www.dartmouthatlas.org/publications/reports.aspx.
14. Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in medicare spending. part 1: the content, quality, and accessibility of care. Ann Intern Med 2003;138:273–87.
15. Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in medicare spending. part 2: health outcomes and satisfaction with care. Ann Intern Med 2003;138:288–98.
16. Abelson R. Heart procedure is off the charts in an Ohio city. New York Times 2006. Accessed 23 Apr 2013 at www.nytimes.com/2006/08/18/business/18stent.html.
17. Akhter N, Milford-Beland S, Roe MT, et al. Gender differences among patients with acute coronary syndromes undergoing percutaneous coronary intervention in the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR). Am Heart J 2009;157:141–8.
18. Blomkalns AL, Chen AY, Hochman JS, et al. Gender disparities in the diagnosis and treatment of non–ST-segment elevation acute coronary syndromesLarge-scale observations from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines) National Quality Improvement Initiative. J Am Coll Cardiol 2005;45:832–7.
19. Daly C, Clemens F, Lopez Sendon JL, et al. Gender differences in the management and clinical outcome of stable angina. Circulation 2006;113:490–8.
20. Groeneveld PW, Heidenreich PA, Garber AM. Racial disparity in cardiac procedures and mortality among long-term survivors of cardiac arrest. Circulation 2003;108:286–91.
21. Hannan EL, Zhong Y, Walford G, et al. Underutilization of percutaneous coronary intervention for ST-elevation myocardial infarction in Medicaid patients relative to private insurance patients. J Intervent Cardiol 2013;26:470–81.
22. Sonel AF, Good CB, Mulgund J, et al. Racial variations in treatment and outcomes of black and white patients with high-risk non–ST-elevation acute coronary syndromes: insights From CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines?). Circulation 2005;111:1225–32.
23. Patel MR, Spertus JA, Brindis RG, et al. ACCF proposed method for evaluating the appropriateness of cardiovascular imaging. J Am Coll Cardiol 2005;46:1606–13.
24. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011;124:2574–609.
25. Campeau L. Letter: Grading of angina pectoris. Circulation 1976;54:522–3.
26. Chan PS, Patel MR, Klein LW, et al. Appropriateness of percutaneous coronary intervention. JAMA 2011;306:53–61.
27. Hannan EL, Cozzens K, Samadashvili Z, et al. Appropriateness of coronary revascularization for patients without acute coronary syndromes. J Am Coll Cardiol 2012;59:1870–6.
28. Bradley SM, Maynard C, Bryson CL. Appropriateness of percutaneous coronary interventions in Washington State. Circ Cardiovasc Qual Outcomes 2012;5:445–53.
29. Nallamothu BK, Tommaso CL, Anderson HV, et al. ACC/AHA/SCAI/AMA–Convened PCPI/NCQA 2013 Performance measures for adults undergoing percutaneous coronary intervention. A report of the American College of Cardiology/American Heart Association Task Force on Performance Measures, the Society for Cardiovascular Angiography and Interventions, the American Medical Association–Convened Physician Consortium for Performance Improvement, and the National Committee for Quality Assurance. J Am Coll Cardiol 2014;63:722–45.
30. Ko DT, Guo H, Wijeysundera HC, et al. Assessing the association of appropriateness of coronary revascularization and clinical outcomes for patients with stable coronary artery disease. J Am Coll Cardiol 2012;60:1876–84.
31. Matlock DD, Groeneveld PW, Sidney S, et al. Geographic variation in cardiovascular procedure use among medicare fee-for-service vs medicare advantage beneficiaries. JAMA 2013;310:155–62.
32. Beltrame JF, Weekes AJ, Morgan C, et al. The prevalence of weekly angina among patients with chronic stable angina in primary care practices: The coronary artery disease in general practice (cadence) study. Arch Intern Med 2009;169:1491–9.
33. Bradley SM, Spertus JA, Nallamothu BK, et al. The association between patient selection for diagnostic coronary angiography and hospital-level PCI appropriateness: Insights from the NCDR. Circ Cardiovasc Qual Outcomes 2013;6:A1. Accessed 20 Nov 2013 at http://circoutcomes.ahajournals.org/cgi/content/short/6/3_MeetingAbstracts/A1?rss=1.
34. Lee J, Chuu K, Spertus J, et al. Patients overestimate the potential benefits of elective percutaneous coronary intervention. Mo Med 2012;109:79.
1. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
2. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013;127:e6–e245.
3. HCUPnet: A tool for identifying, tracking, and analyzing national hospital statistics. Accessed 22 Oct 2013 at http://hcupnet.ahrq.gov/HCUPnet.jsp?Parms=
H4sIAAAAAAAAABXBMQ6AIBAEwC9JAg.gsLAhRvjAnnuXgGihFb9XZwYe3EhLdpN2h2aIcsnQLCp9jQVbLDN3ksq
DnSeqVXzNfIAP9mtmLy0rZhdIAAAA83D0C2BCAE02DD1508408B2C5C094F1ADF6E788C&JS=Y.
4. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13–20.
5. Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–16.
6. Boden WE, O’Rourke RA, Teo KK, et al. Impact of optimal medical therapy with or without percutaneous coronary intervention on long-term cardiovascular end points in patients with stable coronary artery disease (from the COURAGE Trial). Am J Cardiol 2009;104:1–4.
7. Stergiopoulos K, Brown DL. Initial coronary stent implantation with medical therapy vs medical therapy alone for stable coronary artery disease: Meta-analysis of randomized controlled trials. Arch Intern Med 2012;172:312–9.
8. McCullough PA, Adam A, Becker CR, et al. Epidemiology and prognostic implications of contrast-induced nephropathy. Contrast-Induc Nephrop Clin Insights Pract Guid Rep CIN Consens Work Panel 2006;98:5–13.
9. Roe MT, Messenger JC, Weintraub WS, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol 2010;56:254–63.
10. Patel MR, Dehmer GJ, Hirshfeld JW, et al. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: A Report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2009;53:530–53.
11. Patel MR, Dehmer GJ, Hirshfeld JW, et al. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 Appropriate Use Criteria for Coronary Revascularization Focused Update: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2012;59:857–81.
12. Dartmouth Atlas of Health Care. Accessed 8 Jan 2014 at www.dartmouthatlas.org.
13. Dartmouth Atlas of Health Care: Studies of surgical variation. Cardiac surgery report. 2005. Accessed 8 Jan 2014 at www.dartmouthatlas.org/publications/reports.aspx.
14. Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in medicare spending. part 1: the content, quality, and accessibility of care. Ann Intern Med 2003;138:273–87.
15. Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in medicare spending. part 2: health outcomes and satisfaction with care. Ann Intern Med 2003;138:288–98.
16. Abelson R. Heart procedure is off the charts in an Ohio city. New York Times 2006. Accessed 23 Apr 2013 at www.nytimes.com/2006/08/18/business/18stent.html.
17. Akhter N, Milford-Beland S, Roe MT, et al. Gender differences among patients with acute coronary syndromes undergoing percutaneous coronary intervention in the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR). Am Heart J 2009;157:141–8.
18. Blomkalns AL, Chen AY, Hochman JS, et al. Gender disparities in the diagnosis and treatment of non–ST-segment elevation acute coronary syndromesLarge-scale observations from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines) National Quality Improvement Initiative. J Am Coll Cardiol 2005;45:832–7.
19. Daly C, Clemens F, Lopez Sendon JL, et al. Gender differences in the management and clinical outcome of stable angina. Circulation 2006;113:490–8.
20. Groeneveld PW, Heidenreich PA, Garber AM. Racial disparity in cardiac procedures and mortality among long-term survivors of cardiac arrest. Circulation 2003;108:286–91.
21. Hannan EL, Zhong Y, Walford G, et al. Underutilization of percutaneous coronary intervention for ST-elevation myocardial infarction in Medicaid patients relative to private insurance patients. J Intervent Cardiol 2013;26:470–81.
22. Sonel AF, Good CB, Mulgund J, et al. Racial variations in treatment and outcomes of black and white patients with high-risk non–ST-elevation acute coronary syndromes: insights From CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines?). Circulation 2005;111:1225–32.
23. Patel MR, Spertus JA, Brindis RG, et al. ACCF proposed method for evaluating the appropriateness of cardiovascular imaging. J Am Coll Cardiol 2005;46:1606–13.
24. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011;124:2574–609.
25. Campeau L. Letter: Grading of angina pectoris. Circulation 1976;54:522–3.
26. Chan PS, Patel MR, Klein LW, et al. Appropriateness of percutaneous coronary intervention. JAMA 2011;306:53–61.
27. Hannan EL, Cozzens K, Samadashvili Z, et al. Appropriateness of coronary revascularization for patients without acute coronary syndromes. J Am Coll Cardiol 2012;59:1870–6.
28. Bradley SM, Maynard C, Bryson CL. Appropriateness of percutaneous coronary interventions in Washington State. Circ Cardiovasc Qual Outcomes 2012;5:445–53.
29. Nallamothu BK, Tommaso CL, Anderson HV, et al. ACC/AHA/SCAI/AMA–Convened PCPI/NCQA 2013 Performance measures for adults undergoing percutaneous coronary intervention. A report of the American College of Cardiology/American Heart Association Task Force on Performance Measures, the Society for Cardiovascular Angiography and Interventions, the American Medical Association–Convened Physician Consortium for Performance Improvement, and the National Committee for Quality Assurance. J Am Coll Cardiol 2014;63:722–45.
30. Ko DT, Guo H, Wijeysundera HC, et al. Assessing the association of appropriateness of coronary revascularization and clinical outcomes for patients with stable coronary artery disease. J Am Coll Cardiol 2012;60:1876–84.
31. Matlock DD, Groeneveld PW, Sidney S, et al. Geographic variation in cardiovascular procedure use among medicare fee-for-service vs medicare advantage beneficiaries. JAMA 2013;310:155–62.
32. Beltrame JF, Weekes AJ, Morgan C, et al. The prevalence of weekly angina among patients with chronic stable angina in primary care practices: The coronary artery disease in general practice (cadence) study. Arch Intern Med 2009;169:1491–9.
33. Bradley SM, Spertus JA, Nallamothu BK, et al. The association between patient selection for diagnostic coronary angiography and hospital-level PCI appropriateness: Insights from the NCDR. Circ Cardiovasc Qual Outcomes 2013;6:A1. Accessed 20 Nov 2013 at http://circoutcomes.ahajournals.org/cgi/content/short/6/3_MeetingAbstracts/A1?rss=1.
34. Lee J, Chuu K, Spertus J, et al. Patients overestimate the potential benefits of elective percutaneous coronary intervention. Mo Med 2012;109:79.