User login
SAN DIEGO – People with relapsing multiple sclerosis who switched from an injectable disease-modifying therapy to fingolimod after the randomized phase of the PREFERMS trial experienced improved annualized relapse rates, exposure-adjusted percentage brain volume loss, and satisfaction, compared with study participants who did not switch.
Importantly, there were no differences between switchers who were treatment-naive or previously treated with one injectable therapy class at baseline in the phase 4, active-controlled, open-label, and multicenter PREFERMS trial, according to the researchers.
Of the 875 patients with relapsing multiple sclerosis, 254 or 58% of those initially randomized to an injectable disease-modifying therapy (iDMT) switched to fingolimod (Gilenya) during the 12-month study. Participants in the trial were permitted to switch therapy once after at least 3 months, or sooner if warranted for safety or efficacy reasons.
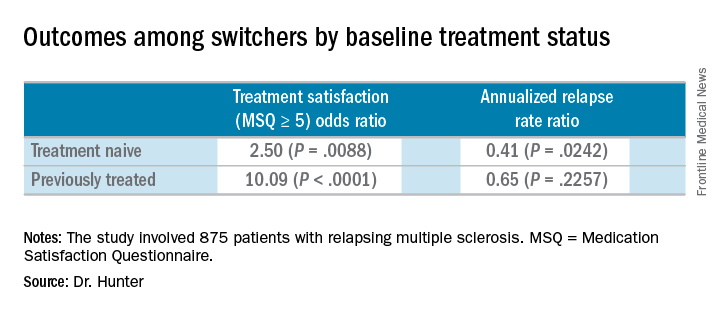
Compared with the end the randomization phase, patients who switched from an iDMT to fingolimod were more satisfied, defined as a Medication Satisfaction Questionnaire score of 5 or greater (odds ratio, 5.65; P less than .001) at 12 months. At the same time, their annualized relapse rate decreased (ARR ratio, 0.53; P = .0158) and their exposure-adjusted brain volume loss decreased from 0.874% to 0.463%.
The benefit of these switches was observed regardless of previous treatment status, Dr. Hunter said during a poster presentation at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
At the start of the PREFERMS trial, patients were randomized 1:1 to fingolimod 0.5 mg/day or a preselected iDMT. Just more than half, 54%, were treatment-naive and 46% had previous interferon or glatiramer acetate treatment.
“Generally, because most of these people were switched, and we’re looking at people who were treatment-naive or previously treated, when you look at brain volume loss, it’s very low on fingolimod versus high while they’re on injectable therapies,” Dr. Hunter said.
Dr. Hunter and his colleagues reported similar relapse rates and brain volume loss outcomes among the 254 patients who switched to fingolimod mid-study according to baseline treatment history. The investigators noted, however, that these post hoc analyses and P values are intended for hypothesis generation only.
In the 99 treatment-naive switchers, brain volume loss was 0.54% at the end of the study, compared with 0.84% at the end of the randomization period. Among the 155 previously treated switchers, brain volume loss also decreased, from 0.90% at the end of randomization to 0.42% at study end.
Dr. Hunter disclosed that he is an investigator for Novartis, the sponsor of the study.
SOURCE: Hunter S et al. ACTRIMS Forum 2018 Abstract P019.
SAN DIEGO – People with relapsing multiple sclerosis who switched from an injectable disease-modifying therapy to fingolimod after the randomized phase of the PREFERMS trial experienced improved annualized relapse rates, exposure-adjusted percentage brain volume loss, and satisfaction, compared with study participants who did not switch.
Importantly, there were no differences between switchers who were treatment-naive or previously treated with one injectable therapy class at baseline in the phase 4, active-controlled, open-label, and multicenter PREFERMS trial, according to the researchers.
Of the 875 patients with relapsing multiple sclerosis, 254 or 58% of those initially randomized to an injectable disease-modifying therapy (iDMT) switched to fingolimod (Gilenya) during the 12-month study. Participants in the trial were permitted to switch therapy once after at least 3 months, or sooner if warranted for safety or efficacy reasons.
Compared with the end the randomization phase, patients who switched from an iDMT to fingolimod were more satisfied, defined as a Medication Satisfaction Questionnaire score of 5 or greater (odds ratio, 5.65; P less than .001) at 12 months. At the same time, their annualized relapse rate decreased (ARR ratio, 0.53; P = .0158) and their exposure-adjusted brain volume loss decreased from 0.874% to 0.463%.
The benefit of these switches was observed regardless of previous treatment status, Dr. Hunter said during a poster presentation at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.

At the start of the PREFERMS trial, patients were randomized 1:1 to fingolimod 0.5 mg/day or a preselected iDMT. Just more than half, 54%, were treatment-naive and 46% had previous interferon or glatiramer acetate treatment.
“Generally, because most of these people were switched, and we’re looking at people who were treatment-naive or previously treated, when you look at brain volume loss, it’s very low on fingolimod versus high while they’re on injectable therapies,” Dr. Hunter said.
Dr. Hunter and his colleagues reported similar relapse rates and brain volume loss outcomes among the 254 patients who switched to fingolimod mid-study according to baseline treatment history. The investigators noted, however, that these post hoc analyses and P values are intended for hypothesis generation only.
In the 99 treatment-naive switchers, brain volume loss was 0.54% at the end of the study, compared with 0.84% at the end of the randomization period. Among the 155 previously treated switchers, brain volume loss also decreased, from 0.90% at the end of randomization to 0.42% at study end.
Dr. Hunter disclosed that he is an investigator for Novartis, the sponsor of the study.
SOURCE: Hunter S et al. ACTRIMS Forum 2018 Abstract P019.
SAN DIEGO – People with relapsing multiple sclerosis who switched from an injectable disease-modifying therapy to fingolimod after the randomized phase of the PREFERMS trial experienced improved annualized relapse rates, exposure-adjusted percentage brain volume loss, and satisfaction, compared with study participants who did not switch.
Importantly, there were no differences between switchers who were treatment-naive or previously treated with one injectable therapy class at baseline in the phase 4, active-controlled, open-label, and multicenter PREFERMS trial, according to the researchers.
Of the 875 patients with relapsing multiple sclerosis, 254 or 58% of those initially randomized to an injectable disease-modifying therapy (iDMT) switched to fingolimod (Gilenya) during the 12-month study. Participants in the trial were permitted to switch therapy once after at least 3 months, or sooner if warranted for safety or efficacy reasons.
Compared with the end the randomization phase, patients who switched from an iDMT to fingolimod were more satisfied, defined as a Medication Satisfaction Questionnaire score of 5 or greater (odds ratio, 5.65; P less than .001) at 12 months. At the same time, their annualized relapse rate decreased (ARR ratio, 0.53; P = .0158) and their exposure-adjusted brain volume loss decreased from 0.874% to 0.463%.
The benefit of these switches was observed regardless of previous treatment status, Dr. Hunter said during a poster presentation at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.

At the start of the PREFERMS trial, patients were randomized 1:1 to fingolimod 0.5 mg/day or a preselected iDMT. Just more than half, 54%, were treatment-naive and 46% had previous interferon or glatiramer acetate treatment.
“Generally, because most of these people were switched, and we’re looking at people who were treatment-naive or previously treated, when you look at brain volume loss, it’s very low on fingolimod versus high while they’re on injectable therapies,” Dr. Hunter said.
Dr. Hunter and his colleagues reported similar relapse rates and brain volume loss outcomes among the 254 patients who switched to fingolimod mid-study according to baseline treatment history. The investigators noted, however, that these post hoc analyses and P values are intended for hypothesis generation only.
In the 99 treatment-naive switchers, brain volume loss was 0.54% at the end of the study, compared with 0.84% at the end of the randomization period. Among the 155 previously treated switchers, brain volume loss also decreased, from 0.90% at the end of randomization to 0.42% at study end.
Dr. Hunter disclosed that he is an investigator for Novartis, the sponsor of the study.
SOURCE: Hunter S et al. ACTRIMS Forum 2018 Abstract P019.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point:
Major finding: Patients who switched from an injectable disease-modifying therapy to fingolimod were more satisfied, defined as a Medication Satisfaction Questionnaire score of 5 or greater (odds ratio, 5.65; P less than .001).
Study details: Post hoc analyses of the extension study of the PREFERMS trial.
Disclosures: Dr. Hunter disclosed that he is an investigator for Novartis, the sponsor of the study.
Source: Hunter S et al. ACTRIMS Forum 2018 Abstract P019.