User login
Influenza pandemics like the one we had last year are uncommon, and mounting an effective response was a difficult challenge. The pandemic hit early and hard. Physicians and the public health system responded well, administering a seasonal flu vaccine as well as a new H1N1 vaccine that was approved, produced, and distributed in record time. Before the end of the season, approximately 30% of the population had received an H1N1 vaccine and 40% a seasonal vaccine.1
What happened last year
The influenza attack rate in 2009-2010 exceeded that of a normal influenza season and the age groups most affected were also different, with those over the age of 65 largely spared.2 Virtually all the influenza last year was caused by the pandemic H1N1 strain.2 Fortuitously, the virus was not especially virulent and the death rates were below what was initially expected. TABLE 1 lists the population death rates that occurred for different age groups.2 Most of the more than 2000 deaths were among those with high-risk conditions.3 Those conditions are listed in TABLE 2.
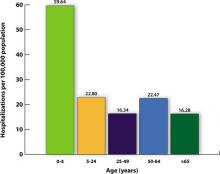
There were, however, 269 deaths by late March among children, which far exceeded the number of deaths in this age group for the previous 3 influenza seasons.2 For the most part, these higher mortality rates were due to higher attack rates, rather than higher case fatality rates. This is evident from hospitalization rates for children younger than age 5, which exceeded those of other age groups, as shown in FIGURE 1.
TABLE 1
2009-2010 Influenza death rates by age
| Age group, years | Death rate/100,000 |
|---|---|
| 0-4 | 0.43 |
| 5-18 | 0.36 |
| 19-24 | 0.54 |
| 25-49 | 0.87 |
| 50-64 | 1.56 |
| ≥65 | 0.95 |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.2 | |
TABLE 2
Individuals at higher risk for influenza complications (or who may spread infection to those at higher risk)
|
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4 |
FIGURE 1
Cumulative lab-confirmed hospitalization rate by age group, 2009 H1N1, April 2009-February 13, 2010*
*Based on 35 states reporting (n=49,516).
Source: Finelli L, et al. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. 2010.3
The task will be simpler this year
While it’s not possible to predict what will happen in the upcoming season, 2 developments should simplify the family physician’s task of adhering to official recommendations:
- Only 1 vaccine formulation will be available, and
- For the first time, the recommendation is to vaccinate everyone who does not have a contraindication.4
The vaccine for the 2010-2011 season will contain 3 antigens: the pandemic H1N1 virus, an H3N2 A strain (A/Perth/16/2009), and a B virus (B/Brisbane/60/2008).2 The decision on which antigens to include is made 6 months in advance of the start of the next flu season and is based on information about the most common influenza antigens circulating worldwide at that time.
Immunization for all
This year’s recommendation to immunize everyone who does not have a contraindication is a major change from the age- and risk-based recommendations of past years. The universal recommendation is the culmination of the incremental expansions of recommendation categories that occurred over the past decade, which resulted in suboptimal immunization rates.1 In 2009, only 40% to 50% of adults for whom the seasonal vaccine was recommended received it.5 While the annual influenza vaccine recommendation is now universal, those who should be specially targeted include those in TABLE 2. Most public health authorities believe children should also receive special emphasis because of the high transmission rate among school-age children and their home contacts. Next, of course, come health care workers, who should be vaccinated to protect ourselves, our families, and our patients.4,6
Antivirals for treatment and prevention
There are 2 uses for antivirals to combat influenza: treatment of those infected and chemoprevention for those exposed to someone infected. Treatment is recommended for those with confirmed or suspected influenza who have severe, complicated, or progressive illness or who are hospitalized.7 Treatment should be strongly considered for anyone at higher risk for complications and death from influenza.7
Chemoprevention is now being deemphasized because of a concern for possible development of antiviral resistance. It should be considered for those in the high-risk categories (TABLE 2) with a documented exposure.7
Which antiviral to use will depend on which influenza strains are circulating and their resistance patterns. So far, H1N1 has remained largely sensitive to both neuraminidase inhibitors: oseltamivir and zanamivir. However, oseltamivir resistance has been documented in a few cases and will be monitored carefully.
Family physicians will need to stay informed by state and local health departments about circulating strains and resistance patterns. The latest Centers for Disease Control and Prevention (CDC) guidance on antiviral therapy can be consulted for dosage and other details on the 4 antiviral drugs licensed in the United States.7
What you must know about vaccine safety
Because of increasing public awareness of safety issues, family physicians will frequently need to address patients’ questions about the safety of this year’s vaccine. Last year, multiple reporting systems including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD) Project, the Defense Medical Surveillance System (DMSS), and others, extensively monitored adverse events that could potentially be linked to the H1N1 vaccine.8 Three so-called weak signals—indications of a possible link to a rare, but statistically significant adverse event—were received.
The 3 signals were for Guillain-Barré syndrome (GBS), Bell’s palsy, and thrombocytopenia/idiopathic thrombocytopenic purpura. The status of the investigation of each potential link to the vaccine can be found on the National Vaccine Advisory Committee (NVAC) safety Web site at http://www.hhs.gov/nvpo/nvac/reports/index.html.
The GBS signal has been investigated the most aggressively because this adverse reaction has been linked to the so-called swine flu vaccine of 1976. One analysis has been published in the Morbidity and Mortality Weekly Report.9 Whether GBS has a causal link to the H1N1 vaccine remains in doubt. In the worst-case scenario, if causation is determined, it appears that the vaccine would account for no more than 1 excess case of GBS per million doses.9
In Western Australia, there has been a recent report of an excess of fever and febrile seizures in children 6 months to 5 years of age, and fever in children 5 to 9 years of age who received seasonal influenza vaccine. The rate of febrile seizures in children younger than age 3 was 7 per 1000, which is 7 times the rate normally expected. These adverse reactions were associated with only 1 vaccine product, Fluvax, and Fluvax Junior, manufactured by CSL Biotherapies.10 The CSL product is marketed in the United States by Merck & Co. under the brand name Afluria.
The Advisory Committee on Immunization Practices (ACIP) has issued the following recommendations:11
- Afluria should not be used in children ages 6 months through 8 years. The exception: children who are ages 5 through 8 years who are considered to be at high risk for influenza complications and for whom no other trivalent inactivated vaccine is available.
- Other age-appropriate, licensed seasonal influenza vaccine formulations should be used for prevention of influenza in children ages 6 months through 8 years.
High-dose vaccine for elderly patients
A new seasonal influenza vaccine (Fluzone High-Dose, manufactured by Sanofi Pasteur) is now available for use in people who are 65 years of age and older.12 Fluzone High-Dose contains 4 times the amount of influenza antigen as other inactivated seasonal influenza vaccines. Fluzone High-Dose vaccine produces higher antibody levels in the elderly but also a higher frequency of local reactions. Studies are being conducted to see if the vaccine results in better patient outcomes. ACIP does not state a preference for any of the available influenza vaccines for those who are 65 years of age and older.12
Children younger than age 9: One dose or two?
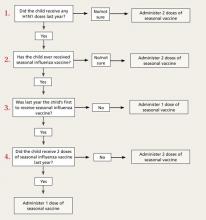
The new recommendations for deciding if a child under the age of 9 years should receive 1 or 2 doses of the vaccine run counter to the trend for simplification in influenza vaccine recommendations. The decision depends on the child’s past immunization history for both seasonal and H1N1 vaccines. To be fully vaccinated with only 1 dose this year, a child must have previously received at least 1 dose of H1N1 vaccine and 2 doses of seasonal vaccine. FIGURE 2 illustrates the process you need to go through to make the dosage choice. When the child’s immunization history is unknown or uncertain, give 2 doses, separated by 4 weeks.4
FIGURE 2
Children younger than 9: Ask 4 questions
Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4
1. Singleton JA. H1N1 vaccination coverage: updated interim results February 24, 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-4-flu-vac.pdf. Accessed July 16, 2010.
2. CDC. Update: influenza activity—United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423-438.
3. Finelli L, Brammer L, Kniss K, et al. Influenza epidemiology and surveillance. ACIP Presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. Accessed July 26, 2010.
4. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. July 29, 2010 (early release);1-62.
5. Harris KM, Maurer J, Uscher-Pines L. Seasonal influenza vaccine use by adults in the US: a snapshot as of mid-November 2009. Available at: http://www.rand.org/pubs/occasional_papers/OP289/. Accessed July 16, 2010.
6. Fiore A. Influenza vaccine workgroup discussions and recommendations, November 2009-February 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-7-flu-vac.pdf. Accessed July 26, 2010.
7. CDC. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed July 16, 2010.
8. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. June 2010. Available at: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html. Accessed July 16, 2010.
9. CDC. Preliminary results: surveillance for Guillain-Barré syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine—United States, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:657-661.
10. McNeil M. Febrile seizures in Australia and CDC monitoring plan for 2010-2011 seasonal influenza vaccine. Available at: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun10/10-8-flu.pdf. Accessed August 19, 2010.
11. CDC. Media statement: ACIP recommendation for use of CSL influenza vaccine. August 6, 2010. Available at: http://www.cdc.gov/media/pressrel/2010/s100806.htm?s_cid=mediarel_s100806. Accessed August 6, 2010.
12. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (Fluzone High-Dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.
Influenza pandemics like the one we had last year are uncommon, and mounting an effective response was a difficult challenge. The pandemic hit early and hard. Physicians and the public health system responded well, administering a seasonal flu vaccine as well as a new H1N1 vaccine that was approved, produced, and distributed in record time. Before the end of the season, approximately 30% of the population had received an H1N1 vaccine and 40% a seasonal vaccine.1
What happened last year
The influenza attack rate in 2009-2010 exceeded that of a normal influenza season and the age groups most affected were also different, with those over the age of 65 largely spared.2 Virtually all the influenza last year was caused by the pandemic H1N1 strain.2 Fortuitously, the virus was not especially virulent and the death rates were below what was initially expected. TABLE 1 lists the population death rates that occurred for different age groups.2 Most of the more than 2000 deaths were among those with high-risk conditions.3 Those conditions are listed in TABLE 2.
There were, however, 269 deaths by late March among children, which far exceeded the number of deaths in this age group for the previous 3 influenza seasons.2 For the most part, these higher mortality rates were due to higher attack rates, rather than higher case fatality rates. This is evident from hospitalization rates for children younger than age 5, which exceeded those of other age groups, as shown in FIGURE 1.
TABLE 1
2009-2010 Influenza death rates by age
| Age group, years | Death rate/100,000 |
|---|---|
| 0-4 | 0.43 |
| 5-18 | 0.36 |
| 19-24 | 0.54 |
| 25-49 | 0.87 |
| 50-64 | 1.56 |
| ≥65 | 0.95 |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.2 | |
TABLE 2
Individuals at higher risk for influenza complications (or who may spread infection to those at higher risk)
|
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4 |
FIGURE 1
Cumulative lab-confirmed hospitalization rate by age group, 2009 H1N1, April 2009-February 13, 2010*
*Based on 35 states reporting (n=49,516).
Source: Finelli L, et al. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. 2010.3
The task will be simpler this year
While it’s not possible to predict what will happen in the upcoming season, 2 developments should simplify the family physician’s task of adhering to official recommendations:
- Only 1 vaccine formulation will be available, and
- For the first time, the recommendation is to vaccinate everyone who does not have a contraindication.4
The vaccine for the 2010-2011 season will contain 3 antigens: the pandemic H1N1 virus, an H3N2 A strain (A/Perth/16/2009), and a B virus (B/Brisbane/60/2008).2 The decision on which antigens to include is made 6 months in advance of the start of the next flu season and is based on information about the most common influenza antigens circulating worldwide at that time.
Immunization for all
This year’s recommendation to immunize everyone who does not have a contraindication is a major change from the age- and risk-based recommendations of past years. The universal recommendation is the culmination of the incremental expansions of recommendation categories that occurred over the past decade, which resulted in suboptimal immunization rates.1 In 2009, only 40% to 50% of adults for whom the seasonal vaccine was recommended received it.5 While the annual influenza vaccine recommendation is now universal, those who should be specially targeted include those in TABLE 2. Most public health authorities believe children should also receive special emphasis because of the high transmission rate among school-age children and their home contacts. Next, of course, come health care workers, who should be vaccinated to protect ourselves, our families, and our patients.4,6
Antivirals for treatment and prevention
There are 2 uses for antivirals to combat influenza: treatment of those infected and chemoprevention for those exposed to someone infected. Treatment is recommended for those with confirmed or suspected influenza who have severe, complicated, or progressive illness or who are hospitalized.7 Treatment should be strongly considered for anyone at higher risk for complications and death from influenza.7
Chemoprevention is now being deemphasized because of a concern for possible development of antiviral resistance. It should be considered for those in the high-risk categories (TABLE 2) with a documented exposure.7
Which antiviral to use will depend on which influenza strains are circulating and their resistance patterns. So far, H1N1 has remained largely sensitive to both neuraminidase inhibitors: oseltamivir and zanamivir. However, oseltamivir resistance has been documented in a few cases and will be monitored carefully.
Family physicians will need to stay informed by state and local health departments about circulating strains and resistance patterns. The latest Centers for Disease Control and Prevention (CDC) guidance on antiviral therapy can be consulted for dosage and other details on the 4 antiviral drugs licensed in the United States.7
What you must know about vaccine safety
Because of increasing public awareness of safety issues, family physicians will frequently need to address patients’ questions about the safety of this year’s vaccine. Last year, multiple reporting systems including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD) Project, the Defense Medical Surveillance System (DMSS), and others, extensively monitored adverse events that could potentially be linked to the H1N1 vaccine.8 Three so-called weak signals—indications of a possible link to a rare, but statistically significant adverse event—were received.
The 3 signals were for Guillain-Barré syndrome (GBS), Bell’s palsy, and thrombocytopenia/idiopathic thrombocytopenic purpura. The status of the investigation of each potential link to the vaccine can be found on the National Vaccine Advisory Committee (NVAC) safety Web site at http://www.hhs.gov/nvpo/nvac/reports/index.html.
The GBS signal has been investigated the most aggressively because this adverse reaction has been linked to the so-called swine flu vaccine of 1976. One analysis has been published in the Morbidity and Mortality Weekly Report.9 Whether GBS has a causal link to the H1N1 vaccine remains in doubt. In the worst-case scenario, if causation is determined, it appears that the vaccine would account for no more than 1 excess case of GBS per million doses.9
In Western Australia, there has been a recent report of an excess of fever and febrile seizures in children 6 months to 5 years of age, and fever in children 5 to 9 years of age who received seasonal influenza vaccine. The rate of febrile seizures in children younger than age 3 was 7 per 1000, which is 7 times the rate normally expected. These adverse reactions were associated with only 1 vaccine product, Fluvax, and Fluvax Junior, manufactured by CSL Biotherapies.10 The CSL product is marketed in the United States by Merck & Co. under the brand name Afluria.
The Advisory Committee on Immunization Practices (ACIP) has issued the following recommendations:11
- Afluria should not be used in children ages 6 months through 8 years. The exception: children who are ages 5 through 8 years who are considered to be at high risk for influenza complications and for whom no other trivalent inactivated vaccine is available.
- Other age-appropriate, licensed seasonal influenza vaccine formulations should be used for prevention of influenza in children ages 6 months through 8 years.
High-dose vaccine for elderly patients
A new seasonal influenza vaccine (Fluzone High-Dose, manufactured by Sanofi Pasteur) is now available for use in people who are 65 years of age and older.12 Fluzone High-Dose contains 4 times the amount of influenza antigen as other inactivated seasonal influenza vaccines. Fluzone High-Dose vaccine produces higher antibody levels in the elderly but also a higher frequency of local reactions. Studies are being conducted to see if the vaccine results in better patient outcomes. ACIP does not state a preference for any of the available influenza vaccines for those who are 65 years of age and older.12
Children younger than age 9: One dose or two?
The new recommendations for deciding if a child under the age of 9 years should receive 1 or 2 doses of the vaccine run counter to the trend for simplification in influenza vaccine recommendations. The decision depends on the child’s past immunization history for both seasonal and H1N1 vaccines. To be fully vaccinated with only 1 dose this year, a child must have previously received at least 1 dose of H1N1 vaccine and 2 doses of seasonal vaccine. FIGURE 2 illustrates the process you need to go through to make the dosage choice. When the child’s immunization history is unknown or uncertain, give 2 doses, separated by 4 weeks.4
FIGURE 2
Children younger than 9: Ask 4 questions
Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4
Influenza pandemics like the one we had last year are uncommon, and mounting an effective response was a difficult challenge. The pandemic hit early and hard. Physicians and the public health system responded well, administering a seasonal flu vaccine as well as a new H1N1 vaccine that was approved, produced, and distributed in record time. Before the end of the season, approximately 30% of the population had received an H1N1 vaccine and 40% a seasonal vaccine.1
What happened last year
The influenza attack rate in 2009-2010 exceeded that of a normal influenza season and the age groups most affected were also different, with those over the age of 65 largely spared.2 Virtually all the influenza last year was caused by the pandemic H1N1 strain.2 Fortuitously, the virus was not especially virulent and the death rates were below what was initially expected. TABLE 1 lists the population death rates that occurred for different age groups.2 Most of the more than 2000 deaths were among those with high-risk conditions.3 Those conditions are listed in TABLE 2.
There were, however, 269 deaths by late March among children, which far exceeded the number of deaths in this age group for the previous 3 influenza seasons.2 For the most part, these higher mortality rates were due to higher attack rates, rather than higher case fatality rates. This is evident from hospitalization rates for children younger than age 5, which exceeded those of other age groups, as shown in FIGURE 1.
TABLE 1
2009-2010 Influenza death rates by age
| Age group, years | Death rate/100,000 |
|---|---|
| 0-4 | 0.43 |
| 5-18 | 0.36 |
| 19-24 | 0.54 |
| 25-49 | 0.87 |
| 50-64 | 1.56 |
| ≥65 | 0.95 |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.2 | |
TABLE 2
Individuals at higher risk for influenza complications (or who may spread infection to those at higher risk)
|
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4 |
FIGURE 1
Cumulative lab-confirmed hospitalization rate by age group, 2009 H1N1, April 2009-February 13, 2010*
*Based on 35 states reporting (n=49,516).
Source: Finelli L, et al. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. 2010.3
The task will be simpler this year
While it’s not possible to predict what will happen in the upcoming season, 2 developments should simplify the family physician’s task of adhering to official recommendations:
- Only 1 vaccine formulation will be available, and
- For the first time, the recommendation is to vaccinate everyone who does not have a contraindication.4
The vaccine for the 2010-2011 season will contain 3 antigens: the pandemic H1N1 virus, an H3N2 A strain (A/Perth/16/2009), and a B virus (B/Brisbane/60/2008).2 The decision on which antigens to include is made 6 months in advance of the start of the next flu season and is based on information about the most common influenza antigens circulating worldwide at that time.
Immunization for all
This year’s recommendation to immunize everyone who does not have a contraindication is a major change from the age- and risk-based recommendations of past years. The universal recommendation is the culmination of the incremental expansions of recommendation categories that occurred over the past decade, which resulted in suboptimal immunization rates.1 In 2009, only 40% to 50% of adults for whom the seasonal vaccine was recommended received it.5 While the annual influenza vaccine recommendation is now universal, those who should be specially targeted include those in TABLE 2. Most public health authorities believe children should also receive special emphasis because of the high transmission rate among school-age children and their home contacts. Next, of course, come health care workers, who should be vaccinated to protect ourselves, our families, and our patients.4,6
Antivirals for treatment and prevention
There are 2 uses for antivirals to combat influenza: treatment of those infected and chemoprevention for those exposed to someone infected. Treatment is recommended for those with confirmed or suspected influenza who have severe, complicated, or progressive illness or who are hospitalized.7 Treatment should be strongly considered for anyone at higher risk for complications and death from influenza.7
Chemoprevention is now being deemphasized because of a concern for possible development of antiviral resistance. It should be considered for those in the high-risk categories (TABLE 2) with a documented exposure.7
Which antiviral to use will depend on which influenza strains are circulating and their resistance patterns. So far, H1N1 has remained largely sensitive to both neuraminidase inhibitors: oseltamivir and zanamivir. However, oseltamivir resistance has been documented in a few cases and will be monitored carefully.
Family physicians will need to stay informed by state and local health departments about circulating strains and resistance patterns. The latest Centers for Disease Control and Prevention (CDC) guidance on antiviral therapy can be consulted for dosage and other details on the 4 antiviral drugs licensed in the United States.7
What you must know about vaccine safety
Because of increasing public awareness of safety issues, family physicians will frequently need to address patients’ questions about the safety of this year’s vaccine. Last year, multiple reporting systems including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD) Project, the Defense Medical Surveillance System (DMSS), and others, extensively monitored adverse events that could potentially be linked to the H1N1 vaccine.8 Three so-called weak signals—indications of a possible link to a rare, but statistically significant adverse event—were received.
The 3 signals were for Guillain-Barré syndrome (GBS), Bell’s palsy, and thrombocytopenia/idiopathic thrombocytopenic purpura. The status of the investigation of each potential link to the vaccine can be found on the National Vaccine Advisory Committee (NVAC) safety Web site at http://www.hhs.gov/nvpo/nvac/reports/index.html.
The GBS signal has been investigated the most aggressively because this adverse reaction has been linked to the so-called swine flu vaccine of 1976. One analysis has been published in the Morbidity and Mortality Weekly Report.9 Whether GBS has a causal link to the H1N1 vaccine remains in doubt. In the worst-case scenario, if causation is determined, it appears that the vaccine would account for no more than 1 excess case of GBS per million doses.9
In Western Australia, there has been a recent report of an excess of fever and febrile seizures in children 6 months to 5 years of age, and fever in children 5 to 9 years of age who received seasonal influenza vaccine. The rate of febrile seizures in children younger than age 3 was 7 per 1000, which is 7 times the rate normally expected. These adverse reactions were associated with only 1 vaccine product, Fluvax, and Fluvax Junior, manufactured by CSL Biotherapies.10 The CSL product is marketed in the United States by Merck & Co. under the brand name Afluria.
The Advisory Committee on Immunization Practices (ACIP) has issued the following recommendations:11
- Afluria should not be used in children ages 6 months through 8 years. The exception: children who are ages 5 through 8 years who are considered to be at high risk for influenza complications and for whom no other trivalent inactivated vaccine is available.
- Other age-appropriate, licensed seasonal influenza vaccine formulations should be used for prevention of influenza in children ages 6 months through 8 years.
High-dose vaccine for elderly patients
A new seasonal influenza vaccine (Fluzone High-Dose, manufactured by Sanofi Pasteur) is now available for use in people who are 65 years of age and older.12 Fluzone High-Dose contains 4 times the amount of influenza antigen as other inactivated seasonal influenza vaccines. Fluzone High-Dose vaccine produces higher antibody levels in the elderly but also a higher frequency of local reactions. Studies are being conducted to see if the vaccine results in better patient outcomes. ACIP does not state a preference for any of the available influenza vaccines for those who are 65 years of age and older.12
Children younger than age 9: One dose or two?
The new recommendations for deciding if a child under the age of 9 years should receive 1 or 2 doses of the vaccine run counter to the trend for simplification in influenza vaccine recommendations. The decision depends on the child’s past immunization history for both seasonal and H1N1 vaccines. To be fully vaccinated with only 1 dose this year, a child must have previously received at least 1 dose of H1N1 vaccine and 2 doses of seasonal vaccine. FIGURE 2 illustrates the process you need to go through to make the dosage choice. When the child’s immunization history is unknown or uncertain, give 2 doses, separated by 4 weeks.4
FIGURE 2
Children younger than 9: Ask 4 questions
Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4
1. Singleton JA. H1N1 vaccination coverage: updated interim results February 24, 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-4-flu-vac.pdf. Accessed July 16, 2010.
2. CDC. Update: influenza activity—United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423-438.
3. Finelli L, Brammer L, Kniss K, et al. Influenza epidemiology and surveillance. ACIP Presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. Accessed July 26, 2010.
4. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. July 29, 2010 (early release);1-62.
5. Harris KM, Maurer J, Uscher-Pines L. Seasonal influenza vaccine use by adults in the US: a snapshot as of mid-November 2009. Available at: http://www.rand.org/pubs/occasional_papers/OP289/. Accessed July 16, 2010.
6. Fiore A. Influenza vaccine workgroup discussions and recommendations, November 2009-February 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-7-flu-vac.pdf. Accessed July 26, 2010.
7. CDC. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed July 16, 2010.
8. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. June 2010. Available at: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html. Accessed July 16, 2010.
9. CDC. Preliminary results: surveillance for Guillain-Barré syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine—United States, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:657-661.
10. McNeil M. Febrile seizures in Australia and CDC monitoring plan for 2010-2011 seasonal influenza vaccine. Available at: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun10/10-8-flu.pdf. Accessed August 19, 2010.
11. CDC. Media statement: ACIP recommendation for use of CSL influenza vaccine. August 6, 2010. Available at: http://www.cdc.gov/media/pressrel/2010/s100806.htm?s_cid=mediarel_s100806. Accessed August 6, 2010.
12. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (Fluzone High-Dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.
1. Singleton JA. H1N1 vaccination coverage: updated interim results February 24, 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-4-flu-vac.pdf. Accessed July 16, 2010.
2. CDC. Update: influenza activity—United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423-438.
3. Finelli L, Brammer L, Kniss K, et al. Influenza epidemiology and surveillance. ACIP Presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. Accessed July 26, 2010.
4. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. July 29, 2010 (early release);1-62.
5. Harris KM, Maurer J, Uscher-Pines L. Seasonal influenza vaccine use by adults in the US: a snapshot as of mid-November 2009. Available at: http://www.rand.org/pubs/occasional_papers/OP289/. Accessed July 16, 2010.
6. Fiore A. Influenza vaccine workgroup discussions and recommendations, November 2009-February 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-7-flu-vac.pdf. Accessed July 26, 2010.
7. CDC. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed July 16, 2010.
8. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. June 2010. Available at: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html. Accessed July 16, 2010.
9. CDC. Preliminary results: surveillance for Guillain-Barré syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine—United States, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:657-661.
10. McNeil M. Febrile seizures in Australia and CDC monitoring plan for 2010-2011 seasonal influenza vaccine. Available at: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun10/10-8-flu.pdf. Accessed August 19, 2010.
11. CDC. Media statement: ACIP recommendation for use of CSL influenza vaccine. August 6, 2010. Available at: http://www.cdc.gov/media/pressrel/2010/s100806.htm?s_cid=mediarel_s100806. Accessed August 6, 2010.
12. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (Fluzone High-Dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.