User login
From Temple University School of Medicine, Philadelphia, PA.
Abstract
- Objective: To outline the use and utility of gastric electric stimulation (GES) as a therapeutic intervention for gastroparesis.
- Methods: Review of the literature.
- Results: Gastroparesis is characterized by delayed gastric emptying, with symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Some patients with gastroparesis do not respond to medical intervention, and for these patients surgical intervention may be warranted. GES utilizes high-frequency gastric neurostimulation to facilitate gastric emptying and reduce symptoms of gastroparesis. It is indicated for patients with idiopathic and diabetic gastroparesis who have nausea and vomiting as their primary symptoms and who have not responded to medical therapy. GES has also been used in postsurgical and pediatric gastroparesis patients. Optimizing the outcome of this surgical treatment through proper patient selection and meticulous surgical technique is essential as there are inherent risks to the procedure. Nonblinded studies of GES for medically refractory gastroparesis have demonstrated therapeutic symptomatic benefit, whereas randomized controlled trials have not. New interventions such as pyloromyotomy and pyloroplasty are reasonable alternatives or addendums to GES.
- Conclusion: GES may be considered among the therapies available for treating patients with refractory symptoms of gastroparesis. More studies, specifically those comparing GES, pyloromyotomy, GES combined with pyloromyotomy, and placebo, are needed to help guide therapy selection for refractory gastroparesis.
Keywords: diabetes; gastroparesis; dysmotility; gastric emptying; electric stimulation.
Gastroparesis is a chronic dysmotility disorder characterized by delayed gastric emptying with associated symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Medical treatments for gastroparesis include dietary modifications, glucose control in those with diabetes, prokinetic medications, antiemetic medications, and symptom modulators, but unfortunately patients frequently do not respond to these treatments. In patients refractory to medical therapy, surgical treatments can be considered.
Gastric electric stimulation (GES; Enterra [Medtronic, Minneapolis, MN]) was approved via a Food and Drug Administration (FDA) Humanitarian Use Device (HUD) exemption for the treatment of medically refractory gastroparesis in 2000. Understanding the indications, risks, outcomes, and alternatives to GES is essential to providing appropriate care for patients with medically refractory gastroparesis. This article outlines the use and utility of GES as a therapeutic intervention for gastroparesis.
Types of Gastroparesis
Gastroparesis is a chronic symptomatic disorder of the stomach manifested by delayed gastric emptying without evidence of gastric outlet obstruction or ulceration.1 The pathophysiology of gastroparesis appears to involve abnormalities in functioning of several elements including the autonomic nervous system, especially the vagus nerve, smooth muscle cells, enteric neurons, and interstitial cells of Cajal.
Idiopathic gastroparesis and diabetic gastroparesis are the 2 most common types of gastroparesis.2 Symptomatic delayed gastric emptying with no primary underlying abnormality predisposing to gastroparesis is categorized as idiopathic gastroparesis.3 A small subset of patients with idiopathic gastroparesis report an initial infectious prodrome such as gastroenteritis or respiratory infection. It has been suggested that this postinfectious gastroparesis results from viral injury to the neural innervation of the stomach or the interstitial cells of Cajal in the stomach.4 Viruses that have been implicated in the development of gastroparesis include cytomegalovirus, Epstein-Barr virus, Norwalk virus, rotavirus, herpes zoster, and varicella zoster.5-9
Diabetic gastroparesis is characterized as onset of symptoms of gastroparesis in patients with diabetes, with concomitant delayed gastric emptying. It is often attributed to chronic hyperglycemia-induced damage to the vagus nerve, and is frequently observed in association with other diabetic complications such as neuropathy, retinopathy, and nephropathy.10
Gastroparesis that develops following surgery is classified as postsurgical gastroparesis. In the past, this form of gastroparesis most commonly occurred after ulcer surgery, often performed with vagotomy. These types of surgeries are performed less frequently in the era of proton pump inhibitor therapy and treatments for Helicobacter pylori. Presently, Nissen fundoplication and bariatric surgery are the more common surgical procedures associated with gastroparesis.3 Long-term use of medications that delay gastric emptying, such as opiate narcotic medications, can lead to gastroparesis and represent another form of iatrogenic gastroparesis. Other forms of gastroparesis (atypical gastroparesis) arise due to various underlying etiologies, including neurological disorders (eg, Parkinson disease, multiple sclerosis), metabolic or endocrine conditions (eg, hypothyroidism), autoimmune disorders, connective tissue and collagen vascular disorders (eg, systemic lupus erythematosus, scleroderma, Sjögren syndrome, Ehlers-Danlos syndrome), or eating disorders (eg, anorexia, bulimia).3
Epidemiology
There is a female preponderance in patients with gastroparesis. Data from the Rochester Epidemiology Project, a database of linked medical records for residents of Olmsted County, MN, showed that the age-adjusted prevalence of definite gastroparesis per 100,000 inhabitants was 37.8 for women and 9.6 for men.11 More recent estimates have suggested a much higher prevalence of probable gastroparesis (approximately 1.8%) in the general population using symptoms suggestive of gastroparesis.12 Hospitalization rates for gastroparesis have increased since 2000, which could reflect rising prevalence and/or the effects of heightened awareness about and better identification of gastroparesis.13 This increase may also be due in part to the rising rate of diabetes leading to more cases of diabetic gastroparesis; withdrawal of some gastroparesis treatments from the market (cisapride, tegaserod) leading to hospitalizations for symptoms not adequately being treated; and hospitalizations needed for insertion of the gastric electric stimulator.
Gastroparesis Symptoms
The main symptoms of gastroparesis are early satiety, postprandial fullness, bloating, nausea, and vomiting.14 Nausea (> 90% of patients) and early satiety (60% of patients) are the most common symptoms.15 Abdominal pain is often present in patients with gastroparesis but is usually not the predominant symptom. The pain can be multifactorial, with somatic, visceral, and neuropathic components.16-18 Moderate to severe abdominal pain has been found more often in patients with idiopathic gastroparesis and in association with opiate use.16 Symptoms of gastroparesis may be persistent or present as episodic flares. Due to the symptoms, some patients will experience weight loss and malnutrition and, in severe cases, dehydration.19
Although the definition of gastroparesis is a delay in gastric emptying along with symptoms, symptoms correlate poorly with the degree of delayed gastric emptying. The symptoms that appear to have the strongest correlation with gastric emptying are nausea, vomiting, early satiety, and postprandial fullness, whereas symptoms such as abdominal pain and bloating have little correlation. Furthermore, improving gastric emptying does not necessarily lead to improved symptoms, and symptom improvement does not always lead to improved gastric emptying times.20 Between 5% and 12% of patients with diabetes report symptoms consistent with gastroparesis, though many of these patients have normal gastric emptying. The symptoms of gastroparesis overlap with those of functional dyspepsia, as both may have motor and sensory alterations.21
The Gastroparesis Cardinal Symptom Index (GCSI), a subset of the Patient Assessment of Gastrointestinal Disorders Symptom Severity Index (PAGI-SYM), is a questionnaire that is commonly used to establish symptom severity in patients with gastroparesis. It is comprised of 3 subscales—nausea and vomiting, postprandial fullness and early satiety, and bloating—which are averaged to provide a total GCSI score. Symptoms over the 2 weeks prior to administration of the questionnaire are assessed and rated from 0 (none) to 5 (very severe).22 Grading the severity of gastroparesis may take into account symptoms, quality of life, and gastric emptying. One commonly used grading system assigns a grade from 1 to 3, with grade 1 being mild gastroparesis, grade 2 being compensated gastroparesis, and grade 3 being gastric failure with refractory symptoms that are uncontrolled.18,23 Quality-of-life surveys also suggest that gastroparesis independent of other factors leads to a worse quality of life.24
Indications for GES
Gastric electric stimulator implantation is a surgical procedure with inherent risks and complications and is reserved for patients with intractable symptoms of gastroparesis who remain symptomatic despite treatment attempts with dietary management, antiemetic agents (eg, compazine, phenergan, and ondansetron), and prokinetic agents (eg, metoclopramide, erythromycin, and domperidone). Symptom modulators such as nortriptyline and mirtazapine are occasionally tried.
Surgical intervention can be considered upon failure of medical treatment measures. At least a year of documented care provided by a physician specializing in gastroparesis is suggested for surgical consideration. The gastric electric neurostimulator is approved by the FDA as a HUD for the care of patients with idiopathic and diabetic gastroparesis, performed on a compassionate basis. GES implantation requires Institutional Review Board approval at the institution, and patients are required to have documented delayed gastric emptying.
It is important to remember that the GES device is incompatible with magnetic resonance imaging (MRI) and explantation of the device is necessary prior to MRI. As such, in patients with anticipated need of frequent MRI, such as those with multiple sclerosis, serious consideration should be given to alternative strategies prior to focusing on this modality.
Device Placement
GES was devised to improve gastric emptying. The Enterra GES system uses high-frequency, low-energy electric stimulation. An alternative method is true gastric pacing that uses high-energy, low-frequency stimulation to entrain the gastric slow waves and subsequent contractions at 3 cycles per minute (cpm). Gastric pacing has greater energy requirements than GES, which makes the size of the stimulator too large to be practical. In pilot animal studies, GES produced an accelerating effect on gastric emptying, but in human studies GES had an inconsistent effect on gastric emptying. Studies have suggested that GES influences the proximal stomach, with a reduction of gastric tone,25 and also that GES has an afferent modulatory mechanism.26
The Enterra GES is placed surgically under general anesthesia, commonly via laparotomy or minimal access surgical techniques (laparoscopically or robotically assisted). Preoperative intravenous antibiotics are given. The system consists of a pair of electrodes connected to a pulse generator. The 2 stimulation leads are inserted into the gastric muscularis propria 1 cm apart along the greater curvature 10 cm proximal to the pylorus. Upper endoscopy is performed to ensure that the leads do not penetrate through the mucosa into the stomach lumen; if this occurs, repositioning of the lead is necessary. A horizontal incision through the skin is made, and the distal ends of the stimulating wires are tunneled through the abdominal wall and connected to the pulse generator. The impedance (resistance) between the wires is measured to ensure the appropriate range (200-800 Ohms). The neurostimulator with the distal ends of the stimulating wires is then placed into the subcutaneous pocket and sutured to the underlying fascia. The pulse generator delivers a high-frequency, low-energy, 0.1-second train of pulses at a frequency of 12 cpm. Within each pulse train, individual pulses oscillate at a frequency of 14 cycles per second. The voltage of the stimulations is set to provide a current of 5 milliamps (mA; remembering that voltage = current × resistance).
Patients are often hospitalized with a recovery time of 1 to 3 days. Immediate postoperative care usually includes intravenous fluids, controlling any postoperative ileus, advancing diet, and providing analgesic pain medications. Hospital length of stay can be impacted by surgical technique.25 Patients are seen several weeks after discharge for assessment of the incision and toleration of diet. Medications for gastroparesis that patients were taking prior to the GES implantation are usually continued postoperatively, with a goal of reducing these medications over time. Patients are then followed every 3 to 12 months, depending on their clinical condition.
At follow-up visits, medications are reviewed and new treatments can be added if appropriate. The gastric stimulator is interrogated to determine if changes in resistance occurred; if necessary, minor readjustments can be made to keep the current at desired levels (5 mA). For persistent symptoms with GES treatment, the stimulator parameters can be adjusted after 3 months of follow up, typically first increasing the current from 5 to 7.5 mA and then to 10 mA. After this, the frequency can be increased from 14 Hz to 28 Hz, and then to 55 Hz. Rarely, the ON duration is increased from 0.1 to 1 second. Increasing the ON time can worsen symptoms in some patients, cause abdominal pain, and decrease the battery life from the usual 7 years.
Complications of GES
In an analysis of the Manufacturer and User Facility Device Experience (MAUDE) databank, Bielefeldt identified 1587 reports of adverse effects related to the gastric electric stimulator from January 2001 to October 2015.27 The most common adverse effects are reviewed here.
Skin erosion/wound dehiscence is one of the most common reported complications; it may be related to superficial placement or inadequate securing of the device to the fascia. Abscess can develop postoperatively due to hematogenous seeding or may be a sign of lead erosion into the lumen, tracking along the leads into subcutaneous tissue.28 It is important to warn patients to protect the area over the device from needle injections as this also can lead to hematoma formation and direct contamination of the device. If the device gets infected, it cannot be salvaged and requires explantation. Implantation of a new device can be attempted once all wound issues resolve.
Device migration/flipping most often occurs because the device is inadequately fixed to the underlying fascia, but occasionally it can occur from patients flipping the device around. Flipping can occur due to superficial pocket location within subcutaneous tissue, especially in obese patients. Migration/flipping can lead to prominence of the contour of the device and discomfort, ultimately requiring surgical correction.
Perforation and erosion of the leads. With time, leads can erode into the stomach, although this is rare. Usually erosion is associated with loss of device function. Endoscopy confirms this finding. In rare cases, infection can track proximally along the lead and present as a surgical site infection at the pulse generator. This complication often requires explantation of the neurostimulator leads and pulse generator.
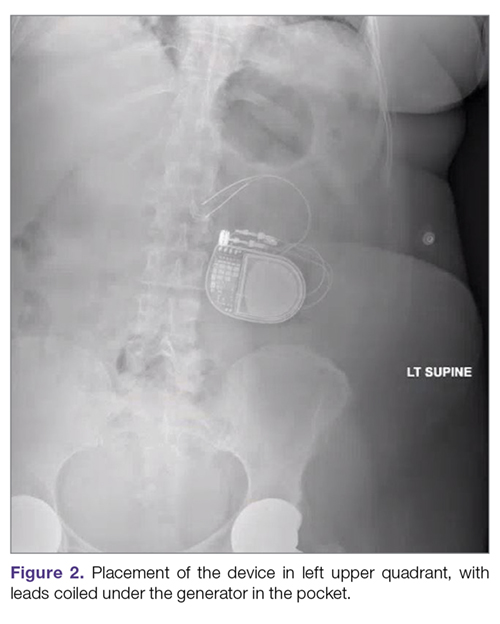
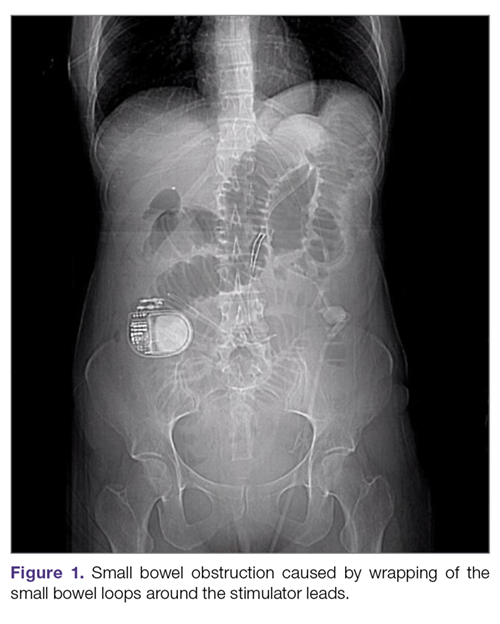
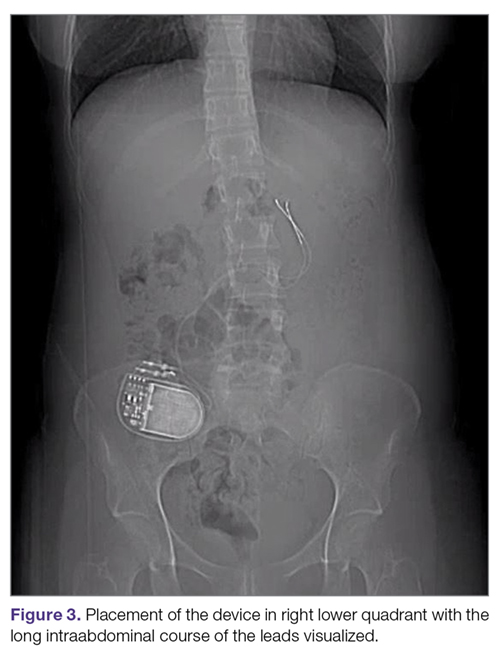
Intestinal obstruction. Although rare, the intestines can get wrapped around the leads of the device, causing different degrees of obstruction (Figure 1). Positioning the device in the left upper quadrant minimizes the intraabdominal length of the leads and pulls them maximally out, coiling under the device (Figure 2). In cases where other locations are used either due to a hostile upper abdominal region (skin infection, presence of gastrostomy or other devices) or surgeon’s preference, the GES device can be implanted in the lower abdomen (Figure 3). In these circumstances, carefully draping the omentum over the bowels might help to prevent this complication. Tacking of the leads to the parietal peritoneum with sutures can also be preventative. In cases of obstruction requiring intervention by laparotomy or minimal access techniques (laparoscopy or robotic assisted surgery), all efforts are made to preserve the neurostimulator leads. In cases that require bowel resection, lead contamination is a serious concern, but lead explantation is not mandatory. Close postoperative monitoring for the development of lead infection is required.
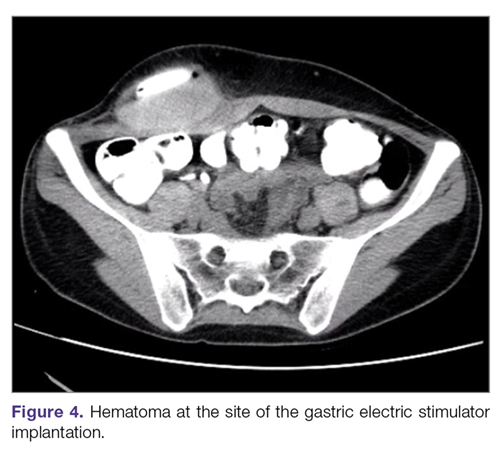
Hematoma and seroma. Postoperative hematomas can occur from inadequate hemostasis, and seromas can occur in the stimulator pocket. Small hematomas may be observed if not complicated (Figure 4). In cases of large hematomas with skin compromise or dehiscence, prompt washout and drainage is required. In ideal cases, the device can be preserved. Relocation to another site might be required if skin necrosis develops. The possibility of device contamination also must be considered; after resolution of wound issues, implantation of a new device may be tried. Seromas at the generator pocket site are a frequent occurrence but are often benign, self-limiting, and generally resolve over 4 to 6 weeks.
Incisional hernia. Hernias can develop after any abdominal surgery and are not unique to GES implantation. Use of minimally invasive technique for the GES implantation minimizes this complication.
Electric shock sensations may occur from breakage of the plastic lining covering the stimulator wires or from fluid buildup around the insertion of the wires into the stimulator. Shocks can also occur due to shortening of the leads on the muscles of the abdominal wall. Patients describe periodic muscle cramps with the frequency of the device (every 5 seconds). To prevent this complication, freshly implanted leads should be covered by an omental flap to isolate them from the abdominal wall. In patients who continue to feel shocks despite all efforts, the possibility of visceral hypersensitivity should be considered. A trial of symptom modulators such as nortriptyline and lowering of the output amperage below the minimal recommended setting of 5 mA can be undertaken. If these interventions do not work, the device must be turned off for a period of time. Occasionally, replacement of the leads or explantation of the device must be considered.
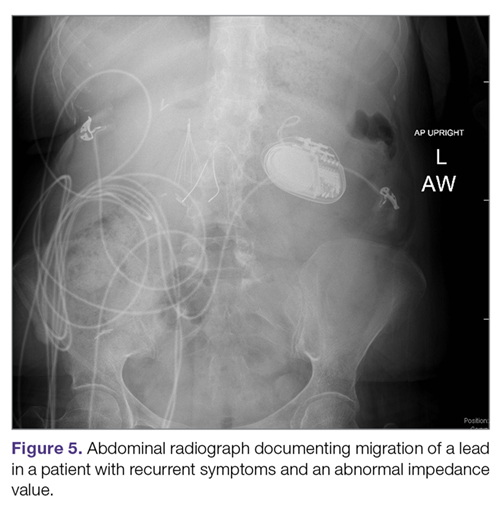
Lack of effect/persistent symptoms. If a patient presents with lack of improvement after device implantation, a thorough workup should be undertaken to ensure that the device is functioning properly. In the case of abnormal impedance values, an abdominal x-ray study can be performed to rule out lead migration (Figure 5). If no abnormalities are detected, the output of the device can be increased. After adjusting device settings, the patient should be assessed for improvement over at least a 1- to 3-month period. One report suggests that in patients not responding to GES, repositioning the location of the stimulator leads on the stomach can be helpful.29
Outcomes of GES
Study results of investigative GES models in animals and select patients were published in 1997.30,31 Following these reports, 2 large multicenter studies were conducted to demonstrate the efficacy of GES for the treatment of refractory gastroparesis. The Gastric Electrical Mechanical Stimulation Study (GEMS) was an open-label, multicenter study of 38 patients who received percutaneous and later permanent GES devices.32 Marked reduction in weekly vomiting and nausea was observed at 4 weeks, with a 90% reduction in nausea and vomiting frequency at 11 months. Following this, a second multicenter study (Worldwide Anti-Vomiting Electrical Stimulation Study [WAVES]) involving a double-blind sham stimulation controlled trial with 33 idiopathic and diabetic gastroparesis patients was performed.33 During the blinded portion of this study, there was a noticeable decrease in vomiting frequency, particularly in the patients with diabetic gastroparesis. Patient preference was for the stimulator ON as compared to OFF. The FDA’s HUD exemption for the Enterra GES device in 2000 was based on these studies.
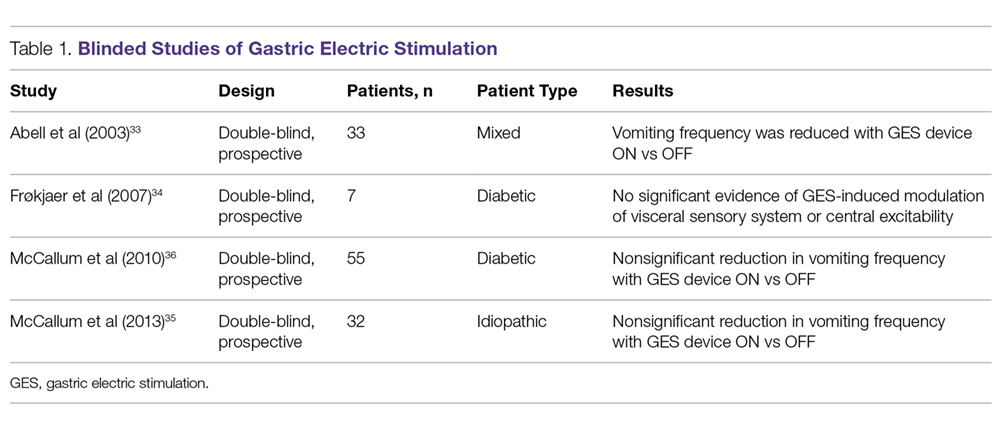
Four independent double-blind studies of GES have been conducted (Table 1).33-37 It has been difficult to demonstrate improvement during the double-blind period with gastric stimulation compared to no stimulation. Despite total symptom severity improvement and individual symptom improvements in these studies, a recent meta-analysis demonstrated a summative insignificant difference between the GES ON versus OFF states.38
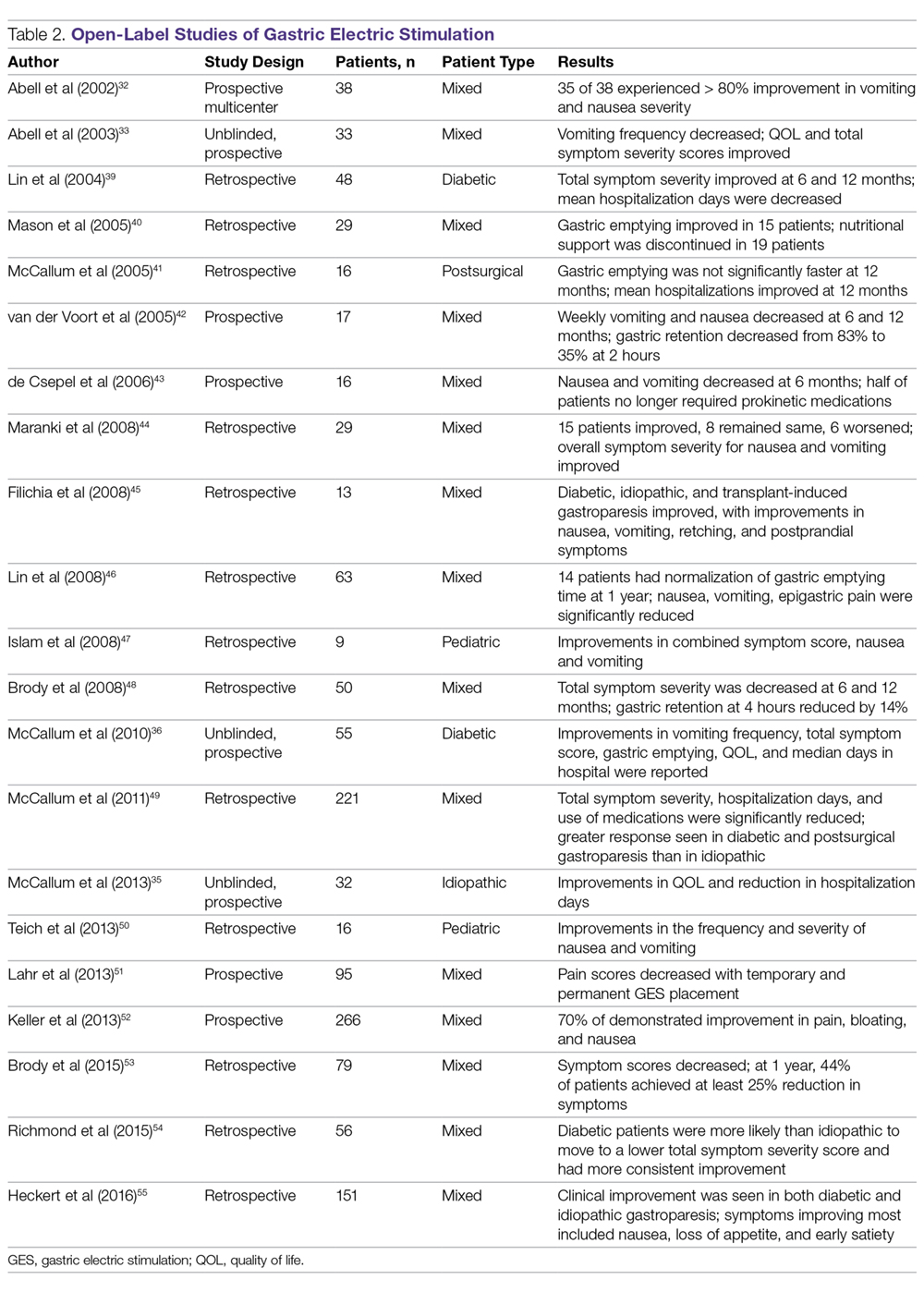
In contrast to the double-blind studies, numerous open-label studies have demonstrated clinical improvements in patients with diabetic and idiopathic gastroparesis (Table 2),32,33,35,36,39-55 leading some to question whether the demonstrable efficacy reflects a placebo effect or regression to the mean. Patients may perceive an operative, aggressive intervention as likely to be effectual in comparison to incremental medication efforts, thus creating a placebo effect. It should also be noted that not all open-label studies have demonstrated improvement with GES. Indeed, Jones et al reported no significant difference in nausea and vomiting at 6-month follow-up, and recommended that physicians exercise caution with GES as a therapeutic strategy given the cost and lack of confirmed demonstrable effect.56 Thus, the clinical successes demonstrated in open-label studies must be weighed not only against the lack of unequivocal improvement, but also against the potential deleterious effects of the surgery.
In an open-label study that employed the GCSI to follow symptoms of gastroparesis, 29 patients underwent GES implantation over an 18-month period, with follow-up in 28 patients.44 GES resulted in clinical improvement in 50% of patients with refractory gastroparesis. The overall GCSI significantly decreased, with improvement in the nausea/vomiting subscore and the post-prandial fullness subscore, but no improvement in the bloating subscore or abdominal pain. The decrease in GCSI was greater for patients with diabetic versus idiopathic gastroparesis. Patients with the main symptom of nausea/vomiting had a greater improvement than patients with the main symptom of abdominal pain. Patients taking narcotic analgesics at the time of implant had a poorer response compared to patients who were not. In this study, 3 clinical parameters were associated with a favorable clinical response: (1) diabetic rather than idiopathic gastroparesis, (2) nausea/vomiting rather than abdominal pain as the primary symptom, and (3) independence from narcotic analgesics prior to stimulator implantation. Knowledge of these 3 factors may allow improved patient selection for GES.
A large prospective study by Heckert et al detailed marked improvements with GES and the patterns of those improvements.55 Nausea, vomiting, loss of appetite, and early satiety improved significantly with stimulator use, with a greater improvement in vomiting in patients with diabetic gastroparesis than in those with the idiopathic form. Although GES improved symptoms in 75% of all patients, patients with diabetes had a post-GES Clinical Patient Grading Assessment score that was statistically higher than the score among patients with idiopathic gastroparesis. This difference is thought to be due to the neuromolecular mechanism of diabetic gastroparesis, where blunting of the enteric nervous system may contribute to symptomatology.
Several studies have demonstrated a clinical response to GES in patients with postsurgical gastroparesis. A study by Oubre et al showed that GES led to weekly vomiting improvements as well as a reduction in total symptom severity score.57 A study by McCallum et al further demonstrated improved symptoms, quality of life, nutritional status, and hospitalization requirements.58 GES has also been shown to improve gastroparesis symptoms in pediatric populations.47,59 Thus, although not a direct indication, GES has been shown to be beneficial in various subtypes of gastroparesis.
Additionally, irrespective of gastroparesis type, the improved symptomatology with GES appears to be durable, with one study showing persistent clinical improvements up to 8 years after device placement.60 The improvements were persistent and incremental. Likewise, McCallum et al showed that continued reductions in total symptom severity scores were evident in all gastroparesis types up to 10 years after stimulator implantation.61 The success of the procedures in part comes from careful selection of patients. Clinical parameters that are associated with favorable clinical response include diabetic gastroparesis subtype, nausea/vomiting predominance, and independence from narcotic analgesics prior to stimulator placement.62
GES has also been noted to improve other patient care metrics besides symptomatology, including nutritional status, reduced need for nutritional supplementation, and improved HbA1c.63-65 Additionally, a study by Cutts et al established that health care resource utilization significantly improved at 12, 24, and 36 months following GES placement, as compared to patients receiving standard medical therapy.66 This decreased resource utilization was also reflected in decreased costs in the GES group compared with the standard care group.
Surgical Alternatives to GES
Pyloric interventions such as pyloroplasty and pyloromyotomy are other surgical treatment modalities offered for gastroparesis. Whereas GES uses neurostimulation to facilitate gastric emptying and potentially improve fundic accommodation, pyloric interventions are intended to increase gastric emptying by reducing outflow resistance from the pyloric sphincter.
Pyloric Interventions
Various studies have shown significant improvements with pyloric interventions, similar to the improvements seen with GES. One such study involving 177 patients demonstrated an 86% improvement in gastric emptying, with symptom severity scores for nausea, vomiting, bloating, abdominal pain, and early satiety decreasing significantly at 3 months following pyloroplasty.67 A significant advantage of pyloric interventions is that pyloromyotomy can be performed endoscopically (gastric peroral endoscopic pyloromyotomy [G-POEM] or peroral pyloromyotomy [POP]), thus minimizing the risks of open surgery. A recent review that included a pooled analysis of 7 studies of G-POEM for gastroparesis demonstrated 100% technical success, with clinical efficacy in 81.5% of the procedures as assessed by the GCSI.68 Additionally, the intraoperative and perioperative complication rates were 6.6% and 7.6%, respectively, suggesting that G-POEM is a safe and clinically beneficial therapeutic option. Few studies comparing the outcomes of pyloric interventions to GES have been performed.
Recently, GES has been combined with pyloric interventions to maximize therapeutic potential. This allows simultaneous neurologic and functional interventions to expedite gastric emptying and improve patient symptomatology. Davis et al demonstrated significant improvement in 21 patients who underwent GES placement and pyloroplasty, with 71% improvement in total symptom severity.69 Notably, dual surgery did not increase the incidence of infection or adverse surgical outcomes. Although this study did not directly compare dual surgery to GES alone, the results are nonetheless favorable. GES provides a strong antiemetic and anti-nausea effect, whereas the pyloromyotomy provides improvement in gastric emptying.
Feeding/Venting Tubes
Feeding jejunostomy tubes and venting gastrostomy tubes can be used alone or in combination with GES. Feeding jejunostomy is performed for malnutrition and weight loss that accompanies the refractory symptoms of early satiety, nausea, and vomiting. Venting gastrostomy tubes allow for removal of retained gastric contents that may cause distension, nausea, and vomiting. Gastrojejunostomy tubes can also be placed endoscopically or by interventional radiology.
Gastrectomy
Gastrectomy can provide therapeutic benefit through elimination of the gastric reservoir function and consequent removal of afferent neural impulses. In select patient populations, outcomes of gastrectomy have compared favorably with those of GES. For example, one study demonstrated favorable outcomes of Roux-en-Y gastrectomy in morbidly obese patients with gastroparesis.70 In another study, favorable outcomes were reported in a cohort of 103 patients, with gastrectomy demonstrating 87% symptom improvement (nausea, vomiting, epigastric pain) compared to just 63% improvement with GES.71 However, the dramatic impact on anatomy and physiology and the invasiveness of the procedure need to be weighed against the therapeutic benefit. For example, in the same study, the 30-day morbidity was 23% for gastrectomy versus just 8% for the GES implant.71
When to Use GES
The gastric electrical neurostimulator (Enterra; Medtronic, Inc.) is approved for treatment of idiopathic and diabetic gastroparesis that is refractory to medical treatment, performed on a compassionate basis. Patients with diabetic gastroparesis respond to GES better than do patients with the idiopathic form. Of the symptoms of gastroparesis, primarily nausea and vomiting improve. Thus, GES favors patients with diabetic gastroparesis who have primarily nausea and vomiting, rather than, for instance, patients with idiopathic gastroparesis who have primarily abdominal pain and may be taking narcotics. Some centers provide GES for postsurgical patients and children with gastroparesis.
The 3 main surgical interventions for medically refractory gastroparesis are GES, pyloric intervention (pyloroplasty or pyloromyotomy), and gastrectomy. Of the 3 interventions, gastrectomy is the most radical given its dramatic effect on anatomy and is thus not preferred. The clinical decision then becomes: GES, pyloric intervention, or both? There are limited data to support a definitive answer to this question.
In a single-center retrospective analysis of prospective data (electronic medical record), Arthur et al compared outcomes of GES patients with medically refractory gastroparesis who received various surgical interventions.72 In total, 33 stimulator, 7 pyloroplasty, 2 gastrectomy, and 16 combined stimulator and pyloroplasty patients were analyzed for postoperative symptom improvement. Pyloroplasty alone demonstrated the least symptom improvement, combination GES and pyloroplasty demonstrated increased improvement, and GES alone demonstrated the most improvement. The results of this study suggest that barring contraindication, placement of a gastric stimulator as the initial treatment is best, with pyloroplasty reserved for patients who do not achieve adequate symptom control. Limitations of the study include its single-center design and low patient numbers for pyloroplasty in isolation.
In contrast, a recent retrospective systematic review synthesized the outcomes of various studies of GES and pyloric interventions for medically refractory gastroparesis.73 A therapeutic effect was found for each surgical intervention, with pyloric surgery patients demonstrating a greater response to intervention than GES patients. Unfortunately, attempts to analyze combination interventions were hindered by a lack of power.
Conclusion
Initial management of gastroparesis is medical (lifestyle and diet changes), with antiemetic and prokinetic agents used in refractory cases. Following failure of this therapy, placement of a GES device is a surgical intervention that has been approved under FDA humanitarian device exemption to help ameliorate symptomatology. Improvement with GES has been demonstrated in nonblinded studies, but the lack of randomized controlled trials demonstrating benefit suggests the possibility of an underlying placebo effect. Additionally, new medical procedures such as G-POEM complicate the decision of which intervention should be attempted first. More studies, specifically comparing GES, pyloric interventions, and combined GES with pyloric intervention to placebo, are needed to fully understand what therapy is best for refractory gastroparesis.
Corresponding author: Henry P. Parkman, MD, Gastroenterology Section, Temple University School of Medicine, 3401 North Broad Street, Philadelphia, PA 19140; [email protected].
Financial disclosures: None.
1. Camilleri M, Parkman HP, Shafi MA, et al. Clinical Guideline: Management of gastroparesis. Am J Gastroenterol. 2013;108:18-37.
2. Jehangir A, Parkman HP. Rome IV Diagnostic Questionnaire Complements Patient Assessment of Gastrointestinal Symptoms for Patients with Gastroparesis Symptoms. Dig Dis Sci. 2018;63:2231-2243.
3. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622.
4. Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101-115.
5. Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501-1504.
6. Kebede D, Barthel JS, Singh A. Transient gastroparesis associated with cutaneous herpes zoster. Dig Dis Sci. 1987;32:318-322.
7. Meeroff JC, Schreiber DS, Trier JS, Blacklow NR. Abnormal gastric motor function in viral gastroenteritis. Ann Intern Med. 1980;92:370-373.
8. Paliwal M, Prasanna KS, Saraswat VA, et al. Varicella zoster cranial polyneuropathy presenting with dysphagia, esophagitis and gastroparesis. J Neurogastroenterol Motil. 2011;17:192-194.
9. Sigurdsson L, Flores A, Putnam PE, et al. Postviral gastroparesis: presentation, treatment, and outcome. J Pediatr. 1997;131:751-754.
10. Kockar MC, Kayahan IK, Bavbek N. Diabetic gastroparesis in association with autonomic neuropathy and microvasculopathy. Acta Med Okayama. 2002;56:237-243.
11. Jung HK, Choung RS, Locke GR III, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225-1233.
12. Rey E, Choung RS, Schleck CD, et al. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18:34-42.
13. Wang YR, Fisher RS. Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol. 2008;103:313-322.
14. Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22:113-133.
15. Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398-2404.
16. Cherian D, Sachdeva P, Fisher RS, Parkman HP. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol. 2010;8:676-681.
17. Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil. 2013;25:427-438.
18. Jehangir A, Abdallah RT, Parkman HP. Characterizing abdominal pain in patients with gastroparesis into neuropathic and nociceptive components. J Clin Gastroenterol. 2018 May 18. doi: 10.1097/MCG.0000000000001059.
19. Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am. 2015;44:1-7.
20. Fosso CL, Quigley EMM. A critical review of the current clinical landscape of gastroparesis. Gastroenterol Hepatol. 2018;14:140-145.
21. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972-1978.
22. Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670-680.
23. Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil. 2012;24:456-463.
24. Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44:9-19.
25. Soffer E, Abell T, Lin Z, et al. Review article: Gastric electrical stimulation for gastroparesis – physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30:681-694.
26. Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on rat thoracic spinal neurons receiving input from the stomach. Neurosci Res. 2007;57:29-39
27. Bielefeldt K. Adverse events of gastric electrical stimulators recorded in the Manufacturer and User Device Experience (MAUDE) Registry. Auton Neurosci. 2017;202:40-44
28. Liu RC, Sabnis AA, Chand B. Erosion of gastric electrical stimulator electrodes: evaluation, management, and laparoscopic techniques. Surg Laparosc Endosc Percutan Tech. 2007;17:438-441.
29. Harrison NS, Williams PA, Walker MR, et al. Evaluation and treatment of gastric stimulator failure in patients with gastroparesis. Surg Innov. 2014;21:244-249.
30. Familoni BO, Abell TL, Nemoto D, et al. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci. 1997;42:885-891.
31. Familoni BO, Abell TL, Nemoto D, et al. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892-897.
32. Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204-212.
33. Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421-428.
34. Frøkjaer JB, Ejskjaer N, Rask P, et al. Central neuronal mechanisms of gastric electrical stimulation in diabetic gastroparesis. Scand J Gastroenterol. 2008;43:1066-1075.
35. McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25:815-836.
36. McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947-954.
37. Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496-503.
38. Levinthal DJ. Systematic review and meta-analysis: Gastric electrical stimulation for gastroparesis. Auton Neurosci. 2017;202:45-55.
39. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
40. Mason RJ, Lipham J, Eckerling G, et al. Gastric electrical stimulation: An alternative surgical therapy for patients with gastroparesis. Arch Surg. 2005;140:841-846.
41. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
42. van der Voort IR, Becker JC, Dietl KH, et al. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diabetes. 2005;113:38-42.
43. de Csepel J, Goldfarb B, Shapsis A, et al. Electrical stimulation for gastroparesis. gastric motility restored. Surg Endosc. 2006;20:302-306.
44. Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072-2078.
45. Filichia LA, Cendan CJ. Small case series of gastric stimulation for the management of transplant-induced gastroparesis. J Surg Res. 2008;148:90-93.
46. Lin Z, Hou Q, Sarosiek I, et al. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil. 2008;20:464-470.
47. Islam S, Vick LR, Runnels MJ, et al. Gastric electrical stimulation for children with intractable nausea and gastroparesis. J Pediatr Surg. 2008;43:437-442.
48. Brody F, Vaziri K, Saddler A, et al. Gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2008;207:533-538.
49. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
50. Teich S, Mousa HM, Punati J, Di Lorenzo C. Efficacy of permanent gastric electrical stimulation for the treatment of gastroparesis and functional dyspepsia in children and adolescents. J Pediatr Surg. 2013;48:178-183.
51. Lahr CJ, Griffith J, Subramony C, et al. Gastric electrical stimulation for abdominal pain in patients with symptoms of gastroparesis. Am Surg. 2013;79:457-464.
52. Keller DS, Parkman HP, Boucek DO, et al. Surgical outcomes after gastric electric stimulator placement for refractory gastroparesis. J Gastrointest Surg. 2013;17:620-626.
53. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
54. Richmond B, Chong B, Modak A, et al. Gastric electrical stimulation for refractory gastroparesis: Predictors of response and redefining a successful outcome. Am Surg. 2015;81:467-471.
55. Heckert J, Sankineni A, Hughes WB, et al. Gastric electric stimulation for refractory gastroparesis: A prospective analysis of 151 patients at a single center. Dig Dis Sci. 2016;61:168-175.
56. Jones MP, Ebert CC, Murayama K. Enterra for gastroparesis. Am J Gastroenterol. 2003;98:2578.
57. Oubre B, Luo J, Al-Juburi A, et al. Pilot study on gastric electrical stimulation on surgery-associated gastroparesis: Long-term outcome. South Med J. 2005;98:693-697.
58. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
59. Islam S, McLaughlin J, Pierson J, et al. Long-term outcomes of gastric electrical stimulation in children with gastroparesis. J Pediatr Surg. 2016;51:67-71.
60. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
61. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
62. Maranki J, Lytes V, Meilahn JE, et al. Dig Dis Sci. 2008 53:2072-2078.
63. Abell T, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27:277-281.
64. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
65. Lin Z, McElhinney C, Sarosiek I, et al. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50:1328-1334.
66. Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35-43.
67. Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc. 2016;30:1326-1332.
68. Khoury T, Mizrahi M, Mahamid M, et al. State of the art review with literature summary on gastric peroral endoscopic pyloromyotomy for gastroparesis. J Gastroenterol Hepatol. 2018;33:1829-1833.
69. Davis BR, Sarosiek I, Bashashati M, et al. The long-term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg. 2017;21:222-227.
70. Sun Z, Rodriguez J, McMichael J, et al. Surgical treatment of medically refractory gastroparesis in the morbidly obese. Surg Endosc. 2015;29:2683-2689.
71. Zehetner J, Ravari F, Ayazi S, et al. Minimally invasive surgical approach for the treatment of gastroparesis. Surg Endosc. 2013;27:61-66.
72. Arthur LE, Slattery L, Richardson W. Tailored approach to gastroparesis significantly improves symptoms. Surg Endosc. 2017;32:977-982.
73. Zoll B, Zhao H, Edwards MA, et al. Outcomes of surgical intervention for refractory gastroparesis: A systematic review. J Surg Res. 2018;231:263-269.
From Temple University School of Medicine, Philadelphia, PA.
Abstract
- Objective: To outline the use and utility of gastric electric stimulation (GES) as a therapeutic intervention for gastroparesis.
- Methods: Review of the literature.
- Results: Gastroparesis is characterized by delayed gastric emptying, with symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Some patients with gastroparesis do not respond to medical intervention, and for these patients surgical intervention may be warranted. GES utilizes high-frequency gastric neurostimulation to facilitate gastric emptying and reduce symptoms of gastroparesis. It is indicated for patients with idiopathic and diabetic gastroparesis who have nausea and vomiting as their primary symptoms and who have not responded to medical therapy. GES has also been used in postsurgical and pediatric gastroparesis patients. Optimizing the outcome of this surgical treatment through proper patient selection and meticulous surgical technique is essential as there are inherent risks to the procedure. Nonblinded studies of GES for medically refractory gastroparesis have demonstrated therapeutic symptomatic benefit, whereas randomized controlled trials have not. New interventions such as pyloromyotomy and pyloroplasty are reasonable alternatives or addendums to GES.
- Conclusion: GES may be considered among the therapies available for treating patients with refractory symptoms of gastroparesis. More studies, specifically those comparing GES, pyloromyotomy, GES combined with pyloromyotomy, and placebo, are needed to help guide therapy selection for refractory gastroparesis.
Keywords: diabetes; gastroparesis; dysmotility; gastric emptying; electric stimulation.
Gastroparesis is a chronic dysmotility disorder characterized by delayed gastric emptying with associated symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Medical treatments for gastroparesis include dietary modifications, glucose control in those with diabetes, prokinetic medications, antiemetic medications, and symptom modulators, but unfortunately patients frequently do not respond to these treatments. In patients refractory to medical therapy, surgical treatments can be considered.
Gastric electric stimulation (GES; Enterra [Medtronic, Minneapolis, MN]) was approved via a Food and Drug Administration (FDA) Humanitarian Use Device (HUD) exemption for the treatment of medically refractory gastroparesis in 2000. Understanding the indications, risks, outcomes, and alternatives to GES is essential to providing appropriate care for patients with medically refractory gastroparesis. This article outlines the use and utility of GES as a therapeutic intervention for gastroparesis.
Types of Gastroparesis
Gastroparesis is a chronic symptomatic disorder of the stomach manifested by delayed gastric emptying without evidence of gastric outlet obstruction or ulceration.1 The pathophysiology of gastroparesis appears to involve abnormalities in functioning of several elements including the autonomic nervous system, especially the vagus nerve, smooth muscle cells, enteric neurons, and interstitial cells of Cajal.
Idiopathic gastroparesis and diabetic gastroparesis are the 2 most common types of gastroparesis.2 Symptomatic delayed gastric emptying with no primary underlying abnormality predisposing to gastroparesis is categorized as idiopathic gastroparesis.3 A small subset of patients with idiopathic gastroparesis report an initial infectious prodrome such as gastroenteritis or respiratory infection. It has been suggested that this postinfectious gastroparesis results from viral injury to the neural innervation of the stomach or the interstitial cells of Cajal in the stomach.4 Viruses that have been implicated in the development of gastroparesis include cytomegalovirus, Epstein-Barr virus, Norwalk virus, rotavirus, herpes zoster, and varicella zoster.5-9
Diabetic gastroparesis is characterized as onset of symptoms of gastroparesis in patients with diabetes, with concomitant delayed gastric emptying. It is often attributed to chronic hyperglycemia-induced damage to the vagus nerve, and is frequently observed in association with other diabetic complications such as neuropathy, retinopathy, and nephropathy.10
Gastroparesis that develops following surgery is classified as postsurgical gastroparesis. In the past, this form of gastroparesis most commonly occurred after ulcer surgery, often performed with vagotomy. These types of surgeries are performed less frequently in the era of proton pump inhibitor therapy and treatments for Helicobacter pylori. Presently, Nissen fundoplication and bariatric surgery are the more common surgical procedures associated with gastroparesis.3 Long-term use of medications that delay gastric emptying, such as opiate narcotic medications, can lead to gastroparesis and represent another form of iatrogenic gastroparesis. Other forms of gastroparesis (atypical gastroparesis) arise due to various underlying etiologies, including neurological disorders (eg, Parkinson disease, multiple sclerosis), metabolic or endocrine conditions (eg, hypothyroidism), autoimmune disorders, connective tissue and collagen vascular disorders (eg, systemic lupus erythematosus, scleroderma, Sjögren syndrome, Ehlers-Danlos syndrome), or eating disorders (eg, anorexia, bulimia).3
Epidemiology
There is a female preponderance in patients with gastroparesis. Data from the Rochester Epidemiology Project, a database of linked medical records for residents of Olmsted County, MN, showed that the age-adjusted prevalence of definite gastroparesis per 100,000 inhabitants was 37.8 for women and 9.6 for men.11 More recent estimates have suggested a much higher prevalence of probable gastroparesis (approximately 1.8%) in the general population using symptoms suggestive of gastroparesis.12 Hospitalization rates for gastroparesis have increased since 2000, which could reflect rising prevalence and/or the effects of heightened awareness about and better identification of gastroparesis.13 This increase may also be due in part to the rising rate of diabetes leading to more cases of diabetic gastroparesis; withdrawal of some gastroparesis treatments from the market (cisapride, tegaserod) leading to hospitalizations for symptoms not adequately being treated; and hospitalizations needed for insertion of the gastric electric stimulator.
Gastroparesis Symptoms
The main symptoms of gastroparesis are early satiety, postprandial fullness, bloating, nausea, and vomiting.14 Nausea (> 90% of patients) and early satiety (60% of patients) are the most common symptoms.15 Abdominal pain is often present in patients with gastroparesis but is usually not the predominant symptom. The pain can be multifactorial, with somatic, visceral, and neuropathic components.16-18 Moderate to severe abdominal pain has been found more often in patients with idiopathic gastroparesis and in association with opiate use.16 Symptoms of gastroparesis may be persistent or present as episodic flares. Due to the symptoms, some patients will experience weight loss and malnutrition and, in severe cases, dehydration.19
Although the definition of gastroparesis is a delay in gastric emptying along with symptoms, symptoms correlate poorly with the degree of delayed gastric emptying. The symptoms that appear to have the strongest correlation with gastric emptying are nausea, vomiting, early satiety, and postprandial fullness, whereas symptoms such as abdominal pain and bloating have little correlation. Furthermore, improving gastric emptying does not necessarily lead to improved symptoms, and symptom improvement does not always lead to improved gastric emptying times.20 Between 5% and 12% of patients with diabetes report symptoms consistent with gastroparesis, though many of these patients have normal gastric emptying. The symptoms of gastroparesis overlap with those of functional dyspepsia, as both may have motor and sensory alterations.21
The Gastroparesis Cardinal Symptom Index (GCSI), a subset of the Patient Assessment of Gastrointestinal Disorders Symptom Severity Index (PAGI-SYM), is a questionnaire that is commonly used to establish symptom severity in patients with gastroparesis. It is comprised of 3 subscales—nausea and vomiting, postprandial fullness and early satiety, and bloating—which are averaged to provide a total GCSI score. Symptoms over the 2 weeks prior to administration of the questionnaire are assessed and rated from 0 (none) to 5 (very severe).22 Grading the severity of gastroparesis may take into account symptoms, quality of life, and gastric emptying. One commonly used grading system assigns a grade from 1 to 3, with grade 1 being mild gastroparesis, grade 2 being compensated gastroparesis, and grade 3 being gastric failure with refractory symptoms that are uncontrolled.18,23 Quality-of-life surveys also suggest that gastroparesis independent of other factors leads to a worse quality of life.24
Indications for GES
Gastric electric stimulator implantation is a surgical procedure with inherent risks and complications and is reserved for patients with intractable symptoms of gastroparesis who remain symptomatic despite treatment attempts with dietary management, antiemetic agents (eg, compazine, phenergan, and ondansetron), and prokinetic agents (eg, metoclopramide, erythromycin, and domperidone). Symptom modulators such as nortriptyline and mirtazapine are occasionally tried.
Surgical intervention can be considered upon failure of medical treatment measures. At least a year of documented care provided by a physician specializing in gastroparesis is suggested for surgical consideration. The gastric electric neurostimulator is approved by the FDA as a HUD for the care of patients with idiopathic and diabetic gastroparesis, performed on a compassionate basis. GES implantation requires Institutional Review Board approval at the institution, and patients are required to have documented delayed gastric emptying.
It is important to remember that the GES device is incompatible with magnetic resonance imaging (MRI) and explantation of the device is necessary prior to MRI. As such, in patients with anticipated need of frequent MRI, such as those with multiple sclerosis, serious consideration should be given to alternative strategies prior to focusing on this modality.
Device Placement
GES was devised to improve gastric emptying. The Enterra GES system uses high-frequency, low-energy electric stimulation. An alternative method is true gastric pacing that uses high-energy, low-frequency stimulation to entrain the gastric slow waves and subsequent contractions at 3 cycles per minute (cpm). Gastric pacing has greater energy requirements than GES, which makes the size of the stimulator too large to be practical. In pilot animal studies, GES produced an accelerating effect on gastric emptying, but in human studies GES had an inconsistent effect on gastric emptying. Studies have suggested that GES influences the proximal stomach, with a reduction of gastric tone,25 and also that GES has an afferent modulatory mechanism.26
The Enterra GES is placed surgically under general anesthesia, commonly via laparotomy or minimal access surgical techniques (laparoscopically or robotically assisted). Preoperative intravenous antibiotics are given. The system consists of a pair of electrodes connected to a pulse generator. The 2 stimulation leads are inserted into the gastric muscularis propria 1 cm apart along the greater curvature 10 cm proximal to the pylorus. Upper endoscopy is performed to ensure that the leads do not penetrate through the mucosa into the stomach lumen; if this occurs, repositioning of the lead is necessary. A horizontal incision through the skin is made, and the distal ends of the stimulating wires are tunneled through the abdominal wall and connected to the pulse generator. The impedance (resistance) between the wires is measured to ensure the appropriate range (200-800 Ohms). The neurostimulator with the distal ends of the stimulating wires is then placed into the subcutaneous pocket and sutured to the underlying fascia. The pulse generator delivers a high-frequency, low-energy, 0.1-second train of pulses at a frequency of 12 cpm. Within each pulse train, individual pulses oscillate at a frequency of 14 cycles per second. The voltage of the stimulations is set to provide a current of 5 milliamps (mA; remembering that voltage = current × resistance).
Patients are often hospitalized with a recovery time of 1 to 3 days. Immediate postoperative care usually includes intravenous fluids, controlling any postoperative ileus, advancing diet, and providing analgesic pain medications. Hospital length of stay can be impacted by surgical technique.25 Patients are seen several weeks after discharge for assessment of the incision and toleration of diet. Medications for gastroparesis that patients were taking prior to the GES implantation are usually continued postoperatively, with a goal of reducing these medications over time. Patients are then followed every 3 to 12 months, depending on their clinical condition.
At follow-up visits, medications are reviewed and new treatments can be added if appropriate. The gastric stimulator is interrogated to determine if changes in resistance occurred; if necessary, minor readjustments can be made to keep the current at desired levels (5 mA). For persistent symptoms with GES treatment, the stimulator parameters can be adjusted after 3 months of follow up, typically first increasing the current from 5 to 7.5 mA and then to 10 mA. After this, the frequency can be increased from 14 Hz to 28 Hz, and then to 55 Hz. Rarely, the ON duration is increased from 0.1 to 1 second. Increasing the ON time can worsen symptoms in some patients, cause abdominal pain, and decrease the battery life from the usual 7 years.
Complications of GES
In an analysis of the Manufacturer and User Facility Device Experience (MAUDE) databank, Bielefeldt identified 1587 reports of adverse effects related to the gastric electric stimulator from January 2001 to October 2015.27 The most common adverse effects are reviewed here.
Skin erosion/wound dehiscence is one of the most common reported complications; it may be related to superficial placement or inadequate securing of the device to the fascia. Abscess can develop postoperatively due to hematogenous seeding or may be a sign of lead erosion into the lumen, tracking along the leads into subcutaneous tissue.28 It is important to warn patients to protect the area over the device from needle injections as this also can lead to hematoma formation and direct contamination of the device. If the device gets infected, it cannot be salvaged and requires explantation. Implantation of a new device can be attempted once all wound issues resolve.
Device migration/flipping most often occurs because the device is inadequately fixed to the underlying fascia, but occasionally it can occur from patients flipping the device around. Flipping can occur due to superficial pocket location within subcutaneous tissue, especially in obese patients. Migration/flipping can lead to prominence of the contour of the device and discomfort, ultimately requiring surgical correction.

Perforation and erosion of the leads. With time, leads can erode into the stomach, although this is rare. Usually erosion is associated with loss of device function. Endoscopy confirms this finding. In rare cases, infection can track proximally along the lead and present as a surgical site infection at the pulse generator. This complication often requires explantation of the neurostimulator leads and pulse generator.

Intestinal obstruction. Although rare, the intestines can get wrapped around the leads of the device, causing different degrees of obstruction (Figure 1). Positioning the device in the left upper quadrant minimizes the intraabdominal length of the leads and pulls them maximally out, coiling under the device (Figure 2). In cases where other locations are used either due to a hostile upper abdominal region (skin infection, presence of gastrostomy or other devices) or surgeon’s preference, the GES device can be implanted in the lower abdomen (Figure 3). In these circumstances, carefully draping the omentum over the bowels might help to prevent this complication. Tacking of the leads to the parietal peritoneum with sutures can also be preventative. In cases of obstruction requiring intervention by laparotomy or minimal access techniques (laparoscopy or robotic assisted surgery), all efforts are made to preserve the neurostimulator leads. In cases that require bowel resection, lead contamination is a serious concern, but lead explantation is not mandatory. Close postoperative monitoring for the development of lead infection is required.

Hematoma and seroma. Postoperative hematomas can occur from inadequate hemostasis, and seromas can occur in the stimulator pocket. Small hematomas may be observed if not complicated (Figure 4). In cases of large hematomas with skin compromise or dehiscence, prompt washout and drainage is required. In ideal cases, the device can be preserved. Relocation to another site might be required if skin necrosis develops. The possibility of device contamination also must be considered; after resolution of wound issues, implantation of a new device may be tried. Seromas at the generator pocket site are a frequent occurrence but are often benign, self-limiting, and generally resolve over 4 to 6 weeks.

Incisional hernia. Hernias can develop after any abdominal surgery and are not unique to GES implantation. Use of minimally invasive technique for the GES implantation minimizes this complication.
Electric shock sensations may occur from breakage of the plastic lining covering the stimulator wires or from fluid buildup around the insertion of the wires into the stimulator. Shocks can also occur due to shortening of the leads on the muscles of the abdominal wall. Patients describe periodic muscle cramps with the frequency of the device (every 5 seconds). To prevent this complication, freshly implanted leads should be covered by an omental flap to isolate them from the abdominal wall. In patients who continue to feel shocks despite all efforts, the possibility of visceral hypersensitivity should be considered. A trial of symptom modulators such as nortriptyline and lowering of the output amperage below the minimal recommended setting of 5 mA can be undertaken. If these interventions do not work, the device must be turned off for a period of time. Occasionally, replacement of the leads or explantation of the device must be considered.
Lack of effect/persistent symptoms. If a patient presents with lack of improvement after device implantation, a thorough workup should be undertaken to ensure that the device is functioning properly. In the case of abnormal impedance values, an abdominal x-ray study can be performed to rule out lead migration (Figure 5). If no abnormalities are detected, the output of the device can be increased. After adjusting device settings, the patient should be assessed for improvement over at least a 1- to 3-month period. One report suggests that in patients not responding to GES, repositioning the location of the stimulator leads on the stomach can be helpful.29

Outcomes of GES
Study results of investigative GES models in animals and select patients were published in 1997.30,31 Following these reports, 2 large multicenter studies were conducted to demonstrate the efficacy of GES for the treatment of refractory gastroparesis. The Gastric Electrical Mechanical Stimulation Study (GEMS) was an open-label, multicenter study of 38 patients who received percutaneous and later permanent GES devices.32 Marked reduction in weekly vomiting and nausea was observed at 4 weeks, with a 90% reduction in nausea and vomiting frequency at 11 months. Following this, a second multicenter study (Worldwide Anti-Vomiting Electrical Stimulation Study [WAVES]) involving a double-blind sham stimulation controlled trial with 33 idiopathic and diabetic gastroparesis patients was performed.33 During the blinded portion of this study, there was a noticeable decrease in vomiting frequency, particularly in the patients with diabetic gastroparesis. Patient preference was for the stimulator ON as compared to OFF. The FDA’s HUD exemption for the Enterra GES device in 2000 was based on these studies.
Four independent double-blind studies of GES have been conducted (Table 1).33-37 It has been difficult to demonstrate improvement during the double-blind period with gastric stimulation compared to no stimulation. Despite total symptom severity improvement and individual symptom improvements in these studies, a recent meta-analysis demonstrated a summative insignificant difference between the GES ON versus OFF states.38

In contrast to the double-blind studies, numerous open-label studies have demonstrated clinical improvements in patients with diabetic and idiopathic gastroparesis (Table 2),32,33,35,36,39-55 leading some to question whether the demonstrable efficacy reflects a placebo effect or regression to the mean. Patients may perceive an operative, aggressive intervention as likely to be effectual in comparison to incremental medication efforts, thus creating a placebo effect. It should also be noted that not all open-label studies have demonstrated improvement with GES. Indeed, Jones et al reported no significant difference in nausea and vomiting at 6-month follow-up, and recommended that physicians exercise caution with GES as a therapeutic strategy given the cost and lack of confirmed demonstrable effect.56 Thus, the clinical successes demonstrated in open-label studies must be weighed not only against the lack of unequivocal improvement, but also against the potential deleterious effects of the surgery.

In an open-label study that employed the GCSI to follow symptoms of gastroparesis, 29 patients underwent GES implantation over an 18-month period, with follow-up in 28 patients.44 GES resulted in clinical improvement in 50% of patients with refractory gastroparesis. The overall GCSI significantly decreased, with improvement in the nausea/vomiting subscore and the post-prandial fullness subscore, but no improvement in the bloating subscore or abdominal pain. The decrease in GCSI was greater for patients with diabetic versus idiopathic gastroparesis. Patients with the main symptom of nausea/vomiting had a greater improvement than patients with the main symptom of abdominal pain. Patients taking narcotic analgesics at the time of implant had a poorer response compared to patients who were not. In this study, 3 clinical parameters were associated with a favorable clinical response: (1) diabetic rather than idiopathic gastroparesis, (2) nausea/vomiting rather than abdominal pain as the primary symptom, and (3) independence from narcotic analgesics prior to stimulator implantation. Knowledge of these 3 factors may allow improved patient selection for GES.
A large prospective study by Heckert et al detailed marked improvements with GES and the patterns of those improvements.55 Nausea, vomiting, loss of appetite, and early satiety improved significantly with stimulator use, with a greater improvement in vomiting in patients with diabetic gastroparesis than in those with the idiopathic form. Although GES improved symptoms in 75% of all patients, patients with diabetes had a post-GES Clinical Patient Grading Assessment score that was statistically higher than the score among patients with idiopathic gastroparesis. This difference is thought to be due to the neuromolecular mechanism of diabetic gastroparesis, where blunting of the enteric nervous system may contribute to symptomatology.
Several studies have demonstrated a clinical response to GES in patients with postsurgical gastroparesis. A study by Oubre et al showed that GES led to weekly vomiting improvements as well as a reduction in total symptom severity score.57 A study by McCallum et al further demonstrated improved symptoms, quality of life, nutritional status, and hospitalization requirements.58 GES has also been shown to improve gastroparesis symptoms in pediatric populations.47,59 Thus, although not a direct indication, GES has been shown to be beneficial in various subtypes of gastroparesis.
Additionally, irrespective of gastroparesis type, the improved symptomatology with GES appears to be durable, with one study showing persistent clinical improvements up to 8 years after device placement.60 The improvements were persistent and incremental. Likewise, McCallum et al showed that continued reductions in total symptom severity scores were evident in all gastroparesis types up to 10 years after stimulator implantation.61 The success of the procedures in part comes from careful selection of patients. Clinical parameters that are associated with favorable clinical response include diabetic gastroparesis subtype, nausea/vomiting predominance, and independence from narcotic analgesics prior to stimulator placement.62
GES has also been noted to improve other patient care metrics besides symptomatology, including nutritional status, reduced need for nutritional supplementation, and improved HbA1c.63-65 Additionally, a study by Cutts et al established that health care resource utilization significantly improved at 12, 24, and 36 months following GES placement, as compared to patients receiving standard medical therapy.66 This decreased resource utilization was also reflected in decreased costs in the GES group compared with the standard care group.
Surgical Alternatives to GES
Pyloric interventions such as pyloroplasty and pyloromyotomy are other surgical treatment modalities offered for gastroparesis. Whereas GES uses neurostimulation to facilitate gastric emptying and potentially improve fundic accommodation, pyloric interventions are intended to increase gastric emptying by reducing outflow resistance from the pyloric sphincter.
Pyloric Interventions
Various studies have shown significant improvements with pyloric interventions, similar to the improvements seen with GES. One such study involving 177 patients demonstrated an 86% improvement in gastric emptying, with symptom severity scores for nausea, vomiting, bloating, abdominal pain, and early satiety decreasing significantly at 3 months following pyloroplasty.67 A significant advantage of pyloric interventions is that pyloromyotomy can be performed endoscopically (gastric peroral endoscopic pyloromyotomy [G-POEM] or peroral pyloromyotomy [POP]), thus minimizing the risks of open surgery. A recent review that included a pooled analysis of 7 studies of G-POEM for gastroparesis demonstrated 100% technical success, with clinical efficacy in 81.5% of the procedures as assessed by the GCSI.68 Additionally, the intraoperative and perioperative complication rates were 6.6% and 7.6%, respectively, suggesting that G-POEM is a safe and clinically beneficial therapeutic option. Few studies comparing the outcomes of pyloric interventions to GES have been performed.
Recently, GES has been combined with pyloric interventions to maximize therapeutic potential. This allows simultaneous neurologic and functional interventions to expedite gastric emptying and improve patient symptomatology. Davis et al demonstrated significant improvement in 21 patients who underwent GES placement and pyloroplasty, with 71% improvement in total symptom severity.69 Notably, dual surgery did not increase the incidence of infection or adverse surgical outcomes. Although this study did not directly compare dual surgery to GES alone, the results are nonetheless favorable. GES provides a strong antiemetic and anti-nausea effect, whereas the pyloromyotomy provides improvement in gastric emptying.
Feeding/Venting Tubes
Feeding jejunostomy tubes and venting gastrostomy tubes can be used alone or in combination with GES. Feeding jejunostomy is performed for malnutrition and weight loss that accompanies the refractory symptoms of early satiety, nausea, and vomiting. Venting gastrostomy tubes allow for removal of retained gastric contents that may cause distension, nausea, and vomiting. Gastrojejunostomy tubes can also be placed endoscopically or by interventional radiology.
Gastrectomy
Gastrectomy can provide therapeutic benefit through elimination of the gastric reservoir function and consequent removal of afferent neural impulses. In select patient populations, outcomes of gastrectomy have compared favorably with those of GES. For example, one study demonstrated favorable outcomes of Roux-en-Y gastrectomy in morbidly obese patients with gastroparesis.70 In another study, favorable outcomes were reported in a cohort of 103 patients, with gastrectomy demonstrating 87% symptom improvement (nausea, vomiting, epigastric pain) compared to just 63% improvement with GES.71 However, the dramatic impact on anatomy and physiology and the invasiveness of the procedure need to be weighed against the therapeutic benefit. For example, in the same study, the 30-day morbidity was 23% for gastrectomy versus just 8% for the GES implant.71
When to Use GES
The gastric electrical neurostimulator (Enterra; Medtronic, Inc.) is approved for treatment of idiopathic and diabetic gastroparesis that is refractory to medical treatment, performed on a compassionate basis. Patients with diabetic gastroparesis respond to GES better than do patients with the idiopathic form. Of the symptoms of gastroparesis, primarily nausea and vomiting improve. Thus, GES favors patients with diabetic gastroparesis who have primarily nausea and vomiting, rather than, for instance, patients with idiopathic gastroparesis who have primarily abdominal pain and may be taking narcotics. Some centers provide GES for postsurgical patients and children with gastroparesis.
The 3 main surgical interventions for medically refractory gastroparesis are GES, pyloric intervention (pyloroplasty or pyloromyotomy), and gastrectomy. Of the 3 interventions, gastrectomy is the most radical given its dramatic effect on anatomy and is thus not preferred. The clinical decision then becomes: GES, pyloric intervention, or both? There are limited data to support a definitive answer to this question.
In a single-center retrospective analysis of prospective data (electronic medical record), Arthur et al compared outcomes of GES patients with medically refractory gastroparesis who received various surgical interventions.72 In total, 33 stimulator, 7 pyloroplasty, 2 gastrectomy, and 16 combined stimulator and pyloroplasty patients were analyzed for postoperative symptom improvement. Pyloroplasty alone demonstrated the least symptom improvement, combination GES and pyloroplasty demonstrated increased improvement, and GES alone demonstrated the most improvement. The results of this study suggest that barring contraindication, placement of a gastric stimulator as the initial treatment is best, with pyloroplasty reserved for patients who do not achieve adequate symptom control. Limitations of the study include its single-center design and low patient numbers for pyloroplasty in isolation.
In contrast, a recent retrospective systematic review synthesized the outcomes of various studies of GES and pyloric interventions for medically refractory gastroparesis.73 A therapeutic effect was found for each surgical intervention, with pyloric surgery patients demonstrating a greater response to intervention than GES patients. Unfortunately, attempts to analyze combination interventions were hindered by a lack of power.
Conclusion
Initial management of gastroparesis is medical (lifestyle and diet changes), with antiemetic and prokinetic agents used in refractory cases. Following failure of this therapy, placement of a GES device is a surgical intervention that has been approved under FDA humanitarian device exemption to help ameliorate symptomatology. Improvement with GES has been demonstrated in nonblinded studies, but the lack of randomized controlled trials demonstrating benefit suggests the possibility of an underlying placebo effect. Additionally, new medical procedures such as G-POEM complicate the decision of which intervention should be attempted first. More studies, specifically comparing GES, pyloric interventions, and combined GES with pyloric intervention to placebo, are needed to fully understand what therapy is best for refractory gastroparesis.
Corresponding author: Henry P. Parkman, MD, Gastroenterology Section, Temple University School of Medicine, 3401 North Broad Street, Philadelphia, PA 19140; [email protected].
Financial disclosures: None.
From Temple University School of Medicine, Philadelphia, PA.
Abstract
- Objective: To outline the use and utility of gastric electric stimulation (GES) as a therapeutic intervention for gastroparesis.
- Methods: Review of the literature.
- Results: Gastroparesis is characterized by delayed gastric emptying, with symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Some patients with gastroparesis do not respond to medical intervention, and for these patients surgical intervention may be warranted. GES utilizes high-frequency gastric neurostimulation to facilitate gastric emptying and reduce symptoms of gastroparesis. It is indicated for patients with idiopathic and diabetic gastroparesis who have nausea and vomiting as their primary symptoms and who have not responded to medical therapy. GES has also been used in postsurgical and pediatric gastroparesis patients. Optimizing the outcome of this surgical treatment through proper patient selection and meticulous surgical technique is essential as there are inherent risks to the procedure. Nonblinded studies of GES for medically refractory gastroparesis have demonstrated therapeutic symptomatic benefit, whereas randomized controlled trials have not. New interventions such as pyloromyotomy and pyloroplasty are reasonable alternatives or addendums to GES.
- Conclusion: GES may be considered among the therapies available for treating patients with refractory symptoms of gastroparesis. More studies, specifically those comparing GES, pyloromyotomy, GES combined with pyloromyotomy, and placebo, are needed to help guide therapy selection for refractory gastroparesis.
Keywords: diabetes; gastroparesis; dysmotility; gastric emptying; electric stimulation.
Gastroparesis is a chronic dysmotility disorder characterized by delayed gastric emptying with associated symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Medical treatments for gastroparesis include dietary modifications, glucose control in those with diabetes, prokinetic medications, antiemetic medications, and symptom modulators, but unfortunately patients frequently do not respond to these treatments. In patients refractory to medical therapy, surgical treatments can be considered.
Gastric electric stimulation (GES; Enterra [Medtronic, Minneapolis, MN]) was approved via a Food and Drug Administration (FDA) Humanitarian Use Device (HUD) exemption for the treatment of medically refractory gastroparesis in 2000. Understanding the indications, risks, outcomes, and alternatives to GES is essential to providing appropriate care for patients with medically refractory gastroparesis. This article outlines the use and utility of GES as a therapeutic intervention for gastroparesis.
Types of Gastroparesis
Gastroparesis is a chronic symptomatic disorder of the stomach manifested by delayed gastric emptying without evidence of gastric outlet obstruction or ulceration.1 The pathophysiology of gastroparesis appears to involve abnormalities in functioning of several elements including the autonomic nervous system, especially the vagus nerve, smooth muscle cells, enteric neurons, and interstitial cells of Cajal.
Idiopathic gastroparesis and diabetic gastroparesis are the 2 most common types of gastroparesis.2 Symptomatic delayed gastric emptying with no primary underlying abnormality predisposing to gastroparesis is categorized as idiopathic gastroparesis.3 A small subset of patients with idiopathic gastroparesis report an initial infectious prodrome such as gastroenteritis or respiratory infection. It has been suggested that this postinfectious gastroparesis results from viral injury to the neural innervation of the stomach or the interstitial cells of Cajal in the stomach.4 Viruses that have been implicated in the development of gastroparesis include cytomegalovirus, Epstein-Barr virus, Norwalk virus, rotavirus, herpes zoster, and varicella zoster.5-9
Diabetic gastroparesis is characterized as onset of symptoms of gastroparesis in patients with diabetes, with concomitant delayed gastric emptying. It is often attributed to chronic hyperglycemia-induced damage to the vagus nerve, and is frequently observed in association with other diabetic complications such as neuropathy, retinopathy, and nephropathy.10
Gastroparesis that develops following surgery is classified as postsurgical gastroparesis. In the past, this form of gastroparesis most commonly occurred after ulcer surgery, often performed with vagotomy. These types of surgeries are performed less frequently in the era of proton pump inhibitor therapy and treatments for Helicobacter pylori. Presently, Nissen fundoplication and bariatric surgery are the more common surgical procedures associated with gastroparesis.3 Long-term use of medications that delay gastric emptying, such as opiate narcotic medications, can lead to gastroparesis and represent another form of iatrogenic gastroparesis. Other forms of gastroparesis (atypical gastroparesis) arise due to various underlying etiologies, including neurological disorders (eg, Parkinson disease, multiple sclerosis), metabolic or endocrine conditions (eg, hypothyroidism), autoimmune disorders, connective tissue and collagen vascular disorders (eg, systemic lupus erythematosus, scleroderma, Sjögren syndrome, Ehlers-Danlos syndrome), or eating disorders (eg, anorexia, bulimia).3
Epidemiology
There is a female preponderance in patients with gastroparesis. Data from the Rochester Epidemiology Project, a database of linked medical records for residents of Olmsted County, MN, showed that the age-adjusted prevalence of definite gastroparesis per 100,000 inhabitants was 37.8 for women and 9.6 for men.11 More recent estimates have suggested a much higher prevalence of probable gastroparesis (approximately 1.8%) in the general population using symptoms suggestive of gastroparesis.12 Hospitalization rates for gastroparesis have increased since 2000, which could reflect rising prevalence and/or the effects of heightened awareness about and better identification of gastroparesis.13 This increase may also be due in part to the rising rate of diabetes leading to more cases of diabetic gastroparesis; withdrawal of some gastroparesis treatments from the market (cisapride, tegaserod) leading to hospitalizations for symptoms not adequately being treated; and hospitalizations needed for insertion of the gastric electric stimulator.
Gastroparesis Symptoms
The main symptoms of gastroparesis are early satiety, postprandial fullness, bloating, nausea, and vomiting.14 Nausea (> 90% of patients) and early satiety (60% of patients) are the most common symptoms.15 Abdominal pain is often present in patients with gastroparesis but is usually not the predominant symptom. The pain can be multifactorial, with somatic, visceral, and neuropathic components.16-18 Moderate to severe abdominal pain has been found more often in patients with idiopathic gastroparesis and in association with opiate use.16 Symptoms of gastroparesis may be persistent or present as episodic flares. Due to the symptoms, some patients will experience weight loss and malnutrition and, in severe cases, dehydration.19
Although the definition of gastroparesis is a delay in gastric emptying along with symptoms, symptoms correlate poorly with the degree of delayed gastric emptying. The symptoms that appear to have the strongest correlation with gastric emptying are nausea, vomiting, early satiety, and postprandial fullness, whereas symptoms such as abdominal pain and bloating have little correlation. Furthermore, improving gastric emptying does not necessarily lead to improved symptoms, and symptom improvement does not always lead to improved gastric emptying times.20 Between 5% and 12% of patients with diabetes report symptoms consistent with gastroparesis, though many of these patients have normal gastric emptying. The symptoms of gastroparesis overlap with those of functional dyspepsia, as both may have motor and sensory alterations.21
The Gastroparesis Cardinal Symptom Index (GCSI), a subset of the Patient Assessment of Gastrointestinal Disorders Symptom Severity Index (PAGI-SYM), is a questionnaire that is commonly used to establish symptom severity in patients with gastroparesis. It is comprised of 3 subscales—nausea and vomiting, postprandial fullness and early satiety, and bloating—which are averaged to provide a total GCSI score. Symptoms over the 2 weeks prior to administration of the questionnaire are assessed and rated from 0 (none) to 5 (very severe).22 Grading the severity of gastroparesis may take into account symptoms, quality of life, and gastric emptying. One commonly used grading system assigns a grade from 1 to 3, with grade 1 being mild gastroparesis, grade 2 being compensated gastroparesis, and grade 3 being gastric failure with refractory symptoms that are uncontrolled.18,23 Quality-of-life surveys also suggest that gastroparesis independent of other factors leads to a worse quality of life.24
Indications for GES
Gastric electric stimulator implantation is a surgical procedure with inherent risks and complications and is reserved for patients with intractable symptoms of gastroparesis who remain symptomatic despite treatment attempts with dietary management, antiemetic agents (eg, compazine, phenergan, and ondansetron), and prokinetic agents (eg, metoclopramide, erythromycin, and domperidone). Symptom modulators such as nortriptyline and mirtazapine are occasionally tried.
Surgical intervention can be considered upon failure of medical treatment measures. At least a year of documented care provided by a physician specializing in gastroparesis is suggested for surgical consideration. The gastric electric neurostimulator is approved by the FDA as a HUD for the care of patients with idiopathic and diabetic gastroparesis, performed on a compassionate basis. GES implantation requires Institutional Review Board approval at the institution, and patients are required to have documented delayed gastric emptying.
It is important to remember that the GES device is incompatible with magnetic resonance imaging (MRI) and explantation of the device is necessary prior to MRI. As such, in patients with anticipated need of frequent MRI, such as those with multiple sclerosis, serious consideration should be given to alternative strategies prior to focusing on this modality.
Device Placement
GES was devised to improve gastric emptying. The Enterra GES system uses high-frequency, low-energy electric stimulation. An alternative method is true gastric pacing that uses high-energy, low-frequency stimulation to entrain the gastric slow waves and subsequent contractions at 3 cycles per minute (cpm). Gastric pacing has greater energy requirements than GES, which makes the size of the stimulator too large to be practical. In pilot animal studies, GES produced an accelerating effect on gastric emptying, but in human studies GES had an inconsistent effect on gastric emptying. Studies have suggested that GES influences the proximal stomach, with a reduction of gastric tone,25 and also that GES has an afferent modulatory mechanism.26
The Enterra GES is placed surgically under general anesthesia, commonly via laparotomy or minimal access surgical techniques (laparoscopically or robotically assisted). Preoperative intravenous antibiotics are given. The system consists of a pair of electrodes connected to a pulse generator. The 2 stimulation leads are inserted into the gastric muscularis propria 1 cm apart along the greater curvature 10 cm proximal to the pylorus. Upper endoscopy is performed to ensure that the leads do not penetrate through the mucosa into the stomach lumen; if this occurs, repositioning of the lead is necessary. A horizontal incision through the skin is made, and the distal ends of the stimulating wires are tunneled through the abdominal wall and connected to the pulse generator. The impedance (resistance) between the wires is measured to ensure the appropriate range (200-800 Ohms). The neurostimulator with the distal ends of the stimulating wires is then placed into the subcutaneous pocket and sutured to the underlying fascia. The pulse generator delivers a high-frequency, low-energy, 0.1-second train of pulses at a frequency of 12 cpm. Within each pulse train, individual pulses oscillate at a frequency of 14 cycles per second. The voltage of the stimulations is set to provide a current of 5 milliamps (mA; remembering that voltage = current × resistance).
Patients are often hospitalized with a recovery time of 1 to 3 days. Immediate postoperative care usually includes intravenous fluids, controlling any postoperative ileus, advancing diet, and providing analgesic pain medications. Hospital length of stay can be impacted by surgical technique.25 Patients are seen several weeks after discharge for assessment of the incision and toleration of diet. Medications for gastroparesis that patients were taking prior to the GES implantation are usually continued postoperatively, with a goal of reducing these medications over time. Patients are then followed every 3 to 12 months, depending on their clinical condition.
At follow-up visits, medications are reviewed and new treatments can be added if appropriate. The gastric stimulator is interrogated to determine if changes in resistance occurred; if necessary, minor readjustments can be made to keep the current at desired levels (5 mA). For persistent symptoms with GES treatment, the stimulator parameters can be adjusted after 3 months of follow up, typically first increasing the current from 5 to 7.5 mA and then to 10 mA. After this, the frequency can be increased from 14 Hz to 28 Hz, and then to 55 Hz. Rarely, the ON duration is increased from 0.1 to 1 second. Increasing the ON time can worsen symptoms in some patients, cause abdominal pain, and decrease the battery life from the usual 7 years.
Complications of GES
In an analysis of the Manufacturer and User Facility Device Experience (MAUDE) databank, Bielefeldt identified 1587 reports of adverse effects related to the gastric electric stimulator from January 2001 to October 2015.27 The most common adverse effects are reviewed here.
Skin erosion/wound dehiscence is one of the most common reported complications; it may be related to superficial placement or inadequate securing of the device to the fascia. Abscess can develop postoperatively due to hematogenous seeding or may be a sign of lead erosion into the lumen, tracking along the leads into subcutaneous tissue.28 It is important to warn patients to protect the area over the device from needle injections as this also can lead to hematoma formation and direct contamination of the device. If the device gets infected, it cannot be salvaged and requires explantation. Implantation of a new device can be attempted once all wound issues resolve.
Device migration/flipping most often occurs because the device is inadequately fixed to the underlying fascia, but occasionally it can occur from patients flipping the device around. Flipping can occur due to superficial pocket location within subcutaneous tissue, especially in obese patients. Migration/flipping can lead to prominence of the contour of the device and discomfort, ultimately requiring surgical correction.

Perforation and erosion of the leads. With time, leads can erode into the stomach, although this is rare. Usually erosion is associated with loss of device function. Endoscopy confirms this finding. In rare cases, infection can track proximally along the lead and present as a surgical site infection at the pulse generator. This complication often requires explantation of the neurostimulator leads and pulse generator.

Intestinal obstruction. Although rare, the intestines can get wrapped around the leads of the device, causing different degrees of obstruction (Figure 1). Positioning the device in the left upper quadrant minimizes the intraabdominal length of the leads and pulls them maximally out, coiling under the device (Figure 2). In cases where other locations are used either due to a hostile upper abdominal region (skin infection, presence of gastrostomy or other devices) or surgeon’s preference, the GES device can be implanted in the lower abdomen (Figure 3). In these circumstances, carefully draping the omentum over the bowels might help to prevent this complication. Tacking of the leads to the parietal peritoneum with sutures can also be preventative. In cases of obstruction requiring intervention by laparotomy or minimal access techniques (laparoscopy or robotic assisted surgery), all efforts are made to preserve the neurostimulator leads. In cases that require bowel resection, lead contamination is a serious concern, but lead explantation is not mandatory. Close postoperative monitoring for the development of lead infection is required.

Hematoma and seroma. Postoperative hematomas can occur from inadequate hemostasis, and seromas can occur in the stimulator pocket. Small hematomas may be observed if not complicated (Figure 4). In cases of large hematomas with skin compromise or dehiscence, prompt washout and drainage is required. In ideal cases, the device can be preserved. Relocation to another site might be required if skin necrosis develops. The possibility of device contamination also must be considered; after resolution of wound issues, implantation of a new device may be tried. Seromas at the generator pocket site are a frequent occurrence but are often benign, self-limiting, and generally resolve over 4 to 6 weeks.

Incisional hernia. Hernias can develop after any abdominal surgery and are not unique to GES implantation. Use of minimally invasive technique for the GES implantation minimizes this complication.
Electric shock sensations may occur from breakage of the plastic lining covering the stimulator wires or from fluid buildup around the insertion of the wires into the stimulator. Shocks can also occur due to shortening of the leads on the muscles of the abdominal wall. Patients describe periodic muscle cramps with the frequency of the device (every 5 seconds). To prevent this complication, freshly implanted leads should be covered by an omental flap to isolate them from the abdominal wall. In patients who continue to feel shocks despite all efforts, the possibility of visceral hypersensitivity should be considered. A trial of symptom modulators such as nortriptyline and lowering of the output amperage below the minimal recommended setting of 5 mA can be undertaken. If these interventions do not work, the device must be turned off for a period of time. Occasionally, replacement of the leads or explantation of the device must be considered.
Lack of effect/persistent symptoms. If a patient presents with lack of improvement after device implantation, a thorough workup should be undertaken to ensure that the device is functioning properly. In the case of abnormal impedance values, an abdominal x-ray study can be performed to rule out lead migration (Figure 5). If no abnormalities are detected, the output of the device can be increased. After adjusting device settings, the patient should be assessed for improvement over at least a 1- to 3-month period. One report suggests that in patients not responding to GES, repositioning the location of the stimulator leads on the stomach can be helpful.29

Outcomes of GES
Study results of investigative GES models in animals and select patients were published in 1997.30,31 Following these reports, 2 large multicenter studies were conducted to demonstrate the efficacy of GES for the treatment of refractory gastroparesis. The Gastric Electrical Mechanical Stimulation Study (GEMS) was an open-label, multicenter study of 38 patients who received percutaneous and later permanent GES devices.32 Marked reduction in weekly vomiting and nausea was observed at 4 weeks, with a 90% reduction in nausea and vomiting frequency at 11 months. Following this, a second multicenter study (Worldwide Anti-Vomiting Electrical Stimulation Study [WAVES]) involving a double-blind sham stimulation controlled trial with 33 idiopathic and diabetic gastroparesis patients was performed.33 During the blinded portion of this study, there was a noticeable decrease in vomiting frequency, particularly in the patients with diabetic gastroparesis. Patient preference was for the stimulator ON as compared to OFF. The FDA’s HUD exemption for the Enterra GES device in 2000 was based on these studies.
Four independent double-blind studies of GES have been conducted (Table 1).33-37 It has been difficult to demonstrate improvement during the double-blind period with gastric stimulation compared to no stimulation. Despite total symptom severity improvement and individual symptom improvements in these studies, a recent meta-analysis demonstrated a summative insignificant difference between the GES ON versus OFF states.38

In contrast to the double-blind studies, numerous open-label studies have demonstrated clinical improvements in patients with diabetic and idiopathic gastroparesis (Table 2),32,33,35,36,39-55 leading some to question whether the demonstrable efficacy reflects a placebo effect or regression to the mean. Patients may perceive an operative, aggressive intervention as likely to be effectual in comparison to incremental medication efforts, thus creating a placebo effect. It should also be noted that not all open-label studies have demonstrated improvement with GES. Indeed, Jones et al reported no significant difference in nausea and vomiting at 6-month follow-up, and recommended that physicians exercise caution with GES as a therapeutic strategy given the cost and lack of confirmed demonstrable effect.56 Thus, the clinical successes demonstrated in open-label studies must be weighed not only against the lack of unequivocal improvement, but also against the potential deleterious effects of the surgery.

In an open-label study that employed the GCSI to follow symptoms of gastroparesis, 29 patients underwent GES implantation over an 18-month period, with follow-up in 28 patients.44 GES resulted in clinical improvement in 50% of patients with refractory gastroparesis. The overall GCSI significantly decreased, with improvement in the nausea/vomiting subscore and the post-prandial fullness subscore, but no improvement in the bloating subscore or abdominal pain. The decrease in GCSI was greater for patients with diabetic versus idiopathic gastroparesis. Patients with the main symptom of nausea/vomiting had a greater improvement than patients with the main symptom of abdominal pain. Patients taking narcotic analgesics at the time of implant had a poorer response compared to patients who were not. In this study, 3 clinical parameters were associated with a favorable clinical response: (1) diabetic rather than idiopathic gastroparesis, (2) nausea/vomiting rather than abdominal pain as the primary symptom, and (3) independence from narcotic analgesics prior to stimulator implantation. Knowledge of these 3 factors may allow improved patient selection for GES.
A large prospective study by Heckert et al detailed marked improvements with GES and the patterns of those improvements.55 Nausea, vomiting, loss of appetite, and early satiety improved significantly with stimulator use, with a greater improvement in vomiting in patients with diabetic gastroparesis than in those with the idiopathic form. Although GES improved symptoms in 75% of all patients, patients with diabetes had a post-GES Clinical Patient Grading Assessment score that was statistically higher than the score among patients with idiopathic gastroparesis. This difference is thought to be due to the neuromolecular mechanism of diabetic gastroparesis, where blunting of the enteric nervous system may contribute to symptomatology.
Several studies have demonstrated a clinical response to GES in patients with postsurgical gastroparesis. A study by Oubre et al showed that GES led to weekly vomiting improvements as well as a reduction in total symptom severity score.57 A study by McCallum et al further demonstrated improved symptoms, quality of life, nutritional status, and hospitalization requirements.58 GES has also been shown to improve gastroparesis symptoms in pediatric populations.47,59 Thus, although not a direct indication, GES has been shown to be beneficial in various subtypes of gastroparesis.
Additionally, irrespective of gastroparesis type, the improved symptomatology with GES appears to be durable, with one study showing persistent clinical improvements up to 8 years after device placement.60 The improvements were persistent and incremental. Likewise, McCallum et al showed that continued reductions in total symptom severity scores were evident in all gastroparesis types up to 10 years after stimulator implantation.61 The success of the procedures in part comes from careful selection of patients. Clinical parameters that are associated with favorable clinical response include diabetic gastroparesis subtype, nausea/vomiting predominance, and independence from narcotic analgesics prior to stimulator placement.62
GES has also been noted to improve other patient care metrics besides symptomatology, including nutritional status, reduced need for nutritional supplementation, and improved HbA1c.63-65 Additionally, a study by Cutts et al established that health care resource utilization significantly improved at 12, 24, and 36 months following GES placement, as compared to patients receiving standard medical therapy.66 This decreased resource utilization was also reflected in decreased costs in the GES group compared with the standard care group.
Surgical Alternatives to GES
Pyloric interventions such as pyloroplasty and pyloromyotomy are other surgical treatment modalities offered for gastroparesis. Whereas GES uses neurostimulation to facilitate gastric emptying and potentially improve fundic accommodation, pyloric interventions are intended to increase gastric emptying by reducing outflow resistance from the pyloric sphincter.
Pyloric Interventions
Various studies have shown significant improvements with pyloric interventions, similar to the improvements seen with GES. One such study involving 177 patients demonstrated an 86% improvement in gastric emptying, with symptom severity scores for nausea, vomiting, bloating, abdominal pain, and early satiety decreasing significantly at 3 months following pyloroplasty.67 A significant advantage of pyloric interventions is that pyloromyotomy can be performed endoscopically (gastric peroral endoscopic pyloromyotomy [G-POEM] or peroral pyloromyotomy [POP]), thus minimizing the risks of open surgery. A recent review that included a pooled analysis of 7 studies of G-POEM for gastroparesis demonstrated 100% technical success, with clinical efficacy in 81.5% of the procedures as assessed by the GCSI.68 Additionally, the intraoperative and perioperative complication rates were 6.6% and 7.6%, respectively, suggesting that G-POEM is a safe and clinically beneficial therapeutic option. Few studies comparing the outcomes of pyloric interventions to GES have been performed.
Recently, GES has been combined with pyloric interventions to maximize therapeutic potential. This allows simultaneous neurologic and functional interventions to expedite gastric emptying and improve patient symptomatology. Davis et al demonstrated significant improvement in 21 patients who underwent GES placement and pyloroplasty, with 71% improvement in total symptom severity.69 Notably, dual surgery did not increase the incidence of infection or adverse surgical outcomes. Although this study did not directly compare dual surgery to GES alone, the results are nonetheless favorable. GES provides a strong antiemetic and anti-nausea effect, whereas the pyloromyotomy provides improvement in gastric emptying.
Feeding/Venting Tubes
Feeding jejunostomy tubes and venting gastrostomy tubes can be used alone or in combination with GES. Feeding jejunostomy is performed for malnutrition and weight loss that accompanies the refractory symptoms of early satiety, nausea, and vomiting. Venting gastrostomy tubes allow for removal of retained gastric contents that may cause distension, nausea, and vomiting. Gastrojejunostomy tubes can also be placed endoscopically or by interventional radiology.
Gastrectomy
Gastrectomy can provide therapeutic benefit through elimination of the gastric reservoir function and consequent removal of afferent neural impulses. In select patient populations, outcomes of gastrectomy have compared favorably with those of GES. For example, one study demonstrated favorable outcomes of Roux-en-Y gastrectomy in morbidly obese patients with gastroparesis.70 In another study, favorable outcomes were reported in a cohort of 103 patients, with gastrectomy demonstrating 87% symptom improvement (nausea, vomiting, epigastric pain) compared to just 63% improvement with GES.71 However, the dramatic impact on anatomy and physiology and the invasiveness of the procedure need to be weighed against the therapeutic benefit. For example, in the same study, the 30-day morbidity was 23% for gastrectomy versus just 8% for the GES implant.71
When to Use GES
The gastric electrical neurostimulator (Enterra; Medtronic, Inc.) is approved for treatment of idiopathic and diabetic gastroparesis that is refractory to medical treatment, performed on a compassionate basis. Patients with diabetic gastroparesis respond to GES better than do patients with the idiopathic form. Of the symptoms of gastroparesis, primarily nausea and vomiting improve. Thus, GES favors patients with diabetic gastroparesis who have primarily nausea and vomiting, rather than, for instance, patients with idiopathic gastroparesis who have primarily abdominal pain and may be taking narcotics. Some centers provide GES for postsurgical patients and children with gastroparesis.
The 3 main surgical interventions for medically refractory gastroparesis are GES, pyloric intervention (pyloroplasty or pyloromyotomy), and gastrectomy. Of the 3 interventions, gastrectomy is the most radical given its dramatic effect on anatomy and is thus not preferred. The clinical decision then becomes: GES, pyloric intervention, or both? There are limited data to support a definitive answer to this question.
In a single-center retrospective analysis of prospective data (electronic medical record), Arthur et al compared outcomes of GES patients with medically refractory gastroparesis who received various surgical interventions.72 In total, 33 stimulator, 7 pyloroplasty, 2 gastrectomy, and 16 combined stimulator and pyloroplasty patients were analyzed for postoperative symptom improvement. Pyloroplasty alone demonstrated the least symptom improvement, combination GES and pyloroplasty demonstrated increased improvement, and GES alone demonstrated the most improvement. The results of this study suggest that barring contraindication, placement of a gastric stimulator as the initial treatment is best, with pyloroplasty reserved for patients who do not achieve adequate symptom control. Limitations of the study include its single-center design and low patient numbers for pyloroplasty in isolation.
In contrast, a recent retrospective systematic review synthesized the outcomes of various studies of GES and pyloric interventions for medically refractory gastroparesis.73 A therapeutic effect was found for each surgical intervention, with pyloric surgery patients demonstrating a greater response to intervention than GES patients. Unfortunately, attempts to analyze combination interventions were hindered by a lack of power.
Conclusion
Initial management of gastroparesis is medical (lifestyle and diet changes), with antiemetic and prokinetic agents used in refractory cases. Following failure of this therapy, placement of a GES device is a surgical intervention that has been approved under FDA humanitarian device exemption to help ameliorate symptomatology. Improvement with GES has been demonstrated in nonblinded studies, but the lack of randomized controlled trials demonstrating benefit suggests the possibility of an underlying placebo effect. Additionally, new medical procedures such as G-POEM complicate the decision of which intervention should be attempted first. More studies, specifically comparing GES, pyloric interventions, and combined GES with pyloric intervention to placebo, are needed to fully understand what therapy is best for refractory gastroparesis.
Corresponding author: Henry P. Parkman, MD, Gastroenterology Section, Temple University School of Medicine, 3401 North Broad Street, Philadelphia, PA 19140; [email protected].
Financial disclosures: None.
1. Camilleri M, Parkman HP, Shafi MA, et al. Clinical Guideline: Management of gastroparesis. Am J Gastroenterol. 2013;108:18-37.
2. Jehangir A, Parkman HP. Rome IV Diagnostic Questionnaire Complements Patient Assessment of Gastrointestinal Symptoms for Patients with Gastroparesis Symptoms. Dig Dis Sci. 2018;63:2231-2243.
3. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622.
4. Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101-115.
5. Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501-1504.
6. Kebede D, Barthel JS, Singh A. Transient gastroparesis associated with cutaneous herpes zoster. Dig Dis Sci. 1987;32:318-322.
7. Meeroff JC, Schreiber DS, Trier JS, Blacklow NR. Abnormal gastric motor function in viral gastroenteritis. Ann Intern Med. 1980;92:370-373.
8. Paliwal M, Prasanna KS, Saraswat VA, et al. Varicella zoster cranial polyneuropathy presenting with dysphagia, esophagitis and gastroparesis. J Neurogastroenterol Motil. 2011;17:192-194.
9. Sigurdsson L, Flores A, Putnam PE, et al. Postviral gastroparesis: presentation, treatment, and outcome. J Pediatr. 1997;131:751-754.
10. Kockar MC, Kayahan IK, Bavbek N. Diabetic gastroparesis in association with autonomic neuropathy and microvasculopathy. Acta Med Okayama. 2002;56:237-243.
11. Jung HK, Choung RS, Locke GR III, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225-1233.
12. Rey E, Choung RS, Schleck CD, et al. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18:34-42.
13. Wang YR, Fisher RS. Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol. 2008;103:313-322.
14. Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22:113-133.
15. Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398-2404.
16. Cherian D, Sachdeva P, Fisher RS, Parkman HP. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol. 2010;8:676-681.
17. Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil. 2013;25:427-438.
18. Jehangir A, Abdallah RT, Parkman HP. Characterizing abdominal pain in patients with gastroparesis into neuropathic and nociceptive components. J Clin Gastroenterol. 2018 May 18. doi: 10.1097/MCG.0000000000001059.
19. Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am. 2015;44:1-7.
20. Fosso CL, Quigley EMM. A critical review of the current clinical landscape of gastroparesis. Gastroenterol Hepatol. 2018;14:140-145.
21. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972-1978.
22. Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670-680.
23. Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil. 2012;24:456-463.
24. Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44:9-19.
25. Soffer E, Abell T, Lin Z, et al. Review article: Gastric electrical stimulation for gastroparesis – physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30:681-694.
26. Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on rat thoracic spinal neurons receiving input from the stomach. Neurosci Res. 2007;57:29-39
27. Bielefeldt K. Adverse events of gastric electrical stimulators recorded in the Manufacturer and User Device Experience (MAUDE) Registry. Auton Neurosci. 2017;202:40-44
28. Liu RC, Sabnis AA, Chand B. Erosion of gastric electrical stimulator electrodes: evaluation, management, and laparoscopic techniques. Surg Laparosc Endosc Percutan Tech. 2007;17:438-441.
29. Harrison NS, Williams PA, Walker MR, et al. Evaluation and treatment of gastric stimulator failure in patients with gastroparesis. Surg Innov. 2014;21:244-249.
30. Familoni BO, Abell TL, Nemoto D, et al. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci. 1997;42:885-891.
31. Familoni BO, Abell TL, Nemoto D, et al. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892-897.
32. Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204-212.
33. Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421-428.
34. Frøkjaer JB, Ejskjaer N, Rask P, et al. Central neuronal mechanisms of gastric electrical stimulation in diabetic gastroparesis. Scand J Gastroenterol. 2008;43:1066-1075.
35. McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25:815-836.
36. McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947-954.
37. Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496-503.
38. Levinthal DJ. Systematic review and meta-analysis: Gastric electrical stimulation for gastroparesis. Auton Neurosci. 2017;202:45-55.
39. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
40. Mason RJ, Lipham J, Eckerling G, et al. Gastric electrical stimulation: An alternative surgical therapy for patients with gastroparesis. Arch Surg. 2005;140:841-846.
41. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
42. van der Voort IR, Becker JC, Dietl KH, et al. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diabetes. 2005;113:38-42.
43. de Csepel J, Goldfarb B, Shapsis A, et al. Electrical stimulation for gastroparesis. gastric motility restored. Surg Endosc. 2006;20:302-306.
44. Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072-2078.
45. Filichia LA, Cendan CJ. Small case series of gastric stimulation for the management of transplant-induced gastroparesis. J Surg Res. 2008;148:90-93.
46. Lin Z, Hou Q, Sarosiek I, et al. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil. 2008;20:464-470.
47. Islam S, Vick LR, Runnels MJ, et al. Gastric electrical stimulation for children with intractable nausea and gastroparesis. J Pediatr Surg. 2008;43:437-442.
48. Brody F, Vaziri K, Saddler A, et al. Gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2008;207:533-538.
49. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
50. Teich S, Mousa HM, Punati J, Di Lorenzo C. Efficacy of permanent gastric electrical stimulation for the treatment of gastroparesis and functional dyspepsia in children and adolescents. J Pediatr Surg. 2013;48:178-183.
51. Lahr CJ, Griffith J, Subramony C, et al. Gastric electrical stimulation for abdominal pain in patients with symptoms of gastroparesis. Am Surg. 2013;79:457-464.
52. Keller DS, Parkman HP, Boucek DO, et al. Surgical outcomes after gastric electric stimulator placement for refractory gastroparesis. J Gastrointest Surg. 2013;17:620-626.
53. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
54. Richmond B, Chong B, Modak A, et al. Gastric electrical stimulation for refractory gastroparesis: Predictors of response and redefining a successful outcome. Am Surg. 2015;81:467-471.
55. Heckert J, Sankineni A, Hughes WB, et al. Gastric electric stimulation for refractory gastroparesis: A prospective analysis of 151 patients at a single center. Dig Dis Sci. 2016;61:168-175.
56. Jones MP, Ebert CC, Murayama K. Enterra for gastroparesis. Am J Gastroenterol. 2003;98:2578.
57. Oubre B, Luo J, Al-Juburi A, et al. Pilot study on gastric electrical stimulation on surgery-associated gastroparesis: Long-term outcome. South Med J. 2005;98:693-697.
58. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
59. Islam S, McLaughlin J, Pierson J, et al. Long-term outcomes of gastric electrical stimulation in children with gastroparesis. J Pediatr Surg. 2016;51:67-71.
60. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
61. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
62. Maranki J, Lytes V, Meilahn JE, et al. Dig Dis Sci. 2008 53:2072-2078.
63. Abell T, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27:277-281.
64. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
65. Lin Z, McElhinney C, Sarosiek I, et al. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50:1328-1334.
66. Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35-43.
67. Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc. 2016;30:1326-1332.
68. Khoury T, Mizrahi M, Mahamid M, et al. State of the art review with literature summary on gastric peroral endoscopic pyloromyotomy for gastroparesis. J Gastroenterol Hepatol. 2018;33:1829-1833.
69. Davis BR, Sarosiek I, Bashashati M, et al. The long-term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg. 2017;21:222-227.
70. Sun Z, Rodriguez J, McMichael J, et al. Surgical treatment of medically refractory gastroparesis in the morbidly obese. Surg Endosc. 2015;29:2683-2689.
71. Zehetner J, Ravari F, Ayazi S, et al. Minimally invasive surgical approach for the treatment of gastroparesis. Surg Endosc. 2013;27:61-66.
72. Arthur LE, Slattery L, Richardson W. Tailored approach to gastroparesis significantly improves symptoms. Surg Endosc. 2017;32:977-982.
73. Zoll B, Zhao H, Edwards MA, et al. Outcomes of surgical intervention for refractory gastroparesis: A systematic review. J Surg Res. 2018;231:263-269.
1. Camilleri M, Parkman HP, Shafi MA, et al. Clinical Guideline: Management of gastroparesis. Am J Gastroenterol. 2013;108:18-37.
2. Jehangir A, Parkman HP. Rome IV Diagnostic Questionnaire Complements Patient Assessment of Gastrointestinal Symptoms for Patients with Gastroparesis Symptoms. Dig Dis Sci. 2018;63:2231-2243.
3. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622.
4. Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101-115.
5. Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501-1504.
6. Kebede D, Barthel JS, Singh A. Transient gastroparesis associated with cutaneous herpes zoster. Dig Dis Sci. 1987;32:318-322.
7. Meeroff JC, Schreiber DS, Trier JS, Blacklow NR. Abnormal gastric motor function in viral gastroenteritis. Ann Intern Med. 1980;92:370-373.
8. Paliwal M, Prasanna KS, Saraswat VA, et al. Varicella zoster cranial polyneuropathy presenting with dysphagia, esophagitis and gastroparesis. J Neurogastroenterol Motil. 2011;17:192-194.
9. Sigurdsson L, Flores A, Putnam PE, et al. Postviral gastroparesis: presentation, treatment, and outcome. J Pediatr. 1997;131:751-754.
10. Kockar MC, Kayahan IK, Bavbek N. Diabetic gastroparesis in association with autonomic neuropathy and microvasculopathy. Acta Med Okayama. 2002;56:237-243.
11. Jung HK, Choung RS, Locke GR III, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225-1233.
12. Rey E, Choung RS, Schleck CD, et al. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18:34-42.
13. Wang YR, Fisher RS. Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol. 2008;103:313-322.
14. Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22:113-133.
15. Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398-2404.
16. Cherian D, Sachdeva P, Fisher RS, Parkman HP. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol. 2010;8:676-681.
17. Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil. 2013;25:427-438.
18. Jehangir A, Abdallah RT, Parkman HP. Characterizing abdominal pain in patients with gastroparesis into neuropathic and nociceptive components. J Clin Gastroenterol. 2018 May 18. doi: 10.1097/MCG.0000000000001059.
19. Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am. 2015;44:1-7.
20. Fosso CL, Quigley EMM. A critical review of the current clinical landscape of gastroparesis. Gastroenterol Hepatol. 2018;14:140-145.
21. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972-1978.
22. Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670-680.
23. Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil. 2012;24:456-463.
24. Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44:9-19.
25. Soffer E, Abell T, Lin Z, et al. Review article: Gastric electrical stimulation for gastroparesis – physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30:681-694.
26. Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on rat thoracic spinal neurons receiving input from the stomach. Neurosci Res. 2007;57:29-39
27. Bielefeldt K. Adverse events of gastric electrical stimulators recorded in the Manufacturer and User Device Experience (MAUDE) Registry. Auton Neurosci. 2017;202:40-44
28. Liu RC, Sabnis AA, Chand B. Erosion of gastric electrical stimulator electrodes: evaluation, management, and laparoscopic techniques. Surg Laparosc Endosc Percutan Tech. 2007;17:438-441.
29. Harrison NS, Williams PA, Walker MR, et al. Evaluation and treatment of gastric stimulator failure in patients with gastroparesis. Surg Innov. 2014;21:244-249.
30. Familoni BO, Abell TL, Nemoto D, et al. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci. 1997;42:885-891.
31. Familoni BO, Abell TL, Nemoto D, et al. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892-897.
32. Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204-212.
33. Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421-428.
34. Frøkjaer JB, Ejskjaer N, Rask P, et al. Central neuronal mechanisms of gastric electrical stimulation in diabetic gastroparesis. Scand J Gastroenterol. 2008;43:1066-1075.
35. McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25:815-836.
36. McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947-954.
37. Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496-503.
38. Levinthal DJ. Systematic review and meta-analysis: Gastric electrical stimulation for gastroparesis. Auton Neurosci. 2017;202:45-55.
39. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
40. Mason RJ, Lipham J, Eckerling G, et al. Gastric electrical stimulation: An alternative surgical therapy for patients with gastroparesis. Arch Surg. 2005;140:841-846.
41. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
42. van der Voort IR, Becker JC, Dietl KH, et al. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diabetes. 2005;113:38-42.
43. de Csepel J, Goldfarb B, Shapsis A, et al. Electrical stimulation for gastroparesis. gastric motility restored. Surg Endosc. 2006;20:302-306.
44. Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072-2078.
45. Filichia LA, Cendan CJ. Small case series of gastric stimulation for the management of transplant-induced gastroparesis. J Surg Res. 2008;148:90-93.
46. Lin Z, Hou Q, Sarosiek I, et al. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil. 2008;20:464-470.
47. Islam S, Vick LR, Runnels MJ, et al. Gastric electrical stimulation for children with intractable nausea and gastroparesis. J Pediatr Surg. 2008;43:437-442.
48. Brody F, Vaziri K, Saddler A, et al. Gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2008;207:533-538.
49. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
50. Teich S, Mousa HM, Punati J, Di Lorenzo C. Efficacy of permanent gastric electrical stimulation for the treatment of gastroparesis and functional dyspepsia in children and adolescents. J Pediatr Surg. 2013;48:178-183.
51. Lahr CJ, Griffith J, Subramony C, et al. Gastric electrical stimulation for abdominal pain in patients with symptoms of gastroparesis. Am Surg. 2013;79:457-464.
52. Keller DS, Parkman HP, Boucek DO, et al. Surgical outcomes after gastric electric stimulator placement for refractory gastroparesis. J Gastrointest Surg. 2013;17:620-626.
53. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
54. Richmond B, Chong B, Modak A, et al. Gastric electrical stimulation for refractory gastroparesis: Predictors of response and redefining a successful outcome. Am Surg. 2015;81:467-471.
55. Heckert J, Sankineni A, Hughes WB, et al. Gastric electric stimulation for refractory gastroparesis: A prospective analysis of 151 patients at a single center. Dig Dis Sci. 2016;61:168-175.
56. Jones MP, Ebert CC, Murayama K. Enterra for gastroparesis. Am J Gastroenterol. 2003;98:2578.
57. Oubre B, Luo J, Al-Juburi A, et al. Pilot study on gastric electrical stimulation on surgery-associated gastroparesis: Long-term outcome. South Med J. 2005;98:693-697.
58. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
59. Islam S, McLaughlin J, Pierson J, et al. Long-term outcomes of gastric electrical stimulation in children with gastroparesis. J Pediatr Surg. 2016;51:67-71.
60. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
61. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
62. Maranki J, Lytes V, Meilahn JE, et al. Dig Dis Sci. 2008 53:2072-2078.
63. Abell T, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27:277-281.
64. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
65. Lin Z, McElhinney C, Sarosiek I, et al. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50:1328-1334.
66. Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35-43.
67. Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc. 2016;30:1326-1332.
68. Khoury T, Mizrahi M, Mahamid M, et al. State of the art review with literature summary on gastric peroral endoscopic pyloromyotomy for gastroparesis. J Gastroenterol Hepatol. 2018;33:1829-1833.
69. Davis BR, Sarosiek I, Bashashati M, et al. The long-term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg. 2017;21:222-227.
70. Sun Z, Rodriguez J, McMichael J, et al. Surgical treatment of medically refractory gastroparesis in the morbidly obese. Surg Endosc. 2015;29:2683-2689.
71. Zehetner J, Ravari F, Ayazi S, et al. Minimally invasive surgical approach for the treatment of gastroparesis. Surg Endosc. 2013;27:61-66.
72. Arthur LE, Slattery L, Richardson W. Tailored approach to gastroparesis significantly improves symptoms. Surg Endosc. 2017;32:977-982.
73. Zoll B, Zhao H, Edwards MA, et al. Outcomes of surgical intervention for refractory gastroparesis: A systematic review. J Surg Res. 2018;231:263-269.