User login
There is growing evidence that gender may be associated with COVID-19 infection, presentation, and prognosis
METHODS
Study methods and definitions are available in Appendix 1 and Appendix 2, respectively, and detailed in a previous paper5 and online on the web page of the study.6
Enrolled patients were divided into two groups according to their gender, then propensity score matching (PSM) analysis was performed (1:1 nearest neighbor matching, caliper = 0.01, without replacement and maximizing execution performance). Our
Statistical analysis methods are outlined in Appendix 1.
RESULTS
Of the 2,798 patients consecutively enrolled in the HOPE registry, 1,111 were women (39.7%) and 1,687 were men (60.3%). Of the 2,375 (84.9%) patients who had a nasopharyngeal swab positive for COVID-19, 962 were women and 1,413 were men.
Baseline Characteristics and Clinical Presentation
The baseline characteristics and clinical presentation of the overall population included in the study are summarized in Appendix Table 1. In the raw population, men had a significantly higher prevalence of conventional cardiovascular risk factors, such as diabetes, dyslipidemia, and smoking history, as well as a history of lung and cardiovascular diseases. On presentation, the most common symptoms for all patients were fever, cough, and dyspnea. Fever was more common in men, whereas vomiting, diarrhea, and upper airway symptoms (eg, sore throat, hyposmia/anosmia, dysgeusia) were more common in women.
Most patients had increased values of acute phase reactants. C-reactive protein (CRP) was elevated in 90.2% and D-dimer in 64.2% of patients, both significantly more often in men. Lymphocytopenia was present in 75.4% of patients, more commonly among men. Bilateral pneumonia occurred in 69.2% of the population, more frequently in men.
After PSM analysis (Appendix Table 2), a higher prevalence of hyposmia/anosmia and gastrointestinal symptoms in women was confirmed, as well as a higher prevalence of fever in men. Laboratory tests in men still presented alterations consistent with a more severe COVID-19 infection (significantly higher CRP, troponin, transaminases, lymphocytopenia, thrombocytopenia, and ferritin). There was no significant difference in the time between onset of symptoms and hospital admission by gender (6.2 ± 7.1 days in women vs 5.9 ± 7.6 days in men; P = .472).
The main findings after PSM analysis are summarized in Appendix Figure 1 and Appendix Figure 2.
In-Hospital Management and Outcomes
The supportive and pharmacologic treatments of study patients and their outcomes are summarized in Appendix Table 3. During the in-hospital stay, men required oxygen supplementation more frequently than women. Noninvasive mechanical ventilation, invasive mechanical ventilation, and pronation were more commonly used in men. Chloroquine/hydroxychloroquine, antivirals, and antibiotics were the medications most widely used in our population (84.5%, 65.8%, and 74.4% of patients, respectively), without significant differences between male and female patients, with the exception of antibiotics, which were used more often in men (76.6% vs 71.1%). Immunomodulators (corticosteroids, tocilizumab, and interferon) were used more often in male patients.
After PSM (Table), men more frequently received immunomodulators (corticosteroids and tocilizumab), antibiotics, and pronation. No differences in invasive and noninvasive mechanical ventilation were observed.
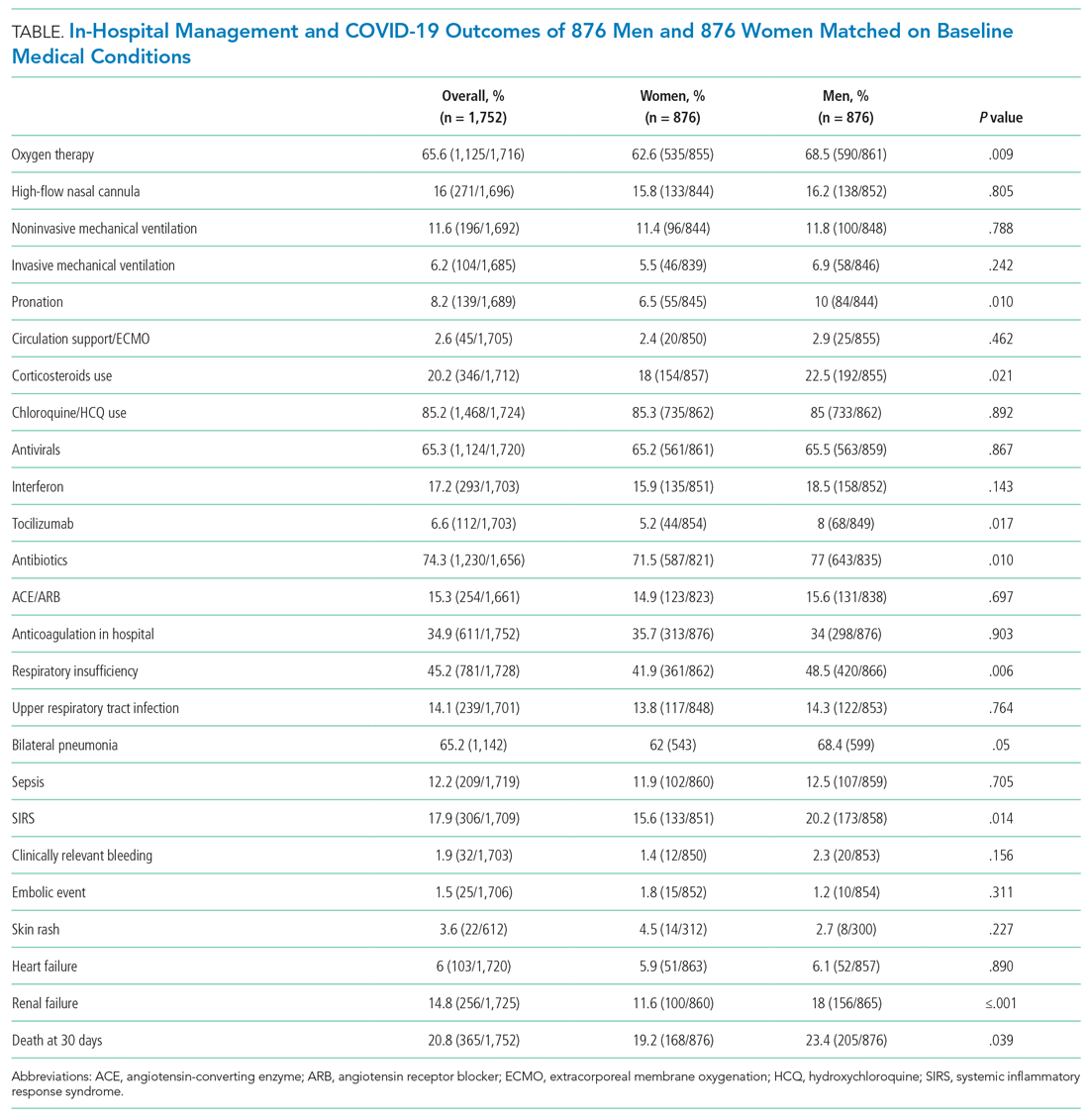
Thirty-day outcome data were available for all patients included in the analysis. During the in-hospital stay, 48% of patients developed respiratory insufficiency, 18.8%
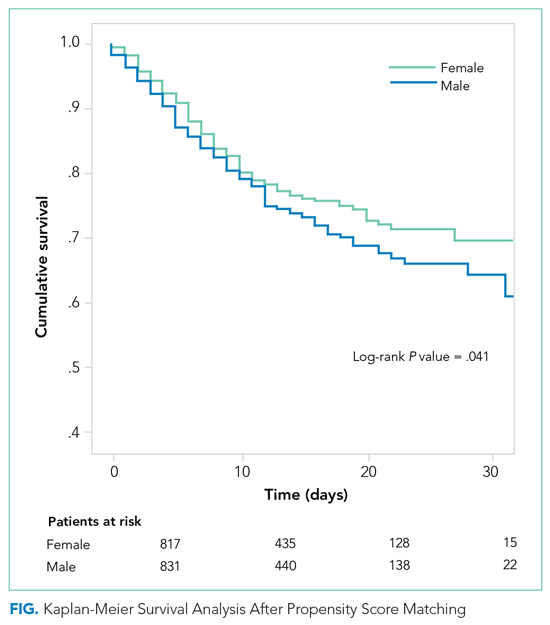
The PSM analysis continued to show a higher 30-day mortality rate among men (Figure), as well as greater need for oxygen, pronation, and use of immunomodulators and antibiotics (Table).
DISCUSSION
The results of our study confirm that among patients with COVID-19, men have a poorer prognosis than women. Because of the design of the study, it is not possible to determine if men are more prone to SARS-CoV-2 infection in our population; however, given the prevalence of men in our unselected, all-comers population, we can assume that men are either infected more often and/or more frequently symptomatic.
After PSM analysis, the 30-day all-cause mortality remained higher among men than women. The poorer prognosis of male patients is attributable not only to a higher burden of cardiovascular risk factors, but may also be related to unmodifiable biological factors, such as sex differences in angiotensin-converting enzyme 2 expression.7,8 The worse prognosis observed in our study confirms the higher incidence of death in male patients that was observed in previous studies.9 Liu et al questioned the role of gender as an independent prognostic factor in COVID-1910; however, that study included fewer patients, who were also younger and had less severe disease.
The clinical presentation of COVID-19 also differed by gender in our study. Gastrointestinal symptoms and hyposmia/anosmia were more common in women, whereas fever was more common in men. The prevalence of olfactory and gustatory dysfunction in women has already been described,11,12 and these symptoms have been linked with milder disease.13 It is possible that women presenting to the hospital had milder forms of COVID-19, or that there were systematic differences in how men and women sought medical care. The results of our study emphasize the need for a high level of suspicion for COVID-19 infection in women, even in the presence of mild mucosal or gastrointestinal symptoms and/or relatively minor laboratory abnormalities.
Laboratory values indicative of more severe COVID-19 infection in men could suggest a higher inflammatory response to the infection. Men also received more immunomodulators and antibiotics in this study. A recent paper from Scully et al14 pointed out the different immune response to viruses observed in men that could partially explain the higher level of inflammation markers and the more severe disease observed in men.
Limitations
Our study has several limitations. As an observational study of hospitalized patients, it may represent patients with more severe COVID-19. Men and women may have sought hospital care differently. Diagnosis, testing, and treatment were not standardized and may have been influenced by patient gender. Although we attempted to match patients on baseline medical conditions, we may not have completely controlled for differences in preexisting health. Finally, gender data were collected as binary and so did not capture other gender categories.
CONCLUSION
In our multicenter cohort of hospitalized COVID-19 patients, men had a higher burden of risk factors; different clinical presentations, with more fever and less olfactory and gastrointestinal symptoms; and a significantly poorer prognosis than women did at 30 days.
Acknowledgments
The authors thank Cardiovascular Excellence SL for their essential support regarding the database and HOPE web page as well as all HOPE researchers. The authors also thank Michael Andrews for his valuable contribution to the English revision.
1. Alkhouli M, Nanjundappa A, Annie F, Bates MC, Bhatt DL. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin Proc. 2020;95(8):1613-1620. https://doi.org/10.1016/j.mayocp.2020.05.014
2. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. https://doi.org/10.1186/s13293-020-00304-9
3. Gausman J, Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health (Larchmt). 2020;29(4):465-466. https://doi.org/10.1089/jwh.2020.8472
4. Walter LA, McGregor AJ. Sex- and gender-specific observations and implications for COVID-19. West J Emerg Med. 2020;21(3):507-509. https://doi.org/10.5811/westjem.2020.4.47536
5. Núñez-Gil IJ, Estrada V, Fernández-Pérez C, et al. Health outcome predictive evaluation for COVID 19 international registry (HOPE COVID-19), rationale and design. Contemp Clin Trials Commun. 2020;20:100654. https://doi.org/10.1016/j.conctc.2020.100654
6. International COVID-19 Clinical Evaluation Registry: HOPE-COVID 19. Accessed February 6, 2021. https://hopeprojectmd.com/en/
7. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37. https://doi.org/10.1038/s41420-020-0276-1
8. Ciaglia E, Vecchione C, Puca AA. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr. 2020;8:206. https://doi.org/10.3389/fped.2020.00206
9. Peckham H, de Gruijter N, Raine C, et al. Sex-bias in COVID-19: a meta-analysis and review of sex differences in disease and immunity. Research Square. April 20, 2020. https://doi.org/10.21203/rs.3.rs-23651/v2
10. Liu J, Zhang L, Chen Y, et al. Association of sex with clinical outcomes in COVID-19 patients: a retrospective analysis of 1190 cases. Respir Med. 2020;173:106159. https://doi.org/10.1016/j.rmed.2020.106159
11. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms—a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722-728. https://doi.org/10.1177/0194599820934380
12. Costa KVTD, Carnaúba ATL, Rocha KW, Andrade KCLD, Ferreira SMS, Menezes PTL. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol. 2020;86(6):781-792. https://doi.org/10.1016/j.bjorl.2020.05.008
13. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251-2261. https://doi.org/10.1007/s00405-020-05965-1
14. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442-447. https://doi.org/10.1038/s41577-020-0348-8
There is growing evidence that gender may be associated with COVID-19 infection, presentation, and prognosis
METHODS
Study methods and definitions are available in Appendix 1 and Appendix 2, respectively, and detailed in a previous paper5 and online on the web page of the study.6
Enrolled patients were divided into two groups according to their gender, then propensity score matching (PSM) analysis was performed (1:1 nearest neighbor matching, caliper = 0.01, without replacement and maximizing execution performance). Our
Statistical analysis methods are outlined in Appendix 1.
RESULTS
Of the 2,798 patients consecutively enrolled in the HOPE registry, 1,111 were women (39.7%) and 1,687 were men (60.3%). Of the 2,375 (84.9%) patients who had a nasopharyngeal swab positive for COVID-19, 962 were women and 1,413 were men.
Baseline Characteristics and Clinical Presentation
The baseline characteristics and clinical presentation of the overall population included in the study are summarized in Appendix Table 1. In the raw population, men had a significantly higher prevalence of conventional cardiovascular risk factors, such as diabetes, dyslipidemia, and smoking history, as well as a history of lung and cardiovascular diseases. On presentation, the most common symptoms for all patients were fever, cough, and dyspnea. Fever was more common in men, whereas vomiting, diarrhea, and upper airway symptoms (eg, sore throat, hyposmia/anosmia, dysgeusia) were more common in women.
Most patients had increased values of acute phase reactants. C-reactive protein (CRP) was elevated in 90.2% and D-dimer in 64.2% of patients, both significantly more often in men. Lymphocytopenia was present in 75.4% of patients, more commonly among men. Bilateral pneumonia occurred in 69.2% of the population, more frequently in men.
After PSM analysis (Appendix Table 2), a higher prevalence of hyposmia/anosmia and gastrointestinal symptoms in women was confirmed, as well as a higher prevalence of fever in men. Laboratory tests in men still presented alterations consistent with a more severe COVID-19 infection (significantly higher CRP, troponin, transaminases, lymphocytopenia, thrombocytopenia, and ferritin). There was no significant difference in the time between onset of symptoms and hospital admission by gender (6.2 ± 7.1 days in women vs 5.9 ± 7.6 days in men; P = .472).
The main findings after PSM analysis are summarized in Appendix Figure 1 and Appendix Figure 2.
In-Hospital Management and Outcomes
The supportive and pharmacologic treatments of study patients and their outcomes are summarized in Appendix Table 3. During the in-hospital stay, men required oxygen supplementation more frequently than women. Noninvasive mechanical ventilation, invasive mechanical ventilation, and pronation were more commonly used in men. Chloroquine/hydroxychloroquine, antivirals, and antibiotics were the medications most widely used in our population (84.5%, 65.8%, and 74.4% of patients, respectively), without significant differences between male and female patients, with the exception of antibiotics, which were used more often in men (76.6% vs 71.1%). Immunomodulators (corticosteroids, tocilizumab, and interferon) were used more often in male patients.
After PSM (Table), men more frequently received immunomodulators (corticosteroids and tocilizumab), antibiotics, and pronation. No differences in invasive and noninvasive mechanical ventilation were observed.

Thirty-day outcome data were available for all patients included in the analysis. During the in-hospital stay, 48% of patients developed respiratory insufficiency, 18.8%
The PSM analysis continued to show a higher 30-day mortality rate among men (Figure), as well as greater need for oxygen, pronation, and use of immunomodulators and antibiotics (Table).

DISCUSSION
The results of our study confirm that among patients with COVID-19, men have a poorer prognosis than women. Because of the design of the study, it is not possible to determine if men are more prone to SARS-CoV-2 infection in our population; however, given the prevalence of men in our unselected, all-comers population, we can assume that men are either infected more often and/or more frequently symptomatic.
After PSM analysis, the 30-day all-cause mortality remained higher among men than women. The poorer prognosis of male patients is attributable not only to a higher burden of cardiovascular risk factors, but may also be related to unmodifiable biological factors, such as sex differences in angiotensin-converting enzyme 2 expression.7,8 The worse prognosis observed in our study confirms the higher incidence of death in male patients that was observed in previous studies.9 Liu et al questioned the role of gender as an independent prognostic factor in COVID-1910; however, that study included fewer patients, who were also younger and had less severe disease.
The clinical presentation of COVID-19 also differed by gender in our study. Gastrointestinal symptoms and hyposmia/anosmia were more common in women, whereas fever was more common in men. The prevalence of olfactory and gustatory dysfunction in women has already been described,11,12 and these symptoms have been linked with milder disease.13 It is possible that women presenting to the hospital had milder forms of COVID-19, or that there were systematic differences in how men and women sought medical care. The results of our study emphasize the need for a high level of suspicion for COVID-19 infection in women, even in the presence of mild mucosal or gastrointestinal symptoms and/or relatively minor laboratory abnormalities.
Laboratory values indicative of more severe COVID-19 infection in men could suggest a higher inflammatory response to the infection. Men also received more immunomodulators and antibiotics in this study. A recent paper from Scully et al14 pointed out the different immune response to viruses observed in men that could partially explain the higher level of inflammation markers and the more severe disease observed in men.
Limitations
Our study has several limitations. As an observational study of hospitalized patients, it may represent patients with more severe COVID-19. Men and women may have sought hospital care differently. Diagnosis, testing, and treatment were not standardized and may have been influenced by patient gender. Although we attempted to match patients on baseline medical conditions, we may not have completely controlled for differences in preexisting health. Finally, gender data were collected as binary and so did not capture other gender categories.
CONCLUSION
In our multicenter cohort of hospitalized COVID-19 patients, men had a higher burden of risk factors; different clinical presentations, with more fever and less olfactory and gastrointestinal symptoms; and a significantly poorer prognosis than women did at 30 days.
Acknowledgments
The authors thank Cardiovascular Excellence SL for their essential support regarding the database and HOPE web page as well as all HOPE researchers. The authors also thank Michael Andrews for his valuable contribution to the English revision.
There is growing evidence that gender may be associated with COVID-19 infection, presentation, and prognosis
METHODS
Study methods and definitions are available in Appendix 1 and Appendix 2, respectively, and detailed in a previous paper5 and online on the web page of the study.6
Enrolled patients were divided into two groups according to their gender, then propensity score matching (PSM) analysis was performed (1:1 nearest neighbor matching, caliper = 0.01, without replacement and maximizing execution performance). Our
Statistical analysis methods are outlined in Appendix 1.
RESULTS
Of the 2,798 patients consecutively enrolled in the HOPE registry, 1,111 were women (39.7%) and 1,687 were men (60.3%). Of the 2,375 (84.9%) patients who had a nasopharyngeal swab positive for COVID-19, 962 were women and 1,413 were men.
Baseline Characteristics and Clinical Presentation
The baseline characteristics and clinical presentation of the overall population included in the study are summarized in Appendix Table 1. In the raw population, men had a significantly higher prevalence of conventional cardiovascular risk factors, such as diabetes, dyslipidemia, and smoking history, as well as a history of lung and cardiovascular diseases. On presentation, the most common symptoms for all patients were fever, cough, and dyspnea. Fever was more common in men, whereas vomiting, diarrhea, and upper airway symptoms (eg, sore throat, hyposmia/anosmia, dysgeusia) were more common in women.
Most patients had increased values of acute phase reactants. C-reactive protein (CRP) was elevated in 90.2% and D-dimer in 64.2% of patients, both significantly more often in men. Lymphocytopenia was present in 75.4% of patients, more commonly among men. Bilateral pneumonia occurred in 69.2% of the population, more frequently in men.
After PSM analysis (Appendix Table 2), a higher prevalence of hyposmia/anosmia and gastrointestinal symptoms in women was confirmed, as well as a higher prevalence of fever in men. Laboratory tests in men still presented alterations consistent with a more severe COVID-19 infection (significantly higher CRP, troponin, transaminases, lymphocytopenia, thrombocytopenia, and ferritin). There was no significant difference in the time between onset of symptoms and hospital admission by gender (6.2 ± 7.1 days in women vs 5.9 ± 7.6 days in men; P = .472).
The main findings after PSM analysis are summarized in Appendix Figure 1 and Appendix Figure 2.
In-Hospital Management and Outcomes
The supportive and pharmacologic treatments of study patients and their outcomes are summarized in Appendix Table 3. During the in-hospital stay, men required oxygen supplementation more frequently than women. Noninvasive mechanical ventilation, invasive mechanical ventilation, and pronation were more commonly used in men. Chloroquine/hydroxychloroquine, antivirals, and antibiotics were the medications most widely used in our population (84.5%, 65.8%, and 74.4% of patients, respectively), without significant differences between male and female patients, with the exception of antibiotics, which were used more often in men (76.6% vs 71.1%). Immunomodulators (corticosteroids, tocilizumab, and interferon) were used more often in male patients.
After PSM (Table), men more frequently received immunomodulators (corticosteroids and tocilizumab), antibiotics, and pronation. No differences in invasive and noninvasive mechanical ventilation were observed.

Thirty-day outcome data were available for all patients included in the analysis. During the in-hospital stay, 48% of patients developed respiratory insufficiency, 18.8%
The PSM analysis continued to show a higher 30-day mortality rate among men (Figure), as well as greater need for oxygen, pronation, and use of immunomodulators and antibiotics (Table).

DISCUSSION
The results of our study confirm that among patients with COVID-19, men have a poorer prognosis than women. Because of the design of the study, it is not possible to determine if men are more prone to SARS-CoV-2 infection in our population; however, given the prevalence of men in our unselected, all-comers population, we can assume that men are either infected more often and/or more frequently symptomatic.
After PSM analysis, the 30-day all-cause mortality remained higher among men than women. The poorer prognosis of male patients is attributable not only to a higher burden of cardiovascular risk factors, but may also be related to unmodifiable biological factors, such as sex differences in angiotensin-converting enzyme 2 expression.7,8 The worse prognosis observed in our study confirms the higher incidence of death in male patients that was observed in previous studies.9 Liu et al questioned the role of gender as an independent prognostic factor in COVID-1910; however, that study included fewer patients, who were also younger and had less severe disease.
The clinical presentation of COVID-19 also differed by gender in our study. Gastrointestinal symptoms and hyposmia/anosmia were more common in women, whereas fever was more common in men. The prevalence of olfactory and gustatory dysfunction in women has already been described,11,12 and these symptoms have been linked with milder disease.13 It is possible that women presenting to the hospital had milder forms of COVID-19, or that there were systematic differences in how men and women sought medical care. The results of our study emphasize the need for a high level of suspicion for COVID-19 infection in women, even in the presence of mild mucosal or gastrointestinal symptoms and/or relatively minor laboratory abnormalities.
Laboratory values indicative of more severe COVID-19 infection in men could suggest a higher inflammatory response to the infection. Men also received more immunomodulators and antibiotics in this study. A recent paper from Scully et al14 pointed out the different immune response to viruses observed in men that could partially explain the higher level of inflammation markers and the more severe disease observed in men.
Limitations
Our study has several limitations. As an observational study of hospitalized patients, it may represent patients with more severe COVID-19. Men and women may have sought hospital care differently. Diagnosis, testing, and treatment were not standardized and may have been influenced by patient gender. Although we attempted to match patients on baseline medical conditions, we may not have completely controlled for differences in preexisting health. Finally, gender data were collected as binary and so did not capture other gender categories.
CONCLUSION
In our multicenter cohort of hospitalized COVID-19 patients, men had a higher burden of risk factors; different clinical presentations, with more fever and less olfactory and gastrointestinal symptoms; and a significantly poorer prognosis than women did at 30 days.
Acknowledgments
The authors thank Cardiovascular Excellence SL for their essential support regarding the database and HOPE web page as well as all HOPE researchers. The authors also thank Michael Andrews for his valuable contribution to the English revision.
1. Alkhouli M, Nanjundappa A, Annie F, Bates MC, Bhatt DL. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin Proc. 2020;95(8):1613-1620. https://doi.org/10.1016/j.mayocp.2020.05.014
2. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. https://doi.org/10.1186/s13293-020-00304-9
3. Gausman J, Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health (Larchmt). 2020;29(4):465-466. https://doi.org/10.1089/jwh.2020.8472
4. Walter LA, McGregor AJ. Sex- and gender-specific observations and implications for COVID-19. West J Emerg Med. 2020;21(3):507-509. https://doi.org/10.5811/westjem.2020.4.47536
5. Núñez-Gil IJ, Estrada V, Fernández-Pérez C, et al. Health outcome predictive evaluation for COVID 19 international registry (HOPE COVID-19), rationale and design. Contemp Clin Trials Commun. 2020;20:100654. https://doi.org/10.1016/j.conctc.2020.100654
6. International COVID-19 Clinical Evaluation Registry: HOPE-COVID 19. Accessed February 6, 2021. https://hopeprojectmd.com/en/
7. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37. https://doi.org/10.1038/s41420-020-0276-1
8. Ciaglia E, Vecchione C, Puca AA. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr. 2020;8:206. https://doi.org/10.3389/fped.2020.00206
9. Peckham H, de Gruijter N, Raine C, et al. Sex-bias in COVID-19: a meta-analysis and review of sex differences in disease and immunity. Research Square. April 20, 2020. https://doi.org/10.21203/rs.3.rs-23651/v2
10. Liu J, Zhang L, Chen Y, et al. Association of sex with clinical outcomes in COVID-19 patients: a retrospective analysis of 1190 cases. Respir Med. 2020;173:106159. https://doi.org/10.1016/j.rmed.2020.106159
11. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms—a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722-728. https://doi.org/10.1177/0194599820934380
12. Costa KVTD, Carnaúba ATL, Rocha KW, Andrade KCLD, Ferreira SMS, Menezes PTL. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol. 2020;86(6):781-792. https://doi.org/10.1016/j.bjorl.2020.05.008
13. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251-2261. https://doi.org/10.1007/s00405-020-05965-1
14. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442-447. https://doi.org/10.1038/s41577-020-0348-8
1. Alkhouli M, Nanjundappa A, Annie F, Bates MC, Bhatt DL. Sex differences in case fatality rate of COVID-19: insights from a multinational registry. Mayo Clin Proc. 2020;95(8):1613-1620. https://doi.org/10.1016/j.mayocp.2020.05.014
2. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. https://doi.org/10.1186/s13293-020-00304-9
3. Gausman J, Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health (Larchmt). 2020;29(4):465-466. https://doi.org/10.1089/jwh.2020.8472
4. Walter LA, McGregor AJ. Sex- and gender-specific observations and implications for COVID-19. West J Emerg Med. 2020;21(3):507-509. https://doi.org/10.5811/westjem.2020.4.47536
5. Núñez-Gil IJ, Estrada V, Fernández-Pérez C, et al. Health outcome predictive evaluation for COVID 19 international registry (HOPE COVID-19), rationale and design. Contemp Clin Trials Commun. 2020;20:100654. https://doi.org/10.1016/j.conctc.2020.100654
6. International COVID-19 Clinical Evaluation Registry: HOPE-COVID 19. Accessed February 6, 2021. https://hopeprojectmd.com/en/
7. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37. https://doi.org/10.1038/s41420-020-0276-1
8. Ciaglia E, Vecchione C, Puca AA. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr. 2020;8:206. https://doi.org/10.3389/fped.2020.00206
9. Peckham H, de Gruijter N, Raine C, et al. Sex-bias in COVID-19: a meta-analysis and review of sex differences in disease and immunity. Research Square. April 20, 2020. https://doi.org/10.21203/rs.3.rs-23651/v2
10. Liu J, Zhang L, Chen Y, et al. Association of sex with clinical outcomes in COVID-19 patients: a retrospective analysis of 1190 cases. Respir Med. 2020;173:106159. https://doi.org/10.1016/j.rmed.2020.106159
11. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms—a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722-728. https://doi.org/10.1177/0194599820934380
12. Costa KVTD, Carnaúba ATL, Rocha KW, Andrade KCLD, Ferreira SMS, Menezes PTL. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol. 2020;86(6):781-792. https://doi.org/10.1016/j.bjorl.2020.05.008
13. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251-2261. https://doi.org/10.1007/s00405-020-05965-1
14. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442-447. https://doi.org/10.1038/s41577-020-0348-8
© 2021 Society of Hospital Medicine