User login
Obesity and overweight, with or without metabolic dysregulation, pose vexing problems for many patients with mood, anxiety, or psychotic disorders. More than one-half of individuals with severe mental illnesses are obese or overweight,1 resulting from multiple factors that may include psychiatric symptoms (eg, anergia and hyperphagia), poor dietary choices, sedentary lifestyle, underlying inflammatory processes, medical comorbidities, and iatrogenic consequences of certain medications. Unfortunately, numerous psychotropic medications can increase weight and appetite due to a variety of mechanisms, including antihistaminergic effects, direct appetite-stimulating effects, and proclivities to cause insulin resistance. While individual agents can vary, a recent review identified an overall 2-fold increased risk for rapid, significant weight gain during treatment with antipsychotics as a class.2 In addition to lifestyle modifications (diet and exercise), many pharmacologic strategies have been proposed to counter iatrogenic weight gain, including appetite suppressants (eg, pro-dopaminergic agents such as phentermine, stimulants, and amantadine), pro-anorectant anticonvulsants (eg, topiramate or zonisamide), opioid receptor antagonists (eg, olanzapine/samidorphan or naltrexone) and oral hypoglycemics such as metformin. However, the magnitude of impact for most of these agents to reverse iatrogenic weight gain tends to be modest, particularly once significant weight gain (ie, ≥7% of initial body weight) has already occurred.
Pharmacologic strategies to modulate or enhance the effects of insulin hold particular importance for combatting psychotropic-associated weight gain. Insulin transports glucose from the intravascular space to end organs for fuel consumption; to varying degrees, second-generation antipsychotics (SGAs) and some other psychotropic medications can cause insulin resistance. This in turn leads to excessive storage of underutilized glucose in the liver (glycogenesis), the potential for developing fatty liver (ie, nonalcoholic steatohepatitis), and conversion of excess carbohydrates to fatty acids and triglycerides, with subsequent storage in adipose tissue. Medications that can enhance the activity of insulin (so-called incretin mimetics) can help to overcome insulin resistance caused by SGAs (and potentially by other psychotropic medications) and essentially lead to weight loss through enhanced “fuel efficiency.”
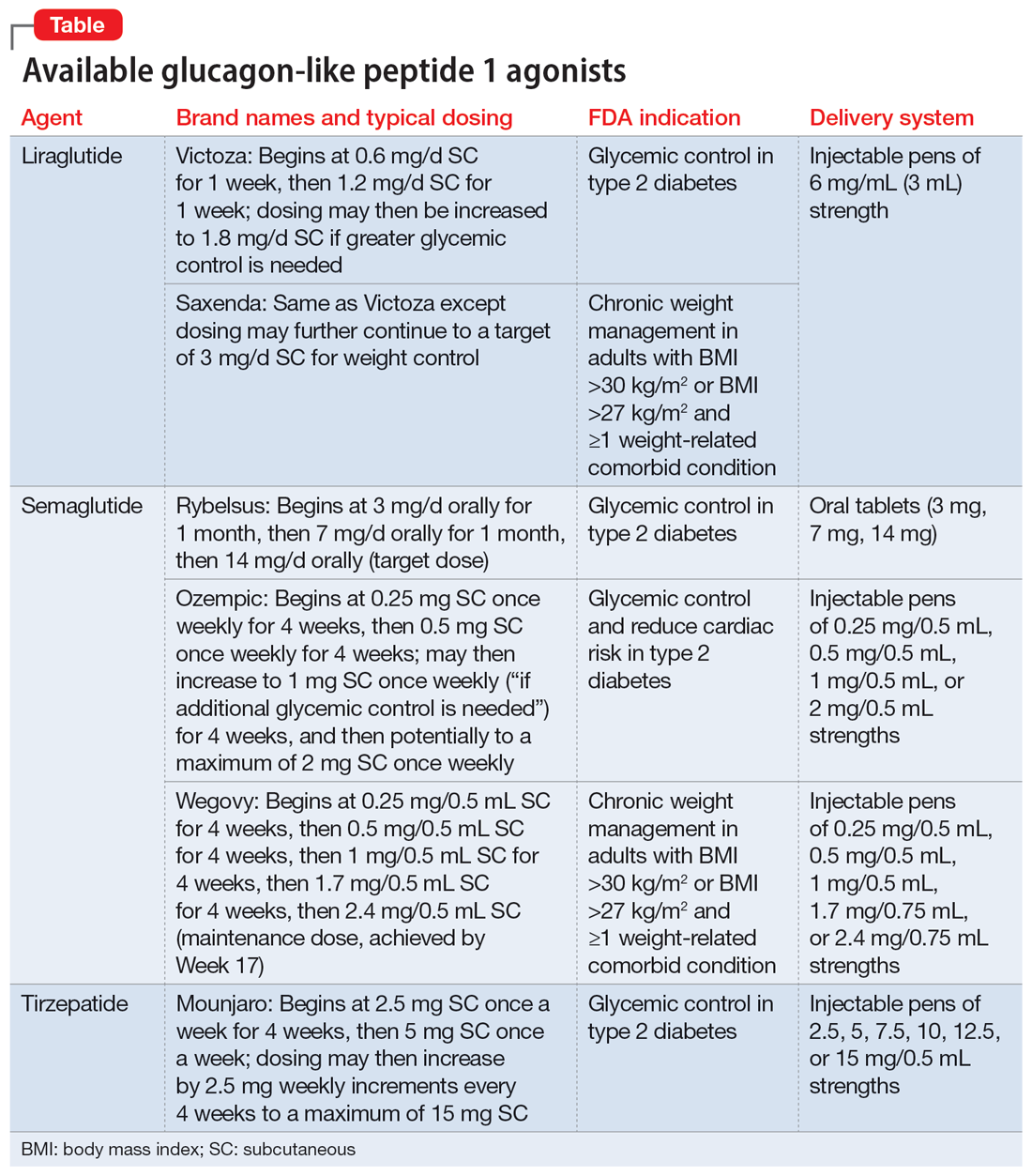
Metformin, typically dosed up to 1,000 mg twice daily with meals, has increasingly become recognized as a first-line strategy to attenuate weight gain and glycemic dysregulation from SGAs via its ability to reduce insulin resistance. Yet meta-analyses have shown that although results are significantly better than placebo, overall long-term weight loss from metformin alone tends to be rather modest (<4 kg) and associated with a reduction in body mass index (BMI) of only approximately 1 point.3 Psychiatrists (and other clinicians who prescribe psychotropic medications that can cause weight gain or metabolic dysregulation) therefore need to become familiar with alternative or adjunctive weight loss options. The use of a relatively new class of incretin mimetics called glucagon-like peptide 1 (GLP-1) agonists (Table) has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation.
What are GLP-1 agonists?
GLP-1 is a hormone secreted by L cells in the intestinal mucosa in response to food. GLP-1 agonists reduce blood sugar by increasing insulin secretion, decreasing glucagon release (thus downregulating further increases in blood sugar), and reducing insulin resistance. GLP-1 agonists also reduce appetite by directly stimulating the satiety center and slowing gastric emptying and GI motility. In addition to GLP-1 agonism, some medications in this family (notably tirzepatide) also agonize a second hormone, glucose-dependent insulinotropic polypeptide, which can further induce insulin secretion as well as decrease stomach acid secretion, potentially delivering an even more substantial reduction in appetite and weight.
Routes of administration and FDA indications
Due to limited bioavailability, most GLP-1 agonists require subcutaneous (SC) injections (the sole exception is the Rybelsus brand of semaglutide, which comes in a daily pill form). Most are FDA-approved not specifically for weight loss but for patients with type 2 diabetes (defined as a hemoglobin A1C ≥6.5% or a fasting blood glucose level ≥126 mg/dL). Weight loss represents a secondary outcome for GLP-1 agonists FDA-approved for glycemic control in patients with type 2 diabetes. The 2 current exceptions to this classification are the Wegovy brand of semaglutide (ie, dosing of 2.4 mg) and the Saxenda brand of liraglutide, both of which carry FDA indications for chronic weight management alone (when paired with dietary and lifestyle modification) in individuals who are obese (BMI >30 kg/m2) regardless of the presence or absence of diabetes, or for persons who are overweight (BMI >27 kg/m2) and have ≥1 weight-related comorbid condition (eg, hypertension, type 2 diabetes, or dyslipidemia). Although patients at risk for diabetes (ie, prediabetes, defined as a hemoglobin A1C 5.7% to 6.4% or a fasting blood glucose level 100 to 125 mg/dL) were included in FDA registration trials of Saxenda or Wegovy, prediabetes is not an FDA indication for any GLP-1 agonist.
Data in weight loss
Most of the existing empirical data on weight loss with GLP-1 agonists come from studies of individuals who are overweight or obese, with or without type 2 diabetes, rather than from studies using these agents to counteract iatrogenic weight gain. In a retrospective cohort study of patients with type 2 diabetes, coadministration with serotonergic antidepressants (eg, citalopram/escitalopram) was associated with attenuation of the weight loss effects of GLP-1 agonists.4
Liraglutide currently is the sole GLP-1 agonist studied for treating SGA-associated weight gain. A 16-week randomized trial compared once-daily SC injected liraglutide vs placebo in patients with schizophrenia who incurred weight gain and prediabetes after taking olanzapine or clozapine.5 Significantly more patients taking liraglutide than placebo developed normal glucose tolerance (64% vs 16%), and body weight decreased by a mean of 5.3 kg.
Continue to: In studies of semaglutide...
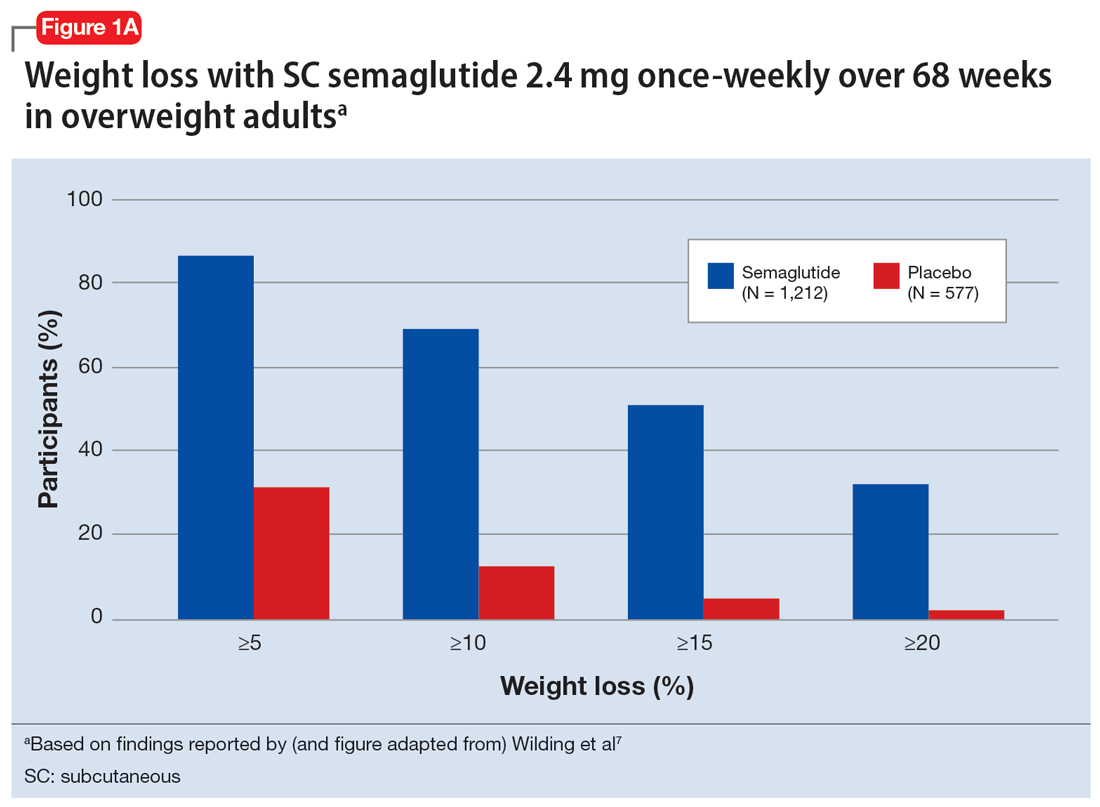
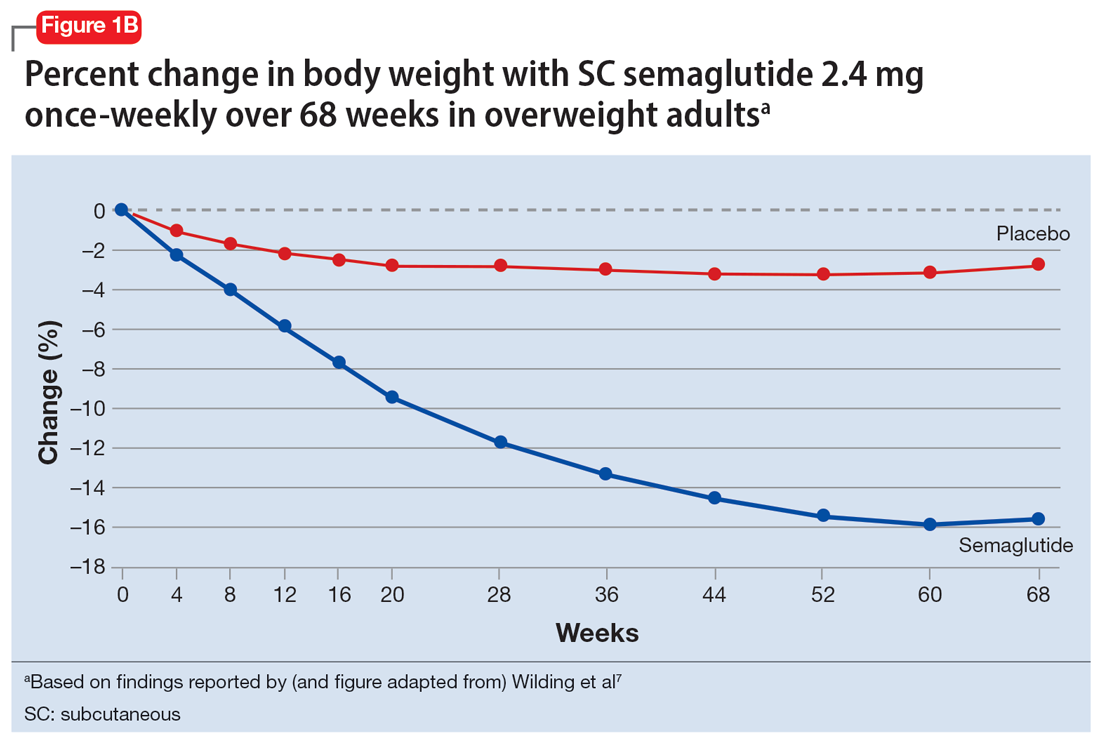
In studies of semaglutide for overweight/obese patients with type 2 diabetes or prediabetes, clinical trials of oral semaglutide (Rybelsus) found a mean weight loss over 26 weeks of -1.0 kg with dosing at 7 mg/d and -2.6 kg with dosing at 14 mg/d.6 A 68-week placebo-controlled trial of semaglutide (dosed at 2.4 mg SC weekly) for overweight/obese adults who did not have diabetes yielded a -15.3 kg weight loss (vs -2.6 kg with placebo); one-half of those who received semaglutide lost 15% of their initial body weight (Figure 1A and Figure 1B).7 Similar findings with semaglutide 2.4 mg SC weekly (Wegovy) were observed in overweight/obese adolescents, with 73% of participants losing ≥5% of their baseline weight.8 A comparative randomized trial in patients with type 2 diabetes also found modestly but significantly greater weight loss with oral semaglutide than with SC liraglutide.9
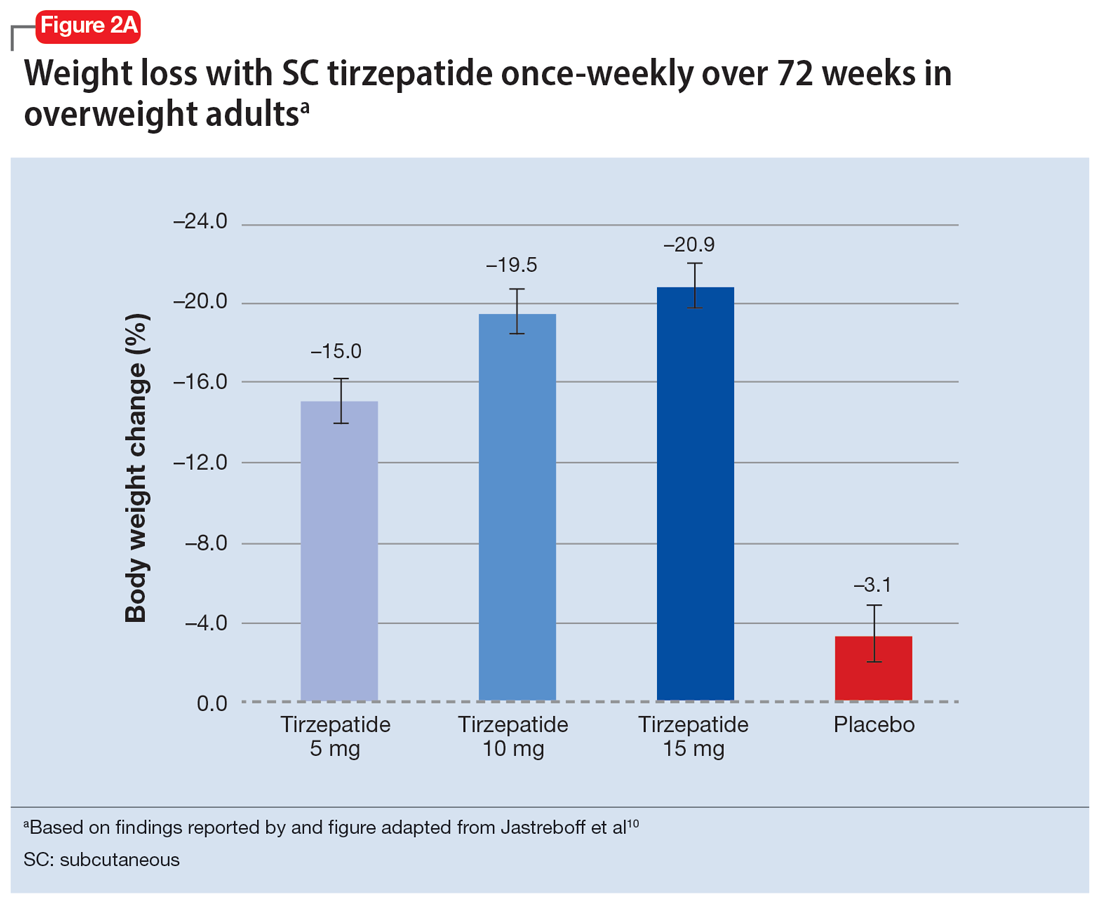
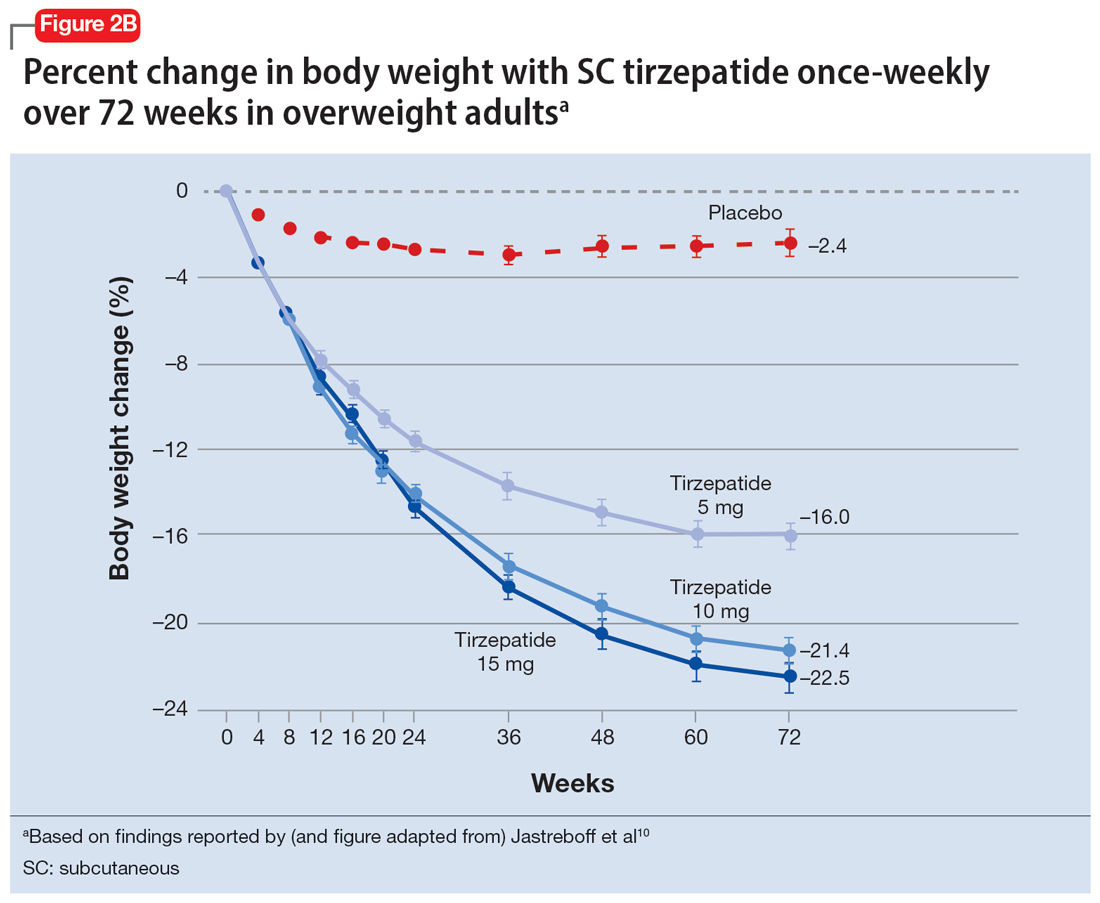
In a 72-week study of tirzepatide specifically for weight loss in nondiabetic patients who were overweight or obese, findings were especially dramatic (Figure 2A and Figure 2B).10 An overall 15% decrease in body weight was observed with 5 mg/week dosing alongside a 19.5% decrease in body weight with 10 mg/week dosing and a 20.9% weight reduction with 15 mg/week dosing.10 As noted in Figure 2B, the observed pattern of weight loss occurred along an exponential decay curve. Notably, a comparative study of tirzepatide vs once-weekly semaglutide (1 mg) in patients with type 2 diabetes11 found significantly greater dose-dependent weight loss with tirzepatide than semaglutide (-1.9 kg at 5 mg, -3.6 kg at 10 mg, and -5.5 kg at 15 mg)—although the somewhat low dosing of semaglutide may have limited its optimal possible weight loss benefit.
Tolerability
Adverse effects with GLP-1 agonists are mainly gastrointestinal (eg, nausea, vomiting, abdominal pain, diarrhea, or constipation)5-11 and generally transient. SC administration is performed in fatty tissue of the abdomen, thigh, or upper arm; site rotation is recommended to minimize injection site pain. All GLP-1 agonists carry manufacturers’ warning and precaution statements identifying the rare potential for acute pancreatitis, acute gall bladder disease, acute kidney injury, and hypoglycemia. Animal studies also have suggested an increased, dose-dependent risk for thyroid C-cell tumors with GLP-1 agonists; this has not been observed in human trials, although postmarketing pharmacovigilance reports have identified cases of medullary thyroid carcinoma in patients who took liraglutide. A manufacturer’s boxed warning indicates that a personal or family history of medullary carcinoma of the thyroid poses a contraindication for taking semaglutide, liraglutide, or tirzepatide.
Initial evidence prompts additional questions
GLP-1 agonists represent an emerging class of novel agents that can modulate glycemic dysregulation and overweight/obesity, often with dramatic results whose magnitude rivals the efficacy of bariatric surgery. Once-weekly formulations of semaglutide (Wegovy) and daily liraglutide (Saxenda) are FDA-approved for weight loss in patients who are overweight or obese while other existing formulations are approved solely for patients with type 2 diabetes, although it is likely that broader indications for weight loss (regardless of glycemic status) are forthcoming. Targeted use of GLP-1 agonists to counteract SGA-associated weight gain is supported by a handful of preliminary reports, with additional studies likely to come. Unanswered questions include:
- When should GLP-1 agonists be considered within a treatment algorithm for iatrogenic weight gain relative to other antidote strategies such as metformin or appetite-suppressing anticonvulsants?
- How effective might GLP-1 agonists be for iatrogenic weight gain from non-SGA psychotropic medications, such as serotonergic antidepressants?
- When and how can GLP-1 agonists be safely coprescribed with other nonincretin mimetic weight loss medications?
- When should psychiatrists prescribe GLP-1 agonists, or do so collaboratively with primary care physicians or endocrinologists, particularly in patients with metabolic syndrome?
Followers of the rapidly emerging literature in this area will likely find themselves best positioned to address these and other questions about optimal management of psychotropic-induced weight gain for the patients they treat.
Bottom Line
The use of glucagon-like peptide 1 (GLP-1) agonists, a relatively new class of incretin mimetics, has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation. Preliminary reports support the potential targeted use of GLP-1 agonists to counteract weight gain associated with second-generation antipsychotics.
Related Resources
- Singh F, Allen A, Ianni A. Managing metabolic syndrome in patients with schizophrenia. Current Psychiatry. 2020;19(12):20-24,26. doi:10.12788/cp.0064
- Ard J, Fitch A, Fruh S, et al. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. 2021;38(6):2821- 2839. doi:10.1007/s12325-021-01710-0
Drug Brand Names
Amantadine • Gocovri
Citalopram • Celexa
Clozapine • Clozaril
Escitalopram • Lexapro
Liraglutide • Victoza, Saxenda
Metformin • Glucophage
Naltrexone • ReVia
Olanzapine • Zyprexa
Olanzapine/samidorphan • Lybalvi
Phentermine • Ionamin
Semaglutide • Rybelsus, Ozempic, Wegovy
Tirzepatide • Mounjaro
Topiramate • Topamax
Zonisamide • Zonegran
1. Afzal M, Siddiqi N, Ahmad B, et al. Prevalence of overweight and obesity in people with severe mental illness: systematic review and meta-analysis. Front Endocrinol (Lausanne). 2021;25;12:769309.
2. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Safety. 2020;19(3):295-314.
3. de Silva AV, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341.
4. Durell N, Franks R, Coon S, et al. Effects of antidepressants on glucagon-like peptide-1 receptor agonist-related weight loss. J Pharm Technol. 2022;38(5):283-288.
5. Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(7):719-728.
6. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724-1732.
7. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
8. Weghuber D, Barrett T, Barrientos-Pérez M, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. Published online November 2, 2022. doi:10.1056/NEJMoa2208601.
9. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomized, double-blind, phase 3a trial. Lancet. 2019;394(10192):39-50.
10. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205-216.
11. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515.
Obesity and overweight, with or without metabolic dysregulation, pose vexing problems for many patients with mood, anxiety, or psychotic disorders. More than one-half of individuals with severe mental illnesses are obese or overweight,1 resulting from multiple factors that may include psychiatric symptoms (eg, anergia and hyperphagia), poor dietary choices, sedentary lifestyle, underlying inflammatory processes, medical comorbidities, and iatrogenic consequences of certain medications. Unfortunately, numerous psychotropic medications can increase weight and appetite due to a variety of mechanisms, including antihistaminergic effects, direct appetite-stimulating effects, and proclivities to cause insulin resistance. While individual agents can vary, a recent review identified an overall 2-fold increased risk for rapid, significant weight gain during treatment with antipsychotics as a class.2 In addition to lifestyle modifications (diet and exercise), many pharmacologic strategies have been proposed to counter iatrogenic weight gain, including appetite suppressants (eg, pro-dopaminergic agents such as phentermine, stimulants, and amantadine), pro-anorectant anticonvulsants (eg, topiramate or zonisamide), opioid receptor antagonists (eg, olanzapine/samidorphan or naltrexone) and oral hypoglycemics such as metformin. However, the magnitude of impact for most of these agents to reverse iatrogenic weight gain tends to be modest, particularly once significant weight gain (ie, ≥7% of initial body weight) has already occurred.
Pharmacologic strategies to modulate or enhance the effects of insulin hold particular importance for combatting psychotropic-associated weight gain. Insulin transports glucose from the intravascular space to end organs for fuel consumption; to varying degrees, second-generation antipsychotics (SGAs) and some other psychotropic medications can cause insulin resistance. This in turn leads to excessive storage of underutilized glucose in the liver (glycogenesis), the potential for developing fatty liver (ie, nonalcoholic steatohepatitis), and conversion of excess carbohydrates to fatty acids and triglycerides, with subsequent storage in adipose tissue. Medications that can enhance the activity of insulin (so-called incretin mimetics) can help to overcome insulin resistance caused by SGAs (and potentially by other psychotropic medications) and essentially lead to weight loss through enhanced “fuel efficiency.”
Metformin, typically dosed up to 1,000 mg twice daily with meals, has increasingly become recognized as a first-line strategy to attenuate weight gain and glycemic dysregulation from SGAs via its ability to reduce insulin resistance. Yet meta-analyses have shown that although results are significantly better than placebo, overall long-term weight loss from metformin alone tends to be rather modest (<4 kg) and associated with a reduction in body mass index (BMI) of only approximately 1 point.3 Psychiatrists (and other clinicians who prescribe psychotropic medications that can cause weight gain or metabolic dysregulation) therefore need to become familiar with alternative or adjunctive weight loss options. The use of a relatively new class of incretin mimetics called glucagon-like peptide 1 (GLP-1) agonists (Table) has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation.

What are GLP-1 agonists?
GLP-1 is a hormone secreted by L cells in the intestinal mucosa in response to food. GLP-1 agonists reduce blood sugar by increasing insulin secretion, decreasing glucagon release (thus downregulating further increases in blood sugar), and reducing insulin resistance. GLP-1 agonists also reduce appetite by directly stimulating the satiety center and slowing gastric emptying and GI motility. In addition to GLP-1 agonism, some medications in this family (notably tirzepatide) also agonize a second hormone, glucose-dependent insulinotropic polypeptide, which can further induce insulin secretion as well as decrease stomach acid secretion, potentially delivering an even more substantial reduction in appetite and weight.
Routes of administration and FDA indications
Due to limited bioavailability, most GLP-1 agonists require subcutaneous (SC) injections (the sole exception is the Rybelsus brand of semaglutide, which comes in a daily pill form). Most are FDA-approved not specifically for weight loss but for patients with type 2 diabetes (defined as a hemoglobin A1C ≥6.5% or a fasting blood glucose level ≥126 mg/dL). Weight loss represents a secondary outcome for GLP-1 agonists FDA-approved for glycemic control in patients with type 2 diabetes. The 2 current exceptions to this classification are the Wegovy brand of semaglutide (ie, dosing of 2.4 mg) and the Saxenda brand of liraglutide, both of which carry FDA indications for chronic weight management alone (when paired with dietary and lifestyle modification) in individuals who are obese (BMI >30 kg/m2) regardless of the presence or absence of diabetes, or for persons who are overweight (BMI >27 kg/m2) and have ≥1 weight-related comorbid condition (eg, hypertension, type 2 diabetes, or dyslipidemia). Although patients at risk for diabetes (ie, prediabetes, defined as a hemoglobin A1C 5.7% to 6.4% or a fasting blood glucose level 100 to 125 mg/dL) were included in FDA registration trials of Saxenda or Wegovy, prediabetes is not an FDA indication for any GLP-1 agonist.
Data in weight loss
Most of the existing empirical data on weight loss with GLP-1 agonists come from studies of individuals who are overweight or obese, with or without type 2 diabetes, rather than from studies using these agents to counteract iatrogenic weight gain. In a retrospective cohort study of patients with type 2 diabetes, coadministration with serotonergic antidepressants (eg, citalopram/escitalopram) was associated with attenuation of the weight loss effects of GLP-1 agonists.4
Liraglutide currently is the sole GLP-1 agonist studied for treating SGA-associated weight gain. A 16-week randomized trial compared once-daily SC injected liraglutide vs placebo in patients with schizophrenia who incurred weight gain and prediabetes after taking olanzapine or clozapine.5 Significantly more patients taking liraglutide than placebo developed normal glucose tolerance (64% vs 16%), and body weight decreased by a mean of 5.3 kg.
Continue to: In studies of semaglutide...
In studies of semaglutide for overweight/obese patients with type 2 diabetes or prediabetes, clinical trials of oral semaglutide (Rybelsus) found a mean weight loss over 26 weeks of -1.0 kg with dosing at 7 mg/d and -2.6 kg with dosing at 14 mg/d.6 A 68-week placebo-controlled trial of semaglutide (dosed at 2.4 mg SC weekly) for overweight/obese adults who did not have diabetes yielded a -15.3 kg weight loss (vs -2.6 kg with placebo); one-half of those who received semaglutide lost 15% of their initial body weight (Figure 1A and Figure 1B).7 Similar findings with semaglutide 2.4 mg SC weekly (Wegovy) were observed in overweight/obese adolescents, with 73% of participants losing ≥5% of their baseline weight.8 A comparative randomized trial in patients with type 2 diabetes also found modestly but significantly greater weight loss with oral semaglutide than with SC liraglutide.9


In a 72-week study of tirzepatide specifically for weight loss in nondiabetic patients who were overweight or obese, findings were especially dramatic (Figure 2A and Figure 2B).10 An overall 15% decrease in body weight was observed with 5 mg/week dosing alongside a 19.5% decrease in body weight with 10 mg/week dosing and a 20.9% weight reduction with 15 mg/week dosing.10 As noted in Figure 2B, the observed pattern of weight loss occurred along an exponential decay curve. Notably, a comparative study of tirzepatide vs once-weekly semaglutide (1 mg) in patients with type 2 diabetes11 found significantly greater dose-dependent weight loss with tirzepatide than semaglutide (-1.9 kg at 5 mg, -3.6 kg at 10 mg, and -5.5 kg at 15 mg)—although the somewhat low dosing of semaglutide may have limited its optimal possible weight loss benefit.


Tolerability
Adverse effects with GLP-1 agonists are mainly gastrointestinal (eg, nausea, vomiting, abdominal pain, diarrhea, or constipation)5-11 and generally transient. SC administration is performed in fatty tissue of the abdomen, thigh, or upper arm; site rotation is recommended to minimize injection site pain. All GLP-1 agonists carry manufacturers’ warning and precaution statements identifying the rare potential for acute pancreatitis, acute gall bladder disease, acute kidney injury, and hypoglycemia. Animal studies also have suggested an increased, dose-dependent risk for thyroid C-cell tumors with GLP-1 agonists; this has not been observed in human trials, although postmarketing pharmacovigilance reports have identified cases of medullary thyroid carcinoma in patients who took liraglutide. A manufacturer’s boxed warning indicates that a personal or family history of medullary carcinoma of the thyroid poses a contraindication for taking semaglutide, liraglutide, or tirzepatide.
Initial evidence prompts additional questions
GLP-1 agonists represent an emerging class of novel agents that can modulate glycemic dysregulation and overweight/obesity, often with dramatic results whose magnitude rivals the efficacy of bariatric surgery. Once-weekly formulations of semaglutide (Wegovy) and daily liraglutide (Saxenda) are FDA-approved for weight loss in patients who are overweight or obese while other existing formulations are approved solely for patients with type 2 diabetes, although it is likely that broader indications for weight loss (regardless of glycemic status) are forthcoming. Targeted use of GLP-1 agonists to counteract SGA-associated weight gain is supported by a handful of preliminary reports, with additional studies likely to come. Unanswered questions include:
- When should GLP-1 agonists be considered within a treatment algorithm for iatrogenic weight gain relative to other antidote strategies such as metformin or appetite-suppressing anticonvulsants?
- How effective might GLP-1 agonists be for iatrogenic weight gain from non-SGA psychotropic medications, such as serotonergic antidepressants?
- When and how can GLP-1 agonists be safely coprescribed with other nonincretin mimetic weight loss medications?
- When should psychiatrists prescribe GLP-1 agonists, or do so collaboratively with primary care physicians or endocrinologists, particularly in patients with metabolic syndrome?
Followers of the rapidly emerging literature in this area will likely find themselves best positioned to address these and other questions about optimal management of psychotropic-induced weight gain for the patients they treat.
Bottom Line
The use of glucagon-like peptide 1 (GLP-1) agonists, a relatively new class of incretin mimetics, has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation. Preliminary reports support the potential targeted use of GLP-1 agonists to counteract weight gain associated with second-generation antipsychotics.
Related Resources
- Singh F, Allen A, Ianni A. Managing metabolic syndrome in patients with schizophrenia. Current Psychiatry. 2020;19(12):20-24,26. doi:10.12788/cp.0064
- Ard J, Fitch A, Fruh S, et al. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. 2021;38(6):2821- 2839. doi:10.1007/s12325-021-01710-0
Drug Brand Names
Amantadine • Gocovri
Citalopram • Celexa
Clozapine • Clozaril
Escitalopram • Lexapro
Liraglutide • Victoza, Saxenda
Metformin • Glucophage
Naltrexone • ReVia
Olanzapine • Zyprexa
Olanzapine/samidorphan • Lybalvi
Phentermine • Ionamin
Semaglutide • Rybelsus, Ozempic, Wegovy
Tirzepatide • Mounjaro
Topiramate • Topamax
Zonisamide • Zonegran
Obesity and overweight, with or without metabolic dysregulation, pose vexing problems for many patients with mood, anxiety, or psychotic disorders. More than one-half of individuals with severe mental illnesses are obese or overweight,1 resulting from multiple factors that may include psychiatric symptoms (eg, anergia and hyperphagia), poor dietary choices, sedentary lifestyle, underlying inflammatory processes, medical comorbidities, and iatrogenic consequences of certain medications. Unfortunately, numerous psychotropic medications can increase weight and appetite due to a variety of mechanisms, including antihistaminergic effects, direct appetite-stimulating effects, and proclivities to cause insulin resistance. While individual agents can vary, a recent review identified an overall 2-fold increased risk for rapid, significant weight gain during treatment with antipsychotics as a class.2 In addition to lifestyle modifications (diet and exercise), many pharmacologic strategies have been proposed to counter iatrogenic weight gain, including appetite suppressants (eg, pro-dopaminergic agents such as phentermine, stimulants, and amantadine), pro-anorectant anticonvulsants (eg, topiramate or zonisamide), opioid receptor antagonists (eg, olanzapine/samidorphan or naltrexone) and oral hypoglycemics such as metformin. However, the magnitude of impact for most of these agents to reverse iatrogenic weight gain tends to be modest, particularly once significant weight gain (ie, ≥7% of initial body weight) has already occurred.
Pharmacologic strategies to modulate or enhance the effects of insulin hold particular importance for combatting psychotropic-associated weight gain. Insulin transports glucose from the intravascular space to end organs for fuel consumption; to varying degrees, second-generation antipsychotics (SGAs) and some other psychotropic medications can cause insulin resistance. This in turn leads to excessive storage of underutilized glucose in the liver (glycogenesis), the potential for developing fatty liver (ie, nonalcoholic steatohepatitis), and conversion of excess carbohydrates to fatty acids and triglycerides, with subsequent storage in adipose tissue. Medications that can enhance the activity of insulin (so-called incretin mimetics) can help to overcome insulin resistance caused by SGAs (and potentially by other psychotropic medications) and essentially lead to weight loss through enhanced “fuel efficiency.”
Metformin, typically dosed up to 1,000 mg twice daily with meals, has increasingly become recognized as a first-line strategy to attenuate weight gain and glycemic dysregulation from SGAs via its ability to reduce insulin resistance. Yet meta-analyses have shown that although results are significantly better than placebo, overall long-term weight loss from metformin alone tends to be rather modest (<4 kg) and associated with a reduction in body mass index (BMI) of only approximately 1 point.3 Psychiatrists (and other clinicians who prescribe psychotropic medications that can cause weight gain or metabolic dysregulation) therefore need to become familiar with alternative or adjunctive weight loss options. The use of a relatively new class of incretin mimetics called glucagon-like peptide 1 (GLP-1) agonists (Table) has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation.

What are GLP-1 agonists?
GLP-1 is a hormone secreted by L cells in the intestinal mucosa in response to food. GLP-1 agonists reduce blood sugar by increasing insulin secretion, decreasing glucagon release (thus downregulating further increases in blood sugar), and reducing insulin resistance. GLP-1 agonists also reduce appetite by directly stimulating the satiety center and slowing gastric emptying and GI motility. In addition to GLP-1 agonism, some medications in this family (notably tirzepatide) also agonize a second hormone, glucose-dependent insulinotropic polypeptide, which can further induce insulin secretion as well as decrease stomach acid secretion, potentially delivering an even more substantial reduction in appetite and weight.
Routes of administration and FDA indications
Due to limited bioavailability, most GLP-1 agonists require subcutaneous (SC) injections (the sole exception is the Rybelsus brand of semaglutide, which comes in a daily pill form). Most are FDA-approved not specifically for weight loss but for patients with type 2 diabetes (defined as a hemoglobin A1C ≥6.5% or a fasting blood glucose level ≥126 mg/dL). Weight loss represents a secondary outcome for GLP-1 agonists FDA-approved for glycemic control in patients with type 2 diabetes. The 2 current exceptions to this classification are the Wegovy brand of semaglutide (ie, dosing of 2.4 mg) and the Saxenda brand of liraglutide, both of which carry FDA indications for chronic weight management alone (when paired with dietary and lifestyle modification) in individuals who are obese (BMI >30 kg/m2) regardless of the presence or absence of diabetes, or for persons who are overweight (BMI >27 kg/m2) and have ≥1 weight-related comorbid condition (eg, hypertension, type 2 diabetes, or dyslipidemia). Although patients at risk for diabetes (ie, prediabetes, defined as a hemoglobin A1C 5.7% to 6.4% or a fasting blood glucose level 100 to 125 mg/dL) were included in FDA registration trials of Saxenda or Wegovy, prediabetes is not an FDA indication for any GLP-1 agonist.
Data in weight loss
Most of the existing empirical data on weight loss with GLP-1 agonists come from studies of individuals who are overweight or obese, with or without type 2 diabetes, rather than from studies using these agents to counteract iatrogenic weight gain. In a retrospective cohort study of patients with type 2 diabetes, coadministration with serotonergic antidepressants (eg, citalopram/escitalopram) was associated with attenuation of the weight loss effects of GLP-1 agonists.4
Liraglutide currently is the sole GLP-1 agonist studied for treating SGA-associated weight gain. A 16-week randomized trial compared once-daily SC injected liraglutide vs placebo in patients with schizophrenia who incurred weight gain and prediabetes after taking olanzapine or clozapine.5 Significantly more patients taking liraglutide than placebo developed normal glucose tolerance (64% vs 16%), and body weight decreased by a mean of 5.3 kg.
Continue to: In studies of semaglutide...
In studies of semaglutide for overweight/obese patients with type 2 diabetes or prediabetes, clinical trials of oral semaglutide (Rybelsus) found a mean weight loss over 26 weeks of -1.0 kg with dosing at 7 mg/d and -2.6 kg with dosing at 14 mg/d.6 A 68-week placebo-controlled trial of semaglutide (dosed at 2.4 mg SC weekly) for overweight/obese adults who did not have diabetes yielded a -15.3 kg weight loss (vs -2.6 kg with placebo); one-half of those who received semaglutide lost 15% of their initial body weight (Figure 1A and Figure 1B).7 Similar findings with semaglutide 2.4 mg SC weekly (Wegovy) were observed in overweight/obese adolescents, with 73% of participants losing ≥5% of their baseline weight.8 A comparative randomized trial in patients with type 2 diabetes also found modestly but significantly greater weight loss with oral semaglutide than with SC liraglutide.9


In a 72-week study of tirzepatide specifically for weight loss in nondiabetic patients who were overweight or obese, findings were especially dramatic (Figure 2A and Figure 2B).10 An overall 15% decrease in body weight was observed with 5 mg/week dosing alongside a 19.5% decrease in body weight with 10 mg/week dosing and a 20.9% weight reduction with 15 mg/week dosing.10 As noted in Figure 2B, the observed pattern of weight loss occurred along an exponential decay curve. Notably, a comparative study of tirzepatide vs once-weekly semaglutide (1 mg) in patients with type 2 diabetes11 found significantly greater dose-dependent weight loss with tirzepatide than semaglutide (-1.9 kg at 5 mg, -3.6 kg at 10 mg, and -5.5 kg at 15 mg)—although the somewhat low dosing of semaglutide may have limited its optimal possible weight loss benefit.


Tolerability
Adverse effects with GLP-1 agonists are mainly gastrointestinal (eg, nausea, vomiting, abdominal pain, diarrhea, or constipation)5-11 and generally transient. SC administration is performed in fatty tissue of the abdomen, thigh, or upper arm; site rotation is recommended to minimize injection site pain. All GLP-1 agonists carry manufacturers’ warning and precaution statements identifying the rare potential for acute pancreatitis, acute gall bladder disease, acute kidney injury, and hypoglycemia. Animal studies also have suggested an increased, dose-dependent risk for thyroid C-cell tumors with GLP-1 agonists; this has not been observed in human trials, although postmarketing pharmacovigilance reports have identified cases of medullary thyroid carcinoma in patients who took liraglutide. A manufacturer’s boxed warning indicates that a personal or family history of medullary carcinoma of the thyroid poses a contraindication for taking semaglutide, liraglutide, or tirzepatide.
Initial evidence prompts additional questions
GLP-1 agonists represent an emerging class of novel agents that can modulate glycemic dysregulation and overweight/obesity, often with dramatic results whose magnitude rivals the efficacy of bariatric surgery. Once-weekly formulations of semaglutide (Wegovy) and daily liraglutide (Saxenda) are FDA-approved for weight loss in patients who are overweight or obese while other existing formulations are approved solely for patients with type 2 diabetes, although it is likely that broader indications for weight loss (regardless of glycemic status) are forthcoming. Targeted use of GLP-1 agonists to counteract SGA-associated weight gain is supported by a handful of preliminary reports, with additional studies likely to come. Unanswered questions include:
- When should GLP-1 agonists be considered within a treatment algorithm for iatrogenic weight gain relative to other antidote strategies such as metformin or appetite-suppressing anticonvulsants?
- How effective might GLP-1 agonists be for iatrogenic weight gain from non-SGA psychotropic medications, such as serotonergic antidepressants?
- When and how can GLP-1 agonists be safely coprescribed with other nonincretin mimetic weight loss medications?
- When should psychiatrists prescribe GLP-1 agonists, or do so collaboratively with primary care physicians or endocrinologists, particularly in patients with metabolic syndrome?
Followers of the rapidly emerging literature in this area will likely find themselves best positioned to address these and other questions about optimal management of psychotropic-induced weight gain for the patients they treat.
Bottom Line
The use of glucagon-like peptide 1 (GLP-1) agonists, a relatively new class of incretin mimetics, has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation. Preliminary reports support the potential targeted use of GLP-1 agonists to counteract weight gain associated with second-generation antipsychotics.
Related Resources
- Singh F, Allen A, Ianni A. Managing metabolic syndrome in patients with schizophrenia. Current Psychiatry. 2020;19(12):20-24,26. doi:10.12788/cp.0064
- Ard J, Fitch A, Fruh S, et al. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. 2021;38(6):2821- 2839. doi:10.1007/s12325-021-01710-0
Drug Brand Names
Amantadine • Gocovri
Citalopram • Celexa
Clozapine • Clozaril
Escitalopram • Lexapro
Liraglutide • Victoza, Saxenda
Metformin • Glucophage
Naltrexone • ReVia
Olanzapine • Zyprexa
Olanzapine/samidorphan • Lybalvi
Phentermine • Ionamin
Semaglutide • Rybelsus, Ozempic, Wegovy
Tirzepatide • Mounjaro
Topiramate • Topamax
Zonisamide • Zonegran
1. Afzal M, Siddiqi N, Ahmad B, et al. Prevalence of overweight and obesity in people with severe mental illness: systematic review and meta-analysis. Front Endocrinol (Lausanne). 2021;25;12:769309.
2. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Safety. 2020;19(3):295-314.
3. de Silva AV, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341.
4. Durell N, Franks R, Coon S, et al. Effects of antidepressants on glucagon-like peptide-1 receptor agonist-related weight loss. J Pharm Technol. 2022;38(5):283-288.
5. Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(7):719-728.
6. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724-1732.
7. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
8. Weghuber D, Barrett T, Barrientos-Pérez M, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. Published online November 2, 2022. doi:10.1056/NEJMoa2208601.
9. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomized, double-blind, phase 3a trial. Lancet. 2019;394(10192):39-50.
10. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205-216.
11. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515.
1. Afzal M, Siddiqi N, Ahmad B, et al. Prevalence of overweight and obesity in people with severe mental illness: systematic review and meta-analysis. Front Endocrinol (Lausanne). 2021;25;12:769309.
2. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Safety. 2020;19(3):295-314.
3. de Silva AV, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341.
4. Durell N, Franks R, Coon S, et al. Effects of antidepressants on glucagon-like peptide-1 receptor agonist-related weight loss. J Pharm Technol. 2022;38(5):283-288.
5. Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(7):719-728.
6. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724-1732.
7. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
8. Weghuber D, Barrett T, Barrientos-Pérez M, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. Published online November 2, 2022. doi:10.1056/NEJMoa2208601.
9. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomized, double-blind, phase 3a trial. Lancet. 2019;394(10192):39-50.
10. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205-216.
11. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515.
