User login
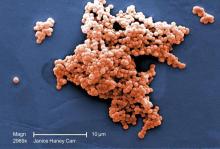
It once was a very a common scenario. A baby born at term looks fine for the first 24 hours of life. Without much warning, the infant develops grunting, tachypnea, and tachycardia. Sepsis is suspected, and within a few hours, group B streptococcus (GBS) is isolated from a blood culture.
According to the CDC, a woman colonized with Group B strep at the time of delivery has a 1 in 200 chance of delivering a baby who will develop GBS disease. Antibiotics during labor drop that risk to 1 in 4,000. It’s not perfect – there are still about 1,000 cases annually in the United States – but is has been a major step forward. In recent years, the incidence of early-onset GBS disease has fallen to just under 0.3 cases per 1,000 live births, and some experts think rates could go even lower with improved adherence to current guidelines.
Reducing late-onset GBS disease requires a different strategy. Efforts to develop a GBS vaccine that could be given to pregnant women continue, and recent phase 2 trials of a trivalent polysaccharide-protein conjugate vaccine looked promising. Fingers crossed that we won’t have to wait until we celebrate the 75th anniversary of Pediatric News to tout the impact of maternal immunization on reducing GBS disease in infants.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
It once was a very a common scenario. A baby born at term looks fine for the first 24 hours of life. Without much warning, the infant develops grunting, tachypnea, and tachycardia. Sepsis is suspected, and within a few hours, group B streptococcus (GBS) is isolated from a blood culture.
According to the CDC, a woman colonized with Group B strep at the time of delivery has a 1 in 200 chance of delivering a baby who will develop GBS disease. Antibiotics during labor drop that risk to 1 in 4,000. It’s not perfect – there are still about 1,000 cases annually in the United States – but is has been a major step forward. In recent years, the incidence of early-onset GBS disease has fallen to just under 0.3 cases per 1,000 live births, and some experts think rates could go even lower with improved adherence to current guidelines.
Reducing late-onset GBS disease requires a different strategy. Efforts to develop a GBS vaccine that could be given to pregnant women continue, and recent phase 2 trials of a trivalent polysaccharide-protein conjugate vaccine looked promising. Fingers crossed that we won’t have to wait until we celebrate the 75th anniversary of Pediatric News to tout the impact of maternal immunization on reducing GBS disease in infants.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
It once was a very a common scenario. A baby born at term looks fine for the first 24 hours of life. Without much warning, the infant develops grunting, tachypnea, and tachycardia. Sepsis is suspected, and within a few hours, group B streptococcus (GBS) is isolated from a blood culture.
According to the CDC, a woman colonized with Group B strep at the time of delivery has a 1 in 200 chance of delivering a baby who will develop GBS disease. Antibiotics during labor drop that risk to 1 in 4,000. It’s not perfect – there are still about 1,000 cases annually in the United States – but is has been a major step forward. In recent years, the incidence of early-onset GBS disease has fallen to just under 0.3 cases per 1,000 live births, and some experts think rates could go even lower with improved adherence to current guidelines.
Reducing late-onset GBS disease requires a different strategy. Efforts to develop a GBS vaccine that could be given to pregnant women continue, and recent phase 2 trials of a trivalent polysaccharide-protein conjugate vaccine looked promising. Fingers crossed that we won’t have to wait until we celebrate the 75th anniversary of Pediatric News to tout the impact of maternal immunization on reducing GBS disease in infants.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].