User login
VIENNA – The investigational interleukin-23 inhibitor guselkumab decisively outperformed adalimumab in a head-to-head comparison for treatment of moderate or severe plaque psoriasis in the pivotal VOYAGE 1 study, Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
VOYAGE 1 was a 48-week, multicenter, international phase III trial in which 837 patients were randomized 2:2:1 to guselkumab, adalimumab (Humira), or placebo, with the placebo group switched to guselkumab at 16 weeks. Roughly three-quarters of patients had moderate psoriasis, the rest had severe disease. One in five had previously been treated with biologic agents; the only biologic disallowed was adalimumab.
The primary endpoints required by regulatory agencies involved efficacy comparisons between guselkumab and placebo at 16 weeks. Those results were a foregone conclusion. Far more arresting were the prespecified secondary endpoints comparing guselkumab to adalimumab at 24 and 48 weeks.
“These are very exciting results. We’re seeing efficacy in this trial that has not ever been seen before in a phase III study,” said Dr. Blauvelt, president of the Oregon Medical Research Center in Portland.
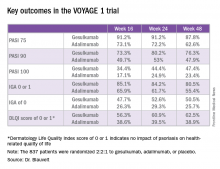
Take, for example, an efficacy yardstick dermatologists are quite familiar with: the PASI 75 response, defined as at least a 75% improvement from baseline in the Psoriasis Area Severity Index score, which averaged 22 at baseline in this trial. The PASI 75 rate in guselkumab-treated patients was 91.2% at 16 weeks, remained at 91.2% at 24 weeks, and was 87.8% at week 48.
“To my knowledge this is the highest PASI 75 response rate that’s been seen in a phase III study of any biologic in psoriasis,” the dermatologist said.
The PASI 75 rates with adalimumab, a tumor necrosis factor–alpha blocker widely prescribed for psoriasis, were markedly lower, although just a few years ago they would have been considered stratospheric: 73.1% at 16 weeks, 72.2% at 24 weeks, and 62.6% at 48 weeks.
The same pattern held for PASI 90, PASI 100, Investigator’s Global Assessment (IGA), and quality-of-life measures.
“There is a clear early separation of guselkumab from adalimumab, sustained over time, curves staying flat, responses not dropping off,” Dr. Blauvelt said in summary.
Guselkumab was dosed at 100 mg subcutaneously at weeks 0 and 4, then every 8 weeks thereafter. Adalimumab was dosed subcutaneously at 80 mg at week 0, 40 mg at week 1, and then 40 mg every other week.
The two coprimary outcomes at week 16 in VOYAGE 1 were the guselkumab and placebo groups’ rates of clear or almost clear skin as defined by an IGA score of 0 or 1, and their PASI 90 response rates. An IGA of 0 or 1 was achieved by 85.1% of the guselkumab group compared with 6.9% on placebo. The week-16 PASI 90 rates – a “high bar” Dr. Blauvelt noted – were 73.3% and 2.9%, respectively.
“Clearly we’re now in an era where PASI 90 is the new PASI 75,” said session cochair Lajos Kemény, MD, professor and chairman of the department of dermatology and allergology at the University of Szeged, Hungary.
Guselkumab is a human monoclonal antibody directed at the p-19 subunit of interleukin-23, thereby preventing the inflammatory cytokine from binding to its receptor. In contrast, ustekinumab (Stelara) blocks both IL-23 and IL-12. Given that ustekinumab has established an excellent long-term safety record in PSOLAR, the Psoriasis Longitudinal Assessment and Registry, it stands to reason that guselkumab should have a favorable safety profile, too, since it targets only one of the two cytokines (J Drugs Dermatol. 2015 Jul;14[7]:706-14). And this indeed proved to be the case through 48 weeks in VOYAGE 1, according to Dr. Blauvelt.
Infections treated with antibiotics occurred in 6.1% of the guselkumab group, 7.2% of patients on adalimumab, and 7.5% on placebo. Mild to moderate injection site reactions occurred in 2.4% of patients on guselkumab and 7.5% on adalimumab. One patient on each of the biologics experienced an acute MI. Two malignancies occurred, both in the guselkumab group. One was prostate cancer, the other was a case of male breast cancer in a patient with a breast mass present at enrollment.
Results of two additional pivotal phase III trials, VOYAGE 2 and NAVIGATE, will be presented at future meetings. NAVIGATE is looking specifically at guselkumab’s performance in psoriasis patients with an inadequate response to ustekinumab.
“Those results look promising. It appears that patients who didn’t clear adequately on ustekinumab do well on guselkumab,” Dr. Blauvelt said in response to an audience question.
A phase II study of guselkumab in treating moderate to severe psoriatic arthritis is ongoing.
VOYAGE 1 was funded by Janssen, which is developing guselkumab. Dr. Blauvelt reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.
VIENNA – The investigational interleukin-23 inhibitor guselkumab decisively outperformed adalimumab in a head-to-head comparison for treatment of moderate or severe plaque psoriasis in the pivotal VOYAGE 1 study, Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
VOYAGE 1 was a 48-week, multicenter, international phase III trial in which 837 patients were randomized 2:2:1 to guselkumab, adalimumab (Humira), or placebo, with the placebo group switched to guselkumab at 16 weeks. Roughly three-quarters of patients had moderate psoriasis, the rest had severe disease. One in five had previously been treated with biologic agents; the only biologic disallowed was adalimumab.
The primary endpoints required by regulatory agencies involved efficacy comparisons between guselkumab and placebo at 16 weeks. Those results were a foregone conclusion. Far more arresting were the prespecified secondary endpoints comparing guselkumab to adalimumab at 24 and 48 weeks.
“These are very exciting results. We’re seeing efficacy in this trial that has not ever been seen before in a phase III study,” said Dr. Blauvelt, president of the Oregon Medical Research Center in Portland.
Take, for example, an efficacy yardstick dermatologists are quite familiar with: the PASI 75 response, defined as at least a 75% improvement from baseline in the Psoriasis Area Severity Index score, which averaged 22 at baseline in this trial. The PASI 75 rate in guselkumab-treated patients was 91.2% at 16 weeks, remained at 91.2% at 24 weeks, and was 87.8% at week 48.
“To my knowledge this is the highest PASI 75 response rate that’s been seen in a phase III study of any biologic in psoriasis,” the dermatologist said.
The PASI 75 rates with adalimumab, a tumor necrosis factor–alpha blocker widely prescribed for psoriasis, were markedly lower, although just a few years ago they would have been considered stratospheric: 73.1% at 16 weeks, 72.2% at 24 weeks, and 62.6% at 48 weeks.
The same pattern held for PASI 90, PASI 100, Investigator’s Global Assessment (IGA), and quality-of-life measures.
“There is a clear early separation of guselkumab from adalimumab, sustained over time, curves staying flat, responses not dropping off,” Dr. Blauvelt said in summary.
Guselkumab was dosed at 100 mg subcutaneously at weeks 0 and 4, then every 8 weeks thereafter. Adalimumab was dosed subcutaneously at 80 mg at week 0, 40 mg at week 1, and then 40 mg every other week.
The two coprimary outcomes at week 16 in VOYAGE 1 were the guselkumab and placebo groups’ rates of clear or almost clear skin as defined by an IGA score of 0 or 1, and their PASI 90 response rates. An IGA of 0 or 1 was achieved by 85.1% of the guselkumab group compared with 6.9% on placebo. The week-16 PASI 90 rates – a “high bar” Dr. Blauvelt noted – were 73.3% and 2.9%, respectively.
“Clearly we’re now in an era where PASI 90 is the new PASI 75,” said session cochair Lajos Kemény, MD, professor and chairman of the department of dermatology and allergology at the University of Szeged, Hungary.
Guselkumab is a human monoclonal antibody directed at the p-19 subunit of interleukin-23, thereby preventing the inflammatory cytokine from binding to its receptor. In contrast, ustekinumab (Stelara) blocks both IL-23 and IL-12. Given that ustekinumab has established an excellent long-term safety record in PSOLAR, the Psoriasis Longitudinal Assessment and Registry, it stands to reason that guselkumab should have a favorable safety profile, too, since it targets only one of the two cytokines (J Drugs Dermatol. 2015 Jul;14[7]:706-14). And this indeed proved to be the case through 48 weeks in VOYAGE 1, according to Dr. Blauvelt.
Infections treated with antibiotics occurred in 6.1% of the guselkumab group, 7.2% of patients on adalimumab, and 7.5% on placebo. Mild to moderate injection site reactions occurred in 2.4% of patients on guselkumab and 7.5% on adalimumab. One patient on each of the biologics experienced an acute MI. Two malignancies occurred, both in the guselkumab group. One was prostate cancer, the other was a case of male breast cancer in a patient with a breast mass present at enrollment.
Results of two additional pivotal phase III trials, VOYAGE 2 and NAVIGATE, will be presented at future meetings. NAVIGATE is looking specifically at guselkumab’s performance in psoriasis patients with an inadequate response to ustekinumab.
“Those results look promising. It appears that patients who didn’t clear adequately on ustekinumab do well on guselkumab,” Dr. Blauvelt said in response to an audience question.
A phase II study of guselkumab in treating moderate to severe psoriatic arthritis is ongoing.
VOYAGE 1 was funded by Janssen, which is developing guselkumab. Dr. Blauvelt reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.
VIENNA – The investigational interleukin-23 inhibitor guselkumab decisively outperformed adalimumab in a head-to-head comparison for treatment of moderate or severe plaque psoriasis in the pivotal VOYAGE 1 study, Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
VOYAGE 1 was a 48-week, multicenter, international phase III trial in which 837 patients were randomized 2:2:1 to guselkumab, adalimumab (Humira), or placebo, with the placebo group switched to guselkumab at 16 weeks. Roughly three-quarters of patients had moderate psoriasis, the rest had severe disease. One in five had previously been treated with biologic agents; the only biologic disallowed was adalimumab.
The primary endpoints required by regulatory agencies involved efficacy comparisons between guselkumab and placebo at 16 weeks. Those results were a foregone conclusion. Far more arresting were the prespecified secondary endpoints comparing guselkumab to adalimumab at 24 and 48 weeks.
“These are very exciting results. We’re seeing efficacy in this trial that has not ever been seen before in a phase III study,” said Dr. Blauvelt, president of the Oregon Medical Research Center in Portland.
Take, for example, an efficacy yardstick dermatologists are quite familiar with: the PASI 75 response, defined as at least a 75% improvement from baseline in the Psoriasis Area Severity Index score, which averaged 22 at baseline in this trial. The PASI 75 rate in guselkumab-treated patients was 91.2% at 16 weeks, remained at 91.2% at 24 weeks, and was 87.8% at week 48.
“To my knowledge this is the highest PASI 75 response rate that’s been seen in a phase III study of any biologic in psoriasis,” the dermatologist said.
The PASI 75 rates with adalimumab, a tumor necrosis factor–alpha blocker widely prescribed for psoriasis, were markedly lower, although just a few years ago they would have been considered stratospheric: 73.1% at 16 weeks, 72.2% at 24 weeks, and 62.6% at 48 weeks.
The same pattern held for PASI 90, PASI 100, Investigator’s Global Assessment (IGA), and quality-of-life measures.
“There is a clear early separation of guselkumab from adalimumab, sustained over time, curves staying flat, responses not dropping off,” Dr. Blauvelt said in summary.
Guselkumab was dosed at 100 mg subcutaneously at weeks 0 and 4, then every 8 weeks thereafter. Adalimumab was dosed subcutaneously at 80 mg at week 0, 40 mg at week 1, and then 40 mg every other week.
The two coprimary outcomes at week 16 in VOYAGE 1 were the guselkumab and placebo groups’ rates of clear or almost clear skin as defined by an IGA score of 0 or 1, and their PASI 90 response rates. An IGA of 0 or 1 was achieved by 85.1% of the guselkumab group compared with 6.9% on placebo. The week-16 PASI 90 rates – a “high bar” Dr. Blauvelt noted – were 73.3% and 2.9%, respectively.
“Clearly we’re now in an era where PASI 90 is the new PASI 75,” said session cochair Lajos Kemény, MD, professor and chairman of the department of dermatology and allergology at the University of Szeged, Hungary.
Guselkumab is a human monoclonal antibody directed at the p-19 subunit of interleukin-23, thereby preventing the inflammatory cytokine from binding to its receptor. In contrast, ustekinumab (Stelara) blocks both IL-23 and IL-12. Given that ustekinumab has established an excellent long-term safety record in PSOLAR, the Psoriasis Longitudinal Assessment and Registry, it stands to reason that guselkumab should have a favorable safety profile, too, since it targets only one of the two cytokines (J Drugs Dermatol. 2015 Jul;14[7]:706-14). And this indeed proved to be the case through 48 weeks in VOYAGE 1, according to Dr. Blauvelt.
Infections treated with antibiotics occurred in 6.1% of the guselkumab group, 7.2% of patients on adalimumab, and 7.5% on placebo. Mild to moderate injection site reactions occurred in 2.4% of patients on guselkumab and 7.5% on adalimumab. One patient on each of the biologics experienced an acute MI. Two malignancies occurred, both in the guselkumab group. One was prostate cancer, the other was a case of male breast cancer in a patient with a breast mass present at enrollment.
Results of two additional pivotal phase III trials, VOYAGE 2 and NAVIGATE, will be presented at future meetings. NAVIGATE is looking specifically at guselkumab’s performance in psoriasis patients with an inadequate response to ustekinumab.
“Those results look promising. It appears that patients who didn’t clear adequately on ustekinumab do well on guselkumab,” Dr. Blauvelt said in response to an audience question.
A phase II study of guselkumab in treating moderate to severe psoriatic arthritis is ongoing.
VOYAGE 1 was funded by Janssen, which is developing guselkumab. Dr. Blauvelt reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.
Key clinical point:
Major finding: The PASI 90 response rate at 24 weeks was 80% in psoriasis patients on guselkumab compared with 53% in those on adalimumab.
Data source: A randomized, multinational, 48-week, pivotal phase III clinical trial involving 837 psoriasis patients assigned to guselkumab, adalimumab, or placebo.
Disclosures: The VOYAGE 1 trial was funded by Janssen, which is developing guselkumab. The study presenter reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.