User login
Polycythemia vera (PV) is a rare myeloproliferative neoplasm affecting 44 to 57 individuals per 100,000 in the United States.1,2 It is characterized by somatic mutations in the hematopoietic stem cell, resulting in hyperproliferation of mature myeloid lineage cells.2 Sustained erythrocytosis is a hallmark of PV, although many patients also have leukocytosis and thrombocytosis.2,3 These patients have increased inherent thrombotic risk with arterial events reported to occur at rates of 7 to 21/1000 person-years and venous thrombotic events at 5 to 20/1000 person-years.4-7 Thrombotic and cardiovascular events are leading causes of morbidity and mortality, resulting in a reduced overall survival of patients with PV compared with the general population.3,8-10
Blood Cell Counts and Thrombotic Events in PV
Treatment strategies for patients with PV mainly aim to prevent or manage thrombotic and bleeding complications through normalization of blood counts.11 Hematocrit (Hct) control has been reported to be associated with reduced thrombotic risk in patients with PV. This was shown and popularized by the prospective, randomized Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial in which participants were randomized 1:1 to maintaining either a low (< 45%) or high (45%-50%) Hct for 5 years to examine the long-term effects of more- or less-intensive cytoreductive therapy.12 Patients in the low-Hct group were found to have a lower rate of death from cardiovascular events or major thrombosis (1.1/100 person-years in the low-Hct group vs 4.4 in the high-Hct group; hazard ratio [HR], 3.91; 95% confidence interval [CI], 1.45-10.53; P = .007). Likewise, cardiovascular events occurred at a lower rate in patients in the low-Hct group compared with the high-Hct group (4.4% vs 10.9% of patients, respectively; HR, 2.69; 95% CI, 1.19-6.12; P = .02).12
Leukocytosis has also been linked to elevated risk for vascular events as shown in several studies, including the real-world European Collaboration on Low-Dose Aspirin in PV (ECLAP) observational study and a post hoc subanalysis of the CYTO-PV study.13,14 In a multivariate, time-dependent analysis in ECLAP, patients with white blood cell (WBC) counts > 15 × 109/L had a significant increase in the risk of thrombosis compared with those who had lower WBC counts, with higher WBC count more strongly associated with arterial than venous thromboembolism.13 In CYTO-PV, a significant correlation between elevated WBC count (≥ 11 × 109/L vs reference level of < 7 × 109/L) and time-dependent risk of major thrombosis was shown (HR, 3.9; 95% CI, 1.24-12.3; P = .02).14 Likewise, WBC count ≥ 11 × 109/L was found to be a predictor of subsequent venous events in a separate single-center multivariate analysis of patients with PV.8
Although CYTO-PV remains one of the largest prospective landmark studies in PV demonstrating the impact of Hct control on thrombosis, it is worthwhile to note that the patients in the high-Hct group who received less frequent myelosuppressive therapy with hydroxyurea than the low-Hct group also had higher WBC counts.12,15 Work is needed to determine the relative effects of high Hct and high WBC counts on PV independent of each other.
The Veteran Population with PV
Two recently published retrospective analyses from Parasuraman and colleagues used data from the Veterans Health Administration (VHA), the largest integrated health care system in the US, with an aim to replicate findings from CYTO-PV in a real-world population.16,17 The 2 analyses focused independently on the effects of Hct control and WBC count on the risk of a thrombotic event in patients with PV.
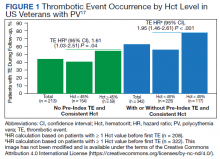
In the first retrospective analysis, 213 patients with PV and no prior thrombosis were placed into groups based on whether Hct levels were consistently either < 45% or ≥ 45% throughout the study period.17 The mean follow-up time was 2.3 years, during which 44.1% of patients experienced a thrombotic event (Figure 1). Patients with Hct levels < 45% had a lower rate of thrombotic events compared to those with levels ≥ 45% (40.3% vs 54.2%, respectively; HR, 1.61; 95% CI, 1.03-2.51; P = .04). In a sensitivity analysis that included patients with pre-index thrombotic events (N = 342), similar results were noted (55.6% vs 76.9% between the < 45% and ≥ 45% groups, respectively; HR, 1.95; 95% CI, 1.46-2.61; P < .001).
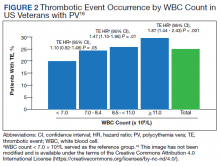
In the second analysis, the authors investigated the relationship between WBC counts and thrombotic events.16 Evaluable patients (N = 1565) were grouped into 1 of 4 cohorts based on the last WBC measurement taken during the study period before a thrombotic event or through the end of follow-up: (1) WBC < 7.0 × 109/L, (2) 7.0 to 8.4 × 109/L, (3) 8.5 to < 11.0 × 109/L, or (4) ≥ 11.0 × 109/L. Mean follow-up time ranged from 3.6 to 4.5 years among WBC count cohorts, during which 24.9% of patients experienced a thrombotic event. Compared with the reference cohort (WBC < 7.0 × 109/L), a significant positive association between WBC counts and thrombotic event occurrence was observed among patients with WBC counts of 8.5 to < 11.0 × 109/L (HR, 1.47; 95% CI, 1.10-1.96; P < .01) and ≥ 11 × 109/L (HR, 1.87; 95% CI, 1.44-2.43; P < .001) (Figure 2).16 When including all patients in a sensitivity analysis regardless of whether they experienced thrombotic events before the index date (N = 1876), similar results were obtained (7.0-8.4 × 109/L group: HR, 1.22; 95% CI, 0.97-1.55; P = .0959; 8.5 - 11.0 × 109/L group: HR, 1.41; 95% CI, 1.10-1.81; P = .0062; ≥ 11.0 × 109/L group: HR, 1.53; 95% CI, 1.23-1.91; P < .001; compared with < 7.0 × 109/L reference group). Rates of phlebotomy and cytoreductive treatments were similar across groups.16
Some limitations to these studies are attributable to their retrospective design, reliance on health records, and the VHA population characteristics, which differ from the general population. For example, in this analysis, patients with PV in the VHA population had significantly increased risk of thrombotic events, even at a lower WBC count threshold (≥ 8.5 × 109/L) compared with those reported in CYTO-PV (≥ 11 × 109/L). Furthermore, approximately one-third of patients had elevated WBC levels, compared with 25.5% in the CYTO-PV study.14,16 This is most likely due to the unique nature of the VHA patient population, who are predominantly older adult men and generally have a higher comorbidity burden. A notable pre-index comorbidity burden was reported in the VHA population in the Hct analysis, even when compared to patients with PV in the general US population (Charlson Comorbidity Index score, 1.3 vs 0.8).6,17 Comorbid conditions such as hypertension, diabetes, and tobacco use, which are most common among the VHA population, are independently associated with higher risk of cardiovascular and thrombotic events.18,19 However, whether these higher levels of comorbidities affected the type of treatments they received was not elucidated, and the effectiveness of treatments to maintain target Hct levels was not addressed in the study.
Current PV Management and Future Implications
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology in myeloproliferative neoplasms recommend maintaining Hct levels < 45% in patients with PV.11 Patients with high-risk disease (age ≥ 60 years and/or history of thrombosis) are monitored for new thrombosis or bleeding and are managed for their cardiovascular risk factors. In addition, they receive low-dose aspirin (81-100 mg/day), undergo phlebotomy to maintain an Hct < 45%, and are managed with pharmacologic cytoreductive therapy. Cytoreductive therapy primarily consists of hydroxyurea or peginterferon alfa-2a for younger patients. Ruxolitinib, a Janus kinase (JAK1)/JAK2 inhibitor, is now approved by the US Food and Drug Administration as second-line treatment for those with PV that is intolerant or unresponsive to hydroxyurea or peginterferon alfa-2a treatments.11,20 However, the role of cytoreductive therapy is not clear for patients with low-risk disease (age < 60 years and no history of thrombosis). These patients are managed for their cardiovascular risk factors, undergo phlebotomy to maintain an Hct < 45%, are maintained on low-dose aspirin (81-100 mg/day), and are monitored for indications for cytoreductive therapy, which include any new thrombosis or disease-related major bleeding, frequent or persistent need for phlebotomy with poor tolerance for the procedure, splenomegaly, thrombocytosis, leukocytosis, and disease-related symptoms (eg, aquagenic pruritus, night sweats, fatigue).
Even though the current guidelines recommend maintaining a target Hct of < 45% in patients with high-risk PV, the role of Hct as the main determinant of thrombotic risk in patients with PV is still debated.21 In JAK2V617F-positive essential thrombocythemia, Hct levels are usually normal but risk of thrombosis is nevertheless still significant.22 The risk of thrombosis is significantly lower in primary familial and congenital polycythemia and much lower in secondary erythrocytosis such as cyanotic heart disease, long-term native dwellers of high altitude, and those with high-oxygen–affinity hemoglobins.21,23 In secondary erythrocytosis from hypoxia or upregulated hypoxic pathway such as hypoxia inducible factor-2α (HIF-2α) mutation and Chuvash erythrocytosis, the risk of thrombosis is more associated with the upregulated HIF pathway and its downstream consequences, rather than the elevated Hct level.24
However, most current literature supports the association of increased risk of thrombosis with higher Hct and high WBC count in patients with PV. In addition, the underlying mechanism of thrombogenesis still remains elusive; it is likely a complex process that involves interactions among multiple components, including elevated blood counts arising from clonal hematopoiesis, JAK2V617F allele burden, and platelet and WBC activation and their interaction with endothelial cells and inflammatory cytokines.25
Nevertheless, Hct control and aspirin use are current standard of care for patients with PV to mitigate thrombotic risk, and the results from the 2 analyses by Parasuraman and colleagues, using real-world data from the VHA, support the current practice guidelines to maintain Hct < 45% in these patients. They also provide additional support for considering WBC counts when determining patient risk and treatment plans. Although treatment response criteria from the European LeukemiaNet include achieving normal WBC levels to decrease the risk of thrombosis, current NCCN guidelines do not include WBC counts as a component for establishing patient risk or provide a target WBC count to guide patient management.11,26,27 Updates to these practice guidelines may be warranted. In addition, further study is needed to understand the mechanism of thrombogenesis in PV and other myeloproliferative disorders in order to develop novel therapeutic targets and improve patient outcomes.
Acknowledgments
Writing assistance was provided by Tania Iqbal, PhD, an employee of ICON (North Wales, PA), and was funded by Incyte Corporation (Wilmington, DE).
1. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595-600. doi:10.3109/10428194.2013.813500
2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. doi:10.1182/blood-2016-03-643544
3. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874-1881. doi:10.1038/leu.2013.163
4. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224-2232. doi:10.1200/JCO.2005.07.062
5. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110(3):840-846. doi:10.1182/blood-2006-12-064287
6. Goyal RK, Davis KL, Cote I, Mounedji N, Kaye JA. Increased incidence of thromboembolic event rates in patients diagnosed with polycythemia vera: results from an observational cohort study. Blood (ASH Annual Meeting Abstracts). 2014;124:4840. doi:10.1182/blood.V124.21.4840.4840
7. Barbui T, Carobbio A, Rumi E, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124(19):3021-3023. doi:10.1182/blood-2014-07-591610 8. Cerquozzi S, Barraco D, Lasho T, et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer J. 2017;7(12):662. doi:10.1038/s41408-017-0035-6
9. Stein BL, Moliterno AR, Tiu RV. Polycythemia vera disease burden: contributing factors, impact on quality of life, and emerging treatment options. Ann Hematol. 2014;93(12):1965-1976. doi:10.1007/s00277-014-2205-y
10. Hultcrantz M, Kristinsson SY, Andersson TM-L, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995-3001. doi:10.1200/JCO.2012.42.1925
11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in myeloproliferative neoplasms (Version 1.2020). Accessed March 3, 2022. https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
12. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. doi:10.1056/NEJMoa1208500
13. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446-2452. doi:10.1182/blood-2006-08-042515
14. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126(4):560-561. doi:10.1182/blood-2015-04-638593
15. Prchal JT, Gordeuk VR. Treatment target in polycythemia vera. N Engl J Med. 2013;368(16):1555-1556. doi:10.1056/NEJMc1301262
16. Parasuraman S, Yu J, Paranagama D, et al. Elevated white blood cell levels and thrombotic events in patients with polycythemia vera: a real-world analysis of Veterans Health Administration data. Clin Lymphoma Myeloma Leuk. 2020;20(2):63-69. doi:10.1016/j.clml.2019.11.010
17. Parasuraman S, Yu J, Paranagama D, et al. Hematocrit levels and thrombotic events in patients with polycythemia vera: an analysis of Veterans Health Administration data. Ann Hematol. 2019;98(11):2533-2539. doi:10.1007/s00277-019-03793-w
18. WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332-e1345. doi:10.1016/S2214-109X(19)30318-3.
19. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. doi:10.1161/CIRCULATIONAHA.107.699579
20. Jakafi. Package insert. Incyte Corporation; 2020.
21. Gordeuk VR, Key NS, Prchal JT. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica. 2019;104(4):653-658. doi:10.3324/haematol.2018.210732
22. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857-5859. doi:10.1182/blood-2011-02-339002
23. Perloff JK, Marelli AJ, Miner PD. Risk of stroke in adults with cyanotic congenital heart disease. Circulation. 1993;87(6):1954-1959. doi:10.1161/01.cir.87.6.1954
24. Gordeuk VR, Miasnikova GY, Sergueeva AI, et al. Thrombotic risk in congenital erythrocytosis due to up-regulated hypoxia sensing is not associated with elevated hematocrit. Haematologica. 2020;105(3):e87-e90. doi:10.3324/haematol.2019.216267
25. Kroll MH, Michaelis LC, Verstovsek S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev. 2015;29(4):215-221. doi:10.1016/j.blre.2014.12.002
26. Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057-1069. doi:10.1038/s41375-018-0077-1
27. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778-4781. doi:10.1182/blood-2013-01-478891
Polycythemia vera (PV) is a rare myeloproliferative neoplasm affecting 44 to 57 individuals per 100,000 in the United States.1,2 It is characterized by somatic mutations in the hematopoietic stem cell, resulting in hyperproliferation of mature myeloid lineage cells.2 Sustained erythrocytosis is a hallmark of PV, although many patients also have leukocytosis and thrombocytosis.2,3 These patients have increased inherent thrombotic risk with arterial events reported to occur at rates of 7 to 21/1000 person-years and venous thrombotic events at 5 to 20/1000 person-years.4-7 Thrombotic and cardiovascular events are leading causes of morbidity and mortality, resulting in a reduced overall survival of patients with PV compared with the general population.3,8-10
Blood Cell Counts and Thrombotic Events in PV
Treatment strategies for patients with PV mainly aim to prevent or manage thrombotic and bleeding complications through normalization of blood counts.11 Hematocrit (Hct) control has been reported to be associated with reduced thrombotic risk in patients with PV. This was shown and popularized by the prospective, randomized Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial in which participants were randomized 1:1 to maintaining either a low (< 45%) or high (45%-50%) Hct for 5 years to examine the long-term effects of more- or less-intensive cytoreductive therapy.12 Patients in the low-Hct group were found to have a lower rate of death from cardiovascular events or major thrombosis (1.1/100 person-years in the low-Hct group vs 4.4 in the high-Hct group; hazard ratio [HR], 3.91; 95% confidence interval [CI], 1.45-10.53; P = .007). Likewise, cardiovascular events occurred at a lower rate in patients in the low-Hct group compared with the high-Hct group (4.4% vs 10.9% of patients, respectively; HR, 2.69; 95% CI, 1.19-6.12; P = .02).12
Leukocytosis has also been linked to elevated risk for vascular events as shown in several studies, including the real-world European Collaboration on Low-Dose Aspirin in PV (ECLAP) observational study and a post hoc subanalysis of the CYTO-PV study.13,14 In a multivariate, time-dependent analysis in ECLAP, patients with white blood cell (WBC) counts > 15 × 109/L had a significant increase in the risk of thrombosis compared with those who had lower WBC counts, with higher WBC count more strongly associated with arterial than venous thromboembolism.13 In CYTO-PV, a significant correlation between elevated WBC count (≥ 11 × 109/L vs reference level of < 7 × 109/L) and time-dependent risk of major thrombosis was shown (HR, 3.9; 95% CI, 1.24-12.3; P = .02).14 Likewise, WBC count ≥ 11 × 109/L was found to be a predictor of subsequent venous events in a separate single-center multivariate analysis of patients with PV.8
Although CYTO-PV remains one of the largest prospective landmark studies in PV demonstrating the impact of Hct control on thrombosis, it is worthwhile to note that the patients in the high-Hct group who received less frequent myelosuppressive therapy with hydroxyurea than the low-Hct group also had higher WBC counts.12,15 Work is needed to determine the relative effects of high Hct and high WBC counts on PV independent of each other.
The Veteran Population with PV
Two recently published retrospective analyses from Parasuraman and colleagues used data from the Veterans Health Administration (VHA), the largest integrated health care system in the US, with an aim to replicate findings from CYTO-PV in a real-world population.16,17 The 2 analyses focused independently on the effects of Hct control and WBC count on the risk of a thrombotic event in patients with PV.
In the first retrospective analysis, 213 patients with PV and no prior thrombosis were placed into groups based on whether Hct levels were consistently either < 45% or ≥ 45% throughout the study period.17 The mean follow-up time was 2.3 years, during which 44.1% of patients experienced a thrombotic event (Figure 1). Patients with Hct levels < 45% had a lower rate of thrombotic events compared to those with levels ≥ 45% (40.3% vs 54.2%, respectively; HR, 1.61; 95% CI, 1.03-2.51; P = .04). In a sensitivity analysis that included patients with pre-index thrombotic events (N = 342), similar results were noted (55.6% vs 76.9% between the < 45% and ≥ 45% groups, respectively; HR, 1.95; 95% CI, 1.46-2.61; P < .001).
In the second analysis, the authors investigated the relationship between WBC counts and thrombotic events.16 Evaluable patients (N = 1565) were grouped into 1 of 4 cohorts based on the last WBC measurement taken during the study period before a thrombotic event or through the end of follow-up: (1) WBC < 7.0 × 109/L, (2) 7.0 to 8.4 × 109/L, (3) 8.5 to < 11.0 × 109/L, or (4) ≥ 11.0 × 109/L. Mean follow-up time ranged from 3.6 to 4.5 years among WBC count cohorts, during which 24.9% of patients experienced a thrombotic event. Compared with the reference cohort (WBC < 7.0 × 109/L), a significant positive association between WBC counts and thrombotic event occurrence was observed among patients with WBC counts of 8.5 to < 11.0 × 109/L (HR, 1.47; 95% CI, 1.10-1.96; P < .01) and ≥ 11 × 109/L (HR, 1.87; 95% CI, 1.44-2.43; P < .001) (Figure 2).16 When including all patients in a sensitivity analysis regardless of whether they experienced thrombotic events before the index date (N = 1876), similar results were obtained (7.0-8.4 × 109/L group: HR, 1.22; 95% CI, 0.97-1.55; P = .0959; 8.5 - 11.0 × 109/L group: HR, 1.41; 95% CI, 1.10-1.81; P = .0062; ≥ 11.0 × 109/L group: HR, 1.53; 95% CI, 1.23-1.91; P < .001; compared with < 7.0 × 109/L reference group). Rates of phlebotomy and cytoreductive treatments were similar across groups.16
Some limitations to these studies are attributable to their retrospective design, reliance on health records, and the VHA population characteristics, which differ from the general population. For example, in this analysis, patients with PV in the VHA population had significantly increased risk of thrombotic events, even at a lower WBC count threshold (≥ 8.5 × 109/L) compared with those reported in CYTO-PV (≥ 11 × 109/L). Furthermore, approximately one-third of patients had elevated WBC levels, compared with 25.5% in the CYTO-PV study.14,16 This is most likely due to the unique nature of the VHA patient population, who are predominantly older adult men and generally have a higher comorbidity burden. A notable pre-index comorbidity burden was reported in the VHA population in the Hct analysis, even when compared to patients with PV in the general US population (Charlson Comorbidity Index score, 1.3 vs 0.8).6,17 Comorbid conditions such as hypertension, diabetes, and tobacco use, which are most common among the VHA population, are independently associated with higher risk of cardiovascular and thrombotic events.18,19 However, whether these higher levels of comorbidities affected the type of treatments they received was not elucidated, and the effectiveness of treatments to maintain target Hct levels was not addressed in the study.
Current PV Management and Future Implications
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology in myeloproliferative neoplasms recommend maintaining Hct levels < 45% in patients with PV.11 Patients with high-risk disease (age ≥ 60 years and/or history of thrombosis) are monitored for new thrombosis or bleeding and are managed for their cardiovascular risk factors. In addition, they receive low-dose aspirin (81-100 mg/day), undergo phlebotomy to maintain an Hct < 45%, and are managed with pharmacologic cytoreductive therapy. Cytoreductive therapy primarily consists of hydroxyurea or peginterferon alfa-2a for younger patients. Ruxolitinib, a Janus kinase (JAK1)/JAK2 inhibitor, is now approved by the US Food and Drug Administration as second-line treatment for those with PV that is intolerant or unresponsive to hydroxyurea or peginterferon alfa-2a treatments.11,20 However, the role of cytoreductive therapy is not clear for patients with low-risk disease (age < 60 years and no history of thrombosis). These patients are managed for their cardiovascular risk factors, undergo phlebotomy to maintain an Hct < 45%, are maintained on low-dose aspirin (81-100 mg/day), and are monitored for indications for cytoreductive therapy, which include any new thrombosis or disease-related major bleeding, frequent or persistent need for phlebotomy with poor tolerance for the procedure, splenomegaly, thrombocytosis, leukocytosis, and disease-related symptoms (eg, aquagenic pruritus, night sweats, fatigue).
Even though the current guidelines recommend maintaining a target Hct of < 45% in patients with high-risk PV, the role of Hct as the main determinant of thrombotic risk in patients with PV is still debated.21 In JAK2V617F-positive essential thrombocythemia, Hct levels are usually normal but risk of thrombosis is nevertheless still significant.22 The risk of thrombosis is significantly lower in primary familial and congenital polycythemia and much lower in secondary erythrocytosis such as cyanotic heart disease, long-term native dwellers of high altitude, and those with high-oxygen–affinity hemoglobins.21,23 In secondary erythrocytosis from hypoxia or upregulated hypoxic pathway such as hypoxia inducible factor-2α (HIF-2α) mutation and Chuvash erythrocytosis, the risk of thrombosis is more associated with the upregulated HIF pathway and its downstream consequences, rather than the elevated Hct level.24
However, most current literature supports the association of increased risk of thrombosis with higher Hct and high WBC count in patients with PV. In addition, the underlying mechanism of thrombogenesis still remains elusive; it is likely a complex process that involves interactions among multiple components, including elevated blood counts arising from clonal hematopoiesis, JAK2V617F allele burden, and platelet and WBC activation and their interaction with endothelial cells and inflammatory cytokines.25
Nevertheless, Hct control and aspirin use are current standard of care for patients with PV to mitigate thrombotic risk, and the results from the 2 analyses by Parasuraman and colleagues, using real-world data from the VHA, support the current practice guidelines to maintain Hct < 45% in these patients. They also provide additional support for considering WBC counts when determining patient risk and treatment plans. Although treatment response criteria from the European LeukemiaNet include achieving normal WBC levels to decrease the risk of thrombosis, current NCCN guidelines do not include WBC counts as a component for establishing patient risk or provide a target WBC count to guide patient management.11,26,27 Updates to these practice guidelines may be warranted. In addition, further study is needed to understand the mechanism of thrombogenesis in PV and other myeloproliferative disorders in order to develop novel therapeutic targets and improve patient outcomes.
Acknowledgments
Writing assistance was provided by Tania Iqbal, PhD, an employee of ICON (North Wales, PA), and was funded by Incyte Corporation (Wilmington, DE).
Polycythemia vera (PV) is a rare myeloproliferative neoplasm affecting 44 to 57 individuals per 100,000 in the United States.1,2 It is characterized by somatic mutations in the hematopoietic stem cell, resulting in hyperproliferation of mature myeloid lineage cells.2 Sustained erythrocytosis is a hallmark of PV, although many patients also have leukocytosis and thrombocytosis.2,3 These patients have increased inherent thrombotic risk with arterial events reported to occur at rates of 7 to 21/1000 person-years and venous thrombotic events at 5 to 20/1000 person-years.4-7 Thrombotic and cardiovascular events are leading causes of morbidity and mortality, resulting in a reduced overall survival of patients with PV compared with the general population.3,8-10
Blood Cell Counts and Thrombotic Events in PV
Treatment strategies for patients with PV mainly aim to prevent or manage thrombotic and bleeding complications through normalization of blood counts.11 Hematocrit (Hct) control has been reported to be associated with reduced thrombotic risk in patients with PV. This was shown and popularized by the prospective, randomized Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial in which participants were randomized 1:1 to maintaining either a low (< 45%) or high (45%-50%) Hct for 5 years to examine the long-term effects of more- or less-intensive cytoreductive therapy.12 Patients in the low-Hct group were found to have a lower rate of death from cardiovascular events or major thrombosis (1.1/100 person-years in the low-Hct group vs 4.4 in the high-Hct group; hazard ratio [HR], 3.91; 95% confidence interval [CI], 1.45-10.53; P = .007). Likewise, cardiovascular events occurred at a lower rate in patients in the low-Hct group compared with the high-Hct group (4.4% vs 10.9% of patients, respectively; HR, 2.69; 95% CI, 1.19-6.12; P = .02).12
Leukocytosis has also been linked to elevated risk for vascular events as shown in several studies, including the real-world European Collaboration on Low-Dose Aspirin in PV (ECLAP) observational study and a post hoc subanalysis of the CYTO-PV study.13,14 In a multivariate, time-dependent analysis in ECLAP, patients with white blood cell (WBC) counts > 15 × 109/L had a significant increase in the risk of thrombosis compared with those who had lower WBC counts, with higher WBC count more strongly associated with arterial than venous thromboembolism.13 In CYTO-PV, a significant correlation between elevated WBC count (≥ 11 × 109/L vs reference level of < 7 × 109/L) and time-dependent risk of major thrombosis was shown (HR, 3.9; 95% CI, 1.24-12.3; P = .02).14 Likewise, WBC count ≥ 11 × 109/L was found to be a predictor of subsequent venous events in a separate single-center multivariate analysis of patients with PV.8
Although CYTO-PV remains one of the largest prospective landmark studies in PV demonstrating the impact of Hct control on thrombosis, it is worthwhile to note that the patients in the high-Hct group who received less frequent myelosuppressive therapy with hydroxyurea than the low-Hct group also had higher WBC counts.12,15 Work is needed to determine the relative effects of high Hct and high WBC counts on PV independent of each other.
The Veteran Population with PV
Two recently published retrospective analyses from Parasuraman and colleagues used data from the Veterans Health Administration (VHA), the largest integrated health care system in the US, with an aim to replicate findings from CYTO-PV in a real-world population.16,17 The 2 analyses focused independently on the effects of Hct control and WBC count on the risk of a thrombotic event in patients with PV.
In the first retrospective analysis, 213 patients with PV and no prior thrombosis were placed into groups based on whether Hct levels were consistently either < 45% or ≥ 45% throughout the study period.17 The mean follow-up time was 2.3 years, during which 44.1% of patients experienced a thrombotic event (Figure 1). Patients with Hct levels < 45% had a lower rate of thrombotic events compared to those with levels ≥ 45% (40.3% vs 54.2%, respectively; HR, 1.61; 95% CI, 1.03-2.51; P = .04). In a sensitivity analysis that included patients with pre-index thrombotic events (N = 342), similar results were noted (55.6% vs 76.9% between the < 45% and ≥ 45% groups, respectively; HR, 1.95; 95% CI, 1.46-2.61; P < .001).
In the second analysis, the authors investigated the relationship between WBC counts and thrombotic events.16 Evaluable patients (N = 1565) were grouped into 1 of 4 cohorts based on the last WBC measurement taken during the study period before a thrombotic event or through the end of follow-up: (1) WBC < 7.0 × 109/L, (2) 7.0 to 8.4 × 109/L, (3) 8.5 to < 11.0 × 109/L, or (4) ≥ 11.0 × 109/L. Mean follow-up time ranged from 3.6 to 4.5 years among WBC count cohorts, during which 24.9% of patients experienced a thrombotic event. Compared with the reference cohort (WBC < 7.0 × 109/L), a significant positive association between WBC counts and thrombotic event occurrence was observed among patients with WBC counts of 8.5 to < 11.0 × 109/L (HR, 1.47; 95% CI, 1.10-1.96; P < .01) and ≥ 11 × 109/L (HR, 1.87; 95% CI, 1.44-2.43; P < .001) (Figure 2).16 When including all patients in a sensitivity analysis regardless of whether they experienced thrombotic events before the index date (N = 1876), similar results were obtained (7.0-8.4 × 109/L group: HR, 1.22; 95% CI, 0.97-1.55; P = .0959; 8.5 - 11.0 × 109/L group: HR, 1.41; 95% CI, 1.10-1.81; P = .0062; ≥ 11.0 × 109/L group: HR, 1.53; 95% CI, 1.23-1.91; P < .001; compared with < 7.0 × 109/L reference group). Rates of phlebotomy and cytoreductive treatments were similar across groups.16
Some limitations to these studies are attributable to their retrospective design, reliance on health records, and the VHA population characteristics, which differ from the general population. For example, in this analysis, patients with PV in the VHA population had significantly increased risk of thrombotic events, even at a lower WBC count threshold (≥ 8.5 × 109/L) compared with those reported in CYTO-PV (≥ 11 × 109/L). Furthermore, approximately one-third of patients had elevated WBC levels, compared with 25.5% in the CYTO-PV study.14,16 This is most likely due to the unique nature of the VHA patient population, who are predominantly older adult men and generally have a higher comorbidity burden. A notable pre-index comorbidity burden was reported in the VHA population in the Hct analysis, even when compared to patients with PV in the general US population (Charlson Comorbidity Index score, 1.3 vs 0.8).6,17 Comorbid conditions such as hypertension, diabetes, and tobacco use, which are most common among the VHA population, are independently associated with higher risk of cardiovascular and thrombotic events.18,19 However, whether these higher levels of comorbidities affected the type of treatments they received was not elucidated, and the effectiveness of treatments to maintain target Hct levels was not addressed in the study.
Current PV Management and Future Implications
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology in myeloproliferative neoplasms recommend maintaining Hct levels < 45% in patients with PV.11 Patients with high-risk disease (age ≥ 60 years and/or history of thrombosis) are monitored for new thrombosis or bleeding and are managed for their cardiovascular risk factors. In addition, they receive low-dose aspirin (81-100 mg/day), undergo phlebotomy to maintain an Hct < 45%, and are managed with pharmacologic cytoreductive therapy. Cytoreductive therapy primarily consists of hydroxyurea or peginterferon alfa-2a for younger patients. Ruxolitinib, a Janus kinase (JAK1)/JAK2 inhibitor, is now approved by the US Food and Drug Administration as second-line treatment for those with PV that is intolerant or unresponsive to hydroxyurea or peginterferon alfa-2a treatments.11,20 However, the role of cytoreductive therapy is not clear for patients with low-risk disease (age < 60 years and no history of thrombosis). These patients are managed for their cardiovascular risk factors, undergo phlebotomy to maintain an Hct < 45%, are maintained on low-dose aspirin (81-100 mg/day), and are monitored for indications for cytoreductive therapy, which include any new thrombosis or disease-related major bleeding, frequent or persistent need for phlebotomy with poor tolerance for the procedure, splenomegaly, thrombocytosis, leukocytosis, and disease-related symptoms (eg, aquagenic pruritus, night sweats, fatigue).
Even though the current guidelines recommend maintaining a target Hct of < 45% in patients with high-risk PV, the role of Hct as the main determinant of thrombotic risk in patients with PV is still debated.21 In JAK2V617F-positive essential thrombocythemia, Hct levels are usually normal but risk of thrombosis is nevertheless still significant.22 The risk of thrombosis is significantly lower in primary familial and congenital polycythemia and much lower in secondary erythrocytosis such as cyanotic heart disease, long-term native dwellers of high altitude, and those with high-oxygen–affinity hemoglobins.21,23 In secondary erythrocytosis from hypoxia or upregulated hypoxic pathway such as hypoxia inducible factor-2α (HIF-2α) mutation and Chuvash erythrocytosis, the risk of thrombosis is more associated with the upregulated HIF pathway and its downstream consequences, rather than the elevated Hct level.24
However, most current literature supports the association of increased risk of thrombosis with higher Hct and high WBC count in patients with PV. In addition, the underlying mechanism of thrombogenesis still remains elusive; it is likely a complex process that involves interactions among multiple components, including elevated blood counts arising from clonal hematopoiesis, JAK2V617F allele burden, and platelet and WBC activation and their interaction with endothelial cells and inflammatory cytokines.25
Nevertheless, Hct control and aspirin use are current standard of care for patients with PV to mitigate thrombotic risk, and the results from the 2 analyses by Parasuraman and colleagues, using real-world data from the VHA, support the current practice guidelines to maintain Hct < 45% in these patients. They also provide additional support for considering WBC counts when determining patient risk and treatment plans. Although treatment response criteria from the European LeukemiaNet include achieving normal WBC levels to decrease the risk of thrombosis, current NCCN guidelines do not include WBC counts as a component for establishing patient risk or provide a target WBC count to guide patient management.11,26,27 Updates to these practice guidelines may be warranted. In addition, further study is needed to understand the mechanism of thrombogenesis in PV and other myeloproliferative disorders in order to develop novel therapeutic targets and improve patient outcomes.
Acknowledgments
Writing assistance was provided by Tania Iqbal, PhD, an employee of ICON (North Wales, PA), and was funded by Incyte Corporation (Wilmington, DE).
1. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595-600. doi:10.3109/10428194.2013.813500
2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. doi:10.1182/blood-2016-03-643544
3. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874-1881. doi:10.1038/leu.2013.163
4. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224-2232. doi:10.1200/JCO.2005.07.062
5. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110(3):840-846. doi:10.1182/blood-2006-12-064287
6. Goyal RK, Davis KL, Cote I, Mounedji N, Kaye JA. Increased incidence of thromboembolic event rates in patients diagnosed with polycythemia vera: results from an observational cohort study. Blood (ASH Annual Meeting Abstracts). 2014;124:4840. doi:10.1182/blood.V124.21.4840.4840
7. Barbui T, Carobbio A, Rumi E, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124(19):3021-3023. doi:10.1182/blood-2014-07-591610 8. Cerquozzi S, Barraco D, Lasho T, et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer J. 2017;7(12):662. doi:10.1038/s41408-017-0035-6
9. Stein BL, Moliterno AR, Tiu RV. Polycythemia vera disease burden: contributing factors, impact on quality of life, and emerging treatment options. Ann Hematol. 2014;93(12):1965-1976. doi:10.1007/s00277-014-2205-y
10. Hultcrantz M, Kristinsson SY, Andersson TM-L, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995-3001. doi:10.1200/JCO.2012.42.1925
11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in myeloproliferative neoplasms (Version 1.2020). Accessed March 3, 2022. https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
12. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. doi:10.1056/NEJMoa1208500
13. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446-2452. doi:10.1182/blood-2006-08-042515
14. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126(4):560-561. doi:10.1182/blood-2015-04-638593
15. Prchal JT, Gordeuk VR. Treatment target in polycythemia vera. N Engl J Med. 2013;368(16):1555-1556. doi:10.1056/NEJMc1301262
16. Parasuraman S, Yu J, Paranagama D, et al. Elevated white blood cell levels and thrombotic events in patients with polycythemia vera: a real-world analysis of Veterans Health Administration data. Clin Lymphoma Myeloma Leuk. 2020;20(2):63-69. doi:10.1016/j.clml.2019.11.010
17. Parasuraman S, Yu J, Paranagama D, et al. Hematocrit levels and thrombotic events in patients with polycythemia vera: an analysis of Veterans Health Administration data. Ann Hematol. 2019;98(11):2533-2539. doi:10.1007/s00277-019-03793-w
18. WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332-e1345. doi:10.1016/S2214-109X(19)30318-3.
19. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. doi:10.1161/CIRCULATIONAHA.107.699579
20. Jakafi. Package insert. Incyte Corporation; 2020.
21. Gordeuk VR, Key NS, Prchal JT. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica. 2019;104(4):653-658. doi:10.3324/haematol.2018.210732
22. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857-5859. doi:10.1182/blood-2011-02-339002
23. Perloff JK, Marelli AJ, Miner PD. Risk of stroke in adults with cyanotic congenital heart disease. Circulation. 1993;87(6):1954-1959. doi:10.1161/01.cir.87.6.1954
24. Gordeuk VR, Miasnikova GY, Sergueeva AI, et al. Thrombotic risk in congenital erythrocytosis due to up-regulated hypoxia sensing is not associated with elevated hematocrit. Haematologica. 2020;105(3):e87-e90. doi:10.3324/haematol.2019.216267
25. Kroll MH, Michaelis LC, Verstovsek S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev. 2015;29(4):215-221. doi:10.1016/j.blre.2014.12.002
26. Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057-1069. doi:10.1038/s41375-018-0077-1
27. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778-4781. doi:10.1182/blood-2013-01-478891
1. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595-600. doi:10.3109/10428194.2013.813500
2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. doi:10.1182/blood-2016-03-643544
3. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874-1881. doi:10.1038/leu.2013.163
4. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224-2232. doi:10.1200/JCO.2005.07.062
5. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110(3):840-846. doi:10.1182/blood-2006-12-064287
6. Goyal RK, Davis KL, Cote I, Mounedji N, Kaye JA. Increased incidence of thromboembolic event rates in patients diagnosed with polycythemia vera: results from an observational cohort study. Blood (ASH Annual Meeting Abstracts). 2014;124:4840. doi:10.1182/blood.V124.21.4840.4840
7. Barbui T, Carobbio A, Rumi E, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124(19):3021-3023. doi:10.1182/blood-2014-07-591610 8. Cerquozzi S, Barraco D, Lasho T, et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer J. 2017;7(12):662. doi:10.1038/s41408-017-0035-6
9. Stein BL, Moliterno AR, Tiu RV. Polycythemia vera disease burden: contributing factors, impact on quality of life, and emerging treatment options. Ann Hematol. 2014;93(12):1965-1976. doi:10.1007/s00277-014-2205-y
10. Hultcrantz M, Kristinsson SY, Andersson TM-L, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995-3001. doi:10.1200/JCO.2012.42.1925
11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in myeloproliferative neoplasms (Version 1.2020). Accessed March 3, 2022. https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
12. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. doi:10.1056/NEJMoa1208500
13. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446-2452. doi:10.1182/blood-2006-08-042515
14. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126(4):560-561. doi:10.1182/blood-2015-04-638593
15. Prchal JT, Gordeuk VR. Treatment target in polycythemia vera. N Engl J Med. 2013;368(16):1555-1556. doi:10.1056/NEJMc1301262
16. Parasuraman S, Yu J, Paranagama D, et al. Elevated white blood cell levels and thrombotic events in patients with polycythemia vera: a real-world analysis of Veterans Health Administration data. Clin Lymphoma Myeloma Leuk. 2020;20(2):63-69. doi:10.1016/j.clml.2019.11.010
17. Parasuraman S, Yu J, Paranagama D, et al. Hematocrit levels and thrombotic events in patients with polycythemia vera: an analysis of Veterans Health Administration data. Ann Hematol. 2019;98(11):2533-2539. doi:10.1007/s00277-019-03793-w
18. WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332-e1345. doi:10.1016/S2214-109X(19)30318-3.
19. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. doi:10.1161/CIRCULATIONAHA.107.699579
20. Jakafi. Package insert. Incyte Corporation; 2020.
21. Gordeuk VR, Key NS, Prchal JT. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica. 2019;104(4):653-658. doi:10.3324/haematol.2018.210732
22. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857-5859. doi:10.1182/blood-2011-02-339002
23. Perloff JK, Marelli AJ, Miner PD. Risk of stroke in adults with cyanotic congenital heart disease. Circulation. 1993;87(6):1954-1959. doi:10.1161/01.cir.87.6.1954
24. Gordeuk VR, Miasnikova GY, Sergueeva AI, et al. Thrombotic risk in congenital erythrocytosis due to up-regulated hypoxia sensing is not associated with elevated hematocrit. Haematologica. 2020;105(3):e87-e90. doi:10.3324/haematol.2019.216267
25. Kroll MH, Michaelis LC, Verstovsek S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev. 2015;29(4):215-221. doi:10.1016/j.blre.2014.12.002
26. Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057-1069. doi:10.1038/s41375-018-0077-1
27. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778-4781. doi:10.1182/blood-2013-01-478891