User login
Heparin-induced thrombocytopenia (HIT) is an immune-mediated drug reaction that requires prompt detection and treatment in order to minimize patient morbidity and mortality.1 HIT is caused by the development of antibodies to platelet factor 4 (PF4), although it is important to note that not all patients who develop PF4 antibodies will experience the clinical syndrome of HIT.2-4 In fact, about 50% of patients who undergo cardiovascular surgery develop PF4 antibodies, but only 1% to 2% of patients with antibodies actually experience HIT.5-7 There is currently no explanation for the phenomenon of HIT.8
In 2012, with an intent to limit HIT-associated morbidity and mortality, the American College of Chest Physicians (ACCP) unveiled the ninth edition of its evidence-based practice guidelines for the detection of HIT and appropriate treatment.5 Much of the information provided in this article emerged from these guidelines.
Epidemiology
Of the 12 million patients treated each year with either unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH), 600,000 (0.5%) will develop HIT. Among these patients, 300,000 will develop thrombosis, and 90,000 will die. In 2009 alone, the HIT-associated cost to the US health care system was estimated at $100 million.1
As growing numbers of patients require anticoagulation therapy, it becomes increasingly important for clinicians to understand the importance of screening for deep vein thrombosis (DVT), one of the two most common thromboses; the other is pulmonary embolism.9,10 Continuing to administer heparin or warfarin to patients with undetected HIT predisposes them to severe complications, including venous and arterial thromboses and gangrenous skin lesions—which can result in loss of life and/or limb.1,11,12
Risk Factors for HIT
Several factors influence a patient's risk for HIT, including the type and dosing regimen of the heparin being administered. Generally, the risk for HIT is about 10-fold in patients treated with UFH (3% to 5%), compared with those receiving LMWH (0.5%).5,13 The risk for HIT is also greater in patients receiving UFH of bovine origin, compared with those taking porcine-derived UFH.8,14,15
In a recent meta-analysis of postsurgical patients who underwent heparin thromboprophylaxis, those given LMWH had a 76% relative risk reduction for HIT, compared with patients taking UFH.16 The incidence of HIT increases among patients receiving LMWH if they have been treated with UFH within the previous 100 days.9 HIT onset may be delayed for several days in patients given heparin for the first time (or for the first time in several months), whereas previously exposed patients who have already developed antiheparin PF4 antibodies can experience severe HIT within hours.9
Patient-Specific Risk Factors
Certain patient characteristics also have an impact on HIT risk. For example, the risk for HIT is approximately doubled in women, compared with men,1,5,15 and the incidence of HIT is greater in surgical patients than in medical patients.7,17 Among surgical patients, 5% of orthopedic patients have been reported to develop HIT, compared with 3% of cardiac patients and 1% of patients undergoing surgery for vascular illnesses.1 The reasons for these differences are poorly understood, but current theory focuses on the inflammatory response of individual patients and the degree of associated platelet activation.2,12
Patient Presentation and History
The typical patient with HIT presents with a new or progressing thrombosis between days 5 and 14 of heparin therapy (with day 0 representing the day the first dose is administered); thrombosis can be venous or arterial, although venous thrombosis occurs much more frequently.1,5,9,15 As patients rarely remain hospitalized for such a long period, it is imperative that providers in clinic and emergency settings obtain detailed histories for patients who present with thrombocytopenia and/or thrombosis. HIT should be suspected in any such patient whose history shows heparin use within the previous two weeks (even if the drug has been discontinued).15
Two forms of atypical HIT are rapid-onset HIT and delayed-onset HIT. Rapid-onset HIT is defined by a platelet count that falls within 24 hours of exposure to heparin. This form is usually associated with previous heparin exposure (ie, within the previous 100 days, but most commonly within the previous 30 days). Affected patients have already developed circulating antiheparin PF4 antibodies, causing an immediate reaction when the patient is re-exposed to the drug.1,15
The less common delayed-onset HIT occurs in patients in whom heparin has been discontinued for as long as 40 days. Delayed-onset HIT carries the greatest risk for severe thrombosis.1,15
Atypically, a patient may present with bleeding, skin necrosis, venous gangrene, or anaphylaxis,9 but skin necrosis at the site of heparin injection is strongly suggestive of HIT.12However, neither physical signs nor symptoms, nor a thrombotic event is required to make a diagnosis of HIT. In fact, the preference is for a diagnosis to be made before thrombosis formation.5
The major manifestation of HIT is thrombocytopenia itself2,18(see "Laboratory Findings"). Nevertheless, if physical signs and symptoms are evident, they will be related to the thrombosis, and the components of the physical exam will proceed accordingly.
Laboratory Findings
Platelet count monitoring and HIT antibody testing are the laboratory tests most commonly used when HIT is suspected. Although 25% of patients with HIT will experience a thrombotic event before the platelet count falls, monitoring the platelet count is considered the most effective means to identify patients with HIT.5 HIT antibody testing is not recommended unless the health care provider has a strong suspicion for HIT.19
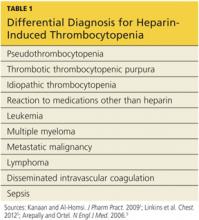
Thrombocytopenia is a common abnormality, especially in hospitalized patients, and its causes are numerous.11 Table 11,5,9 lists the differential diagnosis that the clinician who suspects HIT should consider.
Nevertheless, the ACCP guidelines5 recommend platelet count monitoring for all patients receiving heparin, beginning on day 4 of heparin therapy, then continuing every two to three days until treatment day 14 or heparin discontinuation, whichever occurs first.5,15 A platelet count decrease of 50% or more should raise a suspicion for HIT.15
The ACCP makes two principal exceptions to these recommendations.5 The first involves patients who have received UFH within the previous 100 days. These patients should undergo a baseline platelet count before heparin is administered, followed by a repeat platelet count within 24 hours. Any patient who experiences an anaphylactic reaction to UFH should undergo an immediate platelet count; a decrease in these patients is often transient.
The second exception pertains to medical and obstetric patients. Those receiving LMWH or UFH only to maintain line patency do not require platelet monitoring, as their risk for HIT is relatively low.1,5
Laboratory Interpretation
In HIT, thrombocytopenia is defined as a platelet count below 150,000/mm3 or a platelet count reduction of 50% or more from baseline, even if the platelet count remains above 150,000/mm3.9 (The patient's baseline platelet count is defined as the highest count recorded in the previous two weeks.1,5,15) The thrombocytopenia associated with HIT is rarely severe and can be easily overlooked.1
Once the platelet count suggests a diagnosis of HIT, heparin-dependent antibodies can be identified through immunologic or functional assays.1,9,15 Immunologic assays should be ordered immediately upon suspicion of HIT since they are simple tests with relatively rapid results. Immunologic assays detect immunoglobulin G (IgG), IgA, and IgM antibodies.9Though lacking in specificity, the immunologic assay is highly sensitive.2,12,20 The most frequently used immunologic assay is the enzyme-linked immunosorbent assay (ELISA).1,2,9,20The ELISA, which detects antiheparin PF4 antibodies, has a sensitivity greater than 97% but a specificity of only 74% to 86%.8,12
Functional assays,which are technically demanding, test the ability of PF4 antibodies to activate platelets in the presence of heparin. The functional assay is used to confirm the diagnosis of HIT when a positive ELISA result is obtained.1,9,15 Among the functional assays, the serotonin release assay (SRA) has been most completely studied. Though very expensive, the SRA is 89% to 100% specific in diagnosing HIT.2,12
Diagnosis
The diagnosis of HIT is determined by combining clinical and serologic assessment. HIT should be suspected in any patient who is in day 5 to 14 of heparin therapy and experiences a drop in platelet count of at least 50%, or in whom a new thrombotic event occurs (even if the patient is no longer receiving heparin therapy). The interpretation of all diagnostic information must be made in the context of the patient's clinical probability of HIT.15
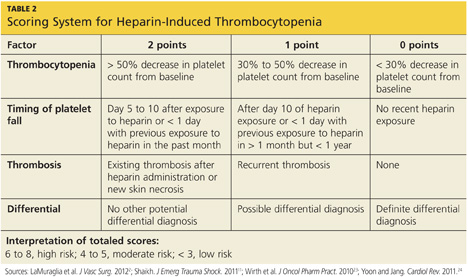
A scoring system referred to as the 4Ts (thrombocytopenia, timing of platelet fall, thrombosis or other sequelae, and test interpretation) is used to help determine the patient's probability of HIT5,21,22 (similar to the scoring strategy shown in Table 22,11,23,24).
The patient diagnosed with HIT must be positive for HIT antibodies and meet at least one additional criterion:
• A platelet count decrease of 30% to 50% below baseline, regardless of the actual value
• A venous or arterial thrombosis
• A skin lesion at the heparin injection site; and/or
• An anaphylactic reaction after IV bolus administration of heparin.15
Treatment/Management
The goal in management of HIT is to reduce the likelihood, then the severity, of thrombosis.9 Treatment should be started as soon as HIT is suspected, before laboratory confirmation.25 Treatment for HIT comprises two steps: stopping all exposure to heparin, and administering an alternative, non-heparin anticoagulant.
Discontinuation of Heparin Exposure
Stopping heparin exposure is the mainstay of treatment for HIT. This includes all potential sources of heparin exposure, including "flushes" that may be used to promote patency of central IV catheters, use of UFH-coated catheters, or addition of any heparin to total parenteral nutrition.9,25
Use of a Non-Heparin Anticoagulant
In addition to discontinuation of all heparin exposure, the patient must be started on a non-heparin anticoagulant, whether or not thrombosis is present.25 Forty percent to 50% of patients without thrombosis will develop a thrombosis within 30 days if alternative anticoagulation is not started.12,18,26
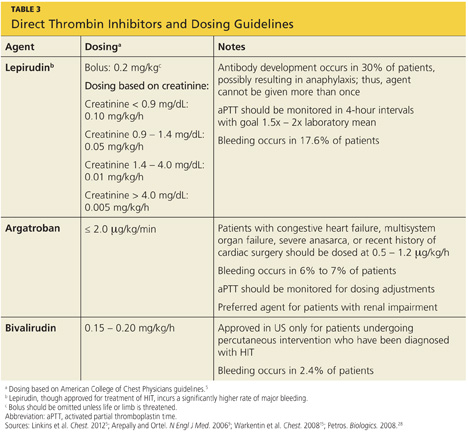
The principal choices for a non-heparin anticoagulant are the direct thrombin inhibitors (DTIs): lepirudin, argatroban, and bivalirudin.1,9,15,25,27 DTIs are the treatment of choice for patients with known or suspected HIT. The ACCP dosing guidelines for the DTIs are summarized in Table 3.9,5,15,28
The factor Xa inhibitor fondaparinux, though FDA approved for DVT prophylaxis,11 has not yet been systematically investigated for the treatment of HIT; thus, its use for this indication is considered off-label.29 However, small studies have shown no cross-reactivity between fondaparinux and PF4 antibodies.30 Due to the positive risk/benefit ratio, ease of use, and reduced need for monitoring in patients taking fondaparinux, it is considered an attractive alternative to DTIs that may receive approval in the near future.12,18,20,29
Currently, the ACCP limits its recommendation of fondaparinux use to patients with a previous history of HIT who require anticoagulation for an acute thrombotic event unrelated to HIT (grade 2C recommendation).5
The vitamin K antagonist warfarin is absolutely contraindicated in patients with HIT until the platelet count is at least 150,000/mm3, due to the risk for warfarin-induced skin necrosis and venous gangrene.9,15 If a patient is receiving warfarin at diagnosis, vitamin K (10 mg orally or 5 to 10 mg IV) should be administered.15
The patient should remain on the alternative non-heparin anticoagulant until the platelet count has stabilized at or above 150,000/mm3. Warfarin should then be started at a maximum of 5 mg/d.2,5 The non-heparin anticoagulant and warfarin should be continued until a therapeutic international normalized ratio (INR) is reached and maintained for 48 hours, with a minimum 5-day overlap of the two medications. Once the non-heparin anticoagulant is discontinued, the INR should be reevaluated for remaining within the therapeutic range, as DTIs can elevate the INR.2,5Warfarin should be continued for as long as four weeks, with frequent INR monitoring.15
Patient Education
The presence of PF4 antibodies is transient (50 to 80 days); however, concern persists regarding recurrent antibody development with subsequent heparin use. Thus, an alternative anticoagulant should be used whenever possible. Patients who have been diagnosed with HIT should be advised to inform future health care professionals regarding their need for alternative anticoagulation whenever possible.
Patients should also be made aware that when the risk for DTI-associated bleeding is too great (as in the case of cardiac surgery), heparin remains the anticoagulant of choice.9,15
Conclusion
Heparin-induced thrombocytopenia is a transient development of antibodies to heparin. While the condition carries a high risk for morbidity and mortality, early detection and prompt treatment can greatly reduce the associated risk to life and limb.
References
1. Kanaan AO, Al-Homsi AS. Heparin-induced thrombocytopenia: pathophysiology, diagnosis, and review of pharmacotherapy. J Pharm Pract. 2009;22:149-157.
2. LaMuraglia GM, Houbballah R, Laposata M. The identification and management of heparin-induced thrombocytopenia in the vascular patient. J Vasc Surg. 2012;55:562-570.
3. Rauova L, Zhai L, Kowalska MA, et al. Role of platelet surface PF4 antigenic complexes in heparin-induced thrombocytopenia pathogenesis: diagnostic and therapeutic implications. Blood. 2006;107:2346-2353.
4. Suvarna S, Espinasse B, Qi R, et al. Determinants of PF4/heparin immunogenicity. Blood. 2007;110:4253-4260.
5. Linkins LA, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e495S-e530S.
6. Demma LJ, Winkler AM, Levy JH. A diagnosis of heparin-induced thrombocytopenia with combined clinical and laboratory methods in cardiothoracic surgical intensive care unit patients. Anesth Analg. 2011;113:697-702.
7. Demma LJ, Levy JH. Diagnosing heparin-induced thrombocytopenia in cardiac surgical patients: not as easy as you think. Anesth Analg. 2011;112:747-749.
8. Alaraj A, Wallace A, Tesoro E, et al. Heparin-induced thrombocytopenia: diagnosis and management. J Neurointervent Surg. 2010;2:371-378.
9. Arepally GM, Ortel TL. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355:809-817.
10. Sud S, Mittmann N, Cook DJ, et al. Screening and prevention of venous thromboembolism in critically ill patients: a decision analysis and economic evaluation. Am J Resp Crit Care Med. 2011;184:1289-1298.
11. Shaikh N. Heparin-induced thrombocytopenia. J Emerg Trauma Shock. 2011;14:97-102.
12. Cuker A. Heparin-induced thrombocytopenia: present and future. J Thromb Thrombolysis. 2011;31:353-366.
13. Locke CSF, Dooley J, Gerber J. Rates of clinically apparent heparin-induced thrombocytopenia for unfractionated heparin vs low molecular weight heparin in non-surgical patients are low and similar. Thromb J. 2005;3:4.
14. Cuker A. Recent advances in heparin-induced thrombocytopenia. Curr Opin Hematol. 2011;18:315-322.
15. Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest. 2008;133(6 suppl):340S-380S.
16. Junqueira DR, Perini E, Penholati RR, Carvalho MG. Unfractionated heparin versus low molecular weight heparin for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev. 2012;9:CD007557.
17. Berry C, Tcherniantchouk O, Ley EJ, et al. Overdiagnosis of heparin-induced thrombocytopenia in surgical ICU patients. J Am Coll Surg. 2011;213:10-17.
18. Cuker A. Current and emerging therapeutics for heparin-induced thrombocytopenia. Semin Thromb Hemost. 2012;38:31-37.
19. Warkentin TE. New approaches to the diagnosis of heparin-induced thrombocytopenia. Chest. 2005;127(2 suppl):35S-45S.
20. Fennessy-Cooney M. Heparin-induced thrombocytopenia. Nurse Pract. 2011;36:31-37.
21. Bryant A, Low J, Austin S, Joseph JE. Timely diagnosis and management of heparin-induced thrombocytopenia in a frequent request, low incidence single centre using clinical 4T's score and particle gel immunoassay. Br J Haematol. 2008;143:721-726.
22. Lo GK, Juhl D, Warkentin TE, et al. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4:759-765.
23. Wirth SM, Macaulay TE, Armistead JA, et al. Evaluation of a clinical scoring scale to direct early appropriate therapy in heparin-induced thrombocytopenia. J Oncol Pharm Pract. 2010;16:161-166.
24. Yoon JH, Jang IK. Heparin-induced thrombocytopenia in cardiovascular patients: pathophysiology, diagnosis, and treatment. Cardiol Rev. 2011;19:143-153.
25. Bartholomew JR. Heparin-induced thrombocytopenia: 2008 update. Curr Treat Options Cardiovasc Med. 2008;10:117-127.
26. Warkentin TE. Platelet count monitoring and laboratory testing for heparin-induced thrombocytopenia. Arch Pathol Lab Med. 2002;126:1415-1423.
27. Badger NO. Fondaparinux (Arixtra®), a safe alternative for the treatment of patients with heparin-induced thrombocytopenia? J Pharm Pract. 2010;23:235-238.
28. Petros S. Lepirudin in the management of patients with heparin-induced thrombocytopenia. Biologics. 2008;2:481-490.
29. Warkentin TE. How I diagnose and manage HIT. Hematology Am Soc Hematol Educ Program. 2011;2011:143-149.
30. Papadopoulos S, Flynn JD, Lewis DA. Fondaparinux as a treatment option for heparin-induced thrombocytopenia. Pharmacotherapy. 2007; 27:921-926.
Heparin-induced thrombocytopenia (HIT) is an immune-mediated drug reaction that requires prompt detection and treatment in order to minimize patient morbidity and mortality.1 HIT is caused by the development of antibodies to platelet factor 4 (PF4), although it is important to note that not all patients who develop PF4 antibodies will experience the clinical syndrome of HIT.2-4 In fact, about 50% of patients who undergo cardiovascular surgery develop PF4 antibodies, but only 1% to 2% of patients with antibodies actually experience HIT.5-7 There is currently no explanation for the phenomenon of HIT.8
In 2012, with an intent to limit HIT-associated morbidity and mortality, the American College of Chest Physicians (ACCP) unveiled the ninth edition of its evidence-based practice guidelines for the detection of HIT and appropriate treatment.5 Much of the information provided in this article emerged from these guidelines.
Epidemiology
Of the 12 million patients treated each year with either unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH), 600,000 (0.5%) will develop HIT. Among these patients, 300,000 will develop thrombosis, and 90,000 will die. In 2009 alone, the HIT-associated cost to the US health care system was estimated at $100 million.1
As growing numbers of patients require anticoagulation therapy, it becomes increasingly important for clinicians to understand the importance of screening for deep vein thrombosis (DVT), one of the two most common thromboses; the other is pulmonary embolism.9,10 Continuing to administer heparin or warfarin to patients with undetected HIT predisposes them to severe complications, including venous and arterial thromboses and gangrenous skin lesions—which can result in loss of life and/or limb.1,11,12
Risk Factors for HIT
Several factors influence a patient's risk for HIT, including the type and dosing regimen of the heparin being administered. Generally, the risk for HIT is about 10-fold in patients treated with UFH (3% to 5%), compared with those receiving LMWH (0.5%).5,13 The risk for HIT is also greater in patients receiving UFH of bovine origin, compared with those taking porcine-derived UFH.8,14,15
In a recent meta-analysis of postsurgical patients who underwent heparin thromboprophylaxis, those given LMWH had a 76% relative risk reduction for HIT, compared with patients taking UFH.16 The incidence of HIT increases among patients receiving LMWH if they have been treated with UFH within the previous 100 days.9 HIT onset may be delayed for several days in patients given heparin for the first time (or for the first time in several months), whereas previously exposed patients who have already developed antiheparin PF4 antibodies can experience severe HIT within hours.9
Patient-Specific Risk Factors
Certain patient characteristics also have an impact on HIT risk. For example, the risk for HIT is approximately doubled in women, compared with men,1,5,15 and the incidence of HIT is greater in surgical patients than in medical patients.7,17 Among surgical patients, 5% of orthopedic patients have been reported to develop HIT, compared with 3% of cardiac patients and 1% of patients undergoing surgery for vascular illnesses.1 The reasons for these differences are poorly understood, but current theory focuses on the inflammatory response of individual patients and the degree of associated platelet activation.2,12
Patient Presentation and History
The typical patient with HIT presents with a new or progressing thrombosis between days 5 and 14 of heparin therapy (with day 0 representing the day the first dose is administered); thrombosis can be venous or arterial, although venous thrombosis occurs much more frequently.1,5,9,15 As patients rarely remain hospitalized for such a long period, it is imperative that providers in clinic and emergency settings obtain detailed histories for patients who present with thrombocytopenia and/or thrombosis. HIT should be suspected in any such patient whose history shows heparin use within the previous two weeks (even if the drug has been discontinued).15
Two forms of atypical HIT are rapid-onset HIT and delayed-onset HIT. Rapid-onset HIT is defined by a platelet count that falls within 24 hours of exposure to heparin. This form is usually associated with previous heparin exposure (ie, within the previous 100 days, but most commonly within the previous 30 days). Affected patients have already developed circulating antiheparin PF4 antibodies, causing an immediate reaction when the patient is re-exposed to the drug.1,15
The less common delayed-onset HIT occurs in patients in whom heparin has been discontinued for as long as 40 days. Delayed-onset HIT carries the greatest risk for severe thrombosis.1,15
Atypically, a patient may present with bleeding, skin necrosis, venous gangrene, or anaphylaxis,9 but skin necrosis at the site of heparin injection is strongly suggestive of HIT.12However, neither physical signs nor symptoms, nor a thrombotic event is required to make a diagnosis of HIT. In fact, the preference is for a diagnosis to be made before thrombosis formation.5
The major manifestation of HIT is thrombocytopenia itself2,18(see "Laboratory Findings"). Nevertheless, if physical signs and symptoms are evident, they will be related to the thrombosis, and the components of the physical exam will proceed accordingly.
Laboratory Findings
Platelet count monitoring and HIT antibody testing are the laboratory tests most commonly used when HIT is suspected. Although 25% of patients with HIT will experience a thrombotic event before the platelet count falls, monitoring the platelet count is considered the most effective means to identify patients with HIT.5 HIT antibody testing is not recommended unless the health care provider has a strong suspicion for HIT.19
Thrombocytopenia is a common abnormality, especially in hospitalized patients, and its causes are numerous.11 Table 11,5,9 lists the differential diagnosis that the clinician who suspects HIT should consider.
Nevertheless, the ACCP guidelines5 recommend platelet count monitoring for all patients receiving heparin, beginning on day 4 of heparin therapy, then continuing every two to three days until treatment day 14 or heparin discontinuation, whichever occurs first.5,15 A platelet count decrease of 50% or more should raise a suspicion for HIT.15
The ACCP makes two principal exceptions to these recommendations.5 The first involves patients who have received UFH within the previous 100 days. These patients should undergo a baseline platelet count before heparin is administered, followed by a repeat platelet count within 24 hours. Any patient who experiences an anaphylactic reaction to UFH should undergo an immediate platelet count; a decrease in these patients is often transient.
The second exception pertains to medical and obstetric patients. Those receiving LMWH or UFH only to maintain line patency do not require platelet monitoring, as their risk for HIT is relatively low.1,5
Laboratory Interpretation
In HIT, thrombocytopenia is defined as a platelet count below 150,000/mm3 or a platelet count reduction of 50% or more from baseline, even if the platelet count remains above 150,000/mm3.9 (The patient's baseline platelet count is defined as the highest count recorded in the previous two weeks.1,5,15) The thrombocytopenia associated with HIT is rarely severe and can be easily overlooked.1
Once the platelet count suggests a diagnosis of HIT, heparin-dependent antibodies can be identified through immunologic or functional assays.1,9,15 Immunologic assays should be ordered immediately upon suspicion of HIT since they are simple tests with relatively rapid results. Immunologic assays detect immunoglobulin G (IgG), IgA, and IgM antibodies.9Though lacking in specificity, the immunologic assay is highly sensitive.2,12,20 The most frequently used immunologic assay is the enzyme-linked immunosorbent assay (ELISA).1,2,9,20The ELISA, which detects antiheparin PF4 antibodies, has a sensitivity greater than 97% but a specificity of only 74% to 86%.8,12
Functional assays,which are technically demanding, test the ability of PF4 antibodies to activate platelets in the presence of heparin. The functional assay is used to confirm the diagnosis of HIT when a positive ELISA result is obtained.1,9,15 Among the functional assays, the serotonin release assay (SRA) has been most completely studied. Though very expensive, the SRA is 89% to 100% specific in diagnosing HIT.2,12
Diagnosis
The diagnosis of HIT is determined by combining clinical and serologic assessment. HIT should be suspected in any patient who is in day 5 to 14 of heparin therapy and experiences a drop in platelet count of at least 50%, or in whom a new thrombotic event occurs (even if the patient is no longer receiving heparin therapy). The interpretation of all diagnostic information must be made in the context of the patient's clinical probability of HIT.15
A scoring system referred to as the 4Ts (thrombocytopenia, timing of platelet fall, thrombosis or other sequelae, and test interpretation) is used to help determine the patient's probability of HIT5,21,22 (similar to the scoring strategy shown in Table 22,11,23,24).

The patient diagnosed with HIT must be positive for HIT antibodies and meet at least one additional criterion:
• A platelet count decrease of 30% to 50% below baseline, regardless of the actual value
• A venous or arterial thrombosis
• A skin lesion at the heparin injection site; and/or
• An anaphylactic reaction after IV bolus administration of heparin.15
Treatment/Management
The goal in management of HIT is to reduce the likelihood, then the severity, of thrombosis.9 Treatment should be started as soon as HIT is suspected, before laboratory confirmation.25 Treatment for HIT comprises two steps: stopping all exposure to heparin, and administering an alternative, non-heparin anticoagulant.
Discontinuation of Heparin Exposure
Stopping heparin exposure is the mainstay of treatment for HIT. This includes all potential sources of heparin exposure, including "flushes" that may be used to promote patency of central IV catheters, use of UFH-coated catheters, or addition of any heparin to total parenteral nutrition.9,25
Use of a Non-Heparin Anticoagulant
In addition to discontinuation of all heparin exposure, the patient must be started on a non-heparin anticoagulant, whether or not thrombosis is present.25 Forty percent to 50% of patients without thrombosis will develop a thrombosis within 30 days if alternative anticoagulation is not started.12,18,26
The principal choices for a non-heparin anticoagulant are the direct thrombin inhibitors (DTIs): lepirudin, argatroban, and bivalirudin.1,9,15,25,27 DTIs are the treatment of choice for patients with known or suspected HIT. The ACCP dosing guidelines for the DTIs are summarized in Table 3.9,5,15,28

The factor Xa inhibitor fondaparinux, though FDA approved for DVT prophylaxis,11 has not yet been systematically investigated for the treatment of HIT; thus, its use for this indication is considered off-label.29 However, small studies have shown no cross-reactivity between fondaparinux and PF4 antibodies.30 Due to the positive risk/benefit ratio, ease of use, and reduced need for monitoring in patients taking fondaparinux, it is considered an attractive alternative to DTIs that may receive approval in the near future.12,18,20,29
Currently, the ACCP limits its recommendation of fondaparinux use to patients with a previous history of HIT who require anticoagulation for an acute thrombotic event unrelated to HIT (grade 2C recommendation).5
The vitamin K antagonist warfarin is absolutely contraindicated in patients with HIT until the platelet count is at least 150,000/mm3, due to the risk for warfarin-induced skin necrosis and venous gangrene.9,15 If a patient is receiving warfarin at diagnosis, vitamin K (10 mg orally or 5 to 10 mg IV) should be administered.15
The patient should remain on the alternative non-heparin anticoagulant until the platelet count has stabilized at or above 150,000/mm3. Warfarin should then be started at a maximum of 5 mg/d.2,5 The non-heparin anticoagulant and warfarin should be continued until a therapeutic international normalized ratio (INR) is reached and maintained for 48 hours, with a minimum 5-day overlap of the two medications. Once the non-heparin anticoagulant is discontinued, the INR should be reevaluated for remaining within the therapeutic range, as DTIs can elevate the INR.2,5Warfarin should be continued for as long as four weeks, with frequent INR monitoring.15
Patient Education
The presence of PF4 antibodies is transient (50 to 80 days); however, concern persists regarding recurrent antibody development with subsequent heparin use. Thus, an alternative anticoagulant should be used whenever possible. Patients who have been diagnosed with HIT should be advised to inform future health care professionals regarding their need for alternative anticoagulation whenever possible.
Patients should also be made aware that when the risk for DTI-associated bleeding is too great (as in the case of cardiac surgery), heparin remains the anticoagulant of choice.9,15
Conclusion
Heparin-induced thrombocytopenia is a transient development of antibodies to heparin. While the condition carries a high risk for morbidity and mortality, early detection and prompt treatment can greatly reduce the associated risk to life and limb.
References
1. Kanaan AO, Al-Homsi AS. Heparin-induced thrombocytopenia: pathophysiology, diagnosis, and review of pharmacotherapy. J Pharm Pract. 2009;22:149-157.
2. LaMuraglia GM, Houbballah R, Laposata M. The identification and management of heparin-induced thrombocytopenia in the vascular patient. J Vasc Surg. 2012;55:562-570.
3. Rauova L, Zhai L, Kowalska MA, et al. Role of platelet surface PF4 antigenic complexes in heparin-induced thrombocytopenia pathogenesis: diagnostic and therapeutic implications. Blood. 2006;107:2346-2353.
4. Suvarna S, Espinasse B, Qi R, et al. Determinants of PF4/heparin immunogenicity. Blood. 2007;110:4253-4260.
5. Linkins LA, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e495S-e530S.
6. Demma LJ, Winkler AM, Levy JH. A diagnosis of heparin-induced thrombocytopenia with combined clinical and laboratory methods in cardiothoracic surgical intensive care unit patients. Anesth Analg. 2011;113:697-702.
7. Demma LJ, Levy JH. Diagnosing heparin-induced thrombocytopenia in cardiac surgical patients: not as easy as you think. Anesth Analg. 2011;112:747-749.
8. Alaraj A, Wallace A, Tesoro E, et al. Heparin-induced thrombocytopenia: diagnosis and management. J Neurointervent Surg. 2010;2:371-378.
9. Arepally GM, Ortel TL. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355:809-817.
10. Sud S, Mittmann N, Cook DJ, et al. Screening and prevention of venous thromboembolism in critically ill patients: a decision analysis and economic evaluation. Am J Resp Crit Care Med. 2011;184:1289-1298.
11. Shaikh N. Heparin-induced thrombocytopenia. J Emerg Trauma Shock. 2011;14:97-102.
12. Cuker A. Heparin-induced thrombocytopenia: present and future. J Thromb Thrombolysis. 2011;31:353-366.
13. Locke CSF, Dooley J, Gerber J. Rates of clinically apparent heparin-induced thrombocytopenia for unfractionated heparin vs low molecular weight heparin in non-surgical patients are low and similar. Thromb J. 2005;3:4.
14. Cuker A. Recent advances in heparin-induced thrombocytopenia. Curr Opin Hematol. 2011;18:315-322.
15. Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest. 2008;133(6 suppl):340S-380S.
16. Junqueira DR, Perini E, Penholati RR, Carvalho MG. Unfractionated heparin versus low molecular weight heparin for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev. 2012;9:CD007557.
17. Berry C, Tcherniantchouk O, Ley EJ, et al. Overdiagnosis of heparin-induced thrombocytopenia in surgical ICU patients. J Am Coll Surg. 2011;213:10-17.
18. Cuker A. Current and emerging therapeutics for heparin-induced thrombocytopenia. Semin Thromb Hemost. 2012;38:31-37.
19. Warkentin TE. New approaches to the diagnosis of heparin-induced thrombocytopenia. Chest. 2005;127(2 suppl):35S-45S.
20. Fennessy-Cooney M. Heparin-induced thrombocytopenia. Nurse Pract. 2011;36:31-37.
21. Bryant A, Low J, Austin S, Joseph JE. Timely diagnosis and management of heparin-induced thrombocytopenia in a frequent request, low incidence single centre using clinical 4T's score and particle gel immunoassay. Br J Haematol. 2008;143:721-726.
22. Lo GK, Juhl D, Warkentin TE, et al. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4:759-765.
23. Wirth SM, Macaulay TE, Armistead JA, et al. Evaluation of a clinical scoring scale to direct early appropriate therapy in heparin-induced thrombocytopenia. J Oncol Pharm Pract. 2010;16:161-166.
24. Yoon JH, Jang IK. Heparin-induced thrombocytopenia in cardiovascular patients: pathophysiology, diagnosis, and treatment. Cardiol Rev. 2011;19:143-153.
25. Bartholomew JR. Heparin-induced thrombocytopenia: 2008 update. Curr Treat Options Cardiovasc Med. 2008;10:117-127.
26. Warkentin TE. Platelet count monitoring and laboratory testing for heparin-induced thrombocytopenia. Arch Pathol Lab Med. 2002;126:1415-1423.
27. Badger NO. Fondaparinux (Arixtra®), a safe alternative for the treatment of patients with heparin-induced thrombocytopenia? J Pharm Pract. 2010;23:235-238.
28. Petros S. Lepirudin in the management of patients with heparin-induced thrombocytopenia. Biologics. 2008;2:481-490.
29. Warkentin TE. How I diagnose and manage HIT. Hematology Am Soc Hematol Educ Program. 2011;2011:143-149.
30. Papadopoulos S, Flynn JD, Lewis DA. Fondaparinux as a treatment option for heparin-induced thrombocytopenia. Pharmacotherapy. 2007; 27:921-926.
Heparin-induced thrombocytopenia (HIT) is an immune-mediated drug reaction that requires prompt detection and treatment in order to minimize patient morbidity and mortality.1 HIT is caused by the development of antibodies to platelet factor 4 (PF4), although it is important to note that not all patients who develop PF4 antibodies will experience the clinical syndrome of HIT.2-4 In fact, about 50% of patients who undergo cardiovascular surgery develop PF4 antibodies, but only 1% to 2% of patients with antibodies actually experience HIT.5-7 There is currently no explanation for the phenomenon of HIT.8
In 2012, with an intent to limit HIT-associated morbidity and mortality, the American College of Chest Physicians (ACCP) unveiled the ninth edition of its evidence-based practice guidelines for the detection of HIT and appropriate treatment.5 Much of the information provided in this article emerged from these guidelines.
Epidemiology
Of the 12 million patients treated each year with either unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH), 600,000 (0.5%) will develop HIT. Among these patients, 300,000 will develop thrombosis, and 90,000 will die. In 2009 alone, the HIT-associated cost to the US health care system was estimated at $100 million.1
As growing numbers of patients require anticoagulation therapy, it becomes increasingly important for clinicians to understand the importance of screening for deep vein thrombosis (DVT), one of the two most common thromboses; the other is pulmonary embolism.9,10 Continuing to administer heparin or warfarin to patients with undetected HIT predisposes them to severe complications, including venous and arterial thromboses and gangrenous skin lesions—which can result in loss of life and/or limb.1,11,12
Risk Factors for HIT
Several factors influence a patient's risk for HIT, including the type and dosing regimen of the heparin being administered. Generally, the risk for HIT is about 10-fold in patients treated with UFH (3% to 5%), compared with those receiving LMWH (0.5%).5,13 The risk for HIT is also greater in patients receiving UFH of bovine origin, compared with those taking porcine-derived UFH.8,14,15
In a recent meta-analysis of postsurgical patients who underwent heparin thromboprophylaxis, those given LMWH had a 76% relative risk reduction for HIT, compared with patients taking UFH.16 The incidence of HIT increases among patients receiving LMWH if they have been treated with UFH within the previous 100 days.9 HIT onset may be delayed for several days in patients given heparin for the first time (or for the first time in several months), whereas previously exposed patients who have already developed antiheparin PF4 antibodies can experience severe HIT within hours.9
Patient-Specific Risk Factors
Certain patient characteristics also have an impact on HIT risk. For example, the risk for HIT is approximately doubled in women, compared with men,1,5,15 and the incidence of HIT is greater in surgical patients than in medical patients.7,17 Among surgical patients, 5% of orthopedic patients have been reported to develop HIT, compared with 3% of cardiac patients and 1% of patients undergoing surgery for vascular illnesses.1 The reasons for these differences are poorly understood, but current theory focuses on the inflammatory response of individual patients and the degree of associated platelet activation.2,12
Patient Presentation and History
The typical patient with HIT presents with a new or progressing thrombosis between days 5 and 14 of heparin therapy (with day 0 representing the day the first dose is administered); thrombosis can be venous or arterial, although venous thrombosis occurs much more frequently.1,5,9,15 As patients rarely remain hospitalized for such a long period, it is imperative that providers in clinic and emergency settings obtain detailed histories for patients who present with thrombocytopenia and/or thrombosis. HIT should be suspected in any such patient whose history shows heparin use within the previous two weeks (even if the drug has been discontinued).15
Two forms of atypical HIT are rapid-onset HIT and delayed-onset HIT. Rapid-onset HIT is defined by a platelet count that falls within 24 hours of exposure to heparin. This form is usually associated with previous heparin exposure (ie, within the previous 100 days, but most commonly within the previous 30 days). Affected patients have already developed circulating antiheparin PF4 antibodies, causing an immediate reaction when the patient is re-exposed to the drug.1,15
The less common delayed-onset HIT occurs in patients in whom heparin has been discontinued for as long as 40 days. Delayed-onset HIT carries the greatest risk for severe thrombosis.1,15
Atypically, a patient may present with bleeding, skin necrosis, venous gangrene, or anaphylaxis,9 but skin necrosis at the site of heparin injection is strongly suggestive of HIT.12However, neither physical signs nor symptoms, nor a thrombotic event is required to make a diagnosis of HIT. In fact, the preference is for a diagnosis to be made before thrombosis formation.5
The major manifestation of HIT is thrombocytopenia itself2,18(see "Laboratory Findings"). Nevertheless, if physical signs and symptoms are evident, they will be related to the thrombosis, and the components of the physical exam will proceed accordingly.
Laboratory Findings
Platelet count monitoring and HIT antibody testing are the laboratory tests most commonly used when HIT is suspected. Although 25% of patients with HIT will experience a thrombotic event before the platelet count falls, monitoring the platelet count is considered the most effective means to identify patients with HIT.5 HIT antibody testing is not recommended unless the health care provider has a strong suspicion for HIT.19
Thrombocytopenia is a common abnormality, especially in hospitalized patients, and its causes are numerous.11 Table 11,5,9 lists the differential diagnosis that the clinician who suspects HIT should consider.
Nevertheless, the ACCP guidelines5 recommend platelet count monitoring for all patients receiving heparin, beginning on day 4 of heparin therapy, then continuing every two to three days until treatment day 14 or heparin discontinuation, whichever occurs first.5,15 A platelet count decrease of 50% or more should raise a suspicion for HIT.15
The ACCP makes two principal exceptions to these recommendations.5 The first involves patients who have received UFH within the previous 100 days. These patients should undergo a baseline platelet count before heparin is administered, followed by a repeat platelet count within 24 hours. Any patient who experiences an anaphylactic reaction to UFH should undergo an immediate platelet count; a decrease in these patients is often transient.
The second exception pertains to medical and obstetric patients. Those receiving LMWH or UFH only to maintain line patency do not require platelet monitoring, as their risk for HIT is relatively low.1,5
Laboratory Interpretation
In HIT, thrombocytopenia is defined as a platelet count below 150,000/mm3 or a platelet count reduction of 50% or more from baseline, even if the platelet count remains above 150,000/mm3.9 (The patient's baseline platelet count is defined as the highest count recorded in the previous two weeks.1,5,15) The thrombocytopenia associated with HIT is rarely severe and can be easily overlooked.1
Once the platelet count suggests a diagnosis of HIT, heparin-dependent antibodies can be identified through immunologic or functional assays.1,9,15 Immunologic assays should be ordered immediately upon suspicion of HIT since they are simple tests with relatively rapid results. Immunologic assays detect immunoglobulin G (IgG), IgA, and IgM antibodies.9Though lacking in specificity, the immunologic assay is highly sensitive.2,12,20 The most frequently used immunologic assay is the enzyme-linked immunosorbent assay (ELISA).1,2,9,20The ELISA, which detects antiheparin PF4 antibodies, has a sensitivity greater than 97% but a specificity of only 74% to 86%.8,12
Functional assays,which are technically demanding, test the ability of PF4 antibodies to activate platelets in the presence of heparin. The functional assay is used to confirm the diagnosis of HIT when a positive ELISA result is obtained.1,9,15 Among the functional assays, the serotonin release assay (SRA) has been most completely studied. Though very expensive, the SRA is 89% to 100% specific in diagnosing HIT.2,12
Diagnosis
The diagnosis of HIT is determined by combining clinical and serologic assessment. HIT should be suspected in any patient who is in day 5 to 14 of heparin therapy and experiences a drop in platelet count of at least 50%, or in whom a new thrombotic event occurs (even if the patient is no longer receiving heparin therapy). The interpretation of all diagnostic information must be made in the context of the patient's clinical probability of HIT.15
A scoring system referred to as the 4Ts (thrombocytopenia, timing of platelet fall, thrombosis or other sequelae, and test interpretation) is used to help determine the patient's probability of HIT5,21,22 (similar to the scoring strategy shown in Table 22,11,23,24).

The patient diagnosed with HIT must be positive for HIT antibodies and meet at least one additional criterion:
• A platelet count decrease of 30% to 50% below baseline, regardless of the actual value
• A venous or arterial thrombosis
• A skin lesion at the heparin injection site; and/or
• An anaphylactic reaction after IV bolus administration of heparin.15
Treatment/Management
The goal in management of HIT is to reduce the likelihood, then the severity, of thrombosis.9 Treatment should be started as soon as HIT is suspected, before laboratory confirmation.25 Treatment for HIT comprises two steps: stopping all exposure to heparin, and administering an alternative, non-heparin anticoagulant.
Discontinuation of Heparin Exposure
Stopping heparin exposure is the mainstay of treatment for HIT. This includes all potential sources of heparin exposure, including "flushes" that may be used to promote patency of central IV catheters, use of UFH-coated catheters, or addition of any heparin to total parenteral nutrition.9,25
Use of a Non-Heparin Anticoagulant
In addition to discontinuation of all heparin exposure, the patient must be started on a non-heparin anticoagulant, whether or not thrombosis is present.25 Forty percent to 50% of patients without thrombosis will develop a thrombosis within 30 days if alternative anticoagulation is not started.12,18,26
The principal choices for a non-heparin anticoagulant are the direct thrombin inhibitors (DTIs): lepirudin, argatroban, and bivalirudin.1,9,15,25,27 DTIs are the treatment of choice for patients with known or suspected HIT. The ACCP dosing guidelines for the DTIs are summarized in Table 3.9,5,15,28

The factor Xa inhibitor fondaparinux, though FDA approved for DVT prophylaxis,11 has not yet been systematically investigated for the treatment of HIT; thus, its use for this indication is considered off-label.29 However, small studies have shown no cross-reactivity between fondaparinux and PF4 antibodies.30 Due to the positive risk/benefit ratio, ease of use, and reduced need for monitoring in patients taking fondaparinux, it is considered an attractive alternative to DTIs that may receive approval in the near future.12,18,20,29
Currently, the ACCP limits its recommendation of fondaparinux use to patients with a previous history of HIT who require anticoagulation for an acute thrombotic event unrelated to HIT (grade 2C recommendation).5
The vitamin K antagonist warfarin is absolutely contraindicated in patients with HIT until the platelet count is at least 150,000/mm3, due to the risk for warfarin-induced skin necrosis and venous gangrene.9,15 If a patient is receiving warfarin at diagnosis, vitamin K (10 mg orally or 5 to 10 mg IV) should be administered.15
The patient should remain on the alternative non-heparin anticoagulant until the platelet count has stabilized at or above 150,000/mm3. Warfarin should then be started at a maximum of 5 mg/d.2,5 The non-heparin anticoagulant and warfarin should be continued until a therapeutic international normalized ratio (INR) is reached and maintained for 48 hours, with a minimum 5-day overlap of the two medications. Once the non-heparin anticoagulant is discontinued, the INR should be reevaluated for remaining within the therapeutic range, as DTIs can elevate the INR.2,5Warfarin should be continued for as long as four weeks, with frequent INR monitoring.15
Patient Education
The presence of PF4 antibodies is transient (50 to 80 days); however, concern persists regarding recurrent antibody development with subsequent heparin use. Thus, an alternative anticoagulant should be used whenever possible. Patients who have been diagnosed with HIT should be advised to inform future health care professionals regarding their need for alternative anticoagulation whenever possible.
Patients should also be made aware that when the risk for DTI-associated bleeding is too great (as in the case of cardiac surgery), heparin remains the anticoagulant of choice.9,15
Conclusion
Heparin-induced thrombocytopenia is a transient development of antibodies to heparin. While the condition carries a high risk for morbidity and mortality, early detection and prompt treatment can greatly reduce the associated risk to life and limb.
References
1. Kanaan AO, Al-Homsi AS. Heparin-induced thrombocytopenia: pathophysiology, diagnosis, and review of pharmacotherapy. J Pharm Pract. 2009;22:149-157.
2. LaMuraglia GM, Houbballah R, Laposata M. The identification and management of heparin-induced thrombocytopenia in the vascular patient. J Vasc Surg. 2012;55:562-570.
3. Rauova L, Zhai L, Kowalska MA, et al. Role of platelet surface PF4 antigenic complexes in heparin-induced thrombocytopenia pathogenesis: diagnostic and therapeutic implications. Blood. 2006;107:2346-2353.
4. Suvarna S, Espinasse B, Qi R, et al. Determinants of PF4/heparin immunogenicity. Blood. 2007;110:4253-4260.
5. Linkins LA, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e495S-e530S.
6. Demma LJ, Winkler AM, Levy JH. A diagnosis of heparin-induced thrombocytopenia with combined clinical and laboratory methods in cardiothoracic surgical intensive care unit patients. Anesth Analg. 2011;113:697-702.
7. Demma LJ, Levy JH. Diagnosing heparin-induced thrombocytopenia in cardiac surgical patients: not as easy as you think. Anesth Analg. 2011;112:747-749.
8. Alaraj A, Wallace A, Tesoro E, et al. Heparin-induced thrombocytopenia: diagnosis and management. J Neurointervent Surg. 2010;2:371-378.
9. Arepally GM, Ortel TL. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355:809-817.
10. Sud S, Mittmann N, Cook DJ, et al. Screening and prevention of venous thromboembolism in critically ill patients: a decision analysis and economic evaluation. Am J Resp Crit Care Med. 2011;184:1289-1298.
11. Shaikh N. Heparin-induced thrombocytopenia. J Emerg Trauma Shock. 2011;14:97-102.
12. Cuker A. Heparin-induced thrombocytopenia: present and future. J Thromb Thrombolysis. 2011;31:353-366.
13. Locke CSF, Dooley J, Gerber J. Rates of clinically apparent heparin-induced thrombocytopenia for unfractionated heparin vs low molecular weight heparin in non-surgical patients are low and similar. Thromb J. 2005;3:4.
14. Cuker A. Recent advances in heparin-induced thrombocytopenia. Curr Opin Hematol. 2011;18:315-322.
15. Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest. 2008;133(6 suppl):340S-380S.
16. Junqueira DR, Perini E, Penholati RR, Carvalho MG. Unfractionated heparin versus low molecular weight heparin for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev. 2012;9:CD007557.
17. Berry C, Tcherniantchouk O, Ley EJ, et al. Overdiagnosis of heparin-induced thrombocytopenia in surgical ICU patients. J Am Coll Surg. 2011;213:10-17.
18. Cuker A. Current and emerging therapeutics for heparin-induced thrombocytopenia. Semin Thromb Hemost. 2012;38:31-37.
19. Warkentin TE. New approaches to the diagnosis of heparin-induced thrombocytopenia. Chest. 2005;127(2 suppl):35S-45S.
20. Fennessy-Cooney M. Heparin-induced thrombocytopenia. Nurse Pract. 2011;36:31-37.
21. Bryant A, Low J, Austin S, Joseph JE. Timely diagnosis and management of heparin-induced thrombocytopenia in a frequent request, low incidence single centre using clinical 4T's score and particle gel immunoassay. Br J Haematol. 2008;143:721-726.
22. Lo GK, Juhl D, Warkentin TE, et al. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4:759-765.
23. Wirth SM, Macaulay TE, Armistead JA, et al. Evaluation of a clinical scoring scale to direct early appropriate therapy in heparin-induced thrombocytopenia. J Oncol Pharm Pract. 2010;16:161-166.
24. Yoon JH, Jang IK. Heparin-induced thrombocytopenia in cardiovascular patients: pathophysiology, diagnosis, and treatment. Cardiol Rev. 2011;19:143-153.
25. Bartholomew JR. Heparin-induced thrombocytopenia: 2008 update. Curr Treat Options Cardiovasc Med. 2008;10:117-127.
26. Warkentin TE. Platelet count monitoring and laboratory testing for heparin-induced thrombocytopenia. Arch Pathol Lab Med. 2002;126:1415-1423.
27. Badger NO. Fondaparinux (Arixtra®), a safe alternative for the treatment of patients with heparin-induced thrombocytopenia? J Pharm Pract. 2010;23:235-238.
28. Petros S. Lepirudin in the management of patients with heparin-induced thrombocytopenia. Biologics. 2008;2:481-490.
29. Warkentin TE. How I diagnose and manage HIT. Hematology Am Soc Hematol Educ Program. 2011;2011:143-149.
30. Papadopoulos S, Flynn JD, Lewis DA. Fondaparinux as a treatment option for heparin-induced thrombocytopenia. Pharmacotherapy. 2007; 27:921-926.