User login
It has been well established that metastatic disease to bone has major significance in the morbidity associated with the diagnosis of cancer.1 More than 75% of patients with metastatic cancer will have bone involvement at the time of death.2-4 Moreover, there is a reported 8% incidence of a pathologic fracture in patients who carry the diagnosis of cancer.5 Common sites of involvement include the spine, ribs, pelvis, and long bones such as humerus and femur.6 Pathologic fracture is fracture caused by disease rather than injury or trauma (referred to here as nonpathologic). In any bone, pathologic fracture will be associated with increased morbidity for the patient, but it is the spine and long bones that frequently require surgical intervention and are associated with high mortality and morbidity. Advanced cancer can also increase fracture risk through increasing falls; in one prospective study of patients with advanced cancer, more than half the patients experienced a fall.7
Based on historical studies of patients who have died from common cancers,4,6 it is commonly believed that breast, lung, thyroid, kidney, and prostate cancers are the most common sources of metastasis to bone, and that other common cancers, such as colorectal carcinoma (CRC), have lower rates of metastasis to bone.6,8,9 It has been inferred from this data that cancers such as CRC thereby have lower rates of pathologic fracture.
Presence of bone metastasis at time of death may be less clinically relevant than occurrence of pathologic fracture and, especially, pathologic fracture requiring hospitalization. The authors are aware of no studies that have determined the number of patients hospitalized as a result of pathologic fracture from common tumors. Despite cadaveric findings, clinical experience dictates that colorectal carcinoma is not an uncommon primary tumor in patients presenting with metastatic disease and pathologic fracture, whereas thyroid carcinoma is more rare.
Despite lower rates of metastasis to bone from CRC, progression to advanced disease is common, with projected 50,000 deaths in the United States in 2014, and tumor progression is associated with metastasis to bone.10 Patterns of health care use and costs associated with skeletal-related events in more common metastatic prostate and breast cancer are well documented.11-13 The authors are aware of no population-based studies examining the burden from metastatic fractures or hospitalization incidence attributed to CRC.
Methods
This is a retrospective study of patients hospitalized in the United States with metastatic disease. Data for this study were obtained from the 2003-2010 National (Nationwide) Inpatient Sample (NIS), the Healthcare Cost and Utilization Project (HCUP), and the Agency for Healthcare Research and Quality.14 The NIS is a stratified sample of approximately 20% of inpatient hospitalization discharges in the United States with more than 7 million hospital stays each year. The dataset contains basic patient demographics, dates of admission, discharge, and procedures, as well as diagnosis and procedure codes for unique hospitalizations. The numbers of new cases of each type of cancer diagnosed in the United States during 2003-2010 were determined from fact sheets published by the American Cancer Society.15
In all, 1,008,641 patients with metastatic disease in the NIS database, were identified by the presence of International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) diagnosis codes 196.0-199.1. Patients were then classified by primary cancer type based on the presence of additional ICD-9-CM codes for a specific cancer type (140.x-189.x) or for a history of a specific cancer type (V10.00 – V10.91). The analysis was limited to the 10 most common types of cancer. Multiple myeloma, leukemia, lymphoma, and primary cancers of bone also cause pathologic fractures, but they were purposefully excluded from the analysis because they do not represent truly metastatic disease. Patients were excluded if they were younger than 18 years (n = 9,425), had been admitted with major significant trauma (Major Diagnostic Category 24; n = 287), or if the cancer type was either not listed in discharge billing data or not one of the 10 most common types (n = 324,249). Therefore, the final study sample consisted of 674,680 hospitalizations.
The primary outcome assessed was pathologic fracture, identified with ICD-9-CM codes 733.10-733,19. Fractures not due to bone metastasis can occur in patients with metastatic disease owing to falls and general debility; therefore, the secondary outcome was nonpathologic fracture, identified with ICD-9-CM codes for fracture (805.0-829.0) in the absence of a code for pathologic fracture. Fractures classified as a “stress fracture” (ICD-9-CM code 733.9x) or where there was a concomitant diagnosis of osteoporosis (ICD-9-CM cod 733.0x) were also considered nonpathologic for the purpose of this study. Thus there were 3 groups of hospitalized patients identified: metastatic disease without fracture (No Fracture); Pathologic Fracture; and Nonpathologic Fracture. The study was limited to the 10 types of cancer with the highest numbers of pathologic fracture, leaving 647,680 hospitalizations for analysis.
Univariate analyses comparing the Pathologic, Nonpathologic, and No Fracture groups were performed with the Student t test for continuous characteristics and chi-square test for categorical characteristics. All analyses were performed with use of Stata 13.1 (StataCorp, College Station, TX).
This study protocol (RSRB00055625) was reviewed by the Office for Human Subject Protection Research Subjects Review Board at the University of Rochester and was determined to meet exemption criteria.
Results
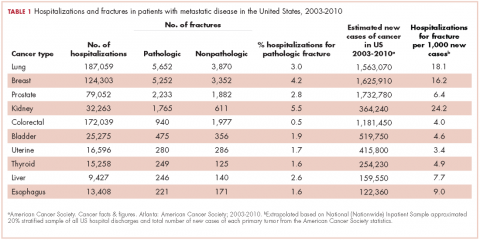
From 2003-2010 there were 674,680 hospitalizations in patients with metastatic cancer that met the inclusion criteria. Hospitalization was most frequent for lung cancer (187,059 admissions), colorectal cancer (172,039), and breast cancer (124,303; Table 1).
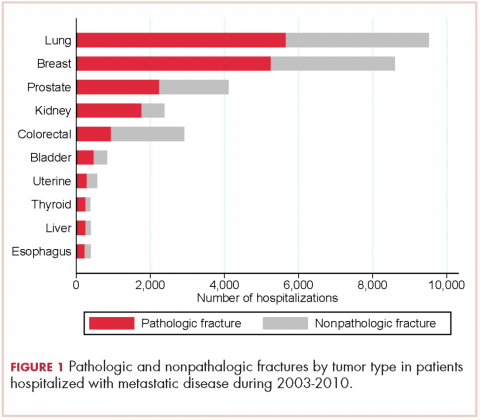
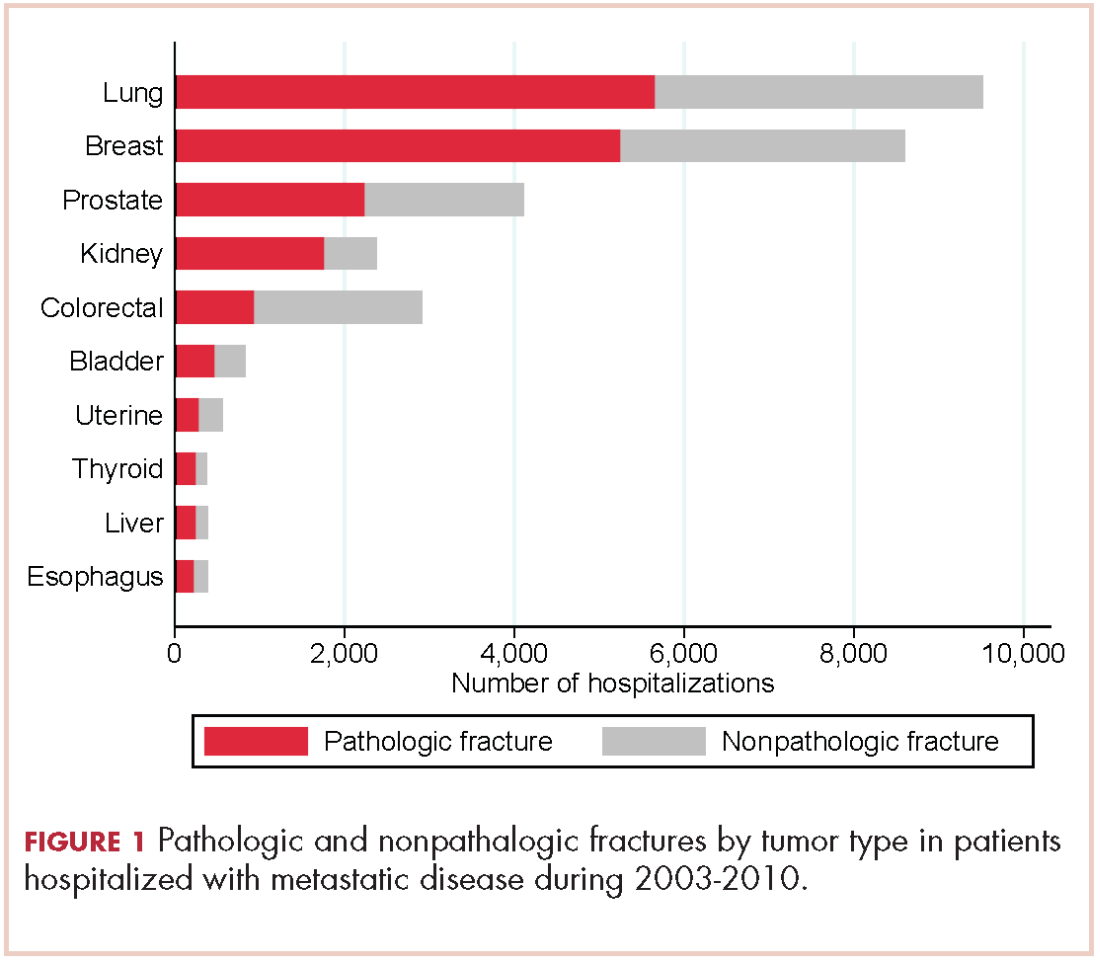
There were 17,303 hospitalizations with pathologic fracture and 12,770 hospitalizations with nonpathologic fracture (Figure 1).
Among the most commonly occurring primary cancers in hospitalizations with pathologic fracture were lung, breast, prostate, kidney, and colorectal cancers (Table 1).
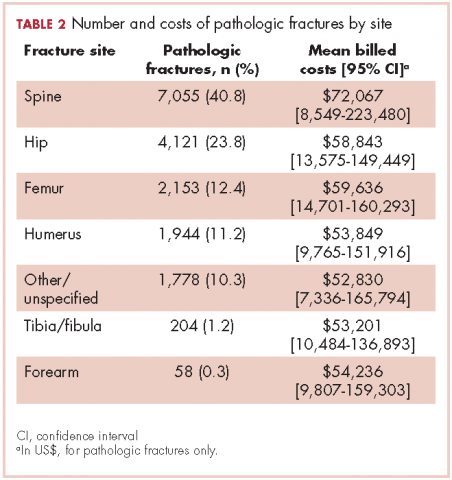
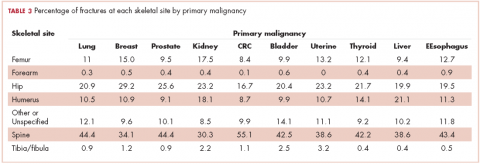
Relative to the annual incidence,15 kidney, lung, and breast cancer had the highest rates of hospital admission for pathologic fracture during the study period. Hospital admission with pathologic fracture was more common than nonpathologic fracture for every type of metastatic disease except colorectal and uterine cancer. Pathologic fracture in patients with metastatic disease was most likely to occur in the spine, hip, and femur (Table 2), and ratio of anatomic sites fractured was relatively consistent across each of the 10 primary malignancies (Table 3).
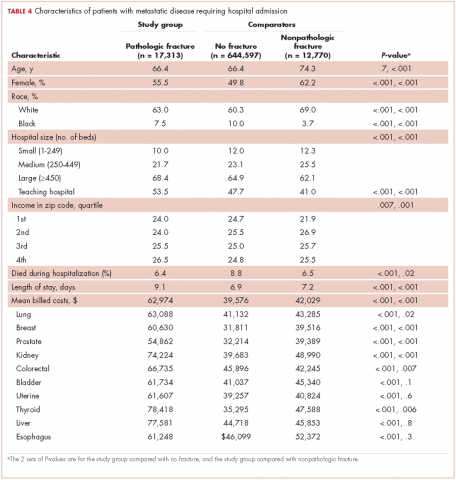
Demographic characteristics of patients in the 3 study groups are shown in Table 4. Patients with pathologic fracture were more likely than those in the no-fracture group to be white (63.0% vs 60.3%, respectively; P < .001) and female (55.5% vs 49.8%; P < .001), but were similar in age (66.4 years; P = 0.7). In-hospital mortality was lower in the pathologic fracture group compared with the no-fracture group (6.4% vs 8.8%; P < .001). People in the pathologic fracture group were more likely than others to be treated at a teaching hospital (P < .001) with ≥450 beds (P < .001), and reside in a zip code with higher income (P < .01).
Pathologic fracture hospitalizations, on average, had higher billed costs and longer length of stay ($62,974, 9.1 days; Table 4), compared with the no-fracture group ($39,576, 6.9 days; both P < .001) and the nonpathologic fracture group ($42,029, 7.2 days; both P < .001). Pathologic fracture in patients with thyroid, liver, and kidney cancer was associated with the highest costs of hospitalization.
In patients with metastatic disease, differences were found between those with pathologic and nonpathologic fractures: those with pathologic fracture were younger (66.4 vs 74.3 years; P < .001), less likely to be white (63.0% vs 69.0%; P < .001), and more commonly treated at a large hospital (68.4% vs 62.1%; P < .001) or a teaching hospital (53.5% vs 41.0%; P < .001).
Discussion
Other investigators have looked at risk factors for pathologic fracture, such as degree of bone involvement, location, and the presence of lytic versus blastic disease, as well as the optimal management of such patients.16-20 In those analyses, there is an emphasis on large, lytic lesions with cortical destruction in weight-bearing long bones, and on functional pain as a key determinant of fracture risk. Although the guidelines outlined by Mirel and others are helpful in predicting fractures, they are not widely applied by practicing oncologists.18 Oncologists and surgeons lack foolproof criteria to predict impending pathologic fracture despite evidence that the pathologic fracture event greatly increases mortality and morbidity.1,4,21,22 As far as we know, this is the first study to determine which types of primary carcinomas were most associated with pathologic fracture requiring hospitalization. This finding will hopefully raise awareness among doctors who care for these patients to be particularly conscientious with patients who present with symptoms of bone pain with activity (functional bone pain) or with lytic disease in the long bones. The results of the present study are similar to those from cadaveric studies, which emphasize the importance of lung, breast, prostate, and kidney cancers as primary tumors that metastasize to bone and lead to pathologic fracture. A novel finding is the nearly 4-fold greater number of pathologic fractures from colorectal carcinoma than thyroid carcinoma.
The importance of detecting patients at risk for pathologic fracture is now more relevant than ever because there are treatment modalities that are readily available to patients with metastatic bone involvement. Two classes of medications, the RANK-ligand inhibitors and bisphosphonates, reduce the number of skeletal events, such as pathologic fracture, in patients with metastatic disease to bone.23-26 However, most of those studies focused on the 3 most common carcinomas (breast, lung, and prostate) to metastasize to bone and cause pathologic fracture. There is greater variability in the prophylactic treatment of other forms of cancer that have metastasized to bone amongst oncologists.
Despite a lower proportion of hospitalizations for fracture in patients with CRC than for thyroid carcinoma (0.5% vs 1.6%, respectively), there were more pathologic fractures from CRC than from thyroid carcinoma because there are far more cases of CRC. SEER data estimate that in 2014 there were 62,000 cases of thyroid cancer and 1,890 deaths, compared with 136,000 cases of CRC and 50,000 deaths.10 Previous findings have shown that bone metastasis from CRC is more common than originally thought, based on autopsies of CRC patients.3 However, the lower rate of bone metastasis in CRC compared with other malignancies has led to a decreased focus on skeletal-related events in CRC. Our results suggest vigilance to bone health is warranted in patients with metastatic CRC. A novel finding is that patients with metastatic CRC also have a high number of hospital admissions for nonpathologic fracture. In establishing that patients with metastatic CRC with bone involvement have a real and significant risk of developing both pathologic and nonpathologic fractures, it may alter the treatment practice for these patients going forward, with greater consideration for an antiresorptive therapy, fall prevention education, or other preventive modalities, such as external-beam radiation therapy after it has been established that patients have metastatic bone disease.
There were some demographic differences between patients with metastatic disease who sustain pathologic fractures and those who do not fracture or sustain nonpathologic fractures. Patients with pathologic fracture were younger than those with nonpathologic fractures, and patients who sustained any fracture were more likely to be white than were patients in the no-fracture group. Known osteoporosis risk factors including older, female, and white with Northern European descent.27 Those findings emphasize the importance of osteoporosis screening and fracture prevention in patients with metastatic disease in general, regardless of the presence of bony metastasis. The present study found that patients who reside in zip codes areas with higher incomes were at slightly increased risk of hospitalization for pathologic fracture. Economic disparities in access to health care and cancer care are well documented,28 and the basis for this finding is a direction for future research.
Both mean billed costs and length of stay were greatest in the pathologic fracture group. The large number of admissions for no-fractured patients may be a final opportunity for intervention and preventative measures in this fragile population. Improved surveillance for bony lesions and attention to pain, especially at night, or unexplained hypercalcemia may help with early diagnosis and prevent some pathologic fractures. Patients with pathologic fracture often undergo additional treatments such as radiation therapy or chemotherapy. These additional treatments may partially explain the higher billed costs associated with inpatient hospitalization; future studies may be able to elucidate treatment differences or other reasons for the increased costs associated with pathologic fractures and identify targets to reduce expenditures.
Limitations
This study is subject to the limitations of a retrospective analysis based on hospital administrative discharge data. It evaluates only billed charges and does not account for costs associated with rehabilitation stays. However, it represents a stratified cross-sample of hospitalizations in the United States, in both teaching and nonteaching hospitals, and is the largest study to date that the authors are aware of looking at the burden of pathologic fractures in patients with metastatic disease.
This study specifically included only patients with metastatic disease, which therefore limits comparisons with the rate of hospitalization for nonpathologic fracture in patients without metastatic disease. Patients with metastatic disease who were not hospitalized during the study period are nevertheless at risk for fracture but would not have been captured in this study. It is also likely that some patients with metastatic disease had multiple hospitalizations, including some that were not for fracture; therefore, this study likely underestimates the percentage of patients with metastatic disease who sustain pathologic and nonpathologic fracture.
Some patients were excluded because we were not able to identify a primary cancer from hospital discharge records. The lack of an included diagnosis may be a result of indeterminate primary during the fracture admission or may represent a failure to accurately code a primary, known cancer. Although the NIS does not permit identification of these patients to determine if a primary cancer was subsequently identified, future studies using other databases may target patients presenting with pathologic fracture and an unknown primary tumor to evaluate subsequent cancer diagnosis.
Summary
The significance of bone metastasis in causing pathologic fractures in lung, breast, prostate, and kidney cancers was confirmed. Colorectal carcinoma has been established as the fifth most common primary cancer in patients with metastatic disease who are hospitalized with pathologic fracture, and a large number of patients with metastatic CRC sustain nonpathologic fractures requiring hospitalization. In patients with metastatic CRC or new skeletal pain, education on fall prevention and increased vigilance should be considered. Further studies are needed to determine the best method for prevention of pathologic fractures in all highly prevalent cancers, with previous hospitalizations without fracture as an appropriate target. Previous paradigms about which cancers metastasize to bone should be reconsidered in the context of which lead to clinically important fractures and hospitalization.
1. Carter JA, Ji X, Botteman MF. Clinical, economic and humanistic burdens of skeletal-related events associated with bone metastases. Expert Rev Pharmacoecon Outcomes Res. 2013;13(4):483-496.
2. Clain A. Secondary malignant disease of bone. Br J Cancer. 1965;19:15-29.
3. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s-6249s.
4. Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588-1594.
5. Higinbotham NL, Marcove RC. The management of pathological fractures. J Trauma. 1965;5(6):792-798.
6. Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624-1633.
7. Stone CA, Lawlor PG, Savva GM, Bennett K, Kenny RA. Prospective study of falls and risk factors for falls in adults with advanced cancer. J Clin Oncol. 2012;30(17):2128-2133.
8. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165-176.
9. Katoh M, Unakami M, Hara M, Fukuchi S. Bone metastasis from colorectal cancer in autopsy cases. J Gastroenterol. 1995;30(5):615-618.
10. Howlader N NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission. Posted April 2014. Accessed January 19, 2018.
11. Hagiwara M, Delea TE, Saville MW, Chung K. Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(1):23-27.
12. Hagiwara M, Delea TE, Chung K. Healthcare costs associated with skeletal-related events in breast cancer patients with bone metastases. J Med Econ. 2014;17(3):223-230.
13. Yong C, Onukwugha E, Mullins CD. Clinical and economic burden of bone metastasis and skeletal-related events in prostate cancer. Curr Opin Oncol. 2014;26(3):274-283.
14. HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2011. Agency for Healthcare Research and Quality R, MD. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Last modified January 17, 2018. Accessed January 18, 2018.
15. American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2003-2010. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2010.html Published January 2010. Accessed January 17, 2018.
16. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1614-1627.
17. Harrington KD. Impending pathologic fractures from metastatic malignancy: evaluation and management. Instr Course Lect. 1986;35:357-381.
18. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
19. Weber KL. Evaluation of the adult patient (aged >40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18(3):169-179.
20. Rougraff BT. Evaluation of the patient with carcinoma of unknown origin metastatic to bone. Clin Orthop Relat Res. 2003(415 Suppl):S105-109.
21. Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61-66.
22. Dijstra S, Wiggers T, van Geel BN, Boxma H. Impending and actual pathological fractures in patients with bone metastases of the long bones. A retrospective study of 233 surgically treated fractures. Eur J Surg. 1994;160(10):535-542.
23. Henry D, Vadhan-Raj S, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679-687.
24. Lorusso V, Duran I, Garzon-Rodriguez C, et al. Health resource utilisation associated with skeletal-related events in European patients with lung cancer: Alpha subgroup analysis from a prospective multinational study. Mol Clin Oncol. 2014;2(5):701-708.
25. Lothgren M, Ribnicsek E, Schmidt L, et al. Cost per patient and potential budget implications of denosumab compared with zoledronic acid in adults with bone metastases from solid tumours who are at risk of skeletal-related events: an analysis for Austria, Sweden and Switzerland. Eu J Hosp Pharm Sci Pract. 2013;20(4):227-231.
26. Luftner D, Lorusso V, Duran I, et al. Health resource utilization associated with skeletal-related events in patients with advanced breast cancer: results from a prospective, multinational observational study. SpringerPlus. 2014;3:328.
27. Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res. 2011;469(7):1891-1899.
28. VanEenwyk J, Campo JS, Ossiander EM. Socioeconomic and demographic disparities in treatment for carcinomas of the colon and rectum. Cancer. 2002;95(1):39-46.
It has been well established that metastatic disease to bone has major significance in the morbidity associated with the diagnosis of cancer.1 More than 75% of patients with metastatic cancer will have bone involvement at the time of death.2-4 Moreover, there is a reported 8% incidence of a pathologic fracture in patients who carry the diagnosis of cancer.5 Common sites of involvement include the spine, ribs, pelvis, and long bones such as humerus and femur.6 Pathologic fracture is fracture caused by disease rather than injury or trauma (referred to here as nonpathologic). In any bone, pathologic fracture will be associated with increased morbidity for the patient, but it is the spine and long bones that frequently require surgical intervention and are associated with high mortality and morbidity. Advanced cancer can also increase fracture risk through increasing falls; in one prospective study of patients with advanced cancer, more than half the patients experienced a fall.7
Based on historical studies of patients who have died from common cancers,4,6 it is commonly believed that breast, lung, thyroid, kidney, and prostate cancers are the most common sources of metastasis to bone, and that other common cancers, such as colorectal carcinoma (CRC), have lower rates of metastasis to bone.6,8,9 It has been inferred from this data that cancers such as CRC thereby have lower rates of pathologic fracture.
Presence of bone metastasis at time of death may be less clinically relevant than occurrence of pathologic fracture and, especially, pathologic fracture requiring hospitalization. The authors are aware of no studies that have determined the number of patients hospitalized as a result of pathologic fracture from common tumors. Despite cadaveric findings, clinical experience dictates that colorectal carcinoma is not an uncommon primary tumor in patients presenting with metastatic disease and pathologic fracture, whereas thyroid carcinoma is more rare.
Despite lower rates of metastasis to bone from CRC, progression to advanced disease is common, with projected 50,000 deaths in the United States in 2014, and tumor progression is associated with metastasis to bone.10 Patterns of health care use and costs associated with skeletal-related events in more common metastatic prostate and breast cancer are well documented.11-13 The authors are aware of no population-based studies examining the burden from metastatic fractures or hospitalization incidence attributed to CRC.
Methods
This is a retrospective study of patients hospitalized in the United States with metastatic disease. Data for this study were obtained from the 2003-2010 National (Nationwide) Inpatient Sample (NIS), the Healthcare Cost and Utilization Project (HCUP), and the Agency for Healthcare Research and Quality.14 The NIS is a stratified sample of approximately 20% of inpatient hospitalization discharges in the United States with more than 7 million hospital stays each year. The dataset contains basic patient demographics, dates of admission, discharge, and procedures, as well as diagnosis and procedure codes for unique hospitalizations. The numbers of new cases of each type of cancer diagnosed in the United States during 2003-2010 were determined from fact sheets published by the American Cancer Society.15
In all, 1,008,641 patients with metastatic disease in the NIS database, were identified by the presence of International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) diagnosis codes 196.0-199.1. Patients were then classified by primary cancer type based on the presence of additional ICD-9-CM codes for a specific cancer type (140.x-189.x) or for a history of a specific cancer type (V10.00 – V10.91). The analysis was limited to the 10 most common types of cancer. Multiple myeloma, leukemia, lymphoma, and primary cancers of bone also cause pathologic fractures, but they were purposefully excluded from the analysis because they do not represent truly metastatic disease. Patients were excluded if they were younger than 18 years (n = 9,425), had been admitted with major significant trauma (Major Diagnostic Category 24; n = 287), or if the cancer type was either not listed in discharge billing data or not one of the 10 most common types (n = 324,249). Therefore, the final study sample consisted of 674,680 hospitalizations.
The primary outcome assessed was pathologic fracture, identified with ICD-9-CM codes 733.10-733,19. Fractures not due to bone metastasis can occur in patients with metastatic disease owing to falls and general debility; therefore, the secondary outcome was nonpathologic fracture, identified with ICD-9-CM codes for fracture (805.0-829.0) in the absence of a code for pathologic fracture. Fractures classified as a “stress fracture” (ICD-9-CM code 733.9x) or where there was a concomitant diagnosis of osteoporosis (ICD-9-CM cod 733.0x) were also considered nonpathologic for the purpose of this study. Thus there were 3 groups of hospitalized patients identified: metastatic disease without fracture (No Fracture); Pathologic Fracture; and Nonpathologic Fracture. The study was limited to the 10 types of cancer with the highest numbers of pathologic fracture, leaving 647,680 hospitalizations for analysis.
Univariate analyses comparing the Pathologic, Nonpathologic, and No Fracture groups were performed with the Student t test for continuous characteristics and chi-square test for categorical characteristics. All analyses were performed with use of Stata 13.1 (StataCorp, College Station, TX).
This study protocol (RSRB00055625) was reviewed by the Office for Human Subject Protection Research Subjects Review Board at the University of Rochester and was determined to meet exemption criteria.
Results
From 2003-2010 there were 674,680 hospitalizations in patients with metastatic cancer that met the inclusion criteria. Hospitalization was most frequent for lung cancer (187,059 admissions), colorectal cancer (172,039), and breast cancer (124,303; Table 1).
There were 17,303 hospitalizations with pathologic fracture and 12,770 hospitalizations with nonpathologic fracture (Figure 1).
Among the most commonly occurring primary cancers in hospitalizations with pathologic fracture were lung, breast, prostate, kidney, and colorectal cancers (Table 1).
Relative to the annual incidence,15 kidney, lung, and breast cancer had the highest rates of hospital admission for pathologic fracture during the study period. Hospital admission with pathologic fracture was more common than nonpathologic fracture for every type of metastatic disease except colorectal and uterine cancer. Pathologic fracture in patients with metastatic disease was most likely to occur in the spine, hip, and femur (Table 2), and ratio of anatomic sites fractured was relatively consistent across each of the 10 primary malignancies (Table 3).
Demographic characteristics of patients in the 3 study groups are shown in Table 4. Patients with pathologic fracture were more likely than those in the no-fracture group to be white (63.0% vs 60.3%, respectively; P < .001) and female (55.5% vs 49.8%; P < .001), but were similar in age (66.4 years; P = 0.7). In-hospital mortality was lower in the pathologic fracture group compared with the no-fracture group (6.4% vs 8.8%; P < .001). People in the pathologic fracture group were more likely than others to be treated at a teaching hospital (P < .001) with ≥450 beds (P < .001), and reside in a zip code with higher income (P < .01).
Pathologic fracture hospitalizations, on average, had higher billed costs and longer length of stay ($62,974, 9.1 days; Table 4), compared with the no-fracture group ($39,576, 6.9 days; both P < .001) and the nonpathologic fracture group ($42,029, 7.2 days; both P < .001). Pathologic fracture in patients with thyroid, liver, and kidney cancer was associated with the highest costs of hospitalization.
In patients with metastatic disease, differences were found between those with pathologic and nonpathologic fractures: those with pathologic fracture were younger (66.4 vs 74.3 years; P < .001), less likely to be white (63.0% vs 69.0%; P < .001), and more commonly treated at a large hospital (68.4% vs 62.1%; P < .001) or a teaching hospital (53.5% vs 41.0%; P < .001).
Discussion
Other investigators have looked at risk factors for pathologic fracture, such as degree of bone involvement, location, and the presence of lytic versus blastic disease, as well as the optimal management of such patients.16-20 In those analyses, there is an emphasis on large, lytic lesions with cortical destruction in weight-bearing long bones, and on functional pain as a key determinant of fracture risk. Although the guidelines outlined by Mirel and others are helpful in predicting fractures, they are not widely applied by practicing oncologists.18 Oncologists and surgeons lack foolproof criteria to predict impending pathologic fracture despite evidence that the pathologic fracture event greatly increases mortality and morbidity.1,4,21,22 As far as we know, this is the first study to determine which types of primary carcinomas were most associated with pathologic fracture requiring hospitalization. This finding will hopefully raise awareness among doctors who care for these patients to be particularly conscientious with patients who present with symptoms of bone pain with activity (functional bone pain) or with lytic disease in the long bones. The results of the present study are similar to those from cadaveric studies, which emphasize the importance of lung, breast, prostate, and kidney cancers as primary tumors that metastasize to bone and lead to pathologic fracture. A novel finding is the nearly 4-fold greater number of pathologic fractures from colorectal carcinoma than thyroid carcinoma.
The importance of detecting patients at risk for pathologic fracture is now more relevant than ever because there are treatment modalities that are readily available to patients with metastatic bone involvement. Two classes of medications, the RANK-ligand inhibitors and bisphosphonates, reduce the number of skeletal events, such as pathologic fracture, in patients with metastatic disease to bone.23-26 However, most of those studies focused on the 3 most common carcinomas (breast, lung, and prostate) to metastasize to bone and cause pathologic fracture. There is greater variability in the prophylactic treatment of other forms of cancer that have metastasized to bone amongst oncologists.
Despite a lower proportion of hospitalizations for fracture in patients with CRC than for thyroid carcinoma (0.5% vs 1.6%, respectively), there were more pathologic fractures from CRC than from thyroid carcinoma because there are far more cases of CRC. SEER data estimate that in 2014 there were 62,000 cases of thyroid cancer and 1,890 deaths, compared with 136,000 cases of CRC and 50,000 deaths.10 Previous findings have shown that bone metastasis from CRC is more common than originally thought, based on autopsies of CRC patients.3 However, the lower rate of bone metastasis in CRC compared with other malignancies has led to a decreased focus on skeletal-related events in CRC. Our results suggest vigilance to bone health is warranted in patients with metastatic CRC. A novel finding is that patients with metastatic CRC also have a high number of hospital admissions for nonpathologic fracture. In establishing that patients with metastatic CRC with bone involvement have a real and significant risk of developing both pathologic and nonpathologic fractures, it may alter the treatment practice for these patients going forward, with greater consideration for an antiresorptive therapy, fall prevention education, or other preventive modalities, such as external-beam radiation therapy after it has been established that patients have metastatic bone disease.
There were some demographic differences between patients with metastatic disease who sustain pathologic fractures and those who do not fracture or sustain nonpathologic fractures. Patients with pathologic fracture were younger than those with nonpathologic fractures, and patients who sustained any fracture were more likely to be white than were patients in the no-fracture group. Known osteoporosis risk factors including older, female, and white with Northern European descent.27 Those findings emphasize the importance of osteoporosis screening and fracture prevention in patients with metastatic disease in general, regardless of the presence of bony metastasis. The present study found that patients who reside in zip codes areas with higher incomes were at slightly increased risk of hospitalization for pathologic fracture. Economic disparities in access to health care and cancer care are well documented,28 and the basis for this finding is a direction for future research.
Both mean billed costs and length of stay were greatest in the pathologic fracture group. The large number of admissions for no-fractured patients may be a final opportunity for intervention and preventative measures in this fragile population. Improved surveillance for bony lesions and attention to pain, especially at night, or unexplained hypercalcemia may help with early diagnosis and prevent some pathologic fractures. Patients with pathologic fracture often undergo additional treatments such as radiation therapy or chemotherapy. These additional treatments may partially explain the higher billed costs associated with inpatient hospitalization; future studies may be able to elucidate treatment differences or other reasons for the increased costs associated with pathologic fractures and identify targets to reduce expenditures.
Limitations
This study is subject to the limitations of a retrospective analysis based on hospital administrative discharge data. It evaluates only billed charges and does not account for costs associated with rehabilitation stays. However, it represents a stratified cross-sample of hospitalizations in the United States, in both teaching and nonteaching hospitals, and is the largest study to date that the authors are aware of looking at the burden of pathologic fractures in patients with metastatic disease.
This study specifically included only patients with metastatic disease, which therefore limits comparisons with the rate of hospitalization for nonpathologic fracture in patients without metastatic disease. Patients with metastatic disease who were not hospitalized during the study period are nevertheless at risk for fracture but would not have been captured in this study. It is also likely that some patients with metastatic disease had multiple hospitalizations, including some that were not for fracture; therefore, this study likely underestimates the percentage of patients with metastatic disease who sustain pathologic and nonpathologic fracture.
Some patients were excluded because we were not able to identify a primary cancer from hospital discharge records. The lack of an included diagnosis may be a result of indeterminate primary during the fracture admission or may represent a failure to accurately code a primary, known cancer. Although the NIS does not permit identification of these patients to determine if a primary cancer was subsequently identified, future studies using other databases may target patients presenting with pathologic fracture and an unknown primary tumor to evaluate subsequent cancer diagnosis.
Summary
The significance of bone metastasis in causing pathologic fractures in lung, breast, prostate, and kidney cancers was confirmed. Colorectal carcinoma has been established as the fifth most common primary cancer in patients with metastatic disease who are hospitalized with pathologic fracture, and a large number of patients with metastatic CRC sustain nonpathologic fractures requiring hospitalization. In patients with metastatic CRC or new skeletal pain, education on fall prevention and increased vigilance should be considered. Further studies are needed to determine the best method for prevention of pathologic fractures in all highly prevalent cancers, with previous hospitalizations without fracture as an appropriate target. Previous paradigms about which cancers metastasize to bone should be reconsidered in the context of which lead to clinically important fractures and hospitalization.
It has been well established that metastatic disease to bone has major significance in the morbidity associated with the diagnosis of cancer.1 More than 75% of patients with metastatic cancer will have bone involvement at the time of death.2-4 Moreover, there is a reported 8% incidence of a pathologic fracture in patients who carry the diagnosis of cancer.5 Common sites of involvement include the spine, ribs, pelvis, and long bones such as humerus and femur.6 Pathologic fracture is fracture caused by disease rather than injury or trauma (referred to here as nonpathologic). In any bone, pathologic fracture will be associated with increased morbidity for the patient, but it is the spine and long bones that frequently require surgical intervention and are associated with high mortality and morbidity. Advanced cancer can also increase fracture risk through increasing falls; in one prospective study of patients with advanced cancer, more than half the patients experienced a fall.7
Based on historical studies of patients who have died from common cancers,4,6 it is commonly believed that breast, lung, thyroid, kidney, and prostate cancers are the most common sources of metastasis to bone, and that other common cancers, such as colorectal carcinoma (CRC), have lower rates of metastasis to bone.6,8,9 It has been inferred from this data that cancers such as CRC thereby have lower rates of pathologic fracture.
Presence of bone metastasis at time of death may be less clinically relevant than occurrence of pathologic fracture and, especially, pathologic fracture requiring hospitalization. The authors are aware of no studies that have determined the number of patients hospitalized as a result of pathologic fracture from common tumors. Despite cadaveric findings, clinical experience dictates that colorectal carcinoma is not an uncommon primary tumor in patients presenting with metastatic disease and pathologic fracture, whereas thyroid carcinoma is more rare.
Despite lower rates of metastasis to bone from CRC, progression to advanced disease is common, with projected 50,000 deaths in the United States in 2014, and tumor progression is associated with metastasis to bone.10 Patterns of health care use and costs associated with skeletal-related events in more common metastatic prostate and breast cancer are well documented.11-13 The authors are aware of no population-based studies examining the burden from metastatic fractures or hospitalization incidence attributed to CRC.
Methods
This is a retrospective study of patients hospitalized in the United States with metastatic disease. Data for this study were obtained from the 2003-2010 National (Nationwide) Inpatient Sample (NIS), the Healthcare Cost and Utilization Project (HCUP), and the Agency for Healthcare Research and Quality.14 The NIS is a stratified sample of approximately 20% of inpatient hospitalization discharges in the United States with more than 7 million hospital stays each year. The dataset contains basic patient demographics, dates of admission, discharge, and procedures, as well as diagnosis and procedure codes for unique hospitalizations. The numbers of new cases of each type of cancer diagnosed in the United States during 2003-2010 were determined from fact sheets published by the American Cancer Society.15
In all, 1,008,641 patients with metastatic disease in the NIS database, were identified by the presence of International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) diagnosis codes 196.0-199.1. Patients were then classified by primary cancer type based on the presence of additional ICD-9-CM codes for a specific cancer type (140.x-189.x) or for a history of a specific cancer type (V10.00 – V10.91). The analysis was limited to the 10 most common types of cancer. Multiple myeloma, leukemia, lymphoma, and primary cancers of bone also cause pathologic fractures, but they were purposefully excluded from the analysis because they do not represent truly metastatic disease. Patients were excluded if they were younger than 18 years (n = 9,425), had been admitted with major significant trauma (Major Diagnostic Category 24; n = 287), or if the cancer type was either not listed in discharge billing data or not one of the 10 most common types (n = 324,249). Therefore, the final study sample consisted of 674,680 hospitalizations.
The primary outcome assessed was pathologic fracture, identified with ICD-9-CM codes 733.10-733,19. Fractures not due to bone metastasis can occur in patients with metastatic disease owing to falls and general debility; therefore, the secondary outcome was nonpathologic fracture, identified with ICD-9-CM codes for fracture (805.0-829.0) in the absence of a code for pathologic fracture. Fractures classified as a “stress fracture” (ICD-9-CM code 733.9x) or where there was a concomitant diagnosis of osteoporosis (ICD-9-CM cod 733.0x) were also considered nonpathologic for the purpose of this study. Thus there were 3 groups of hospitalized patients identified: metastatic disease without fracture (No Fracture); Pathologic Fracture; and Nonpathologic Fracture. The study was limited to the 10 types of cancer with the highest numbers of pathologic fracture, leaving 647,680 hospitalizations for analysis.
Univariate analyses comparing the Pathologic, Nonpathologic, and No Fracture groups were performed with the Student t test for continuous characteristics and chi-square test for categorical characteristics. All analyses were performed with use of Stata 13.1 (StataCorp, College Station, TX).
This study protocol (RSRB00055625) was reviewed by the Office for Human Subject Protection Research Subjects Review Board at the University of Rochester and was determined to meet exemption criteria.
Results
From 2003-2010 there were 674,680 hospitalizations in patients with metastatic cancer that met the inclusion criteria. Hospitalization was most frequent for lung cancer (187,059 admissions), colorectal cancer (172,039), and breast cancer (124,303; Table 1).
There were 17,303 hospitalizations with pathologic fracture and 12,770 hospitalizations with nonpathologic fracture (Figure 1).
Among the most commonly occurring primary cancers in hospitalizations with pathologic fracture were lung, breast, prostate, kidney, and colorectal cancers (Table 1).
Relative to the annual incidence,15 kidney, lung, and breast cancer had the highest rates of hospital admission for pathologic fracture during the study period. Hospital admission with pathologic fracture was more common than nonpathologic fracture for every type of metastatic disease except colorectal and uterine cancer. Pathologic fracture in patients with metastatic disease was most likely to occur in the spine, hip, and femur (Table 2), and ratio of anatomic sites fractured was relatively consistent across each of the 10 primary malignancies (Table 3).
Demographic characteristics of patients in the 3 study groups are shown in Table 4. Patients with pathologic fracture were more likely than those in the no-fracture group to be white (63.0% vs 60.3%, respectively; P < .001) and female (55.5% vs 49.8%; P < .001), but were similar in age (66.4 years; P = 0.7). In-hospital mortality was lower in the pathologic fracture group compared with the no-fracture group (6.4% vs 8.8%; P < .001). People in the pathologic fracture group were more likely than others to be treated at a teaching hospital (P < .001) with ≥450 beds (P < .001), and reside in a zip code with higher income (P < .01).
Pathologic fracture hospitalizations, on average, had higher billed costs and longer length of stay ($62,974, 9.1 days; Table 4), compared with the no-fracture group ($39,576, 6.9 days; both P < .001) and the nonpathologic fracture group ($42,029, 7.2 days; both P < .001). Pathologic fracture in patients with thyroid, liver, and kidney cancer was associated with the highest costs of hospitalization.
In patients with metastatic disease, differences were found between those with pathologic and nonpathologic fractures: those with pathologic fracture were younger (66.4 vs 74.3 years; P < .001), less likely to be white (63.0% vs 69.0%; P < .001), and more commonly treated at a large hospital (68.4% vs 62.1%; P < .001) or a teaching hospital (53.5% vs 41.0%; P < .001).
Discussion
Other investigators have looked at risk factors for pathologic fracture, such as degree of bone involvement, location, and the presence of lytic versus blastic disease, as well as the optimal management of such patients.16-20 In those analyses, there is an emphasis on large, lytic lesions with cortical destruction in weight-bearing long bones, and on functional pain as a key determinant of fracture risk. Although the guidelines outlined by Mirel and others are helpful in predicting fractures, they are not widely applied by practicing oncologists.18 Oncologists and surgeons lack foolproof criteria to predict impending pathologic fracture despite evidence that the pathologic fracture event greatly increases mortality and morbidity.1,4,21,22 As far as we know, this is the first study to determine which types of primary carcinomas were most associated with pathologic fracture requiring hospitalization. This finding will hopefully raise awareness among doctors who care for these patients to be particularly conscientious with patients who present with symptoms of bone pain with activity (functional bone pain) or with lytic disease in the long bones. The results of the present study are similar to those from cadaveric studies, which emphasize the importance of lung, breast, prostate, and kidney cancers as primary tumors that metastasize to bone and lead to pathologic fracture. A novel finding is the nearly 4-fold greater number of pathologic fractures from colorectal carcinoma than thyroid carcinoma.
The importance of detecting patients at risk for pathologic fracture is now more relevant than ever because there are treatment modalities that are readily available to patients with metastatic bone involvement. Two classes of medications, the RANK-ligand inhibitors and bisphosphonates, reduce the number of skeletal events, such as pathologic fracture, in patients with metastatic disease to bone.23-26 However, most of those studies focused on the 3 most common carcinomas (breast, lung, and prostate) to metastasize to bone and cause pathologic fracture. There is greater variability in the prophylactic treatment of other forms of cancer that have metastasized to bone amongst oncologists.
Despite a lower proportion of hospitalizations for fracture in patients with CRC than for thyroid carcinoma (0.5% vs 1.6%, respectively), there were more pathologic fractures from CRC than from thyroid carcinoma because there are far more cases of CRC. SEER data estimate that in 2014 there were 62,000 cases of thyroid cancer and 1,890 deaths, compared with 136,000 cases of CRC and 50,000 deaths.10 Previous findings have shown that bone metastasis from CRC is more common than originally thought, based on autopsies of CRC patients.3 However, the lower rate of bone metastasis in CRC compared with other malignancies has led to a decreased focus on skeletal-related events in CRC. Our results suggest vigilance to bone health is warranted in patients with metastatic CRC. A novel finding is that patients with metastatic CRC also have a high number of hospital admissions for nonpathologic fracture. In establishing that patients with metastatic CRC with bone involvement have a real and significant risk of developing both pathologic and nonpathologic fractures, it may alter the treatment practice for these patients going forward, with greater consideration for an antiresorptive therapy, fall prevention education, or other preventive modalities, such as external-beam radiation therapy after it has been established that patients have metastatic bone disease.
There were some demographic differences between patients with metastatic disease who sustain pathologic fractures and those who do not fracture or sustain nonpathologic fractures. Patients with pathologic fracture were younger than those with nonpathologic fractures, and patients who sustained any fracture were more likely to be white than were patients in the no-fracture group. Known osteoporosis risk factors including older, female, and white with Northern European descent.27 Those findings emphasize the importance of osteoporosis screening and fracture prevention in patients with metastatic disease in general, regardless of the presence of bony metastasis. The present study found that patients who reside in zip codes areas with higher incomes were at slightly increased risk of hospitalization for pathologic fracture. Economic disparities in access to health care and cancer care are well documented,28 and the basis for this finding is a direction for future research.
Both mean billed costs and length of stay were greatest in the pathologic fracture group. The large number of admissions for no-fractured patients may be a final opportunity for intervention and preventative measures in this fragile population. Improved surveillance for bony lesions and attention to pain, especially at night, or unexplained hypercalcemia may help with early diagnosis and prevent some pathologic fractures. Patients with pathologic fracture often undergo additional treatments such as radiation therapy or chemotherapy. These additional treatments may partially explain the higher billed costs associated with inpatient hospitalization; future studies may be able to elucidate treatment differences or other reasons for the increased costs associated with pathologic fractures and identify targets to reduce expenditures.
Limitations
This study is subject to the limitations of a retrospective analysis based on hospital administrative discharge data. It evaluates only billed charges and does not account for costs associated with rehabilitation stays. However, it represents a stratified cross-sample of hospitalizations in the United States, in both teaching and nonteaching hospitals, and is the largest study to date that the authors are aware of looking at the burden of pathologic fractures in patients with metastatic disease.
This study specifically included only patients with metastatic disease, which therefore limits comparisons with the rate of hospitalization for nonpathologic fracture in patients without metastatic disease. Patients with metastatic disease who were not hospitalized during the study period are nevertheless at risk for fracture but would not have been captured in this study. It is also likely that some patients with metastatic disease had multiple hospitalizations, including some that were not for fracture; therefore, this study likely underestimates the percentage of patients with metastatic disease who sustain pathologic and nonpathologic fracture.
Some patients were excluded because we were not able to identify a primary cancer from hospital discharge records. The lack of an included diagnosis may be a result of indeterminate primary during the fracture admission or may represent a failure to accurately code a primary, known cancer. Although the NIS does not permit identification of these patients to determine if a primary cancer was subsequently identified, future studies using other databases may target patients presenting with pathologic fracture and an unknown primary tumor to evaluate subsequent cancer diagnosis.
Summary
The significance of bone metastasis in causing pathologic fractures in lung, breast, prostate, and kidney cancers was confirmed. Colorectal carcinoma has been established as the fifth most common primary cancer in patients with metastatic disease who are hospitalized with pathologic fracture, and a large number of patients with metastatic CRC sustain nonpathologic fractures requiring hospitalization. In patients with metastatic CRC or new skeletal pain, education on fall prevention and increased vigilance should be considered. Further studies are needed to determine the best method for prevention of pathologic fractures in all highly prevalent cancers, with previous hospitalizations without fracture as an appropriate target. Previous paradigms about which cancers metastasize to bone should be reconsidered in the context of which lead to clinically important fractures and hospitalization.
1. Carter JA, Ji X, Botteman MF. Clinical, economic and humanistic burdens of skeletal-related events associated with bone metastases. Expert Rev Pharmacoecon Outcomes Res. 2013;13(4):483-496.
2. Clain A. Secondary malignant disease of bone. Br J Cancer. 1965;19:15-29.
3. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s-6249s.
4. Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588-1594.
5. Higinbotham NL, Marcove RC. The management of pathological fractures. J Trauma. 1965;5(6):792-798.
6. Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624-1633.
7. Stone CA, Lawlor PG, Savva GM, Bennett K, Kenny RA. Prospective study of falls and risk factors for falls in adults with advanced cancer. J Clin Oncol. 2012;30(17):2128-2133.
8. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165-176.
9. Katoh M, Unakami M, Hara M, Fukuchi S. Bone metastasis from colorectal cancer in autopsy cases. J Gastroenterol. 1995;30(5):615-618.
10. Howlader N NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission. Posted April 2014. Accessed January 19, 2018.
11. Hagiwara M, Delea TE, Saville MW, Chung K. Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(1):23-27.
12. Hagiwara M, Delea TE, Chung K. Healthcare costs associated with skeletal-related events in breast cancer patients with bone metastases. J Med Econ. 2014;17(3):223-230.
13. Yong C, Onukwugha E, Mullins CD. Clinical and economic burden of bone metastasis and skeletal-related events in prostate cancer. Curr Opin Oncol. 2014;26(3):274-283.
14. HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2011. Agency for Healthcare Research and Quality R, MD. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Last modified January 17, 2018. Accessed January 18, 2018.
15. American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2003-2010. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2010.html Published January 2010. Accessed January 17, 2018.
16. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1614-1627.
17. Harrington KD. Impending pathologic fractures from metastatic malignancy: evaluation and management. Instr Course Lect. 1986;35:357-381.
18. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
19. Weber KL. Evaluation of the adult patient (aged >40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18(3):169-179.
20. Rougraff BT. Evaluation of the patient with carcinoma of unknown origin metastatic to bone. Clin Orthop Relat Res. 2003(415 Suppl):S105-109.
21. Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61-66.
22. Dijstra S, Wiggers T, van Geel BN, Boxma H. Impending and actual pathological fractures in patients with bone metastases of the long bones. A retrospective study of 233 surgically treated fractures. Eur J Surg. 1994;160(10):535-542.
23. Henry D, Vadhan-Raj S, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679-687.
24. Lorusso V, Duran I, Garzon-Rodriguez C, et al. Health resource utilisation associated with skeletal-related events in European patients with lung cancer: Alpha subgroup analysis from a prospective multinational study. Mol Clin Oncol. 2014;2(5):701-708.
25. Lothgren M, Ribnicsek E, Schmidt L, et al. Cost per patient and potential budget implications of denosumab compared with zoledronic acid in adults with bone metastases from solid tumours who are at risk of skeletal-related events: an analysis for Austria, Sweden and Switzerland. Eu J Hosp Pharm Sci Pract. 2013;20(4):227-231.
26. Luftner D, Lorusso V, Duran I, et al. Health resource utilization associated with skeletal-related events in patients with advanced breast cancer: results from a prospective, multinational observational study. SpringerPlus. 2014;3:328.
27. Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res. 2011;469(7):1891-1899.
28. VanEenwyk J, Campo JS, Ossiander EM. Socioeconomic and demographic disparities in treatment for carcinomas of the colon and rectum. Cancer. 2002;95(1):39-46.
1. Carter JA, Ji X, Botteman MF. Clinical, economic and humanistic burdens of skeletal-related events associated with bone metastases. Expert Rev Pharmacoecon Outcomes Res. 2013;13(4):483-496.
2. Clain A. Secondary malignant disease of bone. Br J Cancer. 1965;19:15-29.
3. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s-6249s.
4. Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588-1594.
5. Higinbotham NL, Marcove RC. The management of pathological fractures. J Trauma. 1965;5(6):792-798.
6. Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624-1633.
7. Stone CA, Lawlor PG, Savva GM, Bennett K, Kenny RA. Prospective study of falls and risk factors for falls in adults with advanced cancer. J Clin Oncol. 2012;30(17):2128-2133.
8. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165-176.
9. Katoh M, Unakami M, Hara M, Fukuchi S. Bone metastasis from colorectal cancer in autopsy cases. J Gastroenterol. 1995;30(5):615-618.
10. Howlader N NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission. Posted April 2014. Accessed January 19, 2018.
11. Hagiwara M, Delea TE, Saville MW, Chung K. Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(1):23-27.
12. Hagiwara M, Delea TE, Chung K. Healthcare costs associated with skeletal-related events in breast cancer patients with bone metastases. J Med Econ. 2014;17(3):223-230.
13. Yong C, Onukwugha E, Mullins CD. Clinical and economic burden of bone metastasis and skeletal-related events in prostate cancer. Curr Opin Oncol. 2014;26(3):274-283.
14. HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2011. Agency for Healthcare Research and Quality R, MD. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Last modified January 17, 2018. Accessed January 18, 2018.
15. American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2003-2010. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2010.html Published January 2010. Accessed January 17, 2018.
16. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1614-1627.
17. Harrington KD. Impending pathologic fractures from metastatic malignancy: evaluation and management. Instr Course Lect. 1986;35:357-381.
18. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
19. Weber KL. Evaluation of the adult patient (aged >40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18(3):169-179.
20. Rougraff BT. Evaluation of the patient with carcinoma of unknown origin metastatic to bone. Clin Orthop Relat Res. 2003(415 Suppl):S105-109.
21. Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61-66.
22. Dijstra S, Wiggers T, van Geel BN, Boxma H. Impending and actual pathological fractures in patients with bone metastases of the long bones. A retrospective study of 233 surgically treated fractures. Eur J Surg. 1994;160(10):535-542.
23. Henry D, Vadhan-Raj S, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679-687.
24. Lorusso V, Duran I, Garzon-Rodriguez C, et al. Health resource utilisation associated with skeletal-related events in European patients with lung cancer: Alpha subgroup analysis from a prospective multinational study. Mol Clin Oncol. 2014;2(5):701-708.
25. Lothgren M, Ribnicsek E, Schmidt L, et al. Cost per patient and potential budget implications of denosumab compared with zoledronic acid in adults with bone metastases from solid tumours who are at risk of skeletal-related events: an analysis for Austria, Sweden and Switzerland. Eu J Hosp Pharm Sci Pract. 2013;20(4):227-231.
26. Luftner D, Lorusso V, Duran I, et al. Health resource utilization associated with skeletal-related events in patients with advanced breast cancer: results from a prospective, multinational observational study. SpringerPlus. 2014;3:328.
27. Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res. 2011;469(7):1891-1899.
28. VanEenwyk J, Campo JS, Ossiander EM. Socioeconomic and demographic disparities in treatment for carcinomas of the colon and rectum. Cancer. 2002;95(1):39-46.