User login
• Measure TSH in any patient >60 years presenting with fatigue, atrial fibrillation, weight loss, and shortness of breath. B
• Achieve faster control of symptoms in elderly patients and those with cardiac disease by pursuing the ablative method with radioactive iodine (RAI). This method is also recommended for patients with toxic multinodular goiter and toxic adenoma. A
• Initiate steroid prophylaxis for patients with Graves’ ophthalmopathy undergoing RAI. A
• Opt for a 12- to 18-month course of an antithyroid drug, rather than a 6-month course. The longer course is associated with a lower relapse rate. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 72-year-old man arrives at the clinic with insomnia and fatigue. His medical history is significant for hypertension, hyperlipidemia, and degenerative joint disease, for which he is taking, respectively, metoprolol 25 mg twice daily, simvastatin 20 mg daily, and acetaminophen as needed for joint pain. He has experienced no weight loss, anxiety, or gastrointestinal or urinary symptoms. He does not smoke or drink alcohol. His blood pressure is 140/75 mm Hg, pulse is 85, respiratory rate is 20, and temperature is 97.1°F. The rest of the physical examination is unremarkable except for 1+ lower extremity edema, unchanged since his previous visit. Routine blood work, however, reveals his thyroid-stimulating hormone (TSH) level to be 0.03 mIU/L.
Clues from the clinical presentation
The subtle, "apathetic presentation" with few symptoms, as described in the case above, is typical of older individuals with hyperthyroidism.1 In contrast, younger patients with hyperthyroidism and those with comorbidities can manifest a number of signs and symptoms (TABLE 1).2
Graves’ disease, the most common cause of hyperthyroidism,3 causes such ocular disturbances as exophthalmos, lid lag, lid retraction, and proptosis in 60% of patients with the condition.3 These findings help differentiate Graves’ disease from other causes of hyperthyroidism. (See “Common [and not so common] causes of hyperthyroidism”.) Palmar sweating, pretibial myxedema, and Plummer’s nails (onycholysis) are also unique for Graves’ disease.4
When you suspect hyperthyroidism, assess the thyroid for size, nodularity, and vascularity. Goiter is less prevalent in the elderly, occurring in less than 50% of patients 61 and older, compared with 77% of patients younger than 60 years.5 Diffuse goiter is typical with Graves’ disease, while a mass with multiple nodules suggests possible toxic multinodular goiter. A solitary palpable nodule could mean toxic adenoma. A thyroid that is tender on palpation may point to subacute thyroiditis, particularly if the patient has had a viral illness recently (TABLE 2).
Measuring a patient’s TSH level is warranted with the above findings. Additionally, measure TSH in any patient older than 60 years presenting with fatigue, atrial fibrillation, weight loss, and shortness of breath.5
TABLE 1
Clinical manifestations of hyperthyroidism2
| Acropachy (swelling of the fingers) |
| Bruit (thyroid) |
| Decreased attention span |
| Diarrhea |
| Edema |
| Exertional dyspnea |
| Fatigue |
| Goiter (smooth or nodular) |
| Gynecomastia |
| Hair loss |
| Heat intolerance |
| Hyperactive deep tendon reflex |
| Hypertension |
| Increased appetite |
| Infertility |
| Insomnia |
| Lid lag, proptosis |
| Muscle weakness |
| Nervousness and irritability |
| Oligomenorrhea |
| Palmar erythema |
| Palpitations |
| Paralysis (sudden) |
| Photophobia, eye irritation, diplopia |
| Pretibial myxedema |
| Tachycardia |
| Tremors |
| Warm, moist skin |
| Weight loss |
Graves’ disease—an autoimmune disorder in which antibodies target thyroid tissue and enzymes and activate thyroid hormone synthesis—affects more than 3 million people in the United States and accounts for 60% of hyperthyroidism cases.3 Remission does occur; however, the recurrence rate is as high as 60%.50 Factors associated with recurrence include tobacco use; male sex; young age; large goiter size or increase in goiter size during treatment; elevated TSH receptor antibodies (TRab); presence of Graves’ ophthalmopathy; markedly elevated thyroid hormones, or delayed treatment.51
Toxic multinodular goiter, also known as Plummer’s disease, is the underlying condition in 15% to 20% of hyperthyroidism cases; it is more common in young patients and in iodine-deficient locations (eg, Denmark).52 However, it also occurs in elderly patients with longstanding goiter.
Toxic adenoma causes just 3% to 5% of cases of hyperthyroidism.53 It, too, occurs more commonly in young patients and in iodine-deficient regions. The radioactive iodine uptake test shows a hot nodule, with suppressed uptake in the surrounding thyroid gland.
Subacute thyroiditis, also known as de Quervain’s thyroiditis, is the reason for 15% to 20% of hyperthyroidism cases; it is usually preceded by viral infection and inflammation that lead to destructive release of preformed thyroid hormone. Symptoms—typically fever, malaise, and tender goiter—usually occur more abruptly than symptoms of Graves’ disease.54 Most cases resolve spontaneously within a few months, and relapse is less common than in Graves’ disease. Other lab abnormalities include increased erythrocyte sedimentation rate and low radioiodine uptake.
Postpartum thyroiditis is an autoimmune disease. Prevalence ranges from 1% to 17% of new mothers.55 It is characterized by a thyroid gland that is painless on palpation and low radioiodine uptake.56 Most cases are reversible with treatment.
Factitious or iatrogenic hyperthyroidism is due to an exogenous intake of thyroid hormone, and typically exhibits a normal or low radioactive iodine uptake and a low thyroglobulin level.
Secondary hyperthyroidism, or TSH-mediated hyperthyroidism, is rare. It is always associated with goiter, and approximately 40% of patients have visual field defects.57
TABLE 2
Clinical and laboratory findings associated with common causes of hyperthyroidism51-57
| Mechanism | Thyroid exam | Lab results | Radioactive iodine uptake | |
|---|---|---|---|---|
| Graves’ disease | Antithyroid antibodies | Diffuse goiter | Low TSH; elevated T3 and/or T4; elevated thyroid antibodies | Diffusely increased |
| Toxic multinodular goiter | Iodine deficiency | Goiter with multiple nodules | Low TSH; elevated T3 and/or T4 | Normal/increased uptake; "hot nodules" with suppression of extranodular tissue |
| Toxic adenoma | Benign thyroid hormone?secreting tumor; iodine deficiency | Palpable nodule | Low TSH; elevated T3 and/or T4 | Normal/increased uptake; functioning "hot nodule" on scan with suppression of surrounding thyroid tissue |
| Subacute thyroiditis | Viral | Tender thyroid on palpation | Low TSH; elevated T3 and/or T4; elevated ESR; elevated thyroid antibodies | Low uptake with poor imaging of the thyroid on scan |
| Factitious hyperthyroidism | Excessive intake of exogenous thyroid hormone | Normal exam | Low TSH; elevated T3 and/or T4; low thyroglobulin level | Low or normal uptake |
| Secondary hyperthyroidism | Excessive pituitary TSH | Goiter | Elevated TSH; elevated T3 and/or T4 | Diffusely increased uptake |
| ESR, erythrocyte sedimentation rate; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone. | ||||
Which laboratory tests to order, and what the results may mean
Rely on second- or third-generation TSH screening (normal=0.5-5 mIU/L), which is more sensitive and specific than measuring free T4 (thyroxine) alone.6
Older patients usually have a higher normal TSH level. In one study, 70% of patients >80 years had a TSH >4.5 mIU/L.7
If the TSH level is low (<0.5 mIU/L), measure free T3 (triiodothyronine) and free T4 levels, which are elevated in hyperthyroidism, and are normal in subclinical hyperthyroidism.
Patients with Graves’ disease tend to have T3 thyrotoxicosis with a T3T4 ratio >20.8 Isolated T4 thyrotoxicosis is more commonly seen with nonthyroidal illness as a result of decreased conversion from T4 to T3, and also in amiodarone-induced hyperthyroidism.9
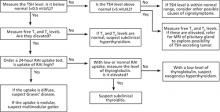
When further testing is needed (FIGURE). If the underlying cause of hyperthyroidism is not established on the basis of clinical findings (eg, diffuse goiter, myxedema, ophthalmopathy), order a 24-hour radioactive iodine (RAI) uptake test.10 Graves’ disease and toxic multinodular goiter exhibit increased RAI uptake that is diffuse and nodular, respectively. Subacute thyroiditis is associated with low RAI uptake (TABLE 2).
If RAI is contraindicated—eg, in pregnancy—testing for elevated levels of thyroid peroxidase antibodies (TPOab), TSH receptor antibodies (TRab), and thyroglobulin may help to differentiate Graves’ disease from multinodular goiter or uncover another autoimmune thyroid disorder.11 If a patient’s TSH, T4, and T3 levels are all elevated, refer him or her for magnetic resonance imaging of the pituitary gland to look for a TSH-secreting adenoma.
FIGURE
Suspect hyperthyroidism? Order these tests6-11
MRI, magnetic resonance imaging; RAI, radioactive iodine; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
Matching treatment to the underlying cause
RAI is usually the treatment of choice for patients without contraindications, although no randomized clinical trials have compared it with antithyroid medications or surgery.12 Each modality has its own risks and benefits (TABLE 3), and treatment selection should be individualized.
TABLE 3
Comparison of treatment modalities for hyperthyroidism12-21
| Antithyroid drugs* | Radioactive iodine* | Surgery | |
|---|---|---|---|
| Recurrence rate | High recurrence rate; no permanent hypothyroidism | Usually permanent hypothyroidism; long-term use of levothyroxine is required | Subtotal thyroidectomy associated with higher rates of recurrence or persistence of hyperthyroidism than total thyroidectomy; permanent hypothyroidism; long-term use of levothyroxine is required |
| Preferred method within treatment modality | MMI is the preferred medication; PTU is used with pregnancy and severe hyperthyroidism not responding to MMI | High ablative dose is preferred in MNG, toxic nodule, cardiac disease, elderly; low calculated dose is preferred in patients with GO | No outcome differences for GO, whether thyroidectomy is total, bilateral subtotal, or unilateral total and contralateral subtotal |
| Setting | Outpatient | Outpatient | Inpatient |
| Risks | No surgical risks | No surgical risks | Reaction to anesthesia, recurrent laryngeal nerve palsy, hypoparathyroidism |
| Adverse effects | ATD adverse effects, including life-threatening agranulocytosis | Worsening of Graves’ ophthalmopathy; transient exacerbation of hyperthyroid symptoms | Permanent hypothyroidism; hypoparathyroidism; anesthesia complications |
| Safety in pregnancy | PTU is used in pregnancy | Contraindicated in pregnancy/lactation | If surgery is indicated in pregnancy, it is best performed in the second trimester |
| ATD, antithyroid drugs; GO, Graves’ ophthalmopathy; MMI, methimazole; MNG, toxic multinodular goiter; PTU, propylthiouracil. *Concomitant use of ATD and RAI is associated with a high failure rate and persistent or recurrent hyperthyroidism. Discontinue ATD 2 weeks before radioactive iodine treatment. | |||
Radioactive iodine
In a 1990 survey, as many as 70% of specialists in the United States used RAI to treat hyperthyroidism, compared with just 22% of specialists in Europe.13 RAI is usually given in a single dose, and its maximal benefit is noted within 3 to 6 months. Two treatment methods are available: the ablative method and the gland-specific dosing method. Both have similar euthyroid state outcomes.14
The ablative method uses a high dose of RAI to achieve permanent hypothyroidism, necessitating lifelong levothyroxine replacement. This method is preferred for the elderly and for patients with cardiac disease, to achieve faster control of symptoms. It is also recommended for patients with toxic multinodular goiter and toxic nodules.
The gland-specific dosing method induces a euthyroid state with a calculated low dose of RAI based on the estimated weight of the patient’s thyroid. The optimal dosage may be difficult to calculate, but it is usually the preferred method for patients with Graves’ ophthalmopathy.
Adverse effects of RAI can include worsening of Graves’ ophthalmopathy and an acute rise in thyroid hormone that increases hyperthyroid symptoms or even causes a thyroid storm associated with increased cardiovascular risk.2 A negative pregnancy test result is a prerequisite for all women of childbearing age before taking RAI, and patients are advised to use contraception for 6 months after RAI administration.
Although RAI is often the initial treatment for hyperthyroidism, in some instances—eg, for older patients with comorbidities—pre-treatment with antithyroid drugs (ATD) is indicated to avoid transient worsening of hyperthyroid symptoms after RAI. However, always discontinue ATD 2 weeks before RAI administration; concomitant use is associated with a higher failure rate and persistent or recurrent hyperthyroidism.15
Antithyroid drugs
Two antithyroid medications are available for use in the United States: propylthiouracil (PTU) and methimazole (MMI). In the United Kingdom, carbimazole is also available.
MMI is the drug of choice.16 Compared with PTU, MMI costs less, has a longer half-life, and causes fewer adverse effects. A starting dose of 15 mg per day for MMI is suitable for mild and moderate hyperthyroidism. For more severe cases, 30 mg per day is the recommended starting dose.16 Reserve PTU for treating hyperthyroidism in pregnancy, during which MMI should be avoided, if possible.
Allergic reactions to ATDs appear in around 5% of patients and usually occur in the first 6 weeks of treatment.17 Agranulocytosis is the main concern, although it occurs in fewer than 1% of patients17 and is reversible by stopping the medication. Measure the leukocyte count 1 week after initiation of treatment and repeat the measurement at 1-month intervals.
Two methods are used to dose these medications: titration and block-and-replace. Titration is as effective as the block-and-replace method and is associated with fewer rashes (6% vs 10% of patients) and less agranulocytosis (0.4 % vs 1.4%). The 2 methods have similar relapse rates (around 50%).18
With titration, MMI is started at a dose of 15 mg per day and titrated upward to the lowest effective dose. Treatment for 12 to 18 months is associated with a lower relapse rate than treatment for 6 months (37% vs 58%).19
The block-and-replace method uses persistently high ATD doses in combination with L-thyroxin replacement to avoid hypothyroidism (MMI 30 mg and levothyroxine 80 mcg).
To monitor effectiveness initially, measure free T4 and T3 levels, because TSH concentration changes slowly and may stay low for a few months. Response to treatment is often temporary.8 More definitive treatment with RAI or surgery is usually necessary.
Surgery
Thyroidectomy creates permanent hypothyroidism, necessitating lifelong thyroxine replacement. In the United States, surgical intervention is reserved for special situations, such as pregnant women with severe disease who are allergic or not responding to antithyroid medications, removal of a clinically suspicious thyroid nodule coexisting with hyperthyroidism, or severe or recurrent Graves’ disease with severe ophthalmopathy.20 Surgical options are total or subtotal thyroidectomy. Hyperthyroidism persists or recurs in 8% of patients with subtotal thyroidectomy.21 Potential complications of thyroidectomy include adverse effects of anesthesia, hypoparathyroidism, and vocal cord paralysis.
Other treatment options
Iodides
Iodides inhibit thyroid hormone release and block conversion of T4 to T3. Use potassium iodide only in combination with ATDs, for patients with severe thyrotoxicosis or as pretreatment for urgent thyroidectomy in patients with Graves’ disease. It has been shown to improve the short-term control of Graves’ hyperthyroidism and is not associated with worsening hyperthyroidism;22 however, potassium iodide should not be used for more than 12 weeks as it can cause paradoxical hyperthyroidism.22
Beta-blockers
Hyperthyroidism is associated with an increased number of beta-adrenergic receptors,23 which explains the symptoms of palpitations, anxiety, and tremors. Nonselective beta-blockers are usually preferred for symptomatic treatment of hyperthyroid symptoms, and propranolol is the most widely used agent.24 If you decide to use a beta-blocker, start it with the ATD and continue it until the patient becomes euthyroid or asymptomatic, then taper it over a period of 4 to 6 weeks. Symptoms may persist, however, and require higher doses of propranolol (80-320 mg/d) given more frequently.
Treating Graves’ ophthalmopathy
Exophthalmos and other eye signs are the hallmark of Graves’ disease and may occur in the absence of hyperthyroidism. Smoking is a significant risk factor for developing ophthalmopathy due to increased orbital connective tissue volume,25 and smoking cessation is recommended.26
Using RAI to treat Graves’ disease increases the risk that ophthalmopathy will develop or worsen. Worsening of Graves’ ophthalmopathy secondary to RAI treatment occurs in 20% of treated patients (transient in 15%; permanent in 5%).27 Steroid prophylaxis is beneficial for patients with ophthalmopathy,28 and prednisone 40 to 80 mg per day tapered over at least 3 months can help reduce the condition.19 In patients with moderate to severe active ophthalmopathy, intravenous corticosteroid therapy has a small but statistically significant advantage over oral therapy and causes significantly fewer adverse events.29
Orbital radiotherapy is also used, and has been shown to decrease diplopia.30 However, the best available evidence recommends combining orbital radiotherapy and oral corticosteroids, which yields efficacy beyond that achievable with either radiotherapy or oral corticosteroids alone.16 Moreover, intravenous methylprednisolone combined with orbital radiotherapy seems to be most efficacious.31 The course of ophthalmopathy is the same whether total or subtotal thyroidectomy is used.32
Prognosis without treatment
Individuals with high-normal thyroid function tests, subclinical hyperthyroidism, and clinical hyperthyroidism are at increased risk for atrial fibrillation.33-35 Hyperthyroidism is also associated with increased risk of heart failure (6% of patients), which might be secondary to coexisting atrial fibrillation or tachycardia-mediated cardiomyopathy.36 Heart failure is usually reversible when the hyperthyroidism is treated.
Patients with overt hyperthyroidism are also at risk for pulmonary hypertension secondary to increased cardiac output and decreased pulmonary vascular resistance.37
In patients with preexisting cardiac disease, hyperthyroidism increases risk of death (hazard ratio [HR]=1.57),38 and might even do so in patients without cardiac disease.39,40 It also increases risk of ischemic stroke (HR=1.44) among adults ages 18 to 44 years.41 Untreated hyperthyroidism also contributes to low bone mineral density and increases the risk of hip fracture.42
Subclinical hyperthyroidism
Subclinical hyperthyroidism occurs in 2% of the US population and is characterized by low serum TSH (<0.1 mIU/L) with normal levels of free T3 and free T4. The causes are similar to overt hyperthyroidism. In addition, it can result from overtreating hypothyroidism with thyroid hormone, thereby inducing a subclinical hyperthyroid state.
The most common endogenous cause of subclinical hyperthyroidism (~60% of patients) is multinodular goiter.43 Subclinical hyperthyroidism carries significant health risks, and yet evidence is lacking on when to treat this condition. Prolonged subclinical hyperthyroidism can lead to atrial fibrillation,24,44 and to systolic and diastolic cardiac dysfunction.45 Subclinical hyperthyroidism is also associated with decreased bone density,46 and an increased risk of dementia.47
The American Association of Clinical Endocrinologists recommends periodic clinical and laboratory assessment for patients with subclinical hyperthyroidism (TSH=0.1-0.5 mIU/mL), including rechecking TSH, free T3 and free T4 at 2- to 4-month intervals.
Treatment of the underlying cause of hyperthyroidism is indicated if serum TSH is <0.1 mIU/mL.
For patients older than 65 years who have persistent subclinical hyperthyroidism, consider treatment in the following scenarios:48
- nodular thyroid disease (due to high conversion rate to overt hyperthyroidism)
- osteopenia or osteoporosis (in women)
- atrial fibrillation
- underlying cardiac disease.
Hyperthyroidism in pregnancy
PTU is the first choice for treating hyperthyroidism in pregnancy. It crosses the placenta less readily than MMI, and is thus less likely to cause fetal hypothyroidism. Additionally, MMI is associated with increased risk of fetal anomalies, such as aplasia cutis and esophageal atresia. MMI may be considered if the patient is intolerant to PTU or fails to become euthyroid while receiving PTU.49 Use the lowest possible dose of either PTU or MMI to maintain thyroid function within the upper limit of normal. The dose of the antithyroid medication is usually decreased as pregnancy progresses and discontinued in the last few weeks, as pregnancy is thought to improve the course of Graves’ disease.
The use of RAI is contraindicated during pregnancy and breastfeeding. Hyperthyroidism symptoms usually resolve after delivery. If symptoms persist, however, the treatment of choice is ATD. Surgery is an option in severe Graves’ disease not responding to ATD.
CORRESPONDENCE
Abdulraouf Ghandour, MD, Department of Family and Community Medicine, University of Missouri-Columbia, One Hospital Drive, Columbia, MO 65212; [email protected]
1. Levy EG. Thyroid disease in the elderly. Med Clin North Am. 1991;75:151-167.
2. Cooper DS. Hyperthyroidism. Lancet. 2003;362:459-468.
3. Weetman AP. Graves’ disease. N Engl J Med. 2000;343:1236-1248.
4. Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
5. Boelaert K, Torlinska B. Older subjects with hyperthyroidism present with a paucity of symptoms and signs: a large cross-sectional study. J Clin Endocrinol Metab. 2010;95:2715-2726.
6. Danese MD, Powe NR, Sawin CT, et al. Screening of mild thyroid failure at the periodic health examination: a decision and cost-effectiveness analysis. JAMA. 1996;276:285-292.
7. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
8. Amino N, Yabu Y, Miki T, et al. Serum ratio of triiodothyronine to thyroxine and thyroxine binding globulin and calcitonin concentrations in Graves’ disease and destruction-induced thyrotoxicosis. J Clin Endocrinol Metab. 1981;53:113-116.
9. Bambini G, Aghini-Lombardi F, Rosner W, et al. Serum sex hormone-binding globulin in amiodarone-treated patients. A marker for tissue thyrotoxicosis. Arch Intern Med. 1987;147:1781-1785.
10. Fogelman I, Cooke SG, Maisey MN. The role of thyroid scanning in hyperthyroidism. Eur J Nucl Med. 1986;11:397-400.
11. Costagliola S, Morgenthaler NG, Hoermann R, et al. Second generation assay for thyrotropin receptor antibodies has superior diagnostic sensitivity for Graves’ disease. J Clin Endocrinol Metab. 1999;84:90-97.
12. Streetman DD, Khanderia U. Diagnosis and treatment of Graves’ disease. Ann Pharmacother. 2003;37:1100-1109.
13. Wartofsky L, Glinoer D, Solomon B, et al. Differences and similarities in the diagnosis and treatment of Graves’ disease in Europe, Japan, and the United States. Thyroid. 1991;1:129-135.
14. de Rooij A, Vandenbroucke JP. Clinical outcomes after estimated versus calculated activity of radioiodine for the treatment of hyperthyroidism: systematic review and meta-analysis. Eur J Endocrinol. 2009;161:771-777.
15. Walter MA, Briel M, Christ-Crain M, et al. Effects of antithyroid drugs on radioiodine treatment: systematic review and meta-analysis of randomised controlled trials. BMJ. 2007;334:514.-
16. Nakamura H, Noh JY. Comparison of methimazole and propylthiouracil in patients with hyperthyroidism caused by Graves’ disease. J Clin Endocrinol Metab. 2007;92:2157-2162.
17. Cooper DS. Antithyroid drugs. N Engl J Med. 2005;352:905-917.
18. Abraham P, Avenell A. A systematic review of drug therapy for Graves’ hyperthyroidism. Eur J Endocrinol. 2005;153:489-498.
19. Abraham P, Avenell A, McGeoch SC, et al. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Sys Rev. 2010;(1):CD003420.-
20. Stalberg P, Svensson A. Surgical treatment of Graves’ disease: evidence-based approach. World J Surg. 2008;32:1269-1277.
21. Palit TK, Miller CC, Miltenburg DM. The efficacy of thyroidectomy for Graves’ disease: a meta-analysis. J Surg Res. 2000;90:161-165.
22. Takata K, Amino N, Kubota S. Benefit of short-term iodide supplementation to antithyroid drug treatment of thyrotoxicosis due to Graves’ disease. Clin Endocrinol. 2010;72:845-850.
23. Bilezikian JP, Loeb JN. The influence of hyperthyroidism and hypothyroidism on alpha- and beta-adrenergic receptor systems and adrenergic responsiveness. Endocr Rev. 1983;4:378-388.
24. Jansson S, Lie-Karlsen K, Stenqvist O, et al. Oxygen consumption in patients with hyperthyroidism before and after treatment with beta-blockade versus thyrostatic treatment: a prospective randomized study. Ann Surg. 2001;233:60-64.
25. Zucs-Frkas Z, Toth J, Kollar J, et al. Volume changes in intra- and extraorbital compartments in patients with Graves’ ophthalmopathy: effect of smoking. Thyroid. 2005;15:146-151.
26. Träisk F, Tallstedt L. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700-3707.
27. Bartalena L, Marcocci C, Bogazzi F, et al. Relation between therapy of hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med. 1998;338:73-78.
28. Acharya SH, Avenell A. Radioiodine therapy (RAI) for Graves’ disease (GD) and the effect on ophthalmopathy: a systematic review. Clin Endocrinol (Oxf). 2008;69:943-950.
29. Stiebel-Kalish H, Robenshtok E. Treatment modalities for Graves’ ophthalmopathy: systematic review and meta-analysis. J Clin Endocrinol Metab. 2009;94:2708-2716.
30. Bradley EA, Gower EW. Orbital radiation for graves ophthalmopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115:398-409.
31. Wei RL, Cheng JW. The use of orbital radiotherapy for Graves’ ophthalmopathy: quantitative review of the evidence. Ophthalmologica. 2008;222:27-31.
32. Witte J, Goretzki PE, Dotzenrath C, et al. Surgery for Graves’ disease: total versus subtotal thyroidectomy–result of a prospective randomized trial. World J Surg. 2000;24:1303-1311.
33. Heeringa J, Hoogendoorn EH. High-normal thyroid function and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med. 2008;168:2219-2224.
34. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249-1252.
35. Cappola AR, Fried LP. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033-1041.
36. Siu CW, Yeung CY, Lau CP, et al. Incidence, clinical characteristics and outcome of congestive heart failure as the initial presentation in patients with primary hyperthyroidism. Heart. 2007;93:483-487.
37. Lozano HF, Sharma CN. Reversible pulmonary hypertension, tricuspid regurgitation and right-sided heart failure associated with hyperthyroidism: case report and review of the literature. Cardiol Rev. 2004;12:299-305.
38. Iervasi G, Molinaro S. Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med. 2007;167:1526-1532.
39. Parle JV, Maisonneuve P, Sheppard MC, et al. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet. 2001;358:861-865.
40. Flynn RW, McDonald TM, Jung RT, et al. Mortality and vascular outcomes in patients treated for thyroid dysfunction. J Clin Endocrinol Metab. 2006;91:2169-2164.
41. Sheu JJ, Kang JH. Hyperthyroidism and risk of ischemic stroke in young adults: a 5-year follow-up study. Stroke. 2010;41:961-966.
42. Vestergaard P, Mosekilde L. Hyperthyroidism, bone mineral, and fracture risk—a meta-analysis. Thyroid. 2003;13:585-593.
43. Diez JJ. Hyperthyroidism in patients older than 55 years: an analysis of the etiology and management. Gerontology. 2003;49:316-323.
44. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249-1252.
45. Abdulrahman RM, Delgado V. Abnormal cardiac contractility in long-term exogenous subclinical hyperthyroid patients as demonstrated by two-dimensional echocardiography speckle tracking imaging. Eur J Endocrinol. 2010;163:435-441.
46. Faber J, Jensen IW, Petersen L, et al. Normalization of serum thyrotrophin by means of radioiodine treatment in subclinical hyperthyroidism: effect on bone loss in postmenopausal women. Clin Endocrinol (Oxf). 1998;48:285-290.
47. Tan ZS, Beiser A, Vasan RS, et al. Thyroid function and the risk of Alzheimer disease: Framingham study. Arch Intern Med. 2008;168:1514-1520.
48. American Association of Clinical Endocrinologists. Medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. 2006. Available at: https://www.aace.com/sites/default/files/hypo_hyper.pdf. Accessed July 9, 2010.
49. Chattaway JM, Klepser TB. Propylthiouracil versus methimazole in treatment of Grave’s disease during pregnancy. Ann Pharmacother. 2007;41:1018-1022.
50. Lucas A, Salinas I. Medical therapy of Graves’ disease: does thyroxine prevent recurrence of hyperthyroidism? J Clin Endocrinol Metab. 1997;82:2410-2413.
51. Vitti P, Rago T, Chiovato L, et al. Clinical features of patients with Graves’ disease undergoing remission after antithyroid drug treatment. Thyroid. 1997;7:369-375.
52. Laurberg P, Bulow Pedersen I, Pedersen KM, et al. Low incidence rate of overt hypothyroidism compared with hyperthyroidism in an area with moderately low iodine intake. Thyroid. 1999;9:33-38.
53. Siegel RD, Lee SL. Toxic nodular goiter: Toxic adenoma and toxic multinodular goiter. Endocrinol Metab Clin North Am. 1998;27:151-168.
54. Volpe R. Subacute (de Quervain’s) thyroiditis. Clin Endocrinol Metab. 1979;8:81-95.
55. Nicholson WK, Robinson KA, Smallridge RC, et al. Prevalence of postpartum thyroid dysfunction: a quantitative review. Thyroid. 2006;16:573-582.
56. Roti E, Emerson CH. Clinical review 29: postpartum thyroiditis. J Clin Endocrinol Metab. 1992;74:3-5.
57. Beck-Peccoz P, Brucker-Davis F, Persani L, et al. Thyrotropin-secreting pituitary tumors. Endocr Rev. 1996;17:610-638.
• Measure TSH in any patient >60 years presenting with fatigue, atrial fibrillation, weight loss, and shortness of breath. B
• Achieve faster control of symptoms in elderly patients and those with cardiac disease by pursuing the ablative method with radioactive iodine (RAI). This method is also recommended for patients with toxic multinodular goiter and toxic adenoma. A
• Initiate steroid prophylaxis for patients with Graves’ ophthalmopathy undergoing RAI. A
• Opt for a 12- to 18-month course of an antithyroid drug, rather than a 6-month course. The longer course is associated with a lower relapse rate. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 72-year-old man arrives at the clinic with insomnia and fatigue. His medical history is significant for hypertension, hyperlipidemia, and degenerative joint disease, for which he is taking, respectively, metoprolol 25 mg twice daily, simvastatin 20 mg daily, and acetaminophen as needed for joint pain. He has experienced no weight loss, anxiety, or gastrointestinal or urinary symptoms. He does not smoke or drink alcohol. His blood pressure is 140/75 mm Hg, pulse is 85, respiratory rate is 20, and temperature is 97.1°F. The rest of the physical examination is unremarkable except for 1+ lower extremity edema, unchanged since his previous visit. Routine blood work, however, reveals his thyroid-stimulating hormone (TSH) level to be 0.03 mIU/L.
Clues from the clinical presentation
The subtle, "apathetic presentation" with few symptoms, as described in the case above, is typical of older individuals with hyperthyroidism.1 In contrast, younger patients with hyperthyroidism and those with comorbidities can manifest a number of signs and symptoms (TABLE 1).2
Graves’ disease, the most common cause of hyperthyroidism,3 causes such ocular disturbances as exophthalmos, lid lag, lid retraction, and proptosis in 60% of patients with the condition.3 These findings help differentiate Graves’ disease from other causes of hyperthyroidism. (See “Common [and not so common] causes of hyperthyroidism”.) Palmar sweating, pretibial myxedema, and Plummer’s nails (onycholysis) are also unique for Graves’ disease.4
When you suspect hyperthyroidism, assess the thyroid for size, nodularity, and vascularity. Goiter is less prevalent in the elderly, occurring in less than 50% of patients 61 and older, compared with 77% of patients younger than 60 years.5 Diffuse goiter is typical with Graves’ disease, while a mass with multiple nodules suggests possible toxic multinodular goiter. A solitary palpable nodule could mean toxic adenoma. A thyroid that is tender on palpation may point to subacute thyroiditis, particularly if the patient has had a viral illness recently (TABLE 2).
Measuring a patient’s TSH level is warranted with the above findings. Additionally, measure TSH in any patient older than 60 years presenting with fatigue, atrial fibrillation, weight loss, and shortness of breath.5
TABLE 1
Clinical manifestations of hyperthyroidism2
| Acropachy (swelling of the fingers) |
| Bruit (thyroid) |
| Decreased attention span |
| Diarrhea |
| Edema |
| Exertional dyspnea |
| Fatigue |
| Goiter (smooth or nodular) |
| Gynecomastia |
| Hair loss |
| Heat intolerance |
| Hyperactive deep tendon reflex |
| Hypertension |
| Increased appetite |
| Infertility |
| Insomnia |
| Lid lag, proptosis |
| Muscle weakness |
| Nervousness and irritability |
| Oligomenorrhea |
| Palmar erythema |
| Palpitations |
| Paralysis (sudden) |
| Photophobia, eye irritation, diplopia |
| Pretibial myxedema |
| Tachycardia |
| Tremors |
| Warm, moist skin |
| Weight loss |
Graves’ disease—an autoimmune disorder in which antibodies target thyroid tissue and enzymes and activate thyroid hormone synthesis—affects more than 3 million people in the United States and accounts for 60% of hyperthyroidism cases.3 Remission does occur; however, the recurrence rate is as high as 60%.50 Factors associated with recurrence include tobacco use; male sex; young age; large goiter size or increase in goiter size during treatment; elevated TSH receptor antibodies (TRab); presence of Graves’ ophthalmopathy; markedly elevated thyroid hormones, or delayed treatment.51
Toxic multinodular goiter, also known as Plummer’s disease, is the underlying condition in 15% to 20% of hyperthyroidism cases; it is more common in young patients and in iodine-deficient locations (eg, Denmark).52 However, it also occurs in elderly patients with longstanding goiter.
Toxic adenoma causes just 3% to 5% of cases of hyperthyroidism.53 It, too, occurs more commonly in young patients and in iodine-deficient regions. The radioactive iodine uptake test shows a hot nodule, with suppressed uptake in the surrounding thyroid gland.
Subacute thyroiditis, also known as de Quervain’s thyroiditis, is the reason for 15% to 20% of hyperthyroidism cases; it is usually preceded by viral infection and inflammation that lead to destructive release of preformed thyroid hormone. Symptoms—typically fever, malaise, and tender goiter—usually occur more abruptly than symptoms of Graves’ disease.54 Most cases resolve spontaneously within a few months, and relapse is less common than in Graves’ disease. Other lab abnormalities include increased erythrocyte sedimentation rate and low radioiodine uptake.
Postpartum thyroiditis is an autoimmune disease. Prevalence ranges from 1% to 17% of new mothers.55 It is characterized by a thyroid gland that is painless on palpation and low radioiodine uptake.56 Most cases are reversible with treatment.
Factitious or iatrogenic hyperthyroidism is due to an exogenous intake of thyroid hormone, and typically exhibits a normal or low radioactive iodine uptake and a low thyroglobulin level.
Secondary hyperthyroidism, or TSH-mediated hyperthyroidism, is rare. It is always associated with goiter, and approximately 40% of patients have visual field defects.57
TABLE 2
Clinical and laboratory findings associated with common causes of hyperthyroidism51-57
| Mechanism | Thyroid exam | Lab results | Radioactive iodine uptake | |
|---|---|---|---|---|
| Graves’ disease | Antithyroid antibodies | Diffuse goiter | Low TSH; elevated T3 and/or T4; elevated thyroid antibodies | Diffusely increased |
| Toxic multinodular goiter | Iodine deficiency | Goiter with multiple nodules | Low TSH; elevated T3 and/or T4 | Normal/increased uptake; "hot nodules" with suppression of extranodular tissue |
| Toxic adenoma | Benign thyroid hormone?secreting tumor; iodine deficiency | Palpable nodule | Low TSH; elevated T3 and/or T4 | Normal/increased uptake; functioning "hot nodule" on scan with suppression of surrounding thyroid tissue |
| Subacute thyroiditis | Viral | Tender thyroid on palpation | Low TSH; elevated T3 and/or T4; elevated ESR; elevated thyroid antibodies | Low uptake with poor imaging of the thyroid on scan |
| Factitious hyperthyroidism | Excessive intake of exogenous thyroid hormone | Normal exam | Low TSH; elevated T3 and/or T4; low thyroglobulin level | Low or normal uptake |
| Secondary hyperthyroidism | Excessive pituitary TSH | Goiter | Elevated TSH; elevated T3 and/or T4 | Diffusely increased uptake |
| ESR, erythrocyte sedimentation rate; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone. | ||||
Which laboratory tests to order, and what the results may mean
Rely on second- or third-generation TSH screening (normal=0.5-5 mIU/L), which is more sensitive and specific than measuring free T4 (thyroxine) alone.6
Older patients usually have a higher normal TSH level. In one study, 70% of patients >80 years had a TSH >4.5 mIU/L.7
If the TSH level is low (<0.5 mIU/L), measure free T3 (triiodothyronine) and free T4 levels, which are elevated in hyperthyroidism, and are normal in subclinical hyperthyroidism.
Patients with Graves’ disease tend to have T3 thyrotoxicosis with a T3T4 ratio >20.8 Isolated T4 thyrotoxicosis is more commonly seen with nonthyroidal illness as a result of decreased conversion from T4 to T3, and also in amiodarone-induced hyperthyroidism.9
When further testing is needed (FIGURE). If the underlying cause of hyperthyroidism is not established on the basis of clinical findings (eg, diffuse goiter, myxedema, ophthalmopathy), order a 24-hour radioactive iodine (RAI) uptake test.10 Graves’ disease and toxic multinodular goiter exhibit increased RAI uptake that is diffuse and nodular, respectively. Subacute thyroiditis is associated with low RAI uptake (TABLE 2).
If RAI is contraindicated—eg, in pregnancy—testing for elevated levels of thyroid peroxidase antibodies (TPOab), TSH receptor antibodies (TRab), and thyroglobulin may help to differentiate Graves’ disease from multinodular goiter or uncover another autoimmune thyroid disorder.11 If a patient’s TSH, T4, and T3 levels are all elevated, refer him or her for magnetic resonance imaging of the pituitary gland to look for a TSH-secreting adenoma.
FIGURE
Suspect hyperthyroidism? Order these tests6-11
MRI, magnetic resonance imaging; RAI, radioactive iodine; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
Matching treatment to the underlying cause
RAI is usually the treatment of choice for patients without contraindications, although no randomized clinical trials have compared it with antithyroid medications or surgery.12 Each modality has its own risks and benefits (TABLE 3), and treatment selection should be individualized.
TABLE 3
Comparison of treatment modalities for hyperthyroidism12-21
| Antithyroid drugs* | Radioactive iodine* | Surgery | |
|---|---|---|---|
| Recurrence rate | High recurrence rate; no permanent hypothyroidism | Usually permanent hypothyroidism; long-term use of levothyroxine is required | Subtotal thyroidectomy associated with higher rates of recurrence or persistence of hyperthyroidism than total thyroidectomy; permanent hypothyroidism; long-term use of levothyroxine is required |
| Preferred method within treatment modality | MMI is the preferred medication; PTU is used with pregnancy and severe hyperthyroidism not responding to MMI | High ablative dose is preferred in MNG, toxic nodule, cardiac disease, elderly; low calculated dose is preferred in patients with GO | No outcome differences for GO, whether thyroidectomy is total, bilateral subtotal, or unilateral total and contralateral subtotal |
| Setting | Outpatient | Outpatient | Inpatient |
| Risks | No surgical risks | No surgical risks | Reaction to anesthesia, recurrent laryngeal nerve palsy, hypoparathyroidism |
| Adverse effects | ATD adverse effects, including life-threatening agranulocytosis | Worsening of Graves’ ophthalmopathy; transient exacerbation of hyperthyroid symptoms | Permanent hypothyroidism; hypoparathyroidism; anesthesia complications |
| Safety in pregnancy | PTU is used in pregnancy | Contraindicated in pregnancy/lactation | If surgery is indicated in pregnancy, it is best performed in the second trimester |
| ATD, antithyroid drugs; GO, Graves’ ophthalmopathy; MMI, methimazole; MNG, toxic multinodular goiter; PTU, propylthiouracil. *Concomitant use of ATD and RAI is associated with a high failure rate and persistent or recurrent hyperthyroidism. Discontinue ATD 2 weeks before radioactive iodine treatment. | |||
Radioactive iodine
In a 1990 survey, as many as 70% of specialists in the United States used RAI to treat hyperthyroidism, compared with just 22% of specialists in Europe.13 RAI is usually given in a single dose, and its maximal benefit is noted within 3 to 6 months. Two treatment methods are available: the ablative method and the gland-specific dosing method. Both have similar euthyroid state outcomes.14
The ablative method uses a high dose of RAI to achieve permanent hypothyroidism, necessitating lifelong levothyroxine replacement. This method is preferred for the elderly and for patients with cardiac disease, to achieve faster control of symptoms. It is also recommended for patients with toxic multinodular goiter and toxic nodules.
The gland-specific dosing method induces a euthyroid state with a calculated low dose of RAI based on the estimated weight of the patient’s thyroid. The optimal dosage may be difficult to calculate, but it is usually the preferred method for patients with Graves’ ophthalmopathy.
Adverse effects of RAI can include worsening of Graves’ ophthalmopathy and an acute rise in thyroid hormone that increases hyperthyroid symptoms or even causes a thyroid storm associated with increased cardiovascular risk.2 A negative pregnancy test result is a prerequisite for all women of childbearing age before taking RAI, and patients are advised to use contraception for 6 months after RAI administration.
Although RAI is often the initial treatment for hyperthyroidism, in some instances—eg, for older patients with comorbidities—pre-treatment with antithyroid drugs (ATD) is indicated to avoid transient worsening of hyperthyroid symptoms after RAI. However, always discontinue ATD 2 weeks before RAI administration; concomitant use is associated with a higher failure rate and persistent or recurrent hyperthyroidism.15
Antithyroid drugs
Two antithyroid medications are available for use in the United States: propylthiouracil (PTU) and methimazole (MMI). In the United Kingdom, carbimazole is also available.
MMI is the drug of choice.16 Compared with PTU, MMI costs less, has a longer half-life, and causes fewer adverse effects. A starting dose of 15 mg per day for MMI is suitable for mild and moderate hyperthyroidism. For more severe cases, 30 mg per day is the recommended starting dose.16 Reserve PTU for treating hyperthyroidism in pregnancy, during which MMI should be avoided, if possible.
Allergic reactions to ATDs appear in around 5% of patients and usually occur in the first 6 weeks of treatment.17 Agranulocytosis is the main concern, although it occurs in fewer than 1% of patients17 and is reversible by stopping the medication. Measure the leukocyte count 1 week after initiation of treatment and repeat the measurement at 1-month intervals.
Two methods are used to dose these medications: titration and block-and-replace. Titration is as effective as the block-and-replace method and is associated with fewer rashes (6% vs 10% of patients) and less agranulocytosis (0.4 % vs 1.4%). The 2 methods have similar relapse rates (around 50%).18
With titration, MMI is started at a dose of 15 mg per day and titrated upward to the lowest effective dose. Treatment for 12 to 18 months is associated with a lower relapse rate than treatment for 6 months (37% vs 58%).19
The block-and-replace method uses persistently high ATD doses in combination with L-thyroxin replacement to avoid hypothyroidism (MMI 30 mg and levothyroxine 80 mcg).
To monitor effectiveness initially, measure free T4 and T3 levels, because TSH concentration changes slowly and may stay low for a few months. Response to treatment is often temporary.8 More definitive treatment with RAI or surgery is usually necessary.
Surgery
Thyroidectomy creates permanent hypothyroidism, necessitating lifelong thyroxine replacement. In the United States, surgical intervention is reserved for special situations, such as pregnant women with severe disease who are allergic or not responding to antithyroid medications, removal of a clinically suspicious thyroid nodule coexisting with hyperthyroidism, or severe or recurrent Graves’ disease with severe ophthalmopathy.20 Surgical options are total or subtotal thyroidectomy. Hyperthyroidism persists or recurs in 8% of patients with subtotal thyroidectomy.21 Potential complications of thyroidectomy include adverse effects of anesthesia, hypoparathyroidism, and vocal cord paralysis.
Other treatment options
Iodides
Iodides inhibit thyroid hormone release and block conversion of T4 to T3. Use potassium iodide only in combination with ATDs, for patients with severe thyrotoxicosis or as pretreatment for urgent thyroidectomy in patients with Graves’ disease. It has been shown to improve the short-term control of Graves’ hyperthyroidism and is not associated with worsening hyperthyroidism;22 however, potassium iodide should not be used for more than 12 weeks as it can cause paradoxical hyperthyroidism.22
Beta-blockers
Hyperthyroidism is associated with an increased number of beta-adrenergic receptors,23 which explains the symptoms of palpitations, anxiety, and tremors. Nonselective beta-blockers are usually preferred for symptomatic treatment of hyperthyroid symptoms, and propranolol is the most widely used agent.24 If you decide to use a beta-blocker, start it with the ATD and continue it until the patient becomes euthyroid or asymptomatic, then taper it over a period of 4 to 6 weeks. Symptoms may persist, however, and require higher doses of propranolol (80-320 mg/d) given more frequently.
Treating Graves’ ophthalmopathy
Exophthalmos and other eye signs are the hallmark of Graves’ disease and may occur in the absence of hyperthyroidism. Smoking is a significant risk factor for developing ophthalmopathy due to increased orbital connective tissue volume,25 and smoking cessation is recommended.26
Using RAI to treat Graves’ disease increases the risk that ophthalmopathy will develop or worsen. Worsening of Graves’ ophthalmopathy secondary to RAI treatment occurs in 20% of treated patients (transient in 15%; permanent in 5%).27 Steroid prophylaxis is beneficial for patients with ophthalmopathy,28 and prednisone 40 to 80 mg per day tapered over at least 3 months can help reduce the condition.19 In patients with moderate to severe active ophthalmopathy, intravenous corticosteroid therapy has a small but statistically significant advantage over oral therapy and causes significantly fewer adverse events.29
Orbital radiotherapy is also used, and has been shown to decrease diplopia.30 However, the best available evidence recommends combining orbital radiotherapy and oral corticosteroids, which yields efficacy beyond that achievable with either radiotherapy or oral corticosteroids alone.16 Moreover, intravenous methylprednisolone combined with orbital radiotherapy seems to be most efficacious.31 The course of ophthalmopathy is the same whether total or subtotal thyroidectomy is used.32
Prognosis without treatment
Individuals with high-normal thyroid function tests, subclinical hyperthyroidism, and clinical hyperthyroidism are at increased risk for atrial fibrillation.33-35 Hyperthyroidism is also associated with increased risk of heart failure (6% of patients), which might be secondary to coexisting atrial fibrillation or tachycardia-mediated cardiomyopathy.36 Heart failure is usually reversible when the hyperthyroidism is treated.
Patients with overt hyperthyroidism are also at risk for pulmonary hypertension secondary to increased cardiac output and decreased pulmonary vascular resistance.37
In patients with preexisting cardiac disease, hyperthyroidism increases risk of death (hazard ratio [HR]=1.57),38 and might even do so in patients without cardiac disease.39,40 It also increases risk of ischemic stroke (HR=1.44) among adults ages 18 to 44 years.41 Untreated hyperthyroidism also contributes to low bone mineral density and increases the risk of hip fracture.42
Subclinical hyperthyroidism
Subclinical hyperthyroidism occurs in 2% of the US population and is characterized by low serum TSH (<0.1 mIU/L) with normal levels of free T3 and free T4. The causes are similar to overt hyperthyroidism. In addition, it can result from overtreating hypothyroidism with thyroid hormone, thereby inducing a subclinical hyperthyroid state.
The most common endogenous cause of subclinical hyperthyroidism (~60% of patients) is multinodular goiter.43 Subclinical hyperthyroidism carries significant health risks, and yet evidence is lacking on when to treat this condition. Prolonged subclinical hyperthyroidism can lead to atrial fibrillation,24,44 and to systolic and diastolic cardiac dysfunction.45 Subclinical hyperthyroidism is also associated with decreased bone density,46 and an increased risk of dementia.47
The American Association of Clinical Endocrinologists recommends periodic clinical and laboratory assessment for patients with subclinical hyperthyroidism (TSH=0.1-0.5 mIU/mL), including rechecking TSH, free T3 and free T4 at 2- to 4-month intervals.
Treatment of the underlying cause of hyperthyroidism is indicated if serum TSH is <0.1 mIU/mL.
For patients older than 65 years who have persistent subclinical hyperthyroidism, consider treatment in the following scenarios:48
- nodular thyroid disease (due to high conversion rate to overt hyperthyroidism)
- osteopenia or osteoporosis (in women)
- atrial fibrillation
- underlying cardiac disease.
Hyperthyroidism in pregnancy
PTU is the first choice for treating hyperthyroidism in pregnancy. It crosses the placenta less readily than MMI, and is thus less likely to cause fetal hypothyroidism. Additionally, MMI is associated with increased risk of fetal anomalies, such as aplasia cutis and esophageal atresia. MMI may be considered if the patient is intolerant to PTU or fails to become euthyroid while receiving PTU.49 Use the lowest possible dose of either PTU or MMI to maintain thyroid function within the upper limit of normal. The dose of the antithyroid medication is usually decreased as pregnancy progresses and discontinued in the last few weeks, as pregnancy is thought to improve the course of Graves’ disease.
The use of RAI is contraindicated during pregnancy and breastfeeding. Hyperthyroidism symptoms usually resolve after delivery. If symptoms persist, however, the treatment of choice is ATD. Surgery is an option in severe Graves’ disease not responding to ATD.
CORRESPONDENCE
Abdulraouf Ghandour, MD, Department of Family and Community Medicine, University of Missouri-Columbia, One Hospital Drive, Columbia, MO 65212; [email protected]
• Measure TSH in any patient >60 years presenting with fatigue, atrial fibrillation, weight loss, and shortness of breath. B
• Achieve faster control of symptoms in elderly patients and those with cardiac disease by pursuing the ablative method with radioactive iodine (RAI). This method is also recommended for patients with toxic multinodular goiter and toxic adenoma. A
• Initiate steroid prophylaxis for patients with Graves’ ophthalmopathy undergoing RAI. A
• Opt for a 12- to 18-month course of an antithyroid drug, rather than a 6-month course. The longer course is associated with a lower relapse rate. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 72-year-old man arrives at the clinic with insomnia and fatigue. His medical history is significant for hypertension, hyperlipidemia, and degenerative joint disease, for which he is taking, respectively, metoprolol 25 mg twice daily, simvastatin 20 mg daily, and acetaminophen as needed for joint pain. He has experienced no weight loss, anxiety, or gastrointestinal or urinary symptoms. He does not smoke or drink alcohol. His blood pressure is 140/75 mm Hg, pulse is 85, respiratory rate is 20, and temperature is 97.1°F. The rest of the physical examination is unremarkable except for 1+ lower extremity edema, unchanged since his previous visit. Routine blood work, however, reveals his thyroid-stimulating hormone (TSH) level to be 0.03 mIU/L.
Clues from the clinical presentation
The subtle, "apathetic presentation" with few symptoms, as described in the case above, is typical of older individuals with hyperthyroidism.1 In contrast, younger patients with hyperthyroidism and those with comorbidities can manifest a number of signs and symptoms (TABLE 1).2
Graves’ disease, the most common cause of hyperthyroidism,3 causes such ocular disturbances as exophthalmos, lid lag, lid retraction, and proptosis in 60% of patients with the condition.3 These findings help differentiate Graves’ disease from other causes of hyperthyroidism. (See “Common [and not so common] causes of hyperthyroidism”.) Palmar sweating, pretibial myxedema, and Plummer’s nails (onycholysis) are also unique for Graves’ disease.4
When you suspect hyperthyroidism, assess the thyroid for size, nodularity, and vascularity. Goiter is less prevalent in the elderly, occurring in less than 50% of patients 61 and older, compared with 77% of patients younger than 60 years.5 Diffuse goiter is typical with Graves’ disease, while a mass with multiple nodules suggests possible toxic multinodular goiter. A solitary palpable nodule could mean toxic adenoma. A thyroid that is tender on palpation may point to subacute thyroiditis, particularly if the patient has had a viral illness recently (TABLE 2).
Measuring a patient’s TSH level is warranted with the above findings. Additionally, measure TSH in any patient older than 60 years presenting with fatigue, atrial fibrillation, weight loss, and shortness of breath.5
TABLE 1
Clinical manifestations of hyperthyroidism2
| Acropachy (swelling of the fingers) |
| Bruit (thyroid) |
| Decreased attention span |
| Diarrhea |
| Edema |
| Exertional dyspnea |
| Fatigue |
| Goiter (smooth or nodular) |
| Gynecomastia |
| Hair loss |
| Heat intolerance |
| Hyperactive deep tendon reflex |
| Hypertension |
| Increased appetite |
| Infertility |
| Insomnia |
| Lid lag, proptosis |
| Muscle weakness |
| Nervousness and irritability |
| Oligomenorrhea |
| Palmar erythema |
| Palpitations |
| Paralysis (sudden) |
| Photophobia, eye irritation, diplopia |
| Pretibial myxedema |
| Tachycardia |
| Tremors |
| Warm, moist skin |
| Weight loss |
Graves’ disease—an autoimmune disorder in which antibodies target thyroid tissue and enzymes and activate thyroid hormone synthesis—affects more than 3 million people in the United States and accounts for 60% of hyperthyroidism cases.3 Remission does occur; however, the recurrence rate is as high as 60%.50 Factors associated with recurrence include tobacco use; male sex; young age; large goiter size or increase in goiter size during treatment; elevated TSH receptor antibodies (TRab); presence of Graves’ ophthalmopathy; markedly elevated thyroid hormones, or delayed treatment.51
Toxic multinodular goiter, also known as Plummer’s disease, is the underlying condition in 15% to 20% of hyperthyroidism cases; it is more common in young patients and in iodine-deficient locations (eg, Denmark).52 However, it also occurs in elderly patients with longstanding goiter.
Toxic adenoma causes just 3% to 5% of cases of hyperthyroidism.53 It, too, occurs more commonly in young patients and in iodine-deficient regions. The radioactive iodine uptake test shows a hot nodule, with suppressed uptake in the surrounding thyroid gland.
Subacute thyroiditis, also known as de Quervain’s thyroiditis, is the reason for 15% to 20% of hyperthyroidism cases; it is usually preceded by viral infection and inflammation that lead to destructive release of preformed thyroid hormone. Symptoms—typically fever, malaise, and tender goiter—usually occur more abruptly than symptoms of Graves’ disease.54 Most cases resolve spontaneously within a few months, and relapse is less common than in Graves’ disease. Other lab abnormalities include increased erythrocyte sedimentation rate and low radioiodine uptake.
Postpartum thyroiditis is an autoimmune disease. Prevalence ranges from 1% to 17% of new mothers.55 It is characterized by a thyroid gland that is painless on palpation and low radioiodine uptake.56 Most cases are reversible with treatment.
Factitious or iatrogenic hyperthyroidism is due to an exogenous intake of thyroid hormone, and typically exhibits a normal or low radioactive iodine uptake and a low thyroglobulin level.
Secondary hyperthyroidism, or TSH-mediated hyperthyroidism, is rare. It is always associated with goiter, and approximately 40% of patients have visual field defects.57
TABLE 2
Clinical and laboratory findings associated with common causes of hyperthyroidism51-57
| Mechanism | Thyroid exam | Lab results | Radioactive iodine uptake | |
|---|---|---|---|---|
| Graves’ disease | Antithyroid antibodies | Diffuse goiter | Low TSH; elevated T3 and/or T4; elevated thyroid antibodies | Diffusely increased |
| Toxic multinodular goiter | Iodine deficiency | Goiter with multiple nodules | Low TSH; elevated T3 and/or T4 | Normal/increased uptake; "hot nodules" with suppression of extranodular tissue |
| Toxic adenoma | Benign thyroid hormone?secreting tumor; iodine deficiency | Palpable nodule | Low TSH; elevated T3 and/or T4 | Normal/increased uptake; functioning "hot nodule" on scan with suppression of surrounding thyroid tissue |
| Subacute thyroiditis | Viral | Tender thyroid on palpation | Low TSH; elevated T3 and/or T4; elevated ESR; elevated thyroid antibodies | Low uptake with poor imaging of the thyroid on scan |
| Factitious hyperthyroidism | Excessive intake of exogenous thyroid hormone | Normal exam | Low TSH; elevated T3 and/or T4; low thyroglobulin level | Low or normal uptake |
| Secondary hyperthyroidism | Excessive pituitary TSH | Goiter | Elevated TSH; elevated T3 and/or T4 | Diffusely increased uptake |
| ESR, erythrocyte sedimentation rate; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone. | ||||
Which laboratory tests to order, and what the results may mean
Rely on second- or third-generation TSH screening (normal=0.5-5 mIU/L), which is more sensitive and specific than measuring free T4 (thyroxine) alone.6
Older patients usually have a higher normal TSH level. In one study, 70% of patients >80 years had a TSH >4.5 mIU/L.7
If the TSH level is low (<0.5 mIU/L), measure free T3 (triiodothyronine) and free T4 levels, which are elevated in hyperthyroidism, and are normal in subclinical hyperthyroidism.
Patients with Graves’ disease tend to have T3 thyrotoxicosis with a T3T4 ratio >20.8 Isolated T4 thyrotoxicosis is more commonly seen with nonthyroidal illness as a result of decreased conversion from T4 to T3, and also in amiodarone-induced hyperthyroidism.9
When further testing is needed (FIGURE). If the underlying cause of hyperthyroidism is not established on the basis of clinical findings (eg, diffuse goiter, myxedema, ophthalmopathy), order a 24-hour radioactive iodine (RAI) uptake test.10 Graves’ disease and toxic multinodular goiter exhibit increased RAI uptake that is diffuse and nodular, respectively. Subacute thyroiditis is associated with low RAI uptake (TABLE 2).
If RAI is contraindicated—eg, in pregnancy—testing for elevated levels of thyroid peroxidase antibodies (TPOab), TSH receptor antibodies (TRab), and thyroglobulin may help to differentiate Graves’ disease from multinodular goiter or uncover another autoimmune thyroid disorder.11 If a patient’s TSH, T4, and T3 levels are all elevated, refer him or her for magnetic resonance imaging of the pituitary gland to look for a TSH-secreting adenoma.
FIGURE
Suspect hyperthyroidism? Order these tests6-11
MRI, magnetic resonance imaging; RAI, radioactive iodine; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
Matching treatment to the underlying cause
RAI is usually the treatment of choice for patients without contraindications, although no randomized clinical trials have compared it with antithyroid medications or surgery.12 Each modality has its own risks and benefits (TABLE 3), and treatment selection should be individualized.
TABLE 3
Comparison of treatment modalities for hyperthyroidism12-21
| Antithyroid drugs* | Radioactive iodine* | Surgery | |
|---|---|---|---|
| Recurrence rate | High recurrence rate; no permanent hypothyroidism | Usually permanent hypothyroidism; long-term use of levothyroxine is required | Subtotal thyroidectomy associated with higher rates of recurrence or persistence of hyperthyroidism than total thyroidectomy; permanent hypothyroidism; long-term use of levothyroxine is required |
| Preferred method within treatment modality | MMI is the preferred medication; PTU is used with pregnancy and severe hyperthyroidism not responding to MMI | High ablative dose is preferred in MNG, toxic nodule, cardiac disease, elderly; low calculated dose is preferred in patients with GO | No outcome differences for GO, whether thyroidectomy is total, bilateral subtotal, or unilateral total and contralateral subtotal |
| Setting | Outpatient | Outpatient | Inpatient |
| Risks | No surgical risks | No surgical risks | Reaction to anesthesia, recurrent laryngeal nerve palsy, hypoparathyroidism |
| Adverse effects | ATD adverse effects, including life-threatening agranulocytosis | Worsening of Graves’ ophthalmopathy; transient exacerbation of hyperthyroid symptoms | Permanent hypothyroidism; hypoparathyroidism; anesthesia complications |
| Safety in pregnancy | PTU is used in pregnancy | Contraindicated in pregnancy/lactation | If surgery is indicated in pregnancy, it is best performed in the second trimester |
| ATD, antithyroid drugs; GO, Graves’ ophthalmopathy; MMI, methimazole; MNG, toxic multinodular goiter; PTU, propylthiouracil. *Concomitant use of ATD and RAI is associated with a high failure rate and persistent or recurrent hyperthyroidism. Discontinue ATD 2 weeks before radioactive iodine treatment. | |||
Radioactive iodine
In a 1990 survey, as many as 70% of specialists in the United States used RAI to treat hyperthyroidism, compared with just 22% of specialists in Europe.13 RAI is usually given in a single dose, and its maximal benefit is noted within 3 to 6 months. Two treatment methods are available: the ablative method and the gland-specific dosing method. Both have similar euthyroid state outcomes.14
The ablative method uses a high dose of RAI to achieve permanent hypothyroidism, necessitating lifelong levothyroxine replacement. This method is preferred for the elderly and for patients with cardiac disease, to achieve faster control of symptoms. It is also recommended for patients with toxic multinodular goiter and toxic nodules.
The gland-specific dosing method induces a euthyroid state with a calculated low dose of RAI based on the estimated weight of the patient’s thyroid. The optimal dosage may be difficult to calculate, but it is usually the preferred method for patients with Graves’ ophthalmopathy.
Adverse effects of RAI can include worsening of Graves’ ophthalmopathy and an acute rise in thyroid hormone that increases hyperthyroid symptoms or even causes a thyroid storm associated with increased cardiovascular risk.2 A negative pregnancy test result is a prerequisite for all women of childbearing age before taking RAI, and patients are advised to use contraception for 6 months after RAI administration.
Although RAI is often the initial treatment for hyperthyroidism, in some instances—eg, for older patients with comorbidities—pre-treatment with antithyroid drugs (ATD) is indicated to avoid transient worsening of hyperthyroid symptoms after RAI. However, always discontinue ATD 2 weeks before RAI administration; concomitant use is associated with a higher failure rate and persistent or recurrent hyperthyroidism.15
Antithyroid drugs
Two antithyroid medications are available for use in the United States: propylthiouracil (PTU) and methimazole (MMI). In the United Kingdom, carbimazole is also available.
MMI is the drug of choice.16 Compared with PTU, MMI costs less, has a longer half-life, and causes fewer adverse effects. A starting dose of 15 mg per day for MMI is suitable for mild and moderate hyperthyroidism. For more severe cases, 30 mg per day is the recommended starting dose.16 Reserve PTU for treating hyperthyroidism in pregnancy, during which MMI should be avoided, if possible.
Allergic reactions to ATDs appear in around 5% of patients and usually occur in the first 6 weeks of treatment.17 Agranulocytosis is the main concern, although it occurs in fewer than 1% of patients17 and is reversible by stopping the medication. Measure the leukocyte count 1 week after initiation of treatment and repeat the measurement at 1-month intervals.
Two methods are used to dose these medications: titration and block-and-replace. Titration is as effective as the block-and-replace method and is associated with fewer rashes (6% vs 10% of patients) and less agranulocytosis (0.4 % vs 1.4%). The 2 methods have similar relapse rates (around 50%).18
With titration, MMI is started at a dose of 15 mg per day and titrated upward to the lowest effective dose. Treatment for 12 to 18 months is associated with a lower relapse rate than treatment for 6 months (37% vs 58%).19
The block-and-replace method uses persistently high ATD doses in combination with L-thyroxin replacement to avoid hypothyroidism (MMI 30 mg and levothyroxine 80 mcg).
To monitor effectiveness initially, measure free T4 and T3 levels, because TSH concentration changes slowly and may stay low for a few months. Response to treatment is often temporary.8 More definitive treatment with RAI or surgery is usually necessary.
Surgery
Thyroidectomy creates permanent hypothyroidism, necessitating lifelong thyroxine replacement. In the United States, surgical intervention is reserved for special situations, such as pregnant women with severe disease who are allergic or not responding to antithyroid medications, removal of a clinically suspicious thyroid nodule coexisting with hyperthyroidism, or severe or recurrent Graves’ disease with severe ophthalmopathy.20 Surgical options are total or subtotal thyroidectomy. Hyperthyroidism persists or recurs in 8% of patients with subtotal thyroidectomy.21 Potential complications of thyroidectomy include adverse effects of anesthesia, hypoparathyroidism, and vocal cord paralysis.
Other treatment options
Iodides
Iodides inhibit thyroid hormone release and block conversion of T4 to T3. Use potassium iodide only in combination with ATDs, for patients with severe thyrotoxicosis or as pretreatment for urgent thyroidectomy in patients with Graves’ disease. It has been shown to improve the short-term control of Graves’ hyperthyroidism and is not associated with worsening hyperthyroidism;22 however, potassium iodide should not be used for more than 12 weeks as it can cause paradoxical hyperthyroidism.22
Beta-blockers
Hyperthyroidism is associated with an increased number of beta-adrenergic receptors,23 which explains the symptoms of palpitations, anxiety, and tremors. Nonselective beta-blockers are usually preferred for symptomatic treatment of hyperthyroid symptoms, and propranolol is the most widely used agent.24 If you decide to use a beta-blocker, start it with the ATD and continue it until the patient becomes euthyroid or asymptomatic, then taper it over a period of 4 to 6 weeks. Symptoms may persist, however, and require higher doses of propranolol (80-320 mg/d) given more frequently.
Treating Graves’ ophthalmopathy
Exophthalmos and other eye signs are the hallmark of Graves’ disease and may occur in the absence of hyperthyroidism. Smoking is a significant risk factor for developing ophthalmopathy due to increased orbital connective tissue volume,25 and smoking cessation is recommended.26
Using RAI to treat Graves’ disease increases the risk that ophthalmopathy will develop or worsen. Worsening of Graves’ ophthalmopathy secondary to RAI treatment occurs in 20% of treated patients (transient in 15%; permanent in 5%).27 Steroid prophylaxis is beneficial for patients with ophthalmopathy,28 and prednisone 40 to 80 mg per day tapered over at least 3 months can help reduce the condition.19 In patients with moderate to severe active ophthalmopathy, intravenous corticosteroid therapy has a small but statistically significant advantage over oral therapy and causes significantly fewer adverse events.29
Orbital radiotherapy is also used, and has been shown to decrease diplopia.30 However, the best available evidence recommends combining orbital radiotherapy and oral corticosteroids, which yields efficacy beyond that achievable with either radiotherapy or oral corticosteroids alone.16 Moreover, intravenous methylprednisolone combined with orbital radiotherapy seems to be most efficacious.31 The course of ophthalmopathy is the same whether total or subtotal thyroidectomy is used.32
Prognosis without treatment
Individuals with high-normal thyroid function tests, subclinical hyperthyroidism, and clinical hyperthyroidism are at increased risk for atrial fibrillation.33-35 Hyperthyroidism is also associated with increased risk of heart failure (6% of patients), which might be secondary to coexisting atrial fibrillation or tachycardia-mediated cardiomyopathy.36 Heart failure is usually reversible when the hyperthyroidism is treated.
Patients with overt hyperthyroidism are also at risk for pulmonary hypertension secondary to increased cardiac output and decreased pulmonary vascular resistance.37
In patients with preexisting cardiac disease, hyperthyroidism increases risk of death (hazard ratio [HR]=1.57),38 and might even do so in patients without cardiac disease.39,40 It also increases risk of ischemic stroke (HR=1.44) among adults ages 18 to 44 years.41 Untreated hyperthyroidism also contributes to low bone mineral density and increases the risk of hip fracture.42
Subclinical hyperthyroidism
Subclinical hyperthyroidism occurs in 2% of the US population and is characterized by low serum TSH (<0.1 mIU/L) with normal levels of free T3 and free T4. The causes are similar to overt hyperthyroidism. In addition, it can result from overtreating hypothyroidism with thyroid hormone, thereby inducing a subclinical hyperthyroid state.
The most common endogenous cause of subclinical hyperthyroidism (~60% of patients) is multinodular goiter.43 Subclinical hyperthyroidism carries significant health risks, and yet evidence is lacking on when to treat this condition. Prolonged subclinical hyperthyroidism can lead to atrial fibrillation,24,44 and to systolic and diastolic cardiac dysfunction.45 Subclinical hyperthyroidism is also associated with decreased bone density,46 and an increased risk of dementia.47
The American Association of Clinical Endocrinologists recommends periodic clinical and laboratory assessment for patients with subclinical hyperthyroidism (TSH=0.1-0.5 mIU/mL), including rechecking TSH, free T3 and free T4 at 2- to 4-month intervals.
Treatment of the underlying cause of hyperthyroidism is indicated if serum TSH is <0.1 mIU/mL.
For patients older than 65 years who have persistent subclinical hyperthyroidism, consider treatment in the following scenarios:48
- nodular thyroid disease (due to high conversion rate to overt hyperthyroidism)
- osteopenia or osteoporosis (in women)
- atrial fibrillation
- underlying cardiac disease.
Hyperthyroidism in pregnancy
PTU is the first choice for treating hyperthyroidism in pregnancy. It crosses the placenta less readily than MMI, and is thus less likely to cause fetal hypothyroidism. Additionally, MMI is associated with increased risk of fetal anomalies, such as aplasia cutis and esophageal atresia. MMI may be considered if the patient is intolerant to PTU or fails to become euthyroid while receiving PTU.49 Use the lowest possible dose of either PTU or MMI to maintain thyroid function within the upper limit of normal. The dose of the antithyroid medication is usually decreased as pregnancy progresses and discontinued in the last few weeks, as pregnancy is thought to improve the course of Graves’ disease.
The use of RAI is contraindicated during pregnancy and breastfeeding. Hyperthyroidism symptoms usually resolve after delivery. If symptoms persist, however, the treatment of choice is ATD. Surgery is an option in severe Graves’ disease not responding to ATD.
CORRESPONDENCE
Abdulraouf Ghandour, MD, Department of Family and Community Medicine, University of Missouri-Columbia, One Hospital Drive, Columbia, MO 65212; [email protected]
1. Levy EG. Thyroid disease in the elderly. Med Clin North Am. 1991;75:151-167.
2. Cooper DS. Hyperthyroidism. Lancet. 2003;362:459-468.
3. Weetman AP. Graves’ disease. N Engl J Med. 2000;343:1236-1248.
4. Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
5. Boelaert K, Torlinska B. Older subjects with hyperthyroidism present with a paucity of symptoms and signs: a large cross-sectional study. J Clin Endocrinol Metab. 2010;95:2715-2726.
6. Danese MD, Powe NR, Sawin CT, et al. Screening of mild thyroid failure at the periodic health examination: a decision and cost-effectiveness analysis. JAMA. 1996;276:285-292.
7. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
8. Amino N, Yabu Y, Miki T, et al. Serum ratio of triiodothyronine to thyroxine and thyroxine binding globulin and calcitonin concentrations in Graves’ disease and destruction-induced thyrotoxicosis. J Clin Endocrinol Metab. 1981;53:113-116.
9. Bambini G, Aghini-Lombardi F, Rosner W, et al. Serum sex hormone-binding globulin in amiodarone-treated patients. A marker for tissue thyrotoxicosis. Arch Intern Med. 1987;147:1781-1785.
10. Fogelman I, Cooke SG, Maisey MN. The role of thyroid scanning in hyperthyroidism. Eur J Nucl Med. 1986;11:397-400.
11. Costagliola S, Morgenthaler NG, Hoermann R, et al. Second generation assay for thyrotropin receptor antibodies has superior diagnostic sensitivity for Graves’ disease. J Clin Endocrinol Metab. 1999;84:90-97.
12. Streetman DD, Khanderia U. Diagnosis and treatment of Graves’ disease. Ann Pharmacother. 2003;37:1100-1109.
13. Wartofsky L, Glinoer D, Solomon B, et al. Differences and similarities in the diagnosis and treatment of Graves’ disease in Europe, Japan, and the United States. Thyroid. 1991;1:129-135.
14. de Rooij A, Vandenbroucke JP. Clinical outcomes after estimated versus calculated activity of radioiodine for the treatment of hyperthyroidism: systematic review and meta-analysis. Eur J Endocrinol. 2009;161:771-777.
15. Walter MA, Briel M, Christ-Crain M, et al. Effects of antithyroid drugs on radioiodine treatment: systematic review and meta-analysis of randomised controlled trials. BMJ. 2007;334:514.-
16. Nakamura H, Noh JY. Comparison of methimazole and propylthiouracil in patients with hyperthyroidism caused by Graves’ disease. J Clin Endocrinol Metab. 2007;92:2157-2162.
17. Cooper DS. Antithyroid drugs. N Engl J Med. 2005;352:905-917.
18. Abraham P, Avenell A. A systematic review of drug therapy for Graves’ hyperthyroidism. Eur J Endocrinol. 2005;153:489-498.
19. Abraham P, Avenell A, McGeoch SC, et al. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Sys Rev. 2010;(1):CD003420.-
20. Stalberg P, Svensson A. Surgical treatment of Graves’ disease: evidence-based approach. World J Surg. 2008;32:1269-1277.
21. Palit TK, Miller CC, Miltenburg DM. The efficacy of thyroidectomy for Graves’ disease: a meta-analysis. J Surg Res. 2000;90:161-165.
22. Takata K, Amino N, Kubota S. Benefit of short-term iodide supplementation to antithyroid drug treatment of thyrotoxicosis due to Graves’ disease. Clin Endocrinol. 2010;72:845-850.
23. Bilezikian JP, Loeb JN. The influence of hyperthyroidism and hypothyroidism on alpha- and beta-adrenergic receptor systems and adrenergic responsiveness. Endocr Rev. 1983;4:378-388.
24. Jansson S, Lie-Karlsen K, Stenqvist O, et al. Oxygen consumption in patients with hyperthyroidism before and after treatment with beta-blockade versus thyrostatic treatment: a prospective randomized study. Ann Surg. 2001;233:60-64.
25. Zucs-Frkas Z, Toth J, Kollar J, et al. Volume changes in intra- and extraorbital compartments in patients with Graves’ ophthalmopathy: effect of smoking. Thyroid. 2005;15:146-151.
26. Träisk F, Tallstedt L. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700-3707.
27. Bartalena L, Marcocci C, Bogazzi F, et al. Relation between therapy of hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med. 1998;338:73-78.
28. Acharya SH, Avenell A. Radioiodine therapy (RAI) for Graves’ disease (GD) and the effect on ophthalmopathy: a systematic review. Clin Endocrinol (Oxf). 2008;69:943-950.
29. Stiebel-Kalish H, Robenshtok E. Treatment modalities for Graves’ ophthalmopathy: systematic review and meta-analysis. J Clin Endocrinol Metab. 2009;94:2708-2716.
30. Bradley EA, Gower EW. Orbital radiation for graves ophthalmopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115:398-409.
31. Wei RL, Cheng JW. The use of orbital radiotherapy for Graves’ ophthalmopathy: quantitative review of the evidence. Ophthalmologica. 2008;222:27-31.
32. Witte J, Goretzki PE, Dotzenrath C, et al. Surgery for Graves’ disease: total versus subtotal thyroidectomy–result of a prospective randomized trial. World J Surg. 2000;24:1303-1311.
33. Heeringa J, Hoogendoorn EH. High-normal thyroid function and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med. 2008;168:2219-2224.
34. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249-1252.
35. Cappola AR, Fried LP. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033-1041.
36. Siu CW, Yeung CY, Lau CP, et al. Incidence, clinical characteristics and outcome of congestive heart failure as the initial presentation in patients with primary hyperthyroidism. Heart. 2007;93:483-487.
37. Lozano HF, Sharma CN. Reversible pulmonary hypertension, tricuspid regurgitation and right-sided heart failure associated with hyperthyroidism: case report and review of the literature. Cardiol Rev. 2004;12:299-305.
38. Iervasi G, Molinaro S. Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med. 2007;167:1526-1532.
39. Parle JV, Maisonneuve P, Sheppard MC, et al. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet. 2001;358:861-865.
40. Flynn RW, McDonald TM, Jung RT, et al. Mortality and vascular outcomes in patients treated for thyroid dysfunction. J Clin Endocrinol Metab. 2006;91:2169-2164.
41. Sheu JJ, Kang JH. Hyperthyroidism and risk of ischemic stroke in young adults: a 5-year follow-up study. Stroke. 2010;41:961-966.
42. Vestergaard P, Mosekilde L. Hyperthyroidism, bone mineral, and fracture risk—a meta-analysis. Thyroid. 2003;13:585-593.
43. Diez JJ. Hyperthyroidism in patients older than 55 years: an analysis of the etiology and management. Gerontology. 2003;49:316-323.
44. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249-1252.
45. Abdulrahman RM, Delgado V. Abnormal cardiac contractility in long-term exogenous subclinical hyperthyroid patients as demonstrated by two-dimensional echocardiography speckle tracking imaging. Eur J Endocrinol. 2010;163:435-441.
46. Faber J, Jensen IW, Petersen L, et al. Normalization of serum thyrotrophin by means of radioiodine treatment in subclinical hyperthyroidism: effect on bone loss in postmenopausal women. Clin Endocrinol (Oxf). 1998;48:285-290.
47. Tan ZS, Beiser A, Vasan RS, et al. Thyroid function and the risk of Alzheimer disease: Framingham study. Arch Intern Med. 2008;168:1514-1520.
48. American Association of Clinical Endocrinologists. Medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. 2006. Available at: https://www.aace.com/sites/default/files/hypo_hyper.pdf. Accessed July 9, 2010.
49. Chattaway JM, Klepser TB. Propylthiouracil versus methimazole in treatment of Grave’s disease during pregnancy. Ann Pharmacother. 2007;41:1018-1022.
50. Lucas A, Salinas I. Medical therapy of Graves’ disease: does thyroxine prevent recurrence of hyperthyroidism? J Clin Endocrinol Metab. 1997;82:2410-2413.
51. Vitti P, Rago T, Chiovato L, et al. Clinical features of patients with Graves’ disease undergoing remission after antithyroid drug treatment. Thyroid. 1997;7:369-375.
52. Laurberg P, Bulow Pedersen I, Pedersen KM, et al. Low incidence rate of overt hypothyroidism compared with hyperthyroidism in an area with moderately low iodine intake. Thyroid. 1999;9:33-38.
53. Siegel RD, Lee SL. Toxic nodular goiter: Toxic adenoma and toxic multinodular goiter. Endocrinol Metab Clin North Am. 1998;27:151-168.
54. Volpe R. Subacute (de Quervain’s) thyroiditis. Clin Endocrinol Metab. 1979;8:81-95.
55. Nicholson WK, Robinson KA, Smallridge RC, et al. Prevalence of postpartum thyroid dysfunction: a quantitative review. Thyroid. 2006;16:573-582.
56. Roti E, Emerson CH. Clinical review 29: postpartum thyroiditis. J Clin Endocrinol Metab. 1992;74:3-5.
57. Beck-Peccoz P, Brucker-Davis F, Persani L, et al. Thyrotropin-secreting pituitary tumors. Endocr Rev. 1996;17:610-638.
1. Levy EG. Thyroid disease in the elderly. Med Clin North Am. 1991;75:151-167.
2. Cooper DS. Hyperthyroidism. Lancet. 2003;362:459-468.
3. Weetman AP. Graves’ disease. N Engl J Med. 2000;343:1236-1248.
4. Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
5. Boelaert K, Torlinska B. Older subjects with hyperthyroidism present with a paucity of symptoms and signs: a large cross-sectional study. J Clin Endocrinol Metab. 2010;95:2715-2726.
6. Danese MD, Powe NR, Sawin CT, et al. Screening of mild thyroid failure at the periodic health examination: a decision and cost-effectiveness analysis. JAMA. 1996;276:285-292.
7. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
8. Amino N, Yabu Y, Miki T, et al. Serum ratio of triiodothyronine to thyroxine and thyroxine binding globulin and calcitonin concentrations in Graves’ disease and destruction-induced thyrotoxicosis. J Clin Endocrinol Metab. 1981;53:113-116.
9. Bambini G, Aghini-Lombardi F, Rosner W, et al. Serum sex hormone-binding globulin in amiodarone-treated patients. A marker for tissue thyrotoxicosis. Arch Intern Med. 1987;147:1781-1785.
10. Fogelman I, Cooke SG, Maisey MN. The role of thyroid scanning in hyperthyroidism. Eur J Nucl Med. 1986;11:397-400.
11. Costagliola S, Morgenthaler NG, Hoermann R, et al. Second generation assay for thyrotropin receptor antibodies has superior diagnostic sensitivity for Graves’ disease. J Clin Endocrinol Metab. 1999;84:90-97.
12. Streetman DD, Khanderia U. Diagnosis and treatment of Graves’ disease. Ann Pharmacother. 2003;37:1100-1109.
13. Wartofsky L, Glinoer D, Solomon B, et al. Differences and similarities in the diagnosis and treatment of Graves’ disease in Europe, Japan, and the United States. Thyroid. 1991;1:129-135.
14. de Rooij A, Vandenbroucke JP. Clinical outcomes after estimated versus calculated activity of radioiodine for the treatment of hyperthyroidism: systematic review and meta-analysis. Eur J Endocrinol. 2009;161:771-777.
15. Walter MA, Briel M, Christ-Crain M, et al. Effects of antithyroid drugs on radioiodine treatment: systematic review and meta-analysis of randomised controlled trials. BMJ. 2007;334:514.-
16. Nakamura H, Noh JY. Comparison of methimazole and propylthiouracil in patients with hyperthyroidism caused by Graves’ disease. J Clin Endocrinol Metab. 2007;92:2157-2162.
17. Cooper DS. Antithyroid drugs. N Engl J Med. 2005;352:905-917.
18. Abraham P, Avenell A. A systematic review of drug therapy for Graves’ hyperthyroidism. Eur J Endocrinol. 2005;153:489-498.
19. Abraham P, Avenell A, McGeoch SC, et al. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Sys Rev. 2010;(1):CD003420.-
20. Stalberg P, Svensson A. Surgical treatment of Graves’ disease: evidence-based approach. World J Surg. 2008;32:1269-1277.
21. Palit TK, Miller CC, Miltenburg DM. The efficacy of thyroidectomy for Graves’ disease: a meta-analysis. J Surg Res. 2000;90:161-165.
22. Takata K, Amino N, Kubota S. Benefit of short-term iodide supplementation to antithyroid drug treatment of thyrotoxicosis due to Graves’ disease. Clin Endocrinol. 2010;72:845-850.
23. Bilezikian JP, Loeb JN. The influence of hyperthyroidism and hypothyroidism on alpha- and beta-adrenergic receptor systems and adrenergic responsiveness. Endocr Rev. 1983;4:378-388.
24. Jansson S, Lie-Karlsen K, Stenqvist O, et al. Oxygen consumption in patients with hyperthyroidism before and after treatment with beta-blockade versus thyrostatic treatment: a prospective randomized study. Ann Surg. 2001;233:60-64.
25. Zucs-Frkas Z, Toth J, Kollar J, et al. Volume changes in intra- and extraorbital compartments in patients with Graves’ ophthalmopathy: effect of smoking. Thyroid. 2005;15:146-151.
26. Träisk F, Tallstedt L. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700-3707.
27. Bartalena L, Marcocci C, Bogazzi F, et al. Relation between therapy of hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med. 1998;338:73-78.
28. Acharya SH, Avenell A. Radioiodine therapy (RAI) for Graves’ disease (GD) and the effect on ophthalmopathy: a systematic review. Clin Endocrinol (Oxf). 2008;69:943-950.
29. Stiebel-Kalish H, Robenshtok E. Treatment modalities for Graves’ ophthalmopathy: systematic review and meta-analysis. J Clin Endocrinol Metab. 2009;94:2708-2716.
30. Bradley EA, Gower EW. Orbital radiation for graves ophthalmopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115:398-409.
31. Wei RL, Cheng JW. The use of orbital radiotherapy for Graves’ ophthalmopathy: quantitative review of the evidence. Ophthalmologica. 2008;222:27-31.
32. Witte J, Goretzki PE, Dotzenrath C, et al. Surgery for Graves’ disease: total versus subtotal thyroidectomy–result of a prospective randomized trial. World J Surg. 2000;24:1303-1311.
33. Heeringa J, Hoogendoorn EH. High-normal thyroid function and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med. 2008;168:2219-2224.
34. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249-1252.
35. Cappola AR, Fried LP. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033-1041.
36. Siu CW, Yeung CY, Lau CP, et al. Incidence, clinical characteristics and outcome of congestive heart failure as the initial presentation in patients with primary hyperthyroidism. Heart. 2007;93:483-487.
37. Lozano HF, Sharma CN. Reversible pulmonary hypertension, tricuspid regurgitation and right-sided heart failure associated with hyperthyroidism: case report and review of the literature. Cardiol Rev. 2004;12:299-305.
38. Iervasi G, Molinaro S. Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med. 2007;167:1526-1532.
39. Parle JV, Maisonneuve P, Sheppard MC, et al. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet. 2001;358:861-865.
40. Flynn RW, McDonald TM, Jung RT, et al. Mortality and vascular outcomes in patients treated for thyroid dysfunction. J Clin Endocrinol Metab. 2006;91:2169-2164.
41. Sheu JJ, Kang JH. Hyperthyroidism and risk of ischemic stroke in young adults: a 5-year follow-up study. Stroke. 2010;41:961-966.
42. Vestergaard P, Mosekilde L. Hyperthyroidism, bone mineral, and fracture risk—a meta-analysis. Thyroid. 2003;13:585-593.
43. Diez JJ. Hyperthyroidism in patients older than 55 years: an analysis of the etiology and management. Gerontology. 2003;49:316-323.
44. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249-1252.
45. Abdulrahman RM, Delgado V. Abnormal cardiac contractility in long-term exogenous subclinical hyperthyroid patients as demonstrated by two-dimensional echocardiography speckle tracking imaging. Eur J Endocrinol. 2010;163:435-441.
46. Faber J, Jensen IW, Petersen L, et al. Normalization of serum thyrotrophin by means of radioiodine treatment in subclinical hyperthyroidism: effect on bone loss in postmenopausal women. Clin Endocrinol (Oxf). 1998;48:285-290.
47. Tan ZS, Beiser A, Vasan RS, et al. Thyroid function and the risk of Alzheimer disease: Framingham study. Arch Intern Med. 2008;168:1514-1520.
48. American Association of Clinical Endocrinologists. Medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. 2006. Available at: https://www.aace.com/sites/default/files/hypo_hyper.pdf. Accessed July 9, 2010.
49. Chattaway JM, Klepser TB. Propylthiouracil versus methimazole in treatment of Grave’s disease during pregnancy. Ann Pharmacother. 2007;41:1018-1022.
50. Lucas A, Salinas I. Medical therapy of Graves’ disease: does thyroxine prevent recurrence of hyperthyroidism? J Clin Endocrinol Metab. 1997;82:2410-2413.
51. Vitti P, Rago T, Chiovato L, et al. Clinical features of patients with Graves’ disease undergoing remission after antithyroid drug treatment. Thyroid. 1997;7:369-375.
52. Laurberg P, Bulow Pedersen I, Pedersen KM, et al. Low incidence rate of overt hypothyroidism compared with hyperthyroidism in an area with moderately low iodine intake. Thyroid. 1999;9:33-38.
53. Siegel RD, Lee SL. Toxic nodular goiter: Toxic adenoma and toxic multinodular goiter. Endocrinol Metab Clin North Am. 1998;27:151-168.
54. Volpe R. Subacute (de Quervain’s) thyroiditis. Clin Endocrinol Metab. 1979;8:81-95.
55. Nicholson WK, Robinson KA, Smallridge RC, et al. Prevalence of postpartum thyroid dysfunction: a quantitative review. Thyroid. 2006;16:573-582.
56. Roti E, Emerson CH. Clinical review 29: postpartum thyroiditis. J Clin Endocrinol Metab. 1992;74:3-5.
57. Beck-Peccoz P, Brucker-Davis F, Persani L, et al. Thyrotropin-secreting pituitary tumors. Endocr Rev. 1996;17:610-638.