User login
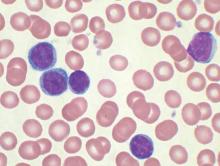
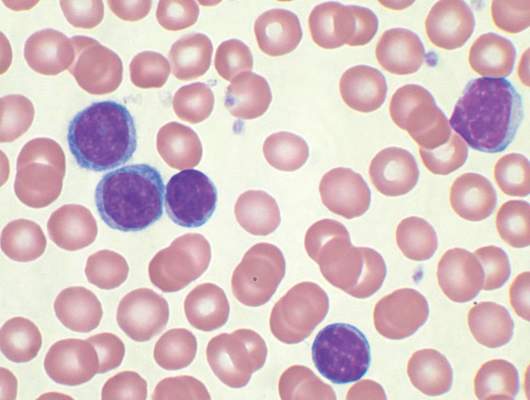
Ibrutinib (Imbruvica) has been approved as a first-line treatment for patients with chronic lymphocytic leukemia (CLL), irrespective of their treatment history, according to a statement issued by the manufacturer, AbbVie.
The approval is based on data from the randomized, multicenter, open-label phase III RESONATE-2 trial, which evaluated the use of ibrutinib versus chlorambucil in 269 treatment-naive patients with CLL or small lymphocytic lymphoma (SLL) aged 65 years or older. The RESONATE-2 data were presented at the 2015 annual meeting of the American Society of Hematology.
“The progression-free survival data seen in these previously untreated CLL patients are strong and encouraging,” Dr. Jan Burger of the University of Texas MD Anderson Cancer Center, Houston, and the lead investigator of RESONATE-2, said in the AbbVie statement. “This is especially important for first-line CLL patients, when considering the safety profile. This treatment represents another option for these patients.”
The National Comprehensive Cancer Network also recently published an update to its clinical practice guidelines for non-Hodgkin’s lymphomas, granting Imbruvica a category 1 recommendation for certain CLL patients, including as a first-line treatment option for frail CLL patients with significant comorbidities, as well as for CLL patients with or without del 17p or the genetic mutation TP53 who are 70 years or older, or younger patients with significant comorbidities, the AbbVie statement noted.
Imbruvica has been jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and by Janssen Biotech.
Ibrutinib (Imbruvica) has been approved as a first-line treatment for patients with chronic lymphocytic leukemia (CLL), irrespective of their treatment history, according to a statement issued by the manufacturer, AbbVie.
The approval is based on data from the randomized, multicenter, open-label phase III RESONATE-2 trial, which evaluated the use of ibrutinib versus chlorambucil in 269 treatment-naive patients with CLL or small lymphocytic lymphoma (SLL) aged 65 years or older. The RESONATE-2 data were presented at the 2015 annual meeting of the American Society of Hematology.
“The progression-free survival data seen in these previously untreated CLL patients are strong and encouraging,” Dr. Jan Burger of the University of Texas MD Anderson Cancer Center, Houston, and the lead investigator of RESONATE-2, said in the AbbVie statement. “This is especially important for first-line CLL patients, when considering the safety profile. This treatment represents another option for these patients.”
The National Comprehensive Cancer Network also recently published an update to its clinical practice guidelines for non-Hodgkin’s lymphomas, granting Imbruvica a category 1 recommendation for certain CLL patients, including as a first-line treatment option for frail CLL patients with significant comorbidities, as well as for CLL patients with or without del 17p or the genetic mutation TP53 who are 70 years or older, or younger patients with significant comorbidities, the AbbVie statement noted.
Imbruvica has been jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and by Janssen Biotech.
Ibrutinib (Imbruvica) has been approved as a first-line treatment for patients with chronic lymphocytic leukemia (CLL), irrespective of their treatment history, according to a statement issued by the manufacturer, AbbVie.
The approval is based on data from the randomized, multicenter, open-label phase III RESONATE-2 trial, which evaluated the use of ibrutinib versus chlorambucil in 269 treatment-naive patients with CLL or small lymphocytic lymphoma (SLL) aged 65 years or older. The RESONATE-2 data were presented at the 2015 annual meeting of the American Society of Hematology.
“The progression-free survival data seen in these previously untreated CLL patients are strong and encouraging,” Dr. Jan Burger of the University of Texas MD Anderson Cancer Center, Houston, and the lead investigator of RESONATE-2, said in the AbbVie statement. “This is especially important for first-line CLL patients, when considering the safety profile. This treatment represents another option for these patients.”
The National Comprehensive Cancer Network also recently published an update to its clinical practice guidelines for non-Hodgkin’s lymphomas, granting Imbruvica a category 1 recommendation for certain CLL patients, including as a first-line treatment option for frail CLL patients with significant comorbidities, as well as for CLL patients with or without del 17p or the genetic mutation TP53 who are 70 years or older, or younger patients with significant comorbidities, the AbbVie statement noted.
Imbruvica has been jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and by Janssen Biotech.