User login
More than one-third of Americans and > 20% of veterans have obesity with a body mass index (BMI) ≥ 30 kg/m2.1,2 It is well documented that patients with obesity have altered lipid metabolism, drug distribution, and drug clearance.3-5 As many as 8.2 million Americans may receive statin (3-hydroxymethylglutaryl coenzyme A reductase inhibitors) prescriptions if the American College of Cardiology/American Heart Association 2013 Cholesterol Guidelines are followed; therefore, it is important to examine how the efficacy of these drugs is altered in patients with obesity.6
Multiple studies have examined the benefits of statin therapy through lowering low-density lipoprotein cholesterol (LDL-C); however, few have examined the impact of obesity on statin efficacy. For example, only 18% of subjects in the Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) trial were classified as having obesity, and subjects in the Scandinavian Simvastatin Survival Study (4S) trial had a mean BMI of only 26 kg/m2.7,8 Though statins decreased mortality in both of these studies, it is unknown whether the lipid-lowering effects were the same for participants with and without obesity. The Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS) demonstrated a decrease in major cardiovascular events and all-cause mortality with atorvastatin 10 mg daily therapy in a sample where more than one-third of subjects had obesity.9 However, the mean baseline BMI of subjects in both study groups was only 28 kg/m2, and outcomes for those with and without obesity were not compared.9
Studies that have examined statin efficacy in those with and without obesity include the Heart Protection Study (HPS), a post hoc analysis of the West of Scotland Coronary Prevention Study (WOSCOPS), and a meta-analysis by Blassetto and colleagues. The HPS examined the event rate of vascular events with simvastatin 40 mg daily in patients with diabetes mellitus (DM).10 Though these subgroups were compared in HPS, no statistical difference was demonstrated between these groups for the rate of vascular events among those with and without DM.10 However, the obesity subgroup’s event rate ratios were consistently higher than were those for the nonobese group.10
A post hoc analysis of WOSCOPS examined obesity as a factor for change in LDL-C with pravastatin 40 mg therapy.11 Though the authors found that no significant difference was present between those with and those without obesity, the data supporting this claim were not disclosed, which makes drawing clinical conclusions from this analysis difficult.11 A meta-analysis by Blassetto and colleagues examined the association between rosuvastatin’s efficacy in lowering LDL-C among the subgroups of hypertension, atherosclerosis, type 2 DM, and obesity.12 Though these subgroups were not compared statistically, the obesity subgroup had the lowest mean percent change in lowering LDL-C. Moreover, patients without obesity were not examined as a subgroup.12
With the expected increase in statin therapy and a significant portion of the U.S. population having obesity, it is necessary to determine if obesity alters the efficacy of statins. This study was conducted to determine the effect of obesity on the percent change in LDL-C with statin therapy within a veteran population.
Methods
This study was a retrospective review examining follow-up data from January 1, 2009 to July 1, 2014 from the VA Midsouth Healthcare Network. This network services more than 350,000 patients each year in Tennessee, Kentucky, and West Virgin
Patients were excluded if they had received treatment for hyperlipidemia (niacin, colestyramine, colestipol, colesevelam, other statins, gemfibrozil, fenofibrate, omega-3 ethyl esters, ezetimibe) during the 6 weeks prior to the initial fill date of the statin prescription. Patients whose simvastatin therapy did not span the follow-up period from the time of filling to the follow-up lipid panel were excluded, as were those who had not filled a simvastatin prescription within 30 days of their baseline lipid panel. Also excluded were patients who were newly established at the VA, pregnant, or receiving concomitant antihyperlipidemia agents, dialysis, or interacting medications (tacrolimus, cyclosporine, atazanavir, darunavir, nelfinavir, saquinavir, ritonavir, indinavir, lopinavir, tipranavir, fosamprenavir, fluconazole, voriconazole, itraconazole, voriconazole, posaconazole, amiodarone, or colchicine). Patients with a BMI < 18 kg/m2, hepatic failure as measured by an aspartate transaminase/alanine transaminase (AST/ALT) ratio > 3 times the upper limit of normal, hepatitis, a history of alcoholism, any change in statin dose prior to follow-up cholesterol values, or no follow-up LDL-C values also were excluded.
The baseline data collected included age, sex, weight, height, BMI, hemoglobin A1c, LDL-C, ALT/AST, and serum creatinine (SCr). All other laboratory results were required to be within 270 days of the time the lipid panel was obtained. The index date was set as the date the initial prescription was filled between February 1, 2009 and April 1, 2014. Follow-up levels for LDL-C were obtained 40 to 95 days after the index date. Direct LDL-C values were preferred unless only calculated values were available. Calculated LDL-C values were determined by using the Friedewald equation. An audit of 150 patient charts was conducted to ensure the integrity of data pulled from the database.
The percent changes in LDL-C were calculated for those with and without obesity for both simvastatin 20 mg daily and simvastatin 40 mg daily. The primary outcome was the percent change in LDL-C from baseline. All laboratory values were compared using independent 2-tailed t tests with α set to .05. To have an 80% chance of detecting a 5% difference in percent change in LDL-C between the experimental and control groups, 129 patients were required. To determine whether an association was present, a correlation between BMI and percent change in LDL-C was conducted. All statistics were conducted using SAS software (Cary, North Carolina).
Results
From January 2009 through July 2014, 35,216 patients were initially screened. The majority of patients did not have a baseline LDL-C value and were excluded. A total of 1,183 patients with simvastatin 20 mg daily (BMI < 30 = 661; BMI ≥ 30 = 1,122) and 478 patients with simvastatin 40 mg daily (BMI < 30 = 259; BMI ≥ 30 = 219) met the inclusion criteria.
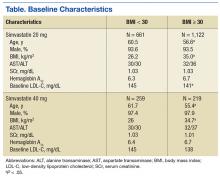
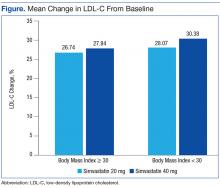
Baseline characteristics were similar between groups except for a slightly higher age in both groups without obesity (Table). Hepatic and renal serum markers indicated a baseline of adequate organ function for drug clearance for all groups. The mean baseline BMI of those without obesity was about 26 kg/m2, which is considered overweight. Baseline LDL-C values were clinically similar for those with and without obesity, though statistically different (145 mg/dL for the nonobese group and 141 mg/dL for the obese group, P < .05). The percent change in LDL-C was not statistically significant for those with and without obesity for simvastatin 20 mg daily (P = .293) or simvastatin 40 mg daily (P = .2773) (Figure). No correlation was found between the continuous percent change in LDL-C and continuous BMI for either simvastatin dosage (r2 = 0.0016 and 0.0028, respectively).
Discussion
In this retrospective chart review, it was determined that obesity did not affect the percent change in LDL-C from baseline with statin therapy. The HPS found similar results as a secondary endpoint, although that study was underpowered.10 In this study, all groups met power, and there was still no difference between those with and without obesity.
Nicholls and colleagues examined REVERSAL study data to determine whether BMI greater than the median BMI impacted inflammatory markers or lipid levels with atorvastatin 80 mg daily or pravastatin 40 mg daily. The REVERSAL study authors found no difference in percent change LDL-C between those above the median BMI compared with those below the median BMI for patients on pravastatin therapy. However, the authors did find a difference in percent change LDL-C with atorvastatin therapy.13 No difference in percent change LDL-C was present with simvastatin therapy in this study. As simvastatin is more lipophilic than is atorvastatin, lipophilicity remains an area for further study for statin therapy in patients with obesity.
The surrogate marker of percent change in LDL-C was used for the primary outcome in this study. The ACC/AHA 2013 guidelines and the National Lipid Association 2014 guidelines recommend an alternative goal of 30% to 50% change in LDL-C from baseline.14,15 Using this clinically relevant marker compensated for differences in baseline LDL-C and limited the effect of these differences on the primary outcome of this study.
Limitations
This study did not include patients who were underweight (BMI < 18 kg/m2), as these patients have previously demonstrated decreased outcomes with statin therapy.16 However, this limits these data to only those patients that have a BMI of at least 18 kg/m2. Limitations of this study also included the inability to consider adherence and lifestyle changes. These limitations were unavoidable due to the nature of a retrospective chart review.
Conclusion
The prevalence of obesity is increasing, and it is a disease that alters pharmacokinetics and lipid metabolism. Though this study did not find a difference between the LDL-C-lowering efficacy of simvastatin in those with and without obesity, continued study of the effect of obesity on the efficacy of medications is vital.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the James H. Qullen VAMC in Mountain Home, Tennessee.
1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806-814.
2. Shen Y, Sambamoorthi U, Rajan M, Miller D, Banerjea R, Pogach L. Obesity and expenditures among elderly Veterans Health Administration users with diabetes. Popul Health Manag. 2009;12(5):255-264.
3. Chan DC, Watts GF, Wang J, Hegele RA, van Bockxmeer FM, Barrett PH. Variation in Niemann-Pick C1-like 1 gene as a determinant of apolipoprotein B-100 kinetics and response to statin therapy in centrally obese men. Clin Endocrinol (Oxf). 2008;69(1):45-51.
4. Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39(3):215-231.
5. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71-87
6. Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370(15):1422-1431.
7. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339(19):1349-1357.
8. Pedersen TR, Kjekshus J, Berg K, et al; Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl. 2004;5(3):81-87.
9. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685-696.
10. Collins R, Armitage J, Parish S, Sleigh P, Peto R; Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005-2016.
11. Streja L, Packard CJ, Shepherd J, Cobbe S, Ford I; WOSCOPS Group. Factors affecting low-density lipoprotein and high-density lipoprotein cholesterol response to pravastatin in the West Of Scotland Coronary Prevention Study (WOSCOPS). Am J Cardiol. 2002;90(7):731-736.
12. Blasetto JW, Stein EA, Brown WV, Chitra R, Raza A. Efficacy of rosuvastatin compared with other statins at selected starting doses in hypercholesterolemic patients and in special population groups. Am J Cardiol. 2003;91(5A):3C-10C; discussion 10C.
13. Nicholls SJ. Tuzcu EM, Sipahi I, et al. Effect of obesity on lipid-lowering, anti-inflammatory, and antiatherosclerotic benefits of atorvastatin or pravastatin in patients with coronary artery disease (from the REVERSAL Study). Am J Cardiol. 2006;97(11):1553-1557.
14. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA Guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk on adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934.
15. Jacobson T, Ito M, Maki K, et al. National Lipid Association recommendation for patient-centered management of dyslipidemia: part 1-full report. J Clin Lipidol. 2015;9(2):129-169.
16. Nylén ES, Faselis C, Kheirbek R, Myers J, Panagiotakos D, Kokkinos P. Statins modulate the mortality risk associated with obesity and cardiorespiratory fitness in diabetics. J Clin Endocrinol Metab. 2013;98(8):33940-3401.
More than one-third of Americans and > 20% of veterans have obesity with a body mass index (BMI) ≥ 30 kg/m2.1,2 It is well documented that patients with obesity have altered lipid metabolism, drug distribution, and drug clearance.3-5 As many as 8.2 million Americans may receive statin (3-hydroxymethylglutaryl coenzyme A reductase inhibitors) prescriptions if the American College of Cardiology/American Heart Association 2013 Cholesterol Guidelines are followed; therefore, it is important to examine how the efficacy of these drugs is altered in patients with obesity.6
Multiple studies have examined the benefits of statin therapy through lowering low-density lipoprotein cholesterol (LDL-C); however, few have examined the impact of obesity on statin efficacy. For example, only 18% of subjects in the Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) trial were classified as having obesity, and subjects in the Scandinavian Simvastatin Survival Study (4S) trial had a mean BMI of only 26 kg/m2.7,8 Though statins decreased mortality in both of these studies, it is unknown whether the lipid-lowering effects were the same for participants with and without obesity. The Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS) demonstrated a decrease in major cardiovascular events and all-cause mortality with atorvastatin 10 mg daily therapy in a sample where more than one-third of subjects had obesity.9 However, the mean baseline BMI of subjects in both study groups was only 28 kg/m2, and outcomes for those with and without obesity were not compared.9
Studies that have examined statin efficacy in those with and without obesity include the Heart Protection Study (HPS), a post hoc analysis of the West of Scotland Coronary Prevention Study (WOSCOPS), and a meta-analysis by Blassetto and colleagues. The HPS examined the event rate of vascular events with simvastatin 40 mg daily in patients with diabetes mellitus (DM).10 Though these subgroups were compared in HPS, no statistical difference was demonstrated between these groups for the rate of vascular events among those with and without DM.10 However, the obesity subgroup’s event rate ratios were consistently higher than were those for the nonobese group.10
A post hoc analysis of WOSCOPS examined obesity as a factor for change in LDL-C with pravastatin 40 mg therapy.11 Though the authors found that no significant difference was present between those with and those without obesity, the data supporting this claim were not disclosed, which makes drawing clinical conclusions from this analysis difficult.11 A meta-analysis by Blassetto and colleagues examined the association between rosuvastatin’s efficacy in lowering LDL-C among the subgroups of hypertension, atherosclerosis, type 2 DM, and obesity.12 Though these subgroups were not compared statistically, the obesity subgroup had the lowest mean percent change in lowering LDL-C. Moreover, patients without obesity were not examined as a subgroup.12
With the expected increase in statin therapy and a significant portion of the U.S. population having obesity, it is necessary to determine if obesity alters the efficacy of statins. This study was conducted to determine the effect of obesity on the percent change in LDL-C with statin therapy within a veteran population.
Methods
This study was a retrospective review examining follow-up data from January 1, 2009 to July 1, 2014 from the VA Midsouth Healthcare Network. This network services more than 350,000 patients each year in Tennessee, Kentucky, and West Virgin
Patients were excluded if they had received treatment for hyperlipidemia (niacin, colestyramine, colestipol, colesevelam, other statins, gemfibrozil, fenofibrate, omega-3 ethyl esters, ezetimibe) during the 6 weeks prior to the initial fill date of the statin prescription. Patients whose simvastatin therapy did not span the follow-up period from the time of filling to the follow-up lipid panel were excluded, as were those who had not filled a simvastatin prescription within 30 days of their baseline lipid panel. Also excluded were patients who were newly established at the VA, pregnant, or receiving concomitant antihyperlipidemia agents, dialysis, or interacting medications (tacrolimus, cyclosporine, atazanavir, darunavir, nelfinavir, saquinavir, ritonavir, indinavir, lopinavir, tipranavir, fosamprenavir, fluconazole, voriconazole, itraconazole, voriconazole, posaconazole, amiodarone, or colchicine). Patients with a BMI < 18 kg/m2, hepatic failure as measured by an aspartate transaminase/alanine transaminase (AST/ALT) ratio > 3 times the upper limit of normal, hepatitis, a history of alcoholism, any change in statin dose prior to follow-up cholesterol values, or no follow-up LDL-C values also were excluded.
The baseline data collected included age, sex, weight, height, BMI, hemoglobin A1c, LDL-C, ALT/AST, and serum creatinine (SCr). All other laboratory results were required to be within 270 days of the time the lipid panel was obtained. The index date was set as the date the initial prescription was filled between February 1, 2009 and April 1, 2014. Follow-up levels for LDL-C were obtained 40 to 95 days after the index date. Direct LDL-C values were preferred unless only calculated values were available. Calculated LDL-C values were determined by using the Friedewald equation. An audit of 150 patient charts was conducted to ensure the integrity of data pulled from the database.
The percent changes in LDL-C were calculated for those with and without obesity for both simvastatin 20 mg daily and simvastatin 40 mg daily. The primary outcome was the percent change in LDL-C from baseline. All laboratory values were compared using independent 2-tailed t tests with α set to .05. To have an 80% chance of detecting a 5% difference in percent change in LDL-C between the experimental and control groups, 129 patients were required. To determine whether an association was present, a correlation between BMI and percent change in LDL-C was conducted. All statistics were conducted using SAS software (Cary, North Carolina).
Results
From January 2009 through July 2014, 35,216 patients were initially screened. The majority of patients did not have a baseline LDL-C value and were excluded. A total of 1,183 patients with simvastatin 20 mg daily (BMI < 30 = 661; BMI ≥ 30 = 1,122) and 478 patients with simvastatin 40 mg daily (BMI < 30 = 259; BMI ≥ 30 = 219) met the inclusion criteria.
Baseline characteristics were similar between groups except for a slightly higher age in both groups without obesity (Table). Hepatic and renal serum markers indicated a baseline of adequate organ function for drug clearance for all groups. The mean baseline BMI of those without obesity was about 26 kg/m2, which is considered overweight. Baseline LDL-C values were clinically similar for those with and without obesity, though statistically different (145 mg/dL for the nonobese group and 141 mg/dL for the obese group, P < .05). The percent change in LDL-C was not statistically significant for those with and without obesity for simvastatin 20 mg daily (P = .293) or simvastatin 40 mg daily (P = .2773) (Figure). No correlation was found between the continuous percent change in LDL-C and continuous BMI for either simvastatin dosage (r2 = 0.0016 and 0.0028, respectively).
Discussion
In this retrospective chart review, it was determined that obesity did not affect the percent change in LDL-C from baseline with statin therapy. The HPS found similar results as a secondary endpoint, although that study was underpowered.10 In this study, all groups met power, and there was still no difference between those with and without obesity.
Nicholls and colleagues examined REVERSAL study data to determine whether BMI greater than the median BMI impacted inflammatory markers or lipid levels with atorvastatin 80 mg daily or pravastatin 40 mg daily. The REVERSAL study authors found no difference in percent change LDL-C between those above the median BMI compared with those below the median BMI for patients on pravastatin therapy. However, the authors did find a difference in percent change LDL-C with atorvastatin therapy.13 No difference in percent change LDL-C was present with simvastatin therapy in this study. As simvastatin is more lipophilic than is atorvastatin, lipophilicity remains an area for further study for statin therapy in patients with obesity.
The surrogate marker of percent change in LDL-C was used for the primary outcome in this study. The ACC/AHA 2013 guidelines and the National Lipid Association 2014 guidelines recommend an alternative goal of 30% to 50% change in LDL-C from baseline.14,15 Using this clinically relevant marker compensated for differences in baseline LDL-C and limited the effect of these differences on the primary outcome of this study.
Limitations
This study did not include patients who were underweight (BMI < 18 kg/m2), as these patients have previously demonstrated decreased outcomes with statin therapy.16 However, this limits these data to only those patients that have a BMI of at least 18 kg/m2. Limitations of this study also included the inability to consider adherence and lifestyle changes. These limitations were unavoidable due to the nature of a retrospective chart review.
Conclusion
The prevalence of obesity is increasing, and it is a disease that alters pharmacokinetics and lipid metabolism. Though this study did not find a difference between the LDL-C-lowering efficacy of simvastatin in those with and without obesity, continued study of the effect of obesity on the efficacy of medications is vital.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the James H. Qullen VAMC in Mountain Home, Tennessee.
More than one-third of Americans and > 20% of veterans have obesity with a body mass index (BMI) ≥ 30 kg/m2.1,2 It is well documented that patients with obesity have altered lipid metabolism, drug distribution, and drug clearance.3-5 As many as 8.2 million Americans may receive statin (3-hydroxymethylglutaryl coenzyme A reductase inhibitors) prescriptions if the American College of Cardiology/American Heart Association 2013 Cholesterol Guidelines are followed; therefore, it is important to examine how the efficacy of these drugs is altered in patients with obesity.6
Multiple studies have examined the benefits of statin therapy through lowering low-density lipoprotein cholesterol (LDL-C); however, few have examined the impact of obesity on statin efficacy. For example, only 18% of subjects in the Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) trial were classified as having obesity, and subjects in the Scandinavian Simvastatin Survival Study (4S) trial had a mean BMI of only 26 kg/m2.7,8 Though statins decreased mortality in both of these studies, it is unknown whether the lipid-lowering effects were the same for participants with and without obesity. The Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS) demonstrated a decrease in major cardiovascular events and all-cause mortality with atorvastatin 10 mg daily therapy in a sample where more than one-third of subjects had obesity.9 However, the mean baseline BMI of subjects in both study groups was only 28 kg/m2, and outcomes for those with and without obesity were not compared.9
Studies that have examined statin efficacy in those with and without obesity include the Heart Protection Study (HPS), a post hoc analysis of the West of Scotland Coronary Prevention Study (WOSCOPS), and a meta-analysis by Blassetto and colleagues. The HPS examined the event rate of vascular events with simvastatin 40 mg daily in patients with diabetes mellitus (DM).10 Though these subgroups were compared in HPS, no statistical difference was demonstrated between these groups for the rate of vascular events among those with and without DM.10 However, the obesity subgroup’s event rate ratios were consistently higher than were those for the nonobese group.10
A post hoc analysis of WOSCOPS examined obesity as a factor for change in LDL-C with pravastatin 40 mg therapy.11 Though the authors found that no significant difference was present between those with and those without obesity, the data supporting this claim were not disclosed, which makes drawing clinical conclusions from this analysis difficult.11 A meta-analysis by Blassetto and colleagues examined the association between rosuvastatin’s efficacy in lowering LDL-C among the subgroups of hypertension, atherosclerosis, type 2 DM, and obesity.12 Though these subgroups were not compared statistically, the obesity subgroup had the lowest mean percent change in lowering LDL-C. Moreover, patients without obesity were not examined as a subgroup.12
With the expected increase in statin therapy and a significant portion of the U.S. population having obesity, it is necessary to determine if obesity alters the efficacy of statins. This study was conducted to determine the effect of obesity on the percent change in LDL-C with statin therapy within a veteran population.
Methods
This study was a retrospective review examining follow-up data from January 1, 2009 to July 1, 2014 from the VA Midsouth Healthcare Network. This network services more than 350,000 patients each year in Tennessee, Kentucky, and West Virgin
Patients were excluded if they had received treatment for hyperlipidemia (niacin, colestyramine, colestipol, colesevelam, other statins, gemfibrozil, fenofibrate, omega-3 ethyl esters, ezetimibe) during the 6 weeks prior to the initial fill date of the statin prescription. Patients whose simvastatin therapy did not span the follow-up period from the time of filling to the follow-up lipid panel were excluded, as were those who had not filled a simvastatin prescription within 30 days of their baseline lipid panel. Also excluded were patients who were newly established at the VA, pregnant, or receiving concomitant antihyperlipidemia agents, dialysis, or interacting medications (tacrolimus, cyclosporine, atazanavir, darunavir, nelfinavir, saquinavir, ritonavir, indinavir, lopinavir, tipranavir, fosamprenavir, fluconazole, voriconazole, itraconazole, voriconazole, posaconazole, amiodarone, or colchicine). Patients with a BMI < 18 kg/m2, hepatic failure as measured by an aspartate transaminase/alanine transaminase (AST/ALT) ratio > 3 times the upper limit of normal, hepatitis, a history of alcoholism, any change in statin dose prior to follow-up cholesterol values, or no follow-up LDL-C values also were excluded.
The baseline data collected included age, sex, weight, height, BMI, hemoglobin A1c, LDL-C, ALT/AST, and serum creatinine (SCr). All other laboratory results were required to be within 270 days of the time the lipid panel was obtained. The index date was set as the date the initial prescription was filled between February 1, 2009 and April 1, 2014. Follow-up levels for LDL-C were obtained 40 to 95 days after the index date. Direct LDL-C values were preferred unless only calculated values were available. Calculated LDL-C values were determined by using the Friedewald equation. An audit of 150 patient charts was conducted to ensure the integrity of data pulled from the database.
The percent changes in LDL-C were calculated for those with and without obesity for both simvastatin 20 mg daily and simvastatin 40 mg daily. The primary outcome was the percent change in LDL-C from baseline. All laboratory values were compared using independent 2-tailed t tests with α set to .05. To have an 80% chance of detecting a 5% difference in percent change in LDL-C between the experimental and control groups, 129 patients were required. To determine whether an association was present, a correlation between BMI and percent change in LDL-C was conducted. All statistics were conducted using SAS software (Cary, North Carolina).
Results
From January 2009 through July 2014, 35,216 patients were initially screened. The majority of patients did not have a baseline LDL-C value and were excluded. A total of 1,183 patients with simvastatin 20 mg daily (BMI < 30 = 661; BMI ≥ 30 = 1,122) and 478 patients with simvastatin 40 mg daily (BMI < 30 = 259; BMI ≥ 30 = 219) met the inclusion criteria.
Baseline characteristics were similar between groups except for a slightly higher age in both groups without obesity (Table). Hepatic and renal serum markers indicated a baseline of adequate organ function for drug clearance for all groups. The mean baseline BMI of those without obesity was about 26 kg/m2, which is considered overweight. Baseline LDL-C values were clinically similar for those with and without obesity, though statistically different (145 mg/dL for the nonobese group and 141 mg/dL for the obese group, P < .05). The percent change in LDL-C was not statistically significant for those with and without obesity for simvastatin 20 mg daily (P = .293) or simvastatin 40 mg daily (P = .2773) (Figure). No correlation was found between the continuous percent change in LDL-C and continuous BMI for either simvastatin dosage (r2 = 0.0016 and 0.0028, respectively).
Discussion
In this retrospective chart review, it was determined that obesity did not affect the percent change in LDL-C from baseline with statin therapy. The HPS found similar results as a secondary endpoint, although that study was underpowered.10 In this study, all groups met power, and there was still no difference between those with and without obesity.
Nicholls and colleagues examined REVERSAL study data to determine whether BMI greater than the median BMI impacted inflammatory markers or lipid levels with atorvastatin 80 mg daily or pravastatin 40 mg daily. The REVERSAL study authors found no difference in percent change LDL-C between those above the median BMI compared with those below the median BMI for patients on pravastatin therapy. However, the authors did find a difference in percent change LDL-C with atorvastatin therapy.13 No difference in percent change LDL-C was present with simvastatin therapy in this study. As simvastatin is more lipophilic than is atorvastatin, lipophilicity remains an area for further study for statin therapy in patients with obesity.
The surrogate marker of percent change in LDL-C was used for the primary outcome in this study. The ACC/AHA 2013 guidelines and the National Lipid Association 2014 guidelines recommend an alternative goal of 30% to 50% change in LDL-C from baseline.14,15 Using this clinically relevant marker compensated for differences in baseline LDL-C and limited the effect of these differences on the primary outcome of this study.
Limitations
This study did not include patients who were underweight (BMI < 18 kg/m2), as these patients have previously demonstrated decreased outcomes with statin therapy.16 However, this limits these data to only those patients that have a BMI of at least 18 kg/m2. Limitations of this study also included the inability to consider adherence and lifestyle changes. These limitations were unavoidable due to the nature of a retrospective chart review.
Conclusion
The prevalence of obesity is increasing, and it is a disease that alters pharmacokinetics and lipid metabolism. Though this study did not find a difference between the LDL-C-lowering efficacy of simvastatin in those with and without obesity, continued study of the effect of obesity on the efficacy of medications is vital.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the James H. Qullen VAMC in Mountain Home, Tennessee.
1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806-814.
2. Shen Y, Sambamoorthi U, Rajan M, Miller D, Banerjea R, Pogach L. Obesity and expenditures among elderly Veterans Health Administration users with diabetes. Popul Health Manag. 2009;12(5):255-264.
3. Chan DC, Watts GF, Wang J, Hegele RA, van Bockxmeer FM, Barrett PH. Variation in Niemann-Pick C1-like 1 gene as a determinant of apolipoprotein B-100 kinetics and response to statin therapy in centrally obese men. Clin Endocrinol (Oxf). 2008;69(1):45-51.
4. Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39(3):215-231.
5. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71-87
6. Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370(15):1422-1431.
7. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339(19):1349-1357.
8. Pedersen TR, Kjekshus J, Berg K, et al; Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl. 2004;5(3):81-87.
9. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685-696.
10. Collins R, Armitage J, Parish S, Sleigh P, Peto R; Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005-2016.
11. Streja L, Packard CJ, Shepherd J, Cobbe S, Ford I; WOSCOPS Group. Factors affecting low-density lipoprotein and high-density lipoprotein cholesterol response to pravastatin in the West Of Scotland Coronary Prevention Study (WOSCOPS). Am J Cardiol. 2002;90(7):731-736.
12. Blasetto JW, Stein EA, Brown WV, Chitra R, Raza A. Efficacy of rosuvastatin compared with other statins at selected starting doses in hypercholesterolemic patients and in special population groups. Am J Cardiol. 2003;91(5A):3C-10C; discussion 10C.
13. Nicholls SJ. Tuzcu EM, Sipahi I, et al. Effect of obesity on lipid-lowering, anti-inflammatory, and antiatherosclerotic benefits of atorvastatin or pravastatin in patients with coronary artery disease (from the REVERSAL Study). Am J Cardiol. 2006;97(11):1553-1557.
14. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA Guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk on adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934.
15. Jacobson T, Ito M, Maki K, et al. National Lipid Association recommendation for patient-centered management of dyslipidemia: part 1-full report. J Clin Lipidol. 2015;9(2):129-169.
16. Nylén ES, Faselis C, Kheirbek R, Myers J, Panagiotakos D, Kokkinos P. Statins modulate the mortality risk associated with obesity and cardiorespiratory fitness in diabetics. J Clin Endocrinol Metab. 2013;98(8):33940-3401.
1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806-814.
2. Shen Y, Sambamoorthi U, Rajan M, Miller D, Banerjea R, Pogach L. Obesity and expenditures among elderly Veterans Health Administration users with diabetes. Popul Health Manag. 2009;12(5):255-264.
3. Chan DC, Watts GF, Wang J, Hegele RA, van Bockxmeer FM, Barrett PH. Variation in Niemann-Pick C1-like 1 gene as a determinant of apolipoprotein B-100 kinetics and response to statin therapy in centrally obese men. Clin Endocrinol (Oxf). 2008;69(1):45-51.
4. Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39(3):215-231.
5. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71-87
6. Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370(15):1422-1431.
7. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339(19):1349-1357.
8. Pedersen TR, Kjekshus J, Berg K, et al; Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl. 2004;5(3):81-87.
9. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685-696.
10. Collins R, Armitage J, Parish S, Sleigh P, Peto R; Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005-2016.
11. Streja L, Packard CJ, Shepherd J, Cobbe S, Ford I; WOSCOPS Group. Factors affecting low-density lipoprotein and high-density lipoprotein cholesterol response to pravastatin in the West Of Scotland Coronary Prevention Study (WOSCOPS). Am J Cardiol. 2002;90(7):731-736.
12. Blasetto JW, Stein EA, Brown WV, Chitra R, Raza A. Efficacy of rosuvastatin compared with other statins at selected starting doses in hypercholesterolemic patients and in special population groups. Am J Cardiol. 2003;91(5A):3C-10C; discussion 10C.
13. Nicholls SJ. Tuzcu EM, Sipahi I, et al. Effect of obesity on lipid-lowering, anti-inflammatory, and antiatherosclerotic benefits of atorvastatin or pravastatin in patients with coronary artery disease (from the REVERSAL Study). Am J Cardiol. 2006;97(11):1553-1557.
14. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA Guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk on adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934.
15. Jacobson T, Ito M, Maki K, et al. National Lipid Association recommendation for patient-centered management of dyslipidemia: part 1-full report. J Clin Lipidol. 2015;9(2):129-169.
16. Nylén ES, Faselis C, Kheirbek R, Myers J, Panagiotakos D, Kokkinos P. Statins modulate the mortality risk associated with obesity and cardiorespiratory fitness in diabetics. J Clin Endocrinol Metab. 2013;98(8):33940-3401.