User login
Due to the growing number of drug shortages and increasing cost of medications, large-scale formulary conversions are becoming more common and necessary for pharmacies to stay within their budget allocations. Statins are widely recognized as first-line therapy for cholesterol lowering and have been proven to reduce cardiovascular morbidity and mortality.1-5 In addition to statin therapy, weight loss and lifestyle changes are often necessary to meet optimum low-density lipoprotein cholesterol (LDL-C) goals.6 According to the Adult Treatment Panel III (ATP III) guidelines, the optimum LDL-C for each patient varies based on the presence of coronary artery disease (CAD), CAD risk equivalents, and other risk factors.7 Patients with CAD or CAD risk equivalents have an LDL-C goal of < 100 mg/dL. Those with multiple risk factors have an LDL-C goal of < 130 mg/dL, and those with 0 to 1 risk factors have an LDL-C goal of < 160 mg/dL.1-3,7,8
Numerous trials have compared the safety and efficacy of the 3-hydroxy-3-methylglutaryl-coenzyme A inhibitors, stating that rosuvastatin 5 to 10 mg per day is equivalent to 20 mg per day of atorvastatin in terms of its ability to lower LDL-C levels.7,9-14 The LUNAR (Limiting Under treatment of lipids in Acute coronary syndrome with Rosuvastatin) study compared the efficacy of rosuvastatin with that of atorvastatin in decreasing LDL-C in patients with acute coronary syndrome.8 Rosuvastatin 40 mg was significantly more successful in lowering LDL-C and increasing high-density lipoprotein cholesterol (HDL-C) compared with atorvastatin 80-mg daily therapy.
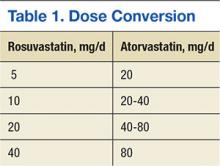
In October 2012, the national VA Pharmacy Benefits Management (PBM) Services released guidance regarding the conversion from rosuvastatin to atorvastatin, including the dosing conversion (Table 1), stating that rosuvastatin 5 mg daily should be considered equivalent and converted to atorvastatin 20 mg daily. In general, adverse events (AEs), such as increased liver enzymes, myopathies, and increased creatinine phosphokinase (CPK), are considered a class effect of the statins.13,14
Related: Statins showed no benefit in reducing risk of recurrent VTE
The recent 2013 American College of Cardiology/American Heart Association (ACC/AHA) Blood Cholesterol Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults has identified a large number of patients as candidates for high-intensity statins, which the authors defined as atorvastatin and rosuvastatin.4 These guidelines do not recommend LDL-C goals and instead use a risk calculator to determine which intensity of statin therapy is appropriate for certain patients. This change in practice will lead to a higher volume of high-intensity statin prescriptions and higher drug costs for some medical centers. Given the volume of prescriptions and the increased use of large-scale formulary conversions to reduce costs, more research is warranted to ensure equivalent dosing. With more data available and equivalent dosing defined, pharmacists may be better able to improve clinical results in patients that are included in these large-scale formulary conversions.
Methods
A retrospective chart review was performed on all LDL-C levels in patients receiving atorvastatin therapy due to the formulary conversion from rosuvastatin to atorvastatin at the Huntington VAMC (HVAMC) in Huntington, West Virginia, per the guidance published by the national VA PBM Services. The number of patients not at their LDL-C goal (as defined by ATP III guidelines) as a result of atorvastatin therapy was determined. Furthermore, AEs due to atorvastatin therapy such as increased liver enzymes, myopathy, and increased CPK were identified and analyzed.
The primary endpoint of this study focused on the rate of treatment failure in LDL-C reduction with atorvastatin treatment in patients previously at their LDL-C goal, as defined by the ATP III guidelines, with rosuvastatin therapy. Secondary endpoints included rate of AEs due to atorvastatin therapy, percentage increase in CPK and liver enzymes as a result of atorvastatin therapy, and percentage LDL-C, HDL-C, total cholesterol (TC), and triglyceride (TGs) changes since conversion from rosuvastatin to atorvastatin.
Patients were included in the study if they were aged 18 to 89 years, previously at LDL-C goal with rosuvastatin therapy for at least 3 months, had never previously received atorvastatin, were converted to atorvastatin therapy as a result of a large-scale formulary conversion at HVAMC, and had a fasting lipid panel completed 1 to 6 months after conversion. Patients were excluded from the study if they received other lipid-lowering medications (eg, bile acid sequestrants, fibrates, niacin, or ezetimibe) in the 12 months before or after receiving statin therapy, had previously documented AEs (eg, myopathy, increased liver enzymes, increased CPK as a result of statin therapy, or history of known homozygous familial hypercholesterolemia) current active liver disease (ALT > 2x ULN [upper limit of normal]), unexplained CPK ≥ 3x ULN, serum creatinine (SCr) > 2 mg/dL, or history of alcohol or drug abuse within the last 5 years.
Results
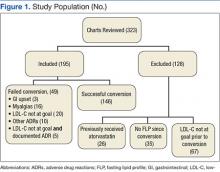
Three hundred twenty-three patients were identified and reviewed as converted from rosuvastatin to atorvastatin during the study period with no prior use of atorvastatin. Of the 323 charts that were reviewed, 195 patients met the study inclusion criteria and were analyzed for rate of treatment failure in terms of lipid goals and rate of AEs. Twenty of 195 patients (10.3%) were no longer at their LDL-C goal after conversion from rosuvastatin to atorvastatin. Of those 195 patients, 29 (14.9%) experienced an adverse drug reaction (ADR) as a result of atorvastatin treatment that was severe enough to result in discontinuation of the drug and switching the patient back to the originally prescribed dose of rosuvastatin. Figure 1 illustrates the number of patients and documented atorvastatin ADRs. The most common ADR documented to atorvastatin was myalgias (8.2%).
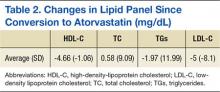
The average change in lipid levels was calculated and atorvastatin therapy was found to result in clinically insignificant changes to the lipid panel (Table 2). A 2-tailed paired t test was used to assess the statistical significance of these changes. Atorvastatin therapy resulted in an average decrease of LDL-C by 5.0 mg/dL (P < .01) in comparison to previous therapy with equivalent rosuvastatin dose. Other noted changes to lipid profile after formulary conversion included TG reduction by 2 mg/dL (P = .69), TC increased by 0.58 mg/dL (P = .80), and HDL-C reduction by 4.66 mg/dL (P < .01).
Although the decrease in LDL-C and HDL-C as a result of the formulary change was found to be statistically significant, they are not thought to result in a clinical difference. Clinically and statistically insignificant changes in liver enzymes and CPK were also discovered as a result of atorvastatin therapy conversion (Table 3). Atorvastatin therapy resulted in an averaged decrease of aspartate aminotransferase by 2.2 IU/L (P = .19) and an increase in alanine aminotransferase by 1.4 IU/L (P = .47). Average change in CPK was -6 IU/L (P = 89).
Related: New Guideline on Dyslipidemia: Less Is More
Discussion
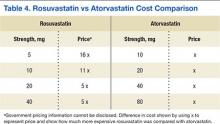
Lipid levels were found to be mostly unchanged and remained at therapy goals, demonstrating use and appropriate equivalent dosing of rosuvastatin and atorvastatin following the formulary conversion defined in Table 1. Documented ADRs were minimal, indicating the ingredient conversion was well tolerated overall by our patients. Following the release of the 2013ACC/AHA guidelines, many patients in the VA required treatment with high-potency statins such as rosuvastatin and atorvastatin. Given the volume of statin prescriptions in the VA and the significant potential for providing the most cost-efficient lipid therapy (Table 4), the formulary conversion from rosuvastatin to atorvastatin was warranted.
Limitations
There are several limitations for extrapolating results from this study to the general population. Due to the retrospective design of the study, no formal assessment of adherence was conducted. A prospective trial, with a researcher monitoring refills and tablet counts would be more accurate to ensure patients adherence with statin therapy.
Many of the patients that were included in the formulary conversion did not have follow-up laboratory work at the time of this study and were therefore excluded, leading to a smaller study population. A larger study population with a similar study design may be able to detect more significant correlations.
There are also several potential complications in regard to formulary conversions, including the inability to ensure the exact period that the patient switched therapies. During the conversion of rosuvastatin to atorvastatin at HVAMC, an attempt was made to minimize this confounder by converting patients on request for a refill of their rosuvastatin therapy. Last, all 185 patients that were studied were male; therefore, it is difficult to extrapolate these results for female patients.
Conclusions
This study shows that the conversion of patients from rosuvastatin therapy to atorvastatin was effective when it targeted a specific LDL-C goal or specific reduction in LDL-C. A similar conversion would likely lead to lower drug costs for many other health systems. Additional studies will be necessary given the recent changes in the national lipid guidelines. Furthermore, studies will be needed to assess concrete clinical endpoints (cardiovascular mortality, all-cause mortality, and cardiac events).
Related: Poor Outcomes for African Americans in Cardiac Rehabilitation
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Krasuski RA, Doeppenschmidt D, Henry JS, et al. Conversion to atorvastatin in patients intolerant or refractory to simvastatin therapy: the CAPISH study. Mayo Clin Proc. 2005;80(9):1163-1168.
2. Clearfield MB, Amerena J, Bassand JP, et al. Comparison of the efficacy and safety of rosuvastatin 10 mg and atorvastatin 20 mg in high-risk patients with hypercholesterolemia--Prospective study to evaluate the Use of Low doses of the Statins Atorvastatin and Rosuvastatin (PULSAR). Trials. 2006;7:35.
3. Park JS, Kim YJ, Choi JY, et al. Comparative study of low doses of rosuvastatin and atorvastatin on lipid and glycemic control in patients with metabolic syndrome and hypercholesterolemia. Korean J Intern Med. 2010;25(1):27-35.
4. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934.
5. Schuster H, Barter PJ, Stender S, et al; Effective Reductions in Cholesterol Using Rosuvastatin Therapy I study group. Effects of switching statin on achievement of lipid goals: Measuring Effective Reductions in Cholesterol Using Rosuvastatin Therapy (MERCURY I) study. Am Heart J. 2004;147(4):705-713.
6. Ballantyne CM, Bertolami M, Garcia HR, et al. Achieving LDL cholesterol, non-HDL cholesterol and apolipoprotein B target levels in high-risk patients: Measuring effective Reductions in Cholesterol Using Rosuvastatin therapY (MERCURY) II. Am Heart J. 2006;151(5):975.e1-975.e9.
7. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
8. Pitt B, Loscalzo J, Monyak J, Miller E, Raichlen J. Comparison of lipid-modifying efficacy of rosuvastatin versus atorvastatin in patients with acute coronary syndrome (from the LUNAR study). Am J Cardiol. 2012;109(9);1239-1246.
9. Takagi H, Niwa M, Mizuno Y, Yamamoto H, Goto SN, Umemoto T. Effects of rosuvastatin versus atorvastatin on small dense low-density lipoprotein: a meta-analysis of randomized trials. Heart Vessels. 2014;29(3);287-299.
10. Fox KM, Gandhi SK, Ohsfeldt RL, Davidson MH. Comparison of low-density lipoprotein cholesterol reduction after switching patients on other statins to rosuvastatin or simvastatin in a real-world clinical practice setting. Am J Manag Care. 2007;13(suppl 10):S270-S275.
11. Taylor AJ, Grace K, Swiecki J, et al. Lipid-lowering efficacy, safety, and costs of a large-scale therapeutic statin formulary conversion program. Pharmacotherapy. 2001;21(9):1130-1139.
12. Bullano MF, Kamat S, Wertz DA, et al. Effectiveness of rosuvastatin versus atorvastatin in reducing lipid levels and achieving low-density-lipoprotein cholesterol goals in a usual care setting. Am J Health Syst Pharm. 2007;64(3):276-284.
13. Palmer MK, Nicholls SJ, Lundman P, Barter PJ, Karison BW. Achievement of LDL-C goals depends on baseline LDL-C and choice and dose of statin: an analysis from the VOYAGER database. Eur J Prev Cardiol. 2013;20(6):1080-1087.
14. Berne C, Siewert-Delle A; URANUS study investigators. Comparison of rosuvastatin and atorvastatin for lipid lowering in patients with type 2 diabetes mellitus: results from the URANUS study. Cardiovasc Diabetol. 2005;4:7.
Due to the growing number of drug shortages and increasing cost of medications, large-scale formulary conversions are becoming more common and necessary for pharmacies to stay within their budget allocations. Statins are widely recognized as first-line therapy for cholesterol lowering and have been proven to reduce cardiovascular morbidity and mortality.1-5 In addition to statin therapy, weight loss and lifestyle changes are often necessary to meet optimum low-density lipoprotein cholesterol (LDL-C) goals.6 According to the Adult Treatment Panel III (ATP III) guidelines, the optimum LDL-C for each patient varies based on the presence of coronary artery disease (CAD), CAD risk equivalents, and other risk factors.7 Patients with CAD or CAD risk equivalents have an LDL-C goal of < 100 mg/dL. Those with multiple risk factors have an LDL-C goal of < 130 mg/dL, and those with 0 to 1 risk factors have an LDL-C goal of < 160 mg/dL.1-3,7,8
Numerous trials have compared the safety and efficacy of the 3-hydroxy-3-methylglutaryl-coenzyme A inhibitors, stating that rosuvastatin 5 to 10 mg per day is equivalent to 20 mg per day of atorvastatin in terms of its ability to lower LDL-C levels.7,9-14 The LUNAR (Limiting Under treatment of lipids in Acute coronary syndrome with Rosuvastatin) study compared the efficacy of rosuvastatin with that of atorvastatin in decreasing LDL-C in patients with acute coronary syndrome.8 Rosuvastatin 40 mg was significantly more successful in lowering LDL-C and increasing high-density lipoprotein cholesterol (HDL-C) compared with atorvastatin 80-mg daily therapy.
In October 2012, the national VA Pharmacy Benefits Management (PBM) Services released guidance regarding the conversion from rosuvastatin to atorvastatin, including the dosing conversion (Table 1), stating that rosuvastatin 5 mg daily should be considered equivalent and converted to atorvastatin 20 mg daily. In general, adverse events (AEs), such as increased liver enzymes, myopathies, and increased creatinine phosphokinase (CPK), are considered a class effect of the statins.13,14
Related: Statins showed no benefit in reducing risk of recurrent VTE
The recent 2013 American College of Cardiology/American Heart Association (ACC/AHA) Blood Cholesterol Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults has identified a large number of patients as candidates for high-intensity statins, which the authors defined as atorvastatin and rosuvastatin.4 These guidelines do not recommend LDL-C goals and instead use a risk calculator to determine which intensity of statin therapy is appropriate for certain patients. This change in practice will lead to a higher volume of high-intensity statin prescriptions and higher drug costs for some medical centers. Given the volume of prescriptions and the increased use of large-scale formulary conversions to reduce costs, more research is warranted to ensure equivalent dosing. With more data available and equivalent dosing defined, pharmacists may be better able to improve clinical results in patients that are included in these large-scale formulary conversions.
Methods
A retrospective chart review was performed on all LDL-C levels in patients receiving atorvastatin therapy due to the formulary conversion from rosuvastatin to atorvastatin at the Huntington VAMC (HVAMC) in Huntington, West Virginia, per the guidance published by the national VA PBM Services. The number of patients not at their LDL-C goal (as defined by ATP III guidelines) as a result of atorvastatin therapy was determined. Furthermore, AEs due to atorvastatin therapy such as increased liver enzymes, myopathy, and increased CPK were identified and analyzed.
The primary endpoint of this study focused on the rate of treatment failure in LDL-C reduction with atorvastatin treatment in patients previously at their LDL-C goal, as defined by the ATP III guidelines, with rosuvastatin therapy. Secondary endpoints included rate of AEs due to atorvastatin therapy, percentage increase in CPK and liver enzymes as a result of atorvastatin therapy, and percentage LDL-C, HDL-C, total cholesterol (TC), and triglyceride (TGs) changes since conversion from rosuvastatin to atorvastatin.
Patients were included in the study if they were aged 18 to 89 years, previously at LDL-C goal with rosuvastatin therapy for at least 3 months, had never previously received atorvastatin, were converted to atorvastatin therapy as a result of a large-scale formulary conversion at HVAMC, and had a fasting lipid panel completed 1 to 6 months after conversion. Patients were excluded from the study if they received other lipid-lowering medications (eg, bile acid sequestrants, fibrates, niacin, or ezetimibe) in the 12 months before or after receiving statin therapy, had previously documented AEs (eg, myopathy, increased liver enzymes, increased CPK as a result of statin therapy, or history of known homozygous familial hypercholesterolemia) current active liver disease (ALT > 2x ULN [upper limit of normal]), unexplained CPK ≥ 3x ULN, serum creatinine (SCr) > 2 mg/dL, or history of alcohol or drug abuse within the last 5 years.
Results
Three hundred twenty-three patients were identified and reviewed as converted from rosuvastatin to atorvastatin during the study period with no prior use of atorvastatin. Of the 323 charts that were reviewed, 195 patients met the study inclusion criteria and were analyzed for rate of treatment failure in terms of lipid goals and rate of AEs. Twenty of 195 patients (10.3%) were no longer at their LDL-C goal after conversion from rosuvastatin to atorvastatin. Of those 195 patients, 29 (14.9%) experienced an adverse drug reaction (ADR) as a result of atorvastatin treatment that was severe enough to result in discontinuation of the drug and switching the patient back to the originally prescribed dose of rosuvastatin. Figure 1 illustrates the number of patients and documented atorvastatin ADRs. The most common ADR documented to atorvastatin was myalgias (8.2%).
The average change in lipid levels was calculated and atorvastatin therapy was found to result in clinically insignificant changes to the lipid panel (Table 2). A 2-tailed paired t test was used to assess the statistical significance of these changes. Atorvastatin therapy resulted in an average decrease of LDL-C by 5.0 mg/dL (P < .01) in comparison to previous therapy with equivalent rosuvastatin dose. Other noted changes to lipid profile after formulary conversion included TG reduction by 2 mg/dL (P = .69), TC increased by 0.58 mg/dL (P = .80), and HDL-C reduction by 4.66 mg/dL (P < .01).
Although the decrease in LDL-C and HDL-C as a result of the formulary change was found to be statistically significant, they are not thought to result in a clinical difference. Clinically and statistically insignificant changes in liver enzymes and CPK were also discovered as a result of atorvastatin therapy conversion (Table 3). Atorvastatin therapy resulted in an averaged decrease of aspartate aminotransferase by 2.2 IU/L (P = .19) and an increase in alanine aminotransferase by 1.4 IU/L (P = .47). Average change in CPK was -6 IU/L (P = 89).
Related: New Guideline on Dyslipidemia: Less Is More
Discussion
Lipid levels were found to be mostly unchanged and remained at therapy goals, demonstrating use and appropriate equivalent dosing of rosuvastatin and atorvastatin following the formulary conversion defined in Table 1. Documented ADRs were minimal, indicating the ingredient conversion was well tolerated overall by our patients. Following the release of the 2013ACC/AHA guidelines, many patients in the VA required treatment with high-potency statins such as rosuvastatin and atorvastatin. Given the volume of statin prescriptions in the VA and the significant potential for providing the most cost-efficient lipid therapy (Table 4), the formulary conversion from rosuvastatin to atorvastatin was warranted.
Limitations
There are several limitations for extrapolating results from this study to the general population. Due to the retrospective design of the study, no formal assessment of adherence was conducted. A prospective trial, with a researcher monitoring refills and tablet counts would be more accurate to ensure patients adherence with statin therapy.
Many of the patients that were included in the formulary conversion did not have follow-up laboratory work at the time of this study and were therefore excluded, leading to a smaller study population. A larger study population with a similar study design may be able to detect more significant correlations.
There are also several potential complications in regard to formulary conversions, including the inability to ensure the exact period that the patient switched therapies. During the conversion of rosuvastatin to atorvastatin at HVAMC, an attempt was made to minimize this confounder by converting patients on request for a refill of their rosuvastatin therapy. Last, all 185 patients that were studied were male; therefore, it is difficult to extrapolate these results for female patients.
Conclusions
This study shows that the conversion of patients from rosuvastatin therapy to atorvastatin was effective when it targeted a specific LDL-C goal or specific reduction in LDL-C. A similar conversion would likely lead to lower drug costs for many other health systems. Additional studies will be necessary given the recent changes in the national lipid guidelines. Furthermore, studies will be needed to assess concrete clinical endpoints (cardiovascular mortality, all-cause mortality, and cardiac events).
Related: Poor Outcomes for African Americans in Cardiac Rehabilitation
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Due to the growing number of drug shortages and increasing cost of medications, large-scale formulary conversions are becoming more common and necessary for pharmacies to stay within their budget allocations. Statins are widely recognized as first-line therapy for cholesterol lowering and have been proven to reduce cardiovascular morbidity and mortality.1-5 In addition to statin therapy, weight loss and lifestyle changes are often necessary to meet optimum low-density lipoprotein cholesterol (LDL-C) goals.6 According to the Adult Treatment Panel III (ATP III) guidelines, the optimum LDL-C for each patient varies based on the presence of coronary artery disease (CAD), CAD risk equivalents, and other risk factors.7 Patients with CAD or CAD risk equivalents have an LDL-C goal of < 100 mg/dL. Those with multiple risk factors have an LDL-C goal of < 130 mg/dL, and those with 0 to 1 risk factors have an LDL-C goal of < 160 mg/dL.1-3,7,8
Numerous trials have compared the safety and efficacy of the 3-hydroxy-3-methylglutaryl-coenzyme A inhibitors, stating that rosuvastatin 5 to 10 mg per day is equivalent to 20 mg per day of atorvastatin in terms of its ability to lower LDL-C levels.7,9-14 The LUNAR (Limiting Under treatment of lipids in Acute coronary syndrome with Rosuvastatin) study compared the efficacy of rosuvastatin with that of atorvastatin in decreasing LDL-C in patients with acute coronary syndrome.8 Rosuvastatin 40 mg was significantly more successful in lowering LDL-C and increasing high-density lipoprotein cholesterol (HDL-C) compared with atorvastatin 80-mg daily therapy.
In October 2012, the national VA Pharmacy Benefits Management (PBM) Services released guidance regarding the conversion from rosuvastatin to atorvastatin, including the dosing conversion (Table 1), stating that rosuvastatin 5 mg daily should be considered equivalent and converted to atorvastatin 20 mg daily. In general, adverse events (AEs), such as increased liver enzymes, myopathies, and increased creatinine phosphokinase (CPK), are considered a class effect of the statins.13,14
Related: Statins showed no benefit in reducing risk of recurrent VTE
The recent 2013 American College of Cardiology/American Heart Association (ACC/AHA) Blood Cholesterol Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults has identified a large number of patients as candidates for high-intensity statins, which the authors defined as atorvastatin and rosuvastatin.4 These guidelines do not recommend LDL-C goals and instead use a risk calculator to determine which intensity of statin therapy is appropriate for certain patients. This change in practice will lead to a higher volume of high-intensity statin prescriptions and higher drug costs for some medical centers. Given the volume of prescriptions and the increased use of large-scale formulary conversions to reduce costs, more research is warranted to ensure equivalent dosing. With more data available and equivalent dosing defined, pharmacists may be better able to improve clinical results in patients that are included in these large-scale formulary conversions.
Methods
A retrospective chart review was performed on all LDL-C levels in patients receiving atorvastatin therapy due to the formulary conversion from rosuvastatin to atorvastatin at the Huntington VAMC (HVAMC) in Huntington, West Virginia, per the guidance published by the national VA PBM Services. The number of patients not at their LDL-C goal (as defined by ATP III guidelines) as a result of atorvastatin therapy was determined. Furthermore, AEs due to atorvastatin therapy such as increased liver enzymes, myopathy, and increased CPK were identified and analyzed.
The primary endpoint of this study focused on the rate of treatment failure in LDL-C reduction with atorvastatin treatment in patients previously at their LDL-C goal, as defined by the ATP III guidelines, with rosuvastatin therapy. Secondary endpoints included rate of AEs due to atorvastatin therapy, percentage increase in CPK and liver enzymes as a result of atorvastatin therapy, and percentage LDL-C, HDL-C, total cholesterol (TC), and triglyceride (TGs) changes since conversion from rosuvastatin to atorvastatin.
Patients were included in the study if they were aged 18 to 89 years, previously at LDL-C goal with rosuvastatin therapy for at least 3 months, had never previously received atorvastatin, were converted to atorvastatin therapy as a result of a large-scale formulary conversion at HVAMC, and had a fasting lipid panel completed 1 to 6 months after conversion. Patients were excluded from the study if they received other lipid-lowering medications (eg, bile acid sequestrants, fibrates, niacin, or ezetimibe) in the 12 months before or after receiving statin therapy, had previously documented AEs (eg, myopathy, increased liver enzymes, increased CPK as a result of statin therapy, or history of known homozygous familial hypercholesterolemia) current active liver disease (ALT > 2x ULN [upper limit of normal]), unexplained CPK ≥ 3x ULN, serum creatinine (SCr) > 2 mg/dL, or history of alcohol or drug abuse within the last 5 years.
Results
Three hundred twenty-three patients were identified and reviewed as converted from rosuvastatin to atorvastatin during the study period with no prior use of atorvastatin. Of the 323 charts that were reviewed, 195 patients met the study inclusion criteria and were analyzed for rate of treatment failure in terms of lipid goals and rate of AEs. Twenty of 195 patients (10.3%) were no longer at their LDL-C goal after conversion from rosuvastatin to atorvastatin. Of those 195 patients, 29 (14.9%) experienced an adverse drug reaction (ADR) as a result of atorvastatin treatment that was severe enough to result in discontinuation of the drug and switching the patient back to the originally prescribed dose of rosuvastatin. Figure 1 illustrates the number of patients and documented atorvastatin ADRs. The most common ADR documented to atorvastatin was myalgias (8.2%).
The average change in lipid levels was calculated and atorvastatin therapy was found to result in clinically insignificant changes to the lipid panel (Table 2). A 2-tailed paired t test was used to assess the statistical significance of these changes. Atorvastatin therapy resulted in an average decrease of LDL-C by 5.0 mg/dL (P < .01) in comparison to previous therapy with equivalent rosuvastatin dose. Other noted changes to lipid profile after formulary conversion included TG reduction by 2 mg/dL (P = .69), TC increased by 0.58 mg/dL (P = .80), and HDL-C reduction by 4.66 mg/dL (P < .01).
Although the decrease in LDL-C and HDL-C as a result of the formulary change was found to be statistically significant, they are not thought to result in a clinical difference. Clinically and statistically insignificant changes in liver enzymes and CPK were also discovered as a result of atorvastatin therapy conversion (Table 3). Atorvastatin therapy resulted in an averaged decrease of aspartate aminotransferase by 2.2 IU/L (P = .19) and an increase in alanine aminotransferase by 1.4 IU/L (P = .47). Average change in CPK was -6 IU/L (P = 89).
Related: New Guideline on Dyslipidemia: Less Is More
Discussion
Lipid levels were found to be mostly unchanged and remained at therapy goals, demonstrating use and appropriate equivalent dosing of rosuvastatin and atorvastatin following the formulary conversion defined in Table 1. Documented ADRs were minimal, indicating the ingredient conversion was well tolerated overall by our patients. Following the release of the 2013ACC/AHA guidelines, many patients in the VA required treatment with high-potency statins such as rosuvastatin and atorvastatin. Given the volume of statin prescriptions in the VA and the significant potential for providing the most cost-efficient lipid therapy (Table 4), the formulary conversion from rosuvastatin to atorvastatin was warranted.
Limitations
There are several limitations for extrapolating results from this study to the general population. Due to the retrospective design of the study, no formal assessment of adherence was conducted. A prospective trial, with a researcher monitoring refills and tablet counts would be more accurate to ensure patients adherence with statin therapy.
Many of the patients that were included in the formulary conversion did not have follow-up laboratory work at the time of this study and were therefore excluded, leading to a smaller study population. A larger study population with a similar study design may be able to detect more significant correlations.
There are also several potential complications in regard to formulary conversions, including the inability to ensure the exact period that the patient switched therapies. During the conversion of rosuvastatin to atorvastatin at HVAMC, an attempt was made to minimize this confounder by converting patients on request for a refill of their rosuvastatin therapy. Last, all 185 patients that were studied were male; therefore, it is difficult to extrapolate these results for female patients.
Conclusions
This study shows that the conversion of patients from rosuvastatin therapy to atorvastatin was effective when it targeted a specific LDL-C goal or specific reduction in LDL-C. A similar conversion would likely lead to lower drug costs for many other health systems. Additional studies will be necessary given the recent changes in the national lipid guidelines. Furthermore, studies will be needed to assess concrete clinical endpoints (cardiovascular mortality, all-cause mortality, and cardiac events).
Related: Poor Outcomes for African Americans in Cardiac Rehabilitation
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Krasuski RA, Doeppenschmidt D, Henry JS, et al. Conversion to atorvastatin in patients intolerant or refractory to simvastatin therapy: the CAPISH study. Mayo Clin Proc. 2005;80(9):1163-1168.
2. Clearfield MB, Amerena J, Bassand JP, et al. Comparison of the efficacy and safety of rosuvastatin 10 mg and atorvastatin 20 mg in high-risk patients with hypercholesterolemia--Prospective study to evaluate the Use of Low doses of the Statins Atorvastatin and Rosuvastatin (PULSAR). Trials. 2006;7:35.
3. Park JS, Kim YJ, Choi JY, et al. Comparative study of low doses of rosuvastatin and atorvastatin on lipid and glycemic control in patients with metabolic syndrome and hypercholesterolemia. Korean J Intern Med. 2010;25(1):27-35.
4. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934.
5. Schuster H, Barter PJ, Stender S, et al; Effective Reductions in Cholesterol Using Rosuvastatin Therapy I study group. Effects of switching statin on achievement of lipid goals: Measuring Effective Reductions in Cholesterol Using Rosuvastatin Therapy (MERCURY I) study. Am Heart J. 2004;147(4):705-713.
6. Ballantyne CM, Bertolami M, Garcia HR, et al. Achieving LDL cholesterol, non-HDL cholesterol and apolipoprotein B target levels in high-risk patients: Measuring effective Reductions in Cholesterol Using Rosuvastatin therapY (MERCURY) II. Am Heart J. 2006;151(5):975.e1-975.e9.
7. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
8. Pitt B, Loscalzo J, Monyak J, Miller E, Raichlen J. Comparison of lipid-modifying efficacy of rosuvastatin versus atorvastatin in patients with acute coronary syndrome (from the LUNAR study). Am J Cardiol. 2012;109(9);1239-1246.
9. Takagi H, Niwa M, Mizuno Y, Yamamoto H, Goto SN, Umemoto T. Effects of rosuvastatin versus atorvastatin on small dense low-density lipoprotein: a meta-analysis of randomized trials. Heart Vessels. 2014;29(3);287-299.
10. Fox KM, Gandhi SK, Ohsfeldt RL, Davidson MH. Comparison of low-density lipoprotein cholesterol reduction after switching patients on other statins to rosuvastatin or simvastatin in a real-world clinical practice setting. Am J Manag Care. 2007;13(suppl 10):S270-S275.
11. Taylor AJ, Grace K, Swiecki J, et al. Lipid-lowering efficacy, safety, and costs of a large-scale therapeutic statin formulary conversion program. Pharmacotherapy. 2001;21(9):1130-1139.
12. Bullano MF, Kamat S, Wertz DA, et al. Effectiveness of rosuvastatin versus atorvastatin in reducing lipid levels and achieving low-density-lipoprotein cholesterol goals in a usual care setting. Am J Health Syst Pharm. 2007;64(3):276-284.
13. Palmer MK, Nicholls SJ, Lundman P, Barter PJ, Karison BW. Achievement of LDL-C goals depends on baseline LDL-C and choice and dose of statin: an analysis from the VOYAGER database. Eur J Prev Cardiol. 2013;20(6):1080-1087.
14. Berne C, Siewert-Delle A; URANUS study investigators. Comparison of rosuvastatin and atorvastatin for lipid lowering in patients with type 2 diabetes mellitus: results from the URANUS study. Cardiovasc Diabetol. 2005;4:7.
1. Krasuski RA, Doeppenschmidt D, Henry JS, et al. Conversion to atorvastatin in patients intolerant or refractory to simvastatin therapy: the CAPISH study. Mayo Clin Proc. 2005;80(9):1163-1168.
2. Clearfield MB, Amerena J, Bassand JP, et al. Comparison of the efficacy and safety of rosuvastatin 10 mg and atorvastatin 20 mg in high-risk patients with hypercholesterolemia--Prospective study to evaluate the Use of Low doses of the Statins Atorvastatin and Rosuvastatin (PULSAR). Trials. 2006;7:35.
3. Park JS, Kim YJ, Choi JY, et al. Comparative study of low doses of rosuvastatin and atorvastatin on lipid and glycemic control in patients with metabolic syndrome and hypercholesterolemia. Korean J Intern Med. 2010;25(1):27-35.
4. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934.
5. Schuster H, Barter PJ, Stender S, et al; Effective Reductions in Cholesterol Using Rosuvastatin Therapy I study group. Effects of switching statin on achievement of lipid goals: Measuring Effective Reductions in Cholesterol Using Rosuvastatin Therapy (MERCURY I) study. Am Heart J. 2004;147(4):705-713.
6. Ballantyne CM, Bertolami M, Garcia HR, et al. Achieving LDL cholesterol, non-HDL cholesterol and apolipoprotein B target levels in high-risk patients: Measuring effective Reductions in Cholesterol Using Rosuvastatin therapY (MERCURY) II. Am Heart J. 2006;151(5):975.e1-975.e9.
7. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
8. Pitt B, Loscalzo J, Monyak J, Miller E, Raichlen J. Comparison of lipid-modifying efficacy of rosuvastatin versus atorvastatin in patients with acute coronary syndrome (from the LUNAR study). Am J Cardiol. 2012;109(9);1239-1246.
9. Takagi H, Niwa M, Mizuno Y, Yamamoto H, Goto SN, Umemoto T. Effects of rosuvastatin versus atorvastatin on small dense low-density lipoprotein: a meta-analysis of randomized trials. Heart Vessels. 2014;29(3);287-299.
10. Fox KM, Gandhi SK, Ohsfeldt RL, Davidson MH. Comparison of low-density lipoprotein cholesterol reduction after switching patients on other statins to rosuvastatin or simvastatin in a real-world clinical practice setting. Am J Manag Care. 2007;13(suppl 10):S270-S275.
11. Taylor AJ, Grace K, Swiecki J, et al. Lipid-lowering efficacy, safety, and costs of a large-scale therapeutic statin formulary conversion program. Pharmacotherapy. 2001;21(9):1130-1139.
12. Bullano MF, Kamat S, Wertz DA, et al. Effectiveness of rosuvastatin versus atorvastatin in reducing lipid levels and achieving low-density-lipoprotein cholesterol goals in a usual care setting. Am J Health Syst Pharm. 2007;64(3):276-284.
13. Palmer MK, Nicholls SJ, Lundman P, Barter PJ, Karison BW. Achievement of LDL-C goals depends on baseline LDL-C and choice and dose of statin: an analysis from the VOYAGER database. Eur J Prev Cardiol. 2013;20(6):1080-1087.
14. Berne C, Siewert-Delle A; URANUS study investigators. Comparison of rosuvastatin and atorvastatin for lipid lowering in patients with type 2 diabetes mellitus: results from the URANUS study. Cardiovasc Diabetol. 2005;4:7.