User login
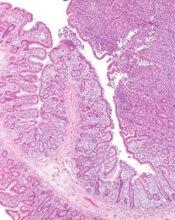
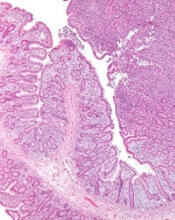
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to cerdulatinib for the treatment of peripheral T-cell lymphoma (PTCL).
Cerdulatinib is an oral Syk/JAK inhibitor being developed by Portola Pharmaceuticals, Inc.
Preclinical data have suggested an important role for Syk and JAK in PTCL tumor survival, and cerdulatinib is currently under evaluation in a phase 2a study of patients with PTCL and other non-Hodgkin lymphomas.
Results from this trial were presented at the 23rd Congress of the European Hematology Association (EHA) earlier this year.
At that time, the trial had enrolled 114 patients, 25 of them with PTCL. The patients received cerdulatinib at 25, 30, or 35 mg twice daily.
The objective response rate was 35% among the PTCL patients. All seven responders had a complete response, and 11 PTCL patients were still on cerdulatinib at the time of the presentation.
Grade 3 or higher adverse events observed in all evaluable patients included lipase increase (18%), neutropenia (17%), pneumonia/lung infection (11%), diarrhea (8%), fatigue (6%), amylase increase (5%), sepsis/septic shock (4%), hypertension (4%), anemia (4%), thrombocytopenia (4%), and hypophosphatemia (4%).
There were five deaths due to sepsis or septic shock (three of which were concomitant with pneumonia) that were considered related to cerdulatinib.
Three of the deaths occurred in patients with chronic lymphocytic leukemia, one in a patient with diffuse large B-cell lymphoma, and one in a patient with follicular lymphoma.
The deaths occurred early on in the trial, and researchers have since taken steps—dose reductions, monitoring, and antibiotic prophylaxis—to prevent additional deaths.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to cerdulatinib for the treatment of peripheral T-cell lymphoma (PTCL).
Cerdulatinib is an oral Syk/JAK inhibitor being developed by Portola Pharmaceuticals, Inc.
Preclinical data have suggested an important role for Syk and JAK in PTCL tumor survival, and cerdulatinib is currently under evaluation in a phase 2a study of patients with PTCL and other non-Hodgkin lymphomas.
Results from this trial were presented at the 23rd Congress of the European Hematology Association (EHA) earlier this year.
At that time, the trial had enrolled 114 patients, 25 of them with PTCL. The patients received cerdulatinib at 25, 30, or 35 mg twice daily.
The objective response rate was 35% among the PTCL patients. All seven responders had a complete response, and 11 PTCL patients were still on cerdulatinib at the time of the presentation.
Grade 3 or higher adverse events observed in all evaluable patients included lipase increase (18%), neutropenia (17%), pneumonia/lung infection (11%), diarrhea (8%), fatigue (6%), amylase increase (5%), sepsis/septic shock (4%), hypertension (4%), anemia (4%), thrombocytopenia (4%), and hypophosphatemia (4%).
There were five deaths due to sepsis or septic shock (three of which were concomitant with pneumonia) that were considered related to cerdulatinib.
Three of the deaths occurred in patients with chronic lymphocytic leukemia, one in a patient with diffuse large B-cell lymphoma, and one in a patient with follicular lymphoma.
The deaths occurred early on in the trial, and researchers have since taken steps—dose reductions, monitoring, and antibiotic prophylaxis—to prevent additional deaths.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to cerdulatinib for the treatment of peripheral T-cell lymphoma (PTCL).
Cerdulatinib is an oral Syk/JAK inhibitor being developed by Portola Pharmaceuticals, Inc.
Preclinical data have suggested an important role for Syk and JAK in PTCL tumor survival, and cerdulatinib is currently under evaluation in a phase 2a study of patients with PTCL and other non-Hodgkin lymphomas.
Results from this trial were presented at the 23rd Congress of the European Hematology Association (EHA) earlier this year.
At that time, the trial had enrolled 114 patients, 25 of them with PTCL. The patients received cerdulatinib at 25, 30, or 35 mg twice daily.
The objective response rate was 35% among the PTCL patients. All seven responders had a complete response, and 11 PTCL patients were still on cerdulatinib at the time of the presentation.
Grade 3 or higher adverse events observed in all evaluable patients included lipase increase (18%), neutropenia (17%), pneumonia/lung infection (11%), diarrhea (8%), fatigue (6%), amylase increase (5%), sepsis/septic shock (4%), hypertension (4%), anemia (4%), thrombocytopenia (4%), and hypophosphatemia (4%).
There were five deaths due to sepsis or septic shock (three of which were concomitant with pneumonia) that were considered related to cerdulatinib.
Three of the deaths occurred in patients with chronic lymphocytic leukemia, one in a patient with diffuse large B-cell lymphoma, and one in a patient with follicular lymphoma.
The deaths occurred early on in the trial, and researchers have since taken steps—dose reductions, monitoring, and antibiotic prophylaxis—to prevent additional deaths.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.