User login
Although it is often taken for granted, the ability to initiate and maintain sleep throughout the night is elusive for many. About one-third of adults experience a troublesome episode of insomnia.1 In most, it is transient, but in 10% to 15% (roughly 30 million people), the problem becomes self-perpetuating and chronic.2 Chronic insomnia is one of the most prevalent conditions that family physicians (FPs) encounter, a function of it being so closely associated with comorbid conditions that FPs deal with every day, such as depression, chronic pain, and polypharmacy.3,4
Insomnia can be vexing for a number of reasons. Because it is not acutely dangerous, patients may present it as an “add-on” concern at the end of an already lengthy visit. And because insomnia is often a symptom of multiple underlying physiologic and psychological factors, it requires the FP to engage in a thorough and time-consuming exploration of possible causes and comorbidities. Finally, standard treatment options have drawbacks: reports show that use of pharmacotherapy is troubling to prescribers primarily because of concerns about adverse effects and dependence;5-7 the other major therapeutic avenue, cognitive behavioral therapy for insomnia (CBT-I), requires training and is time-consuming to deliver in the context of an office visit.8,9
Despite these obstacles, successful evaluation and treatment of insomnia can be highly rewarding. Chronic insomnia is associated with great individual misery and negative consequences for long-term health. Specifically, it is associated with reduced quality of life and daytime functioning,10 depression,11,12 hypertension,13,14 increased workplace accidents and absenteeism,15-17 and exacerbations of chronic pain.18 And while the evaluation and management of insomnia can be laborious, a systematic method can streamline the process.
Insomnia: Symptom or cause?
The International Classification of Sleep Disorders defines chronic insomnia as an inability to sleep sufficiently despite creating adequate opportunity. It occurs at least 3 nights per week for >3 months with perceived negative consequences during the day. Patients typically complain about symptoms including fatigue, diminished cognitive performance, and mood disturbance.19 Acute insomnia triggered by one or more biopsychosocial stresses is, by definition, self-limited and has different underlying mechanisms. As such, it will not be described in this review.
The chief risk factors are female gender, low socioeconomic status, and increasing age.20 However, cohorts of healthy seniors show preserved good sleep; the increase in prevalence of insomnia in the elderly is likely linked more specifically to age-related accumulation of medical/mental health disorders and polypharmacy than aging per se.21
In the past, insomnia was viewed as a symptom, occurring secondarily to an underlying cause, usually an acute biopsychosocial stressor or depression. It was assumed that if the primary cause was effectively treated, then healthy sleep would return.
But research over the past 20 years has changed this paradigm in 2 ways. First, when comorbidities such as depression or chronic pain are present, they have a bidirectional relationship with insomnia rather than a one-way cause and effect. For example, instead of depression being a primary disorder from which insomnia can result, it is now recognized that insomnia can be present first and is a risk factor for new-onset depression. When depression and insomnia coexist, they may exacerbate each other in a bidirectional pattern.
Secondly, an estimated 15% of chronic insomnia sufferers have no targetable comorbidity; rather, they are unable to get sufficient sleep in large part because of a trait-like predisposition to fragile sleep, called hyperarousal brain physiology.22 These people used to be described as having “primary insomnia,” although the term has been dropped from the 5th edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM).23
Assess comorbidities, obtain sleep logs
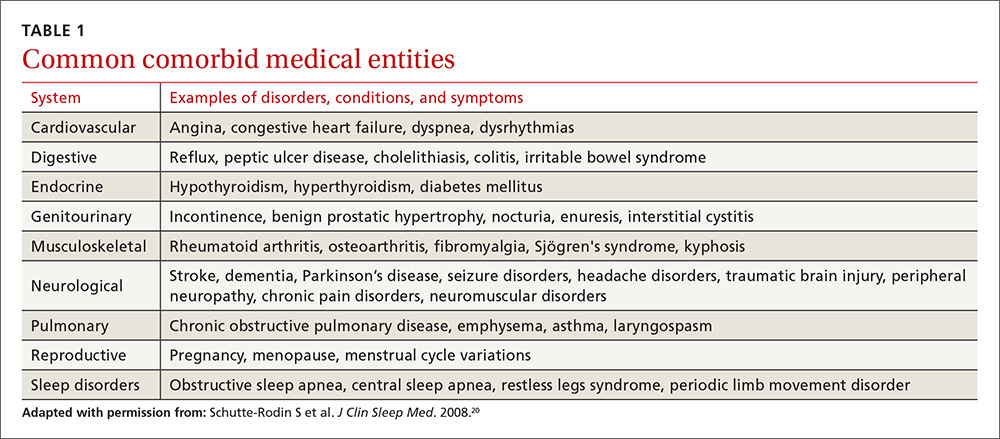
The evaluation of the chronic form of insomnia should begin with a thorough medical history to assess for comorbid conditions that can exacerbate disturbed sleep. These are generally grouped into medical disorders (TABLE 120), medications/substances (eg, antidepressants, stimulants, decongestants, narcotic analgesics, cardiovascular drugs, pulmonary agents, alcohol), and mental health disorders (especially depression and anxiety). It’s important to consider whether such comorbidities are contributing to the insomnia and optimize treatment that addresses them.
Take particular care to evaluate signs and symptoms of comorbid primary sleep disorders such as obstructive sleep apnea, restless legs syndrome (RLS), and circadian rhythm disorders since any of these can present with a complaint of insomnia. RLS, usually classified as a sleep disorder because of its circadian pattern (it is experienced more at night than during the day), is present to a troublesome degree in about 3% to 4% of all adults.24 It is important to inquire about symptoms of RLS (urge to move legs in the night more than during the day; relieved with movement; worsened with inactivity) so as not to miss this treatable cause of insomnia.
The physical exam should focus on signs that suggest sleep-disordered breathing—obesity, large neck girth, hypertension, and crowded oropharynx—because people with sleep apnea often present with the complaint of frequent awakenings.
Sleep logs can present a powerful picture
In addition to a history and physical exam, physicians should ask their patients with chronic insomnia to complete sleep logs for 2 to 3 weeks.20 A sleep log with midnight near the middle of the page is preferred by many because it places the typical sleeping hours in the middle of the page, showing relevant information in a way that can be grasped immediately (FIGURE 1). To save time, nurses can provide sleep logs to patients along with instructions about how to complete them.
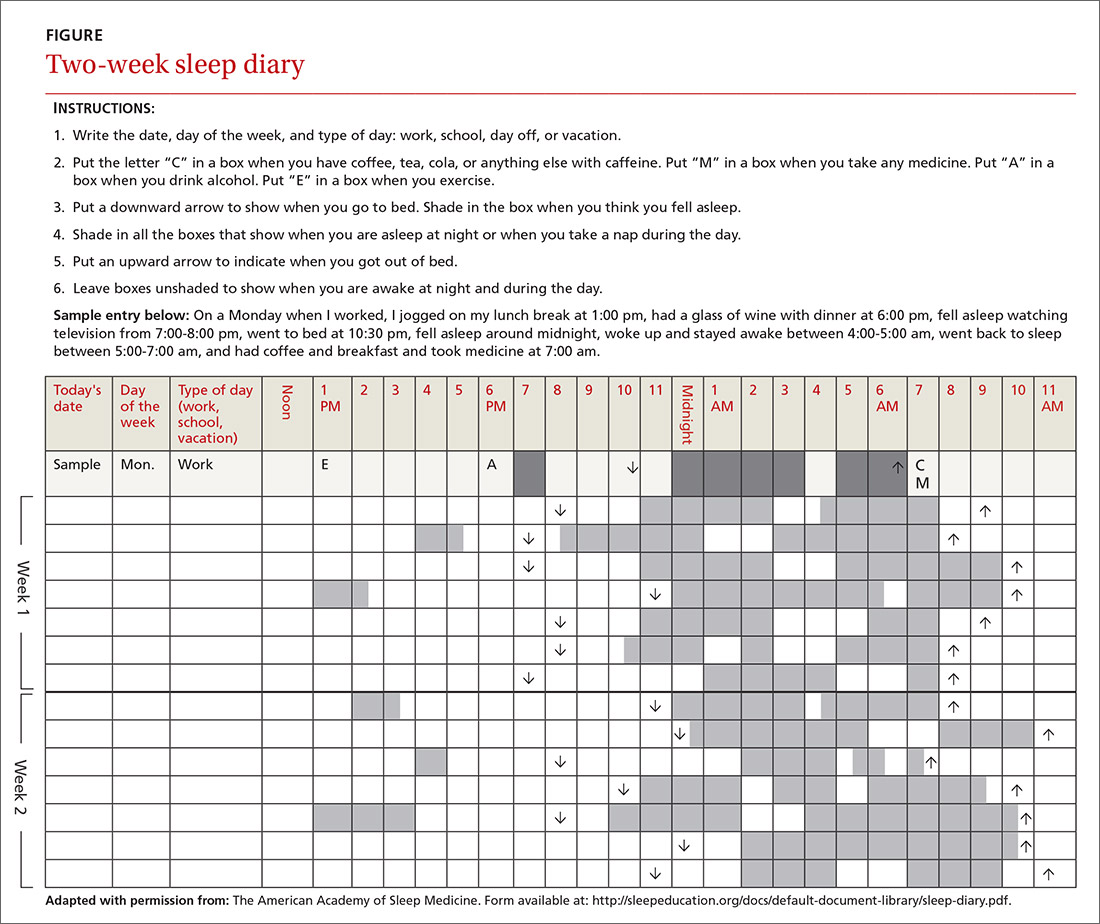
Patient-completed sleep logs often illuminate obvious detrimental behaviors that reinforce insomnia (eg, spending excessive time in bed, having irregular bed/wake times, daytime napping that diminishes sleep drive in the evening). In addition, they sometimes reveal circadian rhythm abnormalities such as delayed sleep phase syndrome in which the patient attempts to sleep at a normal bedtime, but exhibits a marked delay in falling asleep/waking up compared to societal norms. Seeing such information graphically represented is often a powerful learning experience for both physician and patient.
Sleep studies aren't usually warranted
In its most recent (2008) clinical guideline on the evaluation and management of chronic insomnia, the American Academy of Sleep Medicine (AASM) stated that “routine testing in the sleep lab is not warranted for most cases of insomnia.” Instead, it is reserved for individuals in whom there is a suspicion of a comorbid sleep disorder. FPs should refer patients for formal sleep studies only if, in addition to the insomnia complaint, there is suspicion of:20
- obstructive sleep apnea (based upon some combination of loud snoring, obesity, hypertension, and/or excessive daytime sleepiness),
- narcolepsy (based upon excessive daytime sleepiness without a readily identifiable cause), or
- arousals with the potential for self-injurious behavior (parasomnias).
Treatments: Sleep hygiene, CBT-I, and medication
Sleep hygiene, cognitive/behavioral techniques, and pharmacotherapy serve as the core of therapy for chronic insomnia.
Sleep hygiene: Common-sense strategies
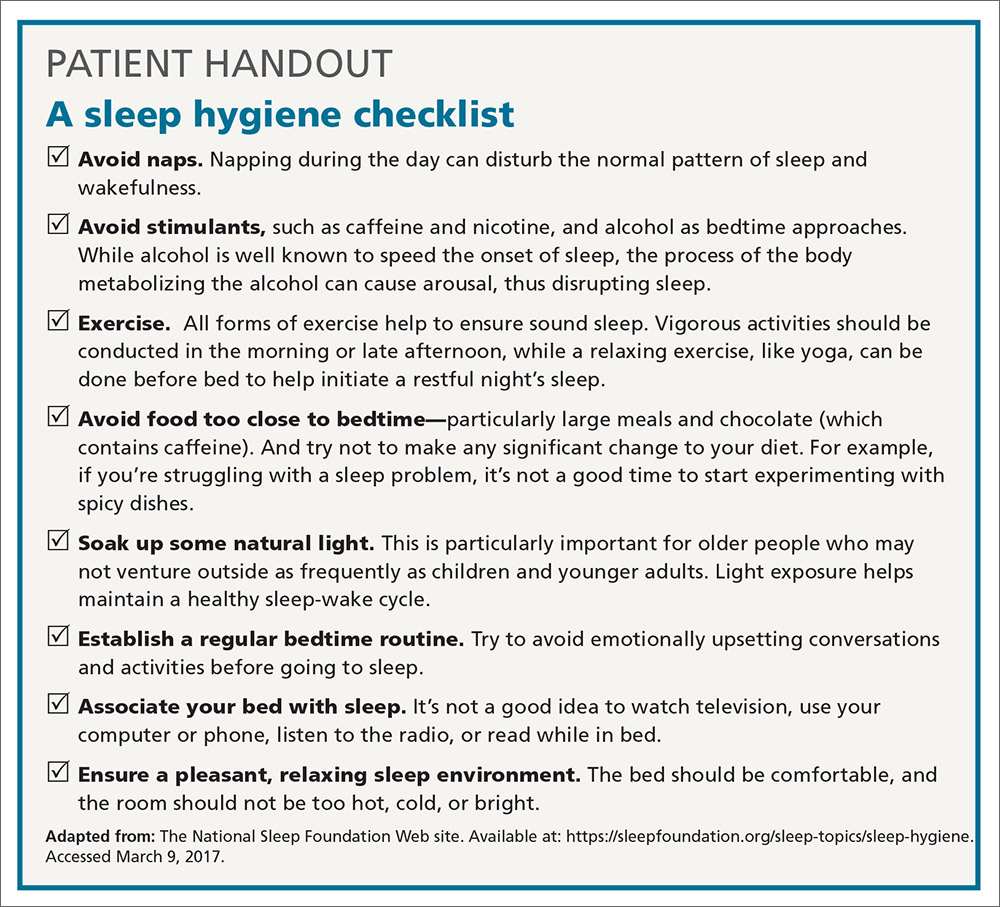
Most FPs are familiar with sleep hygiene instructions; these are simple, common-sense behavioral techniques such as limiting caffeine and screen (television, computer) time at night, avoiding daytime naps, and maintaining regular bed- and out-of-bed times. (See “A sleep hygiene checklist.”) Although it is a logical starting point for behavioral modification, sleep hygiene has not been studied rigorously as a monotherapy for insomnia and, therefore, doesn’t have an evidence rating in terms of effectiveness.20
CBT-I: Treatment of choice
CBT-I seeks to lower cognitive and somatic arousal. Taken together, cognitive and behavioral techniques are effective in 70% to 80% of people, whether they have primary insomnia or comorbidities.25-27 Furthermore, the benefits are sustained with the passage of time.27 CBT-I is regarded as the treatment of choice for chronic insomnia.20
When provided by a highly trained mental health professional, CBT-I usually takes the form of a series of 6 to 8 weekly appointments. Descriptions and manuals for CBT-I abound and online programs have also proliferated.28,29 However, there is a shortage of highly trained providers, and most FPs do not feel proficient to engage fully in CBT-I.8,9 Nevertheless, some behavioral elements of CBT-I, such as stimulus control and sleep restriction, can be utilized in the family medicine setting and may be effective for a significant subset of patients.
Stimulus control and sleep restriction. Two behavioral techniques for insomnia that can be applied in the family medicine setting are stimulus control and sleep restriction therapy.20
With stimulus control, patients attempt to eliminate stimuli that weaken the psychological association between the bed and successful sleep, namely wakeful activities in bed such as watching television, reading, or even “tossing and turning.” Instead, they are instructed to use their bed only for sleep (and intimacy), to vacate it if awake and not clearly on the verge of sleep, and to avoid looking at a clock during the night. Patients are also advised to sleep only in their own bed and not in other places in their home.
Sleep restriction is predicated on the observation that many people with insomnia habitually spend too much time awake in bed, and this creates a conditioned arousal response to the bed. With sleep restriction, the patient is assigned a narrow window of “allowed time in bed,” usually a 6-hour interval of their choosing, and is instructed to adhere to this schedule for a period of 2 to 4 weeks. Many patients find that they fall asleep more rapidly and stay asleep longer after a few weeks. This experience of “successful” sleep initiation and maintenance is important psychologically; it renews their confidence in their ability to sleep, which is missing in most people with chronic insomnia.
If you use this approach with a patient, be sure to acknowledge that sleep restriction usually engenders some sleep deprivation in the first few weeks. But it is only a short-term intervention designed to change the expectation of nightly insomnia that is so ingrained in these patients. While they engage in sleep restriction, patients should keep sleep logs to track their “sleep efficiency” (ie, estimated time asleep vs time in bed). Once good sleep efficiency (>85%) is achieved, they may gradually lengthen their allowed time in bed by 15 minutes each week until they are obtaining 7 to 9 hours of sleep per night. (See “Breaking the cycle of insomnia by employing sleep restriction.”)
SIDEBAR
Breaking the cycle of insomnia by employing sleep restrictionExplain to patients: “Your sleep logs indicate that you get only 3 to 4 hours of sleep per night in total despite being in bed for 8 to 9 hours. I recommend a trial of 'sleep restriction' to increase the proportion of time spent sleeping to overall time in bed. This often helps to break the pattern of insomnia.”
1. Choose a 6-hour interval. The start time is the time you’ll go to bed each night and the end time is the time you’ll get up. Although this might seem like a drastic reduction in the time that you make for sleep, it is still more time than you are presently spending asleep.
2. Get out of bed and conduct a quiet activity—such as reading—if you find that you are wide awake during the 6-hour interval. Return to your bed only if/when you feel drowsy.
3. Continue to complete sleep logs. If you are consistently asleep 85% of the total time in bed, then you can expand your allowed time in bed by 15 minutes (earlier bedtime or later out-of-bed time) each week.
Cognitive therapy. Cognitive therapies for insomnia are usually provided by psychologists with special training. Three specific techniques that have evidence ratings* from the AASM are:20
- Relaxation training, including progressive muscle relaxation, guided imagery, and abdominal breathing to lower somatic and cognitive arousal states that interfere with sleep (strength of recommendation [SOR]: A).
- Biofeedback therapy trains patients to control some physiologic variable through visual or auditory feedback. The objective is to reduce somatic arousal (SOR: B).
- Paradoxical intention in which the patient is trained to confront the fear of staying awake and its potential effects. The objective is to eliminate a patient’s anxiety about sleep performance (SOR: B).
Pharmacotherapy: Overused? Addictive?
For patients who continue to struggle with insomnia despite attempting CBT-I, or for those who prefer a different approach, pharmacotherapy is a reasonable therapeutic option. While hypnotic medications are no guarantee of success, they sometimes provide meaningful benefit when supplied to a patient who has successfully established good cognitive and behavioral techniques, but is still struggling with insomnia.
Use of hypnotic medications has increased dramatically in recent years. Prescriptions for sleep medications approached 60 million in 2008, up 54% from 2004, with sales topping $2 billion.30,31 A National Health and Nutrition Examination Survey looking at the period between 2005 and 2010 found that about 4% of adults ages 20 and older used prescription sleep aids in the past month.32
Meta-analyses of pharmacotherapy for chronic insomnia show small to moderate effect sizes for sleep variables such as latency to sleep onset, total sleep time, and wake time after sleep onset.33,34 Treatment of chronic insomnia with hypnotic medications is of comparable effectiveness to CBT-I in the early phase, but the benefits of CBT-I are more enduring.27
A controversial approach. The appropriateness of hypnotic medications for chronic insomnia is controversial. While their use by health care professionals has been increasing, some authors have raised concerns about sleeping pills, citing a lack of effectiveness and possible adverse effects such as falls, driving impairment, and the potential for addiction, tolerance, and dependence.33,35 The Beers Criteria of the American Geriatric Society recommends against the use of benzodiazepines in the elderly due to the risks of falls, cognitive impairment, and motor vehicle accidents and advises against the use of benzodiazepine agonists (such as zolpidem) for >90 days.36
Despite these concerns, the potential benefits of hypnotic medications for chronic insomnia should not be dismissed. The common strategy of simply addressing comorbidities and advising good sleep hygiene is insufficient for many patients. And some patients prefer the ease of using a hypnotic agent to the commitment required by CBT-I. Several reports suggest that the risk of hypnotic medication misuse in people with no history of substance abuse is overestimated.37,38 And a panel of insomnia experts convened for the New Clinical Drug Evaluation Unit symposium in 2001 concluded, “Patients with chronic insomnia tend to exhibit therapy-seeking behavior, not drug-seeking behavior.”39
Which hypnotic agent to choose?
US Food and Drug Administration (FDA)-approved hypnotic medications fall into 5 families (TABLE 240): benzodiazepines (BDZs), benzodiazepine agonists (BDZAs, sometimes called “Z drugs”), melatonin agonists (eg, ramelteon), tricyclic antidepressants (eg, low-dose doxepin), and orexin antagonists (eg, suvorexant). BDZs, BDZAs, and melatonin agonists potentiate sleep-promoting systems, while orexin antagonists and antihistaminergics suppress wake-promoting systems.
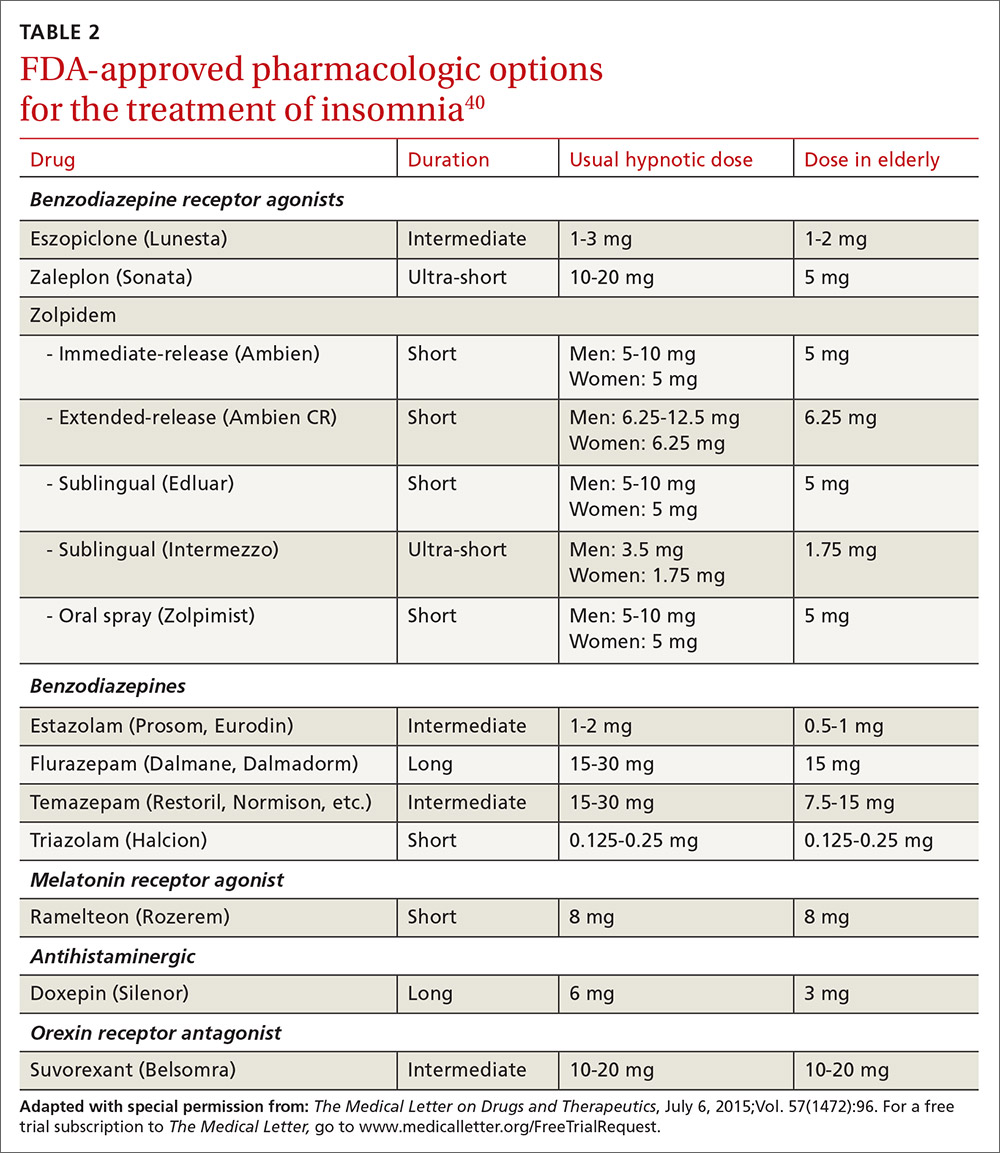
Studies of physician prescribing patterns show that among prescription medications for insomnia, zolpidem is the most popular, followed by trazodone (off-label use), other benzodiazepines, quetiapine (off-label use), and doxepin.41 Overall, over-the-counter melatonin may be more widely used than any of the prescription choices.42
One useful basis for selection of an agent is whether the patient complains of difficulty with sleep initiation at the beginning of the night vs sleep maintenance, or both. For sleep initiation complaints, short-acting/sleep-promoting agents are preferred. For sleep maintenance complaints, longer-acting/wake-inhibiting medications that work at the end of the sleep phase may be necessary.
The AASM has recently concluded an exhaustive review of the literature regarding hypnotic medications for chronic insomnia.43 The authors acknowledge important methodologic limitations, most notably a paucity of data on effectiveness and adverse effects, along with industry sponsorship of most studies and publication bias. Nevertheless, their conclusions favor the use of FDA-approved agents to off-label use of trazodone or over-the-counter use of melatonin or diphenhydramine. To summarize the AASM guidelines:38
- Medications recommended for sleep onset insomnia include: eszopiclone, ramelteon, temazepam, triazolam, zaleplon, and zolpidem.
- Medications recommended for treating sleep maintenance insomnia include: doxepin, eszopiclone, suvorexant, zolpidem, and temazepam.
- Medications not recommended for treating either sleep initiation or sleep maintenance insomnia include: diphenhydramine, melatonin, tiagabine, trazodone, tryptophan, and valerian.
These recommendations are similar to a review of hypnotics published by The Medical Letter in 2015.40
Trazodone, an antidepressant medication with sedating properties, is not FDA-approved for the treatment of insomnia, yet ranks second to zolpidem in the number of prescriptions written for insomnia. Its popularity may be due to a perception of safety implied by its unscheduled FDA status and the lack of restrictions on prescribing duration. However, several reviews point out that its evidence base is weak.44,45 There is only one placebo-controlled study involving trazodone use for "primary insomnia" (other studies have been in people with comorbid depression) and it showed insignificant improvements in sleep parameters and less effectiveness compared to zolpidem.46
Trazodone’s mechanisms of action are thought to be serotonin reuptake inhibition and alpha blockade, which might explain adverse effects such as orthostatic hypotension and psychomotor impairment. The frequency of such adverse effects is difficult to estimate since most studies of trazodone have used higher doses than are commonly used for insomnia in order to address comorbid depression. However, some experts have cautioned against its use—especially in the elderly.
The AASM guidelines recommend against use of trazodone. Others assert that it is probably best reserved for people in whom the complaint of insomnia is linked to comorbid depression.43,44
Is long-term use ever appropriate? There are no published guidelines about dosing strategies for hypnotics and whether nightly or intermittent use is preferred. All FDA-approved hypnotic agents are for short-term use, but this designation stems from a lack of long-term studies demonstrating continuing efficacy rather than actual proof of loss of effect. Although tolerance to over-the-counter sleep aids does occur, it has not been demonstrated to occur with FDA-approved agents. Studies of eszopiclone and zolpidem indicate continuing effectiveness as hypnotics with nightly use over a time-frame of several months to one year.47,48
Regarding the thorny question of long-term use of hypnotics for chronic insomnia, the AASM concluded that long-term use should be reserved for “individuals in whom CBT-I is inaccessible or ineffective, who have been appropriately screened for contraindications to such treatment, who maintain long-term gains with medication, and who are followed regularly.”43
CORRESPONDENCE
Adam J. Sorscher, MD, Dartmouth-Hitchcock Medical Center, 18 Old Etna Road, Lebanon, NH 03766; [email protected].
1. Ellis JG, Perlis ML, Neale LF, et al. The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46:1278-1285.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
3. Shochat T, Umphress J, Israel AG, et al. Insomnia in primary care patients. Sleep. 1999;22:S359-S365.
4. Alattar M, Harrington JJ, Mitchell CM, et al. Sleep problems in primary care: a North Carolina Family Practice Research Network (NC-FP-RN) study. J Am Board Fam Med. 2007;20:365-374.
5. Cook JM, Marshall R, Masci C, et al. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med. 2007;22:303-307.
6. Anthierens S, Habraken H, Petrovic M, et al. The lesser evil? Initiating a benzodiazepine prescription in general practice: a qualitative study on GPs’ perspectives. Scand J Prim Health Care. 2007;25:214-219.
7. Sorscher AJ, Siddiqui AA, Olson A, et al. Pharmacotherapy for chronic insomnia: a brief survey of PCP attitudes and preferences. J Sleep Disor Treat Care. 2016;5.
8. Espie CA. “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549-1558.
9. Anthierens S, Pasteels I, Habraken H, et al. Barriers to nonpharmacologic treatments for stress, anxiety, and insomnia: family physicians’ attitudes toward benzodiazepine prescribing. Can Fam Physician. 2010;56:e398-e406.
10. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. PLoS One. 2015;10:e0137117.
11. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
12. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10-19.
13. Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: The Penn State cohort. Hypertension. 2012;60:929-935.
14. Bathgate CJ, Edinger JD, Wyatt JK, et al. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39:1037-1045.
15. Laugsand LE, Strand LB, Vatten LJ, et al. Insomnia symptoms and risk for unintentional fatal injuries—the HUNT study. Sleep. 2014;37:1777-1786.
16. Leigh JP. Employee and job attributes as predictors of absenteeism in a national sample of workers: the importance of health and dangerous working conditions. Soc Sci Med. 1991;33:127-137.
17. Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65 Suppl 8:13-19.
18. Edwards RR, Almeida DM, Klick B, et al. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202-207.
19. American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed. Darien, IL; American Academy of Sleep Medicine, 2014.
20. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
21. Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999; 22:S366-S372.
22. Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9-15.
23. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
24. Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283-295.
25. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172-1180.
26. Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65 Suppl 16:33-40.
27. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep. 2006;29:1398-1414.
28. Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32:807-815.
29. Wolski CA. 6 online options for insomnia therapy. Sleep Review. December 11, 2014.
30. Petersen A. Dawn of a new sleep drug? The Wall Street Journal. July 19, 2011.
31. Gellene D. Sleeping pill use grows as economy keeps people up at night. Los Angeles Times. March 30, 2009. Available at: www.latimes.com/health/la-he-sleep30-2009mar30-story.html. Accessed March 6, 2017.
32. Chong Y, Fryar CD, Gu Q. Prescription sleep aid use among adults: United States, 2005-2010. Available at: https://www.cdc.gov/nchs/products/databriefs/db127.htm. Accessed March 6, 2017.
33. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335-1350.
34. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-112.
35. Verster JC, Veldhuijzen DS, Patat A, et al. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63-71.
36. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Urol. 2016;195:667-668.
37. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
38. Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333-337.
39. Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 new clinical drug evaluation unit meeting symposium. Sleep Med Rev. 2004;8:7-17.
40. Some hypnotics for insomnia. Med Lett Drugs Ther. 2015;57:95-98.
41. Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343-349.
42. Wu CH, Wang CC, Tsai MT, et al. Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 National Health Interview Surveys. Evid Based Complement Alternat Med. 2014;2014:872320.
43. Sateia M, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:307-349.
44. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469-476.
45. Wiegand MH. Antidepressants for the treatment of insomnia: a suitable approach? Drugs. 2008;68:2411-2417.
46. Walsh JK, Erman M, Erwin CW. Subjective hypnotic efficacy of trazodone and zolpidem in DSMIII-R primary insomnia. Hum Psychopharmacol Clin Exp. 1998;13:191-198.
47. Perlis ML, McCall WV, Krystal AD, et al. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128-1137.
48. Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959-968.
Although it is often taken for granted, the ability to initiate and maintain sleep throughout the night is elusive for many. About one-third of adults experience a troublesome episode of insomnia.1 In most, it is transient, but in 10% to 15% (roughly 30 million people), the problem becomes self-perpetuating and chronic.2 Chronic insomnia is one of the most prevalent conditions that family physicians (FPs) encounter, a function of it being so closely associated with comorbid conditions that FPs deal with every day, such as depression, chronic pain, and polypharmacy.3,4
Insomnia can be vexing for a number of reasons. Because it is not acutely dangerous, patients may present it as an “add-on” concern at the end of an already lengthy visit. And because insomnia is often a symptom of multiple underlying physiologic and psychological factors, it requires the FP to engage in a thorough and time-consuming exploration of possible causes and comorbidities. Finally, standard treatment options have drawbacks: reports show that use of pharmacotherapy is troubling to prescribers primarily because of concerns about adverse effects and dependence;5-7 the other major therapeutic avenue, cognitive behavioral therapy for insomnia (CBT-I), requires training and is time-consuming to deliver in the context of an office visit.8,9
Despite these obstacles, successful evaluation and treatment of insomnia can be highly rewarding. Chronic insomnia is associated with great individual misery and negative consequences for long-term health. Specifically, it is associated with reduced quality of life and daytime functioning,10 depression,11,12 hypertension,13,14 increased workplace accidents and absenteeism,15-17 and exacerbations of chronic pain.18 And while the evaluation and management of insomnia can be laborious, a systematic method can streamline the process.

Insomnia: Symptom or cause?
The International Classification of Sleep Disorders defines chronic insomnia as an inability to sleep sufficiently despite creating adequate opportunity. It occurs at least 3 nights per week for >3 months with perceived negative consequences during the day. Patients typically complain about symptoms including fatigue, diminished cognitive performance, and mood disturbance.19 Acute insomnia triggered by one or more biopsychosocial stresses is, by definition, self-limited and has different underlying mechanisms. As such, it will not be described in this review.
The chief risk factors are female gender, low socioeconomic status, and increasing age.20 However, cohorts of healthy seniors show preserved good sleep; the increase in prevalence of insomnia in the elderly is likely linked more specifically to age-related accumulation of medical/mental health disorders and polypharmacy than aging per se.21
In the past, insomnia was viewed as a symptom, occurring secondarily to an underlying cause, usually an acute biopsychosocial stressor or depression. It was assumed that if the primary cause was effectively treated, then healthy sleep would return.
But research over the past 20 years has changed this paradigm in 2 ways. First, when comorbidities such as depression or chronic pain are present, they have a bidirectional relationship with insomnia rather than a one-way cause and effect. For example, instead of depression being a primary disorder from which insomnia can result, it is now recognized that insomnia can be present first and is a risk factor for new-onset depression. When depression and insomnia coexist, they may exacerbate each other in a bidirectional pattern.
Secondly, an estimated 15% of chronic insomnia sufferers have no targetable comorbidity; rather, they are unable to get sufficient sleep in large part because of a trait-like predisposition to fragile sleep, called hyperarousal brain physiology.22 These people used to be described as having “primary insomnia,” although the term has been dropped from the 5th edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM).23
Assess comorbidities, obtain sleep logs
The evaluation of the chronic form of insomnia should begin with a thorough medical history to assess for comorbid conditions that can exacerbate disturbed sleep. These are generally grouped into medical disorders (TABLE 120), medications/substances (eg, antidepressants, stimulants, decongestants, narcotic analgesics, cardiovascular drugs, pulmonary agents, alcohol), and mental health disorders (especially depression and anxiety). It’s important to consider whether such comorbidities are contributing to the insomnia and optimize treatment that addresses them.

Take particular care to evaluate signs and symptoms of comorbid primary sleep disorders such as obstructive sleep apnea, restless legs syndrome (RLS), and circadian rhythm disorders since any of these can present with a complaint of insomnia. RLS, usually classified as a sleep disorder because of its circadian pattern (it is experienced more at night than during the day), is present to a troublesome degree in about 3% to 4% of all adults.24 It is important to inquire about symptoms of RLS (urge to move legs in the night more than during the day; relieved with movement; worsened with inactivity) so as not to miss this treatable cause of insomnia.
The physical exam should focus on signs that suggest sleep-disordered breathing—obesity, large neck girth, hypertension, and crowded oropharynx—because people with sleep apnea often present with the complaint of frequent awakenings.
Sleep logs can present a powerful picture
In addition to a history and physical exam, physicians should ask their patients with chronic insomnia to complete sleep logs for 2 to 3 weeks.20 A sleep log with midnight near the middle of the page is preferred by many because it places the typical sleeping hours in the middle of the page, showing relevant information in a way that can be grasped immediately (FIGURE 1). To save time, nurses can provide sleep logs to patients along with instructions about how to complete them.

Patient-completed sleep logs often illuminate obvious detrimental behaviors that reinforce insomnia (eg, spending excessive time in bed, having irregular bed/wake times, daytime napping that diminishes sleep drive in the evening). In addition, they sometimes reveal circadian rhythm abnormalities such as delayed sleep phase syndrome in which the patient attempts to sleep at a normal bedtime, but exhibits a marked delay in falling asleep/waking up compared to societal norms. Seeing such information graphically represented is often a powerful learning experience for both physician and patient.
Sleep studies aren't usually warranted
In its most recent (2008) clinical guideline on the evaluation and management of chronic insomnia, the American Academy of Sleep Medicine (AASM) stated that “routine testing in the sleep lab is not warranted for most cases of insomnia.” Instead, it is reserved for individuals in whom there is a suspicion of a comorbid sleep disorder. FPs should refer patients for formal sleep studies only if, in addition to the insomnia complaint, there is suspicion of:20
- obstructive sleep apnea (based upon some combination of loud snoring, obesity, hypertension, and/or excessive daytime sleepiness),
- narcolepsy (based upon excessive daytime sleepiness without a readily identifiable cause), or
- arousals with the potential for self-injurious behavior (parasomnias).
Treatments: Sleep hygiene, CBT-I, and medication
Sleep hygiene, cognitive/behavioral techniques, and pharmacotherapy serve as the core of therapy for chronic insomnia.
Sleep hygiene: Common-sense strategies
Most FPs are familiar with sleep hygiene instructions; these are simple, common-sense behavioral techniques such as limiting caffeine and screen (television, computer) time at night, avoiding daytime naps, and maintaining regular bed- and out-of-bed times. (See “A sleep hygiene checklist.”) Although it is a logical starting point for behavioral modification, sleep hygiene has not been studied rigorously as a monotherapy for insomnia and, therefore, doesn’t have an evidence rating in terms of effectiveness.20

CBT-I: Treatment of choice
CBT-I seeks to lower cognitive and somatic arousal. Taken together, cognitive and behavioral techniques are effective in 70% to 80% of people, whether they have primary insomnia or comorbidities.25-27 Furthermore, the benefits are sustained with the passage of time.27 CBT-I is regarded as the treatment of choice for chronic insomnia.20
When provided by a highly trained mental health professional, CBT-I usually takes the form of a series of 6 to 8 weekly appointments. Descriptions and manuals for CBT-I abound and online programs have also proliferated.28,29 However, there is a shortage of highly trained providers, and most FPs do not feel proficient to engage fully in CBT-I.8,9 Nevertheless, some behavioral elements of CBT-I, such as stimulus control and sleep restriction, can be utilized in the family medicine setting and may be effective for a significant subset of patients.
Stimulus control and sleep restriction. Two behavioral techniques for insomnia that can be applied in the family medicine setting are stimulus control and sleep restriction therapy.20
With stimulus control, patients attempt to eliminate stimuli that weaken the psychological association between the bed and successful sleep, namely wakeful activities in bed such as watching television, reading, or even “tossing and turning.” Instead, they are instructed to use their bed only for sleep (and intimacy), to vacate it if awake and not clearly on the verge of sleep, and to avoid looking at a clock during the night. Patients are also advised to sleep only in their own bed and not in other places in their home.
Sleep restriction is predicated on the observation that many people with insomnia habitually spend too much time awake in bed, and this creates a conditioned arousal response to the bed. With sleep restriction, the patient is assigned a narrow window of “allowed time in bed,” usually a 6-hour interval of their choosing, and is instructed to adhere to this schedule for a period of 2 to 4 weeks. Many patients find that they fall asleep more rapidly and stay asleep longer after a few weeks. This experience of “successful” sleep initiation and maintenance is important psychologically; it renews their confidence in their ability to sleep, which is missing in most people with chronic insomnia.
If you use this approach with a patient, be sure to acknowledge that sleep restriction usually engenders some sleep deprivation in the first few weeks. But it is only a short-term intervention designed to change the expectation of nightly insomnia that is so ingrained in these patients. While they engage in sleep restriction, patients should keep sleep logs to track their “sleep efficiency” (ie, estimated time asleep vs time in bed). Once good sleep efficiency (>85%) is achieved, they may gradually lengthen their allowed time in bed by 15 minutes each week until they are obtaining 7 to 9 hours of sleep per night. (See “Breaking the cycle of insomnia by employing sleep restriction.”)
SIDEBAR
Breaking the cycle of insomnia by employing sleep restrictionExplain to patients: “Your sleep logs indicate that you get only 3 to 4 hours of sleep per night in total despite being in bed for 8 to 9 hours. I recommend a trial of 'sleep restriction' to increase the proportion of time spent sleeping to overall time in bed. This often helps to break the pattern of insomnia.”
1. Choose a 6-hour interval. The start time is the time you’ll go to bed each night and the end time is the time you’ll get up. Although this might seem like a drastic reduction in the time that you make for sleep, it is still more time than you are presently spending asleep.
2. Get out of bed and conduct a quiet activity—such as reading—if you find that you are wide awake during the 6-hour interval. Return to your bed only if/when you feel drowsy.
3. Continue to complete sleep logs. If you are consistently asleep 85% of the total time in bed, then you can expand your allowed time in bed by 15 minutes (earlier bedtime or later out-of-bed time) each week.
Cognitive therapy. Cognitive therapies for insomnia are usually provided by psychologists with special training. Three specific techniques that have evidence ratings* from the AASM are:20
- Relaxation training, including progressive muscle relaxation, guided imagery, and abdominal breathing to lower somatic and cognitive arousal states that interfere with sleep (strength of recommendation [SOR]: A).
- Biofeedback therapy trains patients to control some physiologic variable through visual or auditory feedback. The objective is to reduce somatic arousal (SOR: B).
- Paradoxical intention in which the patient is trained to confront the fear of staying awake and its potential effects. The objective is to eliminate a patient’s anxiety about sleep performance (SOR: B).
Pharmacotherapy: Overused? Addictive?
For patients who continue to struggle with insomnia despite attempting CBT-I, or for those who prefer a different approach, pharmacotherapy is a reasonable therapeutic option. While hypnotic medications are no guarantee of success, they sometimes provide meaningful benefit when supplied to a patient who has successfully established good cognitive and behavioral techniques, but is still struggling with insomnia.
Use of hypnotic medications has increased dramatically in recent years. Prescriptions for sleep medications approached 60 million in 2008, up 54% from 2004, with sales topping $2 billion.30,31 A National Health and Nutrition Examination Survey looking at the period between 2005 and 2010 found that about 4% of adults ages 20 and older used prescription sleep aids in the past month.32
Meta-analyses of pharmacotherapy for chronic insomnia show small to moderate effect sizes for sleep variables such as latency to sleep onset, total sleep time, and wake time after sleep onset.33,34 Treatment of chronic insomnia with hypnotic medications is of comparable effectiveness to CBT-I in the early phase, but the benefits of CBT-I are more enduring.27
A controversial approach. The appropriateness of hypnotic medications for chronic insomnia is controversial. While their use by health care professionals has been increasing, some authors have raised concerns about sleeping pills, citing a lack of effectiveness and possible adverse effects such as falls, driving impairment, and the potential for addiction, tolerance, and dependence.33,35 The Beers Criteria of the American Geriatric Society recommends against the use of benzodiazepines in the elderly due to the risks of falls, cognitive impairment, and motor vehicle accidents and advises against the use of benzodiazepine agonists (such as zolpidem) for >90 days.36
Despite these concerns, the potential benefits of hypnotic medications for chronic insomnia should not be dismissed. The common strategy of simply addressing comorbidities and advising good sleep hygiene is insufficient for many patients. And some patients prefer the ease of using a hypnotic agent to the commitment required by CBT-I. Several reports suggest that the risk of hypnotic medication misuse in people with no history of substance abuse is overestimated.37,38 And a panel of insomnia experts convened for the New Clinical Drug Evaluation Unit symposium in 2001 concluded, “Patients with chronic insomnia tend to exhibit therapy-seeking behavior, not drug-seeking behavior.”39
Which hypnotic agent to choose?
US Food and Drug Administration (FDA)-approved hypnotic medications fall into 5 families (TABLE 240): benzodiazepines (BDZs), benzodiazepine agonists (BDZAs, sometimes called “Z drugs”), melatonin agonists (eg, ramelteon), tricyclic antidepressants (eg, low-dose doxepin), and orexin antagonists (eg, suvorexant). BDZs, BDZAs, and melatonin agonists potentiate sleep-promoting systems, while orexin antagonists and antihistaminergics suppress wake-promoting systems.

Studies of physician prescribing patterns show that among prescription medications for insomnia, zolpidem is the most popular, followed by trazodone (off-label use), other benzodiazepines, quetiapine (off-label use), and doxepin.41 Overall, over-the-counter melatonin may be more widely used than any of the prescription choices.42
One useful basis for selection of an agent is whether the patient complains of difficulty with sleep initiation at the beginning of the night vs sleep maintenance, or both. For sleep initiation complaints, short-acting/sleep-promoting agents are preferred. For sleep maintenance complaints, longer-acting/wake-inhibiting medications that work at the end of the sleep phase may be necessary.
The AASM has recently concluded an exhaustive review of the literature regarding hypnotic medications for chronic insomnia.43 The authors acknowledge important methodologic limitations, most notably a paucity of data on effectiveness and adverse effects, along with industry sponsorship of most studies and publication bias. Nevertheless, their conclusions favor the use of FDA-approved agents to off-label use of trazodone or over-the-counter use of melatonin or diphenhydramine. To summarize the AASM guidelines:38
- Medications recommended for sleep onset insomnia include: eszopiclone, ramelteon, temazepam, triazolam, zaleplon, and zolpidem.
- Medications recommended for treating sleep maintenance insomnia include: doxepin, eszopiclone, suvorexant, zolpidem, and temazepam.
- Medications not recommended for treating either sleep initiation or sleep maintenance insomnia include: diphenhydramine, melatonin, tiagabine, trazodone, tryptophan, and valerian.
These recommendations are similar to a review of hypnotics published by The Medical Letter in 2015.40
Trazodone, an antidepressant medication with sedating properties, is not FDA-approved for the treatment of insomnia, yet ranks second to zolpidem in the number of prescriptions written for insomnia. Its popularity may be due to a perception of safety implied by its unscheduled FDA status and the lack of restrictions on prescribing duration. However, several reviews point out that its evidence base is weak.44,45 There is only one placebo-controlled study involving trazodone use for "primary insomnia" (other studies have been in people with comorbid depression) and it showed insignificant improvements in sleep parameters and less effectiveness compared to zolpidem.46
Trazodone’s mechanisms of action are thought to be serotonin reuptake inhibition and alpha blockade, which might explain adverse effects such as orthostatic hypotension and psychomotor impairment. The frequency of such adverse effects is difficult to estimate since most studies of trazodone have used higher doses than are commonly used for insomnia in order to address comorbid depression. However, some experts have cautioned against its use—especially in the elderly.
The AASM guidelines recommend against use of trazodone. Others assert that it is probably best reserved for people in whom the complaint of insomnia is linked to comorbid depression.43,44
Is long-term use ever appropriate? There are no published guidelines about dosing strategies for hypnotics and whether nightly or intermittent use is preferred. All FDA-approved hypnotic agents are for short-term use, but this designation stems from a lack of long-term studies demonstrating continuing efficacy rather than actual proof of loss of effect. Although tolerance to over-the-counter sleep aids does occur, it has not been demonstrated to occur with FDA-approved agents. Studies of eszopiclone and zolpidem indicate continuing effectiveness as hypnotics with nightly use over a time-frame of several months to one year.47,48
Regarding the thorny question of long-term use of hypnotics for chronic insomnia, the AASM concluded that long-term use should be reserved for “individuals in whom CBT-I is inaccessible or ineffective, who have been appropriately screened for contraindications to such treatment, who maintain long-term gains with medication, and who are followed regularly.”43
CORRESPONDENCE
Adam J. Sorscher, MD, Dartmouth-Hitchcock Medical Center, 18 Old Etna Road, Lebanon, NH 03766; [email protected].
Although it is often taken for granted, the ability to initiate and maintain sleep throughout the night is elusive for many. About one-third of adults experience a troublesome episode of insomnia.1 In most, it is transient, but in 10% to 15% (roughly 30 million people), the problem becomes self-perpetuating and chronic.2 Chronic insomnia is one of the most prevalent conditions that family physicians (FPs) encounter, a function of it being so closely associated with comorbid conditions that FPs deal with every day, such as depression, chronic pain, and polypharmacy.3,4
Insomnia can be vexing for a number of reasons. Because it is not acutely dangerous, patients may present it as an “add-on” concern at the end of an already lengthy visit. And because insomnia is often a symptom of multiple underlying physiologic and psychological factors, it requires the FP to engage in a thorough and time-consuming exploration of possible causes and comorbidities. Finally, standard treatment options have drawbacks: reports show that use of pharmacotherapy is troubling to prescribers primarily because of concerns about adverse effects and dependence;5-7 the other major therapeutic avenue, cognitive behavioral therapy for insomnia (CBT-I), requires training and is time-consuming to deliver in the context of an office visit.8,9
Despite these obstacles, successful evaluation and treatment of insomnia can be highly rewarding. Chronic insomnia is associated with great individual misery and negative consequences for long-term health. Specifically, it is associated with reduced quality of life and daytime functioning,10 depression,11,12 hypertension,13,14 increased workplace accidents and absenteeism,15-17 and exacerbations of chronic pain.18 And while the evaluation and management of insomnia can be laborious, a systematic method can streamline the process.

Insomnia: Symptom or cause?
The International Classification of Sleep Disorders defines chronic insomnia as an inability to sleep sufficiently despite creating adequate opportunity. It occurs at least 3 nights per week for >3 months with perceived negative consequences during the day. Patients typically complain about symptoms including fatigue, diminished cognitive performance, and mood disturbance.19 Acute insomnia triggered by one or more biopsychosocial stresses is, by definition, self-limited and has different underlying mechanisms. As such, it will not be described in this review.
The chief risk factors are female gender, low socioeconomic status, and increasing age.20 However, cohorts of healthy seniors show preserved good sleep; the increase in prevalence of insomnia in the elderly is likely linked more specifically to age-related accumulation of medical/mental health disorders and polypharmacy than aging per se.21
In the past, insomnia was viewed as a symptom, occurring secondarily to an underlying cause, usually an acute biopsychosocial stressor or depression. It was assumed that if the primary cause was effectively treated, then healthy sleep would return.
But research over the past 20 years has changed this paradigm in 2 ways. First, when comorbidities such as depression or chronic pain are present, they have a bidirectional relationship with insomnia rather than a one-way cause and effect. For example, instead of depression being a primary disorder from which insomnia can result, it is now recognized that insomnia can be present first and is a risk factor for new-onset depression. When depression and insomnia coexist, they may exacerbate each other in a bidirectional pattern.
Secondly, an estimated 15% of chronic insomnia sufferers have no targetable comorbidity; rather, they are unable to get sufficient sleep in large part because of a trait-like predisposition to fragile sleep, called hyperarousal brain physiology.22 These people used to be described as having “primary insomnia,” although the term has been dropped from the 5th edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM).23
Assess comorbidities, obtain sleep logs
The evaluation of the chronic form of insomnia should begin with a thorough medical history to assess for comorbid conditions that can exacerbate disturbed sleep. These are generally grouped into medical disorders (TABLE 120), medications/substances (eg, antidepressants, stimulants, decongestants, narcotic analgesics, cardiovascular drugs, pulmonary agents, alcohol), and mental health disorders (especially depression and anxiety). It’s important to consider whether such comorbidities are contributing to the insomnia and optimize treatment that addresses them.

Take particular care to evaluate signs and symptoms of comorbid primary sleep disorders such as obstructive sleep apnea, restless legs syndrome (RLS), and circadian rhythm disorders since any of these can present with a complaint of insomnia. RLS, usually classified as a sleep disorder because of its circadian pattern (it is experienced more at night than during the day), is present to a troublesome degree in about 3% to 4% of all adults.24 It is important to inquire about symptoms of RLS (urge to move legs in the night more than during the day; relieved with movement; worsened with inactivity) so as not to miss this treatable cause of insomnia.
The physical exam should focus on signs that suggest sleep-disordered breathing—obesity, large neck girth, hypertension, and crowded oropharynx—because people with sleep apnea often present with the complaint of frequent awakenings.
Sleep logs can present a powerful picture
In addition to a history and physical exam, physicians should ask their patients with chronic insomnia to complete sleep logs for 2 to 3 weeks.20 A sleep log with midnight near the middle of the page is preferred by many because it places the typical sleeping hours in the middle of the page, showing relevant information in a way that can be grasped immediately (FIGURE 1). To save time, nurses can provide sleep logs to patients along with instructions about how to complete them.

Patient-completed sleep logs often illuminate obvious detrimental behaviors that reinforce insomnia (eg, spending excessive time in bed, having irregular bed/wake times, daytime napping that diminishes sleep drive in the evening). In addition, they sometimes reveal circadian rhythm abnormalities such as delayed sleep phase syndrome in which the patient attempts to sleep at a normal bedtime, but exhibits a marked delay in falling asleep/waking up compared to societal norms. Seeing such information graphically represented is often a powerful learning experience for both physician and patient.
Sleep studies aren't usually warranted
In its most recent (2008) clinical guideline on the evaluation and management of chronic insomnia, the American Academy of Sleep Medicine (AASM) stated that “routine testing in the sleep lab is not warranted for most cases of insomnia.” Instead, it is reserved for individuals in whom there is a suspicion of a comorbid sleep disorder. FPs should refer patients for formal sleep studies only if, in addition to the insomnia complaint, there is suspicion of:20
- obstructive sleep apnea (based upon some combination of loud snoring, obesity, hypertension, and/or excessive daytime sleepiness),
- narcolepsy (based upon excessive daytime sleepiness without a readily identifiable cause), or
- arousals with the potential for self-injurious behavior (parasomnias).
Treatments: Sleep hygiene, CBT-I, and medication
Sleep hygiene, cognitive/behavioral techniques, and pharmacotherapy serve as the core of therapy for chronic insomnia.
Sleep hygiene: Common-sense strategies
Most FPs are familiar with sleep hygiene instructions; these are simple, common-sense behavioral techniques such as limiting caffeine and screen (television, computer) time at night, avoiding daytime naps, and maintaining regular bed- and out-of-bed times. (See “A sleep hygiene checklist.”) Although it is a logical starting point for behavioral modification, sleep hygiene has not been studied rigorously as a monotherapy for insomnia and, therefore, doesn’t have an evidence rating in terms of effectiveness.20

CBT-I: Treatment of choice
CBT-I seeks to lower cognitive and somatic arousal. Taken together, cognitive and behavioral techniques are effective in 70% to 80% of people, whether they have primary insomnia or comorbidities.25-27 Furthermore, the benefits are sustained with the passage of time.27 CBT-I is regarded as the treatment of choice for chronic insomnia.20
When provided by a highly trained mental health professional, CBT-I usually takes the form of a series of 6 to 8 weekly appointments. Descriptions and manuals for CBT-I abound and online programs have also proliferated.28,29 However, there is a shortage of highly trained providers, and most FPs do not feel proficient to engage fully in CBT-I.8,9 Nevertheless, some behavioral elements of CBT-I, such as stimulus control and sleep restriction, can be utilized in the family medicine setting and may be effective for a significant subset of patients.
Stimulus control and sleep restriction. Two behavioral techniques for insomnia that can be applied in the family medicine setting are stimulus control and sleep restriction therapy.20
With stimulus control, patients attempt to eliminate stimuli that weaken the psychological association between the bed and successful sleep, namely wakeful activities in bed such as watching television, reading, or even “tossing and turning.” Instead, they are instructed to use their bed only for sleep (and intimacy), to vacate it if awake and not clearly on the verge of sleep, and to avoid looking at a clock during the night. Patients are also advised to sleep only in their own bed and not in other places in their home.
Sleep restriction is predicated on the observation that many people with insomnia habitually spend too much time awake in bed, and this creates a conditioned arousal response to the bed. With sleep restriction, the patient is assigned a narrow window of “allowed time in bed,” usually a 6-hour interval of their choosing, and is instructed to adhere to this schedule for a period of 2 to 4 weeks. Many patients find that they fall asleep more rapidly and stay asleep longer after a few weeks. This experience of “successful” sleep initiation and maintenance is important psychologically; it renews their confidence in their ability to sleep, which is missing in most people with chronic insomnia.
If you use this approach with a patient, be sure to acknowledge that sleep restriction usually engenders some sleep deprivation in the first few weeks. But it is only a short-term intervention designed to change the expectation of nightly insomnia that is so ingrained in these patients. While they engage in sleep restriction, patients should keep sleep logs to track their “sleep efficiency” (ie, estimated time asleep vs time in bed). Once good sleep efficiency (>85%) is achieved, they may gradually lengthen their allowed time in bed by 15 minutes each week until they are obtaining 7 to 9 hours of sleep per night. (See “Breaking the cycle of insomnia by employing sleep restriction.”)
SIDEBAR
Breaking the cycle of insomnia by employing sleep restrictionExplain to patients: “Your sleep logs indicate that you get only 3 to 4 hours of sleep per night in total despite being in bed for 8 to 9 hours. I recommend a trial of 'sleep restriction' to increase the proportion of time spent sleeping to overall time in bed. This often helps to break the pattern of insomnia.”
1. Choose a 6-hour interval. The start time is the time you’ll go to bed each night and the end time is the time you’ll get up. Although this might seem like a drastic reduction in the time that you make for sleep, it is still more time than you are presently spending asleep.
2. Get out of bed and conduct a quiet activity—such as reading—if you find that you are wide awake during the 6-hour interval. Return to your bed only if/when you feel drowsy.
3. Continue to complete sleep logs. If you are consistently asleep 85% of the total time in bed, then you can expand your allowed time in bed by 15 minutes (earlier bedtime or later out-of-bed time) each week.
Cognitive therapy. Cognitive therapies for insomnia are usually provided by psychologists with special training. Three specific techniques that have evidence ratings* from the AASM are:20
- Relaxation training, including progressive muscle relaxation, guided imagery, and abdominal breathing to lower somatic and cognitive arousal states that interfere with sleep (strength of recommendation [SOR]: A).
- Biofeedback therapy trains patients to control some physiologic variable through visual or auditory feedback. The objective is to reduce somatic arousal (SOR: B).
- Paradoxical intention in which the patient is trained to confront the fear of staying awake and its potential effects. The objective is to eliminate a patient’s anxiety about sleep performance (SOR: B).
Pharmacotherapy: Overused? Addictive?
For patients who continue to struggle with insomnia despite attempting CBT-I, or for those who prefer a different approach, pharmacotherapy is a reasonable therapeutic option. While hypnotic medications are no guarantee of success, they sometimes provide meaningful benefit when supplied to a patient who has successfully established good cognitive and behavioral techniques, but is still struggling with insomnia.
Use of hypnotic medications has increased dramatically in recent years. Prescriptions for sleep medications approached 60 million in 2008, up 54% from 2004, with sales topping $2 billion.30,31 A National Health and Nutrition Examination Survey looking at the period between 2005 and 2010 found that about 4% of adults ages 20 and older used prescription sleep aids in the past month.32
Meta-analyses of pharmacotherapy for chronic insomnia show small to moderate effect sizes for sleep variables such as latency to sleep onset, total sleep time, and wake time after sleep onset.33,34 Treatment of chronic insomnia with hypnotic medications is of comparable effectiveness to CBT-I in the early phase, but the benefits of CBT-I are more enduring.27
A controversial approach. The appropriateness of hypnotic medications for chronic insomnia is controversial. While their use by health care professionals has been increasing, some authors have raised concerns about sleeping pills, citing a lack of effectiveness and possible adverse effects such as falls, driving impairment, and the potential for addiction, tolerance, and dependence.33,35 The Beers Criteria of the American Geriatric Society recommends against the use of benzodiazepines in the elderly due to the risks of falls, cognitive impairment, and motor vehicle accidents and advises against the use of benzodiazepine agonists (such as zolpidem) for >90 days.36
Despite these concerns, the potential benefits of hypnotic medications for chronic insomnia should not be dismissed. The common strategy of simply addressing comorbidities and advising good sleep hygiene is insufficient for many patients. And some patients prefer the ease of using a hypnotic agent to the commitment required by CBT-I. Several reports suggest that the risk of hypnotic medication misuse in people with no history of substance abuse is overestimated.37,38 And a panel of insomnia experts convened for the New Clinical Drug Evaluation Unit symposium in 2001 concluded, “Patients with chronic insomnia tend to exhibit therapy-seeking behavior, not drug-seeking behavior.”39
Which hypnotic agent to choose?
US Food and Drug Administration (FDA)-approved hypnotic medications fall into 5 families (TABLE 240): benzodiazepines (BDZs), benzodiazepine agonists (BDZAs, sometimes called “Z drugs”), melatonin agonists (eg, ramelteon), tricyclic antidepressants (eg, low-dose doxepin), and orexin antagonists (eg, suvorexant). BDZs, BDZAs, and melatonin agonists potentiate sleep-promoting systems, while orexin antagonists and antihistaminergics suppress wake-promoting systems.

Studies of physician prescribing patterns show that among prescription medications for insomnia, zolpidem is the most popular, followed by trazodone (off-label use), other benzodiazepines, quetiapine (off-label use), and doxepin.41 Overall, over-the-counter melatonin may be more widely used than any of the prescription choices.42
One useful basis for selection of an agent is whether the patient complains of difficulty with sleep initiation at the beginning of the night vs sleep maintenance, or both. For sleep initiation complaints, short-acting/sleep-promoting agents are preferred. For sleep maintenance complaints, longer-acting/wake-inhibiting medications that work at the end of the sleep phase may be necessary.
The AASM has recently concluded an exhaustive review of the literature regarding hypnotic medications for chronic insomnia.43 The authors acknowledge important methodologic limitations, most notably a paucity of data on effectiveness and adverse effects, along with industry sponsorship of most studies and publication bias. Nevertheless, their conclusions favor the use of FDA-approved agents to off-label use of trazodone or over-the-counter use of melatonin or diphenhydramine. To summarize the AASM guidelines:38
- Medications recommended for sleep onset insomnia include: eszopiclone, ramelteon, temazepam, triazolam, zaleplon, and zolpidem.
- Medications recommended for treating sleep maintenance insomnia include: doxepin, eszopiclone, suvorexant, zolpidem, and temazepam.
- Medications not recommended for treating either sleep initiation or sleep maintenance insomnia include: diphenhydramine, melatonin, tiagabine, trazodone, tryptophan, and valerian.
These recommendations are similar to a review of hypnotics published by The Medical Letter in 2015.40
Trazodone, an antidepressant medication with sedating properties, is not FDA-approved for the treatment of insomnia, yet ranks second to zolpidem in the number of prescriptions written for insomnia. Its popularity may be due to a perception of safety implied by its unscheduled FDA status and the lack of restrictions on prescribing duration. However, several reviews point out that its evidence base is weak.44,45 There is only one placebo-controlled study involving trazodone use for "primary insomnia" (other studies have been in people with comorbid depression) and it showed insignificant improvements in sleep parameters and less effectiveness compared to zolpidem.46
Trazodone’s mechanisms of action are thought to be serotonin reuptake inhibition and alpha blockade, which might explain adverse effects such as orthostatic hypotension and psychomotor impairment. The frequency of such adverse effects is difficult to estimate since most studies of trazodone have used higher doses than are commonly used for insomnia in order to address comorbid depression. However, some experts have cautioned against its use—especially in the elderly.
The AASM guidelines recommend against use of trazodone. Others assert that it is probably best reserved for people in whom the complaint of insomnia is linked to comorbid depression.43,44
Is long-term use ever appropriate? There are no published guidelines about dosing strategies for hypnotics and whether nightly or intermittent use is preferred. All FDA-approved hypnotic agents are for short-term use, but this designation stems from a lack of long-term studies demonstrating continuing efficacy rather than actual proof of loss of effect. Although tolerance to over-the-counter sleep aids does occur, it has not been demonstrated to occur with FDA-approved agents. Studies of eszopiclone and zolpidem indicate continuing effectiveness as hypnotics with nightly use over a time-frame of several months to one year.47,48
Regarding the thorny question of long-term use of hypnotics for chronic insomnia, the AASM concluded that long-term use should be reserved for “individuals in whom CBT-I is inaccessible or ineffective, who have been appropriately screened for contraindications to such treatment, who maintain long-term gains with medication, and who are followed regularly.”43
CORRESPONDENCE
Adam J. Sorscher, MD, Dartmouth-Hitchcock Medical Center, 18 Old Etna Road, Lebanon, NH 03766; [email protected].
1. Ellis JG, Perlis ML, Neale LF, et al. The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46:1278-1285.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
3. Shochat T, Umphress J, Israel AG, et al. Insomnia in primary care patients. Sleep. 1999;22:S359-S365.
4. Alattar M, Harrington JJ, Mitchell CM, et al. Sleep problems in primary care: a North Carolina Family Practice Research Network (NC-FP-RN) study. J Am Board Fam Med. 2007;20:365-374.
5. Cook JM, Marshall R, Masci C, et al. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med. 2007;22:303-307.
6. Anthierens S, Habraken H, Petrovic M, et al. The lesser evil? Initiating a benzodiazepine prescription in general practice: a qualitative study on GPs’ perspectives. Scand J Prim Health Care. 2007;25:214-219.
7. Sorscher AJ, Siddiqui AA, Olson A, et al. Pharmacotherapy for chronic insomnia: a brief survey of PCP attitudes and preferences. J Sleep Disor Treat Care. 2016;5.
8. Espie CA. “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549-1558.
9. Anthierens S, Pasteels I, Habraken H, et al. Barriers to nonpharmacologic treatments for stress, anxiety, and insomnia: family physicians’ attitudes toward benzodiazepine prescribing. Can Fam Physician. 2010;56:e398-e406.
10. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. PLoS One. 2015;10:e0137117.
11. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
12. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10-19.
13. Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: The Penn State cohort. Hypertension. 2012;60:929-935.
14. Bathgate CJ, Edinger JD, Wyatt JK, et al. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39:1037-1045.
15. Laugsand LE, Strand LB, Vatten LJ, et al. Insomnia symptoms and risk for unintentional fatal injuries—the HUNT study. Sleep. 2014;37:1777-1786.
16. Leigh JP. Employee and job attributes as predictors of absenteeism in a national sample of workers: the importance of health and dangerous working conditions. Soc Sci Med. 1991;33:127-137.
17. Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65 Suppl 8:13-19.
18. Edwards RR, Almeida DM, Klick B, et al. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202-207.
19. American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed. Darien, IL; American Academy of Sleep Medicine, 2014.
20. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
21. Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999; 22:S366-S372.
22. Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9-15.
23. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
24. Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283-295.
25. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172-1180.
26. Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65 Suppl 16:33-40.
27. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep. 2006;29:1398-1414.
28. Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32:807-815.
29. Wolski CA. 6 online options for insomnia therapy. Sleep Review. December 11, 2014.
30. Petersen A. Dawn of a new sleep drug? The Wall Street Journal. July 19, 2011.
31. Gellene D. Sleeping pill use grows as economy keeps people up at night. Los Angeles Times. March 30, 2009. Available at: www.latimes.com/health/la-he-sleep30-2009mar30-story.html. Accessed March 6, 2017.
32. Chong Y, Fryar CD, Gu Q. Prescription sleep aid use among adults: United States, 2005-2010. Available at: https://www.cdc.gov/nchs/products/databriefs/db127.htm. Accessed March 6, 2017.
33. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335-1350.
34. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-112.
35. Verster JC, Veldhuijzen DS, Patat A, et al. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63-71.
36. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Urol. 2016;195:667-668.
37. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
38. Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333-337.
39. Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 new clinical drug evaluation unit meeting symposium. Sleep Med Rev. 2004;8:7-17.
40. Some hypnotics for insomnia. Med Lett Drugs Ther. 2015;57:95-98.
41. Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343-349.
42. Wu CH, Wang CC, Tsai MT, et al. Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 National Health Interview Surveys. Evid Based Complement Alternat Med. 2014;2014:872320.
43. Sateia M, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:307-349.
44. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469-476.
45. Wiegand MH. Antidepressants for the treatment of insomnia: a suitable approach? Drugs. 2008;68:2411-2417.
46. Walsh JK, Erman M, Erwin CW. Subjective hypnotic efficacy of trazodone and zolpidem in DSMIII-R primary insomnia. Hum Psychopharmacol Clin Exp. 1998;13:191-198.
47. Perlis ML, McCall WV, Krystal AD, et al. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128-1137.
48. Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959-968.
1. Ellis JG, Perlis ML, Neale LF, et al. The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46:1278-1285.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
3. Shochat T, Umphress J, Israel AG, et al. Insomnia in primary care patients. Sleep. 1999;22:S359-S365.
4. Alattar M, Harrington JJ, Mitchell CM, et al. Sleep problems in primary care: a North Carolina Family Practice Research Network (NC-FP-RN) study. J Am Board Fam Med. 2007;20:365-374.
5. Cook JM, Marshall R, Masci C, et al. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med. 2007;22:303-307.
6. Anthierens S, Habraken H, Petrovic M, et al. The lesser evil? Initiating a benzodiazepine prescription in general practice: a qualitative study on GPs’ perspectives. Scand J Prim Health Care. 2007;25:214-219.
7. Sorscher AJ, Siddiqui AA, Olson A, et al. Pharmacotherapy for chronic insomnia: a brief survey of PCP attitudes and preferences. J Sleep Disor Treat Care. 2016;5.
8. Espie CA. “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549-1558.
9. Anthierens S, Pasteels I, Habraken H, et al. Barriers to nonpharmacologic treatments for stress, anxiety, and insomnia: family physicians’ attitudes toward benzodiazepine prescribing. Can Fam Physician. 2010;56:e398-e406.
10. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. PLoS One. 2015;10:e0137117.
11. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
12. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10-19.
13. Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: The Penn State cohort. Hypertension. 2012;60:929-935.
14. Bathgate CJ, Edinger JD, Wyatt JK, et al. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39:1037-1045.
15. Laugsand LE, Strand LB, Vatten LJ, et al. Insomnia symptoms and risk for unintentional fatal injuries—the HUNT study. Sleep. 2014;37:1777-1786.
16. Leigh JP. Employee and job attributes as predictors of absenteeism in a national sample of workers: the importance of health and dangerous working conditions. Soc Sci Med. 1991;33:127-137.
17. Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65 Suppl 8:13-19.
18. Edwards RR, Almeida DM, Klick B, et al. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202-207.
19. American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed. Darien, IL; American Academy of Sleep Medicine, 2014.
20. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
21. Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999; 22:S366-S372.
22. Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9-15.
23. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
24. Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283-295.
25. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172-1180.
26. Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65 Suppl 16:33-40.
27. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep. 2006;29:1398-1414.
28. Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32:807-815.
29. Wolski CA. 6 online options for insomnia therapy. Sleep Review. December 11, 2014.
30. Petersen A. Dawn of a new sleep drug? The Wall Street Journal. July 19, 2011.
31. Gellene D. Sleeping pill use grows as economy keeps people up at night. Los Angeles Times. March 30, 2009. Available at: www.latimes.com/health/la-he-sleep30-2009mar30-story.html. Accessed March 6, 2017.
32. Chong Y, Fryar CD, Gu Q. Prescription sleep aid use among adults: United States, 2005-2010. Available at: https://www.cdc.gov/nchs/products/databriefs/db127.htm. Accessed March 6, 2017.
33. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335-1350.
34. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-112.
35. Verster JC, Veldhuijzen DS, Patat A, et al. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63-71.
36. The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Urol. 2016;195:667-668.
37. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
38. Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333-337.
39. Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 new clinical drug evaluation unit meeting symposium. Sleep Med Rev. 2004;8:7-17.
40. Some hypnotics for insomnia. Med Lett Drugs Ther. 2015;57:95-98.
41. Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343-349.
42. Wu CH, Wang CC, Tsai MT, et al. Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 National Health Interview Surveys. Evid Based Complement Alternat Med. 2014;2014:872320.
43. Sateia M, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:307-349.
44. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469-476.
45. Wiegand MH. Antidepressants for the treatment of insomnia: a suitable approach? Drugs. 2008;68:2411-2417.
46. Walsh JK, Erman M, Erwin CW. Subjective hypnotic efficacy of trazodone and zolpidem in DSMIII-R primary insomnia. Hum Psychopharmacol Clin Exp. 1998;13:191-198.
47. Perlis ML, McCall WV, Krystal AD, et al. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128-1137.
48. Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959-968.
PRACTICE RECOMMENDATIONS
› Recommend that patients try cognitive behavioral therapy for insomnia (CBT-I), as it is highly effective and some of its techniques can be employed in a busy family medicine clinic with little time commitment. B
› Consider pharmacotherapy for patients with chronic insomnia that persists despite CBT-I, as long as they are properly screened and followed regularly. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
