User login
› Initiate insulin for patients whose hemoglobin A1c ≥8% despite taking 2 or more oral agents. C
› Prescribe insulin for patients who have not reached their goal one year after diagnosis and initiation of oral therapy. C
› Consider reducing—but do not discontinue—oral agents, such as sulfonylureas and meglitinides, when you initiate insulin therapy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With type 2 diabetes now affecting 8.3% of the US population, most primary care physicians see patients with this disorder every day.1 Based on the concurrent obesity epidemic, aging population, and emergence of type 2 diabetes in children and adolescents, it is estimated that by 2050, the prevalence will have risen from one in 12 Americans to one in 3.1
Type 2 diabetes is a progressive disorder, with a relentless decline in beta cells. By the time of diagnosis, patients typically have lost at least 50% of insulin secretion; within 6 years of diagnosis, insulin secretion decreases to less than 25%.2
The American Association of Clinical Endocrinologists (AACE)3 and the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD)4 have recently published guidelines for the management of type 2 diabetes. While the AACE’s guidelines (available at https://www.aace.com/files/aace_algorithm.pdf) focus on different treatments at different stages of disease and both glycemic and nonglycemic benefits of treatment,3 the ADA/EASD’s guidelines (see http://care.diabetesjournals.org/content/early/2012/04/17/dc12-0413.full.pdf+html) emphasize a patient-centered approach, shared decision making, and individualization of treatment goals based on both patient preference and comorbid disease states.4
One thing both sets of guidelines have in common is a purposeful intensification of therapy every 2 to 3 months, as needed, and the introduction of insulin one year after diagnosis if the patient is still not at goal.3,4 But all too often, this does not occur, particularly in primary care settings.
This article will review the “when” and “how” of insulin initiation. But first, a look at barriers to insulin therapy and evidence in support of earlier use.
Clinical inertia and patient fear are associated with delays
Both the AACE and the ADA/EASD guidelines agree that metformin is best used as early as possible.5,6 With typical use, however, metformin fails to prevent the progression of diabetes, as measured by the climb of hemoglobin A1c (HbA1c), at a failure rate of about 17% of patients per year.5 Physicians have been slow to intensify treatment for type 2 diabetes6—a phenomenon referred to as clinical inertia.
Typically, physicians adopt a stepwise approach, which often results in patients spending more than 10 years with an HbA1c >7% and 5 years with an HbA1c >8% before insulin is started.5 In a recent Veterans Administration study, patients were out of control, with an HbA1c >8%, for an average of 4.6 years before insulin was initiated.7
Both patient and physician factors contribute to the delay. Patient factors include the fear of injection, the belief that insulin will interfere with their lifestyle, and the idea that the use of insulin signifies impending complications or even death.8 But such beliefs are starting to change. In a recent multinational study of patients with type 2 diabetes, less than 20% stated they were unwilling to start insulin.9
For their part, primary care physicians are much less likely to prescribe insulin than clinicians specializing in diabetes.6 Physician-reported barriers to insulin initiation include the time required to train patients to use it correctly; the lack of support, including access to diabetes educators; and the absence of clear guidelines on the use of insulin.10
A case for earlier insulin
There has been recent momentum in favor of earlier initiation of insulin. In fact, some researchers regard intensive insulin as an excellent first treatment for type 2 diabetes,11 based on the belief that early insulin (used for a brief time) can provide not only immediate improvement in glucose control, but also a lasting “legacy” effect. The ADA/EASD guidelines support the use of insulin as a first-line treatment for patients with symptoms of insulin deficiency,4 but do not recommend it for everyone with newly diagnosed type 2 diabetes.
There have also been a number of advances in insulin therapy over the past 2 decades. These include insulin analogs with physiologic profiles that better match daily schedules, as well as improvements in the way insulin is delivered. Insulin pens, smaller needles, disposable devices, and insulin pumps have made it easier to administer and fine-tune insulin delivery. Despite these improvements and recommendations for earlier implementation, the use of insulin in type 2 diabetes is significantly lower today than in the 1990s.12
When to introduce insulin
Insulin is indicated for patients with type 2 diabetes whose disease is not easily controlled. That includes individuals with decompensated type 2 diabetes, those whose HbA1c remains high even with 2 or more oral agents, and individuals who have not reached goal after a year of treatment.
Glucose toxicity. It is generally agreed that insulin is the most effective treatment for patients who present with decompensated type 2 diabetes4—ie, with significant hyperglycemia and catabolic symptoms such as polydipsia, polyuria, and weight loss. Initiation of insulin promotes reversal of glucose toxicity and stabilization of metabolic status. In such cases, insulin can be started at a low dose to expose the patient to the complexities of injection therapy (more about this in a bit), then titrated as needed for stabilization.
HbA1c ≥8% even with 2 or more drugs. In my experience, an oral diabetes drug will lead to a drop in HbA1c of about one percentage point. Generally, the further from goal the patient is, the greater the effect the medication will have. As HbA1c inches closer to 7%, the effect diminishes. And when 2 oral agents fail to lower a patient’s HbA1c adequately, the incremental change expected from the addition of a third, fourth, or fifth agent is small.
Thus, in a patient with an HbA1c ≥8%, there is still a significant fasting hyperglycemic component. In such a case, a basal insulin is likely the best treatment option.
Not at goal at one year. Both the AACE and the ADA/EASD guidelines agree that treatment titration should be considered every 2 to 3 months to achieve metabolic control and that if a patient is not at goal after a year, insulin should be started.3,4 However, traditionally this is not done. The delayed implementation of this recommendation is an example of clinical inertia, which can contribute to further misunderstandings about the role and effect of insulin therapy.
Getting started with basal insulin
Most patients who are started on insulin have global hyperglycemia. But because fasting hyperglycemia can affect pancreatic insulin secretion, it is important to get control of the fasting glucose first. This can often be done with insulin sensitizers (metformin, thiazolidinediones, and incretin-based agents).
Suppression of excessive hepatic glucose production, which is very common in type 2 diabetes, is one of the biggest challenges in normalizing fasting glucose. This is well managed with a basal insulin. When starting basal insulin, however, it is critical that current treatments not be stopped. Oral agents such as sulfonylureas and meglitinides can be reduced to lower the risk of hypoglycemia, but stopping them altogether will only prolong the time it takes to get to goal.
There are 3 insulin formulations that can serve as basal insulin (TABLE 1).13 Neutral protamine Hagedorn (NPH) is a human insulin that can be used 2 to 3 times daily to provide basal insulin coverage. But long-acting basal analog insulins glargine and detemir, typically administered once a day when used by patients with type 2 diabetes, are a better option.14
While all 3 formulations have similar efficacy for lowering HbA1c, the analog basal insulins have numerous advantages: less weight gain, less hypoglycemia for the same level of glucose control, and less frequent dosing. In addition, glargine and detemir are available in a pen or vial, while generic NPH is available only in a vial. The primary disadvantage of the analogs is cost: A month’s supply—one vial—of NPH sells for approximately $25 (generic) or $94 (brand name); in comparison, a month’s supply (one box of 5 3-mL pens) of detemir and glargine costs about $300 and $320, respectively.15 (Humulin N, a brand-name NPH, is available in a pen, at a cost of approximately $315 per box.)
Use a weight-based initial dose
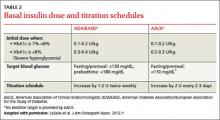
The recommended starting dose is 0.1 to 0.2 U/kg daily for patients with an HbA1c <8%. If HbA1c is ≥8%, the ADA/EASD guidelines recommend a starting dose of 0.3 to 0.4 U/kg daily4(TABLE 2).3,4,16 While basal insulin is most commonly dosed at bedtime, in fact, basal analog insulins can be given at any time that’s convenient for the patient. Morning dosing may be preferable for individuals with a significant fear of hypoglycemia—a phobia that sometimes causes patients to skip insulin doses and engage in “defensive eating” (ie, eating in an attempt to prevent hypoglycemia rather than because of hunger or the need for nutrition).
Teach injection technique
It is critically important that patients get the first shot in the office, guided by a clinician who can teach proper injection technique. This also helps to dispel the apprehension of self-injection.
In addition to being surprised at how easy and painless injection can be, patients have the opportunity to observe the results and gain confidence in insulin’s efficacy. And, in my experience, adherence to an insulin regimen is much greater if the first injection is administered in an office setting.
(Tech-savvy patients may find it helpful to use a smartphone app, such as Glucose Buddy or Dbees.com, to help manage their diabetes. See “The 13 best diabetes iPhone & Android apps of 2013” at http://www.healthline.com/health-slideshow/top-iphone-android-apps-diabetes.)
Establish a titration schedule
It is important, too, to teach the patient how to titrate the insulin dose from the start, rather than waiting until the next visit to address this. Patient titration—facilitated by a clinician-provided titration schedule (available from the AACE and the ADA/EASD3,4)—has been shown to achieve target glucose levels faster than physician titration.17
I usually suggest that patients increase the basal insulin dose by 3 units every 3 days, with an upper limit of 0.5 U/kg/d, until fasting glucose is consistently between 100 and 150 mg/dL. I advise every patient who starts taking insulin to track morning readings and titrate the dose until one of 3 things occurs:
1) the 0.5 U/kg/d limit is reached;
2) the patient has a glucose reading <100 mg/dL; or
3) the patient achieves his or her HbA1c target (<7% for most patients).
In every case, I recommend that the patient call my office for further instruction.
If the patient has any low glucose readings, I reduce the basal insulin by 5 U/kg/d. If he or she is still above goal, I advise the patient to continue titration, but more slowly. If the patient is at goal, I advise continuing at the current dose.
Basal titration vs mealtime coverage. Most people with type 2 diabetes require between 0.2 and 1 U/kg of basal insulin daily. It is currently recommended that when a patient has titrated to a dose of 0.5 U/kg/d, it is time to look at the glucose pattern to determine whether further titrating basal insulin or addressing prandial hyperglycemia should be the next step.4,18 This requires a change in fingerstick pattern.
The patient can stop the first morning glucose check and start checking before meals and 90 to 120 minutes postmeal. This allows for exploration of the mealtime excursion. Generally, a difference of <50 mg/dL is preferred. If the morning glucose level is at target but HbA1c is high, it is likely that postprandial glucose is contributing to this difference. This is particularly true when the HbA1c is between 7% and 8%. If the glucose pattern shows high postmeal glucose readings, it is much safer to address mealtime insulin (not discussed in this article) than to continue to titrate the basal insulin.4,18
Avoid “overbasalization”—ie, titrating basal insulin beyond its normal role to suppress hepatic glucose production and get the fasting glucose to goal. Doing so puts the patient at risk for unexpected hypoglycemia, as the insulin will now try to overcome hyperglycemia with meals, as well. Basal insulins are not designed to meet insulin requirements at meals. If a patient misses a meal yet continues the same dose of basal insulin, the risk of a hypoglycemic episode increases substantially.
In the pipeline. There are a number of new basal insulins in development, including one that has a prolonged duration of action and the potential for every-other-day injections19 and another that uses an attached polyethylene glycol moiety to slow absorption and prolong its effect.20
The nuts and bolts of insulin prescribing
When you prescribe insulin, there are a number of components to consider.
Pen or vial? In addition to deciding whether to order pen or vial, it is essential to consider the volume of insulin needed. Glargine, detemir, and Humulin N are available in 10 mL vials (100 U/mL) and in 3 mL pens (100 U/mL). (Generic NPH is available in vials only.) Most patients prefer insulin pens, which are more convenient and easier to use than a vial and syringe.
The choice also depends on the dosage, however. A patient on a daily dose of 45 units would need one box of 5 pens (each prefilled pen has a 3 mL, or 300 unit, capacity) to have sufficient insulin for a month. Vials would be preferable for an individual who requires a larger single dose than a pen can dispense at one time (80 units of glargine, 60 units of detemir).
Syringe and needle size. If you are ordering insulin vials, you will also need to specify the correct syringe—available in 0.3 mL (which holds 30 units), 0.5 mL (50 units), and 1 mL (100 units) sizes. If the patient requires <50 units, order a small syringe to ensure that the unit markings are clear; a 1 mL syringe is preferable for those using a larger volume of insulin. Order the smallest syringe, which also has half-unit markings, if the patient is a child.
All needles are fine, with a 29 to 31 gauge, and available in regular (12.7 mm), short (8 mm), mini (5 mm), and nano (4 mm). Recent studies have shown that absorption, safety, and adverse events are similar for all needle lengths across a variety of patient factors,21 but patients generally prefer shorter needles.
Remember, too, to specify the maximum daily dose of insulin—a consideration that will be more important when prescribing mealtime insulin but is worth mentioning here.
Finally, tell patients who are getting started on insulin about www.accurateinsulin.org. Hosted by The Endocrine Society in partnership with the American Association of Diabetes Educators, ADA, American Pharmacists Association, and American College of Osteopathic Family Physicians, among other clinical groups, the Web site is designed to help patients (as well as providers) navigate the initiation and adjustment of insulin.18
CORRESPONDENCE
Jay Shubrook, DO, FAAFP, FACOFP, The Diabetes Institute at Ohio University, Athens, OH 45701; [email protected]
1. 2011 CDC National Diabetes Fact Sheet. Centers for Disease Control and Prevention Web site. Available at http://www.cdc.gov/diabetes/pubs/factsheet11.htm. Accessed January 3, 2014.
2. UKPDS Group. Intensive blood glucose control with sulphonylurea or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
3. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19:327-336.
4. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient centered approach. A position statement of the ADA and the EASD. Diabetes Care. 2012;35:1364-1379.
5. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535-1540.
6. Shah BR, Hux JE, Laupacis A, et al. Clinical inertia in response to inadequate glycemic control. Diabetes Care. 2005;28:600-606.
7. Parchman ML, Wang CP. Initiation of insulin among veterans with type 2 diabetes and sustained elevation of HbA1c. Primary Care Diabetes. 2012;6:19-25.
8. Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673-2679.
9. Polonsky WH, Hajos TR, Dain MP, et al. Are patients with type 2 diabetes reluctant to start insulin therapy? An examination of the scope and underpinnings of psychological insulin resistance in a large international trial. Curr Med Res Opin. 2011;27:1169-1174.
10. Kunt T, Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. Int J Clin Pract Suppl. 2009;(164):6-10.
11. Presswala LS, Shubrook JH. Intensive insulin therapy as the primary treatment of type 2 diabetes. Clin Diabetes. 2011;29:151-153.
12. Li, C, Ford ES, Zhao G, et al. Trends of insulin use among US adults with type 2 diabetes: the Behavioral Risk Factor Surveillance System, 1995-2007. J Diabetes Complications. 2012;12:17-22.
13. Monthly Prescribing Reference (MPR). Insulin. Available at: http://www.empr.com/insulins/article/123739/. Accessed January 10, 2014.
14. Monami M, Marchionni N, Mannucci E. Long acting insulin analogs vs. NPH human insulin in Type 1 diabetes. A metaanalysis. Diabetes Obes Metab. 2009;11:372-378.
15. Goodrx Web site. Available at: www.goodrx.com. Accessed January 4, 2014.
16. LaSalle JR, Berria R. Insulin therapy in type 2 diabetes: a practical approach for primary care physicians and other health professionals. J Am Osteopath Assoc. 2013;113:152-162.
17. Davies M, Storms F, Shutler S, et al; ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282-1288.
18. Accurate Insulin Decisions. The Endocrine Society Web site. Available at: http://www.accurateinsulin.org/. Accessed January 4, 2014.
19. Keating GM. Insulin degludec and insulin degludec/insulin aspart: a review of their use in the management of diabetes mellitus. Drugs. 2013;73:575-593.
20. Bergenstal RM, Rosenstock J, Arakaki RF, et al. A randomized, controlled study of once –daily LY2605541, a novel long acting basal insulin, versus insulin glargine in basal Insulin treated with patients in type 2 diabetes. Diabetes Care. 2012;35:2140-2147.
21. Hirsch LJ, Gibney MA, Li L, et al. Glycemic control, reported pain and leakage with a 4 mm × 32 G pen needle in obese and non-obese adults with diabetes: a post hoc analysis. Curr Med Res Opin. 2012;28:1305-1311.
› Initiate insulin for patients whose hemoglobin A1c ≥8% despite taking 2 or more oral agents. C
› Prescribe insulin for patients who have not reached their goal one year after diagnosis and initiation of oral therapy. C
› Consider reducing—but do not discontinue—oral agents, such as sulfonylureas and meglitinides, when you initiate insulin therapy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With type 2 diabetes now affecting 8.3% of the US population, most primary care physicians see patients with this disorder every day.1 Based on the concurrent obesity epidemic, aging population, and emergence of type 2 diabetes in children and adolescents, it is estimated that by 2050, the prevalence will have risen from one in 12 Americans to one in 3.1
Type 2 diabetes is a progressive disorder, with a relentless decline in beta cells. By the time of diagnosis, patients typically have lost at least 50% of insulin secretion; within 6 years of diagnosis, insulin secretion decreases to less than 25%.2
The American Association of Clinical Endocrinologists (AACE)3 and the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD)4 have recently published guidelines for the management of type 2 diabetes. While the AACE’s guidelines (available at https://www.aace.com/files/aace_algorithm.pdf) focus on different treatments at different stages of disease and both glycemic and nonglycemic benefits of treatment,3 the ADA/EASD’s guidelines (see http://care.diabetesjournals.org/content/early/2012/04/17/dc12-0413.full.pdf+html) emphasize a patient-centered approach, shared decision making, and individualization of treatment goals based on both patient preference and comorbid disease states.4
One thing both sets of guidelines have in common is a purposeful intensification of therapy every 2 to 3 months, as needed, and the introduction of insulin one year after diagnosis if the patient is still not at goal.3,4 But all too often, this does not occur, particularly in primary care settings.
This article will review the “when” and “how” of insulin initiation. But first, a look at barriers to insulin therapy and evidence in support of earlier use.
Clinical inertia and patient fear are associated with delays
Both the AACE and the ADA/EASD guidelines agree that metformin is best used as early as possible.5,6 With typical use, however, metformin fails to prevent the progression of diabetes, as measured by the climb of hemoglobin A1c (HbA1c), at a failure rate of about 17% of patients per year.5 Physicians have been slow to intensify treatment for type 2 diabetes6—a phenomenon referred to as clinical inertia.
Typically, physicians adopt a stepwise approach, which often results in patients spending more than 10 years with an HbA1c >7% and 5 years with an HbA1c >8% before insulin is started.5 In a recent Veterans Administration study, patients were out of control, with an HbA1c >8%, for an average of 4.6 years before insulin was initiated.7
Both patient and physician factors contribute to the delay. Patient factors include the fear of injection, the belief that insulin will interfere with their lifestyle, and the idea that the use of insulin signifies impending complications or even death.8 But such beliefs are starting to change. In a recent multinational study of patients with type 2 diabetes, less than 20% stated they were unwilling to start insulin.9
For their part, primary care physicians are much less likely to prescribe insulin than clinicians specializing in diabetes.6 Physician-reported barriers to insulin initiation include the time required to train patients to use it correctly; the lack of support, including access to diabetes educators; and the absence of clear guidelines on the use of insulin.10
A case for earlier insulin
There has been recent momentum in favor of earlier initiation of insulin. In fact, some researchers regard intensive insulin as an excellent first treatment for type 2 diabetes,11 based on the belief that early insulin (used for a brief time) can provide not only immediate improvement in glucose control, but also a lasting “legacy” effect. The ADA/EASD guidelines support the use of insulin as a first-line treatment for patients with symptoms of insulin deficiency,4 but do not recommend it for everyone with newly diagnosed type 2 diabetes.
There have also been a number of advances in insulin therapy over the past 2 decades. These include insulin analogs with physiologic profiles that better match daily schedules, as well as improvements in the way insulin is delivered. Insulin pens, smaller needles, disposable devices, and insulin pumps have made it easier to administer and fine-tune insulin delivery. Despite these improvements and recommendations for earlier implementation, the use of insulin in type 2 diabetes is significantly lower today than in the 1990s.12
When to introduce insulin
Insulin is indicated for patients with type 2 diabetes whose disease is not easily controlled. That includes individuals with decompensated type 2 diabetes, those whose HbA1c remains high even with 2 or more oral agents, and individuals who have not reached goal after a year of treatment.
Glucose toxicity. It is generally agreed that insulin is the most effective treatment for patients who present with decompensated type 2 diabetes4—ie, with significant hyperglycemia and catabolic symptoms such as polydipsia, polyuria, and weight loss. Initiation of insulin promotes reversal of glucose toxicity and stabilization of metabolic status. In such cases, insulin can be started at a low dose to expose the patient to the complexities of injection therapy (more about this in a bit), then titrated as needed for stabilization.
HbA1c ≥8% even with 2 or more drugs. In my experience, an oral diabetes drug will lead to a drop in HbA1c of about one percentage point. Generally, the further from goal the patient is, the greater the effect the medication will have. As HbA1c inches closer to 7%, the effect diminishes. And when 2 oral agents fail to lower a patient’s HbA1c adequately, the incremental change expected from the addition of a third, fourth, or fifth agent is small.
Thus, in a patient with an HbA1c ≥8%, there is still a significant fasting hyperglycemic component. In such a case, a basal insulin is likely the best treatment option.
Not at goal at one year. Both the AACE and the ADA/EASD guidelines agree that treatment titration should be considered every 2 to 3 months to achieve metabolic control and that if a patient is not at goal after a year, insulin should be started.3,4 However, traditionally this is not done. The delayed implementation of this recommendation is an example of clinical inertia, which can contribute to further misunderstandings about the role and effect of insulin therapy.
Getting started with basal insulin
Most patients who are started on insulin have global hyperglycemia. But because fasting hyperglycemia can affect pancreatic insulin secretion, it is important to get control of the fasting glucose first. This can often be done with insulin sensitizers (metformin, thiazolidinediones, and incretin-based agents).
Suppression of excessive hepatic glucose production, which is very common in type 2 diabetes, is one of the biggest challenges in normalizing fasting glucose. This is well managed with a basal insulin. When starting basal insulin, however, it is critical that current treatments not be stopped. Oral agents such as sulfonylureas and meglitinides can be reduced to lower the risk of hypoglycemia, but stopping them altogether will only prolong the time it takes to get to goal.
There are 3 insulin formulations that can serve as basal insulin (TABLE 1).13 Neutral protamine Hagedorn (NPH) is a human insulin that can be used 2 to 3 times daily to provide basal insulin coverage. But long-acting basal analog insulins glargine and detemir, typically administered once a day when used by patients with type 2 diabetes, are a better option.14
While all 3 formulations have similar efficacy for lowering HbA1c, the analog basal insulins have numerous advantages: less weight gain, less hypoglycemia for the same level of glucose control, and less frequent dosing. In addition, glargine and detemir are available in a pen or vial, while generic NPH is available only in a vial. The primary disadvantage of the analogs is cost: A month’s supply—one vial—of NPH sells for approximately $25 (generic) or $94 (brand name); in comparison, a month’s supply (one box of 5 3-mL pens) of detemir and glargine costs about $300 and $320, respectively.15 (Humulin N, a brand-name NPH, is available in a pen, at a cost of approximately $315 per box.)
Use a weight-based initial dose
The recommended starting dose is 0.1 to 0.2 U/kg daily for patients with an HbA1c <8%. If HbA1c is ≥8%, the ADA/EASD guidelines recommend a starting dose of 0.3 to 0.4 U/kg daily4(TABLE 2).3,4,16 While basal insulin is most commonly dosed at bedtime, in fact, basal analog insulins can be given at any time that’s convenient for the patient. Morning dosing may be preferable for individuals with a significant fear of hypoglycemia—a phobia that sometimes causes patients to skip insulin doses and engage in “defensive eating” (ie, eating in an attempt to prevent hypoglycemia rather than because of hunger or the need for nutrition).
Teach injection technique
It is critically important that patients get the first shot in the office, guided by a clinician who can teach proper injection technique. This also helps to dispel the apprehension of self-injection.
In addition to being surprised at how easy and painless injection can be, patients have the opportunity to observe the results and gain confidence in insulin’s efficacy. And, in my experience, adherence to an insulin regimen is much greater if the first injection is administered in an office setting.
(Tech-savvy patients may find it helpful to use a smartphone app, such as Glucose Buddy or Dbees.com, to help manage their diabetes. See “The 13 best diabetes iPhone & Android apps of 2013” at http://www.healthline.com/health-slideshow/top-iphone-android-apps-diabetes.)
Establish a titration schedule
It is important, too, to teach the patient how to titrate the insulin dose from the start, rather than waiting until the next visit to address this. Patient titration—facilitated by a clinician-provided titration schedule (available from the AACE and the ADA/EASD3,4)—has been shown to achieve target glucose levels faster than physician titration.17
I usually suggest that patients increase the basal insulin dose by 3 units every 3 days, with an upper limit of 0.5 U/kg/d, until fasting glucose is consistently between 100 and 150 mg/dL. I advise every patient who starts taking insulin to track morning readings and titrate the dose until one of 3 things occurs:
1) the 0.5 U/kg/d limit is reached;
2) the patient has a glucose reading <100 mg/dL; or
3) the patient achieves his or her HbA1c target (<7% for most patients).
In every case, I recommend that the patient call my office for further instruction.
If the patient has any low glucose readings, I reduce the basal insulin by 5 U/kg/d. If he or she is still above goal, I advise the patient to continue titration, but more slowly. If the patient is at goal, I advise continuing at the current dose.
Basal titration vs mealtime coverage. Most people with type 2 diabetes require between 0.2 and 1 U/kg of basal insulin daily. It is currently recommended that when a patient has titrated to a dose of 0.5 U/kg/d, it is time to look at the glucose pattern to determine whether further titrating basal insulin or addressing prandial hyperglycemia should be the next step.4,18 This requires a change in fingerstick pattern.
The patient can stop the first morning glucose check and start checking before meals and 90 to 120 minutes postmeal. This allows for exploration of the mealtime excursion. Generally, a difference of <50 mg/dL is preferred. If the morning glucose level is at target but HbA1c is high, it is likely that postprandial glucose is contributing to this difference. This is particularly true when the HbA1c is between 7% and 8%. If the glucose pattern shows high postmeal glucose readings, it is much safer to address mealtime insulin (not discussed in this article) than to continue to titrate the basal insulin.4,18
Avoid “overbasalization”—ie, titrating basal insulin beyond its normal role to suppress hepatic glucose production and get the fasting glucose to goal. Doing so puts the patient at risk for unexpected hypoglycemia, as the insulin will now try to overcome hyperglycemia with meals, as well. Basal insulins are not designed to meet insulin requirements at meals. If a patient misses a meal yet continues the same dose of basal insulin, the risk of a hypoglycemic episode increases substantially.
In the pipeline. There are a number of new basal insulins in development, including one that has a prolonged duration of action and the potential for every-other-day injections19 and another that uses an attached polyethylene glycol moiety to slow absorption and prolong its effect.20
The nuts and bolts of insulin prescribing
When you prescribe insulin, there are a number of components to consider.
Pen or vial? In addition to deciding whether to order pen or vial, it is essential to consider the volume of insulin needed. Glargine, detemir, and Humulin N are available in 10 mL vials (100 U/mL) and in 3 mL pens (100 U/mL). (Generic NPH is available in vials only.) Most patients prefer insulin pens, which are more convenient and easier to use than a vial and syringe.
The choice also depends on the dosage, however. A patient on a daily dose of 45 units would need one box of 5 pens (each prefilled pen has a 3 mL, or 300 unit, capacity) to have sufficient insulin for a month. Vials would be preferable for an individual who requires a larger single dose than a pen can dispense at one time (80 units of glargine, 60 units of detemir).
Syringe and needle size. If you are ordering insulin vials, you will also need to specify the correct syringe—available in 0.3 mL (which holds 30 units), 0.5 mL (50 units), and 1 mL (100 units) sizes. If the patient requires <50 units, order a small syringe to ensure that the unit markings are clear; a 1 mL syringe is preferable for those using a larger volume of insulin. Order the smallest syringe, which also has half-unit markings, if the patient is a child.
All needles are fine, with a 29 to 31 gauge, and available in regular (12.7 mm), short (8 mm), mini (5 mm), and nano (4 mm). Recent studies have shown that absorption, safety, and adverse events are similar for all needle lengths across a variety of patient factors,21 but patients generally prefer shorter needles.
Remember, too, to specify the maximum daily dose of insulin—a consideration that will be more important when prescribing mealtime insulin but is worth mentioning here.
Finally, tell patients who are getting started on insulin about www.accurateinsulin.org. Hosted by The Endocrine Society in partnership with the American Association of Diabetes Educators, ADA, American Pharmacists Association, and American College of Osteopathic Family Physicians, among other clinical groups, the Web site is designed to help patients (as well as providers) navigate the initiation and adjustment of insulin.18
CORRESPONDENCE
Jay Shubrook, DO, FAAFP, FACOFP, The Diabetes Institute at Ohio University, Athens, OH 45701; [email protected]
› Initiate insulin for patients whose hemoglobin A1c ≥8% despite taking 2 or more oral agents. C
› Prescribe insulin for patients who have not reached their goal one year after diagnosis and initiation of oral therapy. C
› Consider reducing—but do not discontinue—oral agents, such as sulfonylureas and meglitinides, when you initiate insulin therapy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With type 2 diabetes now affecting 8.3% of the US population, most primary care physicians see patients with this disorder every day.1 Based on the concurrent obesity epidemic, aging population, and emergence of type 2 diabetes in children and adolescents, it is estimated that by 2050, the prevalence will have risen from one in 12 Americans to one in 3.1
Type 2 diabetes is a progressive disorder, with a relentless decline in beta cells. By the time of diagnosis, patients typically have lost at least 50% of insulin secretion; within 6 years of diagnosis, insulin secretion decreases to less than 25%.2
The American Association of Clinical Endocrinologists (AACE)3 and the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD)4 have recently published guidelines for the management of type 2 diabetes. While the AACE’s guidelines (available at https://www.aace.com/files/aace_algorithm.pdf) focus on different treatments at different stages of disease and both glycemic and nonglycemic benefits of treatment,3 the ADA/EASD’s guidelines (see http://care.diabetesjournals.org/content/early/2012/04/17/dc12-0413.full.pdf+html) emphasize a patient-centered approach, shared decision making, and individualization of treatment goals based on both patient preference and comorbid disease states.4
One thing both sets of guidelines have in common is a purposeful intensification of therapy every 2 to 3 months, as needed, and the introduction of insulin one year after diagnosis if the patient is still not at goal.3,4 But all too often, this does not occur, particularly in primary care settings.
This article will review the “when” and “how” of insulin initiation. But first, a look at barriers to insulin therapy and evidence in support of earlier use.
Clinical inertia and patient fear are associated with delays
Both the AACE and the ADA/EASD guidelines agree that metformin is best used as early as possible.5,6 With typical use, however, metformin fails to prevent the progression of diabetes, as measured by the climb of hemoglobin A1c (HbA1c), at a failure rate of about 17% of patients per year.5 Physicians have been slow to intensify treatment for type 2 diabetes6—a phenomenon referred to as clinical inertia.
Typically, physicians adopt a stepwise approach, which often results in patients spending more than 10 years with an HbA1c >7% and 5 years with an HbA1c >8% before insulin is started.5 In a recent Veterans Administration study, patients were out of control, with an HbA1c >8%, for an average of 4.6 years before insulin was initiated.7
Both patient and physician factors contribute to the delay. Patient factors include the fear of injection, the belief that insulin will interfere with their lifestyle, and the idea that the use of insulin signifies impending complications or even death.8 But such beliefs are starting to change. In a recent multinational study of patients with type 2 diabetes, less than 20% stated they were unwilling to start insulin.9
For their part, primary care physicians are much less likely to prescribe insulin than clinicians specializing in diabetes.6 Physician-reported barriers to insulin initiation include the time required to train patients to use it correctly; the lack of support, including access to diabetes educators; and the absence of clear guidelines on the use of insulin.10
A case for earlier insulin
There has been recent momentum in favor of earlier initiation of insulin. In fact, some researchers regard intensive insulin as an excellent first treatment for type 2 diabetes,11 based on the belief that early insulin (used for a brief time) can provide not only immediate improvement in glucose control, but also a lasting “legacy” effect. The ADA/EASD guidelines support the use of insulin as a first-line treatment for patients with symptoms of insulin deficiency,4 but do not recommend it for everyone with newly diagnosed type 2 diabetes.
There have also been a number of advances in insulin therapy over the past 2 decades. These include insulin analogs with physiologic profiles that better match daily schedules, as well as improvements in the way insulin is delivered. Insulin pens, smaller needles, disposable devices, and insulin pumps have made it easier to administer and fine-tune insulin delivery. Despite these improvements and recommendations for earlier implementation, the use of insulin in type 2 diabetes is significantly lower today than in the 1990s.12
When to introduce insulin
Insulin is indicated for patients with type 2 diabetes whose disease is not easily controlled. That includes individuals with decompensated type 2 diabetes, those whose HbA1c remains high even with 2 or more oral agents, and individuals who have not reached goal after a year of treatment.
Glucose toxicity. It is generally agreed that insulin is the most effective treatment for patients who present with decompensated type 2 diabetes4—ie, with significant hyperglycemia and catabolic symptoms such as polydipsia, polyuria, and weight loss. Initiation of insulin promotes reversal of glucose toxicity and stabilization of metabolic status. In such cases, insulin can be started at a low dose to expose the patient to the complexities of injection therapy (more about this in a bit), then titrated as needed for stabilization.
HbA1c ≥8% even with 2 or more drugs. In my experience, an oral diabetes drug will lead to a drop in HbA1c of about one percentage point. Generally, the further from goal the patient is, the greater the effect the medication will have. As HbA1c inches closer to 7%, the effect diminishes. And when 2 oral agents fail to lower a patient’s HbA1c adequately, the incremental change expected from the addition of a third, fourth, or fifth agent is small.
Thus, in a patient with an HbA1c ≥8%, there is still a significant fasting hyperglycemic component. In such a case, a basal insulin is likely the best treatment option.
Not at goal at one year. Both the AACE and the ADA/EASD guidelines agree that treatment titration should be considered every 2 to 3 months to achieve metabolic control and that if a patient is not at goal after a year, insulin should be started.3,4 However, traditionally this is not done. The delayed implementation of this recommendation is an example of clinical inertia, which can contribute to further misunderstandings about the role and effect of insulin therapy.
Getting started with basal insulin
Most patients who are started on insulin have global hyperglycemia. But because fasting hyperglycemia can affect pancreatic insulin secretion, it is important to get control of the fasting glucose first. This can often be done with insulin sensitizers (metformin, thiazolidinediones, and incretin-based agents).
Suppression of excessive hepatic glucose production, which is very common in type 2 diabetes, is one of the biggest challenges in normalizing fasting glucose. This is well managed with a basal insulin. When starting basal insulin, however, it is critical that current treatments not be stopped. Oral agents such as sulfonylureas and meglitinides can be reduced to lower the risk of hypoglycemia, but stopping them altogether will only prolong the time it takes to get to goal.
There are 3 insulin formulations that can serve as basal insulin (TABLE 1).13 Neutral protamine Hagedorn (NPH) is a human insulin that can be used 2 to 3 times daily to provide basal insulin coverage. But long-acting basal analog insulins glargine and detemir, typically administered once a day when used by patients with type 2 diabetes, are a better option.14
While all 3 formulations have similar efficacy for lowering HbA1c, the analog basal insulins have numerous advantages: less weight gain, less hypoglycemia for the same level of glucose control, and less frequent dosing. In addition, glargine and detemir are available in a pen or vial, while generic NPH is available only in a vial. The primary disadvantage of the analogs is cost: A month’s supply—one vial—of NPH sells for approximately $25 (generic) or $94 (brand name); in comparison, a month’s supply (one box of 5 3-mL pens) of detemir and glargine costs about $300 and $320, respectively.15 (Humulin N, a brand-name NPH, is available in a pen, at a cost of approximately $315 per box.)
Use a weight-based initial dose
The recommended starting dose is 0.1 to 0.2 U/kg daily for patients with an HbA1c <8%. If HbA1c is ≥8%, the ADA/EASD guidelines recommend a starting dose of 0.3 to 0.4 U/kg daily4(TABLE 2).3,4,16 While basal insulin is most commonly dosed at bedtime, in fact, basal analog insulins can be given at any time that’s convenient for the patient. Morning dosing may be preferable for individuals with a significant fear of hypoglycemia—a phobia that sometimes causes patients to skip insulin doses and engage in “defensive eating” (ie, eating in an attempt to prevent hypoglycemia rather than because of hunger or the need for nutrition).
Teach injection technique
It is critically important that patients get the first shot in the office, guided by a clinician who can teach proper injection technique. This also helps to dispel the apprehension of self-injection.
In addition to being surprised at how easy and painless injection can be, patients have the opportunity to observe the results and gain confidence in insulin’s efficacy. And, in my experience, adherence to an insulin regimen is much greater if the first injection is administered in an office setting.
(Tech-savvy patients may find it helpful to use a smartphone app, such as Glucose Buddy or Dbees.com, to help manage their diabetes. See “The 13 best diabetes iPhone & Android apps of 2013” at http://www.healthline.com/health-slideshow/top-iphone-android-apps-diabetes.)
Establish a titration schedule
It is important, too, to teach the patient how to titrate the insulin dose from the start, rather than waiting until the next visit to address this. Patient titration—facilitated by a clinician-provided titration schedule (available from the AACE and the ADA/EASD3,4)—has been shown to achieve target glucose levels faster than physician titration.17
I usually suggest that patients increase the basal insulin dose by 3 units every 3 days, with an upper limit of 0.5 U/kg/d, until fasting glucose is consistently between 100 and 150 mg/dL. I advise every patient who starts taking insulin to track morning readings and titrate the dose until one of 3 things occurs:
1) the 0.5 U/kg/d limit is reached;
2) the patient has a glucose reading <100 mg/dL; or
3) the patient achieves his or her HbA1c target (<7% for most patients).
In every case, I recommend that the patient call my office for further instruction.
If the patient has any low glucose readings, I reduce the basal insulin by 5 U/kg/d. If he or she is still above goal, I advise the patient to continue titration, but more slowly. If the patient is at goal, I advise continuing at the current dose.
Basal titration vs mealtime coverage. Most people with type 2 diabetes require between 0.2 and 1 U/kg of basal insulin daily. It is currently recommended that when a patient has titrated to a dose of 0.5 U/kg/d, it is time to look at the glucose pattern to determine whether further titrating basal insulin or addressing prandial hyperglycemia should be the next step.4,18 This requires a change in fingerstick pattern.
The patient can stop the first morning glucose check and start checking before meals and 90 to 120 minutes postmeal. This allows for exploration of the mealtime excursion. Generally, a difference of <50 mg/dL is preferred. If the morning glucose level is at target but HbA1c is high, it is likely that postprandial glucose is contributing to this difference. This is particularly true when the HbA1c is between 7% and 8%. If the glucose pattern shows high postmeal glucose readings, it is much safer to address mealtime insulin (not discussed in this article) than to continue to titrate the basal insulin.4,18
Avoid “overbasalization”—ie, titrating basal insulin beyond its normal role to suppress hepatic glucose production and get the fasting glucose to goal. Doing so puts the patient at risk for unexpected hypoglycemia, as the insulin will now try to overcome hyperglycemia with meals, as well. Basal insulins are not designed to meet insulin requirements at meals. If a patient misses a meal yet continues the same dose of basal insulin, the risk of a hypoglycemic episode increases substantially.
In the pipeline. There are a number of new basal insulins in development, including one that has a prolonged duration of action and the potential for every-other-day injections19 and another that uses an attached polyethylene glycol moiety to slow absorption and prolong its effect.20
The nuts and bolts of insulin prescribing
When you prescribe insulin, there are a number of components to consider.
Pen or vial? In addition to deciding whether to order pen or vial, it is essential to consider the volume of insulin needed. Glargine, detemir, and Humulin N are available in 10 mL vials (100 U/mL) and in 3 mL pens (100 U/mL). (Generic NPH is available in vials only.) Most patients prefer insulin pens, which are more convenient and easier to use than a vial and syringe.
The choice also depends on the dosage, however. A patient on a daily dose of 45 units would need one box of 5 pens (each prefilled pen has a 3 mL, or 300 unit, capacity) to have sufficient insulin for a month. Vials would be preferable for an individual who requires a larger single dose than a pen can dispense at one time (80 units of glargine, 60 units of detemir).
Syringe and needle size. If you are ordering insulin vials, you will also need to specify the correct syringe—available in 0.3 mL (which holds 30 units), 0.5 mL (50 units), and 1 mL (100 units) sizes. If the patient requires <50 units, order a small syringe to ensure that the unit markings are clear; a 1 mL syringe is preferable for those using a larger volume of insulin. Order the smallest syringe, which also has half-unit markings, if the patient is a child.
All needles are fine, with a 29 to 31 gauge, and available in regular (12.7 mm), short (8 mm), mini (5 mm), and nano (4 mm). Recent studies have shown that absorption, safety, and adverse events are similar for all needle lengths across a variety of patient factors,21 but patients generally prefer shorter needles.
Remember, too, to specify the maximum daily dose of insulin—a consideration that will be more important when prescribing mealtime insulin but is worth mentioning here.
Finally, tell patients who are getting started on insulin about www.accurateinsulin.org. Hosted by The Endocrine Society in partnership with the American Association of Diabetes Educators, ADA, American Pharmacists Association, and American College of Osteopathic Family Physicians, among other clinical groups, the Web site is designed to help patients (as well as providers) navigate the initiation and adjustment of insulin.18
CORRESPONDENCE
Jay Shubrook, DO, FAAFP, FACOFP, The Diabetes Institute at Ohio University, Athens, OH 45701; [email protected]
1. 2011 CDC National Diabetes Fact Sheet. Centers for Disease Control and Prevention Web site. Available at http://www.cdc.gov/diabetes/pubs/factsheet11.htm. Accessed January 3, 2014.
2. UKPDS Group. Intensive blood glucose control with sulphonylurea or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
3. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19:327-336.
4. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient centered approach. A position statement of the ADA and the EASD. Diabetes Care. 2012;35:1364-1379.
5. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535-1540.
6. Shah BR, Hux JE, Laupacis A, et al. Clinical inertia in response to inadequate glycemic control. Diabetes Care. 2005;28:600-606.
7. Parchman ML, Wang CP. Initiation of insulin among veterans with type 2 diabetes and sustained elevation of HbA1c. Primary Care Diabetes. 2012;6:19-25.
8. Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673-2679.
9. Polonsky WH, Hajos TR, Dain MP, et al. Are patients with type 2 diabetes reluctant to start insulin therapy? An examination of the scope and underpinnings of psychological insulin resistance in a large international trial. Curr Med Res Opin. 2011;27:1169-1174.
10. Kunt T, Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. Int J Clin Pract Suppl. 2009;(164):6-10.
11. Presswala LS, Shubrook JH. Intensive insulin therapy as the primary treatment of type 2 diabetes. Clin Diabetes. 2011;29:151-153.
12. Li, C, Ford ES, Zhao G, et al. Trends of insulin use among US adults with type 2 diabetes: the Behavioral Risk Factor Surveillance System, 1995-2007. J Diabetes Complications. 2012;12:17-22.
13. Monthly Prescribing Reference (MPR). Insulin. Available at: http://www.empr.com/insulins/article/123739/. Accessed January 10, 2014.
14. Monami M, Marchionni N, Mannucci E. Long acting insulin analogs vs. NPH human insulin in Type 1 diabetes. A metaanalysis. Diabetes Obes Metab. 2009;11:372-378.
15. Goodrx Web site. Available at: www.goodrx.com. Accessed January 4, 2014.
16. LaSalle JR, Berria R. Insulin therapy in type 2 diabetes: a practical approach for primary care physicians and other health professionals. J Am Osteopath Assoc. 2013;113:152-162.
17. Davies M, Storms F, Shutler S, et al; ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282-1288.
18. Accurate Insulin Decisions. The Endocrine Society Web site. Available at: http://www.accurateinsulin.org/. Accessed January 4, 2014.
19. Keating GM. Insulin degludec and insulin degludec/insulin aspart: a review of their use in the management of diabetes mellitus. Drugs. 2013;73:575-593.
20. Bergenstal RM, Rosenstock J, Arakaki RF, et al. A randomized, controlled study of once –daily LY2605541, a novel long acting basal insulin, versus insulin glargine in basal Insulin treated with patients in type 2 diabetes. Diabetes Care. 2012;35:2140-2147.
21. Hirsch LJ, Gibney MA, Li L, et al. Glycemic control, reported pain and leakage with a 4 mm × 32 G pen needle in obese and non-obese adults with diabetes: a post hoc analysis. Curr Med Res Opin. 2012;28:1305-1311.
1. 2011 CDC National Diabetes Fact Sheet. Centers for Disease Control and Prevention Web site. Available at http://www.cdc.gov/diabetes/pubs/factsheet11.htm. Accessed January 3, 2014.
2. UKPDS Group. Intensive blood glucose control with sulphonylurea or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
3. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19:327-336.
4. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient centered approach. A position statement of the ADA and the EASD. Diabetes Care. 2012;35:1364-1379.
5. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535-1540.
6. Shah BR, Hux JE, Laupacis A, et al. Clinical inertia in response to inadequate glycemic control. Diabetes Care. 2005;28:600-606.
7. Parchman ML, Wang CP. Initiation of insulin among veterans with type 2 diabetes and sustained elevation of HbA1c. Primary Care Diabetes. 2012;6:19-25.
8. Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673-2679.
9. Polonsky WH, Hajos TR, Dain MP, et al. Are patients with type 2 diabetes reluctant to start insulin therapy? An examination of the scope and underpinnings of psychological insulin resistance in a large international trial. Curr Med Res Opin. 2011;27:1169-1174.
10. Kunt T, Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. Int J Clin Pract Suppl. 2009;(164):6-10.
11. Presswala LS, Shubrook JH. Intensive insulin therapy as the primary treatment of type 2 diabetes. Clin Diabetes. 2011;29:151-153.
12. Li, C, Ford ES, Zhao G, et al. Trends of insulin use among US adults with type 2 diabetes: the Behavioral Risk Factor Surveillance System, 1995-2007. J Diabetes Complications. 2012;12:17-22.
13. Monthly Prescribing Reference (MPR). Insulin. Available at: http://www.empr.com/insulins/article/123739/. Accessed January 10, 2014.
14. Monami M, Marchionni N, Mannucci E. Long acting insulin analogs vs. NPH human insulin in Type 1 diabetes. A metaanalysis. Diabetes Obes Metab. 2009;11:372-378.
15. Goodrx Web site. Available at: www.goodrx.com. Accessed January 4, 2014.
16. LaSalle JR, Berria R. Insulin therapy in type 2 diabetes: a practical approach for primary care physicians and other health professionals. J Am Osteopath Assoc. 2013;113:152-162.
17. Davies M, Storms F, Shutler S, et al; ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282-1288.
18. Accurate Insulin Decisions. The Endocrine Society Web site. Available at: http://www.accurateinsulin.org/. Accessed January 4, 2014.
19. Keating GM. Insulin degludec and insulin degludec/insulin aspart: a review of their use in the management of diabetes mellitus. Drugs. 2013;73:575-593.
20. Bergenstal RM, Rosenstock J, Arakaki RF, et al. A randomized, controlled study of once –daily LY2605541, a novel long acting basal insulin, versus insulin glargine in basal Insulin treated with patients in type 2 diabetes. Diabetes Care. 2012;35:2140-2147.
21. Hirsch LJ, Gibney MA, Li L, et al. Glycemic control, reported pain and leakage with a 4 mm × 32 G pen needle in obese and non-obese adults with diabetes: a post hoc analysis. Curr Med Res Opin. 2012;28:1305-1311.